Abstract
Background and purpose:
Clostridium perfringens beta-toxin, an important agent of necrotic enteritis, causes plasma extravasation due to the release of a tachykinin NK1 receptor agonist in mouse skin. In this study, we investigated the role of cytokines in beta-toxin-induced plasma extravasation.
Experimental approach:
Male Balb/c, C3H/HeN and C3H/HeJ mice were anaesthetized with pentobarbitone and beta-toxin was injected i.d. into shaved dorsal skin. SR140333, capsaicin, chlorpromazine and pentoxifylline were given as pretreatment when required before the injection of the toxin. Cytokines in the dorsal skin were measured by ELISA.
Key results:
Injection (i.d.) of beta-toxin induced a dose-dependent increase in dermal TNF-α and interleukin (IL)-1β levels with a concomitant increase in plasma extravasation, but not the release of IL-6. SR140333 and capsaicin significantly inhibited the toxin-induced release of TNF-α and IL-1β. The plasma extravasation and the release of TNF-α induced by beta-toxin were significantly inhibited by chlorpromazine and pentoxifylline which inhibit the release of TNF-α. The toxin-induced plasma extravasation in mouse skin was attenuated by pretreatment with a monoclonal antibody against TNF-α, but not anti-IL-1β. Furthermore, the toxin caused an increase in plasma extravasation in both C3H/HeN (TLR4-intact) and C3H/HeJ (TLR4-deficient) mice. In C3H/HeN mice, the toxin-induced leakage was not inhibited by pretreatment with anti-TLR4/MD-2 antibody.
Conclusions and implications:
These observations show that beta-toxin-induced plasma extravasation in mouse skin is related to the release of TNF-α via the mechanism involving tachykinin NK1 receptors, but not via TLR4.
Keywords: Clostridium perfringens, beta-toxin, plasma extravasation, tachykinin NK1 receptor, TNF-α
Introduction
Clostridium perfringens β-toxin is thought to be a significant factor in the pathogenesis of necrotic enteritis caused by the type C strain (Songer, 1996; Sakurai and Nagahama, 2003, 2006). Epidemics of enteritis necroticans (Darmbrand) were first described in northern Germany and Scandinavia after World War II. Later, enteritis necroticans (Pig-bel) was reported in the highlands of Papua New Guinea in 1963. The disease is characterized by an acute sudden onset, severe haemorrhage into the lumen of the small intestine and early death. Furthermore, mortality is reported to be 35–40% and highest in the young and the old. β-Toxin possesses lethal and dermonecrotic activities (Sakurai et al., 1997; Sakurai and Nagahama, 2003, 2006). The monomeric form of the toxin is toxic, but the oligomeric form, which is difficult to dissociate from the monomer, is non-toxic. The toxin changes readily from the active to the inactive form, indicating that it is extremely labile. Moreover, the toxin is extremely susceptible to proteinases and heat.
The deduced amino-acid sequence of β-toxin significantly resembles that of pore-forming toxins, such as Staphylococcus aureus α-toxin, leucocidin and γ-toxin (Sakurai and Nagahama, 2006). Shatursky et al. (2000) and Tweten (2001) reported that the lethal action of C. perfringens β-toxin is based on the formation of cation-selective pores in susceptible cells. We reported that β-toxin induced swelling and lysis of HL-60 cells and that the toxin formed a functional oligomer of 228 kDa, which is linked to the cytotoxicity, in lipid rafts of HL-60 cells (Nagahama et al., 2003a). The characteristics of β-toxin resemble those of pore-forming toxins, suggesting that β-toxin belongs to the same family as the α-toxin, which forms a functional oligomer in membranes (Sakurai and Nagahama, 2006).
We have reported that β-toxin acted on the autonomic nervous system and produced arterial constriction (Sakurai et al., 1981, 1984). We also reported that administration of the toxin to mice induces signs of neurological involvement, such as tetany and opisthotonus (severe convulsions) (Sakurai and Nagahama, 2006). We found that β-toxin causes stimulation of capsaicin-sensitive sensory neurons in mouse skin, leading to neurogenic plasma protein extravasation due to the release of a tachykinin neurokinin-1 (NK1) receptor agonist, which is responsible for the release of histamine from mast cells (Nagahama et al., 2003b). However, little is known about the precise mechanism of the plasma extravasation induced by β-toxin in mouse skin. Plasma extravasation induced by lipopolysaccharide (LPS) (Ishida et al., 2000), Bothrops asper snake venom (Chaves et al., 2005), 12-O-tetra-decanoylphorbol-13-acetate (Murakawa et al., 2006) and IgE (Gibbs et al., 2001) has been reported to be mediated by the release of tumour necrosis factor-α (TNF-α). Moreover, Holzer (1998) reported that neuropeptides released from sensory nerve such as substance P (SP) induce the release of cytokines such as TNF-α and interleukin-1 (IL-1). Furthermore, it has been reported that plasma extravasation induced by a tachykinin NK1 receptor agonist is also mediated through the local release of several cytokines (Holzer, 1998; Roosterman et al., 2006). Therefore, to better understand the actions of β-toxin in vivo, we investigated if β-toxin induces plasma extravasation via a cytokine-dependent mechanism in mouse skin.
