Abstract
It is well known that cAMP, an important intracellular second messenger, is released from many cells upon adenylate cyclase stimulation. Cell surface bound phosphodiesterase together with ecto-5′-nucleotidase may convert the extracellular cAMP to adenosine, which may stimulate in a paracrine and/or autocrine manner cells expressing P1 receptors. In this issue of the British Journal of Pharmacology, Chiavegatti et al. demonstrate the existence of an extracellular cAMP-adenosine cascade in skeletal muscle cells which suggests a link between adrenergic stimulation of contraction, elevated cAMP formation and release and exercise hyperaemia.
Keywords: adenosine, cAMP, ecto-phosphodiesterase, CD73, 5′-nucleotidase
Extracellular nucleotides and their derivatives have long been recognized to regulate many cellular functions including immune responses, exocrine and endocrine secretion, inflammation, platelet aggregation and endothelium-dependent and endothelium-independent vasodilatation. In addition, purinergic and pyrimidinergic signalling modulates cell proliferation and differentiation. These diverse functions are mainly achieved by activation of three receptor families including the G-protein-coupled P1 and P2Y receptors and the ionotropic P2X receptors (Burnstock, 2007). Whereas P1 receptors are activated by adenosine, P2X receptors show the highest affinity for ATP. The P2Y receptor family may also be stimulated by ATP; however, uridine 5'-diphosphate and ADP, respectively, display highest affinities to some of the P2Y family members.
There has been a long-lasting debate in the field of adenosine research concerning the source of these extracellularly acting nucleotides. In general, adenosine is thought to be derived from ATP. Both the cellular release of adenosine formed inside the cell and the extracellular formation of adenosine from ATP, ADP and AMP are possible means of elevating extracellular adenosine levels. The intracellular formation relies on the activity of the cytosolic 5′-nucleotidase and the subsequent release of adenosine into the extracellular compartment via an equilibrative adenosine transporter. However, adenosine kinase and adenosine deaminase compete with the export of adenosine and therefore this way appears to be not a general route to increase interstitial adenosine levels. In most cases, the extracellular formation of adenosine via an enzymatic cascade for the breakdown of ATP, ADP and AMP appears to be the major mechanism that leads to elevated extracellular adenosine. First, ecto-nucleoside triphosphate diphosphohydrolase (eNTPD; CD39) catalyses ATP breakdown to AMP, which is further converted by ecto-5′-nucleotidase (e-5′-NTase; CD73) to adenosine, which may act in an autocrine or paracrine manner on purinergic receptors presented by surrounding cells. Knockout mice with disrupted CD39 and CD73 have underscored the importance of the extracellular adenosine formation, respectively (Enjyoji et al., 1999; Koszalka et al., 2004). Phenotypic analysis of these mice revealed substantial effects on vascular homoeostasis with altered thromboregulation and elevated inflammatory responses. Thus, adenosine formed within blood vessels appears to play a protective role in the vasculature. In addition, the tubulo-glomerular feedback mechanism matching glomerular filtration rate and tubular flow on single nephron basis is known to be mediated by adenosine acting on A1 receptors. As shown by two groups, this regulatory principle was altered in CD73-deficient mice, demonstrating that normal renal function depended at least in part on the extracellular formation of adenosine by CD73 (Castrop et al., 2004; Huang et al., 2006).
What is the source of nucleotides feeding the extracellular pathway? ATP and ADP release from neurons and platelets relies on storage of these nucleotides in vesicles and their release is achieved by vesicle fusion with the cellular membranes. In contrast, other cell types appear to use plasmalemmal exit gates and the ABC transporter CFTR, connexin 43 hemichannels and pannexin have all been proposed to release ATP. Agonists that enhance intracellular Ca2+ levels and also other stimuli such as mechanical forces have been shown to elevate ATP release. This has been demonstrated using hypotonic swelling or shear stress as mechanical stimulus. However, mechanical stimulation appears not to induce a uniform response of ATP release as some cells continuously exposed to mechanical forces, such as cardiac myocytes, do not release ATP upon contraction (Gödecke et al., 2005).
