Abstract
Background and purpose:
Protein transduction domains (PTDs), such as Tat, antennapedia homeoprotein (Antp), Rev and VP22, have been extensively utilized for intracellular delivery of biologically active macromolecules in vitro and in vivo. There is little known, however, about the relative transduction efficacy, cytotoxicity and internalization mechanism of individual PTDs.
Experimental approach:
We examined the cargo delivery efficacies of four major PTDs (Tat, Antp, Rev and VP22) and evaluated their toxicities and cell internalizing pathways in various cell lines.
Key results:
The relative order of the transduction efficacy of these PTDs conjugated to fluorescein was Rev>Antp>Tat>VP22, independent of cell type (HeLa, HaCaT, A431, Jurkat, MOLT-4 and HL60 cells). Antp produced significant toxicity in HeLa and Jurkat cells, and Rev produced significant toxicity in Jurkat cells. Flow cytometric analysis demonstrated that the uptake of PTD–fluorescein conjugate was dose-dependently inhibited by methyl-β-cyclodextrin, cytochalasin D and amiloride, indicating that all four PTDs were internalized by the macropinocytotic pathway. Accordingly, in cells co-treated with ‘Tat-fused' endosome-disruptive HA2 peptides (HA2-Tat) and independent PTD-fluorescent protein conjugates, fluorescence spread throughout the cytosol, indicating that all four PTDs were internalized into the same vesicles as Tat.
Conclusions and implications:
These findings suggest that macropinocytosis-dependent internalization is a crucial step in PTD-mediated molecular transduction. From the viewpoint of developing effective and safe protein transduction technology, although Tat was the most versatile carrier among the peptides studied, PTDs should be selected based on their individual characteristics.
Keywords: protein transduction domains, Tat, antennapedia, Rev, VP22, macropinocytosis
Introduction
A strong focus of research in the post-genomic era is the development of effective therapies for refractory diseases such as cancer and neurodegenerative syndromes (Rhodes and Chinnaiyan, 2005; Brusic et al., 2007; Drabik et al., 2007). Because the therapeutic targets of these diseases generally exist inside the cell, it is necessary to establish drug delivery methods that transfer macromolecules, such as therapeutic proteins or peptide-based drugs, across the cellular membrane (Nori and Kopecek, 2005; Murriel and Dowdy, 2006; Borsello and Forloni, 2007).
Protein transduction is a recently developed method for delivering biologically active proteins directly into mammalian cells with high efficiency (Hawiger, 1999; Schwarze et al., 2000). Recombinant technology is used to modify the biophysical properties of proteins and peptides, particularly with respect to their cell permeability, using the so-called protein transduction domains (PTDs) (Nagahara et al., 1998; Rojas et al., 1998; Schwarze et al., 1999). The HIV-1-derived Tat peptide renders various macromolecules cell permeable. Although the initial reports suggested that protein transduction is energy and temperature independent, these characteristics are now mostly attributed to phenomena such as fixation artefacts (Richard et al., 2003). More recent data indicate that basic PTDs such as Tat enter the cells through a macropinocytotic pathway that is universally active in all cells (Wadia et al., 2004; Kaplan et al., 2005). A series of events involves Tat attachment to an anionic cell surface, followed by the association of these complexes with lipid rafts, which triggers dynamin-independent macropinocytosis. After internalization, the endosome pH falls and Tat apparently destabilizes the membranes, which results in a small amount of the endosome contents escaping into the cytosol. The released fraction of Tat can then exert its biologic activity. Consistent with this model, Tat delivery is enhanced by the influenza virus haemagglutinin fusogenic motif, which further destabilizes endosomal membranes at a low pH (Han et al., 2001; Skehel et al., 2001). In cells cotreated with Tat-conjugated HA2 (HA2-Tat), a greater proportion of endocytosed Tat-fused Cre recombinase escapes into the cytoplasm (Wadia et al., 2004). Other studies suggest that certain cell types might incorporate Tat constructs by clathrin- or caveolin-dependent endocytosis, raising the possibility that transport varies according to the cargo or cell type (Ferrari et al., 2003; Fittipaldi et al., 2003; Richard et al., 2005). In addition to the basic Tat peptide, there are other proteins (fragments), such as antennapedia (Antp), Rev and VP22, that enhance cellular uptake of proteins (Table 1) (Derossi et al., 1994; Elliott and O'Hare, 1997; Futaki et al., 2001; Joliot and Prochiantz, 2004). These four well-known PTDs facilitate the delivery of various biomacromolecules into the cell, but few studies have examined their relative efficacy.
Table 1.
Protein sequences of the PTDs evaluated
| PTD | Origin | Sequence | pI |
|---|---|---|---|
| Tat | HIV-1 | GRKKRRQRRRPPQ | 12.70 |
| Antp | Drosophila | RQIKIWFQNRRMKWKK | 12.31 |
| Rev | HIV-1 | TRQARRNRRRRWRERQF | 12.60 |
| VP22 | HSV | NAKTRRHERRRKLAIER | 12.01 |
The basic amino acids in each sequence are shown in bold.
In the present study, we evaluated the potency and internalizing pathway of four major PTDs to optimize protein transduction technology and to clarify the mechanisms of action. We also evaluated the cytotoxicity of these four PTDs as this is crucial to their utility as effective biomacromolecule carriers. These analyses may help not only to elucidate the mechanism by which the four peptides facilitate the cellular uptake of biomacromolecules, but also provide criteria for their proper use.