Methods
Animals
All animal procedures and experiments were approved by the Institutional Animal Care and Use Committee, Tokushima Bunri University. Male Balb/c, toll-like receptor 4 (TLR4) wild-type C3H/HeN and TLR4-defective C3H/HeJ mice weighing approximately 30 g were obtained from Nippon SLC (Hamamatsu, Japan). The animals were housed in plastic cages under controlled environmental conditions (temperature 22 °C, humidity 55%). Food and water were freely available.
Measurement of plasma extravasation
Mice were anesthetized with sodium pentobarbitone (Sagatal, 50 mg kg−1, intraperitoneally (i.p.)). The dorsal skin was shaved and prepared for intradermal injection (up to four sites per mouse, each in a randomly allocated balanced site pattern). 125I-labelled bovine serum albumin (BSA) was injected intravenously in the tail. After 5 min, β-toxin was injected intradermally (i.d.) (0.05 ml per site) as described previously (Nagahama et al., 2003b). Various agents were given as pretreatments (i.d. or i.p. before intradermal injection of the toxin) when required. After 1 h, a blood sample (0.1 ml) was taken from the tail vein. The mice were killed by cervical dislocation and round pieces of skin 10 mm diameter were punched out. Plasma samples and the skin pieces were placed in a γ-counter (Aloka Basic Scaler; Aloka Co. Ltd, Tokyo, Japan). Plasma extravasation at each site was expressed as microliters of plasma by dividing skin sample 125I counts by 125I counts in 1 μl of plasma (Nagahama et al., 2003b).
Drugs
The effect of various drugs on β-toxin-induced plasma extravasation was examined as follows: SR140333 was dissolved in a small volume of 100% ethanol and diluted in 0.9% NaCl. SR140333 (1 and 5 nmoles per site) was given i.d. 5 min before the injection of the toxin. Chlorpromazine, pentoxifylline and LPS were dissolved in 0.9% NaCl. Chlorpromazine (1 and 5 mg kg−1) and pentoxifylline (1 and 5 mg kg−1) were given i.p. 60 min before the injection of the toxin. All antibodies against cytokines were diluted with 0.9% NaCl. All antibodies were given i.d. 12 h before the injection of the toxin. The doses of these drugs were chosen based on various studies (Neuner et al., 1994, Netea et al., 1995, Wada et al., 2000, Nagahama et al., 2003b).
Capsaicin desensitization protocol
A 10% (w/v) solution of capsaicin was prepared in absolute ethanol (capsaicin stock solution). The solution was further diluted 1:1:8 (capsaicin stock solution:Tween 80:0.15 M NaCl) (Alber et al., 1989). After the dorsal skin of the mice was shaved, it was painted with the capsaicin solution three times on the first day of pretreatment and two times per day during the following four days. As control, the diluent (ethanol and Tween 80) alone was painted on the dorsal skin of mice as described previously (Nagahama et al., 2003b).
Measurement of cytokines
Approximately 500 mg of pulverized skin was weighed and homogenized in 0.5 ml. of PBS containing 0.5% BSA and 0.1% Tween 20 using a glass homogenizer. The homogenate was centrifuged at 10 000 g for 1 h at 4 °C. The amounts of cytokines in the supernatant were determined using an enzyme-linked immunosorbent assay kit (Suganuma et al., 2002).
Statistical analysis
All values were expressed as mean±s.e.mean. Student's unpaired t-test and one-way analysis of variance were used for statistical analysis. P-values less than 0.05 were considered to be statistically significant.
Materials
SR140333 was kindly provided by Sanofi (Toulouse, France). Pentoxifylline, chlorpromazine, capsaicin (8-methyl-N-vanillyl-6-nonenamide), LPS (from Salmonella typhimurium, L6511) and BSA were purchased from Sigma (St Louis, MO, USA). The expression and purification of β-toxin from C. perfringens was carried out as described previously (Sakurai and Fujii, 1987).
