Abstract
Background and purpose:
In gastrointestinal smooth muscle cGMP levels in response to relaxant agonists are regulated by PKG-mediated phosphorylation and activation of phosphodiesterase 5 (PDE5). The aim of the present study was to determine whether contractile agonists modulate cGMP levels by cross-regulating PDE5 activity and to identify the mechanism of action.
Experimental approach:
Dispersed and cultured muscle cells from rabbit stomach were treated with the nitric oxide donor, S-nitrosoglutathione (GSNO), or with a contractile agonist, ACh and GSNO. PDE5 phosphorylation and activity, and cGMP levels were determined.
Key results:
GSNO stimulated PDE5 phosphorylation and activity and increased cGMP levels in gastric smooth muscle cells. Concurrent activation of cells with ACh augmented GSNO-stimulated PDE5 phosphorylation and activity, and attenuated cGMP levels. The effect of ACh was blocked by the m3 receptor antagonist and by inhibitors of protein kinase C (PKC) or RhoA, but not by the m2 receptor antagonist or inhibitors of PI hydrolysis. The effects of ACh on PDE5 phosphorylation and activity, and cGMP levels were mimicked by a low concentration of tautomycin (10 nM), and a high (1 μM) but not low (1 nM) concentration of okadaic acid. PDE5 was associated with protein phosphatase 1 (PP1) and dephosphorylated by the catalytic subunit of PP1 but not PP2A.
Conclusion and implications:
In gastrointestinal smooth muscle cGMP levels are cross-regulated by contractile agonists via a mechanism that involves RhoA-dependent, PKC-mediated inhibition of PP1 activity. This leads to augmentation of PDE5 phosphorylation and activity, and inhibition of cGMP levels.
Keywords: phosphodiesterases, protein kinase G, protein phosphatase, muscle relaxation
Introduction
In the gastrointestinal tract, the main inhibitory transmitters of the myenteric plexus that regulate smooth muscle relaxation are vasoactive intestinal peptide and its homologue, pituitary AC-activating peptide and nitric oxide (NO). These neurotransmitters induce relaxation through the generation of cAMP and cGMP, and activation of cAMP-dependent PKA and cGMP-dependent PKG (Murthy, 2006). PKA and PKG act at various loci in the pathways that mediate contraction to induce dephosphorylation of myosin light chain (MLC20) and muscle relaxation (Murthy and Makhlouf, 1995b; Murthy, 2001b, 2006; Murthy and Zhou, 2003; Murthy et al., 2003b). Concomitant stimulation of AC and soluble GC, and generation of both cAMP and cGMP are the physiological norms in smooth muscle of the gastrointestinal tract.
Cyclic cAMP and cGMP are hydrolysed into inactive 5′AMP and 5′GMP, respectively, by the activities of PDEs and thus the levels of cAMP and cGMP reflect the balance between the activities of cyclases and PDEs. We have previously shown that cAMP and cGMP levels are regulated by a coordinated interplay between cyclases, PDEs and protein kinases. Cyclic AMP levels are regulated by feedback activation of cAMP-specific PDE3A and PDE4D5 and inhibition of AC via PKA-mediated phosphorylation of PDE3A, PDE4D5 and AC V/VI (Murthy et al., 2002). Cyclic GMP levels are regulated by feedback activation of cGMP-specific PDE5 and inhibition of soluble GC via PKA- and PKG-mediated phosphorylation of PDE5 and PKG-mediated phosphorylation of soluble GC (Murthy, 2001a, 2004).
PDEs are encoded by at least 21 different genes and classified into 11 different gene families based on sequence similarity, substrate specificity, inhibitor sensitivity and mode of regulation (Soderling and Beavo, 2000; Francis and Corbin, 2005; Bender and Beavo, 2006). The abundant expression of cGMP-specific PDE5 in smooth muscle and the ability of PDE5 inhibitors (for example, sildenafil) to induce muscle relaxation underline the important role of this enzyme in particular and NO/cGMP pathway in general in the regulation of smooth muscle function (Pyne et al., 1996; Rybalkin et al., 2003b). PDE5 is a dimer containing two allosteric cGMP-binding sites in its regulatory N-terminal domain and a specific cGMP-binding site in its catalytic C-terminal domain that hydrolyses cGMP (Turko et al., 1998; Francis and Corbin, 2005). An increase in cGMP levels not only stimulates PKG, but also augments PDE5 activity by allosteric activation via binding to its regulatory domain (Turko et al., 1998; Rybalkin et al., 2003a). PKG-mediated phosphorylation of PDE5 at a conserved serine residue in the N-terminal region further stabilizes the allosteric activation (Corbin et al., 2000). Phosphorylation of PDE5 by PKG is dependent on the binding of cGMP to the regulatory site, and the phosphorylated enzyme exhibits greater affinity for cGMP binding to the regulatory domain and increased catalytic activity. In many cell types, PKG-mediated phosphorylation of PDE5 parallels activation of PDE5 by cGMP (Wyatt et al., 1998; Gopal et al., 2001; Murthy, 2001a; Rybalkin et al., 2003a). Thus, the allosteric binding and phosphorylation provides a mechanism for feedback regulation of cGMP levels. Other regulatory mechanisms, such as interaction with protein phosphatase/MLC phosphatase and the γ subunit of the PDE6 that control PDE5 activity, have also been reported (Lochhead et al., 1997; Rybalkin et al., 2003a, 2003b).
One of the characteristic features of the smooth muscle in the gastrointestinal tract is its ability to go through rhythmic cycles of contraction and relaxation regulated by excitatory and inhibitory transmitters. The transmitters are released sequentially and their release often overlaps resulting in the cross-regulation of signalling within the smooth muscle. We have speculated that signalling constituents in the pathways that mediate smooth muscle contraction could act to cross-regulate cGMP levels upon subsequent release of relaxant neurotransmitters, via regulation of PDE5 phosphorylation and activation. In this study, we examined whether concurrent activation of muscarinic M3 receptors by ACh regulates PDE5 phosphorylation and activity, and cGMP levels induced by NO donor, S-nitrosoglutathione (GSNO). We have identified a novel mechanism for stimulation of PDE5 phosphorylation and activity by ACh acting via M3 receptors. The mechanism involves activation of PKC downstream of the Gα13/RhoA-dependent pathway and inhibition of protein phosphatase 1 (PP1), resulting in augmentation of PDE5 phosphorylation and activity.