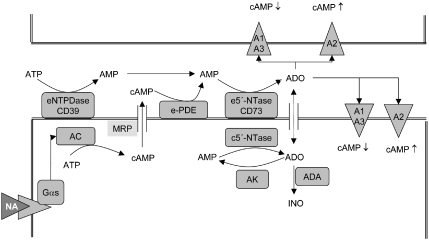
However, there is accumulating evidence that cAMP, a well-known intracellular second messenger, might represent another relevant adenosine precursor. It is well established that stimulation of AC and GC results in the release of the cyclic nucleotides cAMP and cGMP, respectively. The extracellular conversion of cAMP to adenosine had been shown as early as 1976. This conversion is achieved by the sequential activities of ecto-phosphodiesterase and CD73. Thus, CD73 appears to be an important point of convergence for the extracellular formation of adenosine derived from ATP or cAMP. The concept of the extracellular cAMP–adenosine pathway has been studied in many cell types but was explored in most detail in the kidney by Jackson et al. (1997, 2006). Recently, the pancreato-hepatorenal cascade was postulated, which links pancreatic release of glucagon, elevated hepatic cAMP release with renal adenosine formation and altered sodium/phosphate handling. There are two important aspects of the extracellular cAMP–adenosine cascade: (i) in contrast to adenosine, cAMP is stable in plasma and may therefore act at distant sites; (ii) a stimulus such as β1-adrenoceptor activation enhancing cAMP in a target cell may be further processed to modulate cAMP contents in a paracrine and/or autocrine manner (Figure 1). The effect of the extracellular conversion of cAMP to adenosine would thereby depend on the type of adenosine receptors expressed on the target and neighbouring cells, respectively. Whereas A1/A3 receptors would attenuate cAMP levels, A2A and A2B receptors would increase cAMP levels.
Figure 1.
The extracellular cAMP–adenosine cascade. Stimulation of Gαs-coupled receptors such as β1-adrenoceptors by noradrenaline (NA) leads to stimulation of AC. cAMP may leave the cell most likely via multidrug resistance-related proteins (MRP). Ecto-phosphodiesterase (e-PDE) and ecto-5′-nucleotidase (e-5′-NTase/CD73) convert cAMP to adenosine (ADO). Adenosine may act on P1 purinergic A1, A2A, A2B and A3 receptors to modulate cAMP levels in an autocrine and/or paracrine manner. In addition, extracellular adenosine may be enhanced by degradation of ATP by ecto-nucleoside triphosphate dihydrolase (eNTPDase) and CD73 or by release of adenosine from the intracellular compartment via an equilibrative adenosine transporter (indicated by a double arrow). As this transport has to compete with adenosine kinase (AK) and adenosine deaminase (ADA), this pathway represents, in most cases, a minor route to elevation of extracellular adenosine levels.
In this issue of the British Journal of Pharmacology, Chiavegatti et al. (2008) now extend the list of tissues able to use the extracellular cAMP–adenosine pathway to skeletal muscle. The authors show that cultured skeletal muscle fibres derived from satellite cells of newborn rats convert cAMP to adenosine, when it is added exogenously and when it is released from cells in response to the β-adrenoceptor agonist isoprenaline. The increase in cAMP release and adenosine formation was blocked to a large extent by probenecid. Therefore, this study is in line with earlier work that demonstrated that probenecid-sensitive multidrug resistance proteins such as MRP4, MRP5 and MRP8 are able to transport cAMP and cGMP in an ATP-dependent manner out of cells. Thus, these data envisage a link between neurohumoral stimulation of muscular contraction, cAMP synthesis and an elevated adenosine formation at the muscle cell surface. In view of adenosine's function to induce vasodilation, the authors suggest a coupling of elevated cAMP export from muscle and exercise hyperaemia.
