Abstract
Background and purpose:
Cyclooxygenase-2 (COX-2) is highly expressed during inflammation and can promote the progression of colorectal cancer. Interactions between cancer cells and vascular endothelial cells are key events in this process. Recently, the selective COX-2 inhibitor, celecoxib, was shown to inhibit expression of the adhesion molecules, ICAM-1 and VCAM-1, in the human colon cancer cell line HT29 and to inhibit adhesion of HT29 cells to FCS-coated plastic wells. Here, we evaluated the effects of celecoxib on adhesion of HT29 cells to human umbilical vein endothelial cells (HUVEC), mediated by ICAM-1 and VCAM-1, to assess further the potential protective effects of celecoxib on cancer development.
Experimental approach:
Celecoxib was incubated for 4 h with HT29 cells and HUVEC and adhesion was quantified by a computerized micro-imaging system. Expression analysis of ICAM-1 and VCAM-1 cell adhesion molecules was performed by western blot.
Key results:
Celecoxib (1 nM–10 μM) inhibited, with the same potency, adhesion of HT29 cells to resting HUVEC or to HUVEC stimulated by tumour necrosis factor-α (TNF-α), mimicking inflammatory conditions. Analysis of ICAM-1 and VCAM-1 expression showed that celecoxib inhibited expression of both molecules in TNF-α-stimulated HUVEC, but not in resting HUVEC; inhibition was concentration-dependent and maximal (about 50%) at 10 μM celecoxib.
Conclusions and implications:
In conclusion, our data show that celecoxib inhibits HT29 cell adhesion to HUVEC and expression of ICAM-1 and VCAM-1, in stimulated endothelial cells. These effects may contribute to the chemopreventive activity of celecoxib in the development of colorectal cancer.
Keywords: celecoxib, HUVEC, HT29 cells, adhesion, ICAM-1, VCAM-1
Introduction
Colorectal cancer accounts for about 15% of human malignancies and represents one of the most frequent causes of death by cancer in Western countries. To date, the best therapeutic option is surgical resection, but chemoprevention before the occurrence of the malignant tumour is receiving increasing attention as an attractive and plausible approach (Herendeen and Lindley, 2003; Chun and Surh, 2004, Rao and Reddy, 2004).
Chronic inflammation is regarded as an important factor in tumour promotion, and cell adhesion plays a key role in both processes (Kobayashi et al., 2007), as migration of inflammatory and tumour cells involves similar adhesive mechanisms. As with leukocytes, attachment of cancer cells to the vascular endothelium seems to be initiated by the interaction between sialyl Lewis x, a surface carbohydrate, expressed on several cell types including many tumour cells, and E-selectin expressed on vascular endothelial cells (Kannagi et al., 2004; Kobayashi et al., 2007) upon activation by proinflammatory cytokines, such as tumour-necrosis factor-α (TNF-α) or interleukin-1β (Gangopadhyay et al., 1998; Kobayashi et al., 2007). After this initial interaction, other cellular adhesion molecules are recruited to provide firm adhesion. Intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) are members of the immunoglobulin superfamily of proteins and play a key role not only in trafficking of leukocytes across endothelial and epithelial barriers, but also in tumour metastasis formation (Neeson et al., 2003; Yamada et al., 2006). Clinical studies show elevated serum levels of soluble VCAM-1 and ICAM-1 in melanoma patients (Schadendorf et al., 1996; Franzke et al., 1998; Boyano et al., 2000) and these levels correlate with tumour progression. Normal epithelial cells lining the adult colon do not express ICAM-1 and VCAM-1, but these molecules can be expressed upon malignant transformation (Dippold et al., 1993; Vainer et al., 2003; Taglia et al., 2007).
Intercellular adhesion molecule-1 is widely expressed at low basal levels and is upregulated by inflammatory cytokines in leukocytes and endothelial cells. It interacts with several integrins mediating firm adhesion (Archelos et al., 1999), but also with fibrinogen and hyaluronic acid. VCAM-1 is not normally expressed on vascular endothelial cells, but it is expressed after stimulation. It interacts with the α4β1 integrin, also named very late antigen-4, and participates in the firm adhesion of leukocytes to activated endothelium. Moreover, it also transduces intracellular signals in endothelial cells, triggering cell shape changes and favouring leukocyte migration (Matheny et al., 2000). These observations suggest that cellular adhesion molecules may not be simple anchors for cell-to-cell interaction; however, they may play a more active role in modulating cell function and response (Zhou et al., 2007).
The link between inflammation and tumorigenesis can explain why non-steroidal anti-inflammatory drugs have been shown to inhibit malignant transformation in various animal and in vitro models and to halt or regress tumour growth in patients bearing colon polyps (DuBois and Smalley, 1996; Winde et al., 1997; Fujimura et al., 2006; Markowitz, 2007). These effects have been ascribed to pharmacological blockade of COX, the dominant enzyme in the metabolic pathway responsible for conversion of arachidonic acid into prostanoids (DuBois et al., 1998). COX-2 is constitutively expressed in colorectal carcinomas, and experiments with human colorectal carcinoma-derived cell lines showed that COX-2 expression modifies cell adhesion, increases invasiveness, inhibits apoptosis and induces angiogenesis (Tsujii and DuBois, 1995; Tsujii et al., 1997, 1998; Elder et al., 2002; Wendum et al., 2004). Therefore, it is now accepted that most of the chemopreventive effects of non-steroidal anti-inflammatory drugs are predominantly secondary to COX-2 inhibition, although COX-1 also appears to have a role in angiogenesis (Hilmi and Goh, 2006).
Chemopreventive treatment regimens are usually carried out over long periods (months to years), and so determination of a reasonable benefit/risk ratio for this approach is crucial. Long-term use of traditional non-steroidal anti-inflammatory drugs, which inhibit both COX-1 and COX-2, is associated with serious gastrointestinal side effects; on the other hand, COX-2-selective inhibitors are currently under critical investigation because of the increased risk of cardiovascular side effects. These effects prompted Merck and Pfizer to withdraw rofecoxib and valdecoxib, respectively, from the pharmaceutical market (Grösch et al., 2006), but the meta-analysis of Kearney et al. (2006) has shown that there is only a moderate increase in the risk of vascular events, comparable to those with high-dose regimens of ibuprofen and diclofenac. Celecoxib is a selective COX-2 inhibitor drug and is the only non-steroidal anti-inflammatory drug that has been approved by the Food and Drug Administration for adjuvant treatment of patients with familial adenomatous polyposis to date (Chun and Surh, 2004; Rao and Reddy, 2004; Grösch et al., 2006; Half and Arber, 2006; Chan et al., 2007), but the molecular mechanisms of its chemopreventive action are poorly understood and further experimental and clinical studies are needed.