Methods
Preparation of dispersed gastric smooth muscle cells
Dispersed gastric smooth muscle cells were prepared as described previously (Murthy, 2001a; Murthy et al., 2002; Murthy and Zhou, 2003). Briefly, strips of circular muscle from rabbit stomach were dissected and incubated at 31 °C for 30 min in 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid medium containing 120 mM NaCl, 4 mM KCl, 2.6 mM KH2PO4, 0.6 mM MgCl2, 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid, 14 mM glucose, 2.1% Eagle's essential amino acid mixture, 0.1% collagenase and 0.1% soybean trypsin inhibitor. After the partly digested strips were washed twice with 50 ml of enzyme-free medium, the muscle cells were allowed to disperse spontaneously for 30 min. The cells were harvested by filtration through 500-μm Nitex (Tetko Inc., Briarcliff Manor, NY, USA) and centrifuged twice at 350 g for 10 min. For some experiments, muscle cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum until they attained confluence and were then passaged once for use.
Expression of dominant-negative RhoA (N19RhoA) and Gα13 minigene in cultured gastric smooth muscle cell
Dominant-negative RhoA (N19RhoA) was subcloned into the multiple cloning site (EcoR1) of eukaryotic expression vector pEXV vector. Recombinant plasmid DNAs (2 μg) were transiently transfected into muscle cells in primary cultures using Effectene transfection reagent for 48 h. Control cells were transfected with 2 μg of pEXV vector. Activation of Gα13 was blocked by the expression of cDNA encoding the last C-terminal 11 amino acids as described previously (Zhou and Murthy, 2004). The cDNA sequences were amplified by PCR and verified by DNA sequencing. Rabbit-cultured gastric smooth muscle cells were transfected transiently with minigene plasmid DNA using Effectene transfection reagent. The cells were cotransfected with 1 μg of pGreen lantern-1 to monitor expression. Transfection efficiency (∼70%) was monitored microscopically by the expression of green fluorescent protein using FITC filters (Zhou et al., 2003; Zhou and Murthy, 2004).
Assay for PDE5 activity
PDE5 activity was measured in immunoprecipitates of PDE5 by the method of Wyatt et al. (1998) as described previously (Murthy, 2001a). One millilitre aliquots (3 × 106 cells per ml) of muscle cells were incubated with NO donor, GSNO for 5 min in the presence or absence of ACh. PDE5 immunoprecipitates were washed in a medium containing 50 mM Tris (pH 7.5), 200 mM NaCl, 5 mM EDTA and then incubated for 15 min at 30 °C in a reaction mixture containing 100 mM 2-[N-morpholino] ethanesulphonic acid (pH 7.5), 10 mM EDTA, 0.1 M Mg acetate, 0.9 mg ml−1 bovine serum albumin, 20 μM cGMP and [3H]-cGMP. The samples were boiled for 3 min, chilled for 3 min and then incubated at 30 °C for 10 min in 20 mM Tris (pH 7.5) medium containing 10 μl of Crotalus atrox snake venom (10 μg μl−1). The samples were added to DEAE-Sephacel A-25 columns and the radioactivity in the effluent was counted. The results are expressed as counts per min per mg protein (c.p.m. per mg protein).
In experiments using PP1 and PP2A, the immunoprecipitates were washed with a medium containing 50 mM Tris-HCl (pH 7.5), 0.5 mM EDTA, 5 mM β-mercaptoethanol and 0.1% Triton X-100 and incubated for 20 min at 30 °C with purified PP1 (0.3 μg) and PP2A (0.3 μg) in the presence or absence of okadaic acid (10 μM) and calyculin A (10 μM). The phosphatases were then removed by further washes with Tris-HCl medium and PDE5 phosphorylation and activity measured (Murthy, 2001a).
Phosphorylation of PDE5, CPI-17 and PHI-17
Phosphorylation of PDE5, CPI-17 and phosphatase-holoenzyme inhibitor-1 (PHI-1) was measured by immunoblot analysis using phospho-specific antibodies as described previously ((Murthy et al., 2003a). One millilitre aliquots (3 × 106 cells per ml) of samples were incubated with NO donor, GSNO and/or ACh for 5 min and the reaction terminated with an equal volume of lysis buffer and placed on ice for 30 min. The cell lysates were separated from the insoluble material by centrifugation at 13 000 g for 15 min at 4 °C, precleared with 40 μl of protein A-sepharose and incubated with antibodies to PDE5, CPI-17 or PHI-1 for 2 h at 4 °C, and with 40 μl of protein A-sepharose for another 1 h. The immunoprecipitates were washed five times with 1 ml of wash buffer (0.5% Triton X-100, 150 mM NaCl, 10 mM Tris-HCl, pH 7.4), extracted with Laemmli sample buffer and boiled for 15 min, and then separated on 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis followed by transfer to polyvinylidine difluoride membranes. The membranes were incubated for 12 h with phospho-specific antibodies to PDE5 (Ser92), CPI-17 (Thr38) and PHI-1 (Thr57) and then for 1 h with a horseradish peroxidase-conjugated secondary antibody. The bands were identified by enhanced chemiluminescence.
PDE5 immunoprecipitation and PP1 or PP2A immunoblotting
PDE5 immunoprecipitates derived from cells treated with GSNO and/or ACh were separated on 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and then electrophoretically transferred to polyvinylidene difluoride membranes as described above. The membranes were incubated for 12 h with antibody to the catalytic subunit of PP1 or PP2A and then for 1 h with a horseradish peroxidase-conjugated secondary antibody. The bands were identified by enhanced chemiluminescence.
Radioimmunoassay for cGMP
Cyclic GMP production was measured by radioimmunoassay as described previously (Murthy, 2001a; Murthy et al., 2002). Briefly, muscle cells (3 × 106 cells) were treated with GSNO in the presence or absence of ACh for 5 min and the reaction terminated with 10% trichloroacetic acid. After extraction with water-saturated diethyl ether, the lyophilized aqueous phase was reconstituted in 500 μl of 50 mM Na acetate (pH 6.2). The samples were acetylated with triethylamine/acetic anhydride (2:1) for 30 min and cGMP was measured in duplicate using 100 μl aliquots. The results were expressed as picomol per mg protein.
Statistical analysis
All values are expressed as means±s.e.mean, where n represents the number of animal studies. Regression analysis was performed using GraphPad Prism 4. Statistical analysis was performed by Student's unpaired t-test and P<0.05 was considered statistically significant.
Chemical and drugs
[125I]-GMP and [3H]-cGMP were obtained from Amersham Pharmacia Biotech (Piscataway, NJ, USA); collagenase and soybean trypsin inhibitor from Worthington Biochemical Inc. (Freehold, NJ, USA); western blot, chromatography material and protein assay kit from Bio-Rad Laboratories (Hercules, CA, USA); antibody to PDE5 and phospho-antibody to PDE5 (Ser92) were obtained from FabGennix Inc. (Frisco, TX, USA); phospho-antibody to CPI-17 (Thr38) and PHI-1 (Thr57) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA); protein phosphatase 1/2A from Calbiochem (La Jolla, CA, USA); cGMP, Crotalus atrox snake venom and all other chemicals from Sigma Chemical Company (St Louis, MO, USA).
