Abstract
Background and purpose:
Dipyridamole enhances post-occlusive reactive hyperaemia (PORH) in the human forearm vascular bed. We hypothesize that this effect is completely mediated by increased adenosine receptor stimulation. To test this hypothesis, the effect of caffeine (an adenosine receptor antagonist) on dipyridamole-induced augmentation of PORH was explored.
Experimental approach:
The forearm vasodilator responses to three increasing periods of forearm ischaemia (2, 5 and 13 min) were determined during placebo infusion. Forty minutes after the last reperfusion period, this procedure was repeated during intra-arterial infusion of dipyridamole (7.4 nmol min−1 per 100 ml forearm). At least 2 weeks later, this whole procedure was repeated, but now in the presence of caffeine (90 μg min−1 per 100 ml volume).
Key results:
After 2, 5 and 13 min of ischaemia, the average forearm blood flow increased to 5.6±0.7, 9.7±1.3 and 34.5±2.1 ml min−1 per 100 ml. After infusion of dipyridamole into the brachial artery, these numbers were significantly increased to 7.7±0.8, 12.5±1.5 and 41.6±3.1 ml min−1 per 100 ml. This response was abolished by the concomitant infusion of caffeine (6.6±0.5, 10.2±0.6, 35.1±2.2 (caffeine) versus 7.4±0.4, 10.5±0.6, 33.7±2.2 ml min−1per 100 ml (caffeine/dipyridamole)).
Conclusions and implications:
Caffeine prevented the augmenting effect of dipyridamole on PORH. This indicates that dipyridamole-induced augmentation of PORH is mediated via increased adenosine receptor stimulation as a result of elevated extracellular formation of adenosine during ischaemia.
Keywords: adenosine, dipyridamole, caffeine, reactive hyperaemia, ischaemia
Introduction
Adenosine, an endogenous nucleoside, has a variety of effects through different subtypes of adenosine receptors (Rongen et al., 1997; Tabrizchi and Bedi, 2001). Adenosine reduces platelet aggregation, the inflammatory response and the release of noradrenaline from vascular sympathetic nerve endings (Kitakaze et al., 1991; Rongen et al., 1996; Hasko and Cronstein, 2004). Furthermore, adenosine stimulates the release of nitric oxide from the endothelium and modulates vascular wall proliferation (Smits et al., 1995; Burnstock, 2002). These actions of adenosine may act in concert to prevent atherosclerosis, as has been recently proposed by observations in ecto-5′-nucleotidase-deficient mice (Zernecke et al., 2006). In addition, adenosine reduces ischaemia–reperfusion injury in heart, brain and skeletal muscle (De Jong et al., 2000; Liem et al., 2001; Rongen et al., 2005).
Clinical exploitation of these beneficial actions of adenosine are hampered by the rapid cellular uptake of infused adenosine, which limits its availability in the vascular wall and tissue (Moser et al., 1989; Gamboa et al., 2003; Bijlstra et al., 2004), and by the significant chemoreflex activation of the sympathetic nervous system, which may trigger cardiac arrhythmias, activate platelet aggregation and induce adverse vascular remodelling when this nucleoside is infused intravenously (Paul et al., 1990; Smits et al., 1991a; Rongen et al., 1996). Therefore, alternative approaches have been developed to fulfil the cardiovascular promises of adenosine, including pharmacological inhibition of the transmembrane transport of adenosine by the so-called equilibrative nucleoside transporters (ENTs) (Rongen et al., 1995). Indeed, oral treatment with dipyridamole inhibits cellular adenosine uptake (Gamboa et al., 2005; Riksen et al., 2005a) and improves clinical outcome in patients with atherosclerosis (Diener et al., 1996). In addition, treatment with dipyridamole prevents ischaemia–reperfusion injury (Strauer et al., 1996; Heidland et al., 2000; Riksen et al., 2005a). However, it is still a matter of debate whether these clinical benefits of treatment with dipyridamole are mediated by endogenous adenosine. In particular, two areas of uncertainty exist. Firstly, the intracellular concentration of adenosine rises during ischaemia, which reduces or may even reverse the adenosine gradient across the cell membrane. Secondly, dipyridamole may have other actions, including free radical scavenging, prostacyclin release and inhibition of phosphodiesterases, that may affect the incidence of atherothrombosis or ischaemia–reperfusion injury.
We have recently shown that dipyridamole augments forearm post-occlusive reactive hyperaemia (PORH) in healthy volunteers (Riksen et al., 2007). Although this observation suggests that under these conditions dipyridamole increases extracellular adenosine levels, we have not yet ascertained whether endogenous adenosine is fully responsible for this action of dipyridamole. In the present study, we show that dipyridamole-induced augmentation of PORH can be completely abolished by caffeine, a competitive antagonist of A1 and A2 adenosine receptors (Fredholm and Persson, 1982; Smits et al., 1990; Biaggioni et al., 1991). This indicates that dipyridamole-induced augmentation of PORH is mediated through increased adenosine receptor stimulation as a result of elevated extracellular formation of adenosine during ischaemia.
Methods
Subjects
After the study protocol was approved by the Institutional Review Board of the Radboud University Nijmegen Medical Centre, eight healthy volunteers (six females; age 19–24 years) with a normal medical history, physical examination and blood pressure gave written informed consent before entering the study. All volunteers participated twice with a minimum study-free interval of 2 weeks. On both days, volunteers were studied in supine position after at least 24 h of caffeine abstinence. Experiments were performed in a temperature-controlled room (24 °C) in the morning after an overnight fast or in the afternoon after having refrained from food or fluid intake for 6 h. For each volunteer, the two studies were performed at the same time of the day.