Goat anti-mouse IL-1α antibody, goat anti-mouse IL-1β antibody, goat anti-mouse TNF-α antibody and control goat IgG were purchased from R&D Systems (Minneapolis, MN, USA). TNF-α, IL-1β and IL-6 enzyme-linked immunosorbent assay kits were obtained from BioSource International (Camarillo, CA, USA). Functional-grade neutralizing purified anti-mouse TLR4/MD2 MTS5101 (rat IgG2a) and functional grade purified rat IgG2a isotype control were obtained from eBioscience (San Diego, CA, USA). Several approaches were adopted to demonstrate the specificity of the antibody against TLR4 (Akashi et al., 2000, Nomura et al., 2000). Mouse anti-β-toxin antibody was prepared as described previously (Nagahama et al., 1999). IgG from the anti-toxin was isolated by immunoaffinity column chromatography as described previously (Nagahama et al., 1991).
Results
β-Toxin-induced release of cytokines in mouse dorsal skin
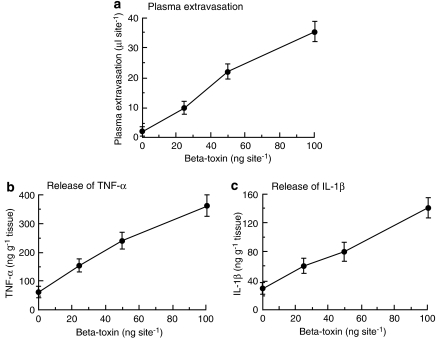
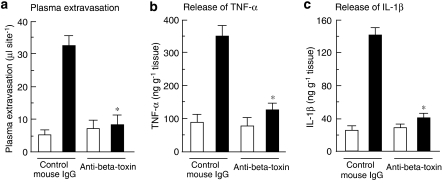
We reported that plasma extravasation induced by β-toxin was induced rapidly within 15 min, and reached a maximum within 120 min (Nagahama et al., 2003b). To investigate the role of cytokines in early stage of β-toxin-induced plasma extravasation, the toxin-induced plasma extravasation and release of TNF-α, IL-1β and IL-6 at the site of injection were evaluated 60 min after the administration of β-toxin. As shown in Figure 1a, the toxin (25–100 ng per site) dose dependently induced the extravasation of plasma and the release of TNF-α and IL-1β (Figures 1b and c), but not that of IL-6 (data not shown). As shown in Figures 1a–c, the dose-dependent increase in the toxin-induced plasma extravasation was similar to that in the toxin-induced release of TNF-α and IL-1β in mouse dorsal skin. In addition, the toxin-induced release of plasma extravasation and cytokines in dermal skin was inhibited by the injection of anti-β-toxin antiserum (Figure 2).
Figure 1.
β-Toxin-induced plasma extravasation and release of cytokines in the dorsal skin of mice. 125I-labelled BSA (0.1 ml of 2.5% solution) was injected into the tail vein. After 5 min, β-toxin (25–100 ng) was injected i.d. (0.05 ml per site). Plasma extravasation was measured 60 min after the injection of β-toxin (a). TNF-α (b) and IL-1β (c) levels were determined in skin samples collected 1 h after the injection of β-toxin. Values are mean±s.e.mean, n=6. BSA, bovine serum albumin; i.d., intradermally; IL-1β, interleukin-1β; TNF-α, tumour necrosis factor-α.
Figure 2.
Effect of anti-β-toxin antibody on β-toxin-induced plasma extravasation and release of cytokines in the dorsal skin of mice. Anti-β-toxin antibody (1 μg per site) and control mouse IgG (1 μg per site) were administered i.d. 5 min before the experiment. β-Toxin (50 ng per site) or saline (0.05 ml per site) was injected i.d. Plasma extravasation and release of cytokines were measured 60 min after the injection of β-toxin or saline. Values are mean±s.e.mean, n=6. *P<0.01, compared with β-toxin with control mouse IgG. I.d., intradermally.
Effect of SR140333 and capsaicin on the toxin-induced release of cytokines
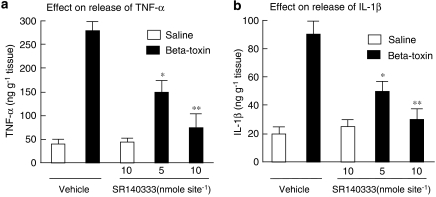
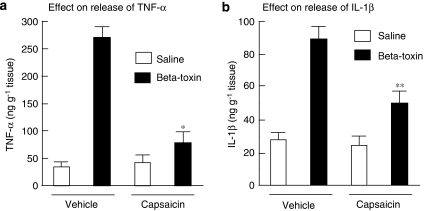
SR140333, a long-lasting non-peptide tachykinin NK1 antagonist, markedly inhibited the toxin-induced leakage of plasma. To test whether the toxin-induced release of cytokines is dependent on the release of tachykinins, the effect of SR140333 was investigated. Figures 3a and b show that the inhibitor (5 and 10 nmoles per site) caused a reduction in the toxin-induced release of TNF-α and IL-1β in a dose-dependent manner. It has been reported that treatment with capsaicin leads to release of neuropeptides (tachykinins such as SP and calcitonin gene-related peptide) and causes depletion of neuropeptides in sensory nerves. We also reported that the leakage of plasma induced by the β-toxin was markedly reduced in capsaicin-pretreated mouse skin (Nagahama et al., 2003b). To investigate the role of endogenous SP in the β-toxin-induced release of cytokines, the effect of a topical administration of capsaicin on the toxin-induced extravasation was tested. Topical administration of capsaicin onto the dorsal back of mice significantly inhibited the toxin-induced release of TNF-α and IL-1β in the skin, as shown in Figures 4a and b. The observation suggests that the toxin-induced release of cytokines in dermal tissue is linked to the release induced by β-toxin of a tachykinin NK1 receptor agonist from sensory nerves.