Materials and methods
Cell lines
HeLa cells (human cervical carcinoma cells) and A431 cells (human epithelial carcinoma cells) were obtained from the American Type Culture Collection (Manassas, VA, USA). HaCaT cells (human keratinocyte cells) were kindly provided by Dr S Inui, Osaka University. Jurkat cells (human leukaemia cells) and MOLT-4 cells (human leukaemia cells) were kindly provided by Hayashibara Biochemical Laboratories Inc. (Okayama, Japan). HL60 cells (human promyelocytic leukaemia cells) were obtained from the Japanese Collection of Research Bioresources (JCRB; Osaka, Japan). HeLa cells were cultured in minimal essential medium (MEMα; Wako Pure Chemicals, Osaka, Japan) medium supplemented with 10% fetal bovine serum (FBS) and antibiotics. A431 cells and HaCaT cells were maintained in Dulbecco's modified Eagle's medium (Wako Pure Chemicals) supplemented with 10% FBS, 1% L-glutamine and antibiotics. Jurkat cells and MOLT-4 cells were maintained in RPMI-1640 medium (Wako Pure Chemicals) supplemented with 10% FBS and antibiotics. HL60 cells were maintained in RPMI-1640 medium (Wako Pure Chemicals) supplemented with 20% FBS and antibiotics. All cells were cultured at 37 °C in 5% CO2.
Synthetic peptides
All peptides used in this study were purchased from the Toray Research Center Inc. (Tokyo, Japan) and had purities above 90%, which was confirmed by high-performance liquid chromatography analysis and mass spectroscopy. The sequences of these peptides were GRKKRRQRRRPPQK-FAM (FAM=carboxyfluorescein) for Tat-conjugated FAM (Tat-FAM), RQIKIWFQNRRMKWKKK-FAM for Antp-conjugated FAM (Antp-FAM), TRQARRNRRRRWRERQRK-FAM for Rev-conjugated FAM (Rev-FAM), NAKTRRHERRRKLAIERK-FAM for VP22-conjugated FAM (VP22-FAM), YGRKKRRQRRRK-biotin for Tat-conjugated biotin, RQIKIWFQNRRMKWKKK-biotin Antp-conjugated biotin, TQRARRNRRRRWRERQRK-biotin for Rev-conjugated biotin, NAKTRRHERRRKLAIERK-biotin for VP22-conjugated biotin and GLFEAIEGFIENGWEGMIDGWYGYGRKKRRQRRR for HA2-conjugated Tat (HA2-Tat). The individual PTD sequences are underlined.
Flow cytometric analysis
HeLa cells, HaCaT cells and A431 cells were cultured in 24-well plates (Nalge Nunc International, Naperville, IL, USA) at 5.0 × 104 cells per well in culture medium and incubated for 24 h at 37 °C. Jurkat cells, MOLT-4 cells and HL60 cells were cultured in 24-well plates (Nalge Nunc International) at 1.0 × 105 cells per well in Opti-MEM I (Invitrogen, Carlsbad, CA, USA). After aspirating the media, FAM-conjugated PTD (PTD-FAM) (10 μM) was added to the cells in Opti-MEM I and the culture dishes were incubated for an additional 3 h. Following incubation, the cells were washed with phosphate-buffered saline and incubated for 5 min with 0.1% trypsin to detach them and to remove surface-bound peptides. After incubation, 2 vol of 10% FBS-containing culture medium was added to stop the trypsin activity and to detach the cells completely. The cell suspension was centrifuged at 800 g, washed with phosphate-buffered saline, centrifuged again and resuspended in 500 μl of 0.4% paraformaldehyde. Fluorescence was analysed on a FACSCalibur flow cytometer, and data were analysed using CellQuest software (both from Becton Dickinson, San Jose, CA, USA). In the low-temperature uptake experiment, cells were preincubated at 4 °C for 1 h in Opti-MEM I prior to adding the PTDs, and all buffers and solutions were equilibrated to 4 °C. To analyse the internalization mechanism, HeLa cells were pretreated for 30 min in Opti-MEM I medium with 0–5 mM methyl-β-cyclodextrin, 0–2.5 μM cytochalasin D or 0–5 mM amiloride (all from Sigma-Aldrich, St Louis, MO, USA). After the PTD-FAMs were added, cells were maintained for 1 h (30 min for amiloride) in the presence of inhibitors and washed several times with phosphate-buffered saline. As a control, the cellular uptake of transferrin fluorescein isothiocyanate (Invitrogen) was also monitored.
Cell proliferation assay
Cell viability was determined using a WST-8 assay kit (Nacalai Tesque, Kyoto, Japan) according to the manufacturer's instructions. The assay is based on the cleavage of the tetrazolium salt WST-8 to formazan by cellular mitochondrial dehydrogenase. HeLa cells were cultured in 96-well plates (Nalge Nunc International) at 5.0 × 103 cells per well in MEMα and incubated for 24 h at 37 °C. Jurkat cells were cultured in 96-well plates at 1.0 × 104 cells per well in Dulbecco's modified Eagle's medium. The cells were treated with various concentrations of PTD-biotin. After 24 h incubation, cell viability was measured using the WST-8 assay kit.
Membrane integrity assay
The lactate dehydrogenase (LDH) leakage assay was used to quantify the membrane integrity of the PTD-treated cells. This assay detects the amount of LDH released into the culture media as a result of plasma membrane disruption after PTD treatment. HeLa cells were cultured in 96-well plates (Nalge Nunc International) at 5.0 × 103 cells per well in MEMα and incubated for 24 h at 37 °C. Jurkat cells were cultured in 96-well plates at 1.0 × 104 cells per well in Dulbecco's modified Eagle's medium. Each cell type was treated with various concentrations of PTD-biotin. After 3 h incubation, the LDH release activity of the peptides was measured using an LDH cytotoxicity test (Wako) according to the manufacturer's instructions.