The HT29 cell line, derived from human colon cancer cells, has been widely used to study several aspects of intestinal cell biology and cancer transformation (Chantret et al., 1988). Recently, we have shown that celecoxib inhibited adhesion of HT29 cells to fetal calf serum (FCS)-coated plastic wells and expression of both ICAM-1 and VCAM-1 on these cells (Gallicchio et al., 2008). The aim of this study was to evaluate the effect of celecoxib on adhesion of HT29 cells to human umbilical vein endothelial cells (HUVECs) and on the expression of the adhesion molecules ICAM-1 and VCAM-1 in HUVECs.
Methods
Cell cultures
The HT29 cell line was isolated from a colorectal adenocarcinoma in 1964 and is a widely utilized cell line that is tumorigenic in nude mice, giving rise to well-differentiated adenocarcinomas, comparable to colonic primary carcinoma (grade 1). The HCT116 colon cancer cell line is also derived from a colorectal carcinoma, but in contrast to HT29 cells, does not express the COX-2 gene (both of them were kind gifts from Emanuela Masini, Department of Preclinical and Clinical Pharmacology, University of Florence). HT29 and HCT116 cells were grown in culture dishes as a monolayer in RPMI-1640 medium supplemented with 10% heat-inactivated FCS (v/v), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin and maintained at 37 °C in 5% CO2 humidified atmosphere. Cells were subcultured following enzymatic digestion using trypsin/EDTA solution. In all assays, tumour cell adhesion to nonstimulated HUVECs was between 2 and 10% of the total amount of tumour cells added.
HUVEC were isolated as described elsewhere (Jaffe et al., 1973) and cultured on gelatin-coated culture dishes in M199 medium supplemented with 20% heat-inactivated bovine calf serum, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, 5 IU ml−1 heparin, 12 μg ml−1 bovine brain extract and 200 mM glutamine. HUVECs were utilized between passages 2 and 5.
Fluorescent labelling of HT29 and HCT116 cells
Commercial fluorescent cell linker kit PKH67 was used for membrane labelling of HT29 and HCT116 cells. The whole procedure was performed at 25 °C. Cells (2.5 × 105) were washed with serum-free RPMI-1640. The cell suspension was centrifuged at 259 g for 5 min to produce a cell pellet. The supernatant was removed, leaving less than 25 μl medium on the pellet. A 500 μl portion of diluent C was added to resuspend the cells. The dye was diluted with diluent C to 4 μM immediately before staining. The cells in diluent C were added rapidly to 500 μl of this dye solution. The cells and dye were mixed by gentle pipetting. The mixture was incubated at 25 °C for 5 min. The staining process was stopped by adding an equal volume of FCS and incubating for 1 min. The stained cells were diluted with an equal volume of complete culture medium, centrifuged at 259 g for 10 min and washed at least three times. Then, the cells were resuspended in fresh complete medium. The staining efficiency was monitored by fluorescent microscopy.
Adhesion assay
HUVEC were grown to confluence in 24-well plates, washed and rested for 1 day in M199 medium plus 10% FCS without bovine brain extract. HT29 or HCT116 cells (7 × 104 cells per well) were labelled as described above and plated in a final volume of 0.25 ml M199 medium on HUVECs untreated or pre-stimulated with 0.01 μM TNF-α for 20 h. Celecoxib (1 nM–0.1 μM) was added simultaneously with HT29 or HCT116 cells and HUVECs and left in place for 4 h at 37 °C in 5% CO2. Direct effects on HT29 cells were assessed by seeding cells on 24-well HUVEC-free plates for 4 h at 37 °C in 5% CO2, in the presence of 10 μM celecoxib. The plates had previously been coated with heat-inactivated calf serum for 3 h to reduce spontaneous adhesion to the plastic wells. After incubation, nonadherent HT29 or HCT116 cells were removed by washing three times (drop-to-drop) with 1 ml M199 medium, as described by Laferrière et al. (2004). To evaluate the effect of ICAM-1 on adhesion of HT29 cells to HUVECs, HT29 cells or HUVECs were incubated with 5 μg ml−1 ICAM-1 and VCAM-1 monoclonal antibodies (mAbs) for 30 min. The ability of this mAb to block adhesion molecules in a selective way was previously tested in our laboratory (data not shown), and the mAbs were used at the concentration that demonstrated saturation in binding assays and maximal inhibitory effects in adhesion assays, in accordance with the instruction of the manufacturer. After incubation, HUVECs were washed and incubated with HT29 cells, or HT29 cells were washed and incubated with HUVECs for 4 h at 37 °C in 5% CO2.
The centre of each well was analysed by fluorescence image analysis (Dianzani et al., 2003). Adherent cells were counted using Image Pro Plus Software for micro-imaging (Media Cybernetics, version 5.0). Single experimental points were assayed in quadruplicate, and the standard error of the four replicates was below 10% in all cases. Data are presented as percentage inhibition versus the control value, control adhesion being measured on untreated HT29 or HCT116 cells.
Cell incubation for western blotting analysis
To evaluate the time course of TNF-α on ICAM-1 and VCAM-1 induction, HUVECs at confluence were treated with 0.01 μM TNF-α from time 0 to 20 h and cells were processed every 4 h for protein extraction. The effect of celecoxib on ICAM-1 and VCAM-1 expression was evaluated in HUVECs pretreated with 0.01 μM TNF-α for 16 h and then incubated with the drug at 0.1–10 μM for 4 h.