Measurements made
After local anaesthesia of the skin (xylocaine 2%), a 20 gauge arterial catheter (Angiocath, Deseret Medical Inc., Sandy, UT, USA) was inserted into the brachial artery of the non-dominant arm for intra-arterial drug administration and blood pressure monitoring. In both arms, forearm blood flow (FBF) was measured simultaneously with venous occlusion plethysmography using mercury-in-silastic strain gauges. These strain gauges (DE Hokanson Inc., Washington, DC, USA) were connected to a 24-bit A/D (analog/digital) converter developed by the technical department of the Radboud University Nijmegen Medical Centre (Fysioflex System, Nijmegen, The Netherlands). Automatic analysis of the plethysmographic signal was performed off-line and manually controlled using MIDAC software. Validation of this recording system was performed by comparison with the traditional Hokanson EC4 system (DE Hokanson Inc.) showing similar FBF results (data not shown). During measurements of FBF, the hand circulation was excluded by inflating a wrist cuff to 200 mm Hg to restrict the measurements to skeletal muscle blood flow as much as possible (Lenders et al., 1991).
Experimental design
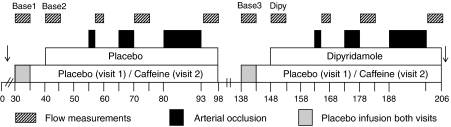
Thirty minutes after cannulation of the brachial artery, normal saline was infused with concomitant measurement of baseline FBF for 5 min (Figure 1). Baseline FBF was measured again after 10 min, followed by determining FBF after three increasing periods of forearm ischaemia (2, 5 and 13 min) recorded during, respectively, 3, 5 and 5 min of post-occlusive reperfusion time. During the last minute of the 13-min ischaemic episode, the subjects performed rhythmic hand gripping as a stimulus to maximize forearm vasodilation (Pedrinelli et al., 1987). Between the last flow measurement and subsequent occlusion of the forearm, 5 min of extra reperfusion allowed the hand circulation to recover from the wrist cuff inflations. After 40 min, baseline measurements of FBF were repeated. Subsequently, intra-arterial infusion of normal saline was replaced by dipyridamole (7.4 nmol min−1 per 100 ml of forearm volume). The administered dose of dipyridamole has previously been shown to augment the vasodilator response to adenosine without relevantly affecting baseline FBF (Bijlstra et al., 2004).
Figure 1.
Schematic overview of the experimental protocol, indicating baseline flow measurements, arterial occlusion and reperfusion flow measurements. Caffeine replaced placebo infusion during the second visit. ↓ Blood sampling for ex vivo determination of adenosine and uridine transport (only performed during the second visit).
The same study protocol was applied on both study days, except that on the second study day, 0.9% saline was replaced by caffeine (90 μg min−1 per 100 ml). First, PORH was determined in the presence of caffeine, followed by determining the effect of caffeine on dipyridamole-enhanced PORH (Figure 1). During all procedures, total intra-arterial infusion rate was kept constant at 100 μl min−1 per 100 ml. Dipyridamole and caffeine infusions were stopped 10 s after initiation of ischaemia and recommenced 10 s before reperfusion. Previously, we performed a time control experiment, which revealed a reproducible hyperaemic response to the used ischaemic stimuli (Bijlstra et al., 1996).
Ex vivo determination of nucleoside uptake inhibition
During their second visit, blood from the venous effluent of the experimental arm was collected in EDTA-containing vacutainer tubes at baseline and during intra-arterial dipyridamole infusion at the end of the experiment for ex vivo determination of transport inhibition of adenosine and uridine (Riksen et al., 2005a). Adenosine and uridine are both specifically transported by ENT, which can be blocked by dipyridamole. After blood sampling, the blood was immediately centrifuged, the erythrocytes washed twice in normal saline and resuspended in morpholinepropanesulphonic acid buffer to obtain a 20% solution.
For uridine transport measurements, a 50 μl uridine solution was added to 100 μl of 10% erythrocytes in morpholinepropanesulphonic acid buffer to obtain a final concentration of 10, 30, 100, 200, 400 and 1000 μM. After 3 s, uridine uptake was completely blocked by 100 μl of 25 μM dipyridamole, and the erythrocytes were isolated by immediate centrifugation through a dibutyl phthalate layer. After removal and washing of the upper layer, the erythrocytes were lysed with Triton X-100 and treated with perchloric acid for protein precipitation. After centrifugation, the uridine concentration in the supernatant was determined by HPLC (see analytical procedures).
To determine adenosine uptake, adenosine (in a final concentration of 3 μM) was added to 100 μl of 1% erythrocytes in Tris-NaCl buffer. After 0, 3, 6, 10 and 15 min, adenosine uptake and deamination were completely blocked by high-dose dipyridamole (10 μM) and erythro-9-(2-hydroxynon-3-yl)-adenine (8 μM), respectively. Subsequently, the cells were separated from the supernatant by centrifugation through a dibutyl phthalate layer, and the adenosine concentration in the supernatant was determined as described below.
