Abstract
Background and purpose:
Transient receptor potential melastatin 2 (TRPM2) is a non-selective Ca2+-permeable cation channel and is known to be activated by adenosine 5′-diphosphoribose (ADP-ribose) and hydrogen peroxide. TRPM2 current responses are reported to be drastically potentiated by the combination of each of these ligands with heat. Furthermore, the combination of cyclic ADP-ribose with heat also activates TRPM2. Although flufenamic acid, antifungal agents (miconazole and clotrimazole), and a phospholipase A2 inhibitor (N-(p-amylcinnamoyl)anthranilic acid) inhibit TRPM2, their inhibition was either gradual or irreversible.
Experimental approach:
To facilitate future research on TRPM2, we screened several compounds to investigate their potential to activate or inhibit the TRPM2 channels using the patch-clamp technique in HEK293 cells, transfected with human TRPM2.
Key results:
2-aminoethoxydiphenyl borate (2-APB) exhibited a rapid and reversible inhibition of TRPM2 channels that had been activated by its ADP-ribose or cADP-ribose and heat in a dose-dependent manner (IC50 about 1 μM). 2-APB also inhibited heat-evoked insulin release from pancreatic islets, isolated from rats.
Conclusions and implications:
2-APB proved to be a powerful and effective tool for studying the function of TRPM2.
Keywords: TRPM2, 2-APB, insulin
Introduction
The transient receptor potential (TRP) superfamily of ion channels in mammals comprises the following six subfamilies: TRPC (canonical or classical), TRPV (vanilloid), TRPM (melastatin), TRPA (ankyrin), TRPP (polycystin) and TRPML (mucolipin) (Clapham, 2003). Among them, to date, nine channels have been reported to be thermosensitive (Bandell et al., 2007). The capsaicin receptor (TRPV1) is the best-known thermosensitive TRP channel, and many specific and nonspecific antagonists of this channel have been reported (Holzer, 2004; Clapham et al., 2005). However, it is difficult to exert an inhibitory effect on the TRPV1-mediated heat-evoked current responses (Tominaga et al., 1998). In fact, only one chemical, SB-705498, has been reported to completely inhibit heat-evoked TRPV1 responses (Rami et al., 2006).
We have recently reported that TRPM2 is one of the thermosensitive TRP channels, and is involved in insulin secretion following ligand activation at body temperature (Togashi et al., 2006). However, there are few membrane-permeable TRPM2-specific agonists, which make further research on TRPM2 difficult. Although hydrogen peroxide (H2O2) has been known to activate TRPM2 from outside (Hara et al., 2002), its action on TRPM2 is presumed to be mediated by ADP-ribose (ADPR) production; additionally, H2O2 is known to exhibit various effects on cellular functions. Several chemicals have been reported to act as TRPM2 antagonists. However, they cannot be easily used for experimental purposes. For example, imidazole derivatives, miconazole and clotrimazole, inhibit TRPM2 in an irreversible manner (Hill et al., 2004b). Another TRPM2 inhibitor, flufenamic acid (FFA), a nonsteroidal anti-inflammatory drug, is not easily dissolved in the aqueous solution, which makes it difficult to prepare a solution containing high concentrations of FFA to inhibit TRPM2 (Hill et al., 2004a). Furthermore, although N-(p-amylcinnamoyl)anthranilic acid (ACA) is an inhibitor of TRPM2 (Kraft et al., 2006), it also functions as a phospholipase A2 inhibitor (Chen et al., 1994).
2-Aminoethoxydiphenyl borate (2-APB) was first reported as an inositol 1,4,5-trisphosphate (InsP3) receptor antagonist (Maruyama et al., 1997) and is known to inhibit InsP3 receptor at relatively low concentrations (Bootman et al., 2002). However, there are several reports proposing that the blocking effects of 2-APB on the increase in intracellular Ca2+ levels results from inhibition of plasma membrane, Ca2+-permeable, cation channels by 2-APB rather than that of Ca2+ release from InsP3-sensitive Ca2+ stores (Dobrydneva and Blackmore, 2001; Bootman et al., 2002). In fact, certain TRPC channels (TRPC1, TRPC3, TRPC5 and TRPC6) and TRPM channels (TRPM3, TRPM7 and TRPM8) have been reported to be inhibited by 2-APB (Ma et al., 2000; Delmas et al., 2002; Hu et al., 2004; Xu et al., 2005; Li et al., 2006). In contrast, some TRPV channels (TRPV1, TRPV2 and TRPV3) are known to be activated by 2-APB (Chung et al., 2004; Hu et al., 2004).
