Abstract
This commentary on the review by DA Saint in the current issue of the British Journal of Pharmacology focuses on the pathological role of late INa in the heart, the evidence supporting inhibition of late INa as a therapeutic target in ischaemic heart disease, and the therapeutic applications and challenges for development of new late INa inhibitors. Recent reports from a large clinical outcome trial (MERLIN) of ranolazine, a drug known to inhibit late INa, indicated that it was safe and reduced recurrent ischaemia and arrhythmic activity. In combination with other results indicating that inhibition of late INa reduces ischaemia, myocardial Ca2+ overload, and electrical and mechanical dysfunction when late INa is increased, the new clinical trial results suggest that reduction of cardiac late INa is safe and therapeutically beneficial.
Keywords: late INa, angina, diastolic dysfunction, ischaemia, ranolazine, Ca2+ overload, sodium channel, heart failure, lusitropy
Introduction
The review by David Saint of the properties of the cardiac persistent late sodium current (INa) (Saint, 2007), and of the potential therapeutic role(s) of drugs that inhibit this current, recognizes that late INa may be increased in ischaemic and failing hearts and thus may be of general pathologic importance in heart disease. The discussion of blockers of late INa in the review is timely, given the recent FDA approval of ranolazine for the treatment of chronic angina (the primary symptom of ischaemic heart disease) and the publication of results from a large clinical outcome trial with the drug (Morrow et al., 2007; Scirica et al., 2007).
Why is enhanced late INa pathologic?
Enhancement of late INa is pathologic because it increases the intracellular concentration of Na+, Na+–Ca2+ exchange (NCX), Ca2+ entry into myocytes and duration of the myocardial action potential (AP). The increase of Ca2+ entry may be of sufficient magnitude to cause Ca2+ overloading with mechanical (e.g., impaired ventricular diastolic relaxation and reduction of diastolic coronary blood flow) and electrical (e.g., early and delayed afterdepolarizations: EADs and DADs) dysfunction (Belardinelli et al., 2006). Enhancement of late INa greatly increases Na+ entry during phase 2 of the ventricular AP (Makielski and Farley, 2006). Because net outward current is small during phase 2, an enhanced late INa reduces repolarization reserve and increases AP duration (APD). When phases 2 and 3 are prolonged, Ca2+ and Na+ channels may reopen, and the subsequent cation influx may lead to further APD prolongation and EADs. Spatial heterogeneity of prolongation of APD in the ventricle due to enhanced late INa may set the stage for triggered and re-entrant arrhythmias.
Enhanced late INa disrupts Na+ and Ca2+ homeostasis. Sodium entry leads to increases of intracellular Na+ concentration, reverse mode (3 Na2+ out for 1 Ca2+ in) NCX and Ca2+ entry. The increase of Ca2+ entry during phase 2 of the AP plateau may not only support systolic contraction, but also cause Ca2+ overload. Acute cellular Ca2+ overloading has negative consequences. Accumulation of Ca2+ by the sarcoplasmic reticulum is increased, but an elevation of both stored Ca2+ and cytosolic Ca2+ concentrations facilitates the occurrence of spontaneous sarcoplasmic reticulum Ca2+ release during diastole (Bers, 2001). Spontaneous release of Ca2+ from the sarcoplasmic reticulum during diastole increases NCX (Bers, 2001). NCX is electrogenic, and its operation in the forward mode (3 Na+ in for 1 Ca2+ out) creates a transient inward current that can generate a DAD and triggered arrhythmic activity, as well as an aftercontraction (i.e., force production during diastole). Thus, both reverse (phases 1 and 2 of the AP) and forward (phases 2 and 3 and diastole) modes of NCX are enhanced subsequent to increases of late INa and intracellular [Na+], although at different times during the cardiac cycle. The late INa-enhanced oscillatory increases and decreases of the forward and reverse modes of the electrogenic NCX may become electrically destabilizing and cause formation of DADs.