Western blotting
Cell dishes were washed with phosphate-buffered saline and treated with ice-cold lysis buffer (20 mM Tris–HCl pH 7.5, 150 mM NaCl, 0.1% sodium dodecyl sulphate, 1% Triton X-100, 1% sodium deoxycholate, 5 mM EDTA, 1 μl ml−1 protease inhibitors, 0.1 mM ZnCl2 and 1 mM phenylmethyl sulphonyl fluoride). Cell lysates were centrifuged at 11 093 g for 20 min and the supernatant was recovered. The protein concentrations were determined using a BCA protein assay, following the manufacturer's directions. HT29 cells and HUVEC lysate samples containing 50 and 18 μg, respectively, were analysed by sodium dodecyl sulphate-poly acrylamide gel electrophoresis using an 8 or 15% gel. Proteins were transferred to a polyvinyldene difluoride membrane and then incubated with SuperBlock blocking buffer.
ICAM-1 and VCAM-1 proteins were detected following incubation, respectively, with a rabbit or goat polyclonal antibody diluted 1:200 in phosphate-buffered saline containing 0.1% Tween 20 for 2 h at room temperature. The secondary antibodies used were horseradish peroxidase-conjugated donkey anti-rabbit IgG for ICAM-1 and horseradish peroxidase-conjugated donkey anti-goat for VCAM-1. Secondary antibodies were diluted 1:10 000 in phosphate-buffered saline containing 0.1% Tween 20 and incubated for 30 min at room temperature. To confirm the homogeneity of the proteins loaded, the membranes were stripped and incubated with β-actin mAb (1:5000) and subsequently with horseradish peroxidase-conjugated sheep anti-mouse IgG (1:10 000) both for 30 min at room temperature. The membranes were overlaid with Western Lightning Chemiluminescence Reagent Plus and then exposed to Hyperfilm ECL film. Protein bands were quantified using the software Gel Pro.Analyser 4.5, 2000. Single experimental points were assayed in triplicate and at three different time points.
Statistical analysis
Data are expressed as means±s.e.m. Statistical analysis was performed with GraphPad Prism 3.0 software. One-way analysis of variance (ANOVA) was performed, and Dunnett's multiple comparison was used to determine significant differences between means. P⩽0.05 was considered significant. The molar concentration of a substance that reduces response to the stimulus by 50% (IC50) was calculated with a nonlinear regression model using Origin version 6.0 software (Microcal Software, Northampton, MA, USA).
Materials
Trypsin was from Difco Laboratories Inc. (Detroit, MI, USA). M199 (endotoxin tested), mouse mAb against β-actin, protease inhibitors cocktail, green fluorescent cell linker kit PKH67, heparin and TNF-α were from Sigma (Chemical Co., St Louis, MO, USA). RPMI-1640 and FCS were from GIBCO BRL (Grand Island, NY, USA). Celecoxib was from Sequoia Research Products Ltd (Pangbourne, UK). The BCA protein assay and SuperBlock blocking buffer were from Pierce Biotechnology Inc. (Rockford, IL, USA); polyvinyldene difluoride was from Millipore (Bedford, MA, USA). Rabbit polyclonal antibodies against human ICAM-1, goat polyclonal antibodies against VCAM-1, and anti-mouse, anti-goat and anti-rabbit immunoglobulin horseradish peroxidase-linked whole antibodies were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Hyperfilm ECL was from Amersham Biosciences Corp. (Piscataway, NJ, USA) and Western Lightning Chemiluminescence Reagent Plus was from PerkinElmer Life Science (Cetus, Norwalk, CT, USA). Mouse mAbs against human ICAM-1 and VCAM-1 were purchased from R&D Systems (Minneapolis, MN, USA).
Gel Pro.Analyser 4.5, 2000 and Image Pro Plus Software for micro-imaging (version 5.0) were from Media Cybernetics Inc. (Leiden, The Netherlands). GraphPad Prism 3.0 software was from GraphPad software (San Diego, CA, USA).
All the other reagents utilized were from Sigma.
Results
Effect of celecoxib on adhesion of HT29 cells to HUVECs
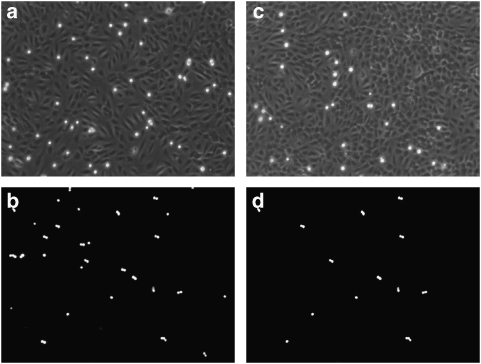
We evaluated the effect of celecoxib on HT29 cell adhesion to primary cultures of HUVECs by coincubating both cell types for 4 h with the drug (10 μM). Figure 1 shows that celecoxib strikingly inhibited HT29 cell adhesion to HUVECs, inhibiting by 53±2% the number of adhering cells (P<0.01; control adhesion of HT29 cells to HUVECs was 65±6 cells per microscopic field; Figures 1a and c; n=15).
Figure 1.
Adhesion of HT29 cells to (a) human umbilical vein endothelial cells (HUVECs) (60 cells) or (b) fetal calf serum (FCS)-coated plastic wells (50 cells). HT29 cells were seeded for 4 h onto monolayers of HUVECs. Attached cancer cells were visualized as bright dots by fluorescent microscopy. The pictures are representative of 1 of 15 individual experiments. Inhibition of HT29 cell adhesion to (c) HUVECs (30 cells) or (d) FCS-coated plastic wells (25 cells) induced by celecoxib. Celecoxib (10 μM) was coincubated with HUVECs and HT29 cells for 4 h. The pictures are representative of one of five individual experiments. Results with FCS-coated wells (b and d) are from our earlier experiments (Gallicchio et al., 2008) and are shown here for comparison.
Figures 1b and d show that celecoxib also inhibited HT29 cell adhesion to FCS-coated plastic wells, inhibiting by 55±3% the number of adhering cells (P<0.01; control adhesion of HT29 cells on FCS-coated plastic wells was 55±6 cells per microscopic field; Figures 1b and d; n=5). These results are taken from our earlier work (Gallicchio et al., 2008) and are shown here for comparison of adhesion to HUVECs or FCS-coated plastic wells. Therefore, the ability of celecoxib to inhibit HT29 cell adhesion is at least partly due to a direct effect on the HT29 cells.