Here, we have screened several compounds to investigate their potential to activate or inhibit the TRPM2 channels, and we observed that 2-APB exerts a strong inhibitory effect on TRPM2 channels that are activated by its ligand and heat. In addition, we observed that 2-APB inhibited heat-evoked insulin release from the pancreatic islets.
Methods
Preparation of HEK cells
Human embryonic kidney cells (HEK293 cells) were maintained in Dulbecco's modified Eagle's medium (Sigma, St Louis, MO, USA; supplemented with 10% fetal bovine serum, penicillin–streptomycin (Gibco, Carlsbad, CA, USA) and heat-stable L-glutamine analogue, GlutaMAX-I (Gibco)) on a culture dish (φ=10 cm) at 37 °C. We confirmed that no TRPM2 is endogenously expressed in HEK293 cells both in western blotting (data not shown) and patch-clamp recordings (Togashi et al., 2006).
HEK293 cells on a culture dish (φ=35 mm) (5 × 105 cells) were transfected with 1.0 μg of human TRPM2 cDNA (generously provided by Dr Mori, Kyoto University) using Lipofectamine and PLUS Reagent (Invitrogen, Carlsbad, CA, USA). Subsequently, the cells were reseeded on 16 15-mm coverslips and maintained at 33 °C for 28–34 h before the experiments. To visually identify cells that expressed exogenous channels in the patch-clamp experiments, we cotransfected the cells with 0.1 μg of pGreen Lantern-1 plasmid (Life Technologies, Carlsbad, CA, USA), which encodes the GFP protein.
Patch-clamp procedures
The standard bath solution (pH 7.4 adjusted with NaOH) for the patch-clamp experiments contained (in mM) 140 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES and 10 glucose. The pipette solution (pH 7.4 adjusted with CsOH) for the recordings contained (in mM) 140 CsCl, 5 EGTA, and 10 HEPES. In the Ca2+-controlled experiments (Figure 4), the pipette solution contained (in mM) 140 CsCl, 5 EGTA, 10 HEPES, 5 ATP and 500 μM of ADPR was included in the pipette solution for TRPM2 activation. In the presence of Ca2+, the Ca2+ concentration was adjusted at 200 nM by adding the appropriate amount of CaCl2 calculated by the CaBuf program (ftp://ftp.cc.kuleuven.ac.be/pub/droogmans/cabuf.zip). Whole-cell recording data were sampled at 10 kHz and filtered at 5 kHz for analysis (AxoPATCH 200B amplifier with pCLAMP 8.2 software, Axon Instruments, Foster City, CA, USA). Whole-cell patch-clamp recordings were performed 1 day after the transfection into the HEK293 cells. All patch-clamp experiments were performed at 25 °C, with the exception of the heat stimulus experiments. In an effort to observe a maximal TRPM2 response to heat, we decided to apply heat ramps approximately 2–3 min after establishing the whole-cell configuration with the pipette solution containing ADPR or cADPR. As the ADPR-evoked currents developed at various time points (approximately 3 min) after establishing the whole-cell configuration, the heat stimuli were applied by increasing the bath temperature at a rate of 1.0 °C s−1 with a preheated solution. When the inactivation of the heat-activated currents began, the temperature of the preheated solution was changed to 25 °C. The chamber temperature was monitored with a thermocouple placed within 100 μm of the patch-clamped cell.