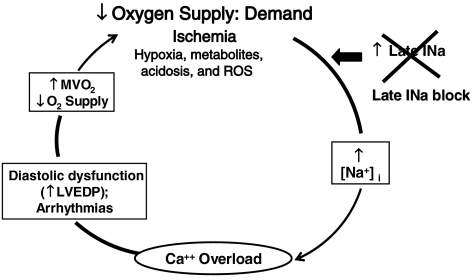
Elevation of the intracellular Ca2+ concentration secondary to an enhanced late INa in ventricular myocytes impairs diastolic relaxation, that is, slows active relaxation of the ventricle (a negative lusitropic effect). Maintenance of contractile tension during a prolonged period of active ventricular relaxation increases myocardial oxygen consumption and reduces coronary blood flow during left-ventricular (LV) isovolumic relaxation, when flow is normally at its peak. A reduction of diastolic coronary flow in a region of the myocardium with already reduced flow reserve only worsens the tenuous balance between oxygen supply and demand (see Figure 1 and Belardinelli et al., 2006). An increase of late INa exacerbates the situation by facilitating AP prolongation, EADs and DADs. These acute electrical events are associated with increases in the cytosolic Ca2+ concentration, force production and delayed or reduced diastolic relaxation that further increase oxygen demand while concurrently reducing diastolic blood flow and myocardial oxygen supply. Thus, increases of late INa and intracellular Ca2+ worsen LV function. Furthermore, although the role of late INa in chronic calcium overloading is unknown, late INa is reported to be increased in myocytes from failing hearts and from post-infarction remodeled myocardium (Huang et al., 2001; Undrovinas et al., 2006). Heart failure appears to be a condition of chronic Ca2+ overloading that is associated with down-regulation of ICa,L, increased NCX, prolongation of APD, decreased contractility and a proarrhythmic state (Bers, 2001).
Figure 1.
Block of late INa interrupts a deleterious positive feedback cycle during ischaemia by reducing [Na+]i-dependent calcium overload.
Regional differences in the magnitude of late INa in ventricular myocardium have been reported, and augmentation of these differences is proarrhythmic (Antzelevitch et al., 1999; Antzelevitch and Belardinelli, 2006). In ischaemic and structurally abnormal hearts, it is doubtful that increases of late INa and cellular Ca2+ overloading occur simultaneously and equally throughout the ventricle. Consequently, cell-to-cell and regional dispersion of increases of late INa and Na+-induced Ca2+ overloading during ischaemia may lead to focal regions of EAD and DAD formation that increase the dispersion of ventricular repolarization. These events alter the conduction and chronology of ventricular electrical (and mechanical) activity, facilitate triggered and re-entrant arrhythmic activity, prolong the time needed for relaxation of systolic contraction and reduce contractile efficiency and diastolic blood flow (oxygenation) in the myocardium.
Much evidence suggests that late INa is enhanced in ischaemia, although quantization of late INa in an intact, blood-perfused heart during ischaemia cannot be done. Myocardial-cell Na+ content increases during ischaemia (Bers, 2001). Myocardial-cell Ca2+ overload and dysfunction caused by ischaemia and reperfusion can be prevented by blockade of membrane Na+ channels or cardiac-specific ablation of NCX, thus implicating increased Na+ entry into cells as a proximate cause of Ca2+ overload (Belardinelli et al., 2006). Hypoxia is known to impair inactivation of Na+ channels and increase late INa (Saint, 2006). Tissue hypoxia and reperfusion of ischaemic myocardium are reported to generate metabolites (e.g., palmitoyl-L-carnitine and lysophosphatidylcholine) and reactive oxygen/nitrogen species (e.g., hydrogen peroxide and nitric oxide) that increase late INa in ventricular myocytes.