Analytical procedures
Plasma caffeine concentrations were determined by the use of reversed-phase HPLC with UV detection set at 273 nm according to Schreiber-Deturmeny and Bruguerolle (1996). In the ex vivo nucleoside uptake experiments, uridine concentration was determined by HPLC with UV detection set at 254 nm using a Polaris C18 column. For the mobile phase, 10 mM TBAHS (tetrabutylammoniumhydrogensulphate) in 0.1% acetic acid was used. Uridine uptake was expressed as nmol per min per mg protein in the membranous fraction, determined according to the well-documented Lowry assay. The adenosine concentration was determined with reversed-phase HPLC with UV detection set at 260 nm. Adenosine was separated by a linear gradient of 2% acetonitrile (in 10 mM TBAHS, 20 mM NH4H2PO4, pH 6.0) to 35% acetonitrile in 15 min at 1 ml min−1.
Statistical analysis
All ex vivo measurements were performed in duplicate and averaged for each subject. The ex vivo uridine uptake was plotted for each subject according to the Michaelis–Menten kinetics. The calculated Vmax (maximum velocity) for uridine uptake and the effect of dipyridamole on adenosine uptake were analysed by paired t-tests.
The last 4 min of baseline FBF measurements were averaged to one value. All FBF measurements during the first 3 min of reperfusion were averaged to one value (FBF3-min). An ANOVA for repeated measures was performed on FBFpeak (maximum FBF during reperfusion) and FBF3-min with caffeine, dipyridamole and arterial occlusion time, as within-subject factors to determine the effect of caffeine on dipyridamole-enhanced PORH. Results are expressed as mean±s.e.mean.
Drugs and solutions
All solutions were freshly prepared. Dipyridamole (Boehringer Ingelheim Espana S.A., Barcelona, Spain) and caffeine (Genfarma, Maarssen, The Netherlands) were diluted to reach final syringe concentrations of 7.4 nmol and 90 μg per 50 μl, respectively.
Results
With the exception of one volunteer on the second study day (1.5 mg l−1, prior to caffeine infusion), the baseline serum caffeine concentrations were below 0.4 mg l−1 during both study days, indicating excellent compliance to the caffeine-free diet. Baseline FBF did not differ between the two study visits. Forty minutes after the first set of ischaemic challenges, FBF remained slightly elevated on both study days as compared with baseline, suggesting some residual vasodilation. Mean arterial pressure slightly increased during the course of the experiment, but this increase did not significantly differ between the two visits (Table 1). Moreover, FBF in the non-infused forearm remained constant throughout the study with the exception of a slight increase during the 13-min ischaemic episode that was not affected by dipyridamole or caffeine infusion (data not shown).
Table 1.
FBF3-min and MAP3-min at baseline and after ischaemia
|
MAP3−min (mm Hg) |
FBF3-min (ml min−1 per 100 ml) |
|||
|---|---|---|---|---|
| Visit 1 (placebo) | Visit 2 (caffeine) | Visit 1 (placebo) | Visit 2 (caffeine) | |
| # | † | # | $ | |
| Base 1 | 82.3±2.3 | 81.8±3.3 | 2.5±0.5 | 2.8±0.4 |
| Base 2 | 82.6±1.8 | 83.6±3.3 | 2.4±0.5 | 3.1±0.4 |
| 2 min ischaemia | 82.3±1.6 | 84.2±3.6 | 5.6±0.7 | 6.6±0.5 |
| 5 min ischaemia | 83.4±1.6 | 84.7±3.9 | 9.7±1.3 | 10.2±0.6 |
| 13 min ischaemia | 83.8±1.7 | 83.9±3.6 | 34.5±2.1 | 35.1±2.2 |
| Base 3 | 85.1±1.6a | 86.8±3.9a | 3.0±0.6a | 3.7±0.5a |
| Dipy | 85.8±1.7a | 87.9±3.9a | 3.4±0.5a | 3.7±0.4a |
| 2 min ischaemia | 86.2±1.6 | 86.7±3.7 | 7.7±0.8 | 7.4±0.4 |
| 5 min ischaemia | 86.2±1.7 | 86.5±3.8 | 12.5±1.5 | 10.5±0.6 |
| 13 min ischaemia | 87.1±2.6 | 85.5±3.7 | 41.6±3.1 | 33.7±2.2 |
Abbreviations: Dipy, baseline during dipyridamole infusion; FBF, forearm blood flow; FBF3-min and MAP3-min, average of 3 min of reperfusion; MAP, mean arterial pressure.
Data are mean±s.e.mean.
P<0.05 versus base 1 and base 2; #P<0.05 for the effect of dipyridamole;
P>0.1 for the interaction between caffeine and dipyridamole points to both P>0.1 and P<0.01 and $P<0.01 for the interaction between caffeine and dipyridamole.
Forearm circulation during reperfusion
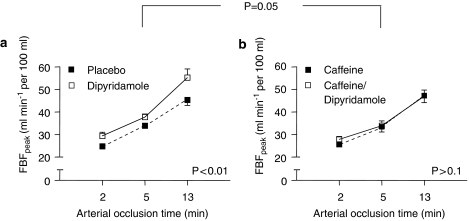
FBFpeak increased as a function of the severity of the ischaemic challenge (Figure 2). This vasodilator state during reperfusion was significantly potentiated by intra-arterial infusion of dipyridamole (Figure 2a). FBF3-min showed a similar pattern (Table 1).
Figure 2.
Peak forearm blood flow (FBFpeak) after ischaemia. Data are mean±s.e.mean. (a) placebo versus dipyridamole and (b) caffeine versus caffeine/dipyridamole. P-value indicates level of significance for the effect of dipyridamole on peak FBF after ischaemia (a and b) and the interaction between caffeine and dipyridamole (a versus b, ANOVA for repeated measures).
