Abstract
It is widely accepted that interleukin-1β (IL-1β), a cytokine produced not only by immune cells but also by glial cells and certain neurons influences brain functions during infectious and inflammatory processes. It is still unclear, however, whether IL-1 production is triggered under nonpathological conditions during activation of a discrete neuronal population and whether this production has functional implications. Here, we show in vivo and in vitro that IL-1β gene expression is substantially increased during long-term potentiation of synaptic transmission, a process considered to underlie certain forms of learning and memory. The increase in gene expression was long lasting, specific to potentiation, and could be prevented by blockade of potentiation with the N-methyl-d-aspartate (NMDA) receptor antagonist, (±)-2-amino-5-phosphonopentanoic acid (AP-5). Furthermore, blockade of IL-1 receptors by the specific interleukin-1 receptor antagonist (IL-1ra) resulted in a reversible impairment of long-term potentiation maintenance without affecting its induction. These results show for the first time that the production of biologically significant amounts of IL-1β in the brain can be induced by a sustained increase in the activity of a discrete population of neurons and suggest a physiological involvement of this cytokine in synaptic plasticity.
Interleukin-1β (IL-1β), which is assigned a key role in the orchestration of the complex immune response to infection and injury (1), was originally described as an immune cell mediator in the periphery. More recently, however, this cytokine has been reported to be synthesized also in the brain by glial cells and certain neurons; and IL-1 receptors were found in different regions of the CNS with the highest abundance in the hippocampus (for review see refs. 2–4). These complementary findings indicate a physiological role of IL-1 in the brain. In support of this, pharmacological and behavioral studies demonstrated the capacity of IL-1β to affect various neuroendocrine functions, to alter the release and turnover rate of certain neurotransmitters and modulators [e.g., norepinephrine, dopamine, gamma-aminobutyric acid (GABA), nitric oxide (NO), corticotropin-releasing hormone (CRH), refs. 5–11], and to induce changes in behavior (12–14). However, in most of these studies relatively high doses of IL-1β were applied or the data were obtained during pathological conditions in which a massive IL-1β production in the CNS occurs. Furthermore, there is as yet no experimental evidence of whether the expression of IL-1β gene is affected by physiological changes in the activity of discrete populations of brain neurons and whether such changes have implications for brain functions. We approached these questions by using long-term potentiation (LTP), a well-established cellular model of synaptic plasticity (15–17). The hallmark of LTP is a sustained enhancement of synaptic transmission and postsynaptic neuronal activity after high frequency stimulation of afferent fibers. Here, we report that induction of LTP in hippocampal slice preparations and in freely moving animals caused a clear and persistent increase of IL-1β gene expression. This increase in gene expression was long lasting, specific to potentiation, and could be prevented by blockade of potentiation with the N-methyl-d-aspartate (NMDA) receptor antagonist (±)-2-amino-5-phosphonopentanoic acid (AP-5). Because application of the specific interleukin-1 receptor antagonist (IL-1ra) resulted in a reversible inhibition of LTP, IL-1β appears to be involved in the maintenance of this process and in synaptic plasticity.
MATERIALS AND METHODS
Animals.
Male Wistar rats (8- to 10-wk-old, 230–330 g) were used for in vitro and in vivo experiments. Animals were fed ad libitum and housed in temperature and light-controlled (12 hr/day) rooms. Rats were treated in accordance to institutional guidelines.
LTP in Vitro.
Animals were killed by decapitation, and the brain was removed, placed in cold, oxygenated artificial cerebrospinal fluid (ACSF; containing 124 mM NaCl/4.9 mM KCl/1.2 mM KH2PO4/1.3 mM MgSO4/2.5 mM CaCl2/25.6 mM NaHCO3/10 mM d-Glucose), and the right hippocampus dissected out. Transverse slices, 400-μm thick, were cut with a tissue chopper and allowed to recover for 5 hr at 33–35°C in oxygenated artificial cerebrospinal fluid. This incubation was necessary because we found that the level of IL-1β transcripts was relatively high in hippocampal slices incubated for shorter periods. This expression was probably caused by tissue injury. After an incubation period of at least 5 hr, IL-1β gene expression declined and remained stable at low levels. Slices were then transferred to a recording chamber, placed underneath a grid of nylon threads adjusted within a U-formed platinum frame, and superfused with oxygenated artificial cerebrospinal fluid (1–2 ml/min). Bipolar silver electrodes were lowered to the stratum radiatum of the CA1 area and population spikes (PS) recorded from the pyramidal layer with glass electrodes (1–4 MΩ). Three test stimuli (pulse width: 200 μs; intertrain interval: 10 s) were applied every 5 min before and every 10 min after tetanization, and the response to the three test stimuli was averaged. The strength of the test stimuli was adjusted to elicit a population spike of ≈30–40% of its maximum amplitude. Tetanic stimulation consisted of three 100-Hz trains for 1 s (pulse width: 400 μs, intertrain interval: 1 min).