The mechanism of action of ranolazine to reduce electrical and mechanical dysfunction of the ischaemic myocardium
Ranolazine, at concentrations within its therapeutic range (⩽10 μM), significantly reduces late INa and reverses or prevents the consequences of increase of late INa (Belardinelli et al., 2006). Ranolazine reduces late INa in mouse myocytes in which late INa is enhanced due to expression of the long-QT syndrome 3 mutant sodium channel, ΔKPQ (Fredj et al., 2006), in ventricular myocytes isolated from normal dogs and from dogs with heart failure (Undrovinas et al., 2006), in guinea pig isolated ventricular myocytes exposed to anemone toxin-II or hydrogen peroxide (H2O2) (Song et al., 2004, 2006) and in HEK293 cells expressing human NaV1.5 channels (Rajamani et al., 2007). The range of values of IC50 (potency) is 5.9–15 μM for ranolazine to reduce late INa in various preparations. Ranolazine (1–10 μM) reduced APD and temporal dispersion of APD, and abolished EADs of guinea pig isolated ventricular myocytes in the presence of either anemone toxin-II or H2O2, both of which increase late INa (Song et al., 2004, 2006), thus supporting the concept that reduction of an increased late INa is anti-arrhythmic. Ranolazine (5–20 μM) reversibly and significantly shortened the APD of myocytes isolated from dogs with heart failure, in which late INa is increased, and reduced variability of APD (Undrovinas et al., 2006). Ranolazine decreased cellular Ca2+ overload caused by anemone toxin-II or ischaemia/reperfusion in rat isolated hearts, and it enhanced recovery of post-ischaemic LV function, coronary flow and coronary vascular conductance (Fraser et al., 2006). As expected, ranolazine had no effect on intracellular diastolic Ca2+ concentration, Ca2+ transient amplitude, LV function, coronary flow or coronary vascular conductance during aerobic conditions (Fraser et al., 2006). Ranolazine-mediated block of late INa was significantly reduced by mutation of a single amino-acid residue (F1760A) in the putative local anaesthetic binding site of the cardiac Na+ channel NaV1.5 (Fredj et al., 2006). Thus, a putative site of ‘binding' of ranolazine in the cardiac Na+ channel has been identified. In sum, the above findings strongly suggest that ranolazine alters the pathophysiology associated with Na+-dependent Ca+2 overloading in myocardium by inhibiting late INa.
The anti-ischaemic effects of ranolazine occur in the absence of haemodynamic changes. Although ranolazine has been reported to antagonize α1- and β-adrenergic antagonist activity in radioligand binding and in vitro tissue assays (Letienne et al., 2001), at therapeutic concentrations (1–10 μM), it does not cause bradycardia or hypotension in animals (Wang et al., 1999) or in resting and exercising humans (Pepine and Wolff, 1999; Chaitman et al., 2004a, 2004b; Rousseau et al., 2005). In dogs that are awake, ranolazine at steady-state concentrations ⩽18 μM did not cause significant slowing of heart rate or lowering of arterial blood pressure and did not affect coronary blood flow, coronary vascular resistance or LV contractility (CV Therapeutics, 2003). These findings suggest that effects of ranolazine in vivo on haemodynamic parameters that are regulated by α1- or β1-adrenergic-mediated activity are not significant in normal dogs with intact autonomic nervous control mechanisms. Furthermore, ranolazine is cardioprotective in ischaemic protocols, wherein hearts are both electrically paced and perfused at constant rates, and in models using cytotoxic ischaemic metabolites (Belardinelli et al., 2006), wherein selective β-adrenergic-receptor-blocking drugs are not cardioprotective. Thus, the data available to date do not indicate any contribution of the sympatholytic action of ranolazine to its anti-ischaemic and anti-anginal effects.
Inhibitions by ranolazine of ion currents other than late INa do not appear to be responsible for the anti-ischaemic and anti-anginal effects of the drug. Inhibition of IKr is likely responsible for the prolongation of the QT interval by ranolazine, but blockade of IKr has not been shown to reduce ischaemic damage or angina. With other drugs, IKr block has been associated with proarrhythmic activity, whereas ranolazine reduces both atrial and ventricular arrhythmic activity in humans (Scirica et al. 2007). The significance of a reported inhibition by ranolazine of late ICa (Antzelevitch et al., 2004) is unknown. Finally, ranolazine (1–10 μM) does not increase the duration of the QRS interval (i.e., does not slow the conduction of electrical activity), a finding consistent with its low potency to reduce peak INa in the ventricle. The potencies (IC50 values) of ranolazine to inhibit peak INa in ventricular myocytes isolated from normal dogs and from dogs with heart failure were 294 and 244 μM, respectively (stimulation rate, 0.1 Hz; holding potential, −140 mV) (Undrovinas et al., 2006). In the same study, the IC50 for ranolazine to inhibit late INa was 6.5 μM. These findings are consistent with the results of a large clinical outcome trial indicating that ranolazine does not increase mortality and reduces arrhythmic activity (Morrow et al., 2007; Scirica et al., 2007).