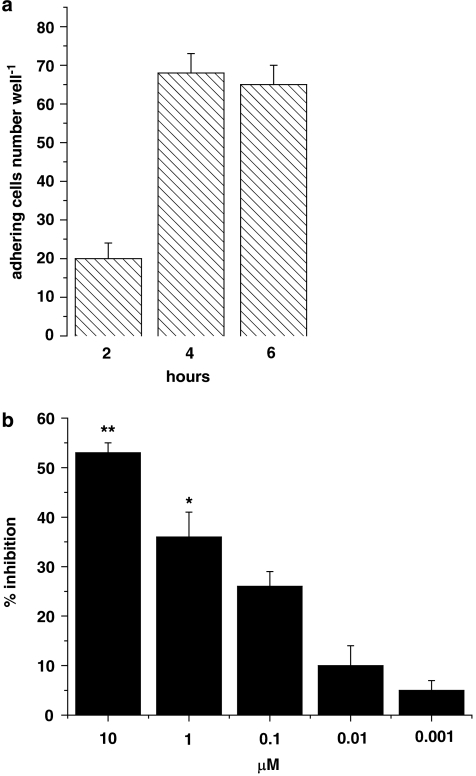
The 4 h incubation time was chosen on the basis of time-course experiments that showed that adhesion of HT29 cells was detectable after 2 h and increased to a plateau at 4–6 h (Figure 2a). We therefore chose 4 h of incubation for all subsequent experiments. To assess whether inhibition was concentration-dependent, the effects of celecoxib on HT29 cell adhesion to HUVECs were evaluated by coincubating both cell types for 4 h with celecoxib over a range of concentrations (1 nM–10 μM). The concentration–response data revealed that the maximum inhibitory effect (about 55%) was reached at 10 μM (Figure 2b); the inhibitory effect decreased at lower concentrations and was not statistically significant at 0.1 μM.
Figure 2.
(a) Time course of HT29 cell adhesion to HUVECs. HT29 cells were incubated with HUVECs for 2, 4 or 6 h. (b) Effects of celecoxib on HT29 cell adhesion to HUVECs. HT29 cells and HUVECs were incubated with celecoxib (1 nM–10 μM) for 4 h (3 μM histogram is not shown). Data are expressed as percentage inhibition of control adhesion, mean±s.e.m., n=5. Asterisks mark statistically significant inhibition of celecoxib versus control (*P<0.05, **P<0.01).
In a smaller series of experiments, under the same conditions as those using HT29 cells, the effects of celecoxib on the adhesion of another colon cancer cell line, HCT116, to HUVECs were assessed. We found that celecoxib (10 μM) also inhibited adhesion of HCT116 cells (43±5% inhibition; n=5) to HUVECs.
Involvement of ICAM-1 and VCAM-1 in adhesion of HT29 cells to HUVECs
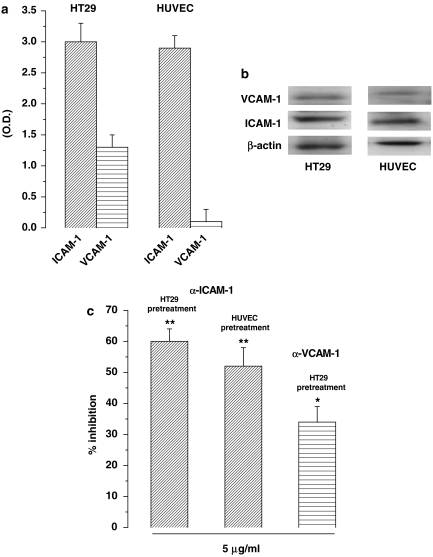
Our static adhesion assay mainly detects firm adhesion interactions such as those mediated by integrins, but cannot detect the weak interactions mediated by selectins. Therefore, candidate adhesion molecules possibly involved in the interaction between HT29 cells and HUVECs are the β2-integrin ligands ICAM-1 and VCAM-1 that are normally expressed on HT29 cells (Zetter, 1993). Moreover, ICAM-1 is also expressed by resting HUVECs. To confirm this expression pattern in our cell lines, we evaluated ICAM-1 and VCAM-1 expression in resting HT29 cells and HUVECs by western blot. Figures 3a and b show that expression of ICAM-1 was similar on HT29 cells and HUVECs, whereas VCAM-1 was weakly expressed on HT29 cells, but was almost absent on resting HUVECs.
Figure 3.
(a) Intercellular adhesion molecule –1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) expression in resting HT29 cells and HUVECs. Cells were processed for analysis by western blot and the results were measured by optical densitometry, normalized with respect to β-actin; mean±s.e.m., n=3. (b) Immunoblot of one experiment representing ICAM-1 and VCAM-1 expression in resting HT29 cells and HUVECs. (c) Effect of ICAM-1 and VCAM-1 mAbs on HT29 cell adhesion to HUVECs. ICAM-1 (5 μg ml−1) mAb was incubated for 30 min with HT29 cells or HUVECs and then further incubated for 4 h with HUVECs or HT29 cells. VCAM-1 mAb (5 μg ml−1) was incubated for 30 min with HT29 cells and then further for 4 h with HUVECs. Data are expressed as percentage inhibition of control adhesion, mean±s.e.m., n=5. Asterisks mark statistically significant inhibition of celecoxib versus control (*P<0.05, **P<0.01).
To investigate the actual role of ICAM-1 and VCAM-1 in the adhesion of HT29 cells to HUVECs, we utilized mAbs that functionally block these adhesion molecules (Figure 3c). Experiments performed by preincubating HT29 cells or HUVECs with a mAb blocking ICAM-1 showed that adhesion of HT29 cells was clearly inhibited, demonstrating a direct involvement of this adhesion molecule in the adhesive interaction between HT29 cells and HUVECs. The mAb blocking VCAM-1 on HT29 cells showed a lower inhibitory effect, demonstrating a lesser involvement of this adhesion molecule.
Effect of celecoxib on HT29 cell adhesion and ICAM-1 and VCAM-1 expression on TNF-α-stimulated HUVECs
Tumours are generally infiltrated with inflammatory cells, which produce cytokines potentiating endothelial cell adhesiveness. ICAM-1 and VCAM-1 expressions are increased in HUVECs upon activation induced by proinflammatory cytokines.
