Abstract
Background and purpose:
Our previous study showed that SM905, a novel artemisinin derivative, exhibited potent immunosuppressive activity. In this study, we evaluate preventive and therapeutic effect of SM905 on collagen-induced arthritis (CIA) in DBA/1 mice, and investigate its mechanisms both in inflammatory and autoimmune aspects of the disease.
Experimental approach:
CIA was induced by type II bovine collagen (CII) in DBA/1 mice. SM905 was given orally either before (continuously 1 day before booster immunization) or after disease onset (continuously 14 days after booster immunization). Disease incidence and severity were monitored, mRNA expression of proinflammatory mediators was determined by real-time PCR, purified T cell proliferation was assessed using [3H]-thymidine incorporated assay, and T helper (Th) 17/Th1/Th2 type cytokine production was examined by ELISA.
Key results:
Oral treatment with SM905 delayed disease onset, reduced arthritis incidence and severity, and suppressed the enhanced expression of pro-inflammatory cytokines, chemokines and chemokine receptors in draining lymph nodes. The CII-induced T cell proliferation and production of interleukin (IL)-17A by T cells were strikingly inhibited. Correspondingly, the mRNA expression of IL-17A and RORγt (a specific transcription factor for Th17) was also reduced. This effect was coupled with a striking reduction of IL-6 production, which has a critical role in Th17 development. In established arthritis, SM905 profoundly inhibited disease progression, reduced IL-17A and RORγt mRNA expression, and suppressed pro-inflammatory mediator expression in arthritic joints.
Conclusions and implications:
SM905 had beneficial effects on CIA by suppressing inflammatory and pathogenic Th17 responses.
Keywords: artemisinin derivative, SM905, collagen-induced arthritis, T cell, inflammation, Th17
Introduction
The antimalarial drug artemisinin is extracted from a Chinese traditional herb Artemisia annua L. (Li and Wu, 2003). Besides having antimalarial activity, artemisinin and its derivatives also exhibit potent immunosuppressive activity (Noori et al., 2004; Zhou et al., 2005; Wang et al., 2007a). In China, artemisinin derivatives have shown promising results when tested as a treatment for rheumatoid arthritis (RA) (Lu 2002; Ding and Hu, 2006). In animal models, artemisinin derivatives suppressed arthritis development through inhibition of nuclear factor-κB activation and proinflammatory cytokine production (Dong et al., 2003; Cuzzocrea et al., 2005; Li et al., 2006; Mirshafiey et al., 2006). Artemisinin derivatives may be developed as an alternative approach to the treatment of RA; however, the efficacy of clinically relevant artemisinin derivatives is limited. SM905 is a novel water-soluble artemisinin derivative identified by our laboratories from a series of new compounds derived from artemisinin, and it shows promising immunosuppressive activity both in vitro and in vivo (Yang et al., 2005, 2006; Wang et al., 2007b). In this study, we provide evidence for the preventive and therapeutic effect of SM905 on collagen-induced arthritis (CIA) and explore its mechanisms in two pathogenic aspects of CIA—inflammation and T-cell immune responses to CII—with a focus on proinflammatory T helper (Th) 17 responses.
RA is a common systemic disorder that is characterized by autoimmunity and chronic inflammation of multiple joints and CIA is a well-established animal model of RA (Myers et al., 1997). CIA is induced in genetically susceptible strains of mice by immunization with type II bovine collagen (CII). Although the effector mechanisms of inflammation ultimately result in pathogenic lesions of joints (inflammatory component of CIA), there is considerable evidence implicating CII-specific CD4+ T cells as primary mediators of disease induction (T-cell immunity component of CIA) (Brand et al., 2003). After antigenic stimulation, naïve CD4+ T cells develop into different types of helper T cells, including Th1, Th2 and Th17, each producing its own set of cytokines that mediate different functions. On the basis of our previous study showing that SM905 exerted a significant inhibitory effect on T-cell activation and proliferation (Wang et al., 2007b), we have further explored here the effect of SM905 on different types of Th cell responses in CIA by analysing the profile of Th1/Th2/Th17 type cytokines. It has been well documented that Th1 cells, which produce interferon (IFN)-γ and interleukin (IL)-2, are associated with the acute phase of CIA (Mauri et al., 1996; Brand et al., 2003), whereas the Th2 type cytokine IL-4 is associated with the remission phase of CIA (Mauri et al., 1996; Doncarli et al., 1997). In addition to Th1 and Th2, a new proinflammatory Th cell lineage, Th17, has been described recently. Th17 cells produce effector cytokines of IL-17 (or IL-17A), IL-6 and tumour necrosis factor (TNF)-α, and have been recently shown to have a crucial role in the immunopathogenesis of CIA (Weaver et al., 2007). Our study focuses on the effect of SM905 on Th17 response, as IL-17, a distinct effector cytokine of Th17, provides communication between the adaptive immune system and the innate immune system, to promote inflammation (Bi et al., 2007). IL-17 has multiple proinflammatory functions, including stimulating the expression of various proinflammatory cytokines and chemokines (Kolls and Lindén, 2004). This is supposed to contribute to severe synovitis, pannus formation and joint destruction in arthritic joints. Therefore, the inhibition of IL-17 production in T cells may be one of the mechanisms by which the new artemisinin derivative SM905 exerts its inhibitory effects in CIA.