Expression and purification of PTD-fused Venus protein
The Venus (variant of yellow fluorescent protein) DNA sequence was kindly provided by Dr A Miyawaki (RIKEN Brain Science Institute). The Tat-Venus DNA sequence was amplified by PCR. At the 5′ end, the primer sequence 5′-TTTAAGAAGGAGATATACATATGGCTTACGGTCGTAAAAAACGTCGCCAGCGTCGCCGTGGTGGCGGCGGTTCCCTCGAGCACCACCATCACCACCATGTGAGCAAGGGCGAGGAGCTGTTCAC-3′ introduced an Nde I site and Tat sequence and at the 3′ end, the primer sequence 5′-GCTTTGTTAGCAGCCGAATTCTTACTTGTACAGCTCGTCCATGCCGAGAGTGATC-3′ introduced an Eco RI site. The PCR product was digested with Nde I and Eco RI and inserted into a protein expression plasmid. Other plasmids expressing Antp-, Rev- or VP22-Venus recombinant proteins were constructed by replacing the Tat-coding region in the Tat-Venus plasmid with the Antp, Rev or VP22 sequences using the Nde I and Xho I restriction sites. These sequences were obtained by annealing the following oligonucleotides with protruding single-strand DNA corresponding to the Nde I and Xho I sites: Antp sense, 5′-TATGGCTCGTCAGATCAAAATCTGGTTCCAGAATCGTCGTATGAAGTGGAAAAAAGGTGGCGGCGGTTCCC-3′; Antp antisense, 5′-TCGAGGGAACCGCCGCCACCTTTTTTCCACTTCATACGACGATTCTGGAACCAGATTTTGATCTGACGAGCCA-3′; Rev sense, 5′-TATGGCTACCCGTCAGGCTCGTCGTAATCGTCGTCGTCGTTGGCGTGAACGTCAGCGTGGTGGCGGCGGTTCCC-3′; Rev antisense, 5′-TCGAGGGAACCGCCGCCACCACGCTGACGTTCACGCCAACGACGACGACGATTACGACGAGCCTGACGGGTAGCCA-3′; VP22 sense, 5′-TATGGCTAACGCTAAAACCCGTCGTCACGAACGTCGTCGTAAACTGGCTATCGAACGTGGTGGCGGCGGTTCCC-3′; VP22 antisense, 5′-TCGAGGGAACCGCCGCCACCACGTTCGATAGCCAGTTTACGACGACGTTCGTGACGACGGGTTTTAGCGTTAGCCA-3′. The plasmids, except for the Antp-Venus expression vector, were transformed into Escherichia coli BL21 Star (DE3) (Invitrogen). The Antp-Venus-expressing vector was transformed into BL21 Star (DE3), in which the plasmid-expressing chaperone (pGro7) was pretransformed. Transformed E. coli was cultured and the cell paste was suspended in BugBuster Master Mix (Novagen, Darmstadt, Germany) and centrifuged. PTD-Venus was recovered in the supernatant and purified by His-tag affinity purification and gel filtration chromatography.
Confocal laser scanning microscopy analysis
HeLa cells were cultured on Lab-Tek II Chambered Coverglass (Nalge Nunc International) at 3.0 × 104 cells per well in MEMα supplemented with 10% FBS and incubated for 24 h at 37 °C. Internalization of PTD-FAM or PTD-Venus was performed as follows: HeLa cells were treated with PTD-FAM or PTD-Venus (10 μM) in Opti-MEM I containing 100 ng ml−1 Hoechst 33342 (Invitrogen) and 6 μg ml−1 FM4-64 (Invitrogen). After incubation at 37 °C for 3 h, the medium was exchanged with fresh medium and fluorescence was observed by confocal laser scanning microscopy (Leica Microsystems GmbH, Wetzlar, Germany) without cell fixation. For cotreatment with HA2-Tat, HeLa cells were cotreated with PTD-FAM (10 μM) and HA2-Tat (2 μM) in Opti MEM I containing 100 ng ml−1 Hoechst 33342. After incubation at 37 °C for 3 h, the medium was exchanged with fresh medium and fluorescence was observed by confocal laser scanning microscopy without cell fixation.
Results
Comparison of transduction efficiency and cytotoxicity of four PTDs
To confirm the intracellular translocation activity of the four selected PTDs, we evaluated the transduction efficiency of Tat-, Antp-, Rev- and VP22-FAM in six cell lines (adherent: HeLa, HaCaT and A431 cells; nonadherent: Jurkat, MOLT-4 and HL60 cells) using flow cytometric analysis (Figure 1). These PTDs contain a large number of basic amino acids (Table 1) and their cationic properties are thought to be important for cell membrane penetration (Futaki et al., 2001; Chauhan et al., 2007). Their positive charge, however, causes them to adsorb nonspecifically to negatively charged cell membranes (Richard et al., 2003). For this reason, the cells were treated with excess trypsin to eliminate nonspecific plasma membrane binding of the PTDs prior to measurement.
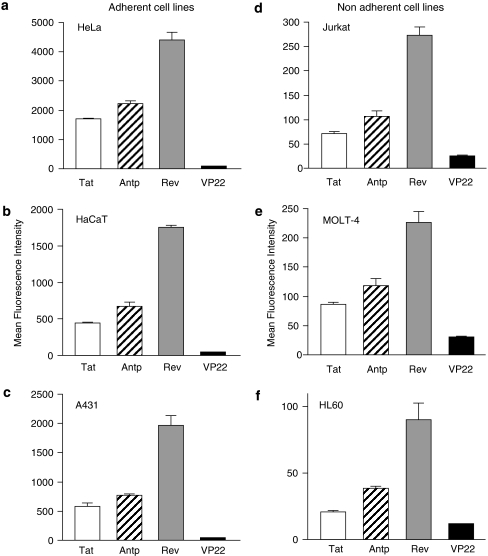
Figure 1.