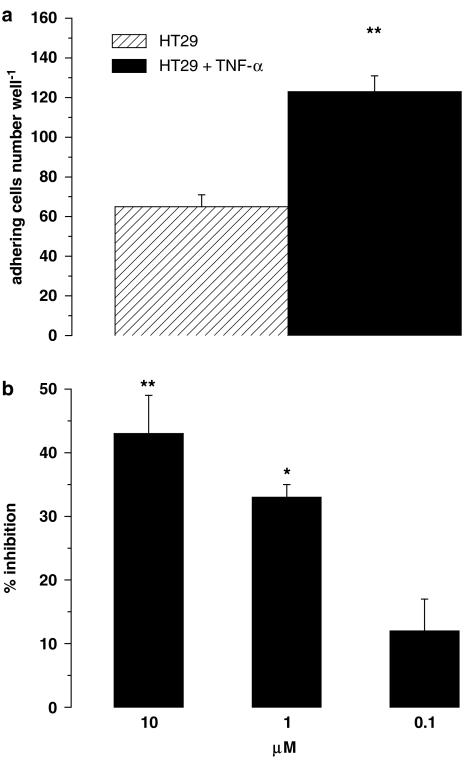
To assess the effect of celecoxib in an inflammatory environment, we performed the adhesion assays using HUVECs pretreated for 16 h with the proinflammatory cytokine TNF-α, known to upregulate expression of several adhesion molecules, such as P- and E-selectin, ICAM-1, ICAM-2 and VCAM-1. The 0.01 μM TNF-α dose was selected as suitable to produce optimal HT29 cell adhesion to HUVECs (ten Kate et al., 2004). TNF-α-induced adhesion was 189±8% of that detected on untreated HUVECs (Figure 4a; P<0.01). Figure 4b shows the concentration-dependent inhibition of celecoxib on HT29 cell adhesion to stimulated HUVECs, and these effects were not significantly different from those reported above for resting HUVECs.
Figure 4.
(a) Effect of tumour necrosis factor-α on HT29 cell adhesion to HUVECs. HUVECs were stimulated with 0.01 μM TNF-α for 16 h. Data are expressed as number of HT29 cells adhering to HUVECs, mean±s.e.m., n=5. Asterisks mark statistically significant increase of adhesion versus control (**P<0.01). (b) Effects of celecoxib on HT29 cell adhesion to stimulated HUVECs. HUVECs were stimulated with 0.01 μM TNF-α for 16 h, then HT29 cells were added simultaneously with 10 μM celecoxib for 4 h. Data are expressed as percentage inhibition of control adhesion, means±s.e.m.; n=5. Asterisks mark statistically significant inhibition of celecoxib versus TNF-α-stimulated HUVECs (*P<0.05, **P< 0.01).
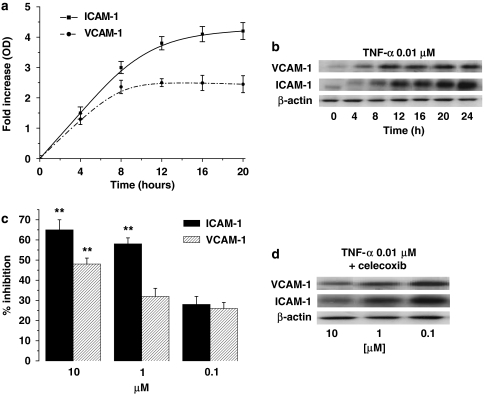
Then, we assessed the effect of celecoxib on the expression of ICAM-1 and VCAM-1 on TNF-α-activated HUVECs. The time-course analysis of ICAM-1 and VCAM-1 expression showed that TNF-α induced a strong upregulation of the expression of both molecules, which achieved a maximum between 16 and 20 h (Figures 5a and d). Celecoxib strongly inhibited ICAM-1 and VCAM-1 expression on stimulated HUVECs (Figures 5c and b). The inhibition was concentration-dependent, with the maximal effect at 10 μM for both ICAM-1 and VCAM-1. Similar experiments performed on unstimulated HUVECs did not detect any variation induced by celecoxib on the expression of ICAM-1 and VCAM-1 on HUVECs (data not shown).
Figure 5.
(a) Time course of TNF-α on ICAM-1 and VCAM-1 expression on HUVECs. Cells were stimulated with 0.01 μM TNF-α from time 0 to 20 h. At the time points indicated, cells were processed for analysis by western blot and measured by densitometry. Data are expressed as fold-increase over normal ICAM-1 and VCAM-1 expression, normalized with respect to β-actin, means±s.e.m.; n=5. (b) Immunoblot of one experiment representing time course of TNF-α on ICAM-1 and VCAM-1 expression on HUVECs. (c, d) Concentration-dependent inhibition by celecoxib of ICAM-1 and VCAM-1 protein induction by TNF-α. (c) HUVECs were stimulated with 0.01 μM TNF-α for 16 h and celecoxib was added for 4 h. Data, normalized with respect to β-actin, are expressed as percentage inhibition of control, mean±s.e.m., n=3. (d) Immunoblot of one experiment representing concentration–response to celecoxib of ICAM-1 and VCAM-1 expression on HUVECs. Asterisks mark statistically significant inhibition of celecoxib versus control (**P<0.01).
Discussion
In colon cancer and adenomas, the high levels of COX-2 expression and the potential cancer-promoting role of its product, PGE2, in epithelial tissues identified celecoxib as a potentially chemopreventive agent (Psaty and Potter, 2006). Reddy et al. (2000) demonstrated its efficacy during the promotion/progression stage of colon carcinogenesis. This suggests that celecoxib may potentially be an effective chemopreventive agent for the secondary prevention of colon cancer in high-risk patients with familial adenomatous polyposis and sporadic polyps (Reddy et al., 2000; Hilmi and Goh, 2006).
The interaction of cancer cells with vascular endothelial cells is mediated by several adhesion molecules (Meyer and Hart, 1998). The potential role of adhesion molecules has undergone a major transition over the last years, as it has become apparent that such molecules play a major role in signalling from outside to the internal milieu of a cell, thereby controlling how a cell is able (or not) to sense and interact with its local environment (Cairns et al., 2003).