The development of Th17 cells depends on their specific transcription factor, the orphan nuclear receptor RORγt (Ivanov et al., 2006). Thus, a Th17 cell population is absent from RORγt-deficient animals, and transduction of naive T cells with an RORγt-encoding retrovirus induces IL-17 production (Ivanov et al., 2006). De novo Th17 differentiation from naive CD4T cell precursor is contingent upon signals from cells of the innate immune system. IL-6, an acute-phase protein induced during inflammation, has been found to induce Th17 generation in the presence of transforming growth factor -β (Bettelli et al., 2006; Veldhoen et al., 2006) and Th17 cells are absent in mice deficient in IL-6 (Ivanov et al., 2006). Moreover, IL-6 development and that of Th17 appear to be tightly linked. Once Th17 cells are induced, IL-17 stimulates IL-6 production, which in turn leads to further differentiation of Th17 cells (Bettelli et al., 2007).
In this study, the effects of the new water-soluble artemisinin derivative, SM905, on the critical events during the initiation and establishment of arthritis were investigated in the CIA model. Our results show that SM905 had a marked protective effect on CIA, suggesting that this new water-soluble artemisinin derivative has a potential to be developed as a new type of treatment of RA.
Materials and methods
Mice
All animal procedures and experiments were carried out according to National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Bioethics Committee of Shanghai Institute of Materia Medica. Male DBA/1 mice (7–8 weeks old, 20–22 g) were obtained from Shanghai Laboratory Animal Center of Chinese Academy of Sciences, and housed in specific pathogen-free conditions.
Induction and assessment of CIA
Bovine CII (Collagen Research Center, Tokyo, Japan) was dissolved in 0.1 M acetic acid and emulsified with Freund's complete adjuvant (Wako Pure Chemical Industries Ltd, Osaka, Japan). Mice were immunized with 0.05 ml of emulsion containing 125 μg of collagen at the tail base, and boosted (as day 0), with the same preparations of collagen plus Freund's complete adjuvant, 21 days after the primary immunization as described previously (Zhou et al., 2006). The severity of arthritis was measured without knowledge of the treatments, by scoring each limb from 0 to 4 grades and by summing up the scores of four limbs: 0=normal; 1=erythema or swelling of one or several digits; 2=erythema and moderate swelling extending from the ankle to the mid-foot (tarsals); 3=erythema and severe swelling extending from the ankle to the metatarsal joints; and 4=complete erythema and swelling encompassing the ankle, foot and digits, resulting in deformity and/or ankylosis. The maximum score for each animal is thus 16.
Drug treatment
SM905, (1-(12β-dihydroartemisinoxy)-2-hydroxy-3-tert-butylaminopropane maleate; C26H43NO10; MW=530; 99% purity), was synthesized and provided by Laboratory of Synthetic Chemistry, Shanghai Institute of Materia Medica. Saline and SM905 (0.25 or 0.5 mg kg−1) was orally administered once daily beginning 1 day before or alternatively 14 days after booster immunization till the end of the experiment.
Histological analysis
On day 28, the mice were killed and the hind paws were collected, fixed with 10% buffered formalin and then decalcified in 5% formic acid and embedded in paraffin. Sections (5 μm) of whole hind paws were stained with haematoxylin and eosin for microscopic evaluation. Histopathological changes were scored (without knowledge of the treatments) using the previously reported parameters (Zhou et al., 2006): 0=normal joint structure; 1=mild changes, synovitis and pannus front with few discrete cartilage focal erosions; 2=moderate changes, accompanying loss of large areas of cartilage, eroding pannus front and synovial hyperplasia with infiltrating mononuclear cells and polymorphonuclear cells; 3=severe synovitis, cartilage and bone erosion; and 4=total destruction of joint architecture.
Cell preparation
B-cell-depleted inguinal and axillary draining lymph node (DLN) cells, purified T cells and enriched antigen-presenting cells (APCs) were prepared as described previously (Wang et al., 2007a). Briefly, B-cell-depleted DLN cells were obtained by using magnetic particles bound to goat anti-mouse Ig (Polysciences Inc., Eppelheim, Germany) to deplete the B cells; T cells were purified from DLN cells using immunomagnetic negative selection to deplete the surface Ig+ cells (B cells) and I-A+ APCs. The purity of the resulting T-cell populations was examined by flow cytometry antibody (BD Biosciences Pharmingen, San Diego, CA, USA), and was consistently >95%; Splenic APC-enriched populations were separated using immunomagnetic negative selection to deplete B cells and T cells. The resulting purified splenic APC-enriched populations contained less than 1% of T and B cells.
Real-time PCR analysis
B-cell-depleted DLN cells, T lymphocytes or joint homogenates were lysed using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and then reverse transcribed. Relative quantification with real-time PCR was performed with SYBR Green PCR Reagents (Qiagen, Valencia, CA, USA) and a Continuous Fluorescence Detection System (MJ Research Inc., Waltham, MA, USA) as previously described (Wang et al., 2007b). The mRNA expression was normalized to that of hypoxanthine-guanine phosphoribosyltransferase for each sample, and the fold changes of each gene against those of normal mice were calculated and are shown in Results.