Results
PKG-mediated phosphorylation and activation of PDE5
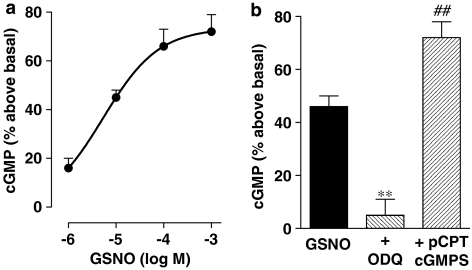
Treatment of dispersed smooth muscle cells with GSNO induced phosphorylation of PDE5 and increased PDE5 activity in a concentration-dependent manner with an EC50 of 10 μM, and a maximal phosphorylation was obtained with 1 mM (Figure 1). The effect of GSNO on PDE5 phosphorylation and activity was blocked by the soluble GC inhibitor, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) (10 μM) or the PKG inhibitor, 8-(4-chlorophenylthio)guanosine 3′,5′-cyclic monophosphate (8-pCPT-cGMPS) (1 μM), suggesting that increased PDE5 activity was mediated by PKG-dependent phosphorylation via cGMP derived from stimulation of soluble GC (Figure 1). The results are consistent with blockade of Na nitroprusside-induced PDE5 phosphorylation by KT5823 ((8R,9S,11s)-(-)-9-methoxy-carbamyl-8-methyl-2,3,9,10-tetrahydro-8,11-epoxy-1H,8H,1H,-2,7b,11a-trizadizobenzo9a,g)cycloocta(c,d,e)-trinden-1-one) and 8-pCPT-cGMPS (Murthy, 2001a).
Figure 1.
PKG-mediated phosphorylation and stimulation of PDE5 activity. (a) Gastric smooth muscle cells were incubated with various concentrations of GSNO (1 μM–1 mM) for 5 min. (b) Cells were incubated with maximal concentrations of GSNO (1 mM) in the presence or absence of soluble GC inhibitor ODQ (10 μM) or PKG inhibitor 8-pCPT-cGMPS (1 μM). Immunoprecipitates derived from 500 μg of protein using PDE5 antibody were separated on sodium dodecyl sulphate-polyacrylamide gel electrophoresis and analysed using phosphoSer92-specific antibody in the western blot. Immunoblot analysis with PDE5 showed equal amounts of loaded protein. PDE5 activity was measured as described in the Methods section using [3H]-cGMP as the substrate. Basal level values (362±78 c.p.m. per mg protein) were subtracted and the results expressed as c.p.m. per mg protein. Values are means±s.e.mean of four experiments. **P<0.001 significant inhibition of PDE5 activity by ODQ and 8-pCPT-cGMPS. GSNO, S-nitrosoglutathione.
Augmentation of PDE5 phosphorylation and activity and inhibition of cGMP levels by M3 receptors via PKC
Cotreatment of muscle cells with ACh significantly (P<0.01) augmented the effect of a submaximal concentration of GSNO (10 μM) on PDE5 phosphorylation and PDE5 activity (Figure 2). The stimulant effect of ACh was blocked by the M3 receptor antagonist, 4-DAMP (0.1 μM) or by the PKC inhibitor, GF109203X (1 μM), but not by the M2 receptor antagonist methoctramine (Figure 2a). Previous studies in smooth muscle cells had demonstrated a high selectivity of 4-DAMP for M3 receptors and methoctramine for M2 receptors (Murthy et al., 2003a). The selective PKA inhibitor myristoylated PKI (1 μM) or the mitogen-activated protein kinase kinase inhibitor, PD98059 had no effect on PDE5 phosphorylation induced by GSNO (data not shown). ACh alone in the absence of GSNO also had no effect on PDE5 phosphorylation (data not shown). These results imply that coactivation of muscarinic M3 receptors augmented PDE5 phosphorylation by the NO donor via PKC, and the stimulant effect of PKC was not due to direct phosphorylation of PDE5.
Figure 2.
Augmentation of GSNO-induced PDE5 activity by PKC. (a) Gastric muscle cells were incubated with submaximal concentrations of GSNO (10 μM) or GSNO plus ACh (0.1 μM) for 5 min in the absence or presence of the M2 receptor antagonist methoctramine (0.1 μM), M3 receptor antagonist 4-DAMP (0.1 μM) or PKC inhibitor GF-109203X (1 μM). (b) Cells were incubated with submaximal concentrations of GSNO (10 μM) or GSNO plus PMA (1 μM) in the presence or absence of GF-109203X (1 μM). Immunoprecipitates derived from 500 μg of protein using PDE5 antibody were separated on sodium dodecyl sulphate-polyacrylamide gel electrophoresis and analysed using phosphoSer92-specific antibody in the western blot. Immunoblot analysis with PDE5 showed equal amounts of loaded protein. PDE5 activity was measured as described in the Methods section using [3H]-cGMP as the substrate. The results are expressed as c.p.m. per mg protein. Values are means±s.e.mean of four experiments. **P<0.001 significant increase in PDE5 activity in the presence of ACh or PMA compared with GSNO alone. GSNO, S-nitrosoglutathione; PMA, phorbol 12-myristate 13-acetate.
Similarly, treatment of cells with a PKC activator, phorbol 12-myristate 13-acetate (PMA, 1 μM) augmented GSNO-induced PDE5 phosphorylation and activity, and the effect of PMA was blocked by GF109203X (Figure 2b).
Treatment of dispersed smooth muscle cells with GSNO increased cGMP levels and the effect was concentration-dependent (Figure 3a). The effect of GSNO (10 μM) on cGMP levels was blocked by ODQ (10 μM) and augmented by 8-pCPT-cGMPS (1 μM), suggesting that cGMP hydrolysis was augmented by PKG-mediated phosphorylation of PDE5. Blockade of phosphorylation with 8-pCPT-cGMPS inhibited PDE5 activity and augmented cAMP levels (Figure 3b).
Figure 3.
PKG-mediated augmentation of cGMP hydrolysis. (a) Gastric smooth muscle cells were incubated with various concentrations of GSNO (1 μM to 1 mM) for 5 min. (b) Cells were incubated with submaximal concentrations of GSNO (10 μM) in the presence or absence of soluble GC inhibitor ODQ (10 μM) or PKG inhibitor 8-pCPT-cGMPS (1 μM). cGMP was measured by radioimmunoassay using [125I]-cGMP as described in the Methods section. The results are expressed as percentage increase above basal levels (0.35±0.06 pmol per mg protein). Values are expressed as means±s.e.mean of four experiments. **P<0.001 significant inhibition of GSNO-induced cGMP levels by ODQ; ##P<0.01 significant augmentation of GSNO-induced cGMP levels by 8-pCPT-cGMPS. GSNO, S-nitrosoglutathione.