Comparison of the cellular uptake of protein transduction domains (PTDs). FAM-labelled Tat (white column), antennapedia (Antp; hatched column), Rev (grey column) and VP22 (black column) were incubated with six cell lines: HeLa (a), HaCaT (b), A431 (c), Jurkat (d), MOLT-4 (e) and HL60 (f) at 10 μM for 3 h. After trypsin treatment to digest PTDs adsorbed on the cell surface, the PTD-transduced cells were harvested and analysed by flow cytometry. Note that the y axis scales for the adherent cell lines are markedly different from that for the nonadherent cell lines. Data shown are the mean±s.d. of triplicate assays.
The relative order of their translocation efficiency (Rev>Antp>Tat>VP22), which was based on mean fluorescence, was independent of the cell type (that is, adherent or nonadherent). Furthermore, using PTD-fused Venus, we confirmed that Rev had the highest transduction efficiency (data not shown). Equally important, the overall translocation efficiency of the PTDs depended markedly on whether the cells were adherent (HeLa, HaCaT and A431 cells) or nonadherent (Jurkat, MOLT-4 and HL60 cells). The transduction efficiency was much higher in the adherent cell lines compared with the nonadherent cell lines (Figure 1); note that that the fluorescence (uptake) was about 8- to 25-fold greater in the adherent, than in nonadherent, cell lines.
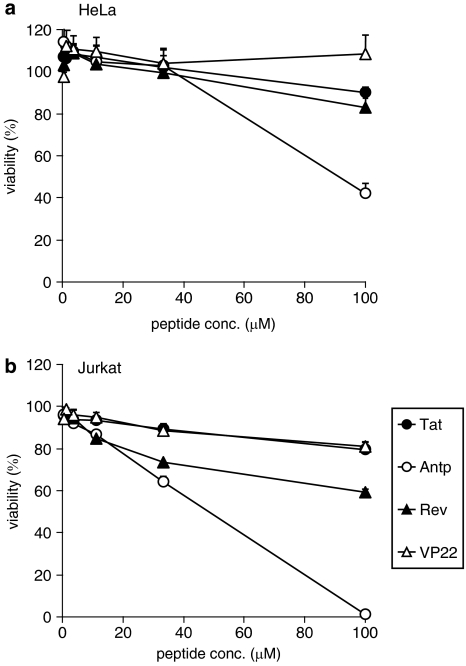
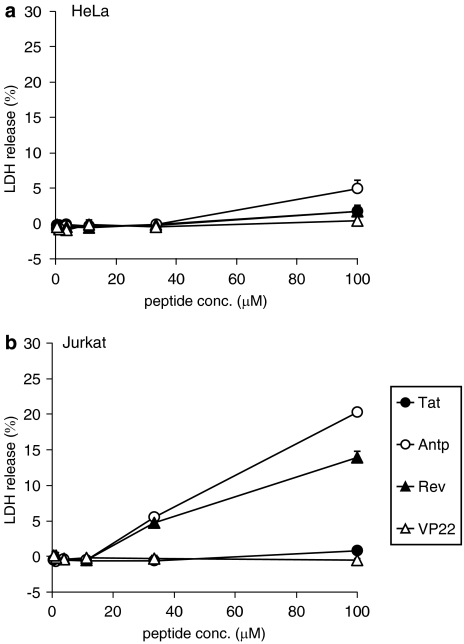
The cytotoxic properties of the four PTDs were evaluated in adherent (HeLa) and nonadherent (Jurkat) cells. To assess the long-term changes in proliferation, mitochondrial dehydrogenase activity was measured using a WST-8 assay 24 h after PTD treatment. In HeLa cells, there was a remarkable decrease in cell viability when the cells were incubated with Antp at 100 μM, whereas other PTDs were not cytotoxic at the higher concentrations (Figure 2a). In contrast in Jurkat cells, Antp was extremely cytotoxic in a dose-dependent manner and Rev reduced cell proliferation by approximately 40% (Figure 2b). Previous reports indicated that amphipathic peptides, such as transportan, induced cytotoxicity by perturbing the cellular membrane (Hallbrink et al., 2001; Jones et al., 2005; Saar et al., 2005; El-Andaloussi et al., 2007). Thus, the membrane integrity of PTD-treated cells was also measured using an LDH leakage assay. Antp and Rev induced significant LDH leakage in Jurkat cells, but only low LDH leakage was detected in Antp-treated HeLa cells (Figure 3). The membrane-perturbing effect of Antp and Rev contributed to the uptake of peptides, which are shown in Figure 1. Jurkat cells appear more sensitive to Antp or Rev treatment than HeLa cells; this difference in cytotoxicity and translocation efficiency may indicate a difference in the PTD-uptake mode.
Figure 2.
Viability of protein transduction domain (PTD)-treated cells. HeLa cells (a) and Jurkat cells (b) were incubated with serially diluted biotin-conjugated Tat, antennapedia (Antp), Rev and VP22 at 37 °C. After 24 h, cell viability was analysed using a WST-8 assay. Data shown are the mean±s.d. of triplicate assays.
Figure 3.
Membrane integrity of protein transduction domain (PTD)-treated cells. HeLa cells (a) and Jurkat cells (b) were incubated with serially diluted biotin-conjugated Tat, antennapedia (Antp), Rev and VP22 at 37 °C. After 3 h, the release of lactate dehydrogenase (LDH) was analysed. Data shown are the mean±s.d. of triplicate assays.