Measurement of T-cell immune responses to CII
Purified T cells (4 × 106 ml−1) from the DLN of CIA mice were cocultured with 30 Gy γ-irradiated APC-enriched cells (1 × 106 ml−1) from splenocytes of normal mice in 96-well flat-bottomed plates for 24, 48, 72 and 96 h with or without 10 μg ml−1 CII. Cells were pulsed with 0.25 μCi per well of -thymidine for 8 h and harvested onto glass fibre filters. The incorporated radioactivity was counted by using a Beta Scintillation Counter (MicroBeta Trilux, PerkinElmer Life Sciences, Boston, MA, USA).
For cytokines, T cells (4 × 106 ml−1) were cocultured with irradiated APC-enriched cells (1 × 106 ml−1) with or without 10 μg ml−1 CII for 48 h in triplicate in 96-well flat-bottomed plates. Cytokine levels in culture supernatants were determined by ELISA (BD PharMingen, San Diego, CA, USA) following the manufacturer's instructions.
Statistical analysis
Data are shown as means±s.e.mean and were analysed using Student's t-test and one-way ANOVA with Newman–Keuls multiple comparisons on post tests. Nonparametric data (arthritis severity score, histological score and gene expression) were analysed using Mann–Whitney U-test; P<0.05 was considered to be statistically significant.
Results
Delay and attenuation of CIA by oral administration of SM905
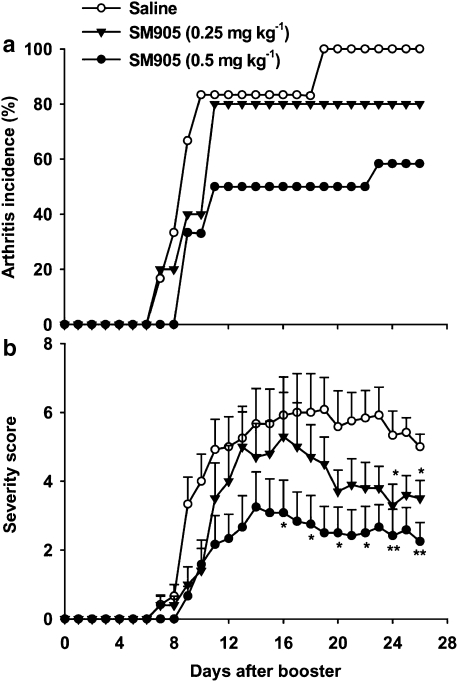
To investigate the effect of SM905 in the progression of CIA, oral administration of SM905 (0.25 and 0.5 mg kg−1) was initiated 1 day before induction of CIA. The doses of SM905 were based on our earlier results using ovalbumin-immunized mice (Wang et al., 2007b). Mice treated with 0.5 mg kg−1 SM905 showed significant reductions in the incidence and severity of CIA (Figure 1). On day 14, when arthritis signs reached their peaks, the arthritis incidence and mean severity score for the 0.5 mg kg−1 SM905 group were 6/12 and 3.3±0.8, respectively, as opposed to 10/12 and 5.7±0.9 for the saline group. At the lower dosage of 0.25 mg kg−1, SM905 exhibited a milder inhibitory effect than with the higher dose, but this treatment significantly suppressed arthritis severity scores only after the acute phase of CIA (Figure 1b). Oral treatment with SM905 at 0.5 mg kg−1 also delayed the onset of disease. Clinical signs of arthritis were observed beginning on day 9 in the 0.5 mg kg−1 SM905 group versus day 7 in the saline control group (Figure 1a). SM905 has low toxicity in vivo for mice with LD50 (medial lethal dose) values of 1.35–1.55 g kg−1 after oral administration (Wang et al., 2007b). To investigate the underlying mechanisms of the effects of SM905 in CIA, all subsequent experiments were performed with the 0.5 mg kg−1 dose of SM905.
Figure 1.
Effects of SM905 on the incidence and severity of CIA. Mice were immunized with CII in CFA and boosted 21 days later to induce CIA (see Materials and methods). Saline or SM905 (0.25 or 0.5 mg kg−1) was given orally 1 day before booster immunization (day −1) and then once daily till the end of the experiment. Arthritis incidence (a) and severity score (b) were assessed from all animals in each group (n=10–12). Severity scores were expressed as mean±s.e.m. for each group for each time point, *P<0.05, **P<0.01 (Mann–Whitney U-test), versus saline-treated CIA group. CFA; Freund's complete adjuvant; CIA, collagen-induced arthritis; CII, type II bovine collagen.
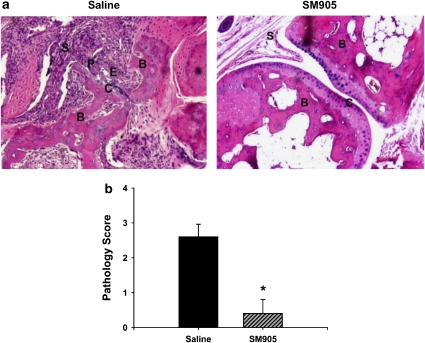
Histopathological analysis was performed on hind paws of CIA mice harvested on day 28 by an investigator without knowledge of the treatments. Representative images of haematoxylin- and eosin-stained joint tissue sections from the groups treated with saline or 0.5 mg kg−1 SM905 are presented in Figure 2a. Treatment with SM905 resulted in significant reductions in cellular infiltration, pannus formation and destruction of cartilage and bone in arthritic joints, with a low pathogenic score of CIA mice (Figure 2b). Thus, oral treatment with SM905 was effective in preventing CIA, based on both clinical and histopathological analyses.