Consistent with the stimulation of PDE5 phosphorylation and activity, cotreatment of muscle cells with ACh significantly (P<0.01) attenuated GSNO-stimulated cGMP levels. The inhibitory effect of ACh was blocked by the M3 receptor antagonist, 4-DAMP (0.1 μM) or by the PKC inhibitor, GF109203X (1 μM), but not by the M2 receptor antagonist methoctramine (Figure 4a). The selective PKA inhibitor myristoylated PKI (1 μM) or the mitogen-activated protein kinase kinase inhibitor, PD98059 had no effect (data not shown). ACh alone in the absence of GSNO also had no effect on cGMP levels. These results imply that coactivation of muscarinic M3 receptors augmented cGMP degradation by stimulating PDE5 activation.
Figure 4.
Attenuation of cGMP levels by PKC. (a) Gastric muscle cells were incubated with submaximal concentrations of GSNO (10 μM) or GSNO plus ACh (0.1 μM) for 5 min in the absence or presence of the M2 receptor antagonist methoctramine (0.1 μM), M3 receptor antagonist 4-DAMP (0.1 μM) or PKC inhibitor GF-109203X (1 μM). (b) Cells were incubated with submaximal concentrations of GSNO (10 μM) or GSNO plus PMA (1 μM) in the presence or absence of GF-109203X (1 μM). cGMP was measured by radioimmunoassay using [125I]-cGMP as described in the Methods section. The results are expressed as percentage increase above basal levels (0.32±0.05 pmol per mg protein). Values are means±s.e.mean of four experiments. **P<0.001 significant decrease in cGMP levels in the presence of ACh or PMA compared with GSNO alone. GSNO, S-nitrosoglutathione; PMA, phorbol 12-myristate 13-acetate.
Similarly, treatment of cells with PMA (1 μM) attenuated GSNO-stimulated cGMP levels, and the effect of PMA was blocked by GF109203X (Figure 4b).
Source of M3-stimulated PKC for augmentation of PDE5 phosphorylation and activity
Previous studies in gastrointestinal smooth muscle have shown that activation of M3 receptors leads to a biphasic increase in PKC activity (Murthy and Makhlouf, 1995a). The initial transient phase reflects DAG derived from stimulation of Gαq-dependent PLC-β activity, whereas the sustained phase reflects DAG derived from stimulation of Gα13/RhoA-dependent PLD activity (Murthy et al., 2001). Hydrolysis of phosphatidylcholine by PLD yields phosphatidic acid, which, in turn, is dephosphorylated to yield DAG. We next identified the source of PKC for stimulation of PDE5 phosphorylation and activity using inhibitors of PLC-β and RhoA activities in freshly dispersed muscle cells, and in cultured muscle cells expressing Gαq or Gα13 minigenes, or dominant-negative mutant RhoA (N19).
ACh-stimulated augmentation of GSNO-induced PDE5 phosphorylation and PDE5 activity and attenuation of cGMP levels in freshly dispersed muscle cells were blocked by the inhibitor of RhoA, C3 exoenzyme, but not by the inhibitor of PI hydrolysis, U73122 (Figure 5). These results were corroborated in cultured muscle cells expressing Gα13 minigene or dominant-negative RhoA(N19). Augmentation of GSNO-induced PDE5 phosphorylation and PDE5 activity and attenuation of cGMP levels by ACh were blocked in cells expressing RhoA(N19) or Gα13 minigene (Figure 6), but not Gαq minigene (data not shown). Previous studies have shown that RhoA activity is blocked by C3 exoenzyme or in cells expressing Gα13 minigene (Zhou and Murthy, 2004). Control studies demonstrated that ACh-stimulated PLC-β activity was blocked by U73122 or in cells expressing Gαq minigene (data not shown). The results imply that augmentation of GSNO-induced PDE5 phosphorylation and activity, and inhibition of cGMP levels by M3 receptors are mediated via PKC derived from stimulation of Gα13-dependent RhoA pathway.
Figure 5.
(a) Augmentation of PDE5 phosphorylation and activity by GSNO and ACh, and attenuation of these effects by the RhoA inhibitor C3 exoenzyme. (b) cGMP levels induced by PKC derived from RhoA activation in these experiments. Freshly dispersed gastric smooth muscle cells incubated with submaximal concentrations of GSNO (10 μM) and GSNO plus ACh in the presence or absence of PLC-β inhibitor U73122 (10 μM) or RhoA inhibitor C3 exoenzyme (2 μg ml−1). PDE5 phosphorylation, PDE5 activity and cGMP levels were measured as described in the Methods section. PDE5 activity is expressed as c.p.m. per mg protein and cGMP levels as percentage increase above basal levels (0.36±0.05 pmol per mg protein). Values are means±s.e.mean of four experiments. **P<0.001 significant increase in PDE5 activity and decrease in cGMP levels in the presence of ACh compared with GSNO alone. GSNO, S-nitrosoglutathione.
Figure 6.
Augmentation of PDE5 phosphorylation and activity, and its attenuation by various drugs and cGMP levels by PKC derived from Gα13/RhoA activation. Cultured muscle cells expressing vector alone (control), Gα13 minigene or dominant-negative RhoA (N19RhoA) were treated with GSNO (10 μM) or GSNO plus ACh. PDE5 phosphorylation (a), PDE5 activity (b) and cGMP levels (c) were measured as described in the Methods section. PDE5 activity is expressed as c.p.m. per mg protein and cGMP levels as percentage increase above basal levels (0.35±0.06 pmol per mg protein). Values are means±s.e.mean of four experiments. **P<0.001 significant increase in PDE5 activity and decrease in cGMP levels in the presence of ACh compared with GSNO alone. GSNO, S-nitrosoglutathione.
Inhibition of PP1 augments GSNO-induced PDE5 phosphorylation and activity
In the absence of direct phosphorylation and activation of PDE5 by PKC, we speculated that augmentation of PDE5 phosphorylation and activity could be mediated by inhibition of the protein phosphatase activity that induces dephosphorylation of PDE5. To examine this notion, we used two approaches: in the first approach we examined the selective inhibitor of PP1 or PP2 on GSNO-induced PDE5 phosphorylation and activity, and in the second approach we used purified PP1 or PP2 to dephosphorylate PDE5 phosphorylation induced by GSNO.
PDE5 activity and PDE5 phosphorylation stimulated by GSNO in freshly dispersed muscle cells were significantly augmented by pretreatment of the cells with a selective PP1 inhibitor tautomycin (10 nM), and by a high concentration of okadaic acid (1 μM) that inhibits both PP1 and PP2A. A low (1 nM) concentration of okadaic acid that selectively inhibits PP2A had no effect (Figure 7).
Figure 7.