Figure 3.
Effect of SR140333 on β-toxin-induced release of cytokines in the dorsal skin of mice. SR140333 (1 or 5 nmoles per site) or vehicle was given i.d. 5 min before the intradermal injection of β-toxin (50 ng per site) or saline (0.05 ml per site). TNF-α (a) and IL-1β (b) levels were determined in skin samples collected 1 h after the injection of β-toxin or saline. Values are mean±s.e.mean, n=6. *P<0.05 and **P<0.01, compared with β-toxin with vehicle. I.d., intradermally; IL-1β, interleukin-1β; TNF-α, tumour necrosis factor-α.
Figure 4.
Effect of capsaicin on β-toxin-induced release of cytokines in the dorsal skin of mice. After the dorsal skin was shaved, capsaicin solution or the diluent alone was painted for 4 days as described in Methods. TNF-α (a) and IL-1β (b) levels were determined in skin samples collected 1 h after the injection of β-toxin (50 ng per site) or saline (0.05 ml per site). Values are mean±s.e.mean, n=6. *P<0.01 and **P<0.05, compared with β-toxin with vehicle. IL-1β, interleukin-1β; TNF-α, tumour necrosis factor-α.
Effect of chlorpromazine and pentoxifylline on the toxin-induced plasma extravasation and release of TNF-α
Chlorpromazine and pentoxifylline inhibit the release of TNF-α. To assess the effect of TNF-α on β-toxin-induced plasma extravasation, we investigated whether chlorpromazine and pentoxifylline affect the toxin-induced extravasation and release of TNF-α. Systemic treatment with chlorpromazine (1 and 5 mg kg−1, i.p., 30 min before) significantly reduced the toxin-induced plasma extravasation and release of TNF-α (Table 1). Pretreatment with pentoxifylline (5 and 10 mg kg−1, i.p., 60 min before) also produced a significant decrease in the toxin-induced plasma extravasation and release of TNF-α (Table 1), suggesting that the release is related to the plasma extravasation.
Table 1.
Effect of chlorpromazine and pentoxifylline on plasma extravasation and release of TNF-α induced by β-toxin in the dorsal skin of mice
| Drugs | Toxin | Plasma extravasation (μl per site) | Production of TNF-α (ng g−1 of tissue) |
|---|---|---|---|
| Vehicle | − | 4.1±1.2 | 48.1±5.1 |
| Vehicle | + | 32.3±5.6 | 232.5±10.1 |
| Chlorpromazine (5 mg kg−1) | − | 5.2±2.2 | 41.3±4.9 |
| Chlorpromazine (1 mg kg−1) | + | 19.5±6.3* | 115.4±10.9* |
| Chlorpromazine (5 mg kg−1) | + | 9.7±4.8** | 76.7±6.3** |
| Pentoxifylline (5 mg kg−1) | − | 5.8±3.1 | 38.8 ±3.9 |
| Pentoxifylline (1 mg kg−1) | + | 15.9±7.5* | 96.1±9.4* |
| Pentoxifylline (5 mg kg−1) | + | 8.2±2.8** | 76.2±6.3** |
Abbreviations: i.d., intradermally; TNF-α, tumour necrosis factor-α.
Mice were injected intraperitoneally with chlorpromazine (1 and 5 mg kg−1), pentoxifylline (1 and 5 mg kg−1) or vehicle 60 min before the injection of the toxin. β-Toxin (50 ng per site) or saline (0.05 ml per site) was injected i.d. Plasma extravasation and TNF-α were evaluated as described in Methods. Values are mean±s.e.mean, n=6.
*P<0.05, compared with vehicle plus toxin.
