Abstract
There is considerable evidence that the potent vasoconstrictor endothelin-1 (ET-1) contributes to the pathogenesis of a variety of cardiovascular diseases. As such, pharmacological manipulation of the ET system might represent a promising therapeutic goal. Many clinical trials have assessed the potential of ET receptor antagonists in cardiovascular disease, the most positive of which have resulted in the licensing of the mixed ET receptor antagonist bosentan, and the selective ETA receptor antagonists, sitaxsentan and ambrisentan, for the treatment of pulmonary arterial hypertension (PAH). In contrast, despite encouraging data from in vitro and animal studies, outcomes in human heart failure have been disappointing, perhaps illustrating the risk of extrapolating preclinical work to man. Many further potential applications of these compounds, including resistant hypertension, chronic kidney disease, connective tissue disease and sub-arachnoid haemorrhage are currently being investigated in the clinic. Furthermore, experience from previous studies should enable improved trial design and scope remains for development of improved compounds and alternative therapeutic strategies. Although ET-converting enzyme inhibitors may represent one such alternative, there have been relatively few suitable compounds developed, and consequently, clinical experience with these agents remains extremely limited. Recent advances, together with an increased understanding of the biology of the ET system provided by improved experimental tools (including cell-specific transgenic deletion of ET receptors), should allow further targeting of clinical trials to diseases in which ET is involved and allow the therapeutic potential for targeting the ET system in cardiovascular disease to be fully realized.
Keywords: endothelin-1, ECE inhibitors, endothelin receptor antagonists, clinical trials, cardiovascular disease
Introduction
The discovery of endothelin-1 (ET-1) almost 20 years ago (Yanagisawa et al., 1988) was rapidly followed by the realization that its release, perhaps from a dysfunctional endothelium, could have a major role in the pathogenesis of a variety of cardiovascular diseases (reviewed by Haynes and Webb, 1992 and Rubanyi and Polokoff, 1994). This offered the tantalizing prospect that pharmacological manipulation of the ET-1 system might provide powerful new treatments for many clinically significant cardiovascular conditions. Considerable research effort has been invested to elucidate the role of ET-1 in cardiovascular disease and develop pharmacological tools that manipulate its activity, including agents that (1) inhibit generation of ET-1 (Jeng et al., 2002) and (2) block the action of ET-1 (Battistini et al., 2006). The rapid identification of such compounds led remarkably quickly to the development of orally active antagonists (Clozel et al., 1994) and their administration to patients (Kiowski et al., 1995). Additional insight into ET physiology has been gained from studies with selective antagonists in man (Haynes and Webb, 1994) and from knockout and transgenic animals, most dramatically revealing the crucial role of ET-1 in development (Kurihara et al., 1994) and regulation of salt excretion (Ahn et al., 2004; Bagnall et al., 2006; Ge et al., 2006). There is now an extensive literature addressing the therapeutic potential of compounds that target the ET system. This article seeks to determine whether the considerable promise of ET-1 manipulation as a therapeutic option has been realized.
Endothelin-1: nature, synthesis and secretion
In this article, only a concise overview of the ET system is possible. A more complete discussion is available in many excellent recent reviews (Masaki, 2004; Motte et al., 2006). The description of a potent endothelium-derived constricting factor released from cultured endothelial cells (ECs) (Hickey et al., 1985; Gillespie et al., 1986) followed the ground-breaking demonstration that the endothelium, rather than being a simple inert barrier between the blood and vascular wall, controls vascular tone by release of an endothelium-derived relaxing factor (Furchgott and Zawadzki, 1980). The structure, generation and cardiovascular effect of ET-1 were subsequently described in a single remarkable paper (Yanagisawa et al., 1988). It is now established that ET-1 is the principal cardiovascular isoform of a family of 21 amino-acid peptides comprising ET-1, ET-2 and ET-3 (Inoue et al., 1989; Bloch et al., 1991) and of which a proposed fourth member was later shown to be the rodent homologue of human ET-2, rather than a truly novel isoform (Bloch et al., 1991). ETs possess striking sequence homology with four further peptides (sarafotoxins) extracted from the venom of Atractaspis engaddensis (Kloog et al., 1988), which also contain the two disulphide bridges (Figure 1) that give these peptides their characteristic ‘open loop' tertiary structure (Janes et al., 1994).
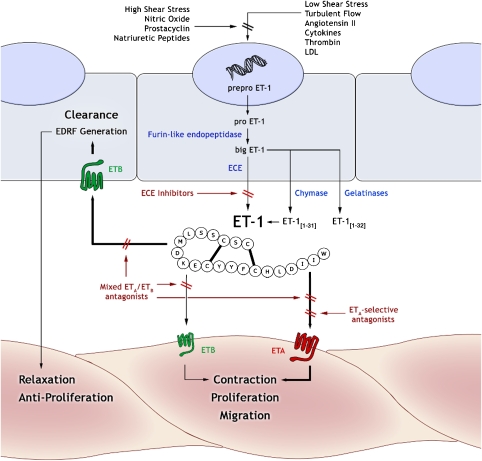
Figure 1.
Generation and action of endothelin-1 (ET-1) in the vascular wall. The 21-amino-acid peptide, ET-1, is the eventual product of a gene on chromosome 6 that encodes preproET-1 protein. This is converted to proET-1 on secretion into the cytoplasm, which itself undergoes enzymatic cleavage by a furin-like endopeptidase to form big ET-1. ET-1 is generated from big ET-1 by endothelin-converting enzymes (ECEs) and is excreted predominantly abluminally into the vessel wall. Alternative enzymatic pathways may also process big ET-1 to yield bioactive 31- and 32-amino acid isoforms. ET-1 stimulates contraction, proliferation and migration of vascular cells by a direct action on VSMC ETA and, to a lesser extent, VSMC ETB receptors. In addition, ET-1 also induces indirect relaxation and anti-proliferation by stimulation of ETB receptors on the endothelium. These actions may be mediated by the release of several distinct dilator compounds (nitric oxide, endothelium-derived hyperpolarizing factor and prostaglandins) but the precise combination of these factors varies between vascular beds. The principal pharmacological approaches in modulating the action of ET-1 are (1) inhibition of synthesis with ECE inhibitors or dual ECE/neutral endopeptidase (NEP) inhibitors and (2) blockade of receptors using antagonists that are selective for either ETA or ETB receptor subtypes or nonselective. EDRF, endothelium-derived relaxing factor.
Endothelin-1 is generated from precursor peptides via a two-step proteolytic pathway (Figure 1). Transcription of a gene on chromosome 6 generates mRNA encoding the 212-amino-acid peptide, preproET-1, which, once translated, is stripped of its signal sequence and secreted into the cytoplasm as proET-1 (Inoue et al., 1989). ProET-1 is further cleaved by a furin-like endopeptidase to the 38-amino-acid precursor molecule big ET-1, which circulates in plasma at low concentration but is not thought to possess significant bioactivity (Yanagisawa et al., 1988). Removal of a further 17 COOH-terminal residues, classically but not exclusively by ET-converting enzymes (ECE), results in formation of the mature 21-amino-acid ET-1 (Hirata et al., 1990).
In humans and rodents, two distinct isozymes of ECE, ECE1 (Schmidt et al., 1994) and ECE2 (Emoto and Yanagisawa, 1995), catalyse the second and rate-limiting step in ET-1 synthesis (Opgenorth et al., 1992). These type II integral membrane proteins have catalytic domains characteristic of zinc metalloproteases and, as such, are closely related to other enzymes of this class, particularly neutral endopeptidase (NEP, EC 3.4.24.11; Ikura et al., 1994). ECE1 has four functionally similar isoforms (ECE1a–d), defined by slight variations in NH2-terminal sequence that determine their discrete sub-cellular localization (Valdenaire et al., 1999) to plasma and intracellular membranes (Russell and Davenport, 1999). Alternative splicing of the ECE1 gene may also give rise to novel variants that exist free in the cytosol (Meidan et al., 2005; Klipper et al., 2006). ECE2 has at least four distinct isoforms but is less well characterized than ECE1 and appears to have a role in the processing of big ET-1 during its transit through intracellular secretory pathways (Harrison et al., 1993). It came as some surprise that ECE1/ECE2 double knockout mice continue to produce significant amounts of ET-1 (Yanagisawa et al., 2000), suggesting substantial redundancy of this pathway exists. Indeed, several other enzymes can cleave big ET-1 to form both ET-1 and isoforms bearing extended COOH-terminal sequences. These include chymase (Wypij et al., 1992) and gelatinolytic matrix metalloproteases (Fernandez-Patron et al., 1999), which mediate the production of ET-1[1−31] and ET-1[1−32], respectively.
In ECs, ET-1 is released through both constitutive and regulated (rapid release) pathways (Russell and Davenport, 1999). Vesicles originating in the trans-Golgi network and Weibel–Palade bodies display immunoreactivity for ET-1, ECE and big ET-1, suggesting that, in addition to their role in transport and storage, they serve as sites of ECE-regulated ET-1 synthesis (Barnes et al., 1998). ET-1 release via the constitutive pathway is regulated principally at the level of gene transcription. Synthesis is enhanced by protein kinase C- and phospholipase C-dependent pathways in response to low-shear stress, turbulent blood flow, hypoxia, cytokines, angiotensin II, adrenaline and low-density lipoprotein. In contrast, increased levels of cyclic nucleotide monophosphates associated with high-shear stress, nitric oxide, vasodilating prostaglandins and natriuretic peptides suppress ET-1 production (Gray and Webb, 1996).
Although the endothelium is the main physiological source of vascular ET-1 in humans and animals (Properzi et al., 1995), it is now recognized that vascular smooth muscle cells (VSMCs), macrophages, leukocytes, cardiomyocytes and fibroblasts are all capable of ET-1 production (Nunez et al., 1990; Resink et al., 1990; Firth and Ratcliffe, 1992). In the kidney, tubular epithelial cells, mesangial cells and podocytes also release ET-1 (Kohan, 1997). Indeed, the kidney contains among the highest ET concentrations of any organ, and in humans, this is principally the ET-1 isoform (Morita et al., 1991).
Clearance of ET-1 from plasma occurs primarily in the lungs (Anggard et al., 1989) by endocytosis and degradation of the ETB receptor-ligand complex (Dupuis et al., 2000). The speed of this clearance, together with the polarized, abluminal nature of ET-1 secretion by ECs emphasize the poor predictive value of plasma ET-1 concentration as a measure of synthesis and has resulted in great difficulty in assessing altered ET-1 activity in disease. EC-specific, but not inner-medullary collecting duct (IMCD) cell-specific, deletion of ETB receptors results in a four-fold increase in plasma ET-1 and impaired clearance of exogenous ET-1, supporting the view that EC ETB receptors contribute much of the capacity for ET-1 clearance (Bagnall et al., 2006; Ge et al., 2006). Enzymatic degradation also contributes to the clearance of ET-1. NEP, probably that located in the brush-border vesicles of the proximal tubule, catabolizes ET-1, and inhibition of this enzyme increases plasma and urinary ET-1 concentrations (Abassi et al., 1992). Indeed, a significant role for the kidney in the clearance of ET-1 is indicated by the demonstration that bilateral nephrectomy impairs removal of exogenous ET-1 in rats (Shi et al., 1994). Recent micro-positron emission tomography studies suggest that renal ET-1 clearance occurs through both receptor-mediated and enzymatic processes and also highlight the liver as a further important site of ET-1 clearance (Johnström et al., 2005).
Endothelin-1: receptors and action in the cardiovascular system
ET receptors
The downstream effects of ET-1 are mediated by two distinct receptor subtypes, ETA and ETB, which belong to the rhodopsin-like G-protein-coupled receptor superfamily. cDNAs encoding both receptors were isolated and cloned within 2 years of the initial characterization of ET-1 and exhibit 59% sequence homology (Arai et al., 1990; Sakurai et al., 1990; Frelin et al., 1991; Sakamoto et al., 1991). Human ETA and ETB bind ET-1 with equal affinity, but ETA possess at least 100-fold less affinity for ET-3 than do ETB receptors (Davenport, 2002). Two additional receptor subtypes identified in dermal melanophores (ETC), and in oocytes and heart (ETAX) of Xenopus laevis, have no mammalian equivalent (Karne et al., 1993; Kumar et al., 1994). Additional classification of the ETB subtype (particularly prompted by the differential sensitivity of specific ETB-mediated functional responses to the antagonist PD142893) has not been supported by detailed kinetic studies or the effects of ETB gene deletion (Gray and Webb, 1996; Mizuguchi et al., 1997). Furthermore, splice variants reported for both subtypes have not been shown to have functional significance (Davenport, 2002).