Preparations of pancreatic islets from rats
All procedures involving the care and use of animals were carried out in accordance with institutional (National Institute for Physiological Science) guidelines. Male Wistar rats (9–10 weeks of age; SLC, Shizuoka, Japan) were used. They were housed in a controlled environment (12 h light/dark cycle, room temperature 22–24 °C, 50–60% relative humidity) with free access to food and water. Rats were deeply anaesthetized with pentobarbital sodium (70 mg kg−1) and killed. The common bile duct was cannulated with a 21-gauge needle and the pancreas was distended with 10 ml of Hanks' balanced salt solution (Gibco) containing collagenase. Subsequently, the pancreas was excised and digested in the Hanks' balanced salt solution at 37 °C. The pancreatic islets were purified on HISTOPAQUE-1077 (Sigma) gradients by a method modified from the one described previously (Sutton et al., 1986; Ban et al., 2000; Togashi et al., 2006). The islets were dispersed in RPMI-1640 medium (with 20% fetal bovine serum, penicillin–streptomycin and GlutaMAX-I); subsequently, they were cultured overnight at 37 °C. Insulin release from the islets was measured following 16-h culture at 37 °C after isolation as previously described (Togashi et al., 2006). The amount of immunoreactive insulin released was determined by ELISA (Morinaga, Yokohama, Japan). The amount of insulin was normalized to the values released from the islets by glucose (3.3 mM). Groups of islets were incubated in Krebs–Ringer bicarbonate buffer with additives for 60 min. At the end of the incubation period, islets were pelleted and aliquots of the buffer were sampled.
Statistical methods
Data are analysed using an unpaired (* or **) or a paired (#) t-test. Values are shown as mean±s.e.mean. P-values <0.05 were considered significant.
Materials
ADP-ribose sodium salt, cyclic ADPR (cADPR), 2-APB and exendin-4 were purchased from Sigma. Stocks of ADPR and cADPR were dissolved in distilled water at concentration of 10 mM, aliquoted into small fractions and frozen at −80 °C. Each aliquot was freshly thawed and diluted in the pipette solution before starting the experiment. The stock of 2-APB was dissolved in 500 mM dimethyl sulphoxide and stored at −20 °C. The stock of exendin-4 was dissolved in 200 μM phosphate-buffered saline with 0.5% BSA, aliquoted into small fractions and frozen at −80 °C. Before the experiment, each stock solution was diluted in the bath solution or Krebs–Ringer bicarbonate buffer.
Results
We first examined the effects of the previously reported TRPM2 antagonists on the ADPR-activated currents. Because the ADPR-activated TRPM2 currents often exhibit some desensitization (Figure 1a) (Togashi et al., 2006), we applied the antagonists just after starting desensitization, although the time course of the compound-induced inhibition was much faster than that of desensitization. Both miconazole (10 μM) and ACA (20 μM) exhibited complete inhibition of ADPR (100 μM)-evoked TRPM2 responses at −60 mV (Figures 1b and c). In contrast, FFA did not exert complete inhibition even at its highest available concentration (200 μM; Figure 1d). We attempted to examine the effects of 2-APB on the TRPM2 currents. ADPR-activated inward currents were inhibited reversibly and rapidly in a dose-dependent manner by 2-APB (Figure 1e), which suggests that the inhibition was not mediated by InsP3 receptor. In either case, the inhibition was much faster than the desensitization. To examine whether 2-APB-induced inhibition of TRPM2 current is voltage dependent, we examined the effects of 2-APB (100 μM) on the TRPM2 currents at different potentials. The ADPR-activated TRPM2 currents did not change during 100-ms-step pulses, and 2-APB caused partial (at 10 μM) or complete (at 100 μM) inhibition of TRPM2 currents at all the membrane potentials examined (Figure 1f), indicating that TRPM2 inhibition by 2-APB was voltage independent.
Figure 1.
