Abstract
Background and purpose:
During the bladder filling phase, the volume of the urinary bladder increases dramatically, with only minimal increases in intravesical pressure. To accomplish this, the smooth muscle of the bladder wall must remain relaxed during bladder filling. However, the mechanisms responsible for the stabilization of bladder excitability during stretch are unclear. We hypothesized that stretch-dependent K+ (TREK) channels in bladder smooth muscle cells may inhibit contraction in response to stretch.
Experimental approaches:
Bladder tissues from mouse, guinea pig and monkey were used for molecular, patch clamp, mechanical, electrical, Ca2+ imaging and cystometric responses to methionine and its derivatives, which are putative blockers of stretch-dependent K+ (SDK) channels.
Key results:
SDK channels are functionally expressed in bladder myocytes. The single channel conductance of SDK channels is 89pS in symmetrical K+ conditions and is blocked by L-methionine. Expressed TREK-1 currents are also inhibited by L-methioninol. All three types of bladder smooth muscle cells from mouse, guinea pig and monkey expressed TREK-1 genes. L-methionine, methioninol and methionine methyl ester but not D-methionine increased contractility in concentration-dependent manner. Methioninol further increased contractility and depolarized the membrane in the presence of blockers of Ca2+-activated K+ conductance. L-methionine induced Ca2+ waves that spread long distances through the tissue under stretched conditions and were associated with strong contractions. In cystometric assays, methioninol injection increased bladder excitability mimicking overactive bladder activity.
Conclusions and implications:
Methioninol-sensitive K+ (SDK, TREK-1) channels appear to be important to prevent spread of excitation through the syncitium during bladder filling.
Keywords: bladder contractility, TREK-1, Ca2+ transients, membrane potentials, BK and SK channels
Introduction
Bladder smooth muscle (BSM) is unique compared to smooth muscle in other organs of the body, as elicited or spontaneous action potentials do not readily spread throughout the tissue (Levin et al., 1986; Brading, 1992). This suggests poor electrical coupling between BSM cells and has the advantage of allowing bladder filling without inducing waves of contraction. Other mechanisms are also involved to maintain a low intravesicular pressure during bladder filling (Levin et al., 1986; Kinder and Mundy, 1987; Brading, 1994; Hashitani et al., 2000); however, the mechanism by which BSM cells prevent stretch-induced excitation is not clear.
A potential myogenic mechanism can be postulated as several types of K+ channel have been identified in detrusor myocytes. K+ channel antagonists are known to prolong action potential duration and promote contractile activity, suggesting a role for K+ channels in BSM excitability. The repolarization phase is mediated, in part, by the activity of large-conductance Ca2+-activated K+ (BK) channels (Klockner and Isenberg, 1985; Heppner et al., 1997). The small-conductance Ca2+-activated K+ (SK) channel blocker, apamin (10−7 M) has also been reported to increase the contraction amplitude and frequency, suggesting that SK channel may also be important to the regulation of spontaneous activity in the bladder (Herrera et al., 2003).
Stretch-dependent K+ (SDK) conductances have been reported in a variety of tissues' (Sackin, 1987; Kim, 1992; Ordway et al., 1995). Their conductance(s) vary from tissue to tissue but fall within the range 20–200 pS in symmetrical K+ conditions. Recently, we reported that SDK channels are expressed in murine and canine colonic myocytes (Koh and Sanders, 2001; Koh et al., 2001). SDK channels are voltage-independent and are activated by negative pressure. The most likely molecular candidate is the TWIK-related K+ (TREK) channel (Koh et al., 2001). SDK channels in colonic myocytes were inhibited by sulphur-containing amino acids, such as methionine, which is a relatively specific blocker of SDK channels (Park et al., 2005). Therefore, in this report, we investigated the molecular identification and functional role of SDK channels in bladder using various methionine derivatives.
Materials and methods
Tissue and cell preparation
The animals used for these studies were maintained, and experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals. All protocols were approved by the Institutional Animal Use and Care Committee at the University of Nevada, Reno. BALB/c mice (60–90 days old) and guinea pigs of either sex (200–350 g) were killed by inhalation of isoflurane (Baxter Healthcare, Deerfield, IL, USA) and CO2, respectively, followed by cervical dislocation. Cynomolgus monkeys (Charles River Laboratories, Sparks, NV, USA) of either sex (2.5–7 years of age) were initially sedated with ketamine (10 mg kg−1; Fort Dodge Animal Health, Fort Dodge, IA, USA), then were given 0.7 ml Beuthanasia-D solution (pentobarbital sodium and phenytoin sodium, Schering-Plough Animal Health Co, NJ, USA), followed by exsanguination. Bladders were cut open from bladder neck to base and rinsed free of urine with Krebs–Ringer bicarbonate buffer (see Park et al., 2005). Tissues were pinned on the base of a Sylgard-coated dish, and the urothelium and suburothelium were dissected away. Muscle strips were used for isometric force, intracellular microelectrode recording and Ca2+-imaging measurements. To obtain isolated smooth muscle cells, detrusor smooth muscle was digested as described previously (Koh and Sanders, 2001).
Isolation of smooth muscle cells
Small strips of smooth muscle layer from the bladder were equilibrated in Ca2+-free Hank's solution for 10 min. The buffer was replaced with an enzyme solution containing (per ml) collagenase 1.3 mg, bovine serum albumin 2 mg, trypsin inhibitor 2 mg and ATP 0.27 mg. The tissue was placed in a 37 °C water bath for 10–12 min without agitation. After three to four washes with Ca2+-free Hank's solution (Koh and Sanders, 2001), the tissue was triturated through a series of three blunt pipettes of decreasing tip diameters. Dispersed cells were stored at 4 °C in Ca2+-free solution. Experiments were performed at room temperature within 6 h of cell dispersion.
Expression of TREK-1 channels
Murine TREK-1 cDNA (NM_010607) was subcloned from pCDNA 3.1 into the retroviral expression vector pLPCX (Clontech Laboratories, Mountain View, CA, USA) by restriction enzyme digestion and ligation at HindIII and NotI. Retrovirus was produced by transfecting the TREK-1/pLPCX construct into RetroPack PT67 packaging cells with TransIT reagent (Mirus Bio, Madison, WI, USA) and repeated selection of surviving clones with 4 μg ml−1 puromycin. A stable COS-7 cell line expressing TREK-1 was produced by transduction with the TREK-1 retrovirus and selection with 7.5 μg ml−1 puromycin. Following selection, surviving clones were maintained in DMEM growth medium supplemented with 10% fetal bovine serum and a single clone was chosen for continued propagation. Cells were trypsinized and replated at 10–15% confluency for 24 h in 35 mm plastic culture dishes before electrophysiological recordings.