Intracellular transduction mechanism of PTDs
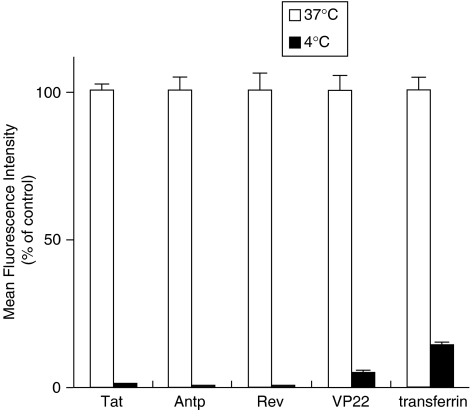
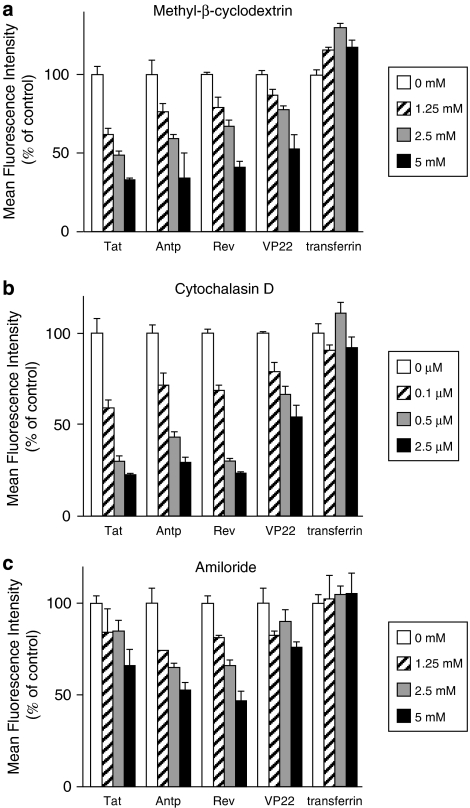
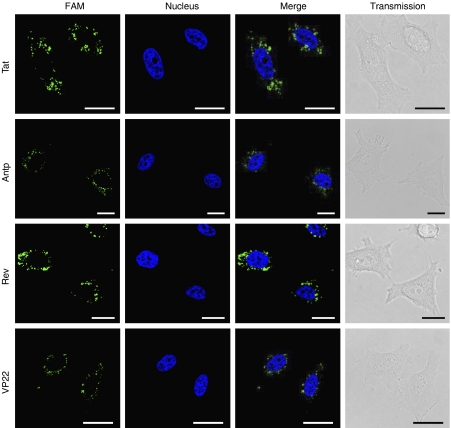
The results of in vitro studies suggest that PTDs enter the cell via an energy-dependent endocytotic pathway (Lundberg et al., 2003; Richard et al., 2003). In particular, studies using various macropinocytosis inhibitors, such as methyl-β-cyclodextrin, to deplete cholesterol from the membrane (Grimmer et al., 2002; Liu et al., 2002), cytochalasin D, to inhibit F-actin elongation (Sampath and Pollard, 1991), or amiloride, to inhibit the Na+-H+ exchanger (West et al., 1989), indicate that Tat is taken up into the cell via lipid raft-dependent macropinocytosis. To the best of our knowledge, however, few comparative studies have analysed the cellular uptake pathway of the four PTDs discussed in this paper. Therefore, we used flow cytometry analysis to determine whether PTD uptake is energy dependent or occurs via lipid raft-mediated macropinocytosis. First, we treated cells with PTD-FAM at 37 or 4 °C and then measured cell fluorescence (Figure 4). At 4 °C, transferrin, which enters cells by clathrin-dependent endocytosis (Schmid, 1997), inhibited the transduction efficiency compared with that at 37 °C. All four PTDs had low transduction ability at 4 °C, indicating that their cellular uptake was energy dependent. We next examined the PTD-FAM uptake efficiency in methyl-β-cyclodextrin-, cytochalasin D- and amiloride-treated HeLa cells. These cell treatments inhibited PTD-FAM incorporation in a dose-dependent manner, but transferrin was not affected (Figure 5). Furthermore, in HeLa cells treated with PTD-FAM, only punctuate fluorescence was observed using confocal laser scanning microscopic analysis (Figure 6). These results indicated that all the PTDs evaluated in this study enter the cell through the macropinocytotic pathway and that most of them were trapped in intracellular vesicles, the macropinosomes.
Figure 4.
Effects of temperature on protein transduction domain (PTD) transduction efficiency. HeLa cells were preincubated at 37 or 4 °C for 1 h prior to adding FAM-labelled PTDs or fluorescein isothiocyanate-labelled transferrin for 3 h. Cells were washed in trypsin and analysed by flow cytometry. Data shown are the mean±s.d. of triplicate assays.
Figure 5.
Effects of endocytosis inhibitors on transduction efficiency of protein transduction domains (PTDs). HeLa cells were pretreated with a range of concentrations of (a) methyl-β-cyclodextrin, (b) cytochalasin D or (c) amiloride for 30 min prior to adding FAM-labelled PTDs or fluorescein isothiocyanate-labelled transferrin for 1 h (a and b) or 30 min (c). Cells were washed in trypsin and analysed by flow cytometry. Data shown are the mean±s.d. of triplicate assays.
Figure 6.
Intracellular behaviour of protein transduction domain (PTD)-FAM in living cells. HeLa cells were treated with 10 μM PTD-FAM for 3 h. Fluorescence images were acquired using confocal laser scanning microscopy and the signals were merged electronically. The nucleus was counterstained with Hoechst 33342 (blue). From top to bottom: Tat-, antennapedia (Antp)-, Rev- and VP22-FAM. From left to right: FAM (green), nucleus (blue), merged fluorescence and transmission image. Scale bars in each microphotograph indicate 20 μm.