Endothelin receptors are expressed in a variety of human tissues. In the vasculature, VSMCs express both ETA and ETB. ECs typically express only ETB (Molenaar et al., 1993), although ETA-like receptors have been reported in the endothelium of the human and rat brain microvasculature (Kawai et al., 1997; Spatz et al., 1997). The relative expression of ETA and ETB by VSMCs is variable but the ETA/ETB ratio is often considered to increase with vessel size (Davenport and Maguire, 1994; Tschudi and Luscher, 1994). In the human heart, cardiomyocytes and fibroblasts predominantly express ETA, although ETB expression is more abundant in cardiac-conducting tissue (Molenaar et al., 1993) and non-ETA/ETB receptors for ET-1 have also been described (Burrell et al., 2000). ETB receptors are highly expressed in the renal medulla (Karet and Davenport, 1996; Kuc and Davenport, 2004) on both epithelial and endothelial (vasa recta) cells, in humans and animals. Renal ETA receptors are less abundant and are predominantly found on the larger renal vessels.
Intracellular signal transduction
Stimulation of ETA and ETB elicits diverse physiological responses, including vasoconstriction, mitogenesis, inflammation, hypertrophy and differentiation. The precise response elicited in a particular tissue is dependent on the identity of the receptor expressed and the second messenger pathways activated. Further complication may result from the formation of ETA/ETB heterodimers giving rise to ligand-dependent differences in functional response and receptor-ligand internalization kinetics (Gregan et al., 2004). A detailed discussion of the intracellular pathways stimulated by ET receptor activation is beyond the scope of this article and has been covered extensively in excellent, detailed reviews (Masaki, 2004; Motte et al., 2006).
Cardiovascular consequences of ET receptor activation
Endothelins are commonly defined by their impressive vasomotor effects (Yanagisawa et al., 1988). Indeed, ET-1 remains one of the most potent and characteristically sustained vasoconstrictors described. Vascular tone is maintained, in part, by the action of endogenous ET-1, and, consequently, systemic ET receptor antagonism in human subjects lowers blood pressure (Haynes et al., 1996). In most species, a bolus of exogenous ET-1 produces short-lived hypotension followed by a sustained increase in blood pressure. The initial ET-1-evoked depressor action, which is notably absent in mice, is mediated by EC ETB receptor-dependent release of nitric oxide and other endothelium-derived vasodilator substances (D'Orleans-Juste et al., 2002). The same pathway may also yield vasoconstrictor mediators (for example, thromboxane A2), but the pressor activity of ET-1 is principally associated with VSMC ETA receptor activation. VSMC ETB receptor-mediated contraction is also well documented in many vascular beds, although it is unlikely to significantly increase vascular tone. Certainly, selective ETB receptor antagonism increases peripheral vascular resistance in healthy humans (Strachan et al., 1999), suggesting that the balance between vasodilator and vasoconstrictor ETB pathways favours vasodilatation. In pathological conditions, however, it is possible that this balance may be altered (Cardillo et al., 1999; Pernow et al., 2000). Deletion of the ETB receptor gene results in salt-sensitive hypertension in rodents (Gariepy et al., 2000), reflecting the importance of renal ETB receptors in the regulation of BP. Furthermore, EC-specific ET-1 overexpressing mice are not hypertensive (Amiri et al., 2004), suggesting that actions of ET-1 beyond vasoconstriction, possibly including upregulation of ETB receptor-mediated vasodilatation and natriuretic pathways, can provide protection against physiological rather than pharmacological increases in ET-1 concentration.
The effects of ET-1 on vascular resistance produce predictable reflex changes in heart rate and cardiac output. Direct effects on the heart including positive inotropy are also reported, although the receptor mediating this effect in humans is disputed (Meyer et al., 1996; Burrell et al., 2000). In many studies, concomitant effects on coronary blood flow limit interpretation of such data.
The kidney is an important target for ET-1. Exogenous ET-1 stimulates regional changes in renal blood flow, typically inducing vasoconstriction in the renal cortex and vasodilatation in the medulla (Rubinstein et al., 1995). Medullary vasodilatation appears to be solely mediated by ETB receptor stimulation, while effects on the larger renal vessels and glomerular afferent and efferent arterioles are both ETA and ETB receptor dependent (Dhaun et al., 2006). In mice, at least, IMCD cell-derived ET-1 exerts an autocrine natriuretic and diuretic effect that is at least partially ETB mediated. Indeed, IMCD cell-specific deletion of ETB, but neither IMCD cell-specific ETA nor EC-specific ETB knockout, results in salt-sensitive hypertension (Bagnall et al., 2006; Ge et al., 2006). The magnitude of hypertension in IMCD cell-specific ETB knockout mice is less than that observed in IMCD cell-specific ET-1 knockout mice (Ge et al., 2006), suggesting that paracrine actions of IMCD-derived ET-1 may also contribute to BP regulation.
Endothelins and cardiovascular pathophysiology
From an early stage, it was proposed that ET-1 might contribute to the development of cardiovascular disease. As previously described (Gray and Webb, 1996), a role for ET-1 might be inferred on the basis of one or, preferably, a combination of three conditions: (1) production of ET-1 or actions at its receptors are altered; (2) administration of ET-1 recapitulates features of the disease; and (3) compounds that reduce ET activity reduce the signs of the disease. On this basis, a role for ET-1 has been suggested in a variety of cardiovascular diseases, predominantly through chronic (for example, hypertension, ischaemic heart disease and congestive heart failure) or acute (for example, cerebral vasospasm following subarachnoid haemorrhage (SAH), Raynaud's syndrome) increases in vessel tone. Pro-inflammatory and pro-fibrotic effects of ET-1 may also contribute to cardiovascular disease pathogenesis (for example, in atherosclerosis). Given the clinical importance and prevalence of these conditions, it is not surprising that the search for new treatments arising from manipulation of ET-1 activity has been the subject of considerable research interest.
Strategies for pharmacological inhibition of ET-1 activity
Analogous to inhibition of the renin–angiotensin–aldosterone system with ACE inhibitors and AT1 receptor antagonists, pharmacological therapeutic manipulation of the ET system has centred on two, potentially complementary strategies: (1) inhibition of ET biosynthesis by ECE and (2) direct antagonism of ET receptors.
ECE inhibition
Pharmacological blockade of ET synthesis, by inhibition of ECE1 (Figure 1), is an intuitive approach in reducing the elevated activity of ET-1 associated with numerous cardiovascular disease states. The viability of this strategy was demonstrated by the ability of phosphoramidon to cause vasodilatation and attenuate big ET-1-induced vasoconstriction in healthy volunteers (Haynes and Webb, 1994). Since then, a variety of animal studies (Jeng et al., 2002) and preliminary work in human patients (Dickstein et al., 2004) have shown potentially beneficial effects of ECE inhibition.
The shortcomings of phosphoramidon (susceptibility to acid-catalysed decomposition, micromolar potency and poor specificity (Jeng et al., 2002)) have prompted the development of novel ECE inhibitors with improved biological stability, oral bioavailability (Table 1) and affinity for ECE1 in the low nanomolar range. While truly selective inhibitors of ECE are now known, the class is still dominated by dual ECE/NEP 24.11 inhibitors. NEP catabolizes endogenous vasoactive peptides, including natriuretic peptides, bradykinin, substance P and ET-1. Although inhibition of vasodilator degradation might be expected to be beneficial, in practice, NEP inhibitors cause ET-dependent vasoconstriction (Ferro et al., 1998). Thus, blockade of ET-1 synthesis and NEP concurrently may unmask these sought after benefits and provide agents with improved clinical efficacy. Triple ACE/ECE/NEP inhibitors have been pursued more recently with the proposition that, since patients with heart failure and hypertension benefit from ACE inhibition, a single compound (such as CGS 35601; Trapani et al., 2004) that blocked ACE, ECE and NEP might provide benefits in cost and compliance. It seems unlikely, however, that at any given dose, a single molecule will possess optimal inhibition kinetics for three distinct enzyme systems.
Table 1.
Comparison of ECE inhibitors in clinical development and experimental use
| Drug | Development phase | Selectivity |
IC50 (nM) |
Reference | ||
|---|---|---|---|---|---|---|
| ECE/NEP | ECEa | NEP | ACE | |||
| TMC 66 | Preclinical | 2900 | Asai et al. (1999) | |||
| Daglutril (SLV 306, KC 12615) | II | Tabrizchi (2003) | ||||
| CGS 26303, CGS 26393 | Preclinical | 0.0033 | 410 | 0.9 | DeLombaert et al. (1994) | |
| Phosphoramidon | Preclinical | 0.0097 | 3500 | 34 | 78 000 | Kukkola et al. (1995) |
| CGS 35601, CGS 37808 | Preclinical | 0.036 | 55 | 2 | 22 | Battistini et al. (2005) |
| CGS 31447 | Preclinical | 0.23 | 21 | 4.8 | Shetty et al. (1998) | |
| SCH 54470 | Preclinical | 1.3 | 70 | 90 | 2.5 | McKittrick et al. (1996) |
| CGS 34043 | Preclinical | 19 | 5.8 | 110 | DeLombaert et al. (2000) | |
| WS 75624 B | Preclinical | 42 | 79 | 3300 | Tsurumi et al. (1995b) | |
| B 90063 | Preclinical | 70 | 1000 | 70 000 | Takaishi et al. (1998) | |
| CGS 35066 | Preclinical | 100 | 22 | 2300 | Trapani et al. (2000) | |
| PD 069185, PD 159790 | Preclinical | >200 | 900 | >180 000 | Ahn et al. (1998) | |
| SM 19712 | Preclinical | >240 | 42 | >10 000 | >10 000 | Umekawa et al. (2000) |
| FR 901533, WS 79089 B | Preclinical | >700 | 140 | >100 000 | Tsurumi et al. (1995a) | |
| Ro 68-7629 | Preclinical | >90 000 | 1.1 | >100 000 | >100 000 | Muller et al. (2002) |
Abbreviations: ACE, angiotensin-converting enzyme; ECE, endothelin converting enzyme; IC50, inhibitory concentration; NEP, neutral endopeptidase.
Where stated, the ECE isoform referred to is ECE-1. Selectivity ratios are displayed to two significant figures. Blank spaces indicate that we were unable to locate appropriate values in the literature.
Endothelin-converting enzyme inhibitors have been eclipsed by ET receptor antagonists in both experimental and clinical use. This may seem surprising when ACE inhibition is preferred to AT1 antagonism to achieve renin–angiotensin–aldosterone system blockade. Captopril, however, enjoyed an 11-year development lead over losartan (Gavras et al., 1978; Whitebread et al., 1989; Gavras and Gavras, 1993), an advantage not shared by orally active ECE/NEP inhibitors. The limited enthusiasm for ECE inhibition may be explicable by theoretical concerns regarding the approach; namely, the redundancy in ET-1-generating pathways and the presence of non-ECE big ET-1-processing enzymes. In addition, should ECE inhibitors successfully reduce ET-1 (despite the presence of alternative synthetic pathways), they would (as with mixed ETA/ETB receptor antagonists) reduce the beneficial ETB-receptor-mediated actions (vasodilation and natriuresis) of endogenous ET-1. While they may also inhibit detrimental ETA/ETB-mediated actions of this peptide, their sole advantage over mixed ET antagonists may be that they do not impair receptor-mediated ET-1 clearance mechanisms. Further, while ECE inhibitors can be expected to share the adverse teratological effects of ET receptor antagonists, additional tolerability issues may arise from the loss of ancillary ECE-mediated functions such as bradykinin degradation. Combined ACE/NEP inhibitors have been associated with a high incidence of treatment-limiting angio-oedema, an effect attributed to loss of two bradykinin catabolic pathways (Jandeleit-Dahm, 2006). Given that bradykinin is also a substrate for ECE, (Hoang and Turner, 1997) combined ECE/NEP inhibitor treatment may also be inappropriate for the large proportion of cardiovascular disease patients treated with ACE inhibitors.
ET receptor antagonism
Nonpeptidic and peptidic ET receptor antagonists were developed from diverse sources in the wake of the initial characterization of ET-1 (Table 2) and have been pivotal in defining the role of ETs in cardiovascular physiology and pathophysiology. Among these, the highly selective ETA receptor antagonist BQ123 (Table 2b), a cyclic pentapeptide derived from structure–activity relationships of microbial products (Ihara et al., 1992), remains the most widely published drug of this class. Further modification of this structure produced the ETB receptor-selective antagonist, BQ788 (Ishikawa et al., 1994), while other lead compounds including PD142893 were derived from similar studies of ET-1 itself (Cody et al., 1992). Screening of chemical libraries and subsequent structural optimization led to the synthesis of many more agents, notably including bosentan, the first ET receptor antagonist to find clinical use (Clozel et al., 1994). Animal and human studies using these compounds demonstrated the ability of ET receptor antagonists to oppose the biological effects of endogenous and exogenous ETs and identified distinct roles for ETA and ETB receptors. The use of peptidic ET receptor antagonists remains restricted to mechanistic studies and acute clinical indications due to rapid proteolytic cleavage in vivo. Orally active, nonpeptide ET receptor antagonists, however, are well suited to chronic administration and many of these feature elimination half-lives compatible with once-a-day dosing (Table 2), an important consideration in a field dominated by therapeutics with similarly optimized pharmacokinetics.
Table 2.