Intra-arterial co-administration of caffeine did not affect reactive hyperaemia in the absence of dipyridamole. However, the augmenting effect of dipyridamole on FBFpeak was almost completely abolished by caffeine (Figure 2b). Similar results were obtained when results are expressed as FBF3-min (Table 1).
Ex vivo determination of nucleoside uptake inhibition
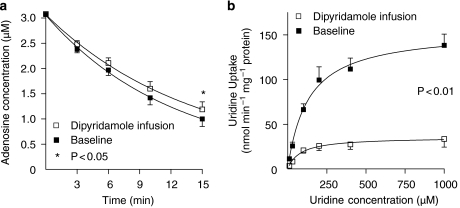
In one volunteer, the ex vivo determination of uridine uptake failed due to technical difficulties. In blood drawn from the remaining seven volunteers, uridine uptake into the erythrocytes was significantly inhibited after dipyridamole infusion compared to baseline. After intra-arterial dipyridamole infusion, Vmax decreased to about 20% of the control value (P<0.01; paired t-test; Figure 3). In concordance, the adenosine concentration in the supernatant, 15 min after ex vivo addition of adenosine, was significantly higher in blood drawn during dipyridamole infusion compared to baseline (P<0.05; Figure 3).
Figure 3.
The ex vivo fall in supernatant adenosine concentration as a result of uptake into erythrocytes and uridine uptake into erythrocytes with and without dipyridamole. (a) Concentration of adenosine as measured in the supernatant up to 15 min after ex vivo addition of adenosine to whole blood that was sampled at baseline and during intra-arterial infusion of dipyridamole (n=8). (b) Uridine uptake as a function of uridine concentration as measured in blood that was collected at baseline and during intra-arterial dipyridamole infusion (n=7). Vmax: 158±20 and 34±8 nmol per min per mg protein in absence and presence of dipyridamole, respectively. Data are mean±s.e.mean. Vmax, maximum velocity.
Discussion
The main new finding of this study is that the enhancing effect of dipyridamole on the vasodilator response to ischaemia is prevented by the adenosine receptor antagonist caffeine. This indicates the involvement of adenosine receptor stimulation by endogenous adenosine in this action of dipyridamole. In addition, intra-arterial infusion of a low dose of dipyridamole appeared sufficient to inhibit the ENT in erythrocytes that were collected from the venous effluent of the infused forearm, confirming the ENT as a target for dipyridamole in this experimental set-up. Taken together, these observations indicate the involvement of adenosine receptors in the dipyridamole-induced augmentation of PORH and support the concept of extracellular formation of adenosine during ischaemia.
Carlsson et al. (1987) previously observed an increased PORH after intravenous infusion of dipyridamole that was inhibited by intravenous theophylline. Although the conclusions of the authors fit well with our observations, this study of Carlsson et al. is difficult to interpret, as both drugs have important effects on blood pressure and heart rate when infused intravenously (Smits et al., 1991b). Unfortunately, they did not report any recordings of blood pressure or heart rate in this study. Drug-related changes in blood pressure could have interfered with their measurements of PORH. Our present study, however, used intra-arterial infusions of both dipyridamole and caffeine at doses that do not induce any significant change in arterial blood pressure. Therefore, the presently observed interaction between caffeine and dipyridamole is not confounded by systemic haemodynamic changes. In addition, Carlsson et al. (1987) used the air-filled latex rubber cuff to measure FBF as opposed to strain gauge plethysmography used in the present study. With this technique, they could not observe a contribution of hand circulation to recorded FBF, nor an incremental effect of prolonged occlusion time (>5 min) on peak post-occlusive flow. Both observations of Carlsson et al (1987) contrast with studies using strain gauge plethysmography (Pedrinelli et al., 1987; Lenders et al., 1991). We believe that the FBF detected with air-filled latex rubber cuffs is less reliable than strain gauge plethysmography as used in our study. Finally, inclusion of the hand in the experimental preparation, as in the experiments of Carlsson et al., increases the contribution of cutaneous blood flow to the measured changes in FBF. In the skin, adenosine-induced vasodilatation via A2 adenosine receptors is significantly reduced by simultaneous stimulation of A1 adenosine receptors (Stojanov and Proctor, 1990). As the contribution of A1 (vasoconstriction) and A2 (vasodilation) receptors may differ between cutaneous and skeletal muscle blood flow, we prefer to limit our measurements to the skeletal muscle circulation as much as possible.