LTP in Vivo.
Experiments were performed on freely moving rats. A monopolar recording electrode (coordinates anterior–posterior −2.8, Lateral 1.8) and a bipolar stimulation electrode (coordinates anterior–posterior −6.9, Lateral 4.1) (18) were implanted stereotaxically under Pentobarbital sodium anesthesia (40 mg/kg, i.p.) into the granule cell layer of the dentate gyrus and into the perforant path, respectively, of the right hemisphere. For drug application, a brass cannula was chronically inserted into the right lateral ventricle (coordinates anterior–posterior −0.8, Lateral 1.6). Animals were allowed to recover from surgery for at least 1 wk. The stimulus intensity was adjusted to evoke 40% of the maximum of PS amplitude. Five test stimuli were applied every 10 min and the responses were averaged for each set of stimulus parameters. Once stable responses were obtained for 45 min, LTP was induced by strong or weak tetanic bursts (strong tetanus: 10 bursts of 15 pulses 200 Hz, 0.2-ms duration each stimulus, interburst interval 10 s; weak tetanus: only three bursts), resulting in a “saturated,” late-LTP and an “unsaturated,” early LTP, respectively. Immediately after tetanization, PS were recorded every 2 and 5 min, respectively, until 15 min post-tetanus. Thereafter, recordings were taken in 15-min intervals for at least 3 hr.
IL-1β Gene Expression in Vitro.
At the times indicated in the figure, hippocampal slices were removed from the recording chamber, immediately homogenized in TRIzol reagent (GIBCO/BRL), and used to determine IL-1β gene expression by semi-quantitative reverse transcriptase–PCR (RT-PCR). Other slices were processed for in situ hybridization (see below).
IL-1β Gene Expression in Vivo.
To study IL-β mRNA expression, rats in which LTP was induced by a strong tetanus were killed 8 hr after tetanic stimulation. To interfere with LTP induction, another group of rats received the NMDA receptor antagonist (±)-2-amino-5-phosphonopentanoic acid (AP-5; 50 μM) injected via the implanted cannula 15 min before tetanic stimulation and also were killed 8 hr later. Control animals were treated in a comparable way but received physiological saline (0.9% NaCl) instead of AP-5. After decapitation, ipsi- and contralateral hippocampi were dissected quickly and processed for the evaluation of IL-1 mRNA.
Determination of IL-1β Gene Expression by Semi-Quantitative RT-PCR.
RNA was extracted from the hippocampi obtained from in vivo and in vitro experiments following standard protocols and reverse transcribed by using a commercial kit (Superscript II RT kit, GIBCO/BRL). Controls performed before semi-quantifying IL-1β by RT-PCR included a parallelism test, the determination of the efficiency of the RT reaction, and the accuracy of the determination of the RNA concentration as described (19). All conditions used in the semi-quantitative RT-PCR by using a multispecific competitive fragment (pRat6) were described (19). The primer sequences were: IL-1β, sense: TCCATGAGCTTTGTACAAGG, and antisense: GGTGCTGATGTACCAGTTGG. Amplicons were separated by agarose gel electrophoresis in 1.5% gels containing 5 μg/ml ethidium bromide and 10% PAGE at 80 V for 3 hr. Bands were visualized in the agarose gels by excitation at 316 nm and then documented. Acrylamide gels were dried, exposed to a phosphor screen, and the signals were quantified by using a PhosphorImager (Molecular Dynamics) with an image quant program (Molecular Dynamics). Background levels