Figure 2.
Preventive effect of SM905 on the histological damage in CIA mice. The hind paws were prepared for histopathological examination on day 28. (a) Representative section of joint histopathology from saline-treated mice (left) and SM905-treated mice (right) are shown (haematoxylin and eosin stained; magnification × 25). Sections from mice with CIA and treated with saline showed cellular infiltration in synovium (S), robust pannus formation (P) and extensive articular cartilage (C) and bone (B) erosion (E). Sections from mice with CIA and treated with SM905 showed significantly reduced pathogenic changes and well-preserved articular cartilage and bone. (b) Pathology scores of each group were calculated and expressed as mean±s.e.m. (n=10). *P<0.05 (Mann–Whitney U-test), versus saline-treated CIA group. CIA, collagen-induced arthritis.
Effects of SM905 on proinflammatory mediator expression in draining lymph nodes
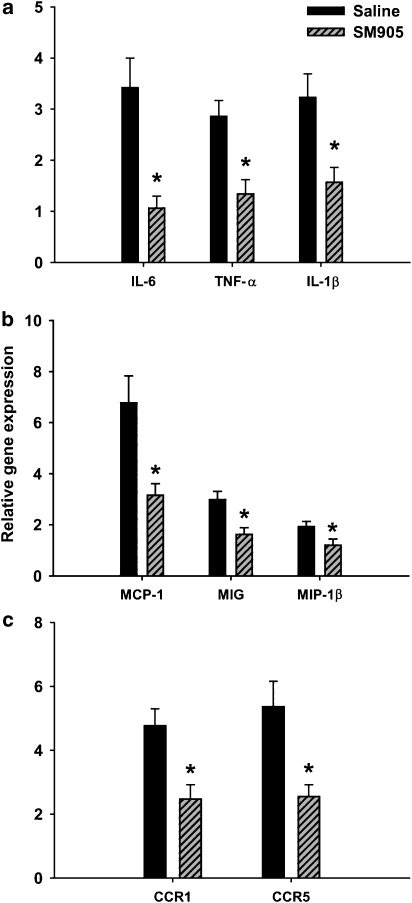
The involvement of a wide array of proinflammatory cytokines and chemokines has been reported in inflammation and in the development of arthritic responses (Veldhoen et al., 2006). To evaluate whether SM905 suppressed the effector mechanisms of inflammation in CIA, the arthritis-induced gene expression of various proinflammatory mediators in CIA mice was tested on day 14 by using real-time PCR. It was observed that the mRNA expression of three major proinflammatory cytokines, IL-6, TNF-α and IL-1β, was markedly reduced in the DLN of SM905-treated mice (Figure 3a). Consistent with these observations, the levels of several inducible chemokines, including monocyte chemoattractant protein-1, monokine induced by interferon γ, macrophage inflammatory protein-1β, chemokine receptor 1 and chemokine receptor 5, were all decreased after SM905 treatment (Figures 3b and c). The expression of two chemokines, macrophage inflammatory protein-1α and RANTES (regulated on activation normally T-cell expressed and secreted), was also observed; however, their mRNA expression was only moderately increased in DLN. Although in SM905-treated mice, there were relative reductions in their gene expression, these effects were not statistically significant (data not shown). Taken together, SM905 exerted a marked inhibitory effect on the systemic inflammatory response in DLN.
Figure 3.
Effects of SM905 on proinflammatory mediator expression in DLN. On day 14, total RNA from B-cell-depleted DLN cells (five mice per group) was extracted and reverse transcribed into cDNA. The relative gene expression of various cytokines was estimated using real-time PCR. Relative gene expression was normalized to HPRT, and the fold changes of gene expression in CIA mice against those of normal mice were presented. (a) The relative gene expression of IL-6, TNF-α and IL-1β. (b) The relative gene expression of monocyte chemoattractant protein-1, MIG and MIP-1β. (c) The relative gene expression of chemokine receptors CCR1 and CCR5. All the results were expressed as mean±s.e.m. of three separate experiments each consisting of 2–4 samples. *P<0.05 (Mann–Whitney U-test), versus saline-treated CIA group. CCR, chemokine receptor; CIA, collagen-induced arthritis; DLN, draining lymph node; HPRT, hypoxanthine-guanine phosphoribosyltransferase; MCP, monocyte chemoattractant protein; MIG, monokine induced by interferon γ; MIP, macrophage inflammatory protein.