Patch clamp techniques
The whole-cell and single-channel patch clamp techniques were used to record membrane currents from dissociated murine colonic smooth muscle cells and COS-7 cells expressing TREK-1. Currents were amplified with an Axopatch 200B (Axon Instruments). Data were digitized with either a 12 or 16-bit analogue to digital converter (Digidata 1322A and Digidata 1320A, respectively, Axon instruments, Foster City, CA, USA). Data were stored directly and digitized online using pClamp software (version 9.0, Axon instrument, Foster City, CA, USA). The data were sampled at 2 KHz for whole-cell and 5 KHz for single-channel recordings with low pass filtered at 1 KHz using an eight-pole Bessel filter. The pipette resistances were 1–3 MΩ for whole-cell and 5–8 MΩ for single-channel recordings. In most experiments, the uncompensated series resistance was between 2 and 4 MΩ, and thus voltage errors could have approached 12 mV. Voltage errors were much smaller during steps to negative potentials. We examined the single-channel slope conductance in symmetrical K+ (140/140 mM). We also used asymmetrical K+ (5/140 mM) solution. Unitary amplitude at various voltages was fitted to Goldman–Hodgkin–Katz equations. Negative pressure was applied to patches by suction via a 1 ml syringe and pressure–volume relationships were calibrated using a pressure transducer. To measure the dependence of SDK channel open probability on negative pressure, we applied atmospheric pressures ranging from −20 to −80 cmH2O to patch membrane.
Mechanical responses of bladder detrusor smooth muscles
Standard organ bath techniques were employed to measure the changes in force generated by BSM strips. One end of BSM strip was attached to a fixed mount and the opposite end to an isometric strain gauge (Fort 10, WPI, Sarasota, FL, USA). A resting force of 100–600 mg was applied to set the muscles at optimum length, and the muscles were allowed to equilibrate for ⩾1 h. Mechanical responses were recorded on a computer running Acqknowlege 3.2.6 (Biopac Systems, Santa Barbara, CA, USA) and measurements of the area under the curve (AUC) obtained. The AUC was determined as the integral values above the baseline of selected area for 10 min recordings (g·s). The AUC for the tissues exposed to tested drugs were compared to the AUC for tissues under control conditions, during an equivalent period of time.
Intracellular microelectrode recordings, Ca2+ imaging analysis in tissues and cells
After preparing the tissue, impalements of cells were made with glass microelectrodes having resistances of 80–120 MΩ. Transmembrane potentials were recorded with a standard electrometer (Duo 773; WPI, Sarasota, FL, USA). Data were recorded on a PC running Acknowledge 3.2.6 software. Calcium imaging methods in tissue and cells used in this study are described in detail by Hennig et al. (2002) and Bayguinov et al. (2000), respectively.
Reverse transcriptase-polymerase chain reaction
Single cells (approximately 60 cells) were collected through applied suction by aspirating them into a wide bore patch clamp pipette (Sutter Instruments, Novato, CA, USA), and stored at −80 °C until use. Total RNA and cDNA preparation and amplification was identical to that reported by Koh et al. (2001). The following PCR primers were used (the GenBank accession number is given in parentheses): for human TREK-1 (KCNK2) (NM00101742Y), nucleotides 1094–1284, amplicon=191 base pairs (bp); for mouse TREK-1 (KCNK2) (NM010607), nucleotides 1485–1664, amplicon=180 bp; for human GAPDH (NM002046), nucleotides 403–572, amplicon=170 bp; for mouse GAPDH (NM608084), nucleotides 345–514, amplicon=170 bp.
Cystometrics
Mice were anaesthetized and subjected to cystometric evaluation. Briefly, the mice were catheterized with a 22 gauge angiocatheter, and a Crede manoeuvre was performed by applying gentle abdominal pressure until the bladders were emptied. The catheter was then replaced with a 24 gauge angiocatheter that was fastened to tubing connected to a three-way stopcock. One arm of the stopcock was connected to a pressure transducer (Model PT300; Astro-Med Grass Instr., West Warwick, RI, USA) and multichannel recorder (Model 7E, Grass Instruments, West Warwick, RI, USA). Krebs was infused (Model 975; Harvard Instruments, Holliston, MA, USA) at a rate of 25 μl min−1 through the other arm of the stopcock and bladder pressure during filling were recorded. Recordings were terminated after a leak was observed.
Statistical analysis of electrophysiological and mechanical responses
Data are expressed as means±s.e.m. The Student's t-test was used where appropriate to evaluate differences in the data. P-values less than 0.05 were taken as a statistically significant differences. The n values reported in the text refer to the number of recordings from the muscle strips, which is equivalent to the number of animals used unless otherwise stated.
Drugs and chemicals
Arachidonic acid, tetraethylammonium (TEA) choloride, methionine methylester, D-methionine RT and apamin were obtained from Sigma Chemical Co (St Louis, MO, USA). Methioninol and L-methionine were purchased from Fluka Biochemika, Buchs, Switzerland. These drugs were dissolved in test solutions directly. Arachidonic acid was dissolved in ethanol (10 mg ml−1) and stored under nitrogen at −20 °C before experiments. The final concentration of ethanol was less than 0.1% and had no effect at this concentration.
Results
The expression of stretch-dependent K+ channels in bladder smooth muscle
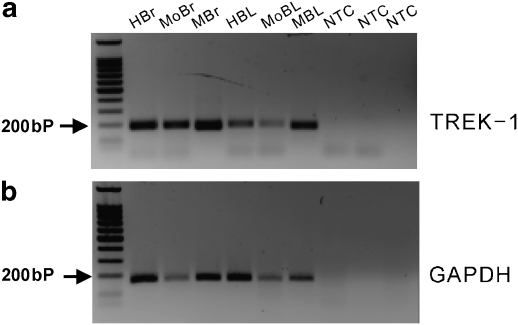
Smooth muscles in organs that have a reservoir function often show a SDK conductance. A possible molecular candidate for this conductance is TREK-1 channel (Koh and Sanders, 2001; Koh et al., 2001). To confirm that BSM cells also express TREK-1, reverse transcription-PCR was performed on isolated BSM cells from murine, human and monkey tissues. TREK-1 was expressed in all BSM cells from three species (Figure 1). TREK-2 and TRAAK, mechanosensitive two-pore K+ channel candidates, were not expressed in murine BSM cells (data not shown).