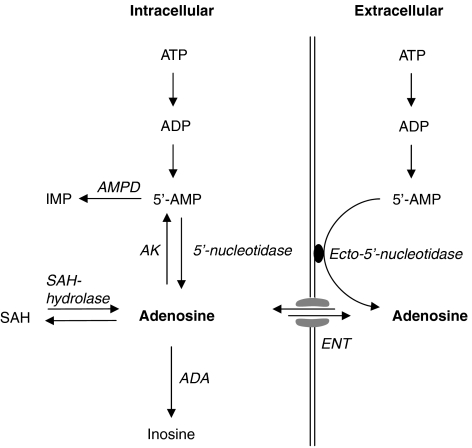
There are currently two known pathways for the production of adenosine (Figure 4), namely dephosphorylation of 5′-AMP and hydrolysis of S-adenosylhomocysteine (SAH) (Schutz et al., 1981). The rate of dephosphorylation of 5′-AMP and hydrolysis of SAH depends on the enzyme activity of 5′-nucleotidase and SAH hydrolase and plasma homocysteine concentration (Ueland, 1982; Darvish et al., 1996; Riksen et al., 2005b). 5′-Nucleotidase is located both in the cytosol and on the extracellular surface of various cell types (so-called ecto-5′-nucleotidase). During well-oxygenated conditions, approximately 90% of adenosine is produced within the cell and is almost entirely derived from SAH (Kroll et al., 1992; Deussen et al., 1999). Owing to the effective intracellular rephosphorylation of adenosine to 5′-AMP by adenosine kinase, the cytosolic adenosine concentration remains very low within the cell resulting in a transmembrane concentration gradient driving extracellular adenosine into the cytosol (Kroll et al., 1993; Deussen et al., 1999). Nucleoside transporters facilitate this diffusion of adenosine across the cell membrane and are responsible for the short half-life of circulating adenosine. The pharmacological actions of ENT inhibitors in well-oxygenated tissues confirm the important role of this transporter in the kinetics of extracellular adenosine (Rongen et al., 1995; Riksen et al., 2005c).
Figure 4.
A schematic presentation of adenosine metabolism. Under normal, well-oxygenated conditions, adenosine kinase (AK) and adenosine deaminase (ADA) activity results in an adenosine concentration gradient across the cell membrane that favours diffusion of adenosine into the cell. This diffusion is facilitated by the equilibrative nucleoside transporter (ENT) and inhibited by dipyridamole. In this study, we provide pharmacological evidence that dipyridamole augments the role of extracellular adenosine in post-occlusive hyperaemia. These observations indicate that during hypoxic conditions, diffusion of adenosine remains directed towards the cytosol and therefore support an extracellular source of adenosine during ischaemia. AMPD, AMP deaminase; IMP, inosine monophosphate; SAH, S-adenosylhomocysteine.
Although the increase in intravascular adenosine concentrations during ischaemia has been well documented (Costa et al., 1999; Saito et al., 1999), controversy exists about the origin of this additional extracellular adenosine. Hypoxia causes an inhibition of adenosine kinase, the enzyme responsible for rephosphorylation of adenosine, in the guinea pig heart (Decking et al., 1997). This reduces the metabolism of intracellular adenosine, which may reduce or even reverse the transmembrane concentration gradient.
Most data from animal studies point towards an important role for 5′-nucleotidase in the formation of extracellular adenosine during ischaemia (Frick and Lowenstein, 1976; Headrick et al., 1992; Kitakaze et al., 1993, 1994), although some data are equivocal (Skinner and Marshall, 1996) possibly due to interspecies differences and variation in experimental set-up. An important issue in this regard is whether the 5′-nucleotidase-mediated formation of adenosine during ischaemia occurs within the cell by cytosolic 5′-nucleotidase or in the extracellular matrix by ecto-5′-nucleotidase. The answer to this question determines the clinical utility of ENT inhibitors to augment extracellular adenosine during ischaemia: cytosolic formation of adenosine during ischaemia requires a functional ENT to increase extracellular adenosine and subsequent stimulation of adenosine receptors, whereas the ENT would limit extracellular adenosine concentrations when this nucleoside is formed in the extracellular matrix during ischaemia.
We have previously shown that dipyridamole augments PORH (Riksen et al., 2007). As dipyridamole blocks adenosine transport across the cell membrane, the enhancing effect on PORH suggests an increased extracellular adenosine formation during ischaemia. However, apart from ENT inhibition, dipyridamole has several other actions, such as inhibition of several phosphodiesterase isoenzymes (Fujishige et al., 1999; Gardner et al., 2000). This can lead to upregulation of cAMP or cGMP-dependent pathways triggered by endogenous substances such as prostacyclin or nitric oxide. Alternatively, increased intracellular adenosine, resulting from cytosolic formation of adenosine during ischaemia in the presence of ENT blockade, might in theory stimulate cellular processes leading to vasodilation without affecting membrane-bound adenosine receptors. These actions of dipyridamole could augment PORH without increasing adenosine levels in the extracellular matrix. Thus, to understand the action of dipyridamole on PORH, it is of importance to ascertain the involvement of adenosine receptor stimulation. Therefore, we used caffeine as a competitive antagonist of A1 and A2 adenosine receptors (Fredholm and Persson, 1982; Smits et al., 1990; Biaggioni et al., 1991). We observed that caffeine completely prevented the augmenting effect of dipyridamole on PORH. In our experimental set-up, caffeine did not affect PORH in the absence of dipyridamole, supporting a specific interaction between dipyridamole and caffeine. In addition, we found that the dose and mode of administration of dipyridamole used here indeed inhibited nucleoside transport in the infused forearm, indicating that the ENT is effectively blocked. We interpret these findings as proof of the concept that ischaemia induces extracellular formation of adenosine resulting in stimulation of adenosine receptors without dependence on intracellular sources of adenosine.
Several potential limitations in our study have to be addressed. As in all volunteers caffeine was administered during the second visit, an order effect could have interfered with our findings. However, an experiment-free interval of 2 weeks is expected to be sufficient to exclude any possible carry over effect. This is further supported by similar baseline blood pressure and FBF and a comparable hyperaemic response to the first set of ischaemic challenges on the two study days. FBF did not return to baseline after a 40-min equilibration period. Caffeine infusion did not influence baseline FBF resulting in a similar baseline FBF prior to the second set of ischaemic challenges on the two study days. Therefore, this small residual vasodilation does not explain the absence of dipyridamole-enhanced reactive hyperaemia in the presence of caffeine.