Whereas in isolated skeletal muscle fibres the whole cAMP–adenosine cascade was shown to be operative, the authors could not yet demonstrate that there was a substantial conversion of cAMP to adenosine in extensor digitorum longus muscle preparations despite an isoprenaline-induced release of cAMP. Thus, an important piece of evidence is still lacking to close the gap in the concept that cAMP elevation in working muscle cells both enhances contractile force in the muscle and modulates muscular blood supply by relaxation of vascular smooth muscle cells via the cAMP–adenosine axis.
Exercise hyperaemia is well known to involve multiple factors for the achievement of maximal vasodilation and adenosine is one among the others. The major problem to address the role of a single factor in this context is the functional redundancy of vasodilatory effects exerted by elevated K+, H+, lactate, NO, vasodilatory prostaglandins, adenosine, endothelium-derived hyperpolarizing factor, and so on. Experimental inactivation of one of these factors is usually without a major effect on total blood flow, suggesting an efficient compensation by an altered release of the other factors. Depending on muscle type, species and extent of exercise, the contribution of adenosine to maximal flow in response to exercise is believed to range between 20 and 40% (Marshall, 2007). Thus, in view of the extensive plasticity of flow regulation, it will be experimentally demanding to explore to what extent the extracellular cAMP–adenosine mechanism contributes to muscular physiology.
Abbreviations
- eNTPD; CD39
ecto-nucleoside triphosphate diphosphohydrolase
- e-5′-NTase/CD73
ecto-5′-nucleotidase
References
- Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrop H, Huang Y, Hashimoto S, Mizel D, Hansen P, Theilig F, et al. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotidase/CD73-deficient mice. J Clin Invest. 2004;114:634–642. doi: 10.1172/JCI21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavegatti T, Costa VL, Jr, Araujo MS, Godinho RO.Skeletal muscle expresses the extracellular cyclic AMP–adenosine pathway Br J Pharmacol 20081531331–1340.this issue [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- Gödecke S, Stumpe T, Schiller H, Schnittler HJ, Schrader J. Do rat cardiac myocytes release ATP on contraction. Am J Physiol Cell Physiol. 2005;289:C609–C616. doi: 10.1152/ajpcell.00065.2005. [DOI] [PubMed] [Google Scholar]
- Huang DY, Vallon V, Zimmermann H, Koszalka P, Schrader J, Osswald H. Ecto-5′-nucleotidase (cd73)-dependent and -independent generation of adenosine participates in the mediation of tubuloglomerular feedback in vivo. Am J Physiol Renal Physiol. 2006;291:F282–F288. doi: 10.1152/ajprenal.00113.2005. [DOI] [PubMed] [Google Scholar]
- Jackson EK, Mi Z, Gillespie DG, Dubey RK. Metabolism of cAMP to adenosine in the renal vasculature. J Pharmacol Exp Ther. 1997;283:177–182. [PubMed] [Google Scholar]
- Jackson EK, Zacharia LC, Zhang M, Gillespie DG, Zhu C, Dubey RK. cAMP–adenosine pathway in the proximal tubule. J Pharmacol Exp Ther. 2006;317:1219–1229. doi: 10.1124/jpet.106.101360. [DOI] [PubMed] [Google Scholar]
- Koszalka P, Özüyaman B, Huo Y, Zernecke A, Flögel U, Braun N, et al. Targeted disruption of cd73/ecto-5′-nucleotidase alters thromboregulation and augments vascular inflammatory response. Circ Res. 2004;95:814–821. doi: 10.1161/01.RES.0000144796.82787.6f. [DOI] [PubMed] [Google Scholar]
- Marshall JM. The roles of adenosine and related substances in exercise hyperaemia. J Physiol. 2007;583:835–845. doi: 10.1113/jphysiol.2007.136416. [DOI] [PMC free article] [PubMed] [Google Scholar]