Inhibition of transient receptor potential melastatin 2 (TRPM2) currents by 2-aminoethoxydiphenyl borate (2-APB) in human embryonic kidney cells (HEK293 cells) expressing TRPM2. (a) A representative trace of TRPM2 current activated by ADP-ribose (ADPR; 100 μM) and its desensitization. (b–d) The known TRPM2 inhibitors—miconazole (10 μM; b), N-(p-amylcinnamoyl)anthranilic acid (ACA, 20 μM; c) and flufenamic acid (FFA, 200 μM; d)—inhibited TRPM2 currents activated by ADPR (100 μM) at room temperature. Bars indicate duration of the compound application. (e) Dose-dependent inhibition of ADPR-activated TRPM2 currents by 2-APB at room temperature. Vh=−60 mV. (f) Current–voltage relation (left) of ADPR-activated whole-cell currents before activation (right upper), during peak activation (right second upper), after inhibition by 10 μM of 2-APB (right third upper) and after inhibition by 100 μM of 2-APB (right lower). The inset on the left indicates the step-pulse protocol.
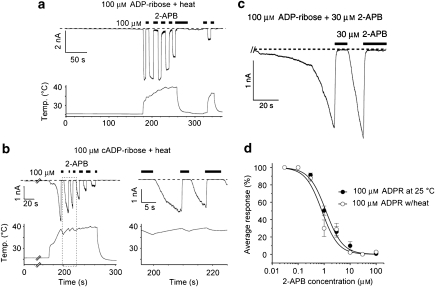
This inhibition of ADPR-evoked TRPM2 currents by 2-APB prompted us to examine the effects of 2-APB on the TRPM2 currents elicited by ADPR with heat. As shown in Figure 2a, 2-APB (100 μM) caused a rapid, complete and reversible inhibition of TRPM2 currents activated by ADPR (100 μM) with heat. Similarly, 2-APB was found to inhibit TRPM2 currents activated by cADPR (100 μM) with heat (Figure 2b). In addition, prolonged heat stimulus resulted in desensitization of TRPM2 currents (Figures 2a and b) similar to that observed in repetitive heat application reported before (Togashi et al., 2006). The rapid and complete inhibition of TRPM2 channels with easy washout by 2-APB (Figure 2b, right) suggests that 2-APB acts on TRPM2 from outside. This interpretation was supported by results from other experiments where TRPM2 currents were activated by ADPR using a pipette solution containing 2-APB (30 μM) and inhibited by 2-APB (30 μM) applied from outside (Figure 2c). Similar IC50 values for 2-APB-induced inhibition of TRPM2 currents activated by ADPR regardless of heat stimulus (0.82±0.2 μM for with heat and 1.17±0.1 μM for without heat) (Figure 2d) suggest identical mechanisms for the inhibitory action of 2-APB, despite the differences in the current size and channel properties, with and without the heat stimulus (Togashi et al., 2006).
Figure 2.
2-Aminoethoxydiphenyl borate (2-APB) completely and reversibly inhibits transient receptor potential melastatin 2 (TRPM2) currents activated by its ligands in combination with heat. (a and b) Representative traces of the 2-APB-induced inhibition of TRPM2 currents activated by ADP-ribose (ADPR; 100 μM) in combination with heat (40 °C; a) or cyclic ADPR (cADPR; 100 μM) in combination with heat (b). A right panel in (b) indicates the magnification of a boxed region in the left panel. Bars indicate duration of the compound application. Vh=−60 mV. (c) A representative trace of TRPM2 current activated by ADPR (100 μM) with a pipette solution containing 2-APB (30 μM) and its inhibition by extracellularly applied 2-APB (30 μM). (d) Concentration-dependent profiles for 2-APB-induced inhibition of TRPM2 currents activated by 100 μM ADPR with or without heat (±s.e.mean). Curves were fitted with the data from 40 and 35 cells for without and with heat, respectively. IC50 values were 0.82±0.2 μM (with heat) and 1.17±0.1 μM (without heat). Hill coefficients with and without heat were 1.38 and 1.34, respectively.