**P<0.01, compared with vehicle plus toxin.
Effect of anti-cytokine antibodies on the toxin-induced plasma extravasation
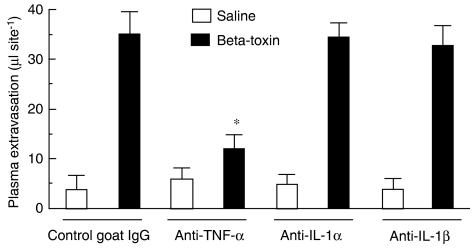
To test whether toxin-induced plasma extravasation is stimulated by TNF-α and IL-1, the effect of anti-cytokine anti-TNF-α, anti-IL-1α and anti-IL-1β on the toxin-induced plasma leakage was investigated. As shown in Figure 5, the β-toxin-induced increase in plasma extravasation was significantly attenuated by pre-injection of the anti-TNF-α antibody, but not the control goat IgG, anti-IL-1α antibody or anti-IL-1β antibody. The result demonstrates that the toxin-induced release of TNF-α in mouse skin plays an important role in the β-toxin-induced plasma extravasation.
Figure 5.
Effect of anti-TNF-α, anti-IL-1α and anti-IL-1β on β-toxin-induced plasma extravasation in the dorsal skin of mice. Anti-TNF-α antibody (1 μg per site), anti-IL-1α antibody (1 μg per site), anti-IL-1β antibody (1 μg per site) antisera and control goat IgG (1 μg per site) were administered i.d. 12 h before the experiment. Mice received β-toxin (50 ng per site) or saline (0.05 ml per site), and plasma extravasation was measured 60 min after the injection of β-toxin. Values are mean±s.e.mean, n=6. *P<0.01, compared with β-toxin with control goat IgG. I.d., intradermally; IL-1α, interleukin-1α; IL-1β, interleukin-1β; TNF-α, tumour necrosis factor-α.
Effect of TLR4 on the toxin-induced release of cytokine
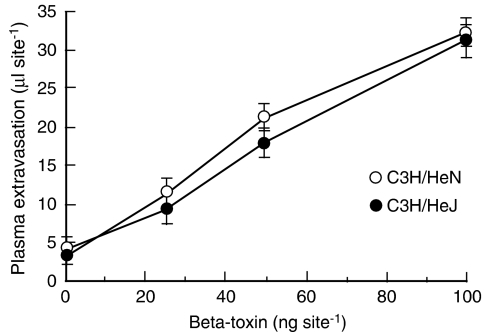
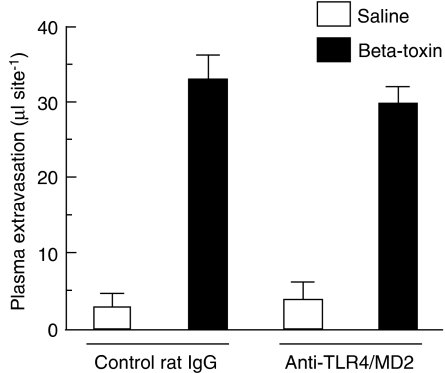
The TLR plays a role in innate immune responses to various microbial infections in mucosal tissue and skin. To investigate the role of TLR4 in the toxin-induced plasma extravasation, we assessed β-toxin-induced plasma extravasation in the dorsal skin of C3H/HeJ and C3H/HeN mice. In preliminary experiments, LPS-induced plasma extravasation was significantly greater in C3H/HeN (36.5±5.1 μl per site) than C3H/HeJ (5.2±2.2 μl per site). On the other hand, as shown in Figure 6, β-toxin increased plasma extravasation in both C3H/HeJ and C3H/HeN mice in a dose-dependent manner (25–100 ng per site). There was no difference in the toxin-induced plasma extravasation between C3H/HeJ and C3H/HeN mice. β-Toxin similarly caused the release of TNF-α in both mice, and anti-TNF-α antibody inhibited the plasma extravasation induced by β-toxin (data not shown). Next, we tested the effect of anti-TLR4/MD-2 antibody on the toxin-induced plasma extravasation in C3H/HeN mice (Figure 7). β-Toxin-induced plasma extravasation was not affected by pre-injection of the anti-TLR4/MD2 antibody or control IgG.
Figure 6.
β-Toxin-induced plasma extravasation in the dorsal skin of C3H/HeN and C3H/HeJ mice. 125I-labelled BSA (0.1 ml of 2.5% solution) was injected intravenously into the tail. After 5 min, β-toxin (25–100 ng) was injected i.d. (0.05 ml per site). Plasma extravasation was measured 60 min after the injection of β-toxin. Values are mean±s.e.mean, n=6. BSA, bovine serum albumin; i.d., intradermally.
Figure 7.
Effect of anti-TLR4/MD-2 antibody on β-toxin-induced plasma extravasation in the dorsal skin of C3H/HeN mice. Anti-TLR4/MD-2 antibody (1 μg per site) and control rat IgG (1 μg per site) were administered i.d. 12 h before the experiment. Mice received β-toxin (50 ng per site) or saline (0.05 ml per site), and plasma extravasation was measured 60 min after the injection. Values are mean±s.e.mean, n=6. I.d., intradermally; TLR4, toll-like receptor 4.