Augmentation of PDE5 phosphorylation and cGMP hydrolysis by PP1. Freshly dispersed gastric smooth muscle cells incubated with submaximal concentrations of GSNO (10 μM) in the presence or absence of tautomycin (10 nM) or okadaic acid (1 nM or 1 μM). (a) PDE5 phosphorylation, PDE5 activity and (b) cGMP levels were measured as described in the Methods section. PDE5 activity is expressed as c.p.m. per mg protein and cGMP levels as percentage increase above basal levels (0.32±0.05 pmol per mg protein). Values are means±s.e.mean of four experiments. **P<0.001 significant increase in PDE5 activity and decrease in cGMP levels in the presence of tautomycin and okadaic acid (1 μM) compared with GSNO alone. GSNO, S-nitrosoglutathione; PP1, protein phosphatase 1.
The increase in cGMP levels induced by GSNO in dispersed muscle cells was significantly attenuated by pretreatment of cells with tautomycin (10 nM) and okadaic acid at a high concentration (1 μM), but not by a low concentration (1 nM) of okadaic acid (Figure 7). These results imply that PDE5 phosphorylation is selectively dephosphorylated by PP1, and inhibition of PP1 results in the augmentation of PDE5 phosphorylation and activity, and a reduction in the levels of cGMP induced by GSNO. This notion was further corroborated by incubating the PDE5 immunoprecipitates derived from cells treated GSNO with the catalytic subunit of PP1 or PP2A. GSNO-induced PDE5 phosphorylation was dephosphorylated by the catalytic subunit of PP1, but not PP2A (Figure 8). From these studies, it was concluded that PDE5 phosphorylation levels are regulated by PP1 activity, and inhibition of PP1 activity could lead to augmentation of PDE5 phosphorylation.
Figure 8.
Dephosphorylation of PDE5 and inhibition of activity by the PP1 catalytic subunit. PDE5 immunoprecipitates derived from cells treated with GSNO were incubated with the catalytic subunits of PP1 or PP2A. PDE5 phosphorylation was measured using phospho-specific (Ser92) antibody as described in the Methods section. Values are means±s.e.mean of four experiments. **P<0.01 significant increase in PDE5 activity. GSNO, S-nitrosoglutathione; PP1, protein phosphatase 1; PP2A, protein phosphatase 2A.
PKC-mediated decrease in the association of PP1 with phosphorylated PDE5
To determine the mechanism of PDE5 augmentation by PKC, we examined the direct phosphorylation of the PP1 catalytic subunit both in vivo (in 32P-labelled cells) and in vitro (using purified catalytic subunit of PP1 and PKC). ACh had no effect on PP1 catalytic subunit phosphorylation in 32P-labelled cells in vivo and PKC did not phosphorylate PP1 catalytic subunit in vitro (data not shown).
To examine whether PP1 interacted with phosphorylated PDE5, muscle cells were treated with GSNO in the presence or absence of ACh and cell lysates were immunoprecipitated by PDE5 antibody, and then subjected to immunoblot analysis using antibody PP1 catalytic subunit. Treatment of cells with GSNO increased the PP1 catalytic subunit in PDE5 immunoprecipitates suggesting an association of PP1 with the phosphorylated PDE5. Cotreatment of cells with ACh attenuated the association of PP1 with PDE5, and this effect of ACh was blocked by 4-DAMP or GF109203X (Figure 9). ACh alone had no effect on the association of PP1 with PDE5 (data not shown). These results suggest that PKC augments PDE5 phosphorylation by inhibiting the interaction of PP1 with PDE5 and thus, PP1-mediated dephosphorylation. The inhibition of PP1 activity was not due to direct phosphorylation of the PP1 catalytic subunit, but could be due to phosphorylation of endogenous inhibitory proteins such as PKC-potentiated inhibitor 17-kDa protein (CPI-17) and PHI-1. Phosphorylation of CPI-17 at Thr38 and PHI-1 at Thr57 by PKC greatly increases their ability to inhibit PP1 (Eto et al., 1999, 2001; Murthy, 2006).
Figure 9.
Decrease in association of PP1 with the phosphorylated PDE5 by PKC. Gastric muscle cells were incubated with a submaximal concentration of GSNO (10 μM) and ACh (0.1 μM) for 5 min in the absence or presence of 4-DAMP or GF-109203X (1 μM). The association of the PP1 catalytic subunit with PDE54 was measured by immunoblot in PDE5 immunoprecipitates. Values are means±s.e.mean of three experiments. **P<0.01 significant increase in PDE5/PP1 association. GSNO, S-nitrosoglutathione; PP1, protein phosphatase 1.
We next examined whether activation of PKC by ACh causes phosphorylation of CPI-17 and PHI-1 using phospho-specific antibodies. Treatment of cells with ACh for 5 min induced phosphorylation of CPI-17 and PHI-17, and this was blocked by 4-DAMP or GF109203X, suggesting that PKC derived from activation of the M3 receptor mediates phosphorylation of CPI-17 and PHI-1 (Figure 10). However, these results do not support a direct causal link between phosphorylation of CPI-17 and PHI-1 and inhibition of PP1, but do corroborate the hypothesis that PKC-mediated augmentation of PDE5 phosphorylation and activity is due to inhibition of PP1 activity.
Figure 10.
Phosphorylation of (a) CPI-17 and (b) PHI-1 by M3 receptors via PKC. Gastric muscle cells were incubated with submaximal concentrations of GSNO (10 μM) or ACh (0.1 μM) for 5 min in the absence or presence of 4-DAMP or GF-109203X (1 μM). Phosphorylation of CPI-17 at Thr38 and PHI-1 at Thr57 was measured using phospho-specific antibodies. Values are means±s.e.mean of three experiments. **P<0.01 significant increase in CPI-17 or PHI-17 phosphorylation. GSNO, S-nitrosoglutathione.
Discussion
In this study, we have demonstrated that in freshly dispersed and cultured smooth muscle cells, concurrent activation of muscarinic M3 receptors attenuates cGMP levels by augmenting PDE5 phosphorylation and activity via activation of PKC, derived from G13/RhoA activation, and inhibition of PP1. This conclusion is based on the following evidence: (1) ACh augmented GSNO-induced PDE5 phosphorylation and activity and caused a concurrent decrease in cGMP levels; (2) the increase in PDE5 phosphorylation and activity and the decrease in cGMP levels were reversed by the M3 receptor antagonist, and inhibitors of RhoA and PKC in freshly dispersed cells and in cells expressing Gα13 minigene and dominant-negative RhoA (N19RhoA); (3) the selective PP1 inhibitors mimicked the ability of PKC to augment PDE5 phosphorylation and activity and inhibit cGMP levels, implying that the effect was mediated by the PKC-dependent inhibition of PP1; (4) in vitro PDE5 phosphorylation was selectively dephosphorylated by purified PP1; and (5) in vivo, PKC did not induce phosphorylation of PP1 or PDE5, but attenuated the association of PP1 with phosphorylated PDE5. These results suggest that augmentation of PDE5 phosphorylation and activity by PKC represents a novel mechanism by which contractile agonists restrain cGMP signalling pathways to terminate relaxation rapidly. Termination of the cGMP signal is also important for restraining the cAMP signal, because recent studies suggest that an increase in cGMP levels augments cAMP levels and PKA activity via inhibition of cAMP-specific PDE3A activity (Murthy et al., 2002).