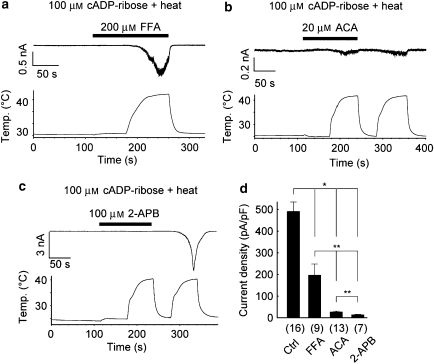
Next, we speculated whether this 2-APB-induced inhibition is specific to TRPM2 currents activated by its ligands with heat. Even in the presence of FFA (200 μM), the cADPR with heat could activate TRPM2 channels although the activated currents were significantly smaller than those without inhibitors (Figures 3a and d). In the presence of ACA (20 μM), slight TRPM2 current activation was observed, and the inhibitory effects lasted even after ACA washout (Figures 3b and d). In contrast, TRPM2 activation was completely inhibited in the presence of 2-APB, and huge inward currents were elicited by cADPR with heat upon 2-APB washout (Figures 3c and d); this clear washout effect was similar to that observed in the short 2-APB application after TRPM2 activation (Figure 2b). These results indicate that 2-APB is a good inhibitor to characterize electrophysiological properties of TRPM2.
Figure 3.
2-Aminoethoxydiphenyl borate (2-APB)-induced inhibition of transient receptor potential melastatin 2 (TRPM2) currents by cyclic ADPR (cADPR) plus heat was complete and reversible. (a–c) Representative traces of TRPM2 currents activated by cADPR in combination with heat in the presence and absence of flufenamic acid (FFA, 200 μM; a), N-(p-amylcinnamoyl)anthranilic acid (ACA, 20 μM; b) or 2-APB (100 μM; c). (d) Comparison of the TRPM2 current densities activated by cADPR in combination with heat in the presence of the indicated compounds. Numbers in parentheses indicate the number of cells tested. *P<0.01, **P<0.05.
There is a report showing that 2-APB (75 and 150 μM) had no effect on TRPM2 currents activated by ADPR although the same concentrations were found to inhibit TRPC5, TRPC6 and TRPM3 (Xu et al., 2005), contradicting our results. They used HEK293 cells having stable tetracycline-regulated expression of cDNA encoding TRPM2, which looks unlikely to explain the difference, and they used a pipette solution containing sodium ATP (5 mM) and 200 nM free Ca2+. To identify the reason why we could see the inhibition of TRPM2 currents by 2-APB, we used an identical pipette solution to that used by Xu et al. with (200 nM) or without Ca2+. However, we could see complete inhibition of TRPM2 currents activated by 500 μM of ADPR (98.8±0.5% inhibition with Ca2+ and 99.0±0.4% inhibition without Ca2+) by 2-APB (30 μM) in either condition, although current activation by ADPR was more rapid in the presence of 200 nM of cytosolic free Ca2+ as previously reported (McHugh et al., 2003) (Figure 4).
Figure 4.
2-Aminoethoxydiphenyl borate (2-APB)-induced inhibition of transient receptor potential melastatin 2 (TRPM2) currents by ADP-ribose (ADPR) was not related to the cytosolic ATP or Ca2+. (a and b) Representative traces of the 2-APB-induced inhibition of TRPM2 currents activated by ADPR (500 μM) in the presence (200 nM; a) and absence (b) of intracellular Ca2+. Whole-cell configuration was established at arrows. (c) Inhibitory effects of 2-APB (30 μM) on TRPM2 currents activated by ADPR (500 μM) in the presence (200 nM) and absence of intracellular Ca2+. Numbers in parentheses indicate the number of cells tested.