Comparison of ET receptor antagonists in clinical use, clinical development and experimental use
| Drug | Trade name | Development phase | Selectivity |
Ki (nM) |
Bioavailability | Elimination t1/2 (hours) | Reference | |
|---|---|---|---|---|---|---|---|---|
| ETA/ETB | ETA | ETB | ||||||
| (a) Mixed ETA/ETB receptor antagonists | ||||||||
| Tezosentan (Ro 61-0612) | Veletri | III | 1.2 | 18 | 21 | i.v. | 2.1 (rat) | Clozel et al. (1999) |
| PD 145065 | Preclinical | 3.8 | 4a | 15a | Doherty et al. (1993) | |||
| PD 142893 | Preclinical | 10 | 15a | 150a | Cody et al. (1992) | |||
| Bosentan (Ro 47-0203) | Tracleer | IV | 20 | 4.7 | 95 | Oral | 3.7–7.5 (human) | Clozel et al. (1994); Weber et al. (1996) |
| TAK 044 | II | 34 | 3.8a | 130a | i.v. | 0.5–1.0 (human) | Watanabe et al. (1995); Haynes et al. (1996) | |
| Avosentan (SPP 301) | III | 50 | Oral | 7.5–15.2 (human) | Dieterle et al. (2004)b | |||
| (b) Selective ETA receptor antagonists | ||||||||
| Enrasentan (SB 217242) | III | 100 | 1.1 | 111 | Oral | 3.3 (rat) | Ohlstein et al. (1996) | |
| Darusentan (LU 135252) | III | 170 | 6 | 1000 | Oral | 12.0 (rat) | Riechers et al. (1996); Cernacek et al. (1998) | |
| Ambrisentan (LU 208075) | Letairis | IV | 200 | 1 | 195 | Oral | 9.0–15.0 (PAH patients) | Riechers et al. (1996); Galié et al. (2005) |
| YM 598 | II | 390 | 3.1a | 1200a | Oral | 2.5 (rat) | Harada et al. (2001) | |
| S 0139 | II | 1000 | 1 | 1000 | Oral | Mihara et al. (1994) | ||
| Clazosentan (Ro 61-1790) | IIb | 1300 | 9.5c | 6.4c | i.v. | 0.8 (monkey) | Roux et al. (1997) | |
| Atrasentan (A 147627) | Xinlay | III | 2000 | 0.069 | 139 | Oral | 2.5 (monkey) | Opgenorth et al. (1996) |
| BQ 123 | Preclinical | 2500 | 7.3a | 18 000a | i.v. | Ihara et al. (1992) | ||
| ZD 4054 | IIa | >4800 | 21a | >100 000a | Oral | 9.1–9.7 (human) | Morris et al. (2005) | |
| Sitaxsentan (TBC 11251) | Thelin | IV | 7000 | 1.4a | 9800a | Oral | 5.9–7.5 (rat) | Wu et al. (1997) |
| BMS 193884 | II | 13 000 | 1.4 | 18 700 | Oral | 9.0 (monkey) | Murugesan et al. (2000) | |
| FR 139317 | Preclinical | 7300 | 1 | 7300 | i.v. | Aramori et al. (1993) | ||
| Edonentan (BMS 207940) | IIa | 81 000 | 0.01 | 810 | Oral | 17.0 (monkey) | Murugesan et al. (2003) | |
| TBC 3711 | I | 440 000 | 0.08a | Oral | 5.3 (rat) | Wu et al. (2004) | ||
| (c) Selective ETB receptor antagonists | ||||||||
| BQ 788 | Preclinical | 1100 | 1300a | 1.2a | i.v. | Ishikawa et al. (1994) | ||
| A 192621 | Preclinical | 1300 | 8200a | 6.4a | Oral | 5.0 (rat) | von Geldern et al. (1999) | |
Abbreviations: ECE, endothelin converting enzyme; ET, endothelin; IC50, inhibitory concentration; Ki, inhibition constant; NEP, neutral endopeptidase; PAH, pulmonary arterial hypertension; t1/2, half-life.
Denotes IC50 (nM).
Avosentan has also has been described as an ETA-selective antagonist but this is, at best, debatable, given the published data. Selectivity ratios are displayed to two significant figures. Blank spaces indicate that we were unable to locate appropriate values in the literature.
Denotes pA2 rather than Ki.
The defining pharmacodynamic variable of ET receptor antagonists is that of selectivity. To be considered ETA selective, antagonists must display at least 100-fold greater affinity for ETA than for ETB (Davenport, 2002), a selectivity ratio superior to that possessed by many so-called β1-selective adrenoceptor antagonists. Thus, at optimal concentration, an ETA-selective compound should occupy >90% of ETA receptors while blocking <10% of ETB receptors. At first glance, the argument in favour of selective ETA antagonists seems compelling since ETB receptors clear ET-1 and mediate beneficial cardiovascular effects (natriuresis, NO release and anti-proliferative and anti-thrombotic effects), a protective role illustrated by both pharmacological and gene knockout approaches. ETB receptor gene deletion, for example, produces salt-sensitive hypertension, vascular oxidative stress and impaired endothelium-dependent vasodilatation (Quaschning et al., 2005) while systemic ETB receptor antagonism in man increases peripheral vascular resistance and plasma ET-1 concentration (Strachan et al., 1999). Certainly, these data emphasize the lack of potential clinical applications for ETB receptor-selective antagonists in cardiovascular disease (though they may hold promise in the treatment of malignant melanoma (Lahav, 2005)). What remains unclear is whether changes in ETB-mediated response in disease may compromise any net beneficial effect of ETB receptor activation (Cardillo et al., 1999; Pernow et al., 2000). Further, it is suggested that cross talk between ET receptors may allow the ETB receptor to adopt functions of the ETA receptor under conditions of selective ETA blockade (Fukuroda et al., 1994; Mickley et al., 1997). The controversial issue of selective ETA vs mixed ETA/ETB receptor antagonism will only be answered when investigated by rigorous head-to-head clinical trials. Furthermore, it is crucial that such studies employ agents that retain ETA receptor selectivity at the doses employed, a widely held concern over many completed trials of ‘selective' ETA receptor antagonists (Kelland and Webb, 2006).
Since gene knockout and pharmacological studies identified the unexpected role of ETs in embryological development, it has been clear that, as with other agents known to cause severe birth defects, use of pharmacological inhibitors of the ET system is to be avoided in women of reproductive potential. Also concerning is the incidence of adverse events observed in clinical use of ET receptor antagonists. While headache, dizziness, nasal congestion and oedema have been reported frequently (probably reflecting vasodilator action), dose-dependent abnormalities of liver function resulting in elevated hepatic transaminases (Yanagisawa et al., 2000) are of greater concern. The incidence of elevated transaminases in bosentan-treated pulmonary arterial hypertension (PAH) patients is ∼9%, with a median onset time of ∼70 days. The suggested mechanism for this phenomenon is impaired bile acid transport resulting in cytotoxic accumulation in hepatocytes (Fattinger et al., 2001). This effect was first reported in trials of bosentan but is observed with both mixed and selective ETA receptor antagonists, though not equally with all agents. The severity of the problem is outlined by two cases of hepatitis following treatment with high-dose sitaxsentan, which resulted in one fatality (Barst et al., 2002). Despite this, with appropriate dosing and close monitoring, adverse effects have proved manageable rather than treatment-limiting in PAH, a condition with a poor outlook and few alternative therapies.
Outcome of clinical trials with endothelin receptor antagonists
Early clinical trials of the ‘-sentan' class of drugs included both selective ETA and mixed ETA/ETB receptor antagonist approaches (Table 2). Thus far, the following indications have been studied in large trials: PAH, scleroderma, heart failure (acute and chronic), essential hypertension, erectile dysfunction and SAH (Table 3). The most encouraging outcome has been the approval of bosentan and, more recently, sitaxsentan and ambrisentan, for treatment of PAH.
Table 3.
Summary and completed clinical trials of ET receptor antagonists in indicated cardiovascular diseases
| Condition | Antagonist | Study | Outcome |
|---|---|---|---|
| Pulmonary arterial hypertension | Bosentan | BREATHE-1 | Improvement in primary and secondary end points, well tolerated |
| BREATHE-4 | Improvement in end points, well tolerated | ||
| Sitaxsentan | Open label | ||
| STRIDE 1–6 | Sustained improvement, well tolerated | ||
| Ambrisentan | AMB-220 | Improvement in end points, sustained for >2 years, well tolerated | |
| ARIES-2 | Improvement in end points, well tolerated | ||
| Heart failure | |||
| Chronic | Bosentan | Pilot | Short-term haemodynamic improvements |
| ENABLE-1; -2 | No benefit, early worsening of symptoms | ||
| REACH-1 | No benefit, toxic effects, trial stopped, trend to reduced mortality | ||
| Enrasentan | ENCOR | No benefit, increased adverse events, trend to increased mortality | |
| Darusentan | EARTH | No benefit, increased adverse events | |
| HEAT-CHF | Some haemodynamic benefit, adverse events (including death) at higher dose | ||
| BMS 193884 | No data, trial probably discontinued | ||
| Edonentan | No data, trial probably discontinued | ||
| Acute | Tezosentan | RITZ-1 | Safety trial, well tolerated, some haemodynamic improvement |
| RITZ-2 | Acute improvement in haemodynamics, dose dependence | ||
| RITZ-3 | Follow-up to RITZ-2, never conducted | ||
| RITZ-4 | No benefit and adverse events | ||
| 204 | Dose-dependent improvements in haemodynamics, decreased urine production | ||
| VERITAS-1, -2 | Limited benefit, trials discontinued | ||
| Hypertension | Bosentan | Reduction of SBP and DBP in essential HT | |
| Darusentan | HEAT-HTN | Dose-dependent reduction of SBP and DBP in essential HT | |
| DAR-201 | Reduction of SBP and DBP in refractory HT | ||
| Aneurysmal SAH | Clazosentan | Pre-CONSCIOUS-1 | Reduced frequency and severity of cerebral vasospasm |
| Erectile dysfunction | BMS 193884 | No improvement over placebo | |
| Scleroderma (ulcers) | Bosentan | RAPIDS-1, -2 | Significant reduction in ulcers, well-tolerated |
Abbreviations: DBP, diastolic blood pressure; ET, endothelin; HT, hypertension; SAH, sub-arachnoid haemorrhage; SBP, systolic blood pressure.
Summarized from Battistini et al. (2006).
Pulmonary arterial hypertension
Pulmonary arterial hypertension is a progressive and fatal condition characterized by a sustained increase in pulmonary vascular resistance leading to right ventricular failure and premature death (median survival ∼2–3 years from diagnosis). ET-1 has emerged as a mediator of increased vascular tone and vascular remodelling (via pro-fibrotic, pro-inflammatory and pro-proliferative effects) in this condition. Circulating and tissue (lung and heart) levels of ET-1 and its precursors are increased in patients with PAH (Stewart et al., 1991) and in animal models (Reinhart et al., 2002), possibly as a result of increased pulmonary generation, or reduced ETB-mediated clearance. In humans, plasma ET-1 levels correlate with markers of disease severity (pulmonary vascular resistance, right atrial pressure and pulmonary artery oxygen saturation) and predict poor prognosis (Stewart et al., 1991). Overexpression of ET-1 in experimental PAH models is localized to distal segments of the pulmonary arterial tree, particularly the medial layers (Takahashi et al., 2001). ETA and ETB are also upregulated (Takahashi et al., 2001), but the role of ETB in PAH is complex: on the one hand, increased ET-1 activity may be ameliorated by upregulation of EC ETB-dependent vasodilator, clearance and anti-proliferative pathways, and PAH in animal models is exaggerated by ETB receptor deficiency (Ivy et al., 2002) or antagonism (Ivy et al., 2000). On the other hand, VSMC and myocardial ETB receptors may mediate detrimental effects of ET-1, such as remodelling and hypertrophy. Indeed, studies of hypoxic rats have suggested a role for ETB in cardiac remodelling since mixed ETA/ETB, but not ETA-selective antagonism attenuates right ventricular hypertrophy (Motte et al., 2006). Overall, studies in animal models have confirmed that both mixed (bosentan) and selective ETA receptor antagonists (sitaxsentan, atrasentan and TBC-3711) are effective in PAH, reducing pulmonary artery pressure and inhibiting vascular remodelling. These effects are conserved across species and are independent of the methodology used to induce PAH. The importance of ETB-mediated pathways in human PAH remains unclear, and both ETA-selective and mixed antagonists are now used clinically.
