Abstract
Background and purpose:
Endocannabinoids (via cannabinoid CB1 receptor activation) are physiological regulators of intestinal motility and food intake. However, their role in the regulation of gastric emptying is largely unexplored. The purpose of the present study was to investigate the involvement of the endocannabinoid system in the regulation of gastric emptying in mice fed either a standard diet (STD) or a high-fat diet (HFD) for 14 weeks.
Experimental approach:
Gastric emptying was evaluated by measuring the amount of phenol red recovered in the stomach after oral challenge; CB1 expression was analysed by quantitative reverse transcription-PCR; endocannabinoid (anandamide and 2-arachidonoyl glycerol) levels were measured by liquid chromatography-mass spectrometry.
Key results:
Gastric emptying was reduced by anandamide, an effect counteracted by the CB1 receptor antagonist rimonabant, but not by the CB2 receptor antagonist SR144528 or by the transient receptor potential vanilloid type 1 (TRPV1) antagonist 5′-iodoresiniferatoxin. The fatty acid amide hydrolase (FAAH) inhibitor N-arachidonoyl-5-hydroxytryptamine (but not the anandamide uptake inhibitor OMDM-2) reduced gastric emptying in a way partly reduced by rimonabant. Compared to STD mice, HFD mice exhibited significantly higher body weight and fasting glucose levels, delayed gastric emptying and lower anandamide and CB1 mRNA levels. N-arachidonoylserotonin (but not rimonabant) affected gastric emptying more efficaciously in HFD than STD mice.
Conclusions and implications:
Gastric emptying is physiologically regulated by the endocannabinoid system, which is downregulated following a HFD leading to overweight.
Keywords: anandamide, anandamide transport, cannabinoid receptors, gastric emptying, gastrointestinal motility, diet-induced obesity, endocannabinoids, high-fat diet, fatty acid amide hydrolase (FAAH), transient receptor potential vanilloid type 1 (TRPV1)
Introduction
A number of reports have suggested that the endocannabinoid system may regulate food intake and energy balance through cannabinoid CB1 receptors in brain and in periphery (Di Marzo and Matias, 2005; Sharkey and Pittman, 2005; Osei-Hyiaman et al., 2006). Consistent with this hypothesis, Phase III clinical trials have shown that a CB1 receptor antagonist reduces body weight and waist circumference to a significantly greater extent than placebo in obese patients (Despres et al., 2005; Van Gaal et al., 2005; Pi-Sunyer et al., 2006; Scheen et al., 2006).
Apart from cannabinoid (CB1 and CB2) receptors, the endocannabinoid system includes endogenous ligands that activate them (that is, the endocannabinoid anandamide (AEA) and 2-arachidonoyl glycerol (2-AG)) and mechanisms for endocannabinoid biosynthesis and inactivation (Bradshaw and Walker, 2005; Di Marzo and Matias, 2005; Mackie, 2006). The latter occurs through cellular reuptake, which might be facilitated by a putative membrane transporter, and enzymatic degradation by hydrolytic enzymes including fatty acid amide hydrolase (FAAH) (Di Marzo and Matias, 2005). CB1 receptors have been identified on enteric nerves, and their activation results in inhibition of excitatory transmission in vitro (Pertwee et al., 1996; Izzo et al., 1998; Guagnini et al., 2006; Hinds et al., 2006) and intestinal propulsion in vivo (Pinto et al., 2002; Carai et al., 2006). Furthermore, there is substantial evidence that the endocannabinoid system is involved in the physiological control of intestinal motility, both in the small and in the large intestine (Coutts and Izzo, 2004; Hornby and Prouty, 2004; Duncan et al., 2005; Massa et al., 2005). Such evidence comes mainly from pharmacological studies in rodents showing prokinetic effects of the CB1 receptor antagonist rimonabant and reduction of intestinal motility by inhibitors of endocannabinoid inactivation (Pinto et al., 2002; Capasso et al., 2005). The involvement of endocannabinoids in the physiological regulation of intestinal motility is consistent with data from Phase III clinical trials, which highlighted diarrhoea as one of the initial adverse events associated with administration of the antiobesity drug rimonabant (Van Gaal et al., 2005). However, the possible involvement of endocannabinoids as physiological modulators of gastric motility has been hardly explored in physiological states and never evaluated in obese animals. Previous studies in which the effect of the CB1 receptor antagonist rimonabant on gastric motility was investigated in vivo in control rats yielded inconclusive results (Izzo et al., 1999; Krowicki et al, 1999; Landi et al., 2002). In addition, the effect on gastric motility of drugs that inhibit endocannabinoid degradation has not been evaluated to date.
The aim of the present study was to evaluate the role of the endocannabinoid system in the regulation of gastric emptying in the mouse in vivo. For this purpose, we used synthetic AEA, the selective CB1 receptor antagonist rimonabant, the selective CB2 receptor antagonist SR144528, the AEA cellular reuptake inhibitor OMDM-2 and the FAAH inhibitor N-arachidonoyl-5-hydroxytryptamine (AA-5-HT). In addition, because obesity may affect gastric motility (Park and Camilleri, 2005) and the CB1 receptor antagonist rimonabant is clinically used in obese patients, additional experiments were performed in mice fed a high-fat diet (HFD) for 14 weeks.
Methods
Animals and diet
Male ICR mice (22–24 g) were generally used. Mice were fed ad libitum with standard mouse food, except for the 12 h period immediately preceding the measurement of gastric emptying. In another set of experiments (that is, HFD-fed mice), male 7-week-old C57Bl/6J mice were used. After 1 week of acclimatization, C57Bl/6J mice were fed a diet containing 25.5% fat (49% of calories), 22% protein and 38.4% carbohydrate (TD97366; Harlan Italy, Correzzana, Milan, Italy) for 14 weeks. Control C57B1/6J mice received a standard diet (STD) containing 5.7% fat (10.9% of calories), 18.9% protein and 57.3% carbohydrate (2018, Harlan Teklad global diet (18% of proteins); Harlan Italy). Mice were fed ad libitum, except for the 12 h period immediately preceding the experiments. Animals were purchased from Harlan Italy. Fasting plasma glucose levels were determined in animals fasted for 12 h by using the glucose test kit with an automatic analyzer (Accu-Chek Active; Roche, Mannheim, Germany) in blood samples obtained from tail vein (Darmani et al., 2005).
Drug administration
Anandamide (1–15 mg kg−1), AA-5-HT (10 mg kg−1), OMDM-2 (10 mg kg−1) or vehicles were injected intraperitoneally 30 min before the administration of the marker (phenol red solution) used to evaluate gastric emptying. In some experiments, rimonabant (0.1 mg kg−1), 5′-iodoresiniferatoxin (I-RTX, 0.75 mg kg−1) or SR144528 (1 mg kg−1) were injected intraperitoneally 10 min before AEA (7.5 mg kg−1) or AA-5-HT (10 mg kg−1). I-RTX and SR144528 doses were selected on the basis of the previous work (Capasso et al., 2005). In some experiments, the effect of either rimonabant (0.1–3 mg kg−1, i.p.), AA-5-HT (2.5–15 mg kg−1, i.p.) or OMDM-2 (10 mg kg−1, i.p.) was evaluated in HFD-fed mice and, for comparison, in aged-matched STD-fed mice.