Figure 1.
mRNA expression of TREK-1 in BSMs cells from mouse, monkey and human bladder. (a) Two per cent agarose/TAE gel showing results of PCR amplifications using probes specific for TREK-1. Lane 1 shows the molecular weight marker used to indicate the size of the PCR fragments. RT-PCR demonstrated the presence of TREK-1 in human brain (HBr, 191 bp), mouse brain (MoBr, 180 bp) and monkey brain (MBr, 191 bp) tissues. TREK-1 was also detected in smooth muscle cells in human bladder (HBL), mouse bladder (MoBL) and monkey bladder (MBL) using the same primers. Human primers were used for monkey tissues. Reverse transcription control on each RNA sample used a cDNA reaction as template for which the reverse transcriptase was not added (NTC), controlling for genomic DNA contamination in the source RNA. (b) GAPDH (170 bp) was used as an internal control to test for DNA contamination in the RNA preparations. BSM, bladder smooth muscle; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RT-PCR, reverse transcription-PCR; TREK, TWIK-related K+.
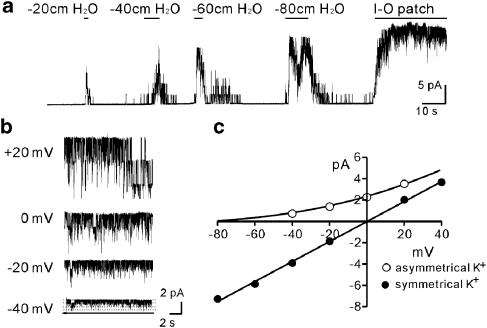
To isolate SDK channel in murine BSM cells, we performed single-channel recordings. Single-channel currents evoked by negative pipette pressure were obtained in cell-attached patches of BSM cells. Patches were exposed to pressures ranging from −20 to −80 cmH2O (holding potential was 0 mV in asymmetrical K+ gradient (5/140 mM) to exclude contamination from nonselective cation conductances). Pipette solutions contained charybdotoxin (200 nM) to block BK and intermediate-conductance Ca2+-activated K+ channels. To be sure that the effects of negative pressure were reversible and lacked desensitization, incremental levels of negative pressure were applied to the same patch. Increasing the level of negative pressure increased NPo (number of channels (N) × open probability (Po)) of the K+ channels. Application of −80 cmH2O to the cell-attached patch caused a maximal increase in NPo (Figure 2a). When atmospheric pressure was restored, the open probability returned to near zero. The cell-free condition (inside-out patch) induced maximal channel openings (Figure 2a). Single-channel conductance in asymmetrical K+ gradient (5/140 mM) at various holding potentials was also measured (Figure 2b). The unitary current amplitude–voltage (I–V) plot of the single-channel conductance was well fitted with the Goldman–Hodgkin–Katz equation, suggesting that currents were due to K+ channels (Figure 2c). The open probability did not differ from negative to positive potentials (Figure 2b), suggesting that regulation of the channel was voltage-independent. Under symmetrical K+ conditions (140/140 mM), the single-channel conductance was 89±8 pS (n=4; Figure 2c). These recordings demonstrate that BSM cells express SDK channels with properties similar to TREK-1 channels (Koh and Sanders, 2001; Koh et al., 2001). These SDK channels in murine BSM cells were not blocked by 4-aminopyridine (up to 5 mM), TEA (up to 10 mM), glibenclamide (1 μM), apamin (300 nM) and charybdotoxin (200 nM) (data not shown).
Figure 2.
Negative pressure activated SDK channel in bladder myocytes. (a) Freshly dispersed bladder myocytes were exposed to pressures ranging from −20 to −80 cmH2O at holding potential of 0 mV in asymmetrical K+ gradient (5/140 mM). I-O patch denotes inside-out patch. (b) Representative traces in asymmetrical K+ gradient (5/140 mM) at various holding potentials from excised patches. Solid line denotes channel close and dotted line channel open. (c) The unitary current amplitude–voltage (I–V) plot of the single-channel conductance was well fitted by the Goldman–Hodgkin–Katz equation in asymmetrical K+ solutions and with linear regression in symmetrical K+ solutions. SDK, stretch-dependent K+ channel.
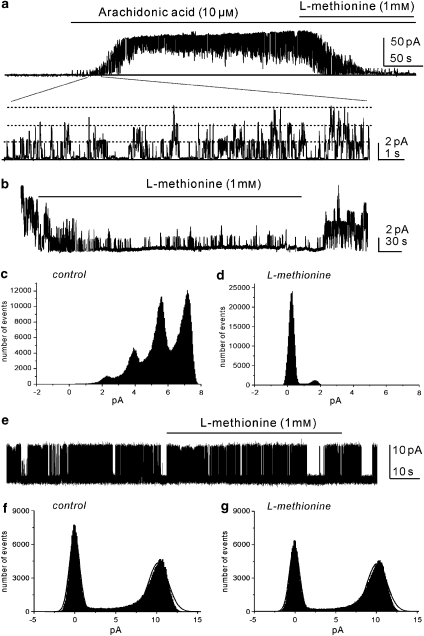
To investigate the role of SDK channels on BSM function, we used the amino acid methionine, which has been shown to block SDK channels (Park et al., 2005) and arachidonic acid known to open TREK-1 channels (Maingret et al., 1999). Cells were held at 0 mV in asymmetrical K+ gradient (5/140 mM). The application of arachidonic acid (10 μM) increased the activity of SDK channels (Figure 3a) and the additional application of L-methionine (1 mM) decreased the open probability of arachidonic acid-activated unitary currents. Excising patches increased the open probability of SDK channels (NPo=3.2±0.7, n=4; Figure 3b). The amplitude histogram in Figures 3c and d was obtained from 1 min of single-channel recording. Application of L-methionine (1 mM) dramatically decreased the open probability of SDK channels (Po=0.1±0.3, P<0.01 compared to control excised patch). The effects of L-methionine were reversible.
Figure 3.
The effect of L-methionine on SDK and BK channels in excised patches. (a) Arachidonic acid increased the open probability of SDK channels and L-methionine decreased the open probability of SDK channels at 0 mV in asymmetrical K+ gradients. The lower panel in (a) shows responses on an extended time scale. Solid line denotes channel closed and dotted line denotes channel openings. (b) Excised patches revealed a high open probability of SDK channels and L-methionine (1 mM) decreased the open probability of SDK channels at 0 mV in asymmetrical K+ gradients. (c and d) The amplitude histogram was obtained from 1 min of channel recording before and after L-methionine. (e) Inside-out patches demonstrated the opening of BK channels at +20 mV in asymmetrical K+ gradients. (f and g) The representative amplitude histogram before and after L-methionine. BK, large-conductance Ca2+-activated K+ channel; SDK, stretch-dependent K+ channel.
