Abstract
Background and purpose:
It has been found that 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) exert various vascular protective effects, beyond their cholesterol-lowering property, including inhibition of platelet-dependent thrombus formation. The objective of the present study was to determine whether the nitric oxide (NO)/cyclic GMP-mediated processes in platelets contribute to the anti-aggregatory activity of simvastatin.
Experimental approach:
After rabbit platelets were incubated with simvastatin for 5 min, aggregation was induced and the platelet aggregation, nitric oxide synthase activity, guanylyl cyclase activity, NO and cyclic GMP formation were measured appropriately.
Key results:
Treatment with simvastatin concentration-dependently inhibited platelet aggregation induced by collagen or arachidonic acid with an IC50 range of 52–158 μM. We also demonstrated that simvastatin (20–80 μM) concentration-dependently further enhanced collagen-induced NO and cyclic GMP formation through increasing NOS activity (from 2.64±0.12 to 3.52±0.21–5.10±0.14 μmol min−1 mg protein−1) and guanylyl cyclase activity (from 142.9±7.2 to 163.5±17.5–283.8±19.5 pmol min−1 mg protein−1) in the platelets. On the contrary, inhibition of platelet aggregation by simvastatin was markedly attenuated (by about 50%) by addition of a nitric oxide synthase inhibitor, a NO scavenger or a NO-sensitive guanylyl cyclase inhibitor. The anti-aggregatory effects of simvastatin were significantly increased by addition of a selective inhibitor of cyclic GMP phosphodiesterase.
Conclusions and implications:
Our findings indicate that enhancement of a NO/cyclic GMP-mediated process plays an important role in the anti-aggregatory activity of simvastatin.
Keywords: platelet aggregation, simvastatin, nitric oxide, cyclic GMP
Introduction
Several clinical trials have shown that inhibition of cholesterol biosynthesis by 3-hydroxy-3-methyl-glutaryl coenzyme A reductase inhibitors (statins) exerts various beneficial vascular effects. However, some of these effects are incompletely explained by the cholesterol-lowering effect alone (Bonetti et al., 2003). It is well known that enhanced platelet activation and aggregation is a crucial event in the development of cardiovascular diseases and thrombosis (Marcus and Safier, 1993; Ruggeri, 2002). Although previous studies have shown that statins have an anti-thrombotic activity in hypercholesterolemic patients and animal thrombotic models (Mayer et al., 1992; Kearney and Fitzgerald, 1999), the inhibition of platelet-dependent thrombus generation in hypercholesterolemic subjects by statins does not correlate with the lipid-lowering effect, suggesting that other lipid-independent effects of statins may contribute to its anti-aggregatory activity (Puccetti et al., 2001; Gaddam et al., 2002). Some possible anti-aggregatory mechanisms of statins, including reducing thromboxane B2 formation and changing cholesterol content of platelet membrane, have been proposed (Osamah et al., 1997; Ma et al., 2002), but the true anti-aggregatory mechanisms involved are still unclear.
Nitric oxide (NO), synthesized from L-arginine by NO synthase (NOS), which then activates intracellular soluble guanylyl cyclase and subsequent guanosine 3′,5′-cyclic guanosine monophosphate (cyclic GMP) formation, plays an important modulatory role in many physiological and pathological conditions (Moncada et al., 1991). Importantly, the platelet-derived NO/cyclic GMP system has been shown to provide an inhibitory pathway regulating platelet adhesion and aggregation (Chou et al., 1999; Emerson et al., 1999). Recent studies have reported that statins can increase NO bioavailability in vascular cells and platelets by enhancing endothelial NOS expression, stabilizing endothelial NOS mRNA and decreasing superoxide anion (O2−) formation (Laufs et al., 1998; Tannous et al., 1999; Kalinowski et al., 2002; Zhou et al., 2004), suggesting that statin-induced NO formation may be involved in their anti-platelet activity. However, whether simvastatin, a statin, can directly affect platelet aggregation and the NO/cyclic GMP pathway in platelets is still unknown. Therefore, in this in vitro study, we tested the hypothesis that simvastatin inhibits platelet aggregation via an enhanced NO/cyclic GMP-mediated process.
The study reported herein demonstrates that simvastatin significantly inhibits platelet aggregation accompanied by an increase in NO and cyclic GMP formation through enhancing platelets NOS and guanylyl cyclase activity. Furthermore, blocking NO or cyclic GMP formation markedly abolished the anti-aggregatory activity of simvastatin. These findings support the notion that enhancement of platelets NO/cyclic GMP pathway may, at least in part, contribute to the anti-aggregatory activity of simvastatin.