Gastric emptying
Gastric emptying in both control and HFD-fed mice (14 weeks of dietary treatment) was performed as described previously (El-Salhy, 2001). Briefly, after an overnight fast, the animals received by gavage 0.2 ml of a solution of 50 mg phenol red in 100 ml 1.5% carboxymethylcellulose, which was constantly stirred and held at 37 °C. After 20 min, mice were killed stomach was quickly ligated at the lower oesophageal sphincter and pyloric region and removed. The stomach was opened and its contents were poured into a test tube and washed with 4 ml distilled water. At the end of the experiment, 2 ml 1 M NaOH was added to each tube to develop the maximum intensity of colour. The solutions were assayed with spectrometer at 560 nm. The percentage of gastric emptying was calculated according to the following formula:
100 × (1−amount of phenol red recovered after 20 min/amount of phenol red recovered after 0 min).
Identification and quantification of endocannabinoids (AEA and 2-AG)
Full-thickness stomachs from HFD-fed mice and aged-matched STD-fed mice (8 and 14 weeks of dietary treatment) were removed and tissue specimens were immediately weighed, immersed into liquid nitrogen and stored at −70 °C until chromatographic separation of endocannabinoids. Tissues were extracted with chloroform/methanol (2:1 (v/v)) containing 100 pmol d8-AEA, synthesized as described previously (Bisogno et al., 1999), and 100 pmol d5-2-AG provided by Cayman Chemicals (Ann Arbor, MI, USA). The lipid extracts were purified by silica column chromatography as described previously (Bisogno et al., 1999; Maione et al., 2007), and the fractions containing AEA and 2-AG were analysed by isotope dilution LC-APCI-MS (liquid chromatography-atmospheric pressure-chemical ionization mass spectrometry) and carried out in the selected monitoring mode as described in detail elsewhere (Maione et al., 2007). Results are expressed as pmol or nmol per g wet tissue. As during tissue extraction/purification, both d8-AEA and native 2-AG were partly transformed into the 1(3)-isomers, and only a limited amount of arachidonic acid was present on the sn-1(3) position of (phospho)glycerides, the amounts of 2-AG shown here represent the combined mono-arachidonyl-glycerol peaks.
Quantitative real-time PCR
Stomach antrum and fundus from control and HFD-fed mice were homogenized separately in 1 ml of Trizol (Invitrogen Carlsbad, CA, USA). Total RNA was extracted according to manufacturer's recommendations, dissolved in RNA storage solution (Ambion, Austin, TX, USA), ultraviolet quantified by a Bio-Photometer (Eppendorf, Hamburg, Germany) and stored at −80 °C. RNA aliquots (6 μg) were digested by RNAse-free DNAse I (Ambion DNA-free kit) in a 20 μl final volume reaction mixture to remove contaminating genomic DNA. After DNAse digestion, concentration and purity of RNA samples were evaluated by the RNA-6000-Nano microchip assay using a 2100 Bioanalyzer equipped with a 2100 Expert Software (Agilent, Santa Clara, CA, USA), following manufacturer's instructions. For all samples tested, the RNA integrity number (RIN) was greater than 6 (relatively to a 0–10 scale); 3 μg of total RNA, as evaluated by the 2100 Bioanalyzer, was reverse transcribed in a 25 μl reaction mixture containing as follows: 50 mM Tris-HCl, pH 8.3, 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol, 1 mM dNTPs, 20 U RNAse inhibitor (Invitrogen), 0.125 A260 units of hexanucleotide mixture (Invitrogen) for random priming and 200 U MoMuLV (Moloney murine leukemia virus) Superscript III reverse transcriptase (Invitrogen). The reaction mixture was incubated in a termocycler iCycler-iQ for a 5 min at 55 °C step, followed by a rapid chilling for 2 min at 4 °C. The protocol was stopped at this step and the MoMuLV reverse transcriptase was added to the samples, except the negative controls (−RT). The incubation was resumed by two thermal steps: 10 min at 20 °C followed by 90 min at 50 °C. Finally, the reaction was terminated by heating at 95 °C for 10 min. Quantitative real-time PCR was performed by an iCycler-iQ in a 25 μl reaction mixture containing: 1 × iQ-SYBR-Green-Supermix (Bio-Rad, Hercules, CA, USA), 20 ng cDNA (calculated on the basis of the retro-transcribed RNA) and 330 nM for each primer. The amplification profile consisted of an initial denaturation of 2 min at 94 °C and 40 cycles of 30 s at 94 °C, annealing for 30 s at TaOpt (optimum annealing temperature—see below) and elongation for 45 s at 68 °C. Fluorescence data were collected during the elongation step. A final extension of 7 min was carried out at 72 °C, followed by melt-curve data analysis. Optimized primers for SYBR Green analysis (and relative TaOpt) were designed by the Beacon Designer software 6.0 version (Biosoft International, Palo Alto, CA, USA) and were synthesized (HPLC purification grade) by MWG Biotech AG (Ebersberg, Germany) (CB1: forward primer CTGATGTTCTGGATCGGAGTC, reverse primer TCTGAGGTGTGAATGATGATGC; β-actin: forward primer CCAGGCATTGCTGACAGG, reverse primer TGGAAGGTGGACAGTGAGG; GADPH: forward primer GCCTTCCGTGTTCCTACC, reverse primer CCTGGTCCTCAGTGTAGC). Assays were performed in quadruplicate (maximum ΔCt of replicate samples <0.5), and a standard curve from consecutive fivefold dilutions (100–0.16 ng) of a cDNA pool representative of all samples was included for PCR efficiency determination. Relative expression analysis, correct for PCR efficiency and normalized with respect to reference genes β-actin and GADPH was performed by GENEX software (Bio-Rad) for groupwise comparison and statistical analysis.
Drugs
Anandamide and I-RTX were purchased from Tocris Cookson (Bristol, UK). AA-5-HT and OMDM-2 ((S)-N-oleoyl-(1′-hydroxybenzyl)-2′-ethanolamine) were synthesized as described previously (Ortar et al., 2003). Rimonabant (5-(p-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-piperidinopyrazole-3-carboxamide hydrochloride) and SR144528 (N-[-1S-endo-1,3,3-trimethyl-bicyclo[2.2.1]heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide) were a kind gift from Drs Madaleine Mossè and Francis Barth (SANOFI Recherche, Montpellier, France).
N-arachidonoyl-5-hydroxytryptamine was dissolved in dimethyl sulphoxide (DMSO)/Tween 80 (1:4), OMDM-2 and I-RTX in DMSO, AEA in Tocrisolve (soya oil/water (1:4 emulsion)). The drug vehicles (10 μl 10 g−1 DMSO/Tween 80, 2 μl 10 g−1 DMSO, 20 μl 10 mg−1 Tocrisolve) had no significant effect on gastric emptying.