We also tested the effects of L-methionine on the open probability of BK channels by omitting charybdotoxin from the pipette solution. In inside-out patches, the open probability of BK channels (free [Ca2+] 5 × 10−7 M) was 0.35±0.1 at 0 mV in asymmetrical K+ gradients (n=4; Figure 3e). L-methionine (1 mM) did not change Po (0.34±0.2; Figures 3f and g). The inactive enantiomer, D-methionine (1 mM) had no effect on SDK channels (data not shown). These data suggest that L-methionine is a specific inhibitor of the SDK channels in bladder myocytes.
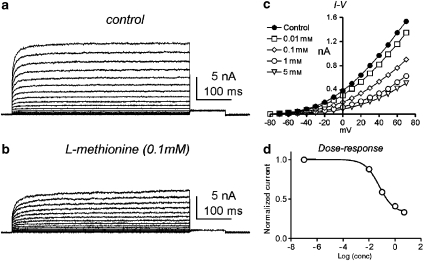
To compare the properties of mTREK-1 cloned from murine smooth muscle with those of the native SDK currents, mTREK-1 was expressed in COS-7 cells. Voltage-clamp recordings from COS-7 cells expressing TREK-1 revealed a rapidly activating and non-inactivating current (Figure 4a). Similar currents were not detected in nontransfected cells. L-methionine decreased TREK-1 currents in a concentration-dependent manner (0.01–5 mM; Figures 4b and c). The data in Figure 4d were derived from the maximum currents at +70 mV of test potential from four cells. The calculated IC50 of the sigmoidal concentration–response function was 0.61 mM with a slope of 0.55. These data support our hypothesis that L-methionine produces depolarization and increased excitability of murine bladder by inhibiting SDK or TREK-1 channels.
Figure 4.
Concentration-dependent response of TREK-1 currents to L-methionine. (a) Representative TREK-1 currents were recorded from COS-7 cells. (b) L-methionine decreased TREK-1 currents. (c) I–V relationship of L-methionine on TREK-1 currents in various concentrations of L-methionine. (d) Dose–response relationship analysed from maximum currents at +70 mV of test potentials and fitted to a sigmoidal concentration–response function. TREK, TWIK-related K+.
The effects of methionine and its derivatives on bladder contractility
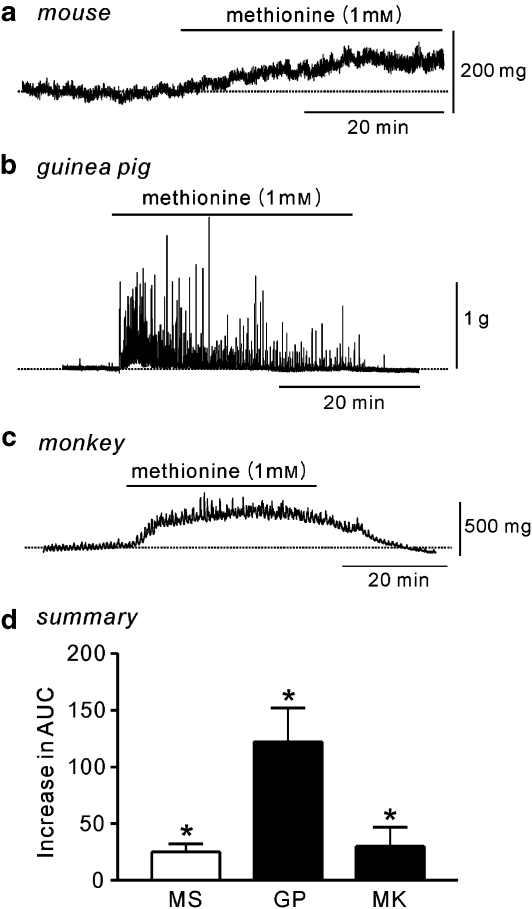
BSM strips, isolated from mouse, guinea pig or monkey, were pretreated with tetrodotoxin (1 μM) to exclude neural activity. At a resting tone of 100 mg, L-methionine (1 mM) caused a slow increase in tone reflected by an increase in the AUC in murine BSM strips (n=9; Figures 5a and d). The inactive enantiomer, D-methionine (1 mM), had no effect (data not shown). Interestingly, in BSM strips from guinea pigs, small spontaneous contractions that varied in amplitude were observed under control conditions. Addition of L-methionine (1 mM) resulted in a large increase in the frequency (from 0.7±0.7 cpm (cycles per minute) to 2.9±0.6 cpm) and amplitude (from 0.1±0.1 to 1.1±0.6 g) along with an increased AUC (n=5; Figures 5b and d). Monkey BSM strips were quiescent in control conditions. Addition of L-methionine resulted in an increase in tone that was maintained as long as the drug remained in the bath, leading to an increased AUC (n=4; Figures 5c and d).
Figure 5.
Effect of L-methionine on contractility of bladder strips. (a) Strips of murine bladder did not display spontaneous contractile activity. Addition of L-methionine (indicated by horizontal black bars) increased the tone. (b) Bladder strips from guinea pig showed spontaneous contractile activity that was greatly enhanced by L-methionine. (c) Bladder strips in monkey rarely showed spontaneous contractile activity. The application of L-methionine increased the tone, amplitude and frequency of contractions. (d) Summarized data show the mean increase in the AUC (mg·s) after L-methionine, compared to control, in mouse (MS), guinea pig (GP) and monkey (MK). All experiments were performed in the presence of tetrodotoxin (1 μM). *Significantly increased over control AUC (P<0.05, Student's t-test). AUC, area under the curve.