Intracellular localization of PTD–protein conjugates
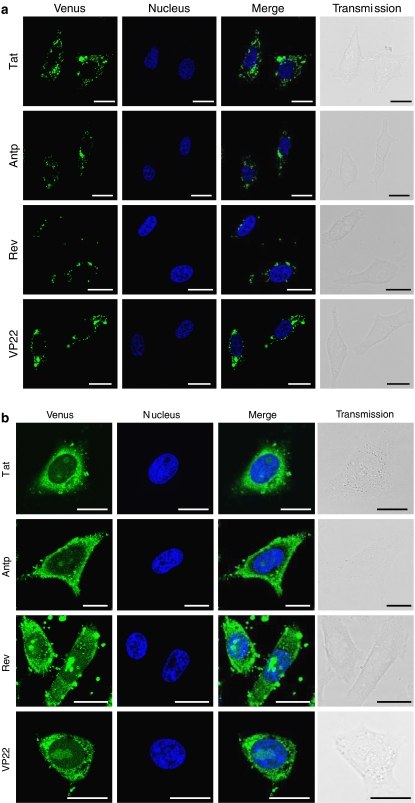
We next examined the intracellular behaviour of the individual PTDs in more detail. To investigate whether individual PTDs are located in the same vesicles, we used Tat-fused HA2 peptide (HA2-Tat), an influenza virus-derived endosome-disrupting peptide. HA2-Tat improves the activity of Tat-fused Cre recombinase (Wadia et al., 2004). Because HA2 alone cannot enter the cell, HA2-Tat is thought to enter the cell in a Tat-dependent manner and to disrupt the membrane of endosomal vesicles in which the Tat cargo is trapped. Thus, if Antp, Rev and VP22 are trapped in the same vesicles as Tat, the fluorescence should spread throughout the cytosol following cotreatment of the cells with HA2-Tat. As predicted, in HeLa cells cotreated with Antp-, Rev- or VP22-Venus and HA2-Tat, the Venus-derived fluorescence spread throughout the cytosol, whereas in the cells treated with Antp-, Rev- or VP22-Venus alone, only punctuate fluorescence was observed (Figure 7). These results suggested that all the PTDs evaluated in this study entered the cell through a macropinocytotic pathway and were trapped in the same vesicles as Tat.
Figure 7.
Intracellular behaviour of protein transduction domain (PTD)-Venus in living cells. HeLa cells were treated with 10 μM PTD-Venus alone (a) or 10 μM HA2-Tat (b) for 3 h. Fluorescence images were acquired using confocal laser scanning microscopy and the signals were merged electronically. The nucleus was counterstained with Hoechst 33342 (blue). From top to bottom: Tat-, antennapedia (Antp)-, Rev- and VP22-Venus. From left to right: Venus (green), nucleus (blue), merged fluorescence and transmission image. Scale bars in each microphotograph indicate 20 μm.
Discussion
In the present study, we have systematically compared PTD-mediated molecular transduction mechanisms. Our findings indicated that individual PTDs have different levels of transduction efficiency and cytotoxicity, suggesting that PTDs are internalized into live cells via different mechanisms. We also examined the internalization pathway and intracellular localization of Tat, Antp, Rev and VP22. Unexpectedly, all the PTDs evaluated in this study entered the cell through the macropinocytotic pathway and were trapped in the same vesicles as Tat. The finding that the intracellular transduction pathways of the four PTDs were the same suggests that the method of cell internalization does not contribute to the differences in the PTD transduction efficiency or cytotoxicity. Although the reason for this phenomenon is not clear, we speculate that the primary structure of the individual PTDs or the cell surface proteins that interact with the individual PTDs contribute to the differences in their transduction efficiency and cytotoxicity.
The initial step in the mechanism of cellular entry of PTDs is thought to be the strong ionic interaction between the amino-acid residues of the PTDs and the plasma membrane constituents. Because the translocation is solely physically mediated, the charge distribution and amphipathicity of the peptide and its interaction with the plasma membrane is critical (Pujals et al., 2006). Although most PTDs, if not all, contain a large number of basic amino acids, such as arginine or lysine, the theoretical isoelectric point (pI) value of each PTD used in this study was essentially identical (Tat, Antp, Rev and VP22 have pI values of 12.70, 12.31, 12.60 and 12.01, respectively). Therefore, the internalization efficiency does not appear to depend on the cationic features of the PTDs.
The amphipathicity of the carrier is probably responsible not only for the strong interaction with the lipid membranes (Yandek et al., 2007), but also for the disruption of the cellular membrane, which results in cell death (Hallbrink et al., 2001; Jones et al., 2005; Saar et al., 2005; El-Andaloussi et al., 2007). In terms of cytotoxicity, our data indicate that Antp and Rev both disrupt the membrane (Figure 3), but Rev does not contain an amphipathic structure. Furthermore, there was no correlation between hydrophobicity and transduction efficiency. Thus, differences in the PTD-mediated transduction efficiency and cytotoxicity might be due to the molecular weight or pI of the conjugated cargo.