In gastrointestinal smooth muscle, ACh initiates distinct signalling pathways to mediate MLC20 phosphorylation and contraction (Murthy et al., 2003a). cGMP via activation of PKG acts at various steps in the contractile pathway either to block phosphorylation and/or promote dephosphorylation of MLC20 resulting in muscle relaxation (Murthy et al., 1993, 2003b; Murthy and Makhlouf, 1995b; Murthy, 2001b). For example, PKG has been shown to decrease IP3 formation, IP3-dependent Ca2+ release and RhoA-dependent signalling. Therefore, rapid and efficient termination of cGMP signalling is essential for apposite signalling by ACh to mediate contraction. ACh regulates cGMP signalling by both decreasing the synthesis and enhancing breakdown of cGMP via inhibition of soluble GC and activation of PDE5, respectively (Murthy, 2004). In the present study, we provide evidence that ACh-induced activation of PKC and subsequent inhibition of PP1 is important for augmentation of PDE5 phosphorylation and activity leading to a decrease in cGMP levels. In smooth muscle cells, PKC derived from activation of M3 receptors via RhoA/PLD pathway can phosphorylate many proteins including CPI-17 and PHI-1, the two endogenous inhibitors of PP1- and PKC-mediated phosphorylation are required to augment their inhibitory potency (Murthy et al., 2003a).
Protein dephosphorylation by PP1 and PP2, often acting in concert with the phosphorylation of proteins that regulate the activity of protein phosphatases, is a fundamental mechanism in controlling the levels of protein phosphorylation. PP1 is the ubiquitous serine/threonine protein phosphatase and its substrate specificity is conferred by a number of regulatory proteins that associate with the catalytic subunit. The activity of the catalytic subunits is regulated by the phosphorylation of regulatory subunits as well as endogenous inhibitory proteins. Four PKC-potentiated endogenous PP1 inhibitors have been identified in humans and mice: CPI-17, PHI-1, GBP1 (gut and brain phosphatase inhibitor) and KEPI (kinase-enhanced phosphatase inhibitor) (Eto et al., 1999, 2001; Liu et al., 2002, 2004). PHI-1 is ubiquitously expressed, whereas GBP1 and CPI-17 are highly expressed in gastrointestinal tissues, and KEPI is highly expressed in heart and skeletal muscle, but not in gastrointestinal tissues (Eto et al., 1995; Liu et al., 2004). These phosphatase inhibitors play an important role in cellular signalling by altering the phosphorylation status of various signalling proteins and are thus, in turn, regulated by phosphorylation in response to receptor activation. In vascular and visceral smooth muscle, sustained MLC20 phosphorylation is maintained by inhibition of PP1δ/MLC phosphatase, which is mediated by phosphorylation of the regulatory protein, MYPT1, and/or phosphorylation of inhibitory proteins, CPI-17 and PHI-1(Somlyo and Somlyo, 2004; Murthy, 2006). Phosphorylation of MYPT1 is mediated by Rho kinase, a downstream effector of RhoA, whereas phosphorylation of CPI-17 and PHI-1 is mediated by PKC derived from Rho-dependent activation of PLD pathway (Murthy et al., 2003a; Somlyo and Somlyo, 2004; Murthy, 2006). Phosphorylation of CPI-17 at Thr38 by PKC has been shown to inhibit myosin-bound PP1Cδ/MLC phosphatase, whereas phosphorylation of PHI-17 at Thr57 inhibits MLC phosphatase and glycogen-bound PP1 (Eto et al., 1995, 1999, 2001). Recent studies have suggested that PP1 activity is also regulated by a novel IP3-binding protein, shown to be similar to PLC-δ, known as PLC-related catalytically inactive proteins (PRIP-1 and PRIP-2) (Uji et al., 2002; Yanagihori et al., 2006). PRIP-1 expression was restricted to the brain, whereas PRIP-2 was expressed ubiquitously. Both PRIP-1 and PRIP-2 bind to and inactivate PP1 suggesting that PRIP acts as a regulatory subunit of PP1. PKA-mediated phosphorylation of PRIP-1 at Thr94 and PRIP-2 at Thr128 induces the release of the PP1 catalytic subunit resulting in the activation of PP1 (Uji et al., 2002). Although the present studies did not identify the mechanism of PP1 inhibition, PKC-mediated phosphorylation of CPI-17 at Thr38 and PHI-1 at Thr57 suggests that CPI-17 and/or PHI-1 are involved in phospho-dependent augmentation of PDE5 phosphorylation by inhibiting PP1 catalytic subunit activity. However, a role of PKC-mediated phosphorylation of the more ubiquitous PRIP-2 and GBP1 in the inhibition of PP1 activity could not be ruled out in smooth muscle cells. The present studies also did not identify the specific PKC isoforms(s) responsible for the inhibition of PP1 and augmentation of PDE5 phosphorylation and activity. On the basis of the inhibition by GF-109203X, conventional and/or novel PKC isoforms are likely to be involved in the inhibition of PP1 activity. Further studies are required to identify the PKC isoforms(s) and the specific inhibitory proteins that are involved in ACh-induced augmentation of PDE5 phosphorylation and activity.
PDE5 is the major cGMP-binding protein, and phosphorylation of PDE5 in the presence of cGMP binding is important for stimulation of PDE5 activity. The phosphorylation site is conserved in murine, canine, bovine and human isoforms and selective for PKG, and thus, phosphorylation of PDE5 could be used as an in vivo indicator for PKG activation (Corbin et al., 2000; Mullershausen et al., 2001; Shimizu-Albergine et al., 2003; Koesling et al., 2005; Zoraghi et al., 2005; Bessay et al., 2007). Stimulation of PDE5 activity by PKG-mediated phosphorylation enhances the rate of cGMP signal termination and therefore, PKG controls its own activation by regulating the levels of cGMP via stimulation of PDE5 activity. The levels of PDE5 phosphorylation and activity are also regulated by other mechanisms. Addition of purified PP1 inhibited phosphorylation of PDE5 by 8-Br-cGMP in human uterine smooth muscle cells, suggesting that PDE5 phosphorylation is regulated by PP1/MLC phosphatase, whereas addition of recombinant PDE6 γ subunit or a peptide corresponding to the amino acids 24–26 of PDE6 γ subunit inhibited activation of PDE5 by PKA in guinea-pig airway smooth muscle cells (Lochhead et al., 1997; Rybalkin et al., 2003a). In airway smooth muscle cells, PDE5 was shown to form a stable complex with 14- and 18-kDA proteins (p14 and p18). Phosphorylation of p14/p18 by a kinase activated by pertussis toxin-sensitive G protein(s) correlates with the reduction in the ability of PKA to activate PDE5 suggesting that an interaction of PDE5 with p14/p18 inhibits phosphorylation-dependent activation (Lochhead et al., 1997). Other mechanisms for regulation of cGMP levels by contractile agonists have been reported. In vascular smooth muscle cells, angiotensin II inhibits cGMP levels by rapid activation of PDE1A via the Ca2+/calmodulin-dependent pathway, and by upregulation of PDE5 expression and activity via extracellular signal-regulated kinase-1/2-dependent pathways (Kim et al., 2001, 2005).