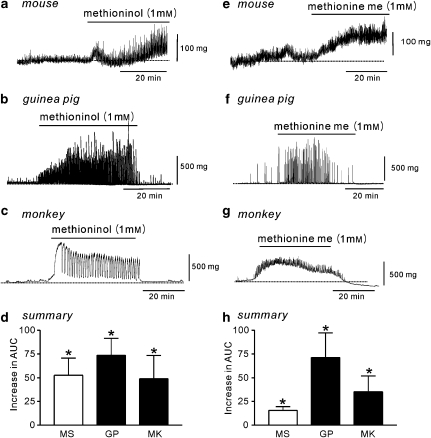
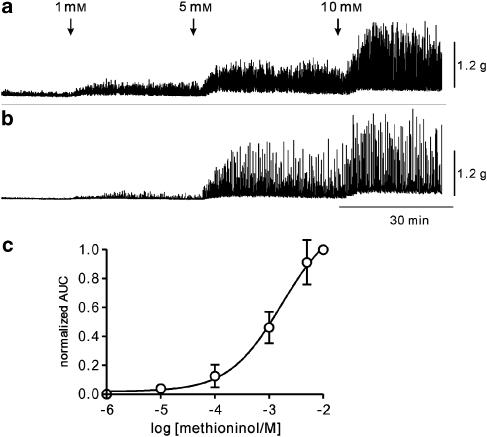
We also investigated the effects of two methionine derivatives methioninol and methionine methyl ester on BSM contractility. Methioninol (1 mM) had an effect similar to that of L-methionine in murine BSM strips (increased AUC, n=5; Figures 6a and d) and guinea pig (increased AUC; frequency increased from 0.8±0.4 to 3.0±0.7 cpm, P<0.05; amplitude increased from 0.7±0.4 to 1.6±0.5 g, P<0.05, n=6; Figures 6b and d). In monkey bladder strips, methioninol also induced large phasic contractions (AUC increased; frequency increased from 0.2±0.26 to 1.2±0.38 cpm, P<0.05; amplitude increased from 0.11±0.07 to 0.23±0.8 g, P<0.05, n=10; Figures 6c and d). Methionine methyl ester also had effects similar to those of L-methionine on BSM contractility in the three species (Figures 6e–h). Furthermore, we obtained concentration-dependent effects of methioninol on murine BSM contractility (Figure 7) and found the EC50 of methioninol was 1.7 mM (0.88 Hill slope; Figure 7c).
Figure 6.
Effects of methioninol and methionine methyl ester on the mechanical activities of bladder strips. (a–c) Methioninol increased contractility in strips of bladder in mouse, guinea pig and monkey (indicated by black bars). (d) Summarized data show the mean increase in the AUC (mg·s) after methioninol compared with control in mouse (MS), guinea pig (GP) and monkey (MK). (e–g) Methionine methyl ester (methionine me) increased contractility in bladder strips from mouse, guinea pig and monkey. (h) Summarized data show mean increase in the AUC (mg·s) after methionine methyl ester compared with control in mouse (MS), guinea pig (GP) and monkey (MK). All experiments were performed in the presence of tetrodotoxin (1 μM). *Significantly increased over control AUC (P<0.05, Student's t-test). AUC, area under the curve.
Figure 7.
Effect of methioninol on murine bladder contractility. (a and b) Representative traces showing the effect of methioninol on bladder contractility in a concentration-dependent manner. (c) The AUC was normalized for the effects of methioninol (10 mM) and a concentration–response curve was fitted (Hill equation; n=6). AUC, area under the curve.
The possible ionic mechanisms of methioninol on bladder excitability
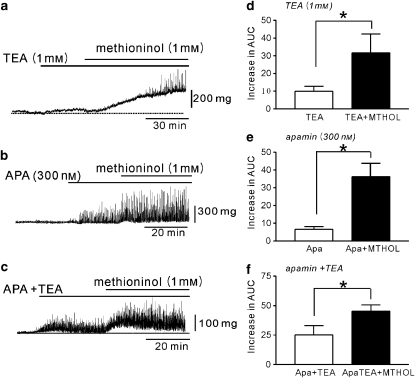
We tested the effects of methioninol on bladder contractility in the presence of TEA and/or apamin. TEA (1 mM) was used to inhibit BK channels in murine BSM strips and caused by itself a small increase in tone (n=5; Figures 8a and d). The addition of methioninol (1 mM) to the TEA-treated strips increased tone further and phasic contractions were observed (Figures 8a and d). The increase in AUC was significantly different from that induced by TEA (1 mM) alone (P<0.05, n=8; Figure 8d). In guinea pig BSM strips, TEA increased the amplitude of contractions from 0.2±0.1 to 0.6±0.3 g (P<0.05) but had no effect on frequency (from 1.6±0.8 to 2.2±0.2 cpm, P=0.10), although the AUC increased (n=5). The addition of methioninol (1 mM) increased contractility as shown by a further increase in AUC (P<0.05 compared with TEA, n=7), frequency (3.8±0.2 cpm, P<0.01 compared with TEA) and amplitude (1.2±0.2 g, P<0.05 compared with TEA). The effect of methioninol in the presence of TEA on monkey bladder strips was similar to those observed in murine or guinea pig tissues (data not shown).
Figure 8.
The effect of methioninol on bladder contractility in the presence of K+ channel blockers. (a) Tetraethyl ammonium (TEA) increased contractility in strips of murine bladder. The application of methioninol further increased contractility in the presence of TEA. (b) Apamin (APA) increased contractility in murine bladder strips. The application of methioninol further increased contractility in the presence of apamin. (c) The application of both TEA (10 mM) and apamin (APA, 300 nM) increased contractility in murine bladder strip. The application of methioninol further increased contractility in the presence of both blockers. (d–f) Summarized data show the mean increase in the AUC (mg·s) after methioninol (MTHOL) in the presence of TEA (d), apamin (Apa) (e) and both blockers (f). All experiments were performed in the presence of tetrodotoxin (1 μM). *Significant difference between means shown; P<0.05, Students' t-test. AUC, area under the curve.
To investigate the effects of methioninol on SK channels, we tested the effect of apamin, a blocker of these channels. Apamin (300 nM) induced spontaneous contractions with a small increase in basal tone in murine bladder strips (n=5; Figures 8b and e). The addition of methioninol (1 mM) to the apamin-exposed strips further increased basal tone (P<0.01; Figures 8b and e). Treatment of murine BSM strips with a combination of TEA and apamin increased tone compared with control strips (n=5; Figures 8c and f), and the further addition of methioninol (1 mM) significantly (P<0.05) increased the overall muscle activity, compared with response with TEA and apamin (Figure 8f).
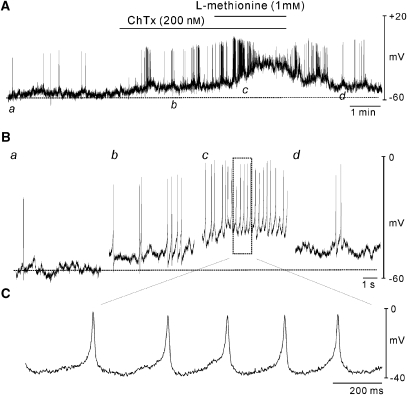
We also performed conventional microelectrode recordings on murine BSM cells to examine the effect of methioninol on membrane potential. Bladder tissues were electrically quiescent (18 of 25 muscle strips). Figure 9 demonstrates typical recordings of electrical activity from murine BSM showing infrequent spikes in control conditions. Charybdotoxin (200 nM) was used to block BK channels and depolarized BSM from −53±2 to −46±1 mV (P<0.05, n=7) and increased the frequency of spontaneous firing from 2.3±0.3 to 8.0±1.7 cpm. The further addition of methioninol (1 mM) caused BSM cells to become more depolarized (to −30±1 mV, P<0.05 compared with charybdotoxin alone) and showed sustained high-frequency spiking activity. Treatment of quiescent BSM with methioninol (1 mM) also induced depolarization from −51±2 to −45±3 mV (P<0.05, n=18).