Statistics
Data are expressed as the mean±s.e.mean of experiments in n mice. To determine statistical significance, Student's t-test was used for comparing a single treatment mean with a control mean, and ANOVA followed by a Tukey–Kramer multiple comparisons test was used for analysis of multiple treatment means (or Bonferroni's for endocannabinoid levels). P-values <0.05 were considered significant.
Results
Gastric emptying in control mice
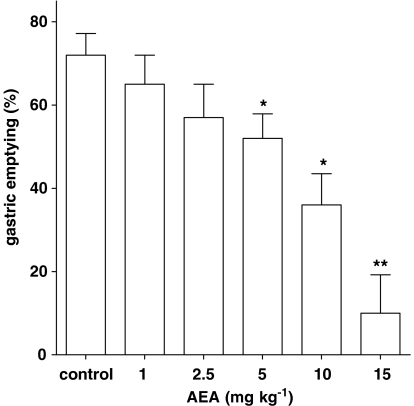
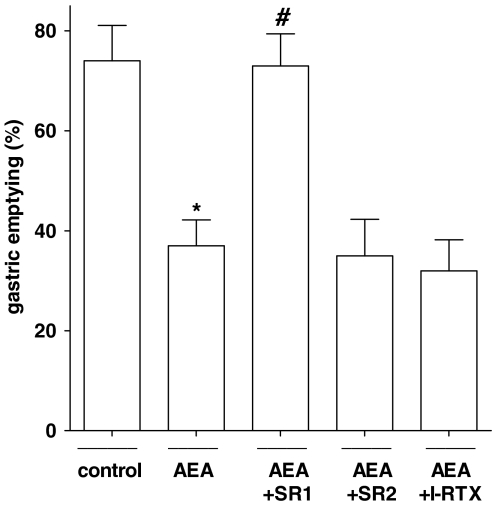
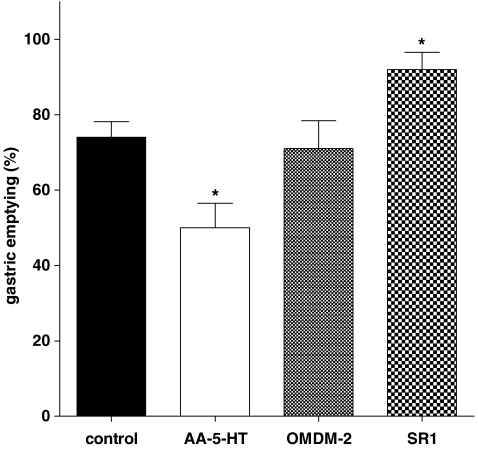
Intraperitoneal administration of AEA (1–15 mg kg−1) produced a dose-dependent inhibition of gastric emptying (Figure 1), with a significant effect starting from the 5 mg kg−1 dose. The inhibitory effect of AEA (7.5 mg kg−1) was counteracted by a non-effective dose per se of the CB1 receptor antagonist rimonabant (0.1 mg kg−1), but not by the CB2 receptor antagonist SR144528 (1 mg kg−1) or the TRPV1 (transient receptor potential vanilloid type 1) antagonist I-RTX (0.75 mg kg−1) (Figure 2). Figure 3 shows that the FAAH inhibitor AA-5-HT (10 mg kg−1) decreased gastric emptying, whereas the CB1 receptor antagonist rimonabant (1 mg kg−1) increased gastric emptying. These results are extrapolated from dose–response curves (data not shown). By contrast, the endocannabinoid reuptake inhibitor OMDM-2 (10 mg kg−1) did not affect gastric emptying significantly (Figure 3). A non-effective dose per se of the CB1 receptor antagonist rimonabant (0.1 mg kg−1), but not of the CB2 receptor antagonist SR144528 (1 mg kg−1) or the TRPV1 antagonist I-RTX (0.75 mg kg−1) significantly reduced the inhibitory effect of AA-5-HT (10 mg kg−1) on gastric emptying (Figure 4). However, AA-5-HT, in the presence of rimonabant, still exerted a significant inhibitory effect (Figure 4).
Figure 1.
Effect of anandamide (AEA), injected intraperitoneally, on gastric emptying in ICR control mice. Columns represent the mean±s.e.mean of 9–11 animals for each experimental group. *P<0.05 and **P<0.01 vs corresponding control.
Figure 2.
Effect of anandamide (AEA, 7.5 mg kg−1, i.p.) alone or in the presence of the CB1 receptor antagonist rimonabant (SR1, 0.1 mg kg−1, i.p.), or the CB2 receptor antagonist SR144528 (SR2, 1 mg kg−1, i.p.) or the TRPV1 antagonist 5′-iodoresiniferatoxin (I-RTX; 0.75 mg kg−1, i.p.) on gastric emptying in ICR control mice. Columns represent the mean±s.e.mean of 7–10 mice for each experimental group. *P<0.05 vs corresponding control and #P<0.05 vs AEA alone. AEA, anandamide.
Figure 3.
Effect of the FAAH inhibitor N-arachidonoyl-5-hydroxytryptamine (AA-5-HT, 10 mg kg−1, i.p.), the anandamide cellular reuptake inhibitor OMDM-2 (10 mg kg−1) or the CB1 receptor antagonist rimonabant (SR1, 1 mg kg−1) on gastric emptying in ICR control mice. Columns represent the mean±s.e.mean of 8–10 mice for each experimental group. *P<0.05 vs control. FAAH, fatty acid amide hydrolase.
Figure 4.
Effect of intraperitoneally injected N-arachidonoyl-5-hydroxytryptamine (AA-5-HT, 10 mg kg−1) on gastric emptying in ICR control mice pretreated (i.p.) with the CB1 receptor antagonist rimonabant (SR1, 0.1 mg kg−1), or the CB2 receptor antagonist SR144528 (SR2, 1 mg kg−1) or the TRPV1 (transient receptor potential vanilloid type 1) antagonist 5′-iodoresiniferatoxin (I-RTX, 0.75 mg kg−1). Columns represent the mean±s.e.mean of 7–9 mice for each experimental group. *P<0.05 vs control and #P<0.05 vs AA-5-HT alone.
Given alone (that is, in absence of any agonist), the CB2 antagonist SR144528 (1 mg kg−1 i.p.) and the TRPV1 antagonist I-RTX (0.75 mg kg−1 i.p.) did not modify significantly gastric emptying (percentage of gastric emptying: control 70.4±6.2, SR144528 68.4±5.2; control 72.0±4.9, I-RTX 64.2±6.7; n=8–10 for each experimental group).