Methods
Preparation of suspensions of washed platelets
The present study was approved by the local Institutional Animal Care and Use Committee. Animals were housed in standard environment and maintained on tap water and rabbit food ad libitum throughout the investigation. Blood was withdrawn from rabbit marginal ear vein, mixed with anticoagulant, ethylenediamine tetraacetic acid (100 mM, 14:1, v/v) and centrifuged at 160 g at 25 °C for 10 min to obtain platelet-rich plasma. Platelet suspension was prepared from the platelet-rich plasma according to the washing procedures described previously, and finally suspended in Tyrode's solution (Chou et al., 1999).
Platelet aggregation
Platelet aggregation was measured turbidimetrically at 37 °C by using an aggregometer (Chrono-Log, Havertown, PA, USA) (Chou et al., 1999). After a 3-min equilibration at 37 °C, the platelet suspension was incubated with simvastatin, dimethyl sulphoxide (DMSO) or in combination with other pharmacological agents for 5 min before the addition of collagen (10 μg ml−1) or arachidonic acid (AA, 100 μM). Platelet aggregation was evaluated by measuring the peak of the aggregation curves. Data were expressed as the percentage of maximal aggregation. To eliminate the effect of the solvent on the platelet aggregation, the final concentration of DMSO was fixed at 0.5%.
Determination of NO formation
Generation of NO was directly monitored by voltametric measurements using a microcomputer-controlled apparatus (IVEC-10; Medical Systems Co., Greenvale, NY, USA) as previously described (Wu et al., 2001; Wu and Chai, 2004). Briefly, a miniature Ag/AgCl reference electrode (200 μm in diameter) was inserted vertically into a detector tube (used to contain the platelet suspension) and fixed with cement to the tube wall. The working electrode, a double carbon fiber filament (each 30 μm in diameter; Textron, Lowell, MA, USA), was held in a manipulator and placed into the tube. The detector tube containing 600 μl of platelet suspension with simvastatin or vehicle was stirred and maintained at 37 °C for 5 min, and then collagen was added. NO release was simultaneously monitored by electrodes. Detection and calibration of NO concentration were carried out by using a mixture of S-nitroso-N-acetyl-DL-penicillamine (0.1–1.0 μM) in 0.1 mM phosphate-buffered saline.
Determination of NOS and guanylyl cyclase activity
After incubation with simvastatin or DMSO for 5 min, followed by addition of collagen for 6 min, the precipitated platelets were collected by centrifugation. Then, the platelets were lysed by sonication for 45 s and kept on ice in 25 mM HEPES buffer (pH 7.5) containing dithiothreitol (1 mM), phenylmethylsulphonyl fluoride (10 μg ml−1), trypsin inhibitor (10 μg ml−1), leupeptin (10 μg ml−1), antipain (10 μg ml−1), chymostatin (10 μg ml−1) and pepstatin (10 μg ml−1). The lysate solution was then centrifuged at 78 000 g for 20 min at 4 °C to obtain supernatant used for activity assay. For NOS activity determination, the cytosol of platelets was resuspended in 300 μl 25 mM HEPES buffer (pH 7.5) containing L-arginine (100 μM), dithiothreitol (1 mM), NADPH (1.5 mM), calmodulin (30 nM), tetrahydrobiopterin (1 μg ml−1), MgCl2 (1 mM), CaCl2 (1 mM) and flavin adenine nucleotide (2.5 μg ml−1) and incubated for 15 min at 37 °C. The reaction was stopped with stop buffer (25 mM HEPES, 2 mM ethylenediamine tetraacetic acid, 2 mM ethylene glycol-bis(â-aminoethyl ether)-N,N,N',N',-tetraacetic acid, pH 5.5). NOS activity was measured by the formation of NOx (nitrite+nitrate) determined by a Sievers Nitric Oxide Analyzer (Sievers 280 NOA, Boulder, CO, USA) and expressed as μmole min−1 per mg of protein (Maurer and Fung, 2000). For determination of guanylyl cyclase activity, 100 μl of platelet cytosol (1 μg protein) was added into the prewarmed (37 °C) buffer (pH 7.2) containing (final concentrations) 25 mM Tris-HCl, 3 mM GTP, 5 mM MgCl2, 1 mM 3-isobutyl-1-methylxanthine for 20 min of incubation. Then, the reaction was terminated by boiling for 3 min, and the amount of cyclic GMP in the mixture was measured by enzyme immunoassay kit. The guanylyl cyclase activity was expressed as pmole min−1 per mg of protein.
Determination of cyclic GMP
In the presence of L-arginine (100 μM), platelet suspensions were incubated with simvastatin or DMSO for 5 min at 37 °C followed by addition of collagen for 6 min. The incubation was stopped by immediately boiling for 3 min and the concentrations of cyclic GMP in the supernatants were determined by electroimmunoassay kit.
Statistical analysis
The experimental results were expressed as mean±s.e.mean. Statistical analyses were performed by one-way analysis of variance or Student's t-test. Results were considered significantly different at a P-value less than 0.05.