Figure 9.
Effects of L-methionine on the membrane potential of murine BSM cells. (A) Intracellular microelectrode recordings were made from intact bladder muscle strips. Charybdotoxin (ChTx) induced depolarization. L-methionine caused further depolarization in the presence of ChTx. (B) Panels a–d: the lower traces (b and c) are expanded from (A). (C) L-methionine induced continuous firing of action potentials. Dashed lines in each panel denote membrane potentials under control conditions. BSM, bladder smooth muscle.
The effects of methioninol on Ca2 activity and cystometry in murine bladder
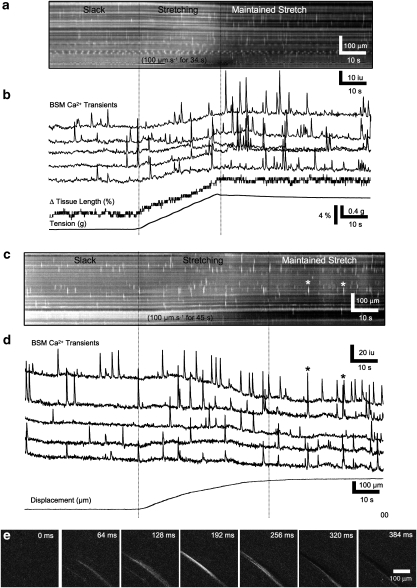
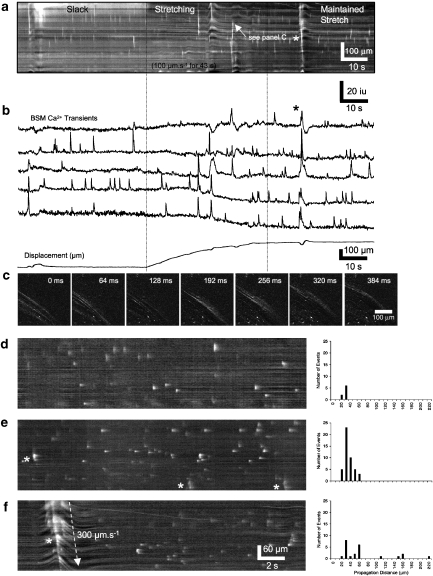
We used Ca2+ imaging to examine whether mechanical stretch of BSM was able to increase intracellular Ca2+ transients or induce Ca2+ waves through the BSM syncitium. Spatio-temporal maps showing the effect of active and maintained stretch on Ca2+ activity in murine BSM are shown in Figure 10. Propagating intercellular Ca2+ waves were rarely seen in control conditions. The majority of events observed consisted of independent Ca2+ transients in individual BSM cells. No propagating Ca2+ waves were observed in control BSM in either slack or stretched conditions (Figures 10a, b and d). After addition of TEA (1 mM), intercellular Ca2+ waves spread rapidly over short distances (<6 cells wide) orthogonal to the long axis of the BSM cells (Figures 10c–e). These waves propagated at a velocity of 27±70 μm s−1 for 31±6 μm (range 16–33 μm; 44 events, n=3; Figure 10e), but these Ca2+ waves did not induce contractions. The further addition of methioninol (1 mM) induced intercellular Ca2+ waves that propagated longer distances across the preparation and were associated with contractions (Figures 11a–c). These waves propagated at a velocity of 241±73 μm s−1 for 62±32 μm (range 16–218 μm, 23 events, n=3; Figures 11d–f).
Figure 10.
Effects of stretch on Ca2+ transients of murine BSM. (a) Shown a spatio-temporal map of Ca2+ transients in a flat-sheet preparation of bladder detrusor muscle during a stretch sequence. At a slack length, isolated individual firing of BSM cells prevailed (see individual Ca2+ transients from five cells in b). During active stretch, the pattern of firing did not change appreciably; however, when the active stretch was stopped and the length maintained, there appeared to be a brief period in which BSM firing was enhanced. (c) The response to stretch in the presence of TEA (1 mM) during which some Ca2+ transients appeared to propagate short distances (see asterisks). (d) Traces of individual BSM cells in TEA (1 mM). (e) Individual frames during a short propagating event (first asterisk in c). BSM, bladder smooth muscle; TEA, tetraethylammonium.
Figure 11.
Effects of stretch on Ca2+ transients in the presence of L-methioninol. (a) A spatio-temporal map of Ca2+ activity in a flat-sheet preparation of bladder detrusor muscle during a stretch sequence in the presence of TEA (1 mM) and L-methioninol (1 mM). In addition to Ca2+ transients in individual BSM cells, during stretch, the frequency and distance of propagation of Ca2+ waves increased dramatically, often spreading across the entire field of view and were associated with large contractions of the tissue (see asterisk in panels a and b). An example of a propagating Ca2+ wave is shown in (c). (d) An example of Ca2+ transients during maintained stretch in control conditions. It was rare to see Ca2+ transients that appeared to propagate. In the presence of TEA during maintained stretch (e), occasional Ca2+ waves appear to propagate short distances (<4 cells; see asterisks in panel e); however, these events did not cause contraction of the tissue. (f) In the presence of TEA and L-methioninol, Ca2+ waves propagated large distances and were associated with considerable movements of the tissue (see asterisk in panel f). The number of events and the distance each Ca2+ propagated in each conditions are shown to the right of each spatio-temporal maps (d–e, n=4). BSM, bladder smooth muscle; TEA, tetraethylammonium.
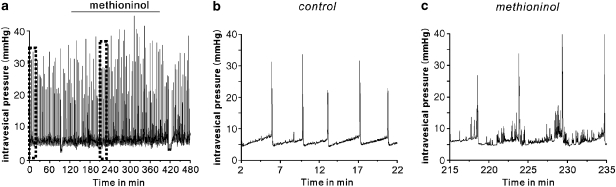
We performed cystometric assays in mice to examine the functional effect of blocking SDK channels in BSM, using methioninol (n=5). Methioninol injection (i.p.; 2 mg g−1) induced increased contractile activity, resembling that of overactive bladder, during the filling phase (Figure 12). These data suggested that SDK (TREK-1) channels were functionally expressed in bladder and facilitated bladder compliance.