The cellular events required for internalization, however, differ between reports and are often conflicting. The first mechanistic studies led to the proposal that PTD internalization occurs rapidly in a receptor- and energy-independent manner, perhaps by destabilizing the lipid bilayer or by the formation of inverted micelles with subsequent release of their contents within the intracellular space (Berlose et al., 1996). More recently, an active mechanism based on vesicular uptake was proposed as the general mode of cell internalization of PTDs. In our experiment, although all four PTDs tended to be present in the same vesicles, the detailed mechanism for this colocalization is not yet known. It has been suggested that PTD internalization requires cell surface heparan sulphate proteoglycans (Tyagi et al., 2001; Console et al., 2003; Ziegler and Seelig, 2004). Because Tat interacts electrostatically with heparan sulphate proteoglycan present on the cell surface, it is possible that some PTDs are taken into the same vesicles when they interact with one heparan sulphate proteoglycan. In contrast, as shown in Figure 7, although fluorescence was observed throughout the cytosol, punctate fluorescence was also observed when the cells were cotreated with PTD-Venus and HA2-Tat. This finding suggested that the PTDs did not all exist in the same vesicles and that some PTDs entered the cell through another pathway. This is just speculation, however, and we are now using proteome analysis, such as liquid chromatography coupled with mass spectrometry or two-dimensional gel electrophoresis, to examine whether there are individual cell surface receptors for different PTDs.
In summary, our data suggest that Antp, Rev, VP22 and Tat cross the plasma membrane and reach the macropinosomes via different mechanisms. Our findings also indicate that several issues, such as endosome entrapment and low cell specificity, which limit the therapeutic activity of the cargo, must be overcome before effective PTD-based drug delivery carriers can be fully developed. We previously reported that cotreatment with HA2-Tat enhances the cytosolic release of Tat-fused peptide-blockers and their biological activities, thereby overcoming the issue of endosome entrapment (Sugita et al., 2007). Furthermore, although the transduction mechanism of PTDs is not yet well understood, these differences led us to explore the possibility of creating novel PTDs. We successfully created novel PTDs that have higher transduction efficiencies than Tat, using a unique phage display-based screening strategy that we previously developed (Mukai et al., 2006; Kamada et al., 2007). Moreover, based on our PTD-screening system, we are currently working to create more useful PTDs with cell type specificity.
Abbreviations
- PTD
protein transduction domain
- Antp
antennapedia
Conflict of interest
The authors state no conflict of interest.
References
- Berlose JP, Convert O, Derossi D, Brunissen A, Chassaing G. Conformational and associative behaviours of the third helix of antennapedia homeodomain in membrane-mimetic environments. Eur J Biochem. 1996;242:372–386. doi: 10.1111/j.1432-1033.1996.0372r.x. [DOI] [PubMed] [Google Scholar]
- Borsello T, Forloni G. JNK signalling: a possible target to prevent neurodegeneration. Curr Pharm Des. 2007;13:1875–1886. doi: 10.2174/138161207780858384. [DOI] [PubMed] [Google Scholar]
- Brusic V, Marina O, Wu CJ, Reinherz EL. Proteome informatics for cancer research: from molecules to clinic. Proteomics. 2007;7:976–991. doi: 10.1002/pmic.200600965. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Tikoo A, Kapur AK, Singh M. The taming of the cell penetrating domain of the HIV Tat: myths and realities. J Control Release. 2007;117:148–162. doi: 10.1016/j.jconrel.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Console S, Marty C, Garcia-Echeverria C, Schwendener R, Ballmer-Hofer K. Antennapedia and HIV transactivator of transcription (TAT) ‘protein transduction domains' promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. J Biol Chem. 2003;278:35109–35114. doi: 10.1074/jbc.M301726200. [DOI] [PubMed] [Google Scholar]
- Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- Drabik A, Bierczynska-Krzysik A, Bodzon-Kulakowska A, Suder P, Kotlinska J, Silberring J. Proteomics in neurosciences. Mass Spectrom Rev. 2007;26:432–450. doi: 10.1002/mas.20131. [DOI] [PubMed] [Google Scholar]
- El-Andaloussi S, Jarver P, Johansson HJ, Langel U. Cargo dependent cytotoxicity and delivery efficacy of cell-penetrating peptides: a comparative study. Biochem J. 2007;407:285–292. doi: 10.1042/BJ20070507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott G, O'Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- Ferrari A, Pellegrini V, Arcangeli C, Fittipaldi A, Giacca M, Beltram F. Caveolae-mediated internalization of extracellular HIV-1 tat fusion proteins visualized in real time. Mol Ther. 2003;8:284–294. doi: 10.1016/s1525-0016(03)00122-9. [DOI] [PubMed] [Google Scholar]
- Fittipaldi A, Ferrari A, Zoppe M, Arcangeli C, Pellegrini V, Beltram F, et al. Cell membrane lipid rafts mediate caveolar endocytosis of HIV-1 Tat fusion proteins. J Biol Chem. 2003;278:34141–34149. doi: 10.1074/jbc.M303045200. [DOI] [PubMed] [Google Scholar]
- Futaki S, Suzuki T, Ohashi W, Yagami T, Tanaka S, Ueda K, et al. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem. 2001;276:5836–5840. doi: 10.1074/jbc.M007540200. [DOI] [PubMed] [Google Scholar]
- Grimmer S, van Deurs B, Sandvig K. Membrane ruffling and macropinocytosis in A431 cells require cholesterol. J Cell Sci. 2002;115:2953–2962. doi: 10.1242/jcs.115.14.2953. [DOI] [PubMed] [Google Scholar]
- Hallbrink M, Floren A, Elmquist A, Pooga M, Bartfai T, Langel U. Cargo delivery kinetics of cell-penetrating peptides. Biochim Biophys Acta. 2001;1515:101–109. doi: 10.1016/s0005-2736(01)00398-4. [DOI] [PubMed] [Google Scholar]