Discussion and conclusions
The present study showed that the toxin-induced plasma extravasation in skin is closely related to the release of TNF-α. This is the first published report on the toxin-induced release of TNF-α in dermal skin.
We reported that β-toxin induces the release of SP, a tachykinin NK1 receptor agonist, which is responsible for the subsequent neurogenic plasma extravasation (Nagahama et al., 2003b). However, SP did not strongly induce plasma extravasation in mouse skin, compared with β-toxin (data not shown), suggesting that β-toxin-induced plasma extravasation is dependent on not only neurogenic inflammation, but also other pathways. Holzer (1998) reported that the release of cytokines in skin is responsible for plasma extravasation. In the present study, the β-toxin induced the release of TNF-α and IL-1β, but not IL-6, when injected into mouse dorsal skin.
The plasma extravasation induced by LPS in the skin of mice is reported to be mediated by proinflammatory mediators such as cytokines, histamine and nitric oxide (Suganuma et al., 2002). TLR4 plays a crucial role in the release of cytokines from mast cells (McCurdy et al., 2001; McInturff et al., 2005) and keratinocytes (Song et al., 2002). When LPS was given to C3H/HeJ, a mouse strain with a mutated TLR4 receptor, and C3H/HeN, a mouse strain with a normal TLR4, the LPS-induced leakage of dye was markedly less extensive in the former mice (Suganuma et al., 2002). TLR4 has therefore been implicated in the release of TNF-α (Beutler, 2004). The present study showed that the β-toxin-induced plasma extravasation in C3H/HeJ mice is almost the same as that in C3H/HeN mice. Moreover, anti-TLR4/MD2 antibody did not inhibit the plasma extravasation induced by the toxin in C3H/HeN mice. Therefore, the plasma extravasation induced by β-toxin in mouse skin is not mediated via a mechanism involving the TLR4 receptor.
We previously reported that plasma extravasation induced by β-toxin was significantly inhibited by SR140333 and capsaicin (Nagahama et al., 2003b). Injection of SR140333 or treatment with capsaicin markedly inhibited the toxin-induced release of TNF-α and IL-1β as well as plasma extravasation. The result showed that the toxin-induced release of cytokines depends on the release of a NK1 receptor agonist induced by the toxin, indicating that the release of cytokines occurs downstream of the release of an NK1 receptor agonist such as SP.
We reported that SP released from sensory nerves induced by β-toxin stimulates mast cells to release histamine (Nagahama et al., 2003b). It has been reported that SP and neurokinin A are capable of activating mast cells and keratinocytes in skin, resulting in the release of proinflammatory cytokines (Ansel et al., 1997; Roosterman et al., 2006), and that SP is capable of mediating the secretion of histamine and TNF-α from mast cells in skin (Holzer, 1998; Roosterman et al., 2006). β-Toxin induced the release of TNF-α and IL-1β in vivo at least.
Neuropeptides are also capable of activating dermal microvascular endothelial cells in vivo by upregulating cell-adhesion molecules such as P-selectin, intercellular adhesion molecules 1 and vascular cell adhesion molecule, 1 which recruit neutrophils and eosinophils (Steinhoff et al., 2003). Therefore, it is possible that injection of β-toxin in mouse skin causes the recruitment of inflammatory cells in the site of the plasma extravasation induced by the toxin.
We have reported that SP released from sensory nerves by β-toxin stimulates mast cells to release histamine (Nagahama et al., 2003b). It is proposed that cutaneous sensory nerves and mast cells can represent a functional unit whereby stimulated nerve fibers may activate local mast cells (Kakurai et al., 2006). SP and neurokinins are reported to induce mast cells to release histamine, TNF-α and other inflammatory mediators (Matsuda et al., 1989; Yano et al., 1989). Therefore, it appeared that β-toxin directly targets sensory nerves as reported previously (Nagahama et al., 2003b).
Chlorpromazine, an inhibitor of the release of TNF-α and IL-1β (Netea et al., 1995), and pentoxifylline, an inhibitor of the release of TNF-α, IL-1β, IL-6 and IL-8 (Neuner et al., 1994), significantly decreased the toxin-induced plasma leakage and release of TNF-α. The β-toxin-induced plasma extravasation was significantly attenuated by antibody against TNF-α but not against IL-1β. These observations suggest that the plasma extravasation induced by β-toxin is closely related to the release of TNF-α in the skin. It therefore appears that NK1 receptor antagonists and inhibitors of TNF-α release may be worth pursuing as a novel therapeutic approach to the treatment of necrotic enteritis caused by C. perfringens type C.