Figure 12.
The effect of methioninol on cystometrograms in mice. (a) Long recordings of cystometrogram before and after methioninol (i.p., 2 mg g−1). (b and c) Cystometrogram displayed on an expanded time scale before and after methioninol, respectively. Representative trace from five experiments.
Discussion and conclusion
The urinary bladder undergoes substantial stretch during filling with only minimal increases in intravesical pressure before a contractile response is elicited. To accomplish this, the BSM must remain relaxed. In addition to neural reflexes, ionic conductances activated by distortion of the plasma membrane may be an important component of the myogenic response. Several mechanosensitive ion channels have been described in visceral smooth muscle, including swelling-activated Cl− channels (Dick et al., 1998), stretch-activated, nonselective cation channels (Wellner and Isenberg, 1993), Cl− channels (Ji et al., 2002) and Ca2+ channels (Farrugia et al., 1999). Activation of any of these channels under physiological ionic gradients results in inward currents, which may lead to depolarization and muscle contraction.
There has been speculation in the literature for at least 20 years that BSMs are ‘stretch receptors' for myogenic responses, but no experimental evidence exists to support this hypothesis. Our group has reported that expression of SDK channels in colonic myocytes also encoded with the TREK-1 gene as a molecular candidate (Koh and Sanders, 2001; Koh et al., 2001). Membrane stretch is an effective activator of SDK and TREK channels (Maingret et al., 1999). Therefore, we hypothesized that SDK (TREK) channels may provide a mechanism to inhibit contraction in response to stretch in BSM cells. In our present experiments, BSM cells from different species exhibited different patterns of electrical activity. In particular, guinea pig bladder strips were extensively studied previously, which revealed regular spontaneous mechanical and electrical activity (Hashitani et al., 2000). However, monkey and murine BSM cells did not exhibit spontaneous contractions. The different contractile patterns in the three species were not due to a species-related expression of TREK-1 channels, as these were equally expressed in all three species. However, monkey and murine BSM cells did not exhibit spontaneous contractions.
We have reported that sulphur-containing amino acids may be useful blockers of SDK channels (Park et al., 2005) and L-methionine was found to be a relatively selective blocker of SDK channels with little or no effect on other known K+ conductances (Park et al., 2005). In present study, L-methionine and its two derivatives, methioninol and methionine methyl ester, were found to inhibit both the native SDK channels and the TREK-1 channels expressed in COS cells, activated by arachidonic acid and stretch. These effects corresponded to the effects of L-methionine or methioninol on BSM excitability in murine, guinea pig and monkey bladders, strongly suggesting that the increased excitability of BSM was causally related to inhibition of the SDK, and probably TREK-1, channels. However, the mechanism of methionine action needs further study.
BK channels have been shown to be involved in the regulation of membrane potential in BSM (Heppner et al., 1997), and these channels are activated by depolarization and by intracellular Ca2+ release through ryanodine receptors (Herrera and Nelson, 2002). To exclude the effects of methioninol on BK channels, we examined its effects on contractility and electrical activity of the SDK channels in the presence of the BK channel blockers, TEA or charybdotoxin. Although both mechanical and electrical events were increased in the presence of the BK channel blockers, addition of methioninol further increased bladder contractility. In patch clamp experiments, L-methionine did not affect the open probability of BK channels, suggesting that the effects of methioninol on contractility and membrane potentials were also independent of BK channels. SK channels are also present in bladder myocytes (Herrera and Nelson, 2002; Herrera et al., 2003), but methioninol in the presence of apamin (a SK channel blocker) further increased contractility, suggesting that the effect of methioninol was independent of SK channels. Furthermore, methioninol also showed significant increase of bladder contractility in the presence of both SK and BK channel blockers. In addition, spontaneous transient outward currents have been previously reported in murine BSM cells (Weidelt and Isenberg, 2000) and intracellular Ca2+ level can alter the open probability of BK or SK channels. However, methioninol did not affect intracellular Ca2+ sparks in isolated cells (data not shown). These observations clearly indicate that the effects of methioninol are not mediated through BK or SK channels
Only a small proportion of murine BSM preparations display phasic electrical activity. We can only speculate as to why some BSM strips were electrically active, whereas others remained quiescent. During the pinning of each strip, variations in the degree of tension may have altered the excitability. Similarly, each preparation may have had a slightly different stretch sensitivity. Extreme stretch can activate nonselective cation channels (Wellner and Isenberg 1993) and induce increased excitability. The mechanisms underlying phasic contractions are likely to involve coupling between BSM cells. Normally, the individual random firing of BSM cells results in an asynchronous generation of tone caused by poor electrical coupling (Turner and Barding, 1997). In those situations where coupling is enhanced between BSM cells (for example, after addition of methionine), muscle action potentials are able to spread through the muscle syncitium causing a synchronous contraction of many muscle cells, giving rising to measurable phasic contractile activity.
Stretch of bladder myocytes has been shown to activate stretch-activated nonselective cation (SAC) channels (Wellner and Isenberg, 1993). This can lead to contraction via Ca2+ influx through SAC, which trigger Ca2+ action potentials in response to depolarization and can induce Ca2+ release from intracellular stores. However, stretch does not activate contraction in bladder, but instead results in stabilization of membrane potential. Thus, if cation influx via SACs is fundamental to myogenic responses to stretch, then influx of these ions is counterbalanced by another mechanism that reduces excitability. This cation influx is not compatible with stabilization of membrane potentials. In our Ca2+ imaging experiments, to examine the response to stretch, we found that Ca2+ release in BSM is minimal in response to moderate levels of tissue stretch. BK channels were likely to be activated by stretch in these preparations as Ca2+ transients were significantly increased in the presence of a BK blocker. At higher degrees of stretch, BSM cells exhibit an increased frequency of Ca2+ transients; however, responses varied between preparations. Although we measured the overall tension on the bladder segment, the complicated non-orthogonal arrangement of muscle fibers in this tissue made it impossible to precisely determine the length/tension of cells within the field of view. Some preparations showed only small increases in BSM firing during active stretch (ramp) compared with maintained stretch and suggests the threshold required to increase the frequency of Ca2+ transients occurred at a higher level during active stretch (Figures 10a and b). In other preparations, an increase in the frequency of BSM Ca2+ transients occurred at much lower levels of stretch and was comparable to the frequency observed during maintained stretch (Figures 11a and b). However, the most interesting finding was the effect of methioninol on stretched BSM, appearing to alter the nature of BSM syncitium, allowing Ca2+ waves to spread long distances through the tissue. Such effects were not seen under control conditions or even after TEA. In the whole bladder, cystometric data suggested that a component of bladder overactivity may involve the inhibition of SDK channel in bladder myocytes.