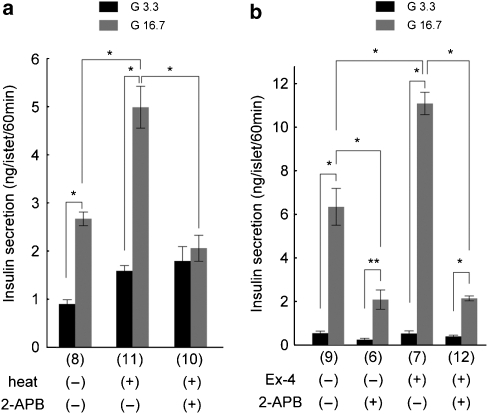
Finally, we examined the effects of 2-APB on insulin release from the rat pancreatic islets because we reported that at physiological body temperature, TRPM2 activation is involved in insulin release (Togashi et al., 2006). Heat stimulus at 40 °C for 5 min caused significant increase in insulin release as previously reported (Figure 5a) (Togashi et al., 2006) and this heat-induced insulin release was almost completely inhibited by 2-APB (100 μM). To examine the 2-APB effects on insulin release under more physiological conditions, we applied 2-APB at 37 °C. 2-APB significantly inhibited insulin release (Figure 5b), thereby suggesting that TRPM2 is involved in insulin release at normal core body temperature as reported earlier (Togashi et al., 2006). We previously reported that TRPM2-mediated action might be involved in glucagon-like peptide 1 (GLP-1)-induced insulin release that involves cAMP activation downstream of glucagon-like peptide 1 receptor (Togashi et al., 2006). Therefore, we examined the effects of 2-APB on the insulin release enhanced by exendin-4, a glucagon-like peptide 1 receptor agonist. 2-APB inhibited the exendin-4-enhanced insulin release to the level similar to that caused by 2-APB without exendin-4 (Figure 5b), suggesting that exendin-4-mediated insulin release involves TRPM2 activation.
Figure 5.
Inhibition of insulin secretion from rat isolated pancreatic islets by 2-aminoethoxydiphenyl borate (2-APB). (a) Heat (40 °C for 5 min)-evoked increase in insulin secretion was inhibited by 2-APB (100 μM). Data from the islets incubated at 29 °C with (+) or without (−) heat stimulation in the presence of 3.3 mM (G3.3) or 16.7 mM (G16.7) glucose. *P<0.01. (b) 2-APB significantly inhibited the insulin secretion in the presence or absence of exendin-4 (Ex-4, 10 nM) at 37 °C. *P<0.01, **P<0.05.
Discussion and conclusions
In the present study, we found that 2-APB could reversibly and completely inhibit TRPM2 currents activated by either ligand alone or a combination of ligands with heat at concentrations comparable to those reported for the known TRPM2 inhibitors (Togashi et al., 2006). In addition, potency of 2-APB to inhibit TRPM2 seems to be higher than those for other channels and proteins (Table 1). Furthermore, 2-APB could clearly inhibit insulin secretion from isolated pancreatic islets, confirming our recent report that TRPM2 activation at body temperature is involved in insulin secretion (Togashi et al., 2006). Given that many mechanisms and pathways are known to be involved in insulin secretion, the significant inhibition of insulin release by 2-APB observed in our study suggests that a mechanism involving TRPM2 plays an important role in insulin secretion in pancreas. The present study also suggests that we should interpret findings with TRPM2 blockers carefully. As ACA, a TRPM2 and phospholipase A2 inhibitor, is known to inhibit insulin secretion (Chen et al., 1994), that secretion would seem to involve both TRPM2 and phospholipase A2. It has been reported that 2-APB (75 and 150 μM) exhibited no effects on ADPR-evoked TRPM2 responses at a whole-cell current level (Xu et al., 2005). To study this phenomenon, we used an identical pipette solution to that used by Xu et al. (2005), and activated TRPM2 by high concentration of ADPR (500 μM). Even under these conditions, 30 μM 2-APB, a lower concentration than those in the report, was found to inhibit the ADPR-activated TRPM2 currents, almost completely regardless of the presence of cytosolic free Ca2+ (Figure 4). Thus, we could not identify the factor(s) responsible for the difference between the two sets of results. Xu et al. (2005) showed no activation process of TRPM2 by ADPR in the experiments examining 2-APB effects, suggesting that some leak components could be present. Alternatively, the tetracycline-regulated expression system with FLAG-epitope-tagged TRPM2, as used in their study might influence the effect of 2-APB.
Table 1.