Reagents
Collagen (type 1, equine tendon), L-arginine, NG-nitro-L-arginine methyl ester (L-NAME), 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO), 1H-[1,2,4] oxadiazolo [4,3-a]quinoxalin-1-one (ODQ), zaprinast and other chemical agents were all purchased from Sigma Chemical Company (St Louis, MO, USA). Cyclic GMP electroimmunoassay kits were purchased from Cayman Chemical Company (Ann Arbor, MI, USA). Simvastatin was kindly provided by Merck Sharp & Dohme (I.A.) Corp. (South Granville, NSW, Australia) and was dissolved in DMSO.
Results
Simvastatin inhibits platelet aggregation
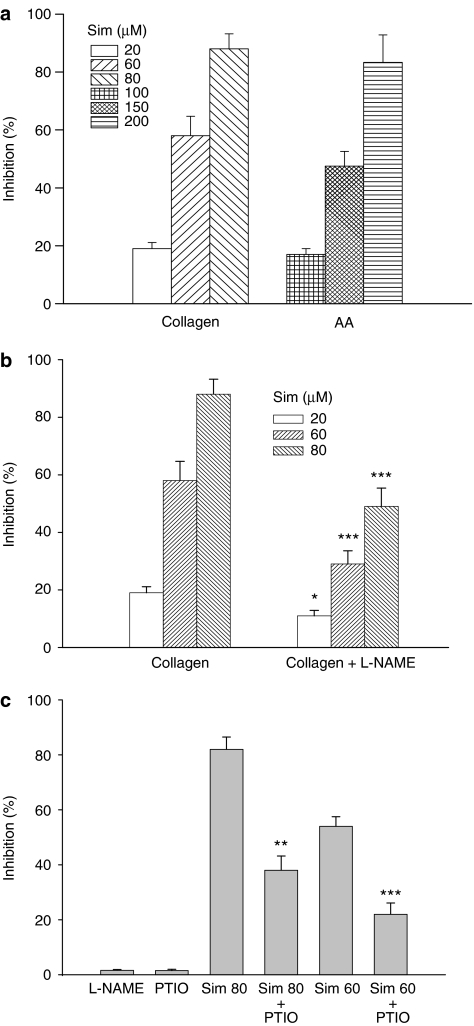
Simvastatin concentration dependently inhibited platelet aggregation induced by collagen or AA with a 50% inhibitory concentration (IC50) of 52.5±6.2 or 158.4±9.1 μM, respectively (Figure 1a). In the presence of L-arginine (100 μM), the inhibitory potency of simvastatin on the platelet aggregation induced by collagen was significantly attenuated by addition of a specific NOS inhibitor, L-NAME (100 μM) (Figure 1b), or PTIO (50 μM), an NO scavenger (Figure 1c), compared with those platelets treated with simvastatin alone. However, by itself neither L-NAME nor PTIO had any significant effect on platelet aggregation induced by collagen.
Figure 1.
Effects of simvastatin and combination with L-NAME or PTIO on platelet aggregation. (a) In the presence of L-arginine (100 μM), washed platelets were preincubated with DMSO or simvastatin (20–200 μM) for 5 min. Then, collagen (10 μg ml−1) or AA (100 μM) was added to trigger platelet aggregation. (b) In the presence of L-arginine, washed platelets were preincubated with simvastatin (20–80 μM) or L-NAME (100 μM) and simvastatin (20–80 μM) for 5 min. Then, collagen was added to trigger platelet aggregation. (c) In the presence of L-arginine, washed platelets were preincubated with simvastatin (60 or 80 μM) or simvastatin (60 or 80 μM)+PTIO (50 μM) for 5 min, followed by addition of collagen. *P<0.05, **P<0.01 and ***P<0.001, as compared with the respective simvastatin or collagen-treated platelets. Percent inhibition of platelet aggregation is presented as means±s.e.mean (n=6). AA, arachidonic acid; DMSO, dimethyl sulphoxide; L-NAME, NG-nitro-L-arginine methyl ester; PTIO, 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide.
Simvastatin enhances NO release and NOS activity
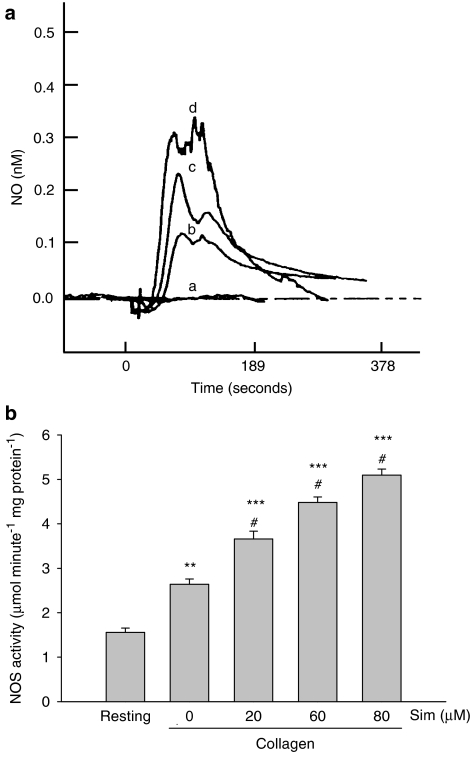
In the presence of L-arginine (100 μM), treatment with simvastatin concentration dependently further enhanced the collagen-induced NO release compared with that of control collagen-stimulated platelets (Figure 2a). To investigate the mechanism by which simvastatin increased NO formation, the NOS activity of the platelets was measured. Although, collagen itself stimulated platelet NOS activity, addition of simvastatin (20–80 μM) further enhanced the platelet NOS activity induced by collagen (Figure 2b).