Gastric emptying in HFD-fed mice
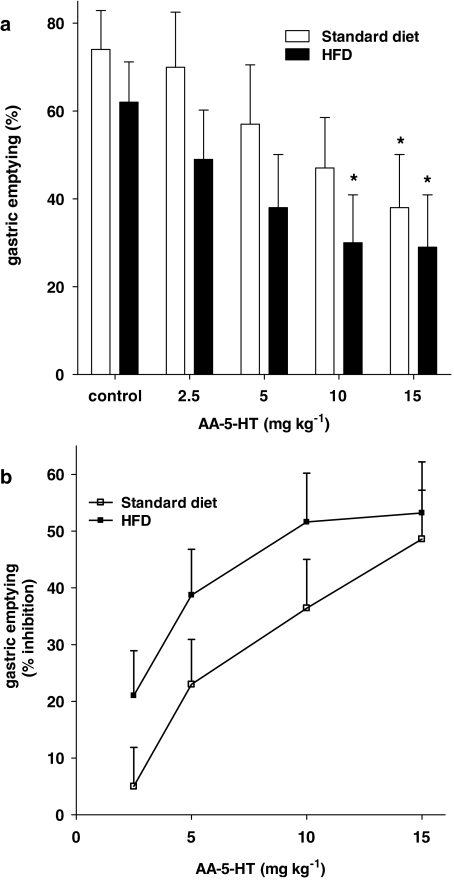
Compared to aged-matched STD-fed animals, HFD-fed mice showed significantly increased body weight (STD 29.6±0.4 g; HFD 41.5±0.7 g, n=38 for each experimental group, P<0.001), higher blood glucose (STD 121.1±5.8; HFD 167.5±4.8 mg per 100 ml, n=7–8 for each experimental group, P<0.05) and delayed gastric emptying (percentage gastric emptying: STD 79.3±4.9; HFD 65.0±3.5, n=9 for each experimental group, P<0.05). Figure 5 shows the effect of rimonabant on gastric emptying in control and HFD-fed mice. Rimonabant increased gastric emptying both in control and HFD-fed mice (Figure 5a). No statistical difference was observed between the curves representing the percentage of increase in control and HFD-fed mice (Figure 5b). By contrast, the FAAH inhibitor AA-5-HT was significantly more effective in reducing gastric emptying in HFD-fed animals compared to control mice (Figure 6). Finally, the endocannabinoid reuptake inhibitor OMDM-2 (10 mg kg−1) did not affect gastric emptying (data not shown).
Figure 5.
Effect of rimonabant (0.1–3 mg kg−1, i.p.) on gastric emptying in C57Bl/6J mice fed for 14 weeks a standard diet or a high-fat diet (HFD). Results (mean±s.e.mean of 4–6 mice for each experimental group) are expressed as a percentage of gastric emptying (a) or a percentage of increase of corresponding control values (b). (a) *P<0.05 vs corresponding control; (b) no statistical differences were observed between the two dose–response curves.
Figure 6.
Effect of N-arachidonoyl-5-hydroxytryptamine (AA-5-HT, 2.5–15 mg kg−1, i.p.) on gastric emptying in C57Bl/6J mice fed for 14 weeks a standard diet or a high-fat diet (HFD). Results (mean±s.e.mean of 3–5 mice for each experimental group) are expressed as a percentage of gastric emptying (a) or as a percentage of inhibition of corresponding control values (b). (a) *P<0.05 vs corresponding control. (b) A significant difference (P<0.05) was observed between the two dose–response curves.
Levels of endocannabinoids
Gastric AEA and 2-AG levels were measured after the periods of 8 and 14 weeks of a HFD or a STD. As shown in Table 1, after 14 weeks of HFD, the levels of AEA were significantly reduced by 25.2%, but no significant changes were observed after 8 weeks. No significant changes were observed for 2-AG levels at both time points.
Table 1.
AEA and 2-AG levels in the stomach of mice fed for up to 8 or 14 weeks with STD or HFD
| Diet |
8 weeks |
14 weeks |
||
|---|---|---|---|---|
| AEA (pmol g−1) | 2-AG (nmol g−1) | AEA (pmol g−1) | 2-AG (nmol g−1) | |
| STD | 72.9±3.1 | 6.2±1.4 | 91.4±4.2 | 10.3±1.9 |
| HFD | 85.5±9.2 | 10.4±2.5 | 68.3±4.6* | 8.4±0.4 |
Abbreviations: AEA, anandamide; 2-AG, 2-arachidonoylglycerol; HFD, high-fat diet; STD, standard diet.
Data are mean±s.e.mean of n=4 animals. *P<0.05 compared to corresponding STD samples.
Expression of cannabinoid CB1 receptors
Results are presented in Table 2. HFD was accompanied by a 12.5-fold decrease in the levels of CB1 receptor mRNA (P<0.05) in the antrum. CB1 expression in the fundus also appeared to be decreased, but these changes did not achieve statistical significance.
Table 2.
Changes in CB1 mRNA expression in the antrum and fundus of the stomach of mice fed a HFD for 14 weeks
| Receptor | Antrum | Fundus |
|---|---|---|
| CB1 | 0.084±0.043* | 0.484±0.170 |
Abbreviations: HFD, high-fat diet; STD, standard diet.
Data are mean±s.e.mean of n=3 determinations from three independent experiments and are expressed as fold changes of mRNA expression in the antrum and fundus of the stomach of mice fed HFD vs STD (control, considered as 1). Refer to Results for expression fold data. *P<0.05 vs control.
Discussion
The possible involvement of endocannabinoids in regulating gastric functions, including gastric motility, is largely unexplored. Immunohistochemical studies have revealed the presence of CB1 receptors on vagal afferents (Burdyga et al., 2004) and myenteric cholinergic nerves innervating rat stomach smooth muscles (Van Sickle et al., 2001; Adami et al., 2002). Previous pharmacological studies have shown that cannabinoid receptor agonists (via CB1) exert gastric antimotility effects in the rat in vivo (Izzo et al., 1999; Krowicki et al., 1999; Landi et al., 2002). In these studies, the CB1 receptor antagonist rimonabant, administered alone, was without effect (Izzo et al., 1999; Landi et al., 2002) or even produced an effect in the same direction as that of cannabinoid agonists (Krowicki et al., 1999), thus preventing the investigators from concluding that endocannabinoids endogenously control gastric motility. Furthermore, the effect on gastric motility of drugs that inhibit endocannabinoid inactivation has never been investigated to date. It is also not known whether endocannabinoids and CB1 receptors undergo any gastric emptying-related adaptive change during HFD-induced overweight. This is an important issue in view of the recently acquired evidence that the endocannabinoid system is overactivated in several peripheral tissues and blood of mice with diet-induced obesity and in obese and hyperglycaemic patients (Matias et al., 2007). This overactivation could also occur in the gastrointestinal tract and might affect the levels of gastrointestinal hormones that control food intake, such as ghrelin (Cani et al., 2004). In the present study, we provide evidence that AEA and CB1 receptors are indeed involved in the inhibition of gastric emptying, both in lean and overweight mice, and that their gastric levels undergo downregulation after a prolonged HFD.