In conclusion, we have shown an enhancing effect of dipyridamole on PORH. This effect could be abolished by the administration of the adenosine receptor antagonist caffeine. This observation indicates the critical involvement of adenosine receptors in dipyridamole-enhanced PORH. From this observation, we conclude that increased levels of extracellular adenosine during ischaemia result from extracellular adenosine formation. This study supports the use of dipyridamole to exploit the beneficial actions of endogenous adenosine. However, our study suggests that this beneficial effect of dipyridamole may be offset by the concomitant use of caffeine. As both caffeine and dipyridamole are distributed worldwide, the combined use of these products is likely to occur. Therefore, future studies should focus on the clinical implication of this interaction, which may ultimately lead to improved tailoring of drug therapy.
Acknowledgments
GA Rongen is an Established Clinical Investigator of the Dutch Heart Foundation (2006 T035). We thank J Verpalen for the analysis of uridine uptake.
Abbreviations
- ENT
equilibrative nucleoside transporter
- FBF
forearm blood flow
- PORH
post-occlusive reactive hyperaemia
- SAH
S-adenosylhomocysteine
Conflict of interest
The authors state no conflict of interest.
References
- Biaggioni I, Paul S, Puckett A, Arzubiagi C. Caffeine and theophylline as adenosine receptor antagonists in humans. J Pharmacol Exp Ther. 1991;258:588–593. [PubMed] [Google Scholar]
- Bijlstra P, Van Ginneken EE, Huls M, Van Dijk R, Smits P, Rongen GA. Glyburide inhibits dipyridamole-induced forearm vasodilation but not adenosine-induced forearm vasodilation. Clin Pharmacol Ther. 2004;75:147–156. doi: 10.1016/j.clpt.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Bijlstra PJ, Den Arend JA, Lutterman JA, Russel FG, Thien T, Smits P. Blockade of vascular ATP-sensitive potassium channels reduces the vasodilator response to ischaemia in humans. Diabetologia. 1996;39:1562–1568. doi: 10.1007/s001250050615. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22:364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- Carlsson I, Sollevia A, Wennmalm A. The role of myogenic relaxation, adenosine and prostaglandins in human forearm reactive hyperaemia. J Physiol. 1987;389:147–161. doi: 10.1113/jphysiol.1987.sp016651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa F, Sulur P, Angel M, Cavalcante J, Haile V, Christman B, et al. Intravascular source of adenosine during forearm ischemia in humans: implications for reactive hyperemia. Hypertension. 1999;33:1453–1457. doi: 10.1161/01.hyp.33.6.1453. [DOI] [PubMed] [Google Scholar]
- Darvish A, Pomerantz RW, Zografides PG, Metting PJ. Contribution of cytosolic and membrane-bound 5′-nucleotidases to cardiac adenosine production. Am J Physiol. 1996;271:H2162–H2167. doi: 10.1152/ajpheart.1996.271.5.H2162. [DOI] [PubMed] [Google Scholar]
- De Jong JW, De Jonge R, Keijzer E, Bradamante S. The role of adenosine in preconditioning. Pharmacol Ther. 2000;87:141–149. doi: 10.1016/s0163-7258(00)00044-9. [DOI] [PubMed] [Google Scholar]
- Decking UK, Schlieper G, Kroll K, Schrader J. Hypoxia-induced inhibition of adenosine kinase potentiates cardiac adenosine release. Circ Res. 1997;81:154–164. doi: 10.1161/01.res.81.2.154. [DOI] [PubMed] [Google Scholar]
- Deussen A, Stappert M, Schafer S, Kelm M. Quantification of extracellular and intracellular adenosine production: understanding the transmembranous concentration gradient. Circulation. 1999;99:2041–2047. doi: 10.1161/01.cir.99.15.2041. [DOI] [PubMed] [Google Scholar]
- Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1–13. doi: 10.1016/s0022-510x(96)00308-5. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Persson CG. Xanthine derivatives as adenosine receptor antagonists. Eur J Pharmacol. 1982;81:673–676. doi: 10.1016/0014-2999(82)90359-4. [DOI] [PubMed] [Google Scholar]
- Frick GP, Lowenstein JM. Studies of 5′-nucleotidase in the perfused rat heart. Including measurements of the enzyme in perfused skeletal muscle and liver. J Biol Chem. 1976;251:6372–6378. [PubMed] [Google Scholar]
- Fujishige K, Kotera J, Michibata H, Yuasa K, Takebayashi S, Okumura K, et al. Cloning and characterization of a novel human phosphodiesterase that hydrolyzes both cAMP and cGMP (PDE10A) J Biol Chem. 1999;274:18438–18445. doi: 10.1074/jbc.274.26.18438. [DOI] [PubMed] [Google Scholar]
- Gamboa A, Abraham R, Diedrich A, Shibao C, Paranjape SY, Farley G, et al. Role of adenosine and nitric oxide on the mechanisms of action of dipyridamole. Stroke. 2005;36:2170–2175. doi: 10.1161/01.STR.0000179044.37760.9d. [DOI] [PubMed] [Google Scholar]