Figure 2.
Effects of simvastatin on NO release and NOS activity in platelets. (a) In the presence of L-arginine (100 μM), typical traces of NO release from platelets shown in the presence of DMSO alone (a) or in the presence of collagen (10 μg ml−1) (b) or in the presence of simvastatin (60 μM) and collagen (10 μg ml−1) (c) or in the presence of simvastatin (80 μM) and collagen (10 μg ml−1) (d). These profiles are representative of five similar experiments. (b) In the presence of L-arginine, washed platelets were preincubated with simvastatin (20–80 μM) or DMSO for 5 min, followed by addition of collagen for 6 min. The homogenized precipitated platelets were used for NOS activity assay by measuring the NOx formation after a 15-min incubation at 37 °C with lysed platelets in reaction buffer. Data from washed platelets only suspended in Tyrode's solution without addition of collagen and other drugs are shown as the resting group. **P<0.01 and ***P<0.001, as compared with the resting group; #P<0.001 compared with the collagen-stimulated platelets. All data are presented as means±s.e.mean (n=5). DMSO, dimethyl sulphoxide; NO, nitric oxide; NOS, nitric oxide synthase.
Simvastatin increases cyclic GMP formation and guanylyl cyclase activity
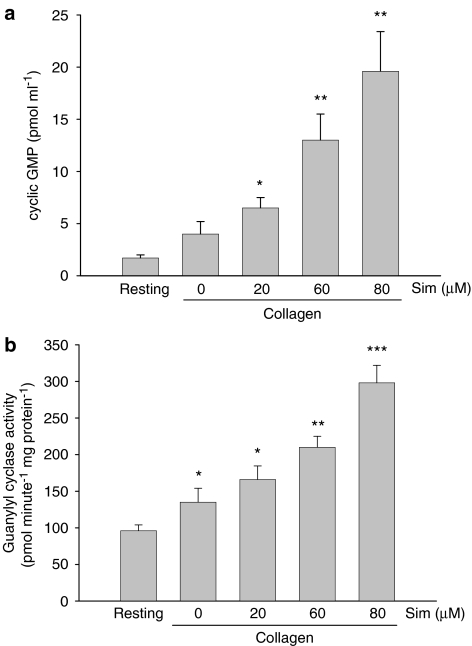
In the presence of L-arginine, simvastatin concentration dependently further increased the formation of cyclic GMP induced by collagen (Figure 3a) and platelet guanylyl cyclase activity (Figure 3b). In resting platelets, simvastatin itself had no significant effect on basal NO release, cyclic GMP formation, platelet NOS and guanylyl cyclase activity (data not shown).
Figure 3.
Effects of simvastatin on cyclic GMP formation and guanylyl cyclase activity in collagen-stimulated platelets. (a) In the presence of L-arginine (100 μM), washed platelets were preincubated with simvastatin (20–80 μM) or DMSO for 5 min, followed by addition of collagen (10 μg ml−1) for 6 min. Then, the supernatant and precipitated platelets were collected for cyclic GMP and guanylyl cyclase activity assay, respectively. The incubation was stopped by immediately boiling for 3 min and the concentrations of cyclic GMP in supernatant were determined. (b) For guanylyl cyclase activity assay, the levels of cyclic GMP were measured following a 20-min incubation at 37 °C with platelet cytosol (1 μg) in reaction buffer. Data from washed platelets only suspended in Tyrode's solution without addition of collagen and other drugs are shown as the resting group. *P<0.05, **P<0.01 and ***P<0.001, as compared with the resting group. All data were presented as means±s.e.mean (n=5). DMSO, dimethyl sulphoxide; cyclic GMP, 3′,5′-cyclic guanosine monophosphate.