Bosentan (‘Tracleer') was granted FDA approval for treatment of patients with NYHA/WHO functional class III or IV symptoms in 2001, based on the results of two trials: ‘study 351' (involving 32 class III patients with either idiopathic PAH or PAH associated with systemic sclerosis (SSc)) (Channick et al., 2001) and the landmark BREATHE-1 (Bosentan Randomised trial of Endothelin Antagonist THErapy for PAH) study (comprising 150 patients with idiopathic PAH, 47 with SSc-associated PAH and 16 with systemic lupus erythematosus-associated PAH) (Rubin et al., 2002). In BREATHE-1, bosentan produced a 44-m placebo-corrected improvement in 6-min walking distance, improved functional class and, importantly, for a condition with such a dire prognosis, increased the time to clinical worsening (a composite of death, hospitalization, lung transplantation, atrial septostomy, lack of clinical improvement or need for epoprostenol treatment). In addition, improvements were noted in echocardiographic parameters (right ventricular function and early left ventricular diastolic filling; Galié et al., 2003). Interestingly, subgroup analysis revealed different response rates according to aetiology. Improvements were documented in idiopathic PAH, but bosentan only prevented deterioration in patients with SSc-associated PAH (considered a positive end point in this condition). Open-label continuation studies in each trial population demonstrated that the effects of bosentan were maintained beyond the initial 12-week study period. Survival at 1 and 2 years in the patients initially treated with bosentan was 96 and 89%, respectively (82 and 66% for SSc-associated PAH), compared with predicted rates in historical controls of 69 and 57%. Broadly, complementary outcomes were obtained in the Sitaxentan (‘Thelin') to Relieve ImpaireD Exercise (STRIDE-1) trial (Barst et al., 2004) with improved exercise capacity, haemodynamic variables (cardiac index and pulmonary vascular resistance) and clinical end points in 178 patients with milder disease (NYHA classes II–III; only one patient in class IV) and different aetiology (∼1/4 of patients had congenital heart disease-associated PAH). Sitaxsentan was granted a licence for use in PAH in 2006. The larger STRIDE-2 trial of 240 patients (reviewed by Battistini et al., 2006) and its open-label extension, STRIDE-2X, used slightly lower doses of sitaxsentan (50 and 100 mg compared with 100 and 300 mg in STRIDE-1; Barst et al., 2004) and included patients who had previously discontinued bosentan treatment because of hepatic side effects (reviewed by Battistini et al., 2006). As expected, with lower doses, the rate of transaminase elevation was lower. Around half the patients receiving sitaxsentan experienced an improvement of at least one functional class, though no clinically significant advantage of an ETA-selective approach over mixed antagonism was demonstrated. Thus, ET antagonists are now firmly established in European and American guidelines for treatment of NYHA class III and IV patients with idiopathic PAH and PAH associated with connective tissue disease, who either do not respond to acute vasodilators or remain class III despite vasodilator responsiveness. Very recently, the relatively ETA-selective antagonist, ambrisentan, has been approved for use in the United States for PAH patients with class II or III symptoms on the basis of the ARIES trials (see Battistini et al., 2006), and a broadening of the indication for this agent is currently under investigation. In this regard, a small open-label, non-randomized trial of bosentan in 16 patients with PAH due to chronic pulmonary venous thromboembolism recently reported continued benefit after 6 months (Bonderman et al., 2005).
A number of questions in this field remain: further studies will be required to determine whether ET antagonists have a role in NYHA class I patients and in the large number with PAH secondary to left ventricular dysfunction and hypoxic lung disease. Whether ET antagonists improve survival in prospective trials (rather than in comparison with historical controls) may now be difficult to assess because of ethical considerations. Furthermore, few data are currently available on the combination of ET-antagonist therapy with other treatments for PAH, such as prostacyclins and phosphodiesterase inhibitors. Safety remains an issue and current recommendations are that individuals treated with ET antagonists should undergo monthly hepatic transaminase surveillance, while those with pre-existing abnormalities of liver function should not receive this class of drug. Patients on warfarin starting sitaxsentan require close monitoring of anti-coagulation because of the drug's inhibition of the CYP2C9 P450 enzyme. Bosentan, in contrast, induces this enzyme and higher warfarin doses may be needed. Following reports of anaemia, haemoglobin checks are also recommended.
Connective tissue disorders
Expanding the indication for ET antagonists to connective tissue disease without PAH, bosentan also reduced digital ulceration in patients with scleroderma in the RAndomised Placebo-controlled study on Prevention of Ischaemic Digital ulcers in Scleroderma (RAPIDS-1 and RAPIDS-2) trials. Furthermore, several investigators have reported positive results with ET antagonists in Raynaud's disease (reviewed by Gayraud, 2007). For example, bosentan improved hand function (Korn et al., 2004), reduced new ulcers and promoted rapid healing of existing ulcers (although the latter is not consistent between investigations) (Humbert and Cabane, 2003; Ramos-Casals et al., 2004; Snyder et al., 2005).
Heart failure
In contrast to the success of ET antagonists in PAH, trials of their use for treatment of congestive cardiac failure have been disappointing. A role for ET in the pathogenesis of heart failure was highlighted in many early investigations (for reviews see Gray and Webb, 1996; Motte et al., 2006). ET-1 was proposed to contribute to acute and chronic increases in vascular resistance, ventricular and vascular remodelling, inflammation and arrhythmogenesis in models of heart failure. Indeed, many of the pathophysiological effects of ET-1 are comparable with hormones, such as noradrenaline and angiotensin II, the inhibition of which have proven successful therapeutic approaches. Human studies were initiated on the basis of a wealth of animal data demonstrating substantial haemodynamic improvement. Preliminary small-scale clinical trials with bosentan (Kiowski et al., 1995), darusentan (Spieker et al., 2000) and BQ-123 (Cowburn et al., 1998) were encouraging, also demonstrating short-term haemodynamic benefits (reduction of right atrial, pulmonary arterial, pulmonary wedge and mean arterial pressures, plus increased cardiac output), even in patients on ACE inhibitors. Similarly, studies of intravenous tezosentan for patients with acutely decompensated heart failure were reported to improve cardiac index and pulmonary capillary wedge pressures (Torre-Amione et al., 2003). Four Randomised, Intravenous TeZosentan (RITZ) trials have since assessed its effectiveness in acute heart failure (RITZ-1; Torre-Amione et al., 2001a; RITZ-2; Cotter et al., 2001; Schalcher et al., 2001), decompensated heart failure associated with acute coronary syndromes (RITZ-4; O'Connor et al., 2002, 2003) and in fulminant pulmonary oedema (RITZ-5; Kaluski et al., 2003). RITZ-3, designed to assess tezosentan in advanced heart failure, appears never to have been performed (Torre-Amione et al., 2001b). In each RITZ trial clinical end points (dyspnoea score, time to death or worsening heart failure) were unaltered by tezosentan and only in the high-dose RITZ-2 study were beneficial effects on dyspnoea, cardiac index and pulmonary wedge pressure reported. Predictably, however, this was associated with an unacceptably high incidence of hepatic transaminase elevation. Further trials (Value of Endothelin Receptor Inhibition with Tezosentan in Acute heart failure Study; VERITAS-1 and -2) of low-dose tezosentan, based on the work of Cotter et al. (2004), were discontinued after ∼1 year, when interim analysis indicated that the mild haemodynamic alterations observed were unlikely to result in a significant improvement in clinical end points. In stable patients with chronic heart failure, each of the four large clinical trials of ET receptor antagonism also failed to demonstrate any benefit in clinically relevant end points (clinical status, mortality or hospitalization for heart failure) and were beset with toxicity problems. The REACH-1 (Randomised Endothelin Antagonism in Chronic Heart Failure) trial of bosentan was stopped early due to problems with anaemia and hepatic transaminase elevation (reflecting the relatively high doses employed) and a tendency for clinical worsening in the early stages of treatment (Mylona and Cleland, 1999). The design of ENABLE-1 (Europe and Australia) and ENABLE-2 (North America) sought to address these issues (Kalra et al., 2002), using a lower dose (125 mg bid) and longer surveillance (9 months). Despite this, the outcome remained that bosentan had no discernable benefit and the early worsening of the condition was still observed. These results have been mirrored in two smaller trials: ENCOR (ENrasentan COoperisedative Randomised evaluation) and EARTH (Endothelin A Receptor antagonist Trial in Heart failure; Anand et al., 2004) using the ET antagonists enrasentan (100-fold ETA selective) and darusentan (170-fold ETA selective), respectively. ENCOR reported both an increase in adverse effects and a trend towards increased mortality, while EARTH found no improvement in a variety of end points and was discontinued because of an increased incidence of adverse events (reviewed by Battistini et al., 2006).
Although disappointing, it is perhaps not surprising that ET antagonists failed to demonstrate any improvement in clinical status or survival in acute and chronic heart failure. In retrospect, not all animal studies produced positive outcomes, and the timing of intervention appeared to be critically important. For example, in myocardial infarction models, early treatment was associated with scar thinning and left ventricular dilatation (Nguyen et al., 1998). Furthermore, clinical experience with β-blockers in patients with heart failure emphasize that acute haemodynamic effects are poorly predictive of long-term improvements in outcome (Grandjean and Rivier, 1968; Packer et al., 1996). Whether ET antagonists may have demonstrated clinical efficacy in patients not taking ACE inhibitors, spironolactone or β-blockers is debatable. The REACH, EARTH, ENCOR and ENABLE trials all appear to have been carried out in patients taking these classes of drugs but the percentage of patients taking each is not clear. While the recognized side effect of ankle oedema with ET antagonists may have been misconstrued as clinical evidence of acute worsening of heart failure, and the problems with toxicity simply a question of dose, none of the trials suggested an improvement in mortality. A key issue that remains to be addressed is whether ETA-selective agents were used at ETA-selective doses (Kelland and Webb, 2006).
Systemic hypertension
Hypertension was an early target for trials of ET antagonists, based on the pressor effects of ET-1 (Yanagisawa et al., 1988), and a number of studies showing that ET antagonists lower blood pressure in animal models (Schiffrin, 1995) and in humans (Haynes et al., 1996). The first large clinical trial for this indication was published in 1998 (Krum et al., 1998) and enrolled patients (n=292) with mild-to-moderate hypertension, who were randomized to either enalapril or bosentan (100, 500, 1000 or 2000 mg day−1). Bosentan lowered systolic and diastolic blood pressure (BP) by ∼10 and 6 mm Hg, respectively, compared with placebo, without activating the sympathetic nervous system or renin–angiotensin–aldosterone system and with a plateau in BP lowering for doses >500 mg. Subsequently, two randomized, double-blind, placebo-controlled trials have reported the effect of the ETA-selective antagonist darusentan on moderate essential and resistant hypertension. The HEAT-HTN (Hypertension Endothelin Antagonist Treatment) study demonstrated a dose-dependent (10, 30 and 100 mg) reduction in systolic blood pressure (SBP) and diastolic BP (DBP; up to 11 and 8 mm Hg, respectively; n=392) after 6 weeks' treatment. Similarly, the DAR-201 trial, with 115 hypertensive patients receiving maximum doses of ⩾3 anti-hypertensive agents, found that the highest dose of darusentan (300 mg day−1) produced a placebo-corrected reduction in both SBP (Δ=11.6 mm Hg) and DBP (Δ=7 mm Hg) (unpublished data; reviewed by Battistini et al., 2006). These trials suggest that ET antagonists may find a niche place in the treatment of patients with difficult to control hypertension, particularly if they can be shown to improve endothelial function, reduce arterial stiffness and moderate the development of atherosclerosis (Dhaun et al., 2006). Indeed, given how common hypertension is as a condition worldwide, this may prove to be a much larger market for ET receptor antagonists than PAH. Perhaps more interesting is the observation that selective ETA receptor antagonists can reduce blood pressure substantially in hypertensive patients with chronic kidney disease (Goddard et al., 2004b) and that this effect is both synergistic with ACE inhibitors (Goddard et al., 2004a) and abolished by significant concurrent ETB receptor blockade. Further, in diabetic and nondiabetic renal disease patients, ET receptor antagonists may produce favourable renal haemodynamic changes that reduce proteinuria, an important surrogate for decline in renal function (Dhaun et al., 2006). Hence, ET antagonists may represent a new class of drug that can offer benefits to patients with chronic kidney disease that extend beyond blood pressure lowering.
Other conditions
Further studies have addressed the role of ET receptor antagonism in erectile dysfunction and aneurysmal SAH, with mixed results. A double-blind pilot study of 53 patients with mild-to-moderate erectile dysfunction demonstrated no benefit of the ETA-selective antagonist BMS-193884 (100 mg by mouth) over placebo (Kim et al., 2002).
The ETA-selective antagonist clazosentan was specifically designed for intravenous use in conditions characterized by cerebral vasoconstriction. Its potential in treating severe aneurysmal SAH has recently been addressed in a phase IIa pilot study for the Clazosentan to Overcome Neurological iSChaemia and Infarction OccUrring after Sub-arachnoid haemorrhage (CONSCIOUS-1) trial (Vajkoczy et al., 2005). This ‘pre-CONSCIOUS-1' study documented a reduction in the frequency and severity of cerebral vasospasm following SAH.