Here, we show that celecoxib inhibits the adhesive interaction between HT29 colon cancer cells and vascular endothelial cells. Our earlier finding that celecoxib also inhibited adhesion of HT29 cells to FCS-coated plastic wells indicates that the drug had a direct effect on these tumour cells (Gallicchio et al., 2008).
The celecoxib-induced inhibition of HT29 cell adhesion to HUVECs occurs also on the TNF-α-activated endothelium, expressing increased levels of several adhesion molecules, such as ICAM-1, VCAM-1, and also E-selectin and P-selectin. This is an important point since microvascular endothelial cells within and around the tumour are generally activated by proinflammatory factors released by tumour-reactive inflammatory cells or mediators released by tumour cells (Laferrière et al., 2004). Moreover, significantly higher levels of TNF-α were found in colon carcinoma than in normal tissue (Kobayashi et al., 2007). Supernatants of colon carcinoma cells increased the production of those cytokines from blood mononuclear cells as well (Salman et al., 2000). Thus, administration of those cytokines partially reconstituted the in vivo environment.
Khatib et al. (2005) also demonstrated that TNF-α proinflammatory responses were tumour-specific and were not observed with some nonmetastatic murine or human carcinoma cells. Our experiments have been performed on HUVECs, providing a simplified model to mimic the tissue microvascular circulation involved in the interactions with tumour cells (Laferrière et al., 2002, 2004). However, it seems to be a suitable model since ten Kate et al. (2004) demonstrated correspondence between HUVECs and HMVEC-L (human microvascular endothelial cells of the lung) in the expression of ICAM-1 and VCAM-1 in resting cells. We have already shown (Gallicchio et al., 2008) that treatment of HT29 cells with 10 μM celecoxib inhibited ICAM-1 and VCAM-1 expression, with a statistically significant decrease between 2 and 6 h. A maximal decrease in expression was detected after 4 h, with about 50 and 40% decrease for ICAM-1 and VCAM-1, respectively. Concentration–response experiments also demonstrated that the effect was concentration-dependent and statistically significant in the range 1–10 μM. In our experimental system, indeed, adhesion inhibition fits with the inhibitory effect of celecoxib on expression of ICAM-1 and VCAM-1 by both HT29 cells (Gallicchio et al., 2008) and activated HUVECs. By contrast, no inhibition of the expression of ICAM-1 and VCAM-1 was detected on resting HUVECs, as they expressed low levels of ICAM-1 and minimal levels of VCAM-1. The change in ICAM-1 and VCAM-1 expression is likely to play an important role in the inhibition of adhesion mediated by celecoxib, as ten Kate et al. (2004) showed that HT29 cells bind endothelial cells through LFA-1 expressed on HT29 cells interacting with ICAM-1 expressed on HUVECs. This possibility is supported by our experiments with specific mAbs blocking ICAM-1 of HT29 cells or HUVECs and VCAM-1 of HT29 cells, demonstrating the involvement of these cellular adhesion molecules in HT29 cell adhesion. There is no evidence that HUVECs express integrins able to bind ICAM-1 and VCAM-1 present on HT29 cells; however, ICAM-1 can also bind to fibrinogen and hyaluronan, which may be involved in HT29 cell adhesion to both HUVECs and FCS-coated plastic wells.
Our data do not rule out that other molecular interactions may also be involved in the effect of celecoxib on cell adhesion. For instance, ten Kate et al. (2004) showed that binding of HT29 cells to endothelial cells also involves the interaction of E-selectin on HUVECs and sialyl Lewis x on HT29 cells. However, this is a dynamic adhesive interaction that was not detected by our experimental system assessing static adhesion only. Moreover, the HT29 cell adhesion to FCS-coated plates might involve also αvβ5, which is the vitronectin receptor on HT29 cells, but this interaction seems not to be involved in HT29 cell adhesion to HUVEC (Laferrière et al., 2004).
A different point is that the effect of celecoxib might be partly mediated by inhibition of cellular adhesion molecules signalling that can modulate cell adhesion, via inside-out signalling. Indeed, binding of colon carcinoma cells to endothelial cells increases tyrosine phosphorylation of several proteins and leads to activation of the p38 mitogen-activated protein kinase. We have already shown that activation of this mitogen-activated protein kinase in HT29 cells was inhibited by celecoxib (Gallicchio et al., 2008).
The quantitatively similar adhesion inhibition exerted by celecoxib on resting and activated HUVECs suggests that the maximal inhibitory effects were reached, as also supported by the observation that higher doses of celecoxib (30 or 100 μM; data not shown) did not induce greater inhibition.
The relatively high concentrations of celecoxib needed to inhibit adhesion is intriguing as they were well above those needed to selectively inhibit COX-2, which is expressed in HT29 cells. This finding suggests that the mechanism activated by celecoxib may not be solely dependent on COX-2. This possibility would be supported by our experiments showing that 10 μM celecoxib also inhibited adhesion to HUVECs of HCT116 cells, a colon cancer cell line not expressing COX-2, but expressing ICAM-1 and VCAM-1 (Cianchi et al., 2004; Corvaisier et al., 2005). On the contrary, a possible role of COX is suggested by the preliminary finding that other drugs inhibiting COX-2 and/or COX-1 are able to inhibit cell adhesion, to a lower extent (rofecoxib and indomethacin; data not shown), which is in agreement with the results of De Menezes et al. (2005), who showed that rats pretreated with celecoxib, rofecoxib or indomethacin displayed a reduced leukocyte migratory response.
In conclusion, this work shows that celecoxib can inhibit the adhesive interaction of HT29 colon cancer cells with HUVECs, possibly by inhibiting expression of adhesion molecules. These findings confirm that this drug might be used as a chemopreventive agent for the secondary prevention of colon cancer in high-risk individuals, such as patients with familial adenomatous polyposis or sporadic polyps, and give new insights into its molecular mechanisms of action.
Acknowledgments
This research was supported by Ministry of Education, Universities and Research (MIUR, 60% and PRIN 2004 Projects). We thank the Pathology Unit and the Obstetrics and Gynecology Unit, Martini Hospital, Turin, for providing human umbilical cords.