In the present study, we propose that the NK1 agonist, SP, released by the β-toxin from sensory neurons induces the release of TNF-α, and these mediators cause plasma extravasation. Further investigation of the mechanism of β-toxin-induced plasma extravasation may reveal a novel mechanism for neurogenic inflammation.
Acknowledgments
We thank K Kobayashi, I Kodama and Y Nakamura for technical assistance. This work was supported by a grant-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, MEXT.HAITEKU, 2003–2007, and the Open Research Center Program of MEXT.
Abbreviations
- BSA
bovine serum albumin
- LPS
lipopolysaccharide
- NK1
neurokinin-1
- SP
substance P
- TLR4
toll-like receptor 4
Conflict of interest
The authors state no conflict of interest.
References
- Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, et al. Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol. 2000;164:3471–3475. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- Alber G, Scheuber PH, Reck B, Sailer-Kramer B, Hartmann A, Hammer DK. Role of substance P in immediate-type skin reactions induced by staphylococcal enterotoxin B in unsensitized monkeys. J Allergy Clin Immunol. 1989;84:880–885. doi: 10.1016/0091-6749(89)90383-7. [DOI] [PubMed] [Google Scholar]
- Ansel JC, Armstrong CA, Song I, Quinlan KL, Olerud JE, Caughman SW, et al. Interactions of the skin and nervous system. J Invest Dermatol Symp Proc. 1997;2:23–26. doi: 10.1038/jidsymp.1997.6. [DOI] [PubMed] [Google Scholar]
- Beutler B. Inferences, questions and possibilities in toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- Chaves F, Teixeira CFP, Gutiérrez JM. Role of TNF-α, IL-1β and IL-6 in the local tissue damage induced by Bothrops asper snake venom: an experimental assessment in mice. Toxicon. 2005;45:171–178. doi: 10.1016/j.toxicon.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Gibbs BF, Wierecky J, Welker P, Henz BM, Wolff HH, Grabbe J. Human skin mast cells rapidly release preformed and newly generated TNF-alpha and IL-8 following stimulation with anti-IgE and other secretagogues. Exp Dermatol. 2001;10:312–320. doi: 10.1034/j.1600-0625.2001.100503.x. [DOI] [PubMed] [Google Scholar]
- Holzer P. Neurogenic vasodilatation and plasma leakage in the skin. Gen Pharmacol. 1998;30:5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- Ishida H, Fujii E, Irie K, Yoshioka T, Muraki T, Ogawa R. Role of inflammatory mediators in lipid A analogue (ONO-4007)-induced vascular permeability change in mouse skin. Br J Pharmacol. 2000;130:1235–1240. doi: 10.1038/sj.bjp.0703425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakurai M, Monteforte R, Suto H, Tsai M, Nakae S, Galli SJ. Mast cell-derived tumor necrosis factor can promote nerve fiber elongation in the skin during contact hypersensitivity in mice. Am J Pathol. 2006;169:1713–1721. doi: 10.2353/ajpath.2006.060602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Kawakita K, Kiso Y, Nakano T, Kitamura Y. Substance P induces granulocyte infiltration through degranulation of mast cells. J Immunol. 1989;142:927–931. [PubMed] [Google Scholar]
- McCurdy JD, Lin TJ, Marshall JS. Toll-like receptor 4-mediated activation of murine mast cells. J Leukoc Biol. 2001;70:977–984. [PubMed] [Google Scholar]
- McInturff JE, Modlin RL, Kim J. The role of toll-like receptors in the pathogenesis and treatment of dermatological disease. J Invest Dermatol. 2005;125:1–8. doi: 10.1111/j.0022-202X.2004.23459.x. [DOI] [PubMed] [Google Scholar]
- Murakawa M, Yamaoka K, Tanaka Y, Fukuda Y. Involvement of tumor necrosis factor (TNF)-α in phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin edema in mice. Biochem Pharmacol. 2006;71:1331–1336. doi: 10.1016/j.bcp.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Nagahama M, Hayashi S, Morimitstu S, Sakurai J. Biological activities and pore formation of Clostridium perfringens beta-toxin in HL 60 cells. J Biol Chem. 2003a;278:36934–36941. doi: 10.1074/jbc.M306562200. [DOI] [PubMed] [Google Scholar]
- Nagahama M, Kihara A, Miyawaki T, Mukai M, Sakaguchi Y, Ochi S, et al. Clostridium perfringens beta-toxin is sensitive to thiol-group modification but does not require a thiol group for lethal activity. Biochim Biophys Acta. 1999;1454:97–105. doi: 10.1016/s0925-4439(99)00026-5. [DOI] [PubMed] [Google Scholar]