Conclusions
The recent licensing of bosentan, sitaxsentan and ambrisentan for treatment of PAH is the most obvious demonstration of the clinical benefit derived from therapeutic manipulation of the ET-1 system in cardiovascular disease. This development of one of the first effective treatments for a condition with poor prognosis has obvious clinical significance and is likely to be extended to include PAH associated with connective tissue disorders. Thus, ET antagonists are already realizing their potential in treatment of cardiovascular diseases, while early clinical data suggest these compounds may prove beneficial in other conditions, such as resistant hypertension, chronic kidney disease and SAH. In contrast, a potential role in conditions associated with vascular remodelling (restenosis, chronic obstructive pulmonary disease and transplant graft rejection) remains speculative and requires further investigation. It should also be noted that the clinical experience with ET antagonists in patients with cardiovascular disease remains relatively limited and the design of new trials could be improved using knowledge gained from previous studies, particularly with regard to drug dose and selectivity.
These successes must obviously be balanced against the failure of ET antagonists to realize their potential in the treatment of heart failure, and the fact that teratogenic effects have restricted their possible use to treatment of conditions where childbearing potential is unlikely to be an issue. Several reasons have been proposed to account for the disappointing outcomes in clinical trials as compared to investigations using animal models of disease, including inadequate models or a bias in publication towards positive outcomes; incorrect dose/timing of administration; the need to show additional benefit over existing treatments; and ET activation being a consequence rather than a cause of the condition. Whatever the reason, this experience urges caution in extrapolating data obtained in vitro and in animals to humans. It is hoped that additional information will emerge from unpublished clinical trials that will shed light on previous failures (Kelland and Webb, 2006), and that the combination of powerful pharmacological and molecular approaches will help us to better understand the role of ETA and ETB receptors in health and disease so as to fully realize the clinical potential created by the identification of the powerful vasoconstrictor peptide, ET-1.
Abbreviations
- BP
blood pressure
- EC
endothelial cell
- ECE
endothelin-converting enzyme
- ET
endothelin
- ET-1
endothelin-1
- ETA/B
ETA/B receptor
- IMCD
inner-medullary collecting duct
- NEP
neutral endopeptidase
- NO
nitric oxide
- NYHA
New York Heart Association
- PAH
pulmonary arterial hypertension
- SAH
subarachnoid haemorrhage
- SSc
systemic sclerosis
- VSMC
vascular smooth muscle cell
Conflict of interest
NSK, PWFH and AJB have no conflicts of interest relevant to this article. DJW has provided consultancy for Abbott Laboratories, Bristol-Myers Squibb Pharmaceuticals, Encysive Pharmaceuticals, F Hoffmann-La Roche and Speedel.
References
- Abassi ZA, Tate JE, Golomb E, Keiser HR. Role of neutral endopeptidase in the metabolism of endothelin. Hypertension. 1992;20:89–95. doi: 10.1161/01.hyp.20.1.89. [DOI] [PubMed] [Google Scholar]
- Ahn D, Ge YQ, Stricklett PK, Gill P, Taylor D, Hughes AK, et al. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest. 2004;114:504–511. doi: 10.1172/JCI21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K, Sisneros AM, Herman SB, Pan SM, Hupe D, Lee C, et al. Novel selective quinazoline inhibitors of endothelin converting enzyme-1. Biochem Biophys Res Commun. 1998;243:184–190. doi: 10.1006/bbrc.1998.8081. [DOI] [PubMed] [Google Scholar]
- Amiri F, Virdis A, Neves MF, Iglarz M, Seidah NG, Touyz RM, et al. Endothelium-restricted over-expression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation. 2004;110:2233–2240. doi: 10.1161/01.CIR.0000144462.08345.B9. [DOI] [PubMed] [Google Scholar]
- Anand I, McMurray J, Cohn JN, Konstam MA, Notter T, Quitzau K, et al. Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the Endothelin(A) Receptor antagonist Trial in Heart failure (EARTH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:347–354. doi: 10.1016/S0140-6736(04)16723-8. [DOI] [PubMed] [Google Scholar]
- Anggard E, Galton S, Rae G, Thomas R, McLoughlin L, DeNucci G, et al. The fate of radioiodinated endothelin-1 and endothelin-3 in the rat. J Cardiovasc Pharmacol. 1989;13:S46–S49. doi: 10.1097/00005344-198900135-00012. [DOI] [PubMed] [Google Scholar]
- Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA-encoding an endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- Aramori I, Nirei H, Shoubo M, Sogabe K, Nakamura K, Kojo H, et al. Subtype selectivity of a novel endothelin antagonist, FR139317, for the two endothelin receptors in transfected Chinese hamster ovary cells. Mol Pharmacol. 1993;43:127–131. [PubMed] [Google Scholar]
- Asai Y, Nonaka N, Suzuki SI, Nishio M, Takahashi K, Shima H, et al. TMC-66, a new endothelin converting enzyme inhibitor produced by Streptomyces sp. A5008. J Antibiot. 1999;52:607–612. doi: 10.7164/antibiotics.52.607. [DOI] [PubMed] [Google Scholar]
- Bagnall AJ, Kelland NF, Gulliver-Sloan F, Davenport AP, Gray GA, Yanagisawa M, et al. Deletion of endothelial cell endothelin B receptors does not affect blood pressure or sensitivity to salt. Hypertension. 2006;48:286–293. doi: 10.1161/01.HYP.0000229907.58470.4c. [DOI] [PubMed] [Google Scholar]
- Barnes K, Brown C, Turner AJ. Endothelin-converting enzyme—ultrastructural localization and its recycling from the cell surface. Hypertension. 1998;31:3–9. doi: 10.1161/01.hyp.31.1.3. [DOI] [PubMed] [Google Scholar]
- Barst RJ, Langleben D, Frost A, Horn EM, Oudiz R, Shapiro S, et al. Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;169:441–447. doi: 10.1164/rccm.200307-957OC. [DOI] [PubMed] [Google Scholar]
- Barst RJ, Rich S, Widlitz A, Horn EM, McLaughlin V, McFarlin J. Clinical efficacy of sitaxsentan, an endothelin-A receptor antagonist, in patients with pulmonary arterial hypertension—open-label pilot study. Chest. 2002;121:1860–1868. doi: 10.1378/chest.121.6.1860. [DOI] [PubMed] [Google Scholar]
- Battistini B, Berthiaume N, Kelland NF, Webb DJ, Kohan DE. Profile of past and current clinical trials involving endothelin receptor antagonists: the novel ‘-sentan' class of drug. Exp Biol Med. 2006;231:653–695. [PubMed] [Google Scholar]
- Battistini B, Daull P, Jeng AY. CGS 35601, a triple inhibitor of angiotensin converting enzyme, neutral endopeptidase and endothelin converting enzyme. Cardiovasc Drug Rev. 2005;23:317–330. doi: 10.1111/j.1527-3466.2005.tb00175.x. [DOI] [PubMed] [Google Scholar]
- Bloch KD, Hong CC, Eddy RL, Shows TB, Quertermous T. cDNA cloning and chromosomal assignment of the endothelin-2 gene—vasoactive intestinal contractor peptide is rat endothelin-2. Genomics. 1991;10:236–242. doi: 10.1016/0888-7543(91)90505-9. [DOI] [PubMed] [Google Scholar]
- Bonderman D, Nowotny R, Skoro-Sajer N, Jakowitsch J, Adlbrecht C, Klepetko A, et al. Bosentan therapy for inoperable chronic thromboembolic pulmonary hypertension. Chest. 2005;128:2599–2603. doi: 10.1378/chest.128.4.2599. [DOI] [PubMed] [Google Scholar]
- Burrell KM, Molenaar P, Dawson PJ, Kaumann AJ. Contractile and arrhythmic effects of endothelin receptor agonists in human heart in vitro: blockade with SB 209670. J Pharmacol Exp Ther. 2000;292:449–459. [PubMed] [Google Scholar]
- Cardillo C, Kilcoyne CM, Waclawiw M, Cannon RO, III, Panza JA. Role of endothelin in the increased vascular tone of patients with essential hypertension. Hypertension. 1999;33:753–758. doi: 10.1161/01.hyp.33.2.753. [DOI] [PubMed] [Google Scholar]
- Cernacek P, Franchi L, Dupuis O, Rouleau JL, Levy M. Radioreceptor assay of an endothelin A receptor antagonist in plasma and urine. Clin Chem. 1998;44:1666–1673. [PubMed] [Google Scholar]
- Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- Clozel M, Breu V, Gray GA, Kalina B, Loffler BM, Burri K, et al. Pharmacological characterization of bosentan, a new potent orally-active nonpeptide endothelin receptor antagonist. J Pharmacol Exp Ther. 1994;270:228–235. [PubMed] [Google Scholar]
- Clozel M, Ramuz H, Clozel JP, Breu V, Hess P, Loffler BM, et al. Pharmacology of tezosentan, new endothelin receptor antagonist designed for parenteral use. J Pharmacol Exp Ther. 1999;290:840–846. [PubMed] [Google Scholar]
- Cody WL, Doherty AM, He JX, Depue PL, Rapundalo ST, Hingorani GA, et al. Design of a functional hexapeptide antagonist of endothelin. J Med Chem. 1992;35:3301–3303. doi: 10.1021/jm00095a029. [DOI] [PubMed] [Google Scholar]
- Cotter G, Kaluski E, Stangl K, Pacher R, Richter C, Milo-Cotter O, et al. The hemodynamic and neuro-hormonal effects of low doses of tezosentan (an endothelin A/B receptor antagonist) in patients with acute heart failure. Eur J Heart Fail. 2004;6:601–609. doi: 10.1016/j.ejheart.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Cotter G, Kiowski W, Kaluski E, Kobrin I, Milovanov O, Marmor A, et al. Tezosentan (an intravenous endothelin receptor A/B antagonist) reduces peripheral resistance and increases cardiac power therefore preventing a steep decrease in blood pressure in patients with congestive heart failure. Eur J Heart Fail. 2001;3:457–461. doi: 10.1016/s1388-9842(01)00168-4. [DOI] [PubMed] [Google Scholar]
- Cowburn PJ, Cleland JGF, McArthur JD, MacLean MR, McMurray JJV, Dargie HJ. Short-term haemodynamic effects of BQ-123, a selective endothelin ETA-receptor antagonist, in chronic heart failure. Lancet. 1998;352:201–202. doi: 10.1016/S0140-6736(05)77807-7. [DOI] [PubMed] [Google Scholar]
- Davenport AP. International union of pharmacology. XXIX. Update on endothelin receptor nomenclature. Pharmacol Rev. 2002;54:219–226. doi: 10.1124/pr.54.2.219. [DOI] [PubMed] [Google Scholar]
- Davenport AP, Maguire JJ. Is endothelin-induced vasoconstriction mediated only by ET(A) receptors in humans. Trends Pharmacol Sci. 1994;15:9–11. doi: 10.1016/0165-6147(94)90120-1. [DOI] [PubMed] [Google Scholar]
- DeLombaert S, Blanchard L, Stamford LB, Tan J, Wallace EM, Satoh Y, et al. Potent and selective non-peptidic inhibitors of endothelin-converting enzyme-1 with sustained duration of action. J Med Chem. 2000;43:488–504. doi: 10.1021/jm990507o. [DOI] [PubMed] [Google Scholar]
- DeLombaert S, Ghai RD, Jeng AY, Trapani AJ, Webb RL. Pharmacological profile of a non-peptidic dual inhibitor of neutral endopeptidase-24.11 and endothelin-converting enzyme. Biochem Biophys Res Commun. 1994;204:407–412. doi: 10.1006/bbrc.1994.2473. [DOI] [PubMed] [Google Scholar]
- Dhaun N, Goddard J, Webb DJ. The endothelin system and its antagonism in chronic kidney disease. J Am Soc Nephrol. 2006;17:943–955. doi: 10.1681/ASN.2005121256. [DOI] [PubMed] [Google Scholar]
- Dickstein K, De Voogd HJ, Miric MP, Willenbrock R, Mitrovic V, Pacher R, et al. Effect of single doses of SLV306, an inhibitor of both neutral endopeptidase and endothelin-converting enzyme, on pulmonary pressures in congestive heart failure. Am J Cardiol. 2004;15:237–239. doi: 10.1016/j.amjcard.2004.03.074. [DOI] [PubMed] [Google Scholar]