The actions of cannabinoids in the digestive tract include inhibition of motility, secretion, inflammation, visceral pain and cell proliferation (reviewed in Duncan et al., 2005; Massa et al., 2005; Di Marzo and Izzo, 2006; Izzo and Capasso, 2006; Izzo, 2007; Sanger, 2007). With regard to gastric functions, previous experimental studies have shown that cannabinoids exert, via CB1 activation, gastric antisecretory (Adami et al., 2002) and antiulcer (Germano et al., 2001; Dembinski et al., 2006; Rutkowska and Fereniec-Goltbiewska, 2006) actions in rats and also inhibit transient lower oesophageal sphincter relaxations in dogs and ferrets (Lehmann et al., 2002; Partosoedarso et al., 2003). Moreover, clinical studies performed in healthy volunteers have demonstrated that oral Δ9-tetrahydrocannabinol significantly retards gastric emptying in humans (McCallum et al., 1999; Esfandyari et al., 2006). Here, we have shown that the endogenous cannabinoid AEA, which exhibits no marked selectivity for CB1 or CB2 receptors (Pertwee, 2006), reduces gastric emptying dose-dependently. This effect is uniquely mediated by CB1 receptors, as the action of AEA was counteracted by the selective CB1 receptor antagonist rimonabant, but not by the CB2 receptor antagonist SR144528; others have shown that AEA reduces cholinergic-mediated twitch contractions in rodents in vitro (Storr et al., 2002; Mulé et al., 2007), an effect that could involve both CB1 and CB2 receptors (Mulé et al., 2007). Anandamide is also an endogenous ligand of the TRPV1 receptor, a non-selective cation channel that belongs to the large family of transient receptor potential ion channels, and is activated by the pungent ingredient of hot chilli peppers, capsaicin (Ross, 2003; Van der Stelt and Di Marzo, 2004). TRPV1 is present in the gastrointestinal tract where it is primarily associated with axons of spinal afferent neurons and, to some extent, vagal afferents (Holzer, 2004). There is evidence that TRPV1 activation by AEA induces ileitis in rats (McVey et al., 2003), increases the release of γ-aminobutyric acid from guinea pig myenteric nerves (Begg et al., 2002) and facilitates spontaneous acetylcholine release from the myenteric plexus of the guinea pig ileum (Mang et al., 2001). However, we have shown here that the inhibitory effect of AEA on gastric motility was not mediated by activation of TRPV1, as the selective and ultrapotent TRPV1 antagonist I-RTX failed to affect endocannabinoid-induced changes in motility. These data are consistent with previous results that showed the lack of involvement of TRPV1 receptors in AEA-induced reduction of small intestinal motility (Izzo et al., 2001). Furthermore, Bartho et al. (2002) found no evidence for AEA activation of TRPV1 receptors in the human-isolated sigmoid colon.
The first step of AEA inactivation is cellular reuptake, which seems to be facilitated by a putative membrane transporter (Ho and Hillard, 2005). Previous studies have shown that an inhibitor of the AEA transporter may reduce motility in the colon (Pinto et al., 2002), but not in the small intestine (Calignano et al., 1997; Mascolo et al., 2002). However, in pathophysiological states, the AEA uptake inhibitor VDM11 was shown to reduce small intestinal motility in the experimental model of ileus induced by acetic acid (Mascolo et al., 2002), to exert anti-inflammatory effects in experimental colitis (D'Argenio et al., 2006) and to reduce cholera toxin-induced hypersecretion (Izzo et al., 2003). In the present study, we have shown that the AEA uptake inhibitor OMDM-2 (de Lago et al., 2004) does not modify gastric emptying in control animals, thus contesting the importance of such a mechanism in the physiological control of gastric motility in vivo. The dose of OMDM-2 used in the present study was previously shown to potentiate the motor inhibitory effects of AEA in vivo (de Lago et al., 2004).
The second step of endocannabinoid inactivation is enzymatic degradation via hydrolytic enzymes, among which the best characterized is FAAH (Jhaveri et al., 2007). In the periphery, the FAAH presence has been found along the small and large intestine (Capasso et al., 2005). In the digestive tract, FAAH inhibition results in anti-inflammatory effects (Massa et al., 2004; D'argenio et al., 2006), inhibition of motility in the small intestine (Capasso et al., 2005) and anticancer effects (Izzo et al., 2007). In the present study, we have shown that the FAAH inhibitor AA-5-HT, at a dose previously shown to increase intestinal endocannabinoid levels (Capasso et al., 2005) and not to activate CB1 receptors, reduces gastric emptying; this effect was attenuated by the CB1 receptor antagonist rimonabant (but not by the TRPV1 antagonist I-RTX), although even in the presence of rimonabant, AA-5-HT exerted a significant inhibitory effect on gastric motility. These results suggest that AA-5-HT, through an increase of endocannabinod levels, could indirectly activate CB1 and hence could reduce gastric emptying. However, a CB1-independent mechanism in AA-5-HT-induced delay in gastric motility also exists. A possible candidate for this CB1-independent mechanism is oleoylethanolamide, an FAAH substrate with the ability to reduce gastric emptying (AA Izzo and V Di Marzo, unpublished observations) and intestinal motility (Capasso et al., 2005), and whose mouse gastric levels, like those of AEA, are increased after the AA-5-HT treatment (AA Izzo and V Di Marzo, unpublished observations).
An ever increasing number of studies have suggested that the endocannabinoid system regulates food intake and energy balance in control and obese animals via CB1 receptors in brain and in periphery (Di Marzo and Matias, 2005; Sharkey and Pittman, 2005; Osei-Hyiaman et al., 2006). These studies have led to the development of the antiobesity drug rimonabant. Phase III clinical trials have shown that this antagonist reduces weight and waist circumference to a significantly greater extent than placebo in obese patients (Despres et al., 2005; Van Gaal et al., 2005; Pi-Sunyer et al., 2006; Scheen et al., 2006). Here, we found that a 14-week HFD increased body weight and glucose blood levels as well as reduced gastric emptying. Our data are in agreement with those of previous investigators who reported delayed gastric emptying in ob/ob mice, a genetic model of obesity and diabetes (Asakawa et al., 2003). The reduced gastric emptying in HFD-fed animals was associated with a modest (30%) but significant decrease of AEA gastric content and of CB1 receptor expression, with no changes in 2-AG levels. These findings indicate that, in contrast to other peripheral organs of mice with high-fat-induced obesity, that is, epididymal adipose tissue, pancreas and liver, where an upregulation was observed (Osei-Hyiaman et al., 2006; Matias et al., 2007), the endocannabinoid system is downregulated in the stomach of HFD-fed mice. This might have consequences on food intake, as it has been suggested that CB1 activation tonically stimulates gastric ghrelin secretion (Cani et al., 2004), and ghrelin in turn disinhibits cholecystokinin-induced suppression of CB1 receptor expression in the nodose ganglion (Burdyga et al., 2006). Thus, suppression of gastric endocannabinoid signalling during HFD-induced obesity might represent an adaptive response aimed at reducing food intake in synergy with reduced gastric emptying. However, it is very unlikely that the decreased AEA and CB1 levels contribute to the reduction of gastric emptying observed in HFD-fed mice, as this signalling system was shown to reduce gastric motility in both lean and obese mice (see above).