Results of a recent ‘proof-of-concept' study of patients with a long-QT syndrome 3 caused by a KPQ deletion in NaV1.5 revealed that ranolazine (1.8–4 μM) significantly shortened the QTc interval and improved measures of the rate of diastolic relaxation of the left ventricle (data on file at CV Therapeutics Inc., Palo Alto, USA). In a different study of patients with a previous transmural myocardial infarction and with ejection fractions ranging from 13–55%, ranolazine improved diastolic function of the non-infarcted myocardium (Hayashida et al., 1994). These results are consistent with the hypothesis that enhanced late INa and its phenotypic manifestations are normalized by therapeutic concentrations of ranolazine in humans, and they provide compelling evidence that ranolazine inhibits late INa of the human heart in vivo.
What are the potential therapeutic applications for an inhibitor of late INa?
Both preclinical experience with late INa inhibitors and the clinical experience with ranolazine indicate that inhibition of late INa improves function of the ischaemic myocardium. Cardiac angina, a symptom of myocardial ischaemia, is relieved by ranolazine (Chaitman et al., 2004a, 2004b; Rousseau et al., 2005). The anti-ischaemic effect of ranolazine, demonstrated in humans by an increased time to 1-mm ST-segment depression during an exercise tolerance test (Pepine and Wolff, 1999; Rousseau et al., 2005), and improved diastolic function (Hayashida et al., 1994) are the likely explanations for the action of ranolazine to alleviate angina in patients with ischaemic heart disease. The observation that late INa is increased in myocytes from failing hearts of dog suggests that late INa may be increased in heart failure, and thus heart failure may yet prove to be another indication for late INa inhibitors (Maltsev et al., 2007). Inhibition of late INa reduces the prolonged ventricular APD (i.e., the QT interval), dispersion of repolarization, arrhythmic activity, Ca2+ loading of myocardial cells and diastolic dysfunction in heart failure (Undrovinas et al., 2006). Diastolic heart function is improved in ΔKPQ long-QT syndrome 3 patients (as noted above), indicating that enhanced late INa may contribute to, and reduction of late INa may alleviate, diastolic dysfunction in some patients. Other potential therapeutic applications of late INa inhibitors are neuronal and muscular disorders in which mutations in various non-cardiac Na+-channel isoforms (e.g., NaV1.7 and NaV1.4) are known to underlie the pathological phenotype, as in neuropathic pain, epilepsy, sudden infant death syndrome and muscular paralysis. The past 10 years have seen remarkable advancements in the identification of pathological effects of enhanced late INa, causal mechanisms of Na+ channelopathies and disease states in which late INa is enhanced. We speculate that increases of late INa will be found to contribute to impairment of the functional status of excitable cells in many other situations. This is not unexpected, given that excessive late INa may disrupt the homeostasis of Na+- and Ca2+-ion concentrations that maintain normal function of myocardial, neural, muscular and secretory cells. Current evidence of a role for reactive oxygen species to increase late INa (Song et al., 2006) suggests that enhancement of late INa may be a common phenomenon in processes such as ischaemia and aging.
What are the challenges in developing an inhibitor of late INa?
Understanding of the roles of late INa in physiology and disease is still unfolding, and development of inhibitors of this current poses several challenges. Ion-channel-blocking drugs used for suppression of cardiac arrhythmias are often unselective for their given ion-channel target (e.g., amiodarone). Na+-channel antagonists at present in therapeutic use (e.g., class I anti-arrhythmic agents), unlike many drugs (e.g., ligands of G-protein-coupled receptors), typically bind to their ion-channel targets with micromolar rather than nanomolar affinity. The goals of high potency, efficacy and selectivity that normally drive drug development may or may not be appropriate to find useful, safe blockers of late INa. In the final analysis, inhibition of ion-channel function should perhaps be rather subtle and readily reversible, and the combination of ion-channel effects of a drug may determine its success or failure.
When inhibiting cardiac late INa, it is preferable to avoid inhibition of peak INa, in the heart as well as in other excitable tissues. Drugs (e.g., flecainide, encainide) that inhibit peak INa in the ventricle were associated with increased mortality in the Cardiac Arrhythmia Suppression Trial (Echt et al., 1991). This finding suggests that late INa-blocking drugs must not alter Na+ channel opening and conductance during the AP upstroke (phase 0), to avoid slowing of impulse conduction and reduction of the wavelength of a re-entrant circuit. Reduction of peak INa by ranolazine increases with increased frequency of stimulation and decreased membrane potential and appears to be tissue (atria vs ventricle; Burashnikov et al., 2007), isoform (i.e., NaV1.1–1.9) and mutation (e.g., late INas of various NaV1.5-mutated channels are differentially responsive to ranolazine) dependent.