Comparison of the potencies of 2-APB in inhibiting a range of channels and proteins
| Proteins | Concentration or IC50* | References |
|---|---|---|
| TRPC1 | More than 80% inhibition at 80 μM | Delmas et al., 2002 |
| TRPC3 | Almost complete inhibition at 75 μM | Ma et al., 2000 |
| TRPC5 | 19 μM* | Xu et al., 2005 |
| TRPC6 | 10.4 μM* | Hu et al., 2004 |
| TRPM2 | 0.82 μM* (ADPR+heat), 1.17 μM* (ADPR) | |
| TRPM3 | 87.5% inhibition at 100 μM | Xu et al., 2005 |
| TRPM7 | 178 μM* | Li et al., 2006 |
| TRPM8 | 7.7 μM* | Hu et al., 2004 |
| InsP3 receptor | 42 μM* | Maruyama et al., 1997 |
| Connexin36 | 3.0 μM* | Bai et al., 2006 |
| Endogenous voltage-gated K+ channel (Limulus) | 5 μM* | Wang et al., 2002 |
Abbreviations: ADPR, ADP-ribose; TRP, transient receptor potential; TRPC, TRP canonical or classical; TRPM, TRP melastatin. *Indicate IC50 values but not significance as shown at the top of Table 1.
Insulin secretion from pancreatic islets was profoundly inhibited by 2-APB, regardless of treatment with exendin-4 (Figure 5b). This effect might be partly explained by the inhibitory effects of 2-APB on other proteins expressed in pancreas, such as connexins, in addition to its effect on TRPM2. For instance, connexin36-mediated coupling is known to contribute to the coordination and synchronization of the function of individual cells within pancreatic islets, particularly in the context of glucose-induced insulin secretion (Nlend et al., 2006) and 2-APB has been reported to inhibit specific gap junction channel subtypes including connexin36 in the micromolar range (Bai et al., 2006). Alternatively, IP3 receptors, K+ channels and other TRP channels, which can be blocked by 2-APB (see Table 1), could also be involved in insulin release.
Structural comparison of the known TRPM2 inhibitors including 2-APB will provide additional information on the structural basis for the TRPM2 gating kinetics. The fact that 2-APB-inhibition of TRPM2 channel is rapid and reversible suggests that 2-APB acts from the outside of the cell membrane. Single-channel analysis will provide us with a more detailed understanding of the mechanism underlying 2-APB inhibition of TRPM2. We conclude that 2-APB is a powerful and effective tool in studying the functions of TRPM2 channels, whose physiological significance can be appreciated in a variety of physiological functions ranging from cell death (Hara et al., 2002) to insulin release.
Acknowledgments
We thank Dr Y Mori for providing us with human TRPM2 cDNA. This work was supported by grants to MT from the Ministry of Education, Culture, Sports, Science and Technology (Japan) and Japan Diabetes Foundation.
Abbreviations
- ACA
N-(p-amylcinnamoyl)anthranilic acid
- ADPR
ADP-ribose
- 2-APB
2-aminoethoxydiphenyl borate
- FFA
flufenamic acid
- HEK293
human embryonic kidney 293 cells
- TRPM2
transient receptor potential melastatin subtype 2
Conflict of interest
The authors state no conflict of interest.
References
- Bai D, del Corsso C, Srinivas M, Spray DC. Block of specific gap junction channel subtypes by 2-aminoethoxydiphenyl borate (2-APB) J Pharmacol Exp Ther. 2006;319:1452–1458. doi: 10.1124/jpet.106.112045. [DOI] [PubMed] [Google Scholar]
- Ban N, Yamada Y, Someya Y, Ihara Y, Adachi T, Kubota A, et al. Activating transcription factor-2 is a positive regulator in CaM kinase IV-induced human insulin gene expression. Diabetes. 2000;49:1142–1148. doi: 10.2337/diabetes.49.7.1142. [DOI] [PubMed] [Google Scholar]
- Bandell M, Macpherson LJ, Patapoutian A. From chills to chilis: mechanisms for thermosensation and chemesthesis via thermoTRPs. Curr Opin Neurobiol. 2007;17:490–497. doi: 10.1016/j.conb.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- Chen TH, Lee B, Hsu WH. Arginine vasopressin-stimulated insulin secretion and elevation of intracellular Ca++ concentration in rat insulinoma cells: influences of a phospholipase C inhibitor 1-[6-[[17 beta-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione (U-73122) and a phospholipase A2 inhibitor N-(p-amylcinnamoyl)anthranilic acid. J Pharmacol Exp Ther. 1994;270:900–904. [PubMed] [Google Scholar]
- Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J Biol Chem. 2004;279:21569–21575. doi: 10.1074/jbc.M401872200. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure–function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- Delmas P, Wanaverbecq N, Abogadie FC, Mistry M, Brown DA. Signaling microdomains define the specificity of receptor-mediated InsP(3) pathways in neurons. Neuron. 2002;34:209–220. doi: 10.1016/s0896-6273(02)00641-4. [DOI] [PubMed] [Google Scholar]
- Dobrydneva Y, Blackmore P. 2-Aminoethoxydiphenyl borate directly inhibits store-operated calcium entry channels in human platelets. Mol Pharmacol. 2001;60:541–552. [PubMed] [Google Scholar]
- Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, et al. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002;9:163–173. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- Hill K, Benham CD, McNulty S, Randall AD. Flufenamic acid is a pH-dependent antagonist of TRPM2 channels. Neuropharmacology. 2004a;47:450–460. doi: 10.1016/j.neuropharm.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Hill K, McNulty S, Randall AD. Inhibition of TRPM2 channels by the antifungal agents clotrimazole and econazole. Naunyn Schmiedebergs Arch Pharmacol. 2004b;370:227–237. doi: 10.1007/s00210-004-0981-y. [DOI] [PubMed] [Google Scholar]
- Holzer P. TRPV1 and the gut: from a tasty receptor for a painful vanilloid to a key player in hyperalgesia. Eur J Pharmacol. 2004;500:231–241. doi: 10.1016/j.ejphar.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, et al. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279:35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- Kraft R, Grimm C, Frenzel H, Harteneck C. Inhibition of TRPM2 cation channels by N-(p-amylcinnamoyl)anthranilic acid. Br J Pharmacol. 2006;148:264–273. doi: 10.1038/sj.bjp.0706739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127:525–537. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HT, Patterson RL, van Rossum DB, Birnbaumer L, Mikoshiba K, Gill DL. Requirement of the inositol trisphosphate receptor for activation of store-operated Ca2+ channels. Science. 2000;287:1647–1651. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem (Tokyo) 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- McHugh D, Flemming R, Xu SZ, Perraud AL, Beech DJ. Critical intracellular Ca2+ dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J Biol Chem. 2003;278:11002–11006. doi: 10.1074/jbc.M210810200. [DOI] [PubMed] [Google Scholar]
- Nlend RN, Michon L, Bavamian S, Boucard N, Caille D, Cancela J, et al. Connexin36 and pancreatic beta-cell functions. Arch Physiol Biochem. 2006;112:74–81. doi: 10.1080/13813450600712019. [DOI] [PubMed] [Google Scholar]
- Rami HK, Thompson M, Stemp G, Fell S, Jerman JC, Stevens AJ, et al. Discovery of SB-705498: a potent, selective and orally bioavailable TRPV1 antagonist suitable for clinical development. Bioorg Med Chem Lett. 2006;16:3287–3291. doi: 10.1016/j.bmcl.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Sutton R, Peters M, McShane P, Gray DW, Morris PJ. Isolation of rat pancreatic islets by ductal injection of collagenase. Transplantation. 1986;42:689–691. doi: 10.1097/00007890-198612000-00022. [DOI] [PubMed] [Google Scholar]
- Togashi K, Hara Y, Tominaga T, Higashi T, Konishi Y, Mori Y, et al. TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J. 2006;25:1804–1815. doi: 10.1038/sj.emboj.7601083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Deshpande M, Payne R. 2-Aminoethoxydiphenyl borate inhibits phototransduction and blocks voltage-gated potassium channels in Limulus ventral photoreceptors. Cell Calcium. 2002;32:209–216. doi: 10.1016/s0143416002001562. [DOI] [PubMed] [Google Scholar]
- Xu SZ, Zeng F, Boulay G, Grimm C, Harteneck C, Beech DJ. Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect. Br J Pharmacol. 2005;145:405–414. doi: 10.1038/sj.bjp.0706197. [DOI] [PMC free article] [PubMed] [Google Scholar]