Effect of cyclic GMP formation on simvastatin-induced anti-aggregatory activity
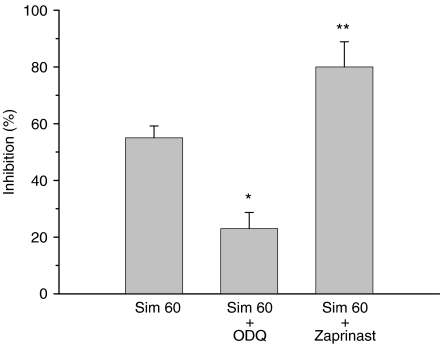
To further evaluate the role of cyclic GMP in the anti-aggregatory activity induced by simvastatin, other inhibitors such as ODQ, an inhibitor of NO-sensitive guanylyl cyclase, or zaprinast, a selective inhibitor of the cyclic GMP phosphodiesterase, was added. As shown in Figure 4, the inhibitory potency of simvastatin (60 μM) on collagen-induced platelet aggregation was significantly attenuated by ODQ, but was further enhanced by zaprinast. ODQ or zaprinast itself did not affect the platelet aggregation induced by collagen (data not shown).
Figure 4.
Effects of ODQ and zaprinast on the inhibitory effect of simvastatin on platelet aggregation. In the presence of L-arginine (100 μM), washed platelets were preincubated with simvastatin (60 μM) alone or in the presence of ODQ (1 μM) or zaprinast (5 μM) for 5 min, and then collagen (10 μg ml−1) was added to trigger platelet aggregation. *P<0.05 and **P<0.01, as compared with the simvastatin and collagen-treated platelets. Percent inhibition of platelet aggregation is presented as the means±s.e.mean (n=5). ODQ, 1H-[1,2,4] oxadiazolo [4,3-a]quinoxalin-1-one.
Discussion
Under pathological conditions, endothelial cells and platelets may become pro-adhesive and pro-coagulant, which may induce platelet adhesion to endothelium and activation of platelet aggregation. Many clinical studies have further demonstrated that increased platelet aggregation plays an important role in the pathogenesis of various cardiovascular and thromboembolic diseases (Andrews and Berndt, 2004). Therefore, prevention of platelet hyperactivity has been considered to be a reasonable therapeutic strategy to alleviate these diseases. Several studies have reported that some so-called cholesterol-independent or ‘pleiotropic' effects of statins, including their anti-thrombotic actions and improving or restoring endothelial function, may be associated with the increase of NO formation from endothelium (Radomski and Moncada, 1993; Dobrucki et al., 2001; Liao, 2004). However, whether statins also directly affect NO formation of platelets and platelet aggregation is still unknown. In this study, we proposed that an increased NO/cyclic GMP-mediated process in platelets might be involved in the anti-aggregatory activity of simvastatin.
There was clear evidence that the anti-aggregatory activity of simvastatin was associated with an enhanced NO/cyclic GMP-mediated process. First, the anti-aggregatory activity of simvastatin was significantly attenuated when NO formation was blocked by an NOS inhibitor, L-NAME, or an NO scavenger, PTIO (Figure 1). Second, the anti-aggregatory activity of simvastatin was also reduced if the biosynthesis of cyclic GMP was inhibited by ODQ, but increased markedly when degradation of cyclic GMP was prevented by zaprinast (Figure 4). Third, the strongest evidence was that simvastatin could directly enhance NO and cyclic GMP production in platelets induced by collagen through increasing NOS and guanylyl cyclase activity (Figures 2 and 3). These findings strongly indicated that the mechanisms by which simvastatin inhibited platelet aggregation might be mediated, in part, by an NO/cyclic GMP-dependent process.
It is noteworthy that in the presence of L-arginine, simvastatin itself did not produce detectable amounts of NO or cyclic GMP, suggesting that in resting platelets the L-arginine-NO–cyclic GMP pathway was not activated by exogenous simvastatin. Following activation of platelets by collagen, NO formation increased, but the amount was not enough to inhibit platelet aggregation. When NO and cyclic GMP formation were further enhanced by simvastatin, collagen-induced platelet aggregation was inhibited significantly, suggesting that increased amounts of NO and cyclic GMP are required to suppress platelet aggregation. Previous studies have reported that the cyclic GMP-dependent kinase I, an important downstream target enzyme of the NO/cyclic GMP-signalling cascade, exerts a potent inhibitory effect on platelet aggregation (Butt et al., 1994; Wang et al., 1998). Therefore, it is very likely that the NO/cyclic GMP/cGK-mediated pathway may be involved in the anti-aggregatory activity of simvastatin.
The exact mechanisms by which simvastatin increases NO formation remain unclear. It is well known that raised intracellular calcium level ([Ca2+]i) plays a crucial role in activating constitutive NOS activity and platelet aggregation (Kroll and Schager, 1989; Fleming and Busse, 2003). A previous study has reported that simvastatin could enhance [Ca2+]i in bovine aortic endothelial cells by increasing Ca2+ entry and Ca2+ release from intracellular stores (Alvarez de and Andriantsitohaina, 2001). Thus, early elevation of platelet [Ca2+]i by simvastatin may be a possible mechanism accounting for the increased platelet NOS activity and subsequent NO production. However, clinical studies also indicated that platelets [Ca2+]i in patients with primary hypercholesterolemia was decreased after a long-term treatment with pravastatin through a cyclic GMP/cyclic GMP-dependent kinase I-mediated pathway (Geiger et al., 1992; Le Quan Sang et al., 1995). These results imply that statins may cause an initial transient elevation of [Ca2+]i to increase NO and cyclic GMP formation, leading to an inhibition of platelet [Ca2+]i rise and aggregation by a subsequent NO/cyclic GMP/cGK-mediated pathway. Other possible mechanisms, such as activation of endothelial NOS via decrease of the expression of caveolin, an inhibitor of endothelial NOS activation, may be also involved in statin actions (Feron et al., 2001).