- Nagahama M, Kobayashi K, Ochi S, Sakurai J. Enzyme-linked immunosorbent assay for rapid detection of toxins from Clostridium perfringens. FEMS Microbiol Lett. 1991;68:41–44. doi: 10.1016/0378-1097(91)90392-n. [DOI] [PubMed] [Google Scholar]
- Nagahama M, Morimitsu S, Kihara A, Akita M, Setsu K, Sakurai J. Involvement of tachykinin receptors in Clostridium perfringens beta-toxin-induced plasma extravasation. Br J Pharmacol. 2003b;138:23–30. doi: 10.1038/sj.bjp.0705022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Blok WL, Kullberg BJ, Bemelmans M, Vogels MT, Buurman WA, et al. Pharmacologic inhibitors of tumor necrosis factor production exert differential effects in lethal endotoxemia and in infection with live microorganisms in mice. J Infect Dis. 1995;171:393–399. doi: 10.1093/infdis/171.2.393. [DOI] [PubMed] [Google Scholar]
- Neuner P, Klosner G, Schauer E, Pourmojib M, Macheiner W, Grunwald C, et al. Pentoxifylline in vivo downregulates the release of IL-1 beta, IL-6, IL-8 and tumour necrosis factor-alpha by human peripheral blood mononuclear cells. Immunology. 1994;83:262–267. [PMC free article] [PubMed] [Google Scholar]
- Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, et al. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with downregulation of surface toll-like receptor 4 expression. J Immunol. 2000;164:3476–3749. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–1379. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- Sakurai J, Fujii Y. Purification and characterization of Clostridium perfringens beta toxin. Toxicon. 1987;25:1301–1310. doi: 10.1016/0041-0101(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Sakurai J, Fujii Y, Dezaki K, Endo K. Effect of Clostridiumperfringens beta toxin on blood pressure of rats. Microbiol Immunol. 1984;28:23–31. doi: 10.1111/j.1348-0421.1984.tb02944.x. [DOI] [PubMed] [Google Scholar]
- Sakurai J, Fujii Y, Matsuura M, Endo K. Pharmacological effect of beta toxin of Clostridium perfringens type C on rats. Microbiol Immunol. 1981;25:423–432. doi: 10.1111/j.1348-0421.1981.tb00045.x. [DOI] [PubMed] [Google Scholar]
- Sakurai J, Nagahama M. Mechanism of action of Clostridiumperfringens beta-toxin. Recent Res Dev Infect Immun. 2003;1:433–449. [Google Scholar]
- Sakurai J, Nagahama M. Clostridium perfringens beta-toxin: characterization and action. Toxin Rev. 2006;25:89–108. [Google Scholar]
- Sakurai J, Nagahama M, Ochi S. Major toxins of Clostridium perfringens. J Toxicol Toxin Rev. 1997;16:195–214. [Google Scholar]
- Shatursky O, Bayles R, Rogers M, Jost BH, Songer JG, Tweten RK. Clostridium perfringens beta-toxin forms potential-dependent, cation-selective channels in lipid bilayers. Infect Immun. 2000;68:5546–5551. doi: 10.1128/iai.68.10.5546-5551.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song PI, Park YM, Abraham T, Harten B, Zivony A, Neparidze N, et al. Human keratinocytes express functional CD14 and toll-like receptor 4. J Invest Dermatol. 2002;119:424–432. doi: 10.1046/j.1523-1747.2002.01847.x. [DOI] [PubMed] [Google Scholar]
- Songer JG. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev. 1996;9:216–234. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Stander S, Seeliger S, Ansel JC, Schmelz M, Luger T. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol. 2003;139:1479–1488. doi: 10.1001/archderm.139.11.1479. [DOI] [PubMed] [Google Scholar]
- Suganuma T, Irie K, Fujii E, Yoshioka T, Muraki T. Effect of heat stress on lipopolysaccharide-induced vascular permeability change in mice. J Pharmacol Exp Ther. 2002;303:656–663. doi: 10.1124/jpet.102.035758. [DOI] [PubMed] [Google Scholar]
- Tweten RK. Clostridium perfringens beta toxin and Clostridium septicum alpha toxin: their mechanisms and possible role in pathogenesis. Vet Microbiol. 2001;82:1–9. doi: 10.1016/s0378-1135(01)00372-8. [DOI] [PubMed] [Google Scholar]
- Wada K, Fujii E, Ishida H, Yoshioka T, Muraki T. Effect of lipoteichoic acid on dermal vascular permeability in mice. J Pharmacol Exp Ther. 2000;294:280–286. [PubMed] [Google Scholar]
- Yano H, Wershil BK, Arizono N, Galli SJ. Substance P-induced augmentation of cutaneous vascular permeability and granulocyte infiltration in mice is mast cell dependent. J Clin Invest. 1989;84:1276–1286. doi: 10.1172/JCI114295. [DOI] [PMC free article] [PubMed] [Google Scholar]