To elucidate the role of the endocannabinoid system also in HFD-treated mice, we investigated the effect of a drug that antagonizes the effect of endocannabinoids at CB1 receptors (that is, the CB1 receptor antagonist rimonabant) and the effect of drugs that inhibit endocannabinoid inactivation (that is, the FAAH inhibitor AA-5-HT and the AEA cellular reuptake inhibitor OMDM-2) on gastric emptying. We found that rimonabant exerted a prokinetic effect in HFD-treated animals, although with the same potency observed in control mice. This confirms that HFD does not cause any increase in endocannabinoid ‘tone' in the stomach, as an antagonist would be more efficacious in the presence of a higher endogenous tone. Indeed, our present data indicate that, if anything, gastric endocannabinoids levels and, particularly, CB1 receptor mRNA expression are reduced after a HFD. The similar efficacy of rimonabant in enhancing gastric motility in lean and obese mice also suggests that the weight loss-inducing effects of this compound, which are observed preferentially in obese animals (Vickers et al., 2003), are unlikely to be effected at the level of gastric emptying. Nevertheless, the prokinetic effect of rimonabant in HFD-fed animals with hyperglycaemia may have some clinical relevance in the light of the observation that diabetic gastroparesis is a well-recognized disturbance in obese patients (Park and Camilleri, 2006) and that rimonabant can produce a clinically meaningful reduction in body weight and improve a number of cardiovascular and metabolic risk factors in overweight or obese patients with type 2 diabetes (Scheen et al., 2006). We also found that, unlike rimonabant, the FAAH inhibitor AA-5-HT was significantly more potent in inhibiting gastric motility in HFD-fed compared to STD-fed mice. The different potency of AA-5-HT could be explained by the observation that FAAH mRNA expression is reduced in the stomach of HFD-fed mice (Aviello et al., 2008) and by the fact that FAAH also catalyzes the hydrolysis of the anti-prokinetic compound oleoylethanolamide, whose gastric levels are increased in overweight animals (Aviello et al., 2008).
Finally, we showed here that the AEA cellular reuptake inhibitor OMDM-2 failed to affect gastric emptying in HFD-fed mice, thus arguing against the importance of this inactivating mechanism in regulating gastric motility in obese animals.
Conclusion
In the present study, we have shown for the first time that the endocannabinoid system is physiologically involved in the regulation of gastric emptying in vivo, both in control and in overweight animals. This conclusion is mainly based on the findings of (a) high amount of endocannabinoids in gastric tissues, (b) a prokinetic effect of the cannabinoid receptor antagonist rimonabant and (c) an inhibitory action on gastric emptying of the FAAH inhibitor AA-5-HT as well as of AEA. We also observed that the endocannabinoid system undergoes adaptive changes after a HFD, as revealed by the changes in AEA and CB1 mRNA levels and by the different potency of the FAAH inhibitor AA-5-HT at delaying gastric emptying after this dietary regime. Targeting the endocannabinoid system might thus offer novel therapeutic strategies in the treatment of gastric motility diseases and has potential for the treatment of diabetic obese patients with dyspepsia.
Acknowledgments
This work was supported by Prin, Regione Campania, ‘Fondazione Enrico ed Enrica Sovena' and Sanofi-Aventis (to VDM).
Abbreviations
- AA-5-HT
N-arachidonoyl-5-hydroxytryptamine
- AEA
anandamide
- 2-AG
2-arachidonoyl glycerol
- DMSO
dimethyl sulphoxide
- FAAH
fatty acid amide hydrolase
- HFD
high-fat diet
- I-RTX
5′-iodoresiniferatoxin
- NAPE-PLD
N-acyl-phosphatidylethanolamine-selective phospholipase D
- RT-PCR
reverse transcription-PCR
- TRPV1
transient receptor potential vanilloid type 1
- STD
standard diet
Conflict of interest
The authors state no conflict of interest.
References
- Adami M, Frati P, Bertini S, Kulkarni-Narla A, Brown DR, de Caro G, et al. Gastric antisecretory role and immunohistochemical localization of cannabinoid receptors in the rat stomach. Br J Pharmacol. 2002;135:1598–1606. doi: 10.1038/sj.bjp.0704625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Ueno N, Makino S, Uemoto M, Fujino MA, et al. Ob/ob mice as a model of delayed gastric emptying. J Diabetes Complicat. 2003;17:27–28. doi: 10.1016/s1056-8727(02)00198-8. [DOI] [PubMed] [Google Scholar]
- Aviello G, Matias I, Capasso R, Petrosino S, Borrelli D, Orlando P, et al. Inhibitory effect of the anorexic compound oleoylethanolamide on gastric emptying in control and overweight mice J Mol Med 2008. 2008, in press [DOI] [PubMed]
- Bartho L, Benko R, Lazar Z, Illenyi L, Horvath OP. Nitric oxide is involved in the relaxant effect of capsaicin in the human sigmoid colon circular muscle. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:496–500. doi: 10.1007/s00210-002-0630-2. [DOI] [PubMed] [Google Scholar]
- Begg M, Molleman A, Parsons M. Modulation of the release of endogenous gamma-aminobutyric acid by cannabinoids in the guinea pig ileum. Eur J Pharmacol. 2002;434:87–94. doi: 10.1016/s0014-2999(01)01530-8. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Berrendero F, Ambrosino G, Cebeira M, Ramos JA, Fernandez-Ruiz JJ, et al. Brain regional distribution of endocannabinoids: implications for their biosynthesis and biological function. Biochem Biophys Res Commun. 1999;256:377–380. doi: 10.1006/bbrc.1999.0254. [DOI] [PubMed] [Google Scholar]
- Bradshaw HB, Walker JM. The expanding field of cannabimimetic and related lipid mediators. Br J Pharmacol. 2005;144:459–465. doi: 10.1038/sj.bjp.0706093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci. 2004;24:2708–2715. doi: 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1289–G1297. doi: 10.1152/ajpgi.00543.2005. [DOI] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Makriyannis A, Lin SY, Beltramo M, Piomelli D. Inhibition of intestinal motility by anandamide, an endogenous cannabinoid. Eur J Pharmacol. 1997;340:R7–R8. [PubMed] [Google Scholar]
- Cani PD, Montoya ML, Neyrinck AM, Delzenne NM, Lambert DM. Potential modulation of plasma ghrelin and glucagon-like peptide-1 by anorexigenic cannabinoid compounds, SR141716A (rimonabant) and oleoylethanolamide. Br J Nutr. 2004;92:757–761. doi: 10.1079/bjn20041256. [DOI] [PubMed] [Google Scholar]