When platelets are activated, the release of AA is increased and this AA is converted to thromboxane A2, a potent inducer of release reaction and platelet aggregation, catalysed by cyclooxygenase and thromboxane synthase (Vezza et al., 2002). Previous studies reported that the NO/cyclic GMP pathway can inhibit the signalling cascade of thromboxane A2 receptor through phosphorylation by cGK and collagen-induced AA release by suppressing Ca2+-activated phospholipase A2 activity (Matsukoka et al., 1989; Wang et al., 1998). Our unpublished data have shown that simvastatin significantly inhibited the formation of AA or collagen-induced thromboxane B2, a stable metabolite of thromboxane A2. Thus, the NO/cyclic GMP-mediated inhibition of thromboxane A2 formation may be another possible anti-aggregatory mechanism of simvastatin. Although we have demonstrated that enhancement of NO and cyclic GMP formation plays a role in the anti-aggregatory activity of simvastatin, it was also observed that there was still inhibition of platelet aggregation by simvastatin, even in the presence of L-NAME or PTIO. This finding suggests that still other mechanisms of simvastatin, including changing the structure of cell membranes, inhibiting the expression of glycoprotein IIb/IIIa on platelet surface (our unpublished data) and the formation of oxidative stress resulting in increasing platelet-derived NO bioavailability (Osamah et al., 1997; Haramaki et al., 2007), may be also involved in its inhibition of platelet aggregation. Many clinical studies have demonstrated that treatment with simvastatin has an anti-platelet activity in hypercholesterolemic patients, which is consistent with the result of this in vitro study (Coumar et al., 1991; Notarbartolo et al., 1995). There is however one study, which showed no influence of simvastatin treatment on platelet function in patients with hypercholesterolemia (Broijersen et al., 1997), and the reasons for this discrepancy are not clear, but the different study design, gender, diagnosis of patients and methodological differences for platelet aggregability in the study of Broijersen et al. (1997) may be possible factors.
In clinical use, doses of simvastatin (20–80 mg per day) are often used. In this study, we demonstrated that the platelet aggregation was concentration dependently inhibited by simvastatin (20–80 μM) in vitro. The dose is similar to that (1–30 μM) of other in vitro studies used to investigate various pharmacological effects of statins (Kaesemeyer et al., 1999; Huang et al., 2003). Since simvastatin is an inactive pro-drug, after administration, simvastatin can be converted into its active metabolite, simvastatin acid, by liver (Mauro, 1993). Because the uptake rate, metabolism and absorption of simvastatin provide major differences between in vivo and in vitro conditions, the concentrations of simvastatin used in vitro, which might correspond to clinical doses, are difficult to define. Theoretically, the in vitro doses of simvastatin required to achieve the same effect may be higher than the clinical doses, and the findings obtained from in vitro studies may be of relevance to the clinical beneficial effects. However, the possibility that simvastatin itself may have a direct effect on platelet function through a 3-hydroxy-3-methyl-glutaryl coenzyme A-independent pathway may not be excluded.
In conclusion, our findings demonstrate that simvastatin enhanced NO and cyclic GMP formation through activating NOS and guanylyl cyclase activity in platelets, which may, at least in part, contribute to its anti-aggregatory actions and provide a new mechanism to explain its vascular protective effects.
Acknowledgments
This work was partially supported by a research grant from the National Science Council of Taiwan, Republic of China (NSC 90-2315-B016-006).
Abbreviations
- AA
arachidonic acid
- cyclic GMP
3′,5′-cyclic guanosine monophosphate
- L-NAME
NG-nitro-L-arginine methyl ester
- NO
nitric oxide
- ODQ
1H-[1,2,4] oxadiazolo [4,3-a]quinoxalin-1-one
- PTIO
2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide
Conflict of interest
The authors state no conflict of interest.