- Dieterle W, Mann J, Kutz K. Pharmacokinetics and pharmacodynamics of the ETA-selective endothelin receptor antagonist SPP301 in healthy human subjects. J Clin Pharmacol. 2004;44:59–66. doi: 10.1177/0091270003261047. [DOI] [PubMed] [Google Scholar]
- Doherty AM, Cody WL, He JX, Depue PL, Cheng XM, Welch KM, et al. In-vitro and in-vivo studies with a series of hexapeptide endothelin antagonists. J Cardiovasc Pharmacol. 1993;22:S98–S102. doi: 10.1097/00005344-199322008-00027. [DOI] [PubMed] [Google Scholar]
- D'Orleans-Juste P, Labonte J, Bkaily G, Choufani S, Plante M, Honore JC. Function of the endothelin(B) receptor in cardiovascular physiology and pathophysiology. Pharmacol Ther. 2002;95:221–238. doi: 10.1016/s0163-7258(02)00235-8. [DOI] [PubMed] [Google Scholar]
- Dupuis J, Jasmin JF, Prie S, Cernacek P. Importance of local production of endothelin-1 and of the ETB receptor in the regulation of pulmonary vascular tone. Pulm Pharmacol Ther. 2000;13:135–140. doi: 10.1006/pupt.2000.0242. [DOI] [PubMed] [Google Scholar]
- Emoto N, Yanagisawa M. Endothelin-converting enzyme-2 is a membrane-bound, phosphoramidon-sensitive metalloprotease with acidic pH optimum. J Biol Chem. 1995;270:15262–15268. doi: 10.1074/jbc.270.25.15262. [DOI] [PubMed] [Google Scholar]
- Fattinger K, Funk C, Pantze M, Weber C, Reichen J, Stieger B, et al. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther. 2001;69:223–231. doi: 10.1067/mcp.2001.114667. [DOI] [PubMed] [Google Scholar]
- Fernandez-Patron C, Radomski MW, Davidge ST. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res. 1999;85:906–911. doi: 10.1161/01.res.85.10.906. [DOI] [PubMed] [Google Scholar]
- Ferro CJ, Spratt JC, Haynes WG, Webb DJ. Inhibition of neutral endopeptidase causes vasoconstriction of human resistance vessels in vivo. Circulation. 1998;97:2323–2330. doi: 10.1161/01.cir.97.23.2323. [DOI] [PubMed] [Google Scholar]
- Firth JD, Ratcliffe PJ. Organ distribution of the 3 rat endothelin messenger RNAs and the effects of ischemia on renal gene expression. J Clin Invest. 1992;90:1023–1031. doi: 10.1172/JCI115915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelin C, Ladoux A, Marsault R, Vigne P. Functional-properties of high-affinity and low-affinity receptor subtypes for endothelin-3. J Cardiovasc Pharmacol. 1991;17:S131–S133. doi: 10.1097/00005344-199100177-00035. [DOI] [PubMed] [Google Scholar]
- Fukuroda T, Ozaki S, Ihara M, Ishikawa K, Yano M, Nishikibe M. Synergistic inhibition by BQ-123 and BQ-788 of endothelin-1-induced contractions of the rabbit pulmonary artery. Br J Pharmacol. 1994;113:336–338. doi: 10.1111/j.1476-5381.1994.tb16901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial-cells in the relaxation of arterial smooth-muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Galié N, Badesch D, Oudiz R, Simonneau G, McGoon MD, Keogh AM, et al. Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol. 2005;46:529–535. doi: 10.1016/j.jacc.2005.04.050. [DOI] [PubMed] [Google Scholar]
- Galié N, Hinderliter AL, Torbicki A, Fourme T, Simonneau G, Pulido T, et al. Effects of the oral endothelin-receptor antagonist bosentan on echocardiographic and Doppler measures in patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2003;41:1380–1386. doi: 10.1016/s0735-1097(03)00121-9. [DOI] [PubMed] [Google Scholar]
- Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest. 2000;105:925–933. doi: 10.1172/JCI8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavras H, Brunner HR, Turini GA, Kershaw GR, Tifft CP, Cuttelod S, et al. Antihypertensive effect of oral angiotensin converting-enzyme inhibitor SQ 14225 in man. N Engl J Med. 1978;298:991–995. doi: 10.1056/NEJM197805042981803. [DOI] [PubMed] [Google Scholar]
- Gavras I, Gavras H.Angiotenisn II—possible adverse effects on arteries, heart, brain and kidney: experimental, clinical and epidemiological evidence The Renin–Angiotensin System 1993Gower Medical Publishing: London; In: Robertson JIS and Nicholls MG (eds). [Google Scholar]
- Gayraud M. Raynaud's phenomenon. Joint bone. Spine. 2007;74:1–8. doi: 10.1016/j.jbspin.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Ge YQ, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, et al. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol. 2006;291:F1274–F1280. doi: 10.1152/ajprenal.00190.2006. [DOI] [PubMed] [Google Scholar]
- Gillespie MN, Owasoyo JO, McMurtry IF, O'Brien RF. Sustained coronary vasoconstriction provoked by a peptidergic substance released from endothelial-cells in culture. J Pharmacol Exp Ther. 1986;236:339–343. [PubMed] [Google Scholar]
- Goddard J, Eckhart C, Johnston NR, Cumming AD, Rankin AJ, Webb DJ. Endothelin A receptor antagonism and angiotensin-converting enzyme inhibition are synergistic via an endothelin B receptor-mediated and nitric oxide-dependent mechanism. J Am Soc Nephrol. 2004a;15:2601–2610. doi: 10.1097/01.ASN.0000141313.84470.4B. [DOI] [PubMed] [Google Scholar]
- Goddard J, Johnston NR, Hand MF, Cumming AD, Rabelink TJ, Rankin AJ, et al. Endothelin-A receptor antagonism reduces blood pressure and increases renal blood flow in hypertensive patients with chronic renal failure—a comparison of selective and combined endothelin receptor blockade. Circulation. 2004b;109:1186–1193. doi: 10.1161/01.CIR.0000118499.69469.51. [DOI] [PubMed] [Google Scholar]
- Grandjean T, Rivier JL. Cardio-circulatory effects of beta-adrenergic blockade in organic heart disease. Comparison between propranolol and CIBA 39 089-Ba. Br Heart J. 1968;30:50–59. doi: 10.1136/hrt.30.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray GA, Webb DJ. The endothelin system and its potential as a therapeutic target in cardiovascular disease. Pharmacol Ther. 1996;72:109–148. doi: 10.1016/s0163-7258(96)00101-5. [DOI] [PubMed] [Google Scholar]
- Gregan B, Jurgensen J, Papsdorf G, Furkert J, Schaefer M, Beyermann M, et al. Ligand-dependent differences in the internalization of endothelin A and endothelin B receptor heterodimers. J Biol Chem. 2004;279:27679–27687. doi: 10.1074/jbc.M403601200. [DOI] [PubMed] [Google Scholar]
- Harada H, Kazami J, Watanuki S, Tsuzuki R, Sudoh K, Fujimori A, et al. Ethenesulfonamide and ethanesulfonamide derivatives, a novel class of orally active endothelin-A receptor antagonists. Bioorg Med Chem. 2001;9:2955–2968. doi: 10.1016/s0968-0896(01)00187-0. [DOI] [PubMed] [Google Scholar]
- Harrison VJ, Corder R, Anggard EE, Vane JR. Evidence for vesicles that transport endothelin-1 in bovine aortic endothelial cells. J Cardiovasc Pharmacol. 1993;22:S57–S60. doi: 10.1097/00005344-199322008-00017. [DOI] [PubMed] [Google Scholar]
- Haynes WG, Ferro CJ, O'Kane KPJ, Somerville D, Lomax CC, Webb DJ. Systemic endothelin receptor blockade decreases peripheral vascular resistance and blood pressure in humans. Circulation. 1996;93:1860–1870. doi: 10.1161/01.cir.93.10.1860. [DOI] [PubMed] [Google Scholar]
- Haynes WG, Webb DJ. Endothelin—a long-acting local constrictor hormone. Br J Hosp Med. 1992;47:340–349. [PubMed] [Google Scholar]
- Haynes WG, Webb DJ. Contribution of endogenous generation of endothelin-1 to basal vascular tone. Lancet. 1994;344:852–854. doi: 10.1016/s0140-6736(94)92827-4. [DOI] [PubMed] [Google Scholar]
- Hickey KA, Rubanyi G, Paul RJ, Highsmith RF. Characterization of a coronary vasoconstrictor produced by cultured endothelial cells. Am J Physiol. 1985;248:C550–C556. doi: 10.1152/ajpcell.1985.248.5.C550. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Yoshimi H, Takagi Y, Kanno K, Eguchi S, Marumo F. Interaction of endothelin isopeptides with vascular endothelin-1 receptor. Biomed Res—Tokyo. 1990;11:195–198. [Google Scholar]
- Hoang MV, Turner AJ. Novel activity of endothelin-converting enzyme: hydrolysis of bradykinin. Biochem J. 1997;327:23–26. doi: 10.1042/bj3270023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert M, Cabane J. Successful treatment of systemic sclerosis digital ulcers and pulmonary arterial hypertension with endothelin receptor antagonist bosentan. Rheumatology. 2003;42:191–193. doi: 10.1093/rheumatology/keg050. [DOI] [PubMed] [Google Scholar]
- Ihara M, Ishikawa K, Fukuroda T, Saeki T, Funabashi K, Fukami T, et al. In vitro biological profile of a highly potent novel endothelin (et) antagonist BQ-123 selective for the ET(A) receptor. J Cardiovasc Pharmacol. 1992;20:S11–S14. doi: 10.1097/00005344-199204002-00005. [DOI] [PubMed] [Google Scholar]
- Ikura T, Sawamura T, Shiraki T, Hosokawa H, Kido T, Hoshikawa H, et al. cDNA cloning and expression of bovine endothelin-converting enzyme. Biochem Biophys Res Commun. 1994;203:1417–1422. doi: 10.1006/bbrc.1994.2343. [DOI] [PubMed] [Google Scholar]
- Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, et al. The human endothelin family—3 structurally and pharmacologically distinct isopeptides predicted by 3 separate genes. Proc Natl Acad Sci USA. 1989;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Ihara M, Noguchi K, Mase T, Mino N, Saeki T, et al. Biochemical and pharmacological profile of a potent and selective endothelin B-receptor antagonist, BQ-788. Proc Natl Acad Sci USA. 1994;91:4892–4896. doi: 10.1073/pnas.91.11.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy DD, Parker TA, Abman SH. Prolonged endothelin B receptor blockade causes pulmonary hypertension in the ovine fetus. Am J Physiol Lung Cell Mol Physiol. 2000;279:L758–L765. doi: 10.1152/ajplung.2000.279.4.L758. [DOI] [PubMed] [Google Scholar]
- Ivy DD, Yanagisawa M, Gariepy CE, Gebb SA, Colvin KL, McMurtry IF. Exaggerated hypoxic pulmonary hypertension in endothelin B receptor-deficient rats. Am J Physiol Lung Cell Mol Physiol. 2002;282:L703–L712. doi: 10.1152/ajplung.00272.2001. [DOI] [PubMed] [Google Scholar]
- Jandeleit-Dahm KAM. Dual ACE/NEP inhibitors—more than playing the ACE card. J Human Hypertens. 2006;20:478–481. doi: 10.1038/sj.jhh.1002018. [DOI] [PubMed] [Google Scholar]
- Janes RW, Peapus DH, Wallace BA. The crystal structure of human endothelin. Nat Struct Biol. 1994;1:311–319. doi: 10.1038/nsb0594-311. [DOI] [PubMed] [Google Scholar]
- Jeng AY, Mulder P, Kwan AL, Battistini B. Non-peptidic endothelin-converting enzyme inhibitors and their potential therapeutic applications. Can J Physiol Pharmacol. 2002;80:440–449. doi: 10.1139/y02-025. [DOI] [PubMed] [Google Scholar]
- Johnström P, Fryer TD, Richards HK, Harris NG, Barret O, Clark JC, et al. Positron emission tomography using 18F-labelled endothelin-1 reveals prevention of binding to cardiac receptors owing to tissue-specific clearance by ET B receptors in vivo. Br J Pharmacol. 2005;144:115–122. doi: 10.1038/sj.bjp.0706064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra PR, Moon JCC, Coats AJS. Do results of the ENABLE (Endothelin Antagonist Bosentan for Lowering Cardiac Events in Heart Failure) study spell the end for non-selective endothelin antagonism in heart failure. Int J Cardiol. 2002;85:195–197. doi: 10.1016/s0167-5273(02)00182-1. [DOI] [PubMed] [Google Scholar]
- Kaluski E, Kobrin I, Zimlichman R, Marmor A, Krakov O, Milo O, et al. RITZ-5: randomized intravenous tezosentan (an endothelin-A/B antagonist) for the treatment of pulmonary edema—a prospective, multicenter, double-blind, placebo-controlled study. J Am Coll Cardiol. 2003;41:204–210. doi: 10.1016/s0735-1097(02)02708-0. [DOI] [PubMed] [Google Scholar]