Inhibition of the HERG current IKr is problematic, and many drugs, including Na+-channel blockers, reduce this current. Reduction of IKr is frequently associated with an increased incidence of torsades de pointes, a potentially fatal ventricular tachycardia. Reduction of late INa offsets the reduction of repolarization reserve and the increase of APD and antagonizes the induction of EADs caused by IKr blockers. Ranolazine reduces IKr and prolongs, although modestly, the APD, but its effect is not rate (use) dependent, and it does not have proarrhythmic activity. The twofold greater potency of ranolazine to inhibit late INa than to inhibit IKr may contribute significantly to the anti-arrhythmic effects and absence of proarrhythmic effects of the drug. In post-myocardial infarction patients without ST-segment elevation but with a QTc interval greater than 450 ms, ranolazine significantly (P<0.001) reduced the incidence of ventricular tachycardia (⩾8 beats at ⩾100 bpm, lasting less than 30 s) during 7 days of Holter monitoring by 47% compared with placebo (Scirica et al., 2007).
Lastly, how much reduction of cardiac late INa is safe? At therapeutic concentrations, ranolazine reduces late INa by about 50%. Would more be better? The answer may depend on the role of late INa in normal cardiac function. For example, Purkinje fibres have a greater late INa than other cells in the heart, and the duration of the Purkinje fibre AP is significantly shortened upon late INa block. Excessive shortening of the Purkinje fibre APD may increase the risk of re-entry. Similar concerns with blockade of physiological roles of late INa in other tissues counsel caution in the development of potent drugs for incompletely understood targets such as late INa. Nevertheless, the observation that ranolazine reduces ischaemia/angina, diastolic dysfunction and arrhythmic activity in a safe manner suggests that reduction of late INa is therapeutically beneficial. However, scientific understanding of late INa is in its infancy, and we remind ourselves that ‘All scientific work is liable to be upset or modified by advancing knowledge' (Hill, 1965).
Abbreviations
- AP
action potential
- APD
action potential duration
- DAD
delayed afterdepolarization
- EAD
early afterdepolarization
- INa
sodium current
- LV
left ventricle
- NCX
Na+–Ca2+ exchange
Conflict of interest
John C Shryock and Luiz Belardinelli are employees of CV Therapeutics Inc., which markets Ranexa (ranolazine).
References
- Antzelevitch C, Belardinelli L. The role of sodium channel current in modulating transmural dispersion of repolarization and arrhythmogenesis. J Cardiovasc Electrophysiol. 2006;17:S79–S85. doi: 10.1111/j.1540-8167.2006.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, et al. Electrophysiological properties of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110:904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C, Shimizu W, Yan G, Sicouri S, Weissenburger J, Nesterenko VV, et al. The M cell: its contribution to the ECG and to normal and abnormal electrical function in the heart. J Cardiovasc Electrophysiol. 1999;10:1124–1152. doi: 10.1111/j.1540-8167.1999.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006;92 Suppl IV:iv6–iv14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. Excitation–Contraction coupling and Cardiac Contractile Force 2001Kluwer Academic Publishers: London; 2nd edn. [Google Scholar]
- Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation. Differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–1457. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaitman BR, Pepine CJ, Parker JO, Skopal J, Chumakova G, Kuch J, et al. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina. JAMA. 2004b;291:309–316. doi: 10.1001/jama.291.3.309. [DOI] [PubMed] [Google Scholar]
- Chaitman BR, Skettino SL, Parker JO, Hanley P, Meluzin J, Kuch J, et al. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol. 2004a;43:1375–1382. doi: 10.1016/j.jacc.2003.11.045. [DOI] [PubMed] [Google Scholar]
- CV Therapeutics Inc. Ranexa (Ranolazine) FDA Review Documents NDA 21-526. CV Therapeutics Inc.: Palo Alto, CA; 2003. [Google Scholar]