In conclusion, concurrent activation of muscarinic M3 receptors attenuates cGMP levels by augmenting PDE5 phosphorylation and activity via PKC-mediated inhibition of PP1. We suggest that the augmentation of PDE5 phosphorylation and activity and subsequent decrease in cGMP levels might play an important role in rapid termination of relaxation of smooth muscle and prime the muscle for optimal contraction. Furthermore, PKC-mediated maintenance of PDE5 phosphorylation also sustains the contractile process by effectively suppressing the inhibitory tone.
Acknowledgments
This work was supported by Grant DK 28300 from the National Institute of Diabetes, and Digestive and Kidney Diseases.
Abbreviations
- GSNO
S-nitrosoglutathione
- 8-pCPT-cGMPS
8-(4-chlorophenylthio)guanosine 3′,5′-cyclic monophosphate
- KT5823
(8R,9S,11s)-(-)-9-methoxy-carbamyl-8-methyl-2,3,9,10-tetrahydro-8,11-epoxy-1H,8H,1H,-2,7b,11a-trizadizobenzo9a,g)cycloocta(c,d,e)-trinden-1-one
- PP1
protein phosphatase 1
Conflict of interest
The authors state no conflict of interest.
References
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- Bessay EP, Zoraghi R, Blount MA, Grimes KA, Beasley A, Francis SH, et al. Phosphorylation of phosphodiesterase-5 is promoted by a conformational change induced by sildenafil, vardenafil, or tadalafil. Front Biosci. 2007;12:1899–1910. doi: 10.2741/2196. [DOI] [PubMed] [Google Scholar]
- Corbin JD, Turko IV, Beasley A, Francis SH. Phosphorylation of phosphodiesterase-5 by cyclic nucleotide-dependent protein kinase alters its catalytic and allosteric cGMP-binding activities. Eur J Biochem. 2000;267:2760–2767. doi: 10.1046/j.1432-1327.2000.01297.x. [DOI] [PubMed] [Google Scholar]
- Eto M, Karginov A, Brautigan DL. A novel phosphoprotein inhibitor of protein type-1 phosphatase holoenzymes. Biochemistry. 1999;38:16952–16957. doi: 10.1021/bi992030o. [DOI] [PubMed] [Google Scholar]
- Eto M, Kitazawa T, Yazawa M, Mukai H, Ono Y, Brautigan DL. Histamine-induced vasoconstriction involves phosphorylation of a specific inhibitor protein for myosin phosphatase by protein kinase C alpha and delta isoforms. J Biol Chem. 2001;276:29072–29078. doi: 10.1074/jbc.M103206200. [DOI] [PubMed] [Google Scholar]
- Eto M, Ohmori T, Suzuki M, Furuya K, Morita F. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C. Isolation from porcine aorta media and characterization. J Biochem (Tokyo) 1995;118:1104–1107. doi: 10.1093/oxfordjournals.jbchem.a124993. [DOI] [PubMed] [Google Scholar]
- Francis SH, Corbin JD. Sildenafil: efficacy, safety, tolerability and mechanism of action in treating erectile dysfunction. Expert Opin Drug Metab Toxicol. 2005;1:283–293. doi: 10.1517/17425255.1.2.283. [DOI] [PubMed] [Google Scholar]
- Gopal VK, Francis SH, Corbin JD. Allosteric sites of phosphodiesterase-5 (PDE5). A potential role in negative feedback regulation of cGMP signaling in corpus cavernosum. Eur J Biochem. 2001;268:3304–3312. doi: 10.1046/j.1432-1327.2001.02233.x. [DOI] [PubMed] [Google Scholar]
- Kim D, Aizawa T, Wei H, Pi X, Rybalkin SD, Berk BC, et al. Angiotensin II increases phosphodiesterase 5A expression in vascular smooth muscle cells: a mechanism by which angiotensin II antagonizes cGMP signaling. J Mol Cell Cardiol. 2005;38:175–184. doi: 10.1016/j.yjmcc.2004.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Rybalkin SD, Pi X, Wang Y, Zhang C, Munzel T, et al. Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation. 2001;104:2338–2343. doi: 10.1161/hc4401.098432. [DOI] [PubMed] [Google Scholar]
- Koesling D, Mullershausen F, Lange A, Friebe A, Mergia E, Wagner C, et al. Negative feedback in NO/cGMP signalling. Biochem Soc Trans. 2005;33:1119–1122. doi: 10.1042/BST20051119. [DOI] [PubMed] [Google Scholar]
- Liu QR, Zhang PW, Lin Z, Li QF, Woods AS, Troncoso J, et al. GBPI, a novel gastrointestinal- and brain-specific PP1-inhibitory protein, is activated by PKC and inactivated by PKA. Biochem J. 2004;377:171–181. doi: 10.1042/BJ20030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QR, Zhang PW, Zhen Q, Walther D, Wang XB, Uhl GR. KEPI, a PKC-dependent protein phosphatase 1 inhibitor regulated by morphine. J Biol Chem. 2002;277:13312–13320. doi: 10.1074/jbc.M107558200. [DOI] [PubMed] [Google Scholar]
- Lochhead A, Nekrasova E, Arshavsky VY, Pyne NJ. The regulation of the cGMP-binding cGMP phosphodiesterase by proteins that are immunologically related to gamma subunit of the photoreceptor cGMP phosphodiesterase. J Biol Chem. 1997;272:18397–18403. doi: 10.1074/jbc.272.29.18397. [DOI] [PubMed] [Google Scholar]
- Mullershausen F, Russwurm M, Thompson WJ, Liu L, Koesling D, Friebe A. Rapid nitric oxide-induced desensitization of the cGMP response is caused by increased activity of phosphodiesterase type 5 paralleled by phosphorylation of the enzyme. J Cell Biol. 2001;155:271–278. doi: 10.1083/jcb.200107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy KS. Activation of phosphodiesterase 5 and inhibition of guanylate cyclase by cGMP-dependent protein kinase in smooth muscle. Biochem J. 2001a;360:199–208. doi: 10.1042/0264-6021:3600199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy KS. cAMP inhibits IP3-dependent Ca2+ release by preferential activation of cGMP-primed PKG. Am J Physiol Gastrointest Liver Physiol. 2001b;281:G1238–G1245. doi: 10.1152/ajpgi.2001.281.5.G1238. [DOI] [PubMed] [Google Scholar]