- Capasso R, Matias I, Lutz B, Borrelli F, Capasso F, Marsicano G, et al. Fatty acid amide hydrolase controls mouse intestinal motility in vivo. Gastroenterology. 2005;129:941–951. doi: 10.1053/j.gastro.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Carai MA, Colombo G, Gessa GL, Yalamanchili R, Basavarajappa BS, Hungund BL. Investigation on the relationship between cannabinoid CB1 and opioid receptors in gastrointestinal motility in mice. Br J Pharmacol. 2006;148:1043–1050. doi: 10.1038/sj.bjp.0706824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts AA, Izzo AA. The gastrointestinal pharmacology of cannabinoids. An update. Curr Opin Pharmacol. 2004;4:572–579. doi: 10.1016/j.coph.2004.05.007. [DOI] [PubMed] [Google Scholar]
- D'Argenio G, Valenti M, Scaglione G, Cosenza V, Sorrentini I, Di Marzo V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006;20:568–570. doi: 10.1096/fj.05-4943fje. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Izzo AA, Degenhardt B, Valenti M, Scaglione G, Capasso R, et al. Involvement of the cannabimimetic compound, N-palmitoyl-ethanolamine, in inflammatory and neuropathic conditions: review of the available pre-clinical data, and first human studies. Neuropharmacology. 2005;48:1154–1163. doi: 10.1016/j.neuropharm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- de Lago E, Ligresti A, Ortar G, Morera E, Cabranes A, Pryce G, et al. In vivo pharmacological actions of two novel inhibitors of anandamide cellular uptake. Eur J Pharmacol. 2004;484:249–257. doi: 10.1016/j.ejphar.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Dembinski A, Warzecha Z, Ceranowicz P, Dembinski M, Cieszkowski J, Pawlik WW, et al. Cannabinoids in acute gastric damage and pancreatitis. J Physiol Pharmacol. 2006;57:137–154. [PubMed] [Google Scholar]
- Despres JP, Golay A, Sjostrom L. Rimonabant in Obesity-Lipids Study Group. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Izzo AA. Endocannabinoid overactivity and intestinal inflammation. Gut. 2006;55:1373–1376. doi: 10.1136/gut.2005.090472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005;8:585–589. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- Duncan M, Davison JS, Sharkey KA. Review article: endocannabinoids and their receptors in the enteric nervous system. Aliment Pharmacol Ther. 2005;22:667–683. doi: 10.1111/j.1365-2036.2005.02648.x. [DOI] [PubMed] [Google Scholar]
- El-Salhy M. Gastric emptying in an animal model of human diabetes type 1: relation to endocrine cells. Acta Diabetol. 2001;38:139–144. doi: 10.1007/s005920170011. [DOI] [PubMed] [Google Scholar]
- Esfandyari T, Camilleri M, Ferber I, Burton D, Baxter K, Zinsmeister AR. Effect of a cannabinoid agonist on gastrointestinal transit and postprandial satiation in healthy human subjects: a randomized, placebo-controlled study. Neurogastroenterol Motil. 2006;18:831–838. doi: 10.1111/j.1365-2982.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- Germano MP, D'Angelo V, Mondello MR, Pergolizzi S, Capasso F, Capasso R, et al. Cannabinoid CB1-mediated inhibition of stress-induced gastric ulcers in rats. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:241–244. doi: 10.1007/s002100000360. [DOI] [PubMed] [Google Scholar]
- Guagnini F, Cogliati P, Mukenge S, Ferla G, Croci T. Tolerance to cannabinoid response on the myenteric plexus of guinea-pig ileum and human small intestinal strips. Br J Pharmacol. 2006;148:1165–1173. doi: 10.1038/sj.bjp.0706813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds NM, Ullrich K, Smid SD. Cannabinoid 1 (CB1) receptors coupled to cholinergic motorneurones inhibit neurogenic circular muscle contractility in the human colon. Br J Pharmacol. 2006;148:191–199. doi: 10.1038/sj.bjp.0706710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Hillard CJ. Modulators of endocannabinoid enzymic hydrolysis and membrane transport. Handb Exp Pharmacol. 2005;168:187–207. doi: 10.1007/3-540-26573-2_6. [DOI] [PubMed] [Google Scholar]
- Holzer P. TRPV1 and the gut: from a tasty receptor for a painful vanilloid to a key player in hyperalgesia. Eur J Pharmacol. 2004;500:231–241. doi: 10.1016/j.ejphar.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Hornby PJ, Prouty SM. Involvement of cannabinoid receptors in gut motility and visceral perception. Br J Pharmacol. 2004;141:1335–1345. doi: 10.1038/sj.bjp.0705783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA. The cannabinoid CB(2) receptor: a good friend in the gut. Neurogastroenterol Motil. 2007;19:704–708. doi: 10.1111/j.1365-2982.2007.00977.x. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Aviello G, Petrosino S, Orlando P, Marsicano G, Lutz B, et al. Increased endocannabinoid levels reduce the development of precancerous lesions in the mouse colon J Mol Med 2007. e-pub ahead of print 6 September 2007 [DOI] [PMC free article] [PubMed]
- Izzo AA, Capasso F. Marijuana for cholera therapy. Trends Pharmacol Sci. 2006;27:7–8. doi: 10.1016/j.tips.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Capasso F, Costagliola A, Bisogno T, Marsicano G, Ligresti A, et al. An endogenous cannabinoid tone attenuates cholera toxin-induced fluid accumulation in mice. Gastroenterology. 2003;125:765–774. doi: 10.1016/s0016-5085(03)00892-8. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Capasso R, Pinto L, Di Carlo G, Mascolo N, Capasso F. Effect of vanilloid drugs on gastrointestinal transit in mice. Br J Pharmacol. 2001;132:1411–1416. doi: 10.1038/sj.bjp.0703975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Mascolo N, Borrelli F, Capasso F. Excitatory transmission to the circular muscle of the guinea-pig ileum: evidence for the involvement of cannabinoid CB1 receptor. Br J Pharmacol. 1998;124:1363–1368. doi: 10.1038/sj.bjp.0701964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Mascolo N, Capasso R, Germano MP, De Pasquale R, Capasso F. Inhibitory effect of cannabinoid agonists on gastric emptying in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:221–223. doi: 10.1007/s002109900054. [DOI] [PubMed] [Google Scholar]
- Jhaveri MD, Richardson D, Chapman V. Endocannabinoid metabolism and uptake: novel targets for neuropathic and inflammatory pain. Br J Pharmacol. 2007;152:624–632. doi: 10.1038/sj.bjp.0707433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krowicki ZK, Moerschbaecher JM, Winsauer PJ, Digavalli SV, Hornby PJ. Delta9-tetrahydrocannabinol inhibits gastric motility in the rat through cannabinoid CB1 receptors. Eur J Pharmacol. 1999;371:187–196. doi: 10.1016/s0014-2999(99)00165-x. [DOI] [PubMed] [Google Scholar]