- Karet FE, Davenport AP. Localization of endothelin peptides in human kidney. Kidney Int. 1996;49:382–387. doi: 10.1038/ki.1996.56. [DOI] [PubMed] [Google Scholar]
- Karne S, Jayawickreme CK, Lerner MR. Cloning and characterization of an endothelin-3 specific receptor (ET(C) receptor) from Xenopus laevis dermal melanophores. J Biol Chem. 1993;268:19126–19133. [PubMed] [Google Scholar]
- Kawai N, Yamamoto T, Yamamoto H, McCarron RM, Spatz M. Functional characterization of endothelin receptors on cultured brain capillary endothelial cells of the rat. Neurochem Int. 1997;31:597–605. doi: 10.1016/s0197-0186(97)00018-1. [DOI] [PubMed] [Google Scholar]
- Kelland NF, Webb DJ. Clinical trials of endothelin antagonists in heart failure: a question of dose. Exp Biol Med. 2006;231:696–699. [PubMed] [Google Scholar]
- Kim NN, Dhir V, Azadzoi KM, Traish AM, Flaherty E, Goldstein I. Pilot study of the endothelin—a receptor selective antagonist BMS-193884 for the treatment of erectile dysfunction. J Androl. 2002;23:76–83. doi: 10.1002/jand.2002.23.1.76. [DOI] [PubMed] [Google Scholar]
- Kiowski W, Sutsch G, Hunziker P, Muller P, Kim J, Oechslin E, et al. Evidence for endothelin-1-mediated vasoconstriction in severe chronic heart failure. Lancet. 1995;346:732–736. doi: 10.1016/s0140-6736(95)91504-4. [DOI] [PubMed] [Google Scholar]
- Klipper E, Levy N, Gilboa T, Muller L, Meidan R. Identification of a novel alternatively spliced variant endothelin converting enzyme-1 lacking a transmembrane domain. Exp Biol Med. 2006;231:723–728. [PubMed] [Google Scholar]
- Kloog Y, Ambar I, Sokolovsky M, Kochva E, Wollberg Z, Bdolah A. Sarafotoxin, a novel vasoconstrictor peptide–phosphoinositide hydrolysis in rat heart and brain. Science. 1988;242:268–270. doi: 10.1126/science.2845579. [DOI] [PubMed] [Google Scholar]
- Kohan DE. Endothelins in the normal and diseased kidney. Am J Kidney Dis. 1997;29:2–26. doi: 10.1016/s0272-6386(97)90004-4. [DOI] [PubMed] [Google Scholar]
- Korn JH, Mayes M, Cerinic MM, Rainisio M, Pope J, Hachulla E, et al. Digital ulcers in systemic sclerosis—prevention by treatment with bosentan, an oral endothelin receptor antagonist. Arthritis Rheum. 2004;50:3985–3993. doi: 10.1002/art.20676. [DOI] [PubMed] [Google Scholar]
- Krum H, Viskoper RJ, Lacourciere Y, Budde M, Charlon V. The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. N Engl J Med. 1998;338:784–790. doi: 10.1056/NEJM199803193381202. [DOI] [PubMed] [Google Scholar]
- Kuc RE, Davenport AP. Comparison of endothelin-A and endothelin-B receptor distribution visualized by radioligand binding versus immunocytochemical localization using subtype selective antisera. J Cardiovasc Pharmacol. 2004;44:S224–S226. doi: 10.1097/01.fjc.0000166260.35099.d5. [DOI] [PubMed] [Google Scholar]
- Kukkola PJ, Savage P, Sakane Y, Berry JC, Bilci NA, Ghai RD, et al. Differential structure–activity relationships of phosphoramidon analogs for inhibition of 3 metalloproteases—endothelin-converting enzyme, neutral endopeptidase, and angiotensin-converting enzyme. J Cardiovasc Pharmacol. 1995;26:S65–S68. [PubMed] [Google Scholar]
- Kumar C, Mwangi V, Nuthulaganti P, Wu HL, Pullen M, Brun K, et al. Cloning and characterization of a novel endothelin receptor from Xenopus heart. J Biol Chem. 1994;269:13414–13420. [PubMed] [Google Scholar]
- Kurihara Y, Kurihara H, Suzuki H, Kodama T, Maemura K, Nagai R, et al. Elevated blood-pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature. 1994;368:703–710. doi: 10.1038/368703a0. [DOI] [PubMed] [Google Scholar]
- Lahav R. Endothelin receptor B is required for the expansion of melanocyte precursors and malignant melanoma. Int J Dev Biol. 2005;49:173–180. doi: 10.1387/ijdb.041951rl. [DOI] [PubMed] [Google Scholar]
- Masaki T. Historical review: endothelin. Trends Pharmacol Sci. 2004;25:219–224. doi: 10.1016/j.tips.2004.02.008. [DOI] [PubMed] [Google Scholar]
- McKittrick BA, Stamford AW, Weng XY, Ma K, Chackalamannil S, Czarniecki M, et al. Design and synthesis of phosphinic acids that triply inhibit endothelin converting enzyme, angiotensin converting enzyme and neutral endopeptidase 24.11. Bioorg Med Chem Lett. 1996;6:1629–1634. [Google Scholar]
- Meidan R, Klipper E, Gilboa T, Muller L, Levy N. Endothelin-converting enzyme-1, abundance of isoforms a-d and identification of a novel alternatively spliced variant lacking a transmembrane domain. J Biol Chem. 2005;280:40867–40874. doi: 10.1074/jbc.M505679200. [DOI] [PubMed] [Google Scholar]
- Meyer M, Lehnart S, Pieske B, Schlottauer K, Munk S, Holubarsch C, et al. Influence of endothelin 1 on human atrial myocardium—myocardial function and subcellular pathways. Basic Res Cardiol. 1996;91:86–93. doi: 10.1007/BF00788869. [DOI] [PubMed] [Google Scholar]
- Mickley EJ, Gray GA, Webb DJ. Activation of endothelin ETA receptors masks the constrictor role of endothelin ETB receptors in rat isolated small mesenteric arteries. Br J Pharmacol. 1997;120:1376–1382. doi: 10.1038/sj.bjp.0701036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara SI, Nakajima S, Matumura S, Kohnoike T, Fujimoto M. Pharmacological characterization of a potent nonpeptide endothelin receptor antagonist, 97–139. J Pharmacol Exp Ther. 1994;268:1122–1128. [PubMed] [Google Scholar]
- Mizuguchi T, Nishiyama M, Moroi K, Tanaka H, Saito T, Masuda Y, et al. Analysis of two pharmacologically predicted endothelin B receptor subtypes by using the endothelin B receptor gene knockout mouse. Br J Pharmacol. 1997;120:1427–1430. doi: 10.1038/sj.bjp.0701054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar P, O'Reilly G, Sharkey A, Kuc RE, Harding DP, Plumpton C, et al. Characterization and localization of endothelin receptor subtypes in the human atrioventricular conducting system and myocardium. Circ Res. 1993;72:526–538. doi: 10.1161/01.res.72.3.526. [DOI] [PubMed] [Google Scholar]
- Morita S, Kitamura K, Yamamoto Y, Eto T, Osada Y, Sumiyoshi A, et al. Immunoreactive endothelin in human kidney. Ann Clin Biochem. 1991;28:267–271. doi: 10.1177/000456329102800312. [DOI] [PubMed] [Google Scholar]
- Morris CD, Rose A, Curwen J, Hughes AM, Wilson DJ, Webb DJ. Specific inhibition of the endothelin A receptor with ZD4054: clinical and pre-clinical evidence. Br J Cancer. 2005;92:2148–2152. doi: 10.1038/sj.bjc.6602676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motte S, McEntee K, Naeije R. Endothelin receptor antagonists. Pharmacol Ther. 2006;110:386–414. doi: 10.1016/j.pharmthera.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Muller DN, Mullally A, Dechend R, Park JK, Fiebeler A, Pilz B, et al. Endothelin-converting enzyme inhibition ameliorates angiotensin II-induced cardiac damage. Hypertension. 2002;40:840–846. doi: 10.1161/01.hyp.0000039748.88581.a0. [DOI] [PubMed] [Google Scholar]
- Murugesan N, Gu ZX, Spergel S, Young M, Chen P, Mathur A, et al. Biphenylsulfonamide endothelin receptor antagonists. 4. Discovery of N-[[2′-[[(4,5-dimethyl-3-isoxazolyl)amino]sulfonyl]-4-(2-oxazolyl)[1,1′-biphenyl]-2-yl]methyl]-N,3,3-trimethylbutanamide (BMS-207940), a highly potent and orally active ETA selective antagonist. J Med Chem. 2003;46:125–137. doi: 10.1021/jm020289q. [DOI] [PubMed] [Google Scholar]
- Murugesan N, Gu ZX, Stein PD, Spergel S, Mathur A, Leith L, et al. Biphenylsulfonamide endothelin receptor antagonists. 2. Discovery of 4′-oxazolyl biphenylsulfonamides as a new class of potent, highly selective ETA antagonists. J Med Chem. 2000;43:3111–3117. doi: 10.1021/jm000105c. [DOI] [PubMed] [Google Scholar]
- Mylona P, Cleland JGF. Update of REACH-1 and MERIT-HF clinical trials in heart failure. Eur J Heart Fail. 1999;1:197–200. doi: 10.1016/s1388-9842(99)00022-7. [DOI] [PubMed] [Google Scholar]
- Nguyen QT, Cernacek P, Calderoni A, Stewart DJ, Picard P, Sirois P, et al. Endothelin A receptor blockade causes adverse left ventricular remodeling but improves pulmonary artery pressure after infarction in the rat. Circulation. 1998;98:2323–2330. doi: 10.1161/01.cir.98.21.2323. [DOI] [PubMed] [Google Scholar]
- Nunez DJR, Brown MJ, Davenport AP, Neylon CB, Schofield JP, Wyse RK. Endothelin-1 messenger-RNA is widely expressed in porcine and human tissues. J Clin Invest. 1990;85:1537–1541. doi: 10.1172/JCI114601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor CM, Gattis WA, Adams KF, Hasselblad V, Chandler B, Frey A, et al. Tezosentan in patients' with acute heart failure and acute coronary syndromes—results of the Randomized Intravenous Tezosentan study (RITZ-4) J Am Coll Cardiol. 2003;41:1452–1457. doi: 10.1016/s0735-1097(03)00194-3. [DOI] [PubMed] [Google Scholar]
- O'Connor CM, Gattis WA, Adams KF, Shah MR, Kobrin I, Frey A, et al. Tezosentan in patients with acute heart failure and acute coronary syndromes: design of the Randomized Intravenous Tezosentan study (RITZ-4) Am Heart J. 2002;144:583–588. doi: 10.1016/s0002-8703(02)00127-8. [DOI] [PubMed] [Google Scholar]
- Ohlstein EH, Nambi P, Lago A, Hay DWP, Beck G, Fong KL, et al. Nonpeptide endothelin receptor antagonists. 6. Pharmacological characterization of SE 217242, a potent and highly bioavailable endothelin receptor antagonist. J Pharmacol Exp Ther. 1996;276:609–615. [PubMed] [Google Scholar]
- Opgenorth TJ, Adler AL, Calzadilla SV, Chiou WJ, Dayton BD, Dixon DB, et al. Pharmacological characterization of A-127722: an orally active and highly potent ET(A)-selective receptor antagonist. J Pharmacol Exp Ther. 1996;276:473–481. [PubMed] [Google Scholar]
- Opgenorth TJ, Wuwong JR, Shiosaki K. Endothelin-converting enzymes. FASEB J. 1992;6:2653–2659. doi: 10.1096/fasebj.6.9.1612289. [DOI] [PubMed] [Google Scholar]
- Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. US Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- Pernow J, Bohm F, Johansson BL, Hedin U, Ryden L. Enhanced vasoconstrictor response to endothelin-B-receptor stimulation in patients with atherosclerosis. J Cardiovasc Pharmacol. 2000;36:S418–S420. doi: 10.1097/00005344-200036051-00122. [DOI] [PubMed] [Google Scholar]
- Properzi G, Terenghi G, Gu XH, Poccia G, Pasqua R, Francavilla S, et al. Early increase precedes a depletion of endothelin-1 but not of von Willebrand factor in cutaneous microvessels of diabetic patients, a quantitative immunohistochemical study. J Pathol. 1995;175:243–252. doi: 10.1002/path.1711750213. [DOI] [PubMed] [Google Scholar]
- Quaschning T, Rebhan B, Wunderlich C, Wanner C, Richter CM, Pfab T, et al. Endothelin B receptor-deficient mice develop endothelial dysfunction independently of salt loading. J Hypertens. 2005;23:979–985. doi: 10.1097/01.hjh.0000166838.55688.7e. [DOI] [PubMed] [Google Scholar]
- Ramos-Casals M, Brito-Zeron P, Nardi N, Claver G, Risco G, Parraga FD, et al. Successful treatment of severe Raynaud's phenomenon with bosentan in four patients with systemic sclerosis. Rheumatology. 2004;43:1454–1456. doi: 10.1093/rheumatology/keh340. [DOI] [PubMed] [Google Scholar]