Effects of SM905 on CII-induced T-cell immune responses
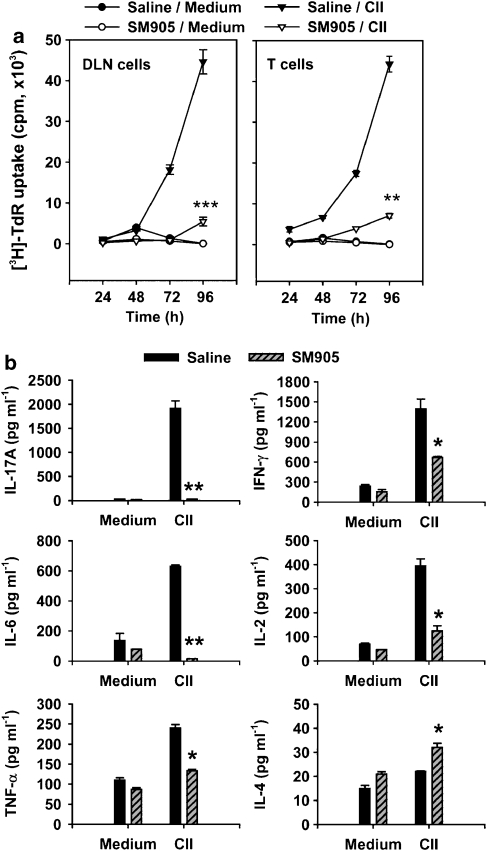
Experiments were performed to determine the ability of SM905 to affect the CII-induced proliferative response of B-cell-depleted DLN cells (containing T cells and APCs) as well as T cells purified from DLN. As shown in Figure 4a, B cell-depleted DLN cells and T cells exhibited an increasing recall response to CII at 24, 48, 72 and 96 h in ex vivo cultures. Treatment with SM905 significantly inhibited CII-induced responses in both sets of cells. Its effect on B-cell-depleted DLN cells paralleled the effect on T cells, suggesting that SM905 directly inhibited T cell responses in DLN.
Figure 4.
Effects of SM905 on Th17/Th1/Th2 responses. (a) B-cell-depleted DLN cells (five mice for each group) were stimulated with CII (10 μg ml−1). T cells purified (4 × 106 ml−1) from DLN (five mice per group) were stimulated with CII (10 μg ml−1) in the presence of 30 Gy γ-irradiated APC-enriched cells (1 × 106 ml−1) from normal mice. Cells were cultured in 96-well flat-bottomed plates in triplicate for 24, 48, 72 and 96 h, and [3H]-thymidine was added for the last 8 h of culture. (b) Cytokine levels in the supernatants of T-cell cultures at 48 h were examined using ELISA. All the results were expressed as mean±s.e.m. (n=3). *P<0.05, **P<0.01, ***P<0.001, versus saline-treated CIA group. APCs, antigen-presenting cells; DLN, draining lymph node.
To evaluate the effect of SM905 on distinct subsets of Th cell responses, the CII-induced production of Th17-, Th1 and Th2 type cytokines by T cells was examined. As shown in Figure 4b, T cells from saline-treated CIA mice produced high levels of IL-17A, IL-6, TNF-α, IFN-γ and IL-2 (Th17 and Th1 type cytokines), and very low levels of IL-4 (Th2 type cytokine). Treatment with SM905 reduced IL-17A, IL-6, TNF-α, IFN-γ and IL-2, while relatively increasing IL-4. Among these cytokines, SM905 exerted the most striking effect on the Th17 type cytokine IL-17A. In SM905-treated CIA mice, CII-specific IL-17A production by T cells was almost completely abolished. These results indicated that treatment with SM905 led to a strong inhibition of Th17-related immune responses in CIA.
SM905 inhibited Th17 development
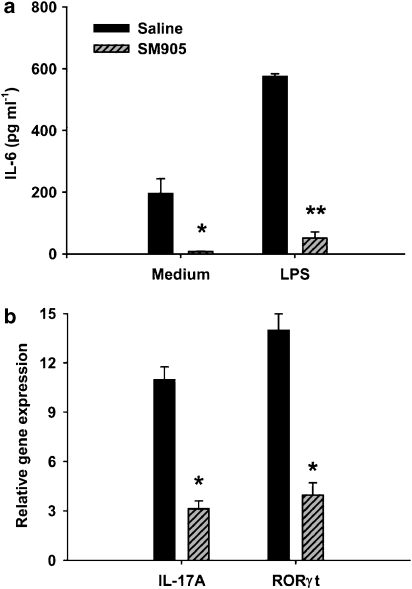
An IL-6 signal is critical for induction of commitment to a Th17 lineage (Bettelli et al., 2006; Veldhoen et al., 2006). To investigate whether SM905 influenced lipopolysaccharide-stimulated IL-6 production by the innate immune system in CIA, purified APCs (containing macrophages and dendritic cells) from the DLN of saline-treated and SM905-treated CIA mice were challenged with or without lipopolysaccharide ex vivo for 24 h in culture. As shown in Figure 5a, IL-6 and lipopolysaccharide-induced IL-6 were significantly inhibited in APCs from SM905-treated CIA mice in comparison to that from saline-treated mice. The result suggested that SM905 could have affected Th17 development through suppression of IL-6 production.
Figure 5.
SM905 inhibited Th17 development. (a) Purified APCs (4 × 106 ml−1) from saline- or SM905-treated CIA mice (five mice per group) were stimulated with or without LPS (1 μg ml−1). Cells were cultured in 96-well flat-bottomed plates in triplicate for 24 h, and IL-6 production was assayed using ELISA. The results were expressed as mean±s.e.m. (n=3). *P<0.05, **P<0.01, versus saline-treated CIA group. (b) Total RNA from T cells of DLN (five mice per group) was extracted and reverse transcribed into cDNA and the expression levels of IL-17A and RORγt mRNA were evaluated using real-time PCR. Relative gene expression was normalized to HPRT. The fold changes of gene expression in CIA mice against those of normal mice were presented as mean±s.e.m. of three separate experiments each consisting of 2–4 samples. *P<0.05 (Mann–Whitney U-test), versus saline-treated CIA mice.APCs, antigen-presenting cells; CIA, collagen-induced arthritis; HPRT, hypoxanthine-guanine phosphoribosyltransferase; LPS, lipopolysaccharide.