Abbreviations
- FCS
fetal calf serum
- HUVECs
human umbilical vein endothelial cells
- ICAM-1
intercellular adhesion molecule-1
- mAbs
monoclonal antibodies
- TNF-α
tumour necrosis factor-α
- VCAM-1
vascular cell adhesion molecule-1
Conflict of interest
The authors state no conflict of intrest.
References
- Archelos JJ, Previtali SC, Hartung HP. The role of integrins in immune-mediated diseases of the nervous system. Trends Neurosci. 1999;22:30–38. doi: 10.1016/s0166-2236(98)01287-9. [DOI] [PubMed] [Google Scholar]
- Boyano MD, Garcia-Vazquez MD, Lopez-Michelena T, Gardeazabal J, Bilbao J, Canavate ML, et al. Soluble interleukin-2 receptor, intercellular adhesion molecule-1 and interleukin-10 serum levels in patients with melanoma. Br J Cancer. 2000;83:847–852. doi: 10.1054/bjoc.2000.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns RA, Khokha R, Hill RP. Molecular mechanisms of tumor invasion and metastasis: an integrated view. Curr Mol Med. 2003;3:659–671. doi: 10.2174/1566524033479447. [DOI] [PubMed] [Google Scholar]
- Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- Chantret I, Barbat A, Dussaulx E, Brattain MG, Zweibaum A. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer Res. 1988;48:1936–1942. [PubMed] [Google Scholar]
- Chun KS, Surh YJ. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem Pharmacol. 2004;68:1089–1100. doi: 10.1016/j.bcp.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Cianchi F, Cortesini C, Fantappiè O, Messerini L, Sardi I, Lasagna N, et al. Cyclooxygenase-2 activation mediates the proangiogenic effect of nitric oxide in colorectal cancer. Clin Cancer Res. 2004;10:2694–2704. doi: 10.1158/1078-0432.ccr-03-0192. [DOI] [PubMed] [Google Scholar]
- Corvaisier M, Moreau-Aubry A, Diez E, Bennouna J, Mosnier J-F, Scotet E, et al. Vγ9Vδ2T cell response to colon carcinoma cells. J Immunol. 2005;175:5481–5488. doi: 10.4049/jimmunol.175.8.5481. [DOI] [PubMed] [Google Scholar]
- De Menezes GB, dos Reis WG, Moreira-Santos JM, Duarte ID, de Francischi J. Inhibition of prostaglandin F2α by selective cyclooxygenase 2 inhibitors accounts for reduced leukocyte migration. Inflammation. 2005;29:163–169. doi: 10.1007/s10753-006-9013-z. [DOI] [PubMed] [Google Scholar]
- Dianzani C, Collino M, Lombardi G, Garbarino G, Fantozzi R. Substance P increases neutrophil adhesion to human umbilical vein endothelial cells. Br J Pharmacol. 2003;139:1103–1110. doi: 10.1038/sj.bjp.0705344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dippold W, Wittig B, Schwaeble W, Mayet W, Meyer Zum Buschenfelde KH. Expression of intercellular adhesion molecule 1 (ICAM-1, CD54) in colonic epithelial cells. Gut. 1993;34:1593–1597. doi: 10.1136/gut.34.11.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- DuBois RN, Smalley WE. Cyclooxygenase, NSAIDs, and colorectal cancer. J Gastroenterol. 1996;31:898–906. doi: 10.1007/BF02358623. [DOI] [PubMed] [Google Scholar]
- Elder DJE, Halton DE, Playle LC, Paraskeva C. The MEK/ERK pathway mediates COX-2-selective NSAID-induced apoptosis and induced COX-2 protein expression in colorectal carcinoma cells. Int J Cancer. 2002;99:323–327. doi: 10.1002/ijc.10330. [DOI] [PubMed] [Google Scholar]
- Franzke A, Probst-Kepper M, Buer J, Duensing S, Hoffman R, Wittke F. Elevated pretreatment serum levels of soluble vascular cell adhesion molecule 1 and lactate dehydrogenase as predictor of survival in cutaneous metastatic malignant melanoma. Br J Cancer. 1998;78:40–45. doi: 10.1038/bjc.1998.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T, Ohta T, Oyama K, Miyashita T, Miwa K. Role of cyclooxygenase-2 in the carcinogenesis of gastrointestinal tract cancers: a review and report of personal experience. World J Gastroenterol. 2006;12:1336–1345. doi: 10.3748/wjg.v12.i9.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallicchio M, Rosa AC, Dianzani C, Brucato L, Collino M, Fantozzi R.Celecoxib decreases expression of the adhesion molecules ICAM-1 and VCAM-1 in a colon cancer cell line (HT29) Br J Pharmacol 2008(in press) [DOI] [PMC free article] [PubMed]
- Gangopadhyay A, Lazure DA, Thomas P. Adhesion of colorectal carcinoma cells to the endothelium is mediated by cytokines from CEA stimulated Kupffer cells. Clin Exp Metastasis. 1998;16:703–712. doi: 10.1023/a:1006576627429. [DOI] [PubMed] [Google Scholar]
- Grösch S, Maier TJ, Schiffmann S, Geisslinger G. Cyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitors. J Natl Cancer Inst. 2006;98:736–747. doi: 10.1093/jnci/djj206. [DOI] [PubMed] [Google Scholar]
- Half E, Arber N. Chemoprevention of colorectal cancer: two steps forward one step back. Future Oncol. 2006;2:697–704. doi: 10.2217/14796694.2.6.697. [DOI] [PubMed] [Google Scholar]
- Herendeen JM, Lindley C. Use of NSAIDs for the chemoprevention of colorectal cancer. Oncology. 2003;37:1664–1674. doi: 10.1345/aph.1C489. [DOI] [PubMed] [Google Scholar]