- Han X, Bushweller JH, Cafiso DS, Tamm LK. Membrane structure and fusion-triggering conformational change of the fusion domain from influenza hemagglutinin. Nat Struct Biol. 2001;8:715–720. doi: 10.1038/90434. [DOI] [PubMed] [Google Scholar]
- Hawiger J. Noninvasive intracellular delivery of functional peptides and proteins. Curr Opin Chem Biol. 1999;3:89–94. doi: 10.1016/s1367-5931(99)80016-7. [DOI] [PubMed] [Google Scholar]
- Joliot A, Prochiantz A. Transduction peptides: from technology to physiology. Nat Cell Biol. 2004;6:189–196. doi: 10.1038/ncb0304-189. [DOI] [PubMed] [Google Scholar]
- Jones SW, Christison R, Bundell K, Voyce CJ, Brockbank SM, Newham P, et al. Characterisation of cell-penetrating peptide-mediated peptide delivery. Br J Pharmacol. 2005;145:1093–1102. doi: 10.1038/sj.bjp.0706279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada H, Okamoto T, Kawamura M, Shibata H, Abe Y, Ohkawa A, et al. Creation of novel cell-penetrating peptides for intracellular drug delivery using systematic phage display technology originated from Tat transduction domain. Biol Pharm Bull. 2007;30:218–223. doi: 10.1248/bpb.30.218. [DOI] [PubMed] [Google Scholar]
- Kaplan IM, Wadia JS, Dowdy SF. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J Control Release. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Liu NQ, Lossinsky AS, Popik W, Li X, Gujuluva C, Kriederman B, et al. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J Virol. 2002;76:6689–6700. doi: 10.1128/JVI.76.13.6689-6700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg M, Wikstrom S, Johansson M. Cell surface adherence and endocytosis of protein transduction domains. Mol Ther. 2003;8:143–150. doi: 10.1016/s1525-0016(03)00135-7. [DOI] [PubMed] [Google Scholar]
- Mukai Y, Sugita T, Yamato T, Yamanada N, Shibata H, Imai S, et al. Creation of novel protein transduction domain (PTD) mutants by a phage display-based high-throughput screening system. Biol Pharm Bull. 2006;29:1570–1574. doi: 10.1248/bpb.29.1570. [DOI] [PubMed] [Google Scholar]
- Murriel CL, Dowdy SF. Influence of protein transduction domains on intracellular delivery of macromolecules. Expert Opin Drug Deliv. 2006;3:739–746. doi: 10.1517/17425247.3.6.739. [DOI] [PubMed] [Google Scholar]
- Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, et al. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med. 1998;4:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- Nori A, Kopecek J. Intracellular targeting of polymer-bound drugs for cancer chemotherapy. Adv Drug Deliv Rev. 2005;57:609–636. doi: 10.1016/j.addr.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Pujals S, Fernandez-Carneado J, Lopez-Iglesias C, Kogan MJ, Giralt E. Mechanistic aspects of CPP-mediated intracellular drug delivery: relevance of CPP self-assembly. Biochim Biophys Acta. 2006;1758:264–279. doi: 10.1016/j.bbamem.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Rhodes DR, Chinnaiyan AM. Integrative analysis of the cancer transcriptome. Nat Genet. 2005;37 Suppl:S31–S37. doi: 10.1038/ng1570. [DOI] [PubMed] [Google Scholar]
- Richard JP, Melikov K, Brooks H, Prevot P, Lebleu B, Chernomordik LV. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. J Biol Chem. 2005;280:15300–15306. doi: 10.1074/jbc.M401604200. [DOI] [PubMed] [Google Scholar]
- Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, et al. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- Rojas M, Donahue JP, Tan Z, Lin YZ. Genetic engineering of proteins with cell membrane permeability. Nat Biotechnol. 1998;16:370–375. doi: 10.1038/nbt0498-370. [DOI] [PubMed] [Google Scholar]
- Saar K, Lindgren M, Hansen M, Eiriksdottir E, Jiang Y, Rosenthal-Aizman K, et al. Cell-penetrating peptides: a comparative membrane toxicity study. Anal Biochem. 2005;345:55–65. doi: 10.1016/j.ab.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Sampath P, Pollard TD. Effects of cytochalasin, phalloidin, and pH on the elongation of actin filaments. Biochemistry. 1991;30:1973–1980. doi: 10.1021/bi00221a034. [DOI] [PubMed] [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Hruska KA, Dowdy SF. Protein transduction: unrestricted delivery into all cells. Trends Cell Biol. 2000;10:290–295. doi: 10.1016/s0962-8924(00)01771-2. [DOI] [PubMed] [Google Scholar]
- Skehel JJ, Cross K, Steinhauer D, Wiley DC. Influenza fusion peptides. Biochem Soc Trans. 2001;29:623–626. doi: 10.1042/bst0290623. [DOI] [PubMed] [Google Scholar]
- Sugita T, Yoshikawa T, Mukai Y, Yamanada N, Imai S, Nagano K, et al. Improved cytosolic translocation and tumor-killing activity of Tat-shepherdin conjugates mediated by co-treatment with Tat-fused endosome-disruptive HA2 peptide. Biochem Biophys Res Commun. 2007;363:1027–1032. doi: 10.1016/j.bbrc.2007.09.077. [DOI] [PubMed] [Google Scholar]
- Tyagi M, Rusnati M, Presta M, Giacca M. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J Biol Chem. 2001;276:3254–3261. doi: 10.1074/jbc.M006701200. [DOI] [PubMed] [Google Scholar]
- Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- West MA, Bretscher MS, Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J Cell Biol. 1989;109:2731–2739. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yandek LE, Pokorny A, Floren A, Knoelke K, Langel U, Almeida PF. Mechanism of the cell-penetrating peptide transportan 10 permeation of lipid bilayers. Biophys J. 2007;92:2434–2444. doi: 10.1529/biophysj.106.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler A, Seelig J. Interaction of the protein transduction domain of HIV-1 TAT with heparan sulfate: binding mechanism and thermodynamic parameters. Biophys J. 2004;86:254–263. doi: 10.1016/S0006-3495(04)74101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]