The gene expression of IL-17A and RORγt, a specific transcription factor for Th17 lineage, was then tested in T cells of DLN to further delineate the influence of SM905 on Th17 development in CIA. Figure 5b shows that IL-17A and RORγt expression in T cells from SM905-treated mice was markedly reduced in comparison to T cells from saline control mice. A potential mechanism by which SM905 improves CIA could be the suppression of pathogenic Th17 development.
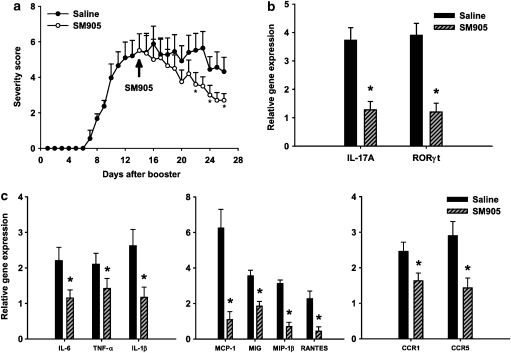
Attenuation of established CIA by oral treatment of SM905
To evaluate the efficacy of the new artemisinin derivative in established disease, SM905 (0.5 mg kg−1, p.o.) was given to mice, beginning 14 days after booster immunization. SM905 treatment led to a marked improvement in arthritis severity scores (Figure 6a) and this effect was linked with a significantly decreased expression of IL-17A and RORγt in T cells of DLN (Figure 6b). In joints, the enhanced expression of proinflammatory cytokines IL-6, TNF-α and IL-1β, inducible chemokine monocyte chemoattractant protein-1, monokine induced by interferon γ, macrophage inflammatory protein-1β and RANTES as well as chemokine receptor 1 and chemokine receptor 5 was correspondingly reduced by SM905 treatment (Figure 6c). These results illustrated a highly protective effect of SM905 on established arthritis, which was correlated with the downregulation of both Th17 and inflammatory responses in CIA.
Figure 6.
Effects of SM905 on established CIA. Mice were immunized with CII in CFA and boosted 21 days later to induce CIA. Saline or 0.5 mg of SM905 was orally administered 14 days after booster immunization (day 14) and then once daily till the end of the experiment. (a) Severity scores of arthritis were assessed from all animals in each group (n=8). The results were expressed as mean±s.e.m. for each group for each time point. *P<0.05 (Mann–Whitney U-test), versus saline-treated CIA group. (b) The relative gene expression of IL-17A and RORγt was examined in T cells from DLN on day 28. (c) Total RNA from joint tissue was extracted on day 28 and reverse transcribed into cDNA. The relative gene expression of the indicated proinflammatory mediators was estimated using real-time PCR. Relative gene expression was normalized to HPRT. The fold changes of the gene expression in CIA mice against those of normal mice are shown. The results were expressed as mean±s.e.m. of three separate experiments each consisting of 2–4 samples. *P<0.05 (Mann–Whitney U-test), versus saline-treated CIA group. CFA, Freund's complete adjuvan; CIA, collagen-induced arthritis; CII, type II bovine collagen; HPRT, hypoxanthine-guanine phosphoribosyltransferase; IL, interleukin.
Discussion
In this study, we show that SM905, a new water-soluble compound derived from artemisinin, provides a highly effective treatment for CIA in mice. Oral treatment of arthritic mice with SM905 decreased the incidence and severity of arthritis, delayed onset and ameliorated joint damage. The therapeutic effect of SM905 on arthritis was associated with a striking reduction of two deleterious components of the disease, the inflammatory and autoimmune responses.
The effector mechanisms of inflammation in the rheumatoid process are mostly dependent on the expression of proinflammatory cytokines and chemokines (Feldmann et al., 1996; Choy and Panayi, 2001). SM905 significantly reduced several proinflammatory agents both in DLN and in inflamed joints of CIA, including IL-6, TNF-α, IL-1β, as well as various chemokines, which have been shown to exacerbate CIA (Arend, 2001). It is well documented that IL-6, TNF-α and IL-1β synergistically mediate synovitis, pannus formation and destruction of cartilage and bone (Feldmann et al., 1996), and chemokines are responsible for migration and accumulation of leukocytes at inflammatory sites. SM905 downregulated the expression of proinflammatory factors and attenuated inflammatory response in the development of arthritis, thereby improving the clinical and pathogenic manifestation of the disease.