- Hilmi I, Goh KL. Chemoprevention of colorectal cancer with nonsteroidal anti-inflammatory drugs. Chin J Dig Dis. 2006;7:1–6. doi: 10.1111/j.1443-9573.2006.00236.x. [DOI] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci. 2004;95:377–384. doi: 10.1111/j.1349-7006.2004.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ. 2006;332:1302–1308. doi: 10.1136/bmj.332.7553.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatib A-M, Auguste P, Fallavollita L, Wang N, Samani A, Kontogiannea M, et al. Characterization of the host proinflammatory response to tumor cells during the initial stages of liver metastasis. Am J Pathol. 2005;167:749–759. doi: 10.1016/S0002-9440(10)62048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Boelte KC, Lin PC. Endothelial cell adhesion molecules and cancer progression. Curr Medicin Chem. 2007;14:377–386. doi: 10.2174/092986707779941032. [DOI] [PubMed] [Google Scholar]
- Laferrière H, Houle F, Hout J. Regulation of the metastatic process by E-selectin and stress-activated protein kinase-2/p38. Ann N Y Acad Sci. 2002;973:562–572. doi: 10.1111/j.1749-6632.2002.tb04702.x. [DOI] [PubMed] [Google Scholar]
- Laferrière H, Houle F, Hout J. Adhesion of HT-29 colon carcinoma cells to endothelial cells requires sequential events involving E-selectin and integrin beta4. Clin Exp Metastasis. 2004;21:257–264. doi: 10.1023/b:clin.0000037708.09420.9a. [DOI] [PubMed] [Google Scholar]
- Markowitz SD. Aspirin and colon cancer-targeting prevention. N Engl J Med. 2007;356:2195–2198. doi: 10.1056/NEJMe078044. [DOI] [PubMed] [Google Scholar]
- Matheny HE, Deem TL, Cook-Millis JM. Lymphocyte migration through monolayers of endothelial cell lines involves VCAM-1 signaling via endothelial cell NADPH oxidase. J Immunol. 2000;164:6550–6559. doi: 10.4049/jimmunol.164.12.6550. [DOI] [PubMed] [Google Scholar]
- Meyer T, Hart IR. Mechanisms of tumour metastasis. Eur J Cancer. 1998;34:214–221. doi: 10.1016/s0959-8049(97)10129-0. [DOI] [PubMed] [Google Scholar]
- Neeson PJ, Thurlow PJ, Jamieson GP, Bradley C. Lymphocyte-facilitated tumour cell adhesion to endothelial cells: the role of high affinity leukocyte integrins. Pathology. 2003;35:50–55. [PubMed] [Google Scholar]
- Psaty BM, Potter JD. Risks and benefits of celecoxib to prevent recurrent adenomas. N Engl J Med. 2006;355:950–952. doi: 10.1056/NEJMe068158. [DOI] [PubMed] [Google Scholar]
- Rao CV, Reddy BS. NSAIDs and chemoprevention. Curr Cancer Targ. 2004;4:29–42. doi: 10.2174/1568009043481632. [DOI] [PubMed] [Google Scholar]
- Reddy BS, Hirose Y, Lubet R, Steele V, Kelloff G, Paulson S, et al. Chemoprevention of colon cancer by specific cyclooxygenase-2 inhibitor, celecoxib, administered during different stages of carcinogenesis. Cancer Res. 2000;60:293–297. [PubMed] [Google Scholar]
- Salman H, Bergman M, Bessler H, Wolloch Y, Puncky I, Djaletti M. Effect of colon carcinoma cell supernatants on cytokine production and phagocytic capacity. Cancer Lett. 2000;159:197–203. doi: 10.1016/s0304-3835(00)00557-7. [DOI] [PubMed] [Google Scholar]
- Schadendorf D, Diehl S, Zuberbier T, Schadendorf C, Henz BM. Quantitative detection of soluble adhesion molecules in sera of melanoma patients correlates with clinical stage. Dermatology. 1996;192:89–93. doi: 10.1159/000246328. [DOI] [PubMed] [Google Scholar]
- Taglia L, Matusiak D, Matkowskyj KA, Benia RV. Gastrin-releasing peptide mediates its morphogenic properties in human colon cancer by upregulating intracellular adhesion protein-1 (ICAM-1) via focal adhesion kinase. Am J Physiol Gastrointest Liver Physiol. 2007;292:182–190. doi: 10.1152/ajpgi.00201.2006. [DOI] [PubMed] [Google Scholar]
- ten Kate M, Hofland LJ, Van Grevenstein WMU, Van Koetsveld PV, Jeekel J, Van Eijck CHJ. Influence of proinflammatory cytokines on the adhesion of human colon carcinoma cells to lung microvascular endothelium. Int J Cancer. 2004;112:943–950. doi: 10.1002/ijc.20506. [DOI] [PubMed] [Google Scholar]
- Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;93:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujii M, Kawano S, Tsujii S, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- Vainer B, Sorensen S, Seidelin J, Nielsen OH, Horn T. Expression of ICAM-1 in colon epithelial cells: an ultrastructural study performed on in vivo and in vitro models. Virchows Arch. 2003;443:774–781. doi: 10.1007/s00428-003-0900-5. [DOI] [PubMed] [Google Scholar]
- Wendum D, Masliah J, Trugnan G, Fléjou J-F. Cyclooxygenase-2 and its role in colorectal cancer development. Virchows Arch. 2004;445:327–333. doi: 10.1007/s00428-004-1105-2. [DOI] [PubMed] [Google Scholar]
- Winde G, Schmid KW, Brandt B, Muller O, Osswald H. Clinical and genomic influence of sulindac on rectal mucosa in familial adenomatous polyposis. Dis Colon Rectum. 1997;31:1156–1168. doi: 10.1007/BF02055161. [DOI] [PubMed] [Google Scholar]
- Yamada M, Yan K, Hasegawa M, Matsushita Y, Horikawa M, Komura K, et al. Regulation of local and metastatic host-mediated anti-tumour mechanisms by L-selectin and intercellular adhesion molecule-1. Clin Exp Immunol. 2006;143:216–227. doi: 10.1111/j.1365-2249.2005.02989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetter BR. Adhesion molecules in tumor metastasis. Semin Cancer Biol. 1993;4:219–229. [PubMed] [Google Scholar]
- Zhou Z, Connel M, MacEwan DJ. TNFR-1-induced NF-κB, but not ERK, p38MAPK or JNK activation, mediates TNF-induced ICAM-1 and VCAM-1 expression on endothelial cells. Cell Signal. 2007;19:1238–1248. doi: 10.1016/j.cellsig.2006.12.013. [DOI] [PubMed] [Google Scholar]