- Gamboa A, Ertl AC, Costa F, Farley G, Manier ML, Hachey DL, et al. Blockade of nucleoside transport is required for delivery of intraarterial adenosine into the interstitium: relevance to therapeutic preconditioning in humans. Circulation. 2003;108:2631–2635. doi: 10.1161/01.CIR.0000101927.70100.41. [DOI] [PubMed] [Google Scholar]
- Gardner C, Robas N, Cawkill D, Fidock M. Cloning and characterization of the human and mouse PDE7B, a novel cAMP-specific cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun. 2000;272:186–192. doi: 10.1006/bbrc.2000.2743. [DOI] [PubMed] [Google Scholar]
- Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Headrick JP, Matherne GP, Berne RM. Myocardial adenosine formation during hypoxia: effects of ecto-5′-nucleotidase inhibition. J Mol Cell Cardiol. 1992;24:295–303. doi: 10.1016/0022-2828(92)93166-h. [DOI] [PubMed] [Google Scholar]
- Heidland UE, Heintzen MP, Michel CJ, Strauer BE. Intracoronary administration of dipyridamole prior to percutaneous transluminal coronary angioplasty provides a protective effect exceeding that of ischemic preconditioning. Coron Artery Dis. 2000;11:607–613. doi: 10.1097/00019501-200012000-00006. [DOI] [PubMed] [Google Scholar]
- Kitakaze M, Hori M, Morioka T, Minamino T, Takashima S, Sato H, et al. Infarct size-limiting effect of ischemic preconditioning is blunted by inhibition of 5′-nucleotidase activity and attenuation of adenosine release. Circulation. 1994;89:1237–1246. doi: 10.1161/01.cir.89.3.1237. [DOI] [PubMed] [Google Scholar]
- Kitakaze M, Hori M, Sato H, Takashima S, Inoue M, Kitabatake A, et al. Endogenous adenosine inhibits platelet aggregation during myocardial ischemia in dogs. Circ Res. 1991;69:1402–1408. doi: 10.1161/01.res.69.5.1402. [DOI] [PubMed] [Google Scholar]
- Kitakaze M, Hori M, Takashima S, Sato H, Inoue M, Kamada T. Ischemic preconditioning increases adenosine release and 5′-nucleotidase activity during myocardial ischemia and reperfusion in dogs. Implications for myocardial salvage. Circulation. 1993;87:208–215. doi: 10.1161/01.cir.87.1.208. [DOI] [PubMed] [Google Scholar]
- Kroll K, Decking UK, Dreikorn K, Schrader J. Rapid turnover of the AMP-adenosine metabolic cycle in the guinea pig heart. Circ Res. 1993;73:846–856. doi: 10.1161/01.res.73.5.846. [DOI] [PubMed] [Google Scholar]
- Kroll K, Deussen A, Sweet IR. Comprehensive model of transport and metabolism of adenosine and S-adenosylhomocysteine in the guinea pig heart. Circ Res. 1992;71:590–604. doi: 10.1161/01.res.71.3.590. [DOI] [PubMed] [Google Scholar]
- Lenders J, Janssen GJ, Smits P, Thien T. Role of the wrist cuff in forearm plethysmography. Clin Sci (Lond) 1991;80:413–417. doi: 10.1042/cs0800413. [DOI] [PubMed] [Google Scholar]
- Liem DA, Van Den Doel MA, De Zeeuw S, Verdouw PD, Duncker DJ. Role of adenosine in ischemic preconditioning in rats depends critically on the duration of the stimulus and involves both A(1) and A(3) receptors. Cardiovasc Res. 2001;51:701–708. doi: 10.1016/s0008-6363(01)00321-2. [DOI] [PubMed] [Google Scholar]
- Moser GH, Schrader J, Deussen A. Turnover of adenosine in plasma of human and dog blood. Am J Physiol. 1989;256:C799–C806. doi: 10.1152/ajpcell.1989.256.4.C799. [DOI] [PubMed] [Google Scholar]
- Paul S, Feoktistov I, Hollister AS, Robertson D, Biaggioni I. Adenosine inhibits the rise in intracellular calcium and platelet aggregation produced by thrombin: evidence that both effects are coupled to adenylate cyclase. Mol Pharmacol. 1990;37:870–875. [PubMed] [Google Scholar]
- Pedrinelli R, Taddei S, Spessot M, Salvetti A. Maximal post-ischaemic forearm vasodilation in human hypertension: a re-assessment of the method. J Hypertension. 1987;5:S431–S433. [Google Scholar]
- Riksen NP, Franke B, Oyen WJ, Borm GF, Van Den Broek BP, Boerman OC, et al. Augmented hyperaemia and reduced tissue injury in response to ischaemia in subjects with the 34C>T variant of the AMPD1 gene. Eur Heart J. 2007;28:1085–1091. doi: 10.1093/eurheartj/ehm032. [DOI] [PubMed] [Google Scholar]
- Riksen NP, Oyen WJ, Ramakers BP, Van Den Broek PH, Engbersen R, Boerman OC, et al. Oral therapy with dipyridamole limits ischemia–reperfusion injury in humans. Clin Pharmacol Ther. 2005a;78:52–59. doi: 10.1016/j.clpt.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Riksen NP, Rongen GA, Boers GH, Blom HJ, Van Den Broek PH, Smits P. Enhanced cellular adenosine uptake limits adenosine receptor stimulation in patients with hyperhomocysteinemia. Arterioscler Thromb Vasc Biol. 2005b;25:109–114. doi: 10.1161/01.ATV.0000150651.85907.69. [DOI] [PubMed] [Google Scholar]