T cells have a crucial role in the initiation and shaping of the autoimmune response in CIA (Fournier, 2005). We showed that SM905 significantly inhibited CII-induced T cell responses in CIA. The inhibitory effect of SM905 on the proliferative response of purified T cells paralleled that of DLN cells, suggesting that treatment with SM905 mainly affected T cell function in CIA. Th cells can be categorized into distinct subsets based on their cytokine profile. The analysis of Th17/Th1/Th2 cytokines revealed that SM905 treatment exerted the most significant suppressive effect on Th17 type cytokine IL-17A. Lines of evidences indicate that IL-17 is a pleiotropic cytokine that mediates the immune-induced tissue injury of CIA (Weaver et al., 2007). Therefore, our follow-up study focused on IL-17 and investigated gene expression of this cytokine and RORγt, a key transcription factor for IL-17. Nevertheless, we observed that SM905 also altered the Th1/Th2 response by decreasing type response and increasing Th2 type response, and the specific transcription factors T-bet for Th1 and GATA3 for Th2 were changed accordingly (data not shown). Consistent with the finding on Th cell cytokine patterns, the T-cell-dependent antigen-specific B cell responses exhibited similar alteration. Anti-CII IgG2a/b were significantly reduced, whereas IgG1 was relatively increased in the serum of SM905-treated CIA mice (Supplementary Figure 1). Treatment with SM905 therefore led to a deficiency in generating the deleterious autoimmune responses of the disease, particularly the pathogenic Th17 response.
Recent studies indicate that IL-6 is critical for the induction of Th17 development from naïve T cells (Bettelli et al., 2006, 2007; Veldhoen et al., 2006). This cytokine induces the expression of RORγt and subsequent differentiation of Th17 cells, together with a ubiquitously available cytokine transforming growth factor -β (Ivanov et al., 2006). Treatment with SM905 abolished IL-6 production and lipopolysaccharide-induced IL-6 production in APCs, suggesting that SM905 had a direct action on APC-derived IL-6 from the innate arm of immune system in CIA and that this effect was independent of adaptive immune response. The lack of IL-6 signal could explain partially the defect of Th17 development in SM905-treated CIA mice. Further experiments are in progress to elucidate a more detailed mechanism of SM905 on effector Th17 lineage development in vitro.
Artemisinin derivatives have been reported to ameliorate arthritis development in animal models by inhibiting nuclear factor-κB activation and proinflammatory cytokine production (Dong et al., 2003; Cuzzocrea et al., 2005; Li et al., 2006; Mirshafiey et al., 2006). In this study, we explored, for the first time, the anti-arthritis mechanisms of the new artemisinin derivative, SM905, not only on the inflammatory aspect but also the CII-specific, T-cell immunity aspects of CIA. This study was based on our previous finding that artemisinin derivatives exerted a significant effect on T cell activation and proliferation both in vitro and in vivo (Wang et al., 2007a, 2007b). In CIA, our results confirmed a significant effect of SM905 on T cell response to CII. More importantly, we further explored the effect of SM905 on different types of Th cell responses in CIA and found that the new artemisinin derivative SM905 exhibited a strikingly inhibitory effect on Th17 response. As IL-17, a characteristic cytokine of Th17 cells, is considered to provide a means of communication between the adaptive and the innate immune system to promote inflammation (Bi et al., 2007), it was a major focus in our study. Our results suggested that, besides the direct inhibition of proinflammatory nuclear factor-κB activation and cytokine production by inflammatory cells as known before, the antiinflammatory effect of this artemisinin derivative in CIA was also closely related to the inhibition of T-cell-mediated inflammatory response, particularly the highly proinflammatory Th17 cell response.
In conclusion, a novel water-soluble artemisinin derivative, SM905, was found to be effective for treating CIA. Our results indicate that very low pharmacological doses of SM905 are needed to exhibit a significant protective effect. The therapeutic effect of SM905 on established disease also fulfilled an essential prerequisite for an antiarthritic agent. The mechanisms of action of SM905 on CIA involved the suppression of inflammatory and pathogenic Th17 responses. These results suggest that SM905 may have therapeutic potential in the treatment of RA and other chronic inflammatory autoimmune disorders.
External data objects
Acknowledgments
This work was supported by the grant of Shanghai Science and Technology Committee (no. 06DZ19006). We thank Dr Hui-Zhen Zhang (Department of Pathology, the 6th Hospital of Shanghai, Shanghai Jiao Tong University) for providing valuable technical assistance in the histological study.