- Murthy KS. Modulation of soluble guanylate cyclase activity by phosphorylation. Neurochem Int. 2004;45:845–851. doi: 10.1016/j.neuint.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:345–374. doi: 10.1146/annurev.physiol.68.040504.094707. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Makhlouf GM. Agonist-mediated activation of phosphatidylcholine-specific phospholipase C and D in intestinal smooth muscle. Mol Pharmacol. 1995a;48:293–304. [PubMed] [Google Scholar]
- Murthy KS, Makhlouf GM. Interaction of cA-kinase and cG-kinase in mediating relaxation of dispersed smooth muscle cells. Am J Physiol. 1995b;268:C171–C180. doi: 10.1152/ajpcell.1995.268.1.C171. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Severi C, Grider JR, Makhlouf GM. Inhibition of IP3 and IP3-dependent Ca2+ mobilization by cyclic nucleotides in isolated gastric muscle cells. Am J Physiol. 1993;264:G967–G974. doi: 10.1152/ajpgi.1993.264.5.G967. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Zhou H. Selective phosphorylation of the IP3R-I in vivo by cGMP-dependent protein kinase in smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2003;284:G221–G230. doi: 10.1152/ajpgi.00401.2002. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Zhou H, Grider JR, Brautigan DL, Eto M, Makhlouf GM. Differential signalling by muscarinic receptors in smooth muscle: m2-mediated inactivation of myosin light chain kinase via Gi3, Cdc42/Rac1 and p21-activated kinase 1 pathway, and m3-mediated MLC20 phosphorylation via Rho-associated kinase/myosin phosphatase targeting subunit 1 and protein kinase C/CPI-17 pathway. Biochem J. 2003a;374:145–155. doi: 10.1042/BJ20021274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy KS, Zhou H, Grider JR, Makhlouf GM. Sequential activation of heterotrimeric and monomeric G proteins mediates PLD activity in smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2001;280:G381–G388. doi: 10.1152/ajpgi.2001.280.3.G381. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Zhou H, Grider JR, Makhlouf GM. Inhibition of sustained smooth muscle contraction by PKA and PKG preferentially mediated by phosphorylation of RhoA. Am J Physiol Gastrointest Liver Physiol. 2003b;284:G1006–G1016. doi: 10.1152/ajpgi.00465.2002. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Zhou H, Makhlouf GM. PKA-dependent activation of PDE3A and PDE4 and inhibition of adenylyl cyclase V/VI in smooth muscle. Am J Physiol Cell Physiol. 2002;282:C508–C517. doi: 10.1152/ajpcell.00373.2001. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, Arshavsky V, Lochhead A. cGMP signal termination. Biochem Soc Trans. 1996;24:1019–1022. doi: 10.1042/bst0241019. [DOI] [PubMed] [Google Scholar]
- Rybalkin SD, Rybalkina IG, Shimizu-Albergine M, Tang XB, Beavo JA. PDE5 is converted to an activated state upon cGMP binding to the GAF A domain. EMBO J. 2003a;22:469–478. doi: 10.1093/emboj/cdg051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybalkin SD, Yan C, Bornfeldt KE, Beavo JA. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003b;93:280–291. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- Shimizu-Albergine M, Rybalkin SD, Rybalkina IG, Feil R, Wolfsgruber W, Hofmann F, et al. Individual cerebellar Purkinje cells express different cGMP phosphodiesterases (PDEs): in vivo phosphorylation of cGMP-specific PDE (PDE5) as an indicator of cGMP-dependent protein kinase (PKG) activation. J Neurosci. 2003;23:6452–6459. doi: 10.1523/JNEUROSCI.23-16-06452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling SH, Beavo JA. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol. 2000;12:174–179. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction through the RhoA/Rho-kinase pathway in smooth muscle. J Muscle Res Cell Motil. 2004;25:613–615. doi: 10.1007/s10974-004-3146-1. [DOI] [PubMed] [Google Scholar]
- Turko IV, Francis SH, Corbin JD. Binding of cGMP to both allosteric sites of cGMP-binding cGMP-specific phosphodiesterase (PDE5) is required for its phosphorylation. Biochem J. 1998;329:505–510. doi: 10.1042/bj3290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uji A, Matsuda M, Kukita T, Maeda K, Kanematsu T, Hirata M. Molecules interacting with PRIP-2, a novel Ins(1,4,5)P3 binding protein type 2: Comparison with PRIP-1. Life Sci. 2002;72:443–453. doi: 10.1016/s0024-3205(02)02275-0. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Naftilan AJ, Francis SH, Corbin JD. ANF elicits phosphorylation of the cGMP phosphodiesterase in vascular smooth muscle cells. Am J Physiol. 1998;274:H448–H455. doi: 10.1152/ajpheart.1998.274.2.H448. [DOI] [PubMed] [Google Scholar]
- Yanagihori S, Terunuma M, Koyano K, Kanematsu T, Ho Ryu S, Hirata M. Protein phosphatase regulation by PRIP, a PLC-related catalytically inactive protein—implications in the phospho-modulation of the GABAA receptor. Adv Enzyme Regul. 2006;46:203–222. doi: 10.1016/j.advenzreg.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Zhou H, Das S, Murthy KS. Erk1/2- and p38 MAP kinase-dependent phosphorylation and activation of cPLA2 by m3 and m2 receptors. Am J Physiol Gastrointest Liver Physiol. 2003;284:G472–G480. doi: 10.1152/ajpgi.00345.2002. [DOI] [PubMed] [Google Scholar]
- Zhou H, Murthy KS. Distinctive G protein-dependent signaling in smooth muscle by sphingosine 1-phosphate receptors S1P1 and S1P2. Am J Physiol Cell Physiol. 2004;286:C1130–C1138. doi: 10.1152/ajpcell.00429.2003. [DOI] [PubMed] [Google Scholar]
- Zoraghi R, Bessay EP, Corbin JD, Francis SH. Structural and functional features in human PDE5A1 regulatory domain that provide for allosteric cGMP binding, dimerization, and regulation. J Biol Chem. 2005;280:12051–12063. doi: 10.1074/jbc.M413611200. [DOI] [PubMed] [Google Scholar]