- Riksen NP, Van Ginneken EE, Van Den Broek PH, Smits P, Rongen GA. In vivo evidence against a role for adenosine in the exercise pressor reflex in humans. J Appl Physiol. 2005c;99:522–527. doi: 10.1152/japplphysiol.00108.2005. [DOI] [PubMed] [Google Scholar]
- Rongen GA, Floras JS, Lenders JW, Thien T, Smits P. Cardiovascular pharmacology of purines. Clin Sci (Lond) 1997;92:13–24. doi: 10.1042/cs0920013. [DOI] [PubMed] [Google Scholar]
- Rongen GA, Lenders JW, Lambrou J, Willemsen JJ, Van Belle H, Thien T, et al. Presynaptic inhibition of norepinephrine release from sympathetic nerve endings by endogenous adenosine. Hypertension. 1996;27:933–938. doi: 10.1161/01.hyp.27.4.933. [DOI] [PubMed] [Google Scholar]
- Rongen GA, Oyen WJ, Ramakers BP, Riksen NP, Boerman OC, Steinmetz N, et al. Annexin A5 scintigraphy of forearm as a novel in vivo model of skeletal muscle preconditioning in humans. Circulation. 2005;111:173–178. doi: 10.1161/01.CIR.0000151612.02223.F2. [DOI] [PubMed] [Google Scholar]
- Rongen GA, Smits P, Ver DK, Willemsen JJ, De Abreu RA, Van Belle H, et al. Hemodynamic and neurohumoral effects of various grades of selective adenosine transport inhibition in humans. Implications for its future role in cardioprotection. J Clin Invest. 1995;95:658–668. doi: 10.1172/JCI117711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H, Nishimura M, Shinano H, Makita H, Tsujino I, Shibuya E, et al. Plasma concentration of adenosine during normoxia and moderate hypoxia in humans. Am J Respir Crit Care Med. 1999;159:1014–1018. doi: 10.1164/ajrccm.159.3.9803100. [DOI] [PubMed] [Google Scholar]
- Schreiber-Deturmeny E, Bruguerolle B. Simultaneous high-performance liquid chromatographic determination of caffeine and theophylline for routine drug monitoring in human plasma. J Chromatogr B Biomed Appl. 1996;677:305–312. doi: 10.1016/0378-4347(95)00383-5. [DOI] [PubMed] [Google Scholar]
- Schutz W, Schrader J, Gerlach E. Different sites of adenosine formation in the heart. Am J Physiol. 1981;240:H963–H970. doi: 10.1152/ajpheart.1981.240.6.H963. [DOI] [PubMed] [Google Scholar]
- Skinner MR, Marshall JM. Studies on the roles of ATP, adenosine and nitric oxide in mediating muscle vasodilatation induced in the rat by acute systemic hypoxia. J Physiol. 1996;495 Pt 2:553–560. doi: 10.1113/jphysiol.1996.sp021615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits P, Lenders JW, Thien T. Caffeine and theophylline attenuate adenosine-induced vasodilation in humans. Clin Pharmacol Ther. 1990;48:410–418. doi: 10.1038/clpt.1990.169. [DOI] [PubMed] [Google Scholar]
- Smits P, Lenders JW, Willemsen JJ, Thien T. Adenosine attenuates the response to sympathetic stimuli in humans. Hypertension. 1991a;18:216–223. doi: 10.1161/01.hyp.18.2.216. [DOI] [PubMed] [Google Scholar]
- Smits P, Straatman C, Pijpers E, Thien T. Dose-dependent inhibition of the hemodynamic response to dipyridamole by caffeine. Clin Pharmacol Ther. 1991b;50:529–537. doi: 10.1038/clpt.1991.178. [DOI] [PubMed] [Google Scholar]
- Smits P, Williams SB, Lipson DE, Banitt P, Rongen GA, Creager MA. Endothelial release of nitric oxide contributes to the vasodilator effect of adenosine in humans. Circulation. 1995;92:2135–2141. doi: 10.1161/01.cir.92.8.2135. [DOI] [PubMed] [Google Scholar]
- Stojanov I, Proctor KG. Temperature-sensitive adenosine-mediated vasoconstriction in the skin microcirculation. J Pharmacol Exp Ther. 1990;253:1083–1089. [PubMed] [Google Scholar]
- Strauer BE, Heidland UE, Heintzen MP, Schwartzkopff B. Pharmacologic myocardial protection during percutaneous transluminal coronary angioplasty by intracoronary application of dipyridamole: impact on hemodynamic function and left ventricular performance. J Am Coll Cardiol. 1996;28:1119–1126. doi: 10.1016/S0735-1097(96)00307-5. [DOI] [PubMed] [Google Scholar]
- Tabrizchi R, Bedi S. Pharmacology of adenosine receptors in the vasculature. Pharmacol Ther. 2001;91:133–147. doi: 10.1016/s0163-7258(01)00152-8. [DOI] [PubMed] [Google Scholar]
- Ueland PM. Pharmacological and biochemical aspects of S-adenosylhomocysteine and S-adenosylhomocysteine hydrolase. Pharmacol Rev. 1982;34:223–253. [PubMed] [Google Scholar]
- Zernecke A, Bidzhekov K, Ozuyaman B, Fraemohs L, Liehn EA, Luscher-Firzlaff JM, et al. CD73/ecto-5′-nucleotidase protects against vascular inflammation and neointima formation. Circulation. 2006;113:2120–2127. doi: 10.1161/CIRCULATIONAHA.105.595249. [DOI] [PubMed] [Google Scholar]