Abbreviations
- CIA
collagen-induced arthritis
- CII
type II bovine collagen
- DLN
draining lymph node
- IFN-γ
interferon-γ
- RA
rheumatoid arthritis
- RANTES
regulated on activation normally T-cell expressed and secreted
- RORγt
retinoid-related orphan receptor γt
- Th
T helper
- TNF
tumour necrosis factor
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Arend WP. Physiology of cytokine pathways in rheumatoid arthritis. Arthritis Care Res. 2001;45:101–106. doi: 10.1002/1529-0131(200102)45:1<101::AID-ANR90>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Bi Y, Liu G, Yang R. Th17 cell induction and immune regulatory effects. J Cell Physiol. 2007;211:273–278. doi: 10.1002/jcp.20973. [DOI] [PubMed] [Google Scholar]
- Brand DD, Kang AH, Rosloniec EF. Immunopathogenesis of collagen arthritis. Springer Semin Immunopathol. 2003;25:3–18. doi: 10.1007/s00281-003-0127-1. [DOI] [PubMed] [Google Scholar]
- Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Saadat F, Di Paola R, Mirshafiey A. Artemether: a new therapeutic strategy in experimental rheumatoid arthritis. Immunopharmacol Immunotoxicol. 2005;27:615–630. doi: 10.1080/08923970500418786. [DOI] [PubMed] [Google Scholar]
- Ding XF, Hu H. Exploring of immune-parmacology effect about artemisia annua curing rheumatoid arthritis. Zhongguo Zhong Yi Ji Chu Yi Xue Za Zhi. 2006;12:75–76. [Google Scholar]
- Doncarli A, Stasiuk LM, Fournier C, Abehsira-Amar O. Conversion in vivo from an early dominant Th0/Th1 response to a Th2 phenotype during the development of collagen-induced arthritis. Eur J Immunol. 1997;27:1451–1460. doi: 10.1002/eji.1830270623. [DOI] [PubMed] [Google Scholar]
- Dong YJ, Li WD, Tu YY. Effect of dihydro-qinghaosu on auto-antibody production, TNF alpha secretion and pathologic change of lupus nephritis in BXSB mice. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2003;23:676–679. [PubMed] [Google Scholar]
- Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Ann Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- Fournier C. Where do T cells stand in rheumatoid arthritis. Joint Bone Spine. 2005;72:527–532. doi: 10.1016/j.jbspin.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of pro-inflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Lindén A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Li WD, Dong YJ, Tu YY, Lin ZB. Dihydroarteannuin ameliorates lupus symptom of BXSB mice by inhibiting production of TNF-alpha and blocking the signaling pathway NF-kappa B translocation. Int Immunopharmacol. 2006;6:1243–1250. doi: 10.1016/j.intimp.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Li Y, Wu YL. An over four millennium story behind qinghaosu (artemisinin)—a fantastic antimalarial drug from a traditional chinese herb. Curr Med Chem. 2003;10:2197–2230. doi: 10.2174/0929867033456710. [DOI] [PubMed] [Google Scholar]
- Lu L. Study on effect of Cordyceps sinensis and artemisinin in preventing recurrence of lupus nephritis. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2002;22:169–171. [PubMed] [Google Scholar]
- Mauri C, Williams RO, Walmsley M, Feldmann M. Relationship between Th1/Th2 cytokine patterns and the arthritogenic response in collagen-induced arthritis. Eur J Immunol. 1996;26:1511–1520. doi: 10.1002/eji.1830260716. [DOI] [PubMed] [Google Scholar]
- Mirshafiey A, Saadat F, Attar M, Di Paola R, Sedaghat R, Cuzzocrea S. Design of a new line in treatment of experimental rheumatoid arthritis by artesunate. Immunopharmacol Immunotoxicol. 2006;28:397–410. doi: 10.1080/08923970600927447. [DOI] [PubMed] [Google Scholar]
- Myers LK, Rosloniec EF, Cremer MA, Kang AH. Collagen-induced arthritis, an animal model of autoimmunity. Life Sci. 1997;61:1861–1872. doi: 10.1016/s0024-3205(97)00480-3. [DOI] [PubMed] [Google Scholar]
- Noori S, Naderi GA, Hassan ZM, Habibi Z, Bathaie SZ, Hashemi SM. Immunosuppressive activity of a molecule isolated from Artemisia annua on DTH responses compared with cyclosporin A. Int Immunopharmacol. 2004;4:1301–1306. doi: 10.1016/j.intimp.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Wang JX, Tang W, Shi LP, Wan J, Zhou R, Ni J, et al. Investigation of the immunosuppressive activity of artemether on T-cell activation and proliferation. Br J Pharmacol. 2007a;150:652–661. doi: 10.1038/sj.bjp.0707137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JX, Tang W, Yang ZS, Wan J, Shi LP, Zhang Y, et al. Suppressive effect of a novel water-soluble artemisinin derivative SM905 on T cell activation and proliferation in vitro and in vivo. Eur J Pharmacol. 2007b;564:211–218. doi: 10.1016/j.ejphar.2007.01.092. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Yang ZS, Wang JX, Zhou Y, Zuo JP, Li Y. Synthesis and immunosuppressive activity of new artemisinin derivatives. Part 2: 2-phenoxyl propionic acids and esters. Bioorg Med Chem. 2006;14:8043–8049. doi: 10.1016/j.bmc.2006.07.038. [DOI] [PubMed] [Google Scholar]
- Yang ZS, Zhou WL, Sui Y, Wang JX, Wu JM, Zhou Y, et al. Synthesis and immunosuppressive activity of new artemisinin derivatives. 1. phen(ox)yl aliphatic acids and esters. J Med Chem. 2005;48:4608–4617. doi: 10.1021/jm048979c. [DOI] [PubMed] [Google Scholar]
- Zhou R, Tang W, Ren YX, He PL, Zhang F, Shi LP, et al. 5R)-5-hydroxytriptolide attenuated collagen-induced arthritis in DBA/1 mice via suppressing interferon-gamma production and its related signaling. J Pharmacol Exp Ther. 2006;318:35–44. doi: 10.1124/jpet.106.101113. [DOI] [PubMed] [Google Scholar]
- Zhou WL, Wu JM, Wu QL, Wang JX, Zhou Y, Zhou R, et al. 3-(12-β-artemisininoxy) phenoxyl succinic acid (SM735), a novel artemisinin derivatives, mediates immunosuppressive effects in vitro and in vivo. Acta Pharmacol Sin. 2005;26:1352–1358. doi: 10.1111/j.1745-7254.2005.00232.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.