References
- Alvarez de S, Andriantsitohaina R. Simvastatin and Ca2+ signaling in endothelial cells: involvement of Rho protein. Biochem Biophys Res Commun. 2001;280:486–490. doi: 10.1006/bbrc.2000.4144. [DOI] [PubMed] [Google Scholar]
- Andrews RK, Berndt MC. Platelet physiology and thrombosis. Thromb Res. 2004;114:447–453. doi: 10.1016/j.thromres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Bonetti PO, Lerman LO, Napoli C, Lerman A. Statin effects beyond lipid lowering-are they clinically relevant. Eur Heart J. 2003;24:225–248. doi: 10.1016/s0195-668x(02)00419-0. [DOI] [PubMed] [Google Scholar]
- Broijersen A, Eriksson M, Leijd B, Angelin B, Hjemdahl P. No influence of simvastatin treatment on platelet function in vivo in patients with hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1997;17:273–278. doi: 10.1161/01.atv.17.2.273. [DOI] [PubMed] [Google Scholar]
- Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, et al. c-AMP and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem. 1994;269:14509–14517. [PubMed] [Google Scholar]
- Chou T-C, Li C-Y, Yen M-H, Ding Y-A. Antiplatelet effect of amlodipine: a possible mechanism through nitric oxide mediated process. Biochem Pharmacol. 1999;58:1657–1663. doi: 10.1016/s0006-2952(99)00235-x. [DOI] [PubMed] [Google Scholar]
- Coumar A, Gill JK, Barradas MA, O'Donoghue S, Jeremy JY, Mikhailidis DP. The effect of treatment with simvastatin on platelet function indices in hypercholesterolemia. J Drug Dev. 1991;4:79–86. [Google Scholar]
- Dobrucki LW, Kalinowski L, Dobrucki IT, Malinski T. Statin-stimulated nitric oxide release from endothelium. Med Sci Monit. 2001;7:622–627. [PubMed] [Google Scholar]
- Emerson M, Momi S, Paul W, Alberti PF, Page C, Gresele P. Endogenous nitric oxide acts as a natural antithrombotic agent in vivo by inhibiting platelet aggregation in the pulmonary vasculature. Thromb Haemost. 1999;81:961–966. [PubMed] [Google Scholar]
- Feron O, Dessy C, Desager JP, Balligand JL. Hydroxy-methylglutaryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation. 2001;103:113–118. doi: 10.1161/01.cir.103.1.113. [DOI] [PubMed] [Google Scholar]
- Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol. 2003;284:R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- Gaddam V, Li DY, Mehta JL. Anti-thrombotic effects of atorvastatin—an effect unrelated to lipid lowering. J Cardiovasc Pharmacol Ther. 2002;7:247–253. doi: 10.1177/107424840200700408. [DOI] [PubMed] [Google Scholar]
- Geiger J, Nolte C, Butt E, Sage SO, Walter U. Role of cGMP and cGMP-dependent protein kinase in nitrovasodilator inhibition of agonist-evoked calcium elevation in human platelets. Proc Natl Acad Sci USA. 1992;89:1031–1035. doi: 10.1073/pnas.89.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramaki N, Ikeda H, Takenaka K, Katoh A, Sugano R, Yamagishi S-I, et al. Fluvastatin alters platelet aggregability in patients with hypercholesterolemia: possible improvement of intraplatelet redox imbalance via HMG-CoA reductase. Arterioscler Thromb Vasc Biol. 2007;27:1471–1477. doi: 10.1161/ATVBAHA.106.128793. [DOI] [PubMed] [Google Scholar]
- Huang K-C, Chen C-W, Chen J-C, Lin W-W. HMG-CoA reductase inhibitors inhibit inducible nitric oxide synthase gene expression in macrophages. J Biomed Sci. 2003;10:396–405. doi: 10.1007/BF02256431. [DOI] [PubMed] [Google Scholar]
- Kaesemeyer WH, Caldwell RB, Huang J, Caldwell W. Pravastatin sodium activates endothelial nitric oxide synthase independent of its cholesterol-lowering actions. J Am Coll Cardiol. 1999;33:234–241. doi: 10.1016/s0735-1097(98)00514-2. [DOI] [PubMed] [Google Scholar]
- Kalinowski L, Dobrucki LW, Brovkovych V, Malinski T. Increased nitric oxide bioavailability in endothelial cells contributes to the pleiotropic effect of cerivastatin. Circulation. 2002;105:933–938. doi: 10.1161/hc0802.104283. [DOI] [PubMed] [Google Scholar]
- Kearney D, Fitzgerald D. The anti-thrombotic effects of statins. J Am Coll Cardiol. 1999;33:1305–1307. doi: 10.1016/s0735-1097(99)00019-4. [DOI] [PubMed] [Google Scholar]
- Kroll M, Schager A. Biochemical mechanisms of platelet activation. Blood. 1989;74:1185–1195. [PubMed] [Google Scholar]
- Laufs U, Fata VL, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG-CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- Le Quan Sang KH, Levenson J, Megnien JL, Simon A, Devynck MA. Platelet cytosolic Ca2+ and membrane dynamics in patients with primary hypercholesterolemia. Effects of pravastatin. Arterioscler Thromb Vasc Biol. 1995;15:759–764. doi: 10.1161/01.atv.15.6.759. [DOI] [PubMed] [Google Scholar]