In conclusion, SDK channels appear to be important to prevent spread of excitation through the syncitium during bladder filling. Further characterization of methionine compounds may be useful to develop a new group of drugs to be used clinically to target bladder excitability.
Acknowledgments
This research was supported by NIH P20-RR18751 to SDK. We express our sincere appreciation to Charles River Laboratories, Preclinical Services for their generous donation of monkey bladder samples.
Abbreviations
- AUC
area under the curve
- BK
large-conductance Ca2+-activated K+ channels
- BSM
bladder smooth muscle
- SAC
stretch-activated nonselective cation channels
- SDK
stretch-dependent K+ channels
- SK
small-conductance Ca2+-activated K+ channel
- TEA
tetraethylammonium
- TREK
TWIK-related K+ channel
Conflict of interest
The authors state no conflict of interest.
References
- Bayguinov O, Hagen B, Bonev AD, Nelson MT, Sanders KM. Intracellular calcium events activated by ATP in murine colonic myocytes. Am J Physiol Cell Physiol. 2000;279:C126–C135. doi: 10.1152/ajpcell.2000.279.1.C126. [DOI] [PubMed] [Google Scholar]
- Brading AF. Ion channels and control of contractile activity in urinary bladder smooth muscle. Jpn J Pharmacol. 1992;58:120P–127P. [PubMed] [Google Scholar]
- Brading AF. The pathophysiological changes in the bladder obstructed by benign prostatic hyperplasia. Br J Urol. 1994;74:133. [PubMed] [Google Scholar]
- Dick GM, Bradley KK, Horowitz B, Hume JR, Sanders KM. Functional and molecular identification of a novel chloride conductance in canine colonic smooth muscle. Am J Physiol. 1998;275:C940–C950. doi: 10.1152/ajpcell.1998.275.4.C940. [DOI] [PubMed] [Google Scholar]
- Farrugia G, Holm AN, Rich A, Sarr MG, Szurszewski JH, Rae JL. A mechanosensitive calcium channel in human intestinal smooth muscle cells. Gastroenterology. 1999;117:900–905. doi: 10.1016/s0016-5085(99)70349-5. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Bramich NJ, Hirst GD. Mechanisms of excitatory neuromuscular transmission in the guinea-pig urinary bladder. J Physiol. 2000;524:565–579. doi: 10.1111/j.1469-7793.2000.t01-2-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig GW, Smith CB, O'Shea DM, Smith TK. Patterns of intracellular and intercellular Ca2+ waves in the longitudinal muscle layer of the murine large intestine in vitro. J Physiol. 2002;543:233–253. doi: 10.1113/jphysiol.2002.018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner TJ, Bonev AD, Nelson MT. Ca2+-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am J Physiol. 1997;273:C110–C117. doi: 10.1152/ajpcell.1997.273.1.C110. [DOI] [PubMed] [Google Scholar]
- Herrera GM, Nelson MT. Differential regulation of SK and BK channels by Ca2+ signals from Ca2+ channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J Physiol. 2002;541:483–492. doi: 10.1113/jphysiol.2002.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, et al. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol. 2003;551:893–903. doi: 10.1113/jphysiol.2003.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Barsotti RJ, Feldman ME, Kotlikoff MI. Stretch-induced calcium release in smooth muscle. J Gen Physiol. 2002;119:533–544. doi: 10.1085/jgp.20028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. A mechanosensitive K+ channel in heart cells. Activation by arachidonic acid. J Gen Physiol. 1992;100:1021–1040. doi: 10.1085/jgp.100.6.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinder RB, Mundy AR. Pathophysiology of idiopathic detrusor instability and detrusor hyper-reflexia. An in vitro study of human detrusor muscle. Br J Urol. 1987;60:509–515. doi: 10.1111/j.1464-410x.1987.tb05031.x. [DOI] [PubMed] [Google Scholar]
- Klockner U, Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig) Pflugers Arch. 1985;405:329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- Koh SD, Monaghan K, Sergeant GP, Ro S, Walker RL, Sanders KM, et al. TREK-1 regulation by nitric oxide and CGMP dependent protein kinase: an essential role in smooth muscle inhibitory neurotransmission. J Biol Chem. 2001;276:44338–44346. doi: 10.1074/jbc.M108125200. [DOI] [PubMed] [Google Scholar]
- Koh SD, Sanders KM. Stretch-dependent potassium channels in murine colonic smooth muscle cells. J Physiol. 2001;533:155–163. doi: 10.1111/j.1469-7793.2001.0155b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin RM, Ruggieri MR, Velagapudi S, Gordon D, Altman B, Wein AJ. Relevance of spontaneous activity to urinary bladder function: an in vitro and in vivo study. J Urol. 1986;136:517–521. doi: 10.1016/s0022-5347(17)44934-2. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- Ordway RW, Petrou S, Kirber MT, Walsh JV, Jr, Singer JJ. Stretch activation of a toad smooth muscle K+ channel may be mediated by fatty acids. J Physiol. 1995;484:331–337. doi: 10.1113/jphysiol.1995.sp020668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KJ, Baker SA, Cho SY, Sanders KM, Koh SD. Sulfur-containing amino acids block stretch-dependent K+ channels and nitrergic responses in the murine colon. Br J Pharmacol. 2005;144:1126–1137. doi: 10.1038/sj.bjp.0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackin H. Stretch-activated potassium channels in renal proximal tubule. Am J Physiol. 1987;253:F1253–F1262. doi: 10.1152/ajprenal.1987.253.6.F1253. [DOI] [PubMed] [Google Scholar]
- Turner WH, Barding AF. Smooth muscle of the bladder in the normal and the diseased state: pathophysiology, diagnosis and treatment. Pharmacol Ther. 1997;75:77–110. doi: 10.1016/s0163-7258(97)00038-7. [DOI] [PubMed] [Google Scholar]
- Weidelt T, Isenberg G. Augmentation of SR Ca2+ release by rapamycin and FK506 causes K+-channel activation and membrane hyperpolarization in bladder smooth muscle. Br J Pharmacol. 2000;129:1293–1300. doi: 10.1038/sj.bjp.0703223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellner MC, Isenberg G. Properties of stretch-activated channels in myocytes from the guinea-pig urinary bladder. J Physiol. 1993;466:213–227. [PMC free article] [PubMed] [Google Scholar]