- Echt DS, Liebson PR, Mitchell LB. Mortality and morbidity in patients receiving encainide, flecainide, or placebo: The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- Fraser H, Belardinelli L, Wang L, Light PE, McVeigh JJ, Clanachan AS. Ranolazine decreases diastolic calcium accumulation caused by ATX-II or ischemia in rat hearts. J Mol Cell Cardiol. 2006;41:1031–1038. doi: 10.1016/j.yjmcc.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Fredj S, Sampson KJ, Liu H, Kass RS. Molecular basis of ranolazine block of LQT-3 mutant sodium channels: evidence for site of action. Br J Pharmacol. 2006;148:16–24. doi: 10.1038/sj.bjp.0706709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida W, van Eyll C, Rousseau MF, Pouleur H. Effects of ranolazine on left ventricular regional diastolic function in patients with ischemic heart disease. Cardiovasc Drugs Ther. 1994;8:741–747. doi: 10.1007/BF00877121. [DOI] [PubMed] [Google Scholar]
- Hill AB. The environment and disease: association or causation. Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, El-Sherif T, Gidh-Jain M, Qin D, El-Sherif N. Alterations of sodium channel kinetics and gene expression in the postinfarction remodeled myocardium. J Cardiovasc Electrophysiol. 2001;12:218–225. doi: 10.1046/j.1540-8167.2001.00218.x. [DOI] [PubMed] [Google Scholar]
- Letienne R, Vie B, Puech A, Vieu S, Le Grand B, John GW. Evidence that ranolazine behaves as a weak β1- and β2-adrenoceptor antagonist in the rat cardiovascular system. Naunyn-Schmiedeberg's Arch Pharmacol. 2001;363:464–471. doi: 10.1007/s002100000378. [DOI] [PubMed] [Google Scholar]
- Makielski JC, Farley AL. Na+ current in human ventricle: implications for sodium loading and homeostasis. J Cardiovasc Electrophysiol. 2006;17:S15–S20. doi: 10.1111/j.1540-8167.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: implications for repolarization variability. Eur J Heart Failure. 2007;9:219–227. doi: 10.1016/j.ejheart.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow DA, Scirica BM, Karwatowska-Prokopczuk E, Murphy SA, Budaj A, Varshavsky S, et al. Effects of ranolazine on recurrent cardiovascular events in patients with non-ST-elevation acute coronary syndromes. The MERLIN-TIMI 36 randomized trial. JAMA. 2007;297:1775–1783. doi: 10.1001/jama.297.16.1775. [DOI] [PubMed] [Google Scholar]
- Pepine CJ, Wolff AA. A controlled trial with a novel anti-ischemic agent, ranolazine, in chronic stable angina pectoris that is responsive to conventional antianginal agents. Am J Cardiol. 1999;84:46–50. doi: 10.1016/s0002-9149(99)00190-3. [DOI] [PubMed] [Google Scholar]
- Rajamani S, Shryock JC, Belardinelli L.Mechanism of ranolazine block of cardiac Na channels Eur Heart J 200728400(abstract) [Google Scholar]
- Rousseau MF, Pouleur H, Cocco G, Wolff AA. Comparative efficacy of ranolazine versus atenolol for chronic angina pectoris. Am J Cardiol. 2005;95:311–316. doi: 10.1016/j.amjcard.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Saint DA. The role of persistent Na+ current during cardiac ischemia and hypoxia. J Cardiovasc Electrophysiol. 2006;17:S96–S103. doi: 10.1111/j.1540-8167.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- Saint DA.The cardiac persistent sodium current: an appealing therapeutic target Br J Pharmacol 2007 10.1038/sj.bjp.0707492[e-pub ahead of print: published online 10 December 2007doi] [DOI] [PMC free article] [PubMed]
- Scirica BM, Morrow DA, Hod H, Murphy SA, Belardinelli L, Hedgepeth CM, et al. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non-ST-segment elevation acute coronary syndrome. Circulation. 2007;116:1647–1652. doi: 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]
- Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther. 2006;318:214–222. doi: 10.1124/jpet.106.101832. [DOI] [PubMed] [Google Scholar]
- Song Y, Shryock JC, Wu L, Belardinelli L. Antagonism by ranolazine of the pro-arrhythmic effects of increasing late INa in guinea pig ventricular myocytes. J Cardiovasc Pharmacol. 2004;44:192–199. doi: 10.1097/00005344-200408000-00008. [DOI] [PubMed] [Google Scholar]
- Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol. 2006;17:S169–S177. doi: 10.1111/j.1540-8167.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J-X, Maruyama K, Murakami M, Endo T, Komatsu H, Akahane M. Antianginal effects of ranolazine in various experimental models of angina. Arzneim-Forsch/Drug Res. 1999;49:193–199. doi: 10.1055/s-0031-1300401. [DOI] [PubMed] [Google Scholar]