- Landi M, Croci T, Rinaldi-Carmona M, Maffrand JP, Le Fur G, Manara L. Modulation of gastric emptying and gastrointestinal transit in rats through intestinal cannabinoid CB(1) receptors. Eur J Pharmacol. 2002;450:77–83. doi: 10.1016/s0014-2999(02)02053-8. [DOI] [PubMed] [Google Scholar]
- Lehmann A, Blackshaw LA, Branden L, Carlsson A, Jensen J, Nygren E, et al. Cannabinoid receptor agonism inhibits transient lower esophageal sphincter relaxations and reflux in dogs. Gastroenterology. 2002;123:1129–1134. doi: 10.1053/gast.2002.36025. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol. 2006;46:101–122. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- Maione S, De Petrocellis L, de Novellis V, Moriello AS, Petrosino S, Palazzo E, et al. Analgesic actions of N-arachidonoyl-serotonin, a fatty acid amide hydrolase inhibitor with antagonistic activity at vanilloid TRPV1 receptors. Br J Pharmacol. 2007;150:766–781. doi: 10.1038/sj.bjp.0707145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang CF, Erbelding D, Kilbinger H. Differential effects of anandamide on acetylcholine release in the guinea-pig ileum mediated via vanilloid and non-CB1 cannabinoid receptors. Br J Pharmacol. 2001;134:161–167. doi: 10.1038/sj.bjp.0704220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascolo N, Izzo AA, Ligresti A, Costagliola A, Pinto L, Cascio MG, et al. The endocannabinoid system and the molecular basis of paralytic ileus in mice. FASEB J. 2002;16:1973–1975. doi: 10.1096/fj.02-0338fje. [DOI] [PubMed] [Google Scholar]
- Massa F, Marsicano G, Hermann H, Cannich A, Krisztina M, Cravatt BF, et al. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest. 2004;113:1202–1209. doi: 10.1172/JCI19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa F, Storr M, Lutz B. The endocannabinoid system in the physiology and pathophysiology of the gastrointestinal tract. J Mol Med. 2005;83:944–954. doi: 10.1007/s00109-005-0698-5. [DOI] [PubMed] [Google Scholar]
- Matias I, Gonthier MP, Petrosino S, Docimo L, Capasso R, Hoareau L, et al. Role and regulation of acylethanolamides in energy balance: focus on adipocytes and beta-cells. Br J Pharmacol. 2007;152:676–690. doi: 10.1038/sj.bjp.0707424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum RW, Soykan I, Sridhar KR, Ricci DA, Lange RC, Plankey MW. Delta-9-tetrahydrocannabinol delays the gastric emptying of solid food in humans: a double-blind, randomized study. Aliment Pharmacol Ther. 1999;13:77–80. doi: 10.1046/j.1365-2036.1999.00441.x. [DOI] [PubMed] [Google Scholar]
- McVey DC, Schmid PC, Schmid HH, Vigna SR. Endocannabinoids induce ileitis in rats via the capsaicin receptor (VR1) J Pharmacol Exp Ther. 2003;304:713–722. doi: 10.1124/jpet.102.043893. [DOI] [PubMed] [Google Scholar]
- Mulé F, Amato A, Baldassano S, Serio R. Involvement of CB1 and CB2 receptors in the modulation of cholinergic neurotransmission in mouse gastric preparations. Pharmacol Res. 2007;56:185–192. doi: 10.1016/j.phrs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Ortar G, Ligresti A, De Petrocellis L, Morera E, Di Marzo V. Novel selective and metabolically stable inhibitors of anandamide cellular uptake. Biochem Pharmacol. 2003;65:1473–1481. doi: 10.1016/s0006-2952(03)00109-6. [DOI] [PubMed] [Google Scholar]
- Osei-Hyiaman D, Harvey-White J, Batkai S, Kunos G. The role of the endocannabinoid system in the control of energy homeostasis. Int J Obes (Lond) 2006;30:S33–S38. doi: 10.1038/sj.ijo.0803276. [DOI] [PubMed] [Google Scholar]
- Park MI, Camilleri M. Gastric motor and sensory functions in obesity. Obes Res. 2005;13:491–500. doi: 10.1038/oby.2005.51. [DOI] [PubMed] [Google Scholar]
- Park MI, Camilleri M. Gastroparesis: clinical update. Am J Gastroenterol. 2006;101:1129–1139. doi: 10.1111/j.1572-0241.2006.00640.x. [DOI] [PubMed] [Google Scholar]
- Partosoedarso ER, Abrahams TP, Scullion RT, Moerschbaecher JM, Hornby PJ. Cannabinoid-1 receptor in the dorsal vagal complex modulates lower oesophageal sphincter relaxation in ferrets. J Physiol. 2003;550 Part 1:149–158. doi: 10.1113/jphysiol.2003.042242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol. 2006;147 Suppl 1:S163–S171. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Fernando SR, Nash JE, Coutts AA. Further evidence for the presence of cannabinoid CB1 receptors in guinea-pig small intestine. Br J Pharmacol. 1996;118:2199–2205. doi: 10.1111/j.1476-5381.1996.tb15663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L, Izzo AA, Cascio MG, Bisogno T, Hospodar-Scott K, Brown DR, et al. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology. 2002;123:227–234. doi: 10.1053/gast.2002.34242. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J, RIO-North America Study Group Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- Ross RA. Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowska M, Fereniec-Goltbiewska L. ACEA (arachidonyl-2-chloroethylamide), the selective cannabinoid CB1 receptor agonist, protects against aspirin-induced gastric ulceration. Pharmazie. 2006;61:341–342. [PubMed] [Google Scholar]
- Sanger GJ. Endocannabinoids and the gastrointestinal tract: what are the key questions. Br J Pharmacol. 2007;152:663–670. doi: 10.1038/sj.bjp.0707422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF, RIO-Diabetes Study Group Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet. 2006;368:1660–1672. doi: 10.1016/S0140-6736(06)69571-8. [DOI] [PubMed] [Google Scholar]
- Sharkey KA, Pittman QJ. Central and peripheral signaling mechanisms involved in endocannabinoid regulation of feeding: a perspective on the munchies. Sci STKE. 2005;2005:pe15. doi: 10.1126/stke.2772005pe15. [DOI] [PubMed] [Google Scholar]
- Storr M, Gaffal E, Saur D, Schusdziarra V, Allescher HD. Effect of cannabinoids on neural transmission in rat gastric fundus. Can J Physiol Pharmacol. 2002;80:67–76. doi: 10.1139/y02-005. [DOI] [PubMed] [Google Scholar]
- Van Der Stelt M, Di Marzo V. Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur J Biochem. 2004;271:1827–1834. doi: 10.1111/j.1432-1033.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S, RIO-Europe Study Group Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Oland LD, Ho W, Hillard CJ, Mackie K, Davison JS, et al. Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology. 2001;121:767–774. doi: 10.1053/gast.2001.28466. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Webster LJ, Wyatt A, Dourish CT, Kennett GA. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology (Berl) 2003;167:103–111. doi: 10.1007/s00213-002-1384-8. [DOI] [PubMed] [Google Scholar]