- Reinhart GA, Preusser LC, Burke SE, Wessale JL, Wegner CD, Opgenorth TJ, et al. Hypertension induced by blockade of ETB receptors in conscious nonhuman primates: role of ETA receptors. Am J Physiol Heart Circ Physiol. 2002;283:H1555–H1561. doi: 10.1152/ajpheart.00346.2002. [DOI] [PubMed] [Google Scholar]
- Resink TJ, Hahn AWA, Scott-Burden T, Powell J, Weber E, Buhler FR. Inducible endothelin messenger RNA expression and peptide secretion in cultured human vascular smooth muscle cells. Biochem Biophys Res Commun. 1990;168:1303–1310. doi: 10.1016/0006-291x(90)91171-n. [DOI] [PubMed] [Google Scholar]
- Riechers H, Albrecht HP, Amberg W, Baumann E, Bernard H, Bohm HJ, et al. Discovery and optimization of a novel class of orally active nonpeptidic endothelin-A receptor antagonists. J Med Chem. 1996;39:2123–2128. doi: 10.1021/jm960274q. [DOI] [PubMed] [Google Scholar]
- Roux S, Breu V, Giller T, Neidhart W, Ramuz H, Coassolo P, et al. Ro 61-1790, a new hydrosoluble endothelin antagonist: general pharmacology and effects on experimental cerebral vasospasm. J Pharmacol Exp Ther. 1997;283:1110–1118. [PubMed] [Google Scholar]
- Rubanyi GM, Polokoff MA. Endothelins—molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev. 1994;46:325–415. [PubMed] [Google Scholar]
- Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- Rubinstein I, Gurbanov K, Hoffman A, Better OS, Winaver J. Differential effect of endothelin-1 on renal regional blood-flow—role of nitric-oxide. J Cardiovasc Pharmacol. 1995;26:S208–S210. [PubMed] [Google Scholar]
- Russell FD, Davenport AP. Secretory pathways in endothelin synthesis. Br J Pharmacol. 1999;126:391–398. doi: 10.1038/sj.bjp.0702315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto A, Yanagisawa M, Sakurai T, Takuwa Y, Yanagisawa H, Masaki T. Cloning and functional expression of human cDNA for the ETB endothelin receptor. Biochem Biophys Res Commun. 1991;178:656–663. doi: 10.1016/0006-291x(91)90158-4. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, et al. Cloning of a cDNA-encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- Schalcher C, Cotter G, Reisin L, Bertel O, Kobrin I, Guyene TT, et al. The dual endothelin receptor antagonist tezosentan acutely improves hemodynamic parameters in patients with advanced heart failure. Am Heart J. 2001;142:340–349. doi: 10.1067/mhj.2001.116760. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL. Endothelin: potential role in hypertension and vascular hypertrophy. Hypertension. 1995;25:1135–1143. doi: 10.1161/01.hyp.25.6.1135. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Kroger B, Jacob E, Seulberger H, Subkowski T, Otter R, et al. Molecular characterization of human and bovine endothelin-converting enzyme (ECE-1) FEBS Lett. 1994;356:238–243. doi: 10.1016/0014-5793(94)01277-6. [DOI] [PubMed] [Google Scholar]
- Shetty SS, Savage P, DelGrande D, De Lombaert S, Jeng AY. Characterization of CGS 31447, a potent and non-peptidic endothelin-converting enzyme inhibitor. J Cardiovasc Pharmacol. 1998;31:S68–S70. doi: 10.1097/00005344-199800001-00022. [DOI] [PubMed] [Google Scholar]
- Shi SJ, Rakugi H, Higashimori K, Jiang BB, Higaki J, Mikami H, et al. Augmentation by converting-enzyme inhibition of accelerated endothelin release from rat mesenteric-arteries following nephrectomy. Biochem Biophys Res Commun. 1994;202:246–251. doi: 10.1006/bbrc.1994.1919. [DOI] [PubMed] [Google Scholar]
- Snyder MJ, Jacobs MR, Grau RG, Wilkes DS, Knox KS. Resolution of severe digital ulceration during a course of bosentan therapy. Ann Intern Med. 2005;142:802–803. doi: 10.7326/0003-4819-142-9-200505030-00029. [DOI] [PubMed] [Google Scholar]
- Spatz M, Kawai N, Merkel N, Bembry J, McCarron RM. Functional properties of cultured endothelial cells derived from large microvessels of human brain. Am J Physiol. 1997;272:C231–C239. doi: 10.1152/ajpcell.1997.272.1.C231. [DOI] [PubMed] [Google Scholar]
- Spieker LE, Mitrovic V, Noll G, Pacher R, Schulze MR, Muntwyler J, et al. Acute hemodynamic and neurohumoral effects of selective ETA receptor blockade in patients with congestive heart failure. J Am Coll Cardiol. 2000;35:1745–1752. doi: 10.1016/s0735-1097(00)00649-5. [DOI] [PubMed] [Google Scholar]
- Stewart DJ, Levy RD, Cernacek P, Langleben D. Increased plasma endothelin-1 in pulmonary-hypertension—marker or mediator of disease. Ann Intern Med. 1991;114:464–469. doi: 10.7326/0003-4819-114-6-464. [DOI] [PubMed] [Google Scholar]
- Strachan FE, Spratt JC, Wilkinson IB, Johnston NR, Gray GA, Webb DJ. Systemic blockade of the endothelin-B receptor increases peripheral vascular resistance in healthy men. Hypertension. 1999;33:581–585. doi: 10.1161/01.hyp.33.1.581. [DOI] [PubMed] [Google Scholar]
- Tabrizchi R. SLV-306. Solvay. Curr Opin Investig Drugs. 2003;4:329–332. [PubMed] [Google Scholar]
- Takahashi H, Soma S, Muramatsu M, Oka M, Ienaga H, Fukuchi Y. Discrepant distribution of big endothelin (ET)-1 and ET receptors in the pulmonary artery. Eur Respir J. 2001;18:5–14. doi: 10.1183/09031936.01.00075501. [DOI] [PubMed] [Google Scholar]
- Takaishi S, Tuchiya N, Sato A, Negishi T, Takamatsu Y, Matsushita Y, et al. B-90063, a novel endothelin converting enzyme inhibitor isolated from a new marine bacterium, Blastobacter sp. SANK 71894. J Antibiot. 1998;51:805–815. doi: 10.7164/antibiotics.51.805. [DOI] [PubMed] [Google Scholar]
- Torre-Amione G, Durand JB, Nagueh S, Vooletich MT, Kobrin I, Pratt C. A pilot safety trial of prolonged (48 h) infusion of the dual endothelin-receptor antagonist tezosentan in patients with advanced heart failure. Chest. 2001a;120:460–466. doi: 10.1378/chest.120.2.460. [DOI] [PubMed] [Google Scholar]
- Torre-Amione G, Young JB, Colucci WS, Lewis BS, Pratt C, Cotter G, et al. Hemodynamic and clinical effects of tezosentan, an intravenous dual endothelin receptor antagonist, in patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2003;42:140–147. doi: 10.1016/s0735-1097(03)00556-4. [DOI] [PubMed] [Google Scholar]
- Torre-Amione G, Young JB, Durand JB, Bozkurt B, Mann DL, Kobrin I, et al. Hemodynamic effects of tezosentan, an intravenous dual endothelin receptor antagonist, in patients with class iii–iv congestive heart failure. Circulation. 2001b;103:973–980. doi: 10.1161/01.cir.103.7.973. [DOI] [PubMed] [Google Scholar]
- Trapani AJ, Beil ME, Bruseo CW, De Lombaert S, Jeng AY. Pharmacological properties of CGS 35066, a potent and selective endothelin-converting enzyme inhibitor, in conscious rats. J Cardiovasc Pharmacol. 2000;36:S40–S43. doi: 10.1097/00005344-200036051-00015. [DOI] [PubMed] [Google Scholar]
- Trapani AJ, Beil ME, Bruseo CW, Savage P, Firooznia F, Jeng AY. CGS 35601 and its orally active prodrug CGS 37808 as triple inhibitors of endothelin-converting enzyme-1, neutral endopeptidase 24.11, and angiotensin-converting enzyme. J Cardiovasc Pharmacol. 2004;44:S211–S215. doi: 10.1097/01.fjc.0000166237.57077.d6. [DOI] [PubMed] [Google Scholar]
- Tschudi MR, Luscher TF. Characterization of contractile endothelin and angiotensin receptors in human resistance arteries—evidence for 2 endothelin and one angiotensin receptor. Biochem Biophys Res Commun. 1994;204:685–690. doi: 10.1006/bbrc.1994.2514. [DOI] [PubMed] [Google Scholar]
- Tsurumi Y, Fujie K, Nishikawa M, Kiyoto S, Okuhara M. Biological and pharmacological properties of highly selective new endothelin-converting enzyme-inhibitor WS79089B isolated from Streptosporangium roseum no-79089. J Antibiot. 1995a;48:169–174. doi: 10.7164/antibiotics.48.169. [DOI] [PubMed] [Google Scholar]
- Tsurumi Y, Ueda H, Hayashi K, Takase S, Nishikawa M, Kiyoto S, et al. WS75624-A and WS75624-B, new endothelin-converting enzyme-inhibitors isolated from Saccharothrix sp. no-75624. 1. Taxonomy, fermentation, isolation, physicochemical properties and biological-activities. J Antibiot. 1995b;48:1066–1072. doi: 10.7164/antibiotics.48.1066. [DOI] [PubMed] [Google Scholar]
- Umekawa K, Hasegawa H, Tsutsumi Y, Sato K, Matsumura Y, Ohashi N. Pharmacological characterization of a novel sulfonylureid-pyrazole derivative, SM-19712, a potent nonpeptidic inhibitor of endothelin converting enzyme. Jpn J Pharmacol. 2000;84:7–15. doi: 10.1254/jjp.84.7. [DOI] [PubMed] [Google Scholar]
- Vajkoczy P, Meyer B, Weidauer S, Raabe A, Thome C, Ringel F, et al. Clazosentan (AXV-034343), a selective endothelin A receptor antagonist, in the prevention of cerebral vasospasm following severe aneurysmal subarachnoid hemorrhage: results of a randomized, double-blind, placebo-controlled, multicenter phase IIa study. J Neurosurg. 2005;103:9–17. doi: 10.3171/jns.2005.103.1.0009. [DOI] [PubMed] [Google Scholar]
- Valdenaire O, Lepailleur-Enouf D, Egidy G, Thouard A, Barret A, Vranckx R, et al. A fourth isoform of endothelin-converting enzyme (ECE-1) is generated from an additional promoter—molecular cloning and characterization. Eur J Biochem. 1999;264:341–349. doi: 10.1046/j.1432-1327.1999.00602.x. [DOI] [PubMed] [Google Scholar]
- von Geldern TW, Tasker AS, Sorensen BK, Winn M, Szczepankiewicz BG, Dixon DB, et al. Pyrrolidine-3-carboxylic acids as endothelin antagonists. 4. Side chain conformational restriction leads to ETB selectivity. J Med Chem. 1999;42:3668–3678. doi: 10.1021/jm990170q. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Awane Y, Ikeda S, Fujiwara S, Kubo K, Kikuchi T, et al. Pharmacology of a nonselective ET(A) and ET(B) receptor antagonist, TAK-044 and the inhibition of myocardial infarct size in rats. Br J Pharmacol. 1995;114:949–954. doi: 10.1111/j.1476-5381.1995.tb13296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Schmitt R, Birnboeck H, Hopfgartner G, vanMarle SP, Peeters PAM, et al. Pharmacokinetics and pharmacodynamics of the endothelin-receptor antagonist bosentan in healthy human subjects. Clin Pharmacol Ther. 1996;60:124–137. doi: 10.1016/S0009-9236(96)90127-7. [DOI] [PubMed] [Google Scholar]
- Whitebread S, Mele M, Kamber B, Degasparo M. Preliminary biochemical characterization of two angiotensin-II receptor subtypes. Biochem Biophys Res Commun. 1989;163:284–291. doi: 10.1016/0006-291x(89)92133-5. [DOI] [PubMed] [Google Scholar]
- Wu CD, Chan MF, Stavros F, Raju B, Okun I, Mong S, et al. Discovery of TBC11251, a potent, long acting, orally active endothelin receptor-A selective antagonist. J Med Chem. 1997;40:1690–1697. doi: 10.1021/jm9700068. [DOI] [PubMed] [Google Scholar]
- Wu CD, Decker ER, Blok N, Bui H, You TJ, Wang JM, et al. Discovery, modeling, and human pharmacokinetics of N-(2-acetyl-4,6-dimethylphenyl)-3-(3,4-dimethylisoxazol-5-sulfamoyl)thiophene-2-carboxamide (TBC3711), a second generation, ETA selective, and orally bioavailable endothelin antagonist. J Med Chem. 2004;47:1969–1986. doi: 10.1021/jm030528p. [DOI] [PubMed] [Google Scholar]
- Wypij DM, Nichols JS, Novak PJ, Stacy DL, Berman J, Wiseman JS. Role of mast-cell chymase in the extracellular processing of big-endothelin-1 to endothelin-1 in the perfused rat lung. Biochem Pharmacol. 1992;43:845–853. doi: 10.1016/0006-2952(92)90252-e. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Hammer RE, Richardson JA, Emoto N, Williams SC, Takeda S, et al. Disruption of ECE-1 and ECE-2 reveals a role for endothelin-converting enzyme-2 in murine cardiac development. J Clin Invest. 2000;105:1373–1382. doi: 10.1172/JCI7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]