- Liao JK. Statin therapy: having the good without the bad. Hypertension. 2004;43:1171–1172. doi: 10.1161/01.HYP.0000126153.80112.5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LP, Nie DN, Hsu SX, Yin SM, Xu LZ, Nunes JV. Inhibition of platelet aggregation and expression of alpha granule membrane protein 140 and thromboxane B2 with pravastatin therapy for hypercholesterolemia. J Assoc Acad Minor Phys. 2002;13:23–26. [PubMed] [Google Scholar]
- Marcus AJ, Safier LB. Thromboregulation: multicellular modulation of platelet reactivity in hemostasis and thrombosis. FASEB J. 1993;7:516–522. doi: 10.1096/fasebj.7.6.8472890. [DOI] [PubMed] [Google Scholar]
- Matsukoka I, Nakahata N, Nakanishi H. Inhibitory effect of 8-bromo cyclic GMP on extracellular Ca2+-dependent arachidonic acid liberation in collagen-stimulated rabbit platelets. Biochem Pharmacol. 1989;38:1841–1847. doi: 10.1016/0006-2952(89)90420-6. [DOI] [PubMed] [Google Scholar]
- Maurer TS, Fung HL. Evaluation of nitric oxide synthase activity and inhibition kinetics by chemiluminescence. Nitric Oxide. 2000;4:372–378. doi: 10.1006/niox.2000.0289. [DOI] [PubMed] [Google Scholar]
- Mauro VF. Clinical pharmacokinetics and practical applications of simvastatin. Clin Pharmacokinet. 1993;24:195–202. doi: 10.2165/00003088-199324030-00002. [DOI] [PubMed] [Google Scholar]
- Mayer J, Eller T, Brauer P, Solleder EM, Schafer RM, Keller F, et al. Effects of long-term treatment with lovastatin on the clotting system and blood platelets. Ann Hematol. 1992;64:196–201. doi: 10.1007/BF01696223. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Notarbartolo A, Davi G, Averna M, Barbagallo CM, Ganci A, Giammarresi C, et al. Inhibition of thromboxane biosynthesis and platelet function by simvastatin in type IIa hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1995;15:247–251. doi: 10.1161/01.atv.15.2.247. [DOI] [PubMed] [Google Scholar]
- Osamah H, Mira R, Sorina S, Shlomo K, Michael A. Reduced platelet aggregation after fluvastatin therapy is associated with altered platelet lipid composition and drug binding to the platelets. Br J Clin Pharmacol. 1997;44:77–83. doi: 10.1046/j.1365-2125.1997.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccetti L, Bruni F, Bova G, Cercignani M, Palazzuoli A, Console E, et al. Effect of diet and treatment with statins on platelet-dependent thrombin generation in hypercholesterolemic subjects. Nutr Metab Cardiovasc Dis. 2001;11:378–387. [PubMed] [Google Scholar]
- Radomski MW, Moncada S. The biological and pharmacological role of nitric oxide in platelet function. Adv Exp Med Biol. 1993;344:251–264. doi: 10.1007/978-1-4615-2994-1_20. [DOI] [PubMed] [Google Scholar]
- Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- Tannous M, Cheung R, Vignini A, Mutus B. Atrovastatin increases ecNOS levels in human platelets of hyperlipidemic subjects. Thromb Haemost. 1999;82:1390–1394. [PubMed] [Google Scholar]
- Vezza R, Mezzasoma AM, Venditti G, Gresele P. Prostaglandin endoperoxides and thromboxane A2 activate the same receptor isoforms in human platelets. Thromb Haemost. 2002;87:114–121. [PubMed] [Google Scholar]
- Wang GR, Zhu Y, Halushka PV, Lincoln TM, Mendelsohn ME. Mechanism of platelet inhibition by nitric oxide: in vivo phosphorylation of thromboxane receptor by cyclic GMP-dependent protein kinase. Proc Natl Acad Sci USA. 1998;95:4888–4893. doi: 10.1073/pnas.95.9.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W-C, Chai C-Y. Nitric oxide release in the nucleus tractus solitarius during and after bilateral common carotid artery occlusion. Clin Exp Pharmacol Physiol. 2004;31:152–158. doi: 10.1111/j.1440-1681.2004.03967.x. [DOI] [PubMed] [Google Scholar]
- Wu W-C, Wang Y, Su C-K, Chai C-Y. The nNOS/cGMP signal transducing system is involved in the cardiovascular responses induced by activation of NMDA receptors in the rostral ventrolateral medulla of cats. Neurosci Lett. 2001;310:121–124. doi: 10.1016/s0304-3940(01)02100-0. [DOI] [PubMed] [Google Scholar]
- Zhou M-S, Jaimes EA, Raij L. Atorvastatin prevents end-organ injury in salt-sensitive hypertension: role of eNOS and oxidant stress. Hypertension. 2004;44:186–190. doi: 10.1161/01.HYP.0000136395.06810.cf. [DOI] [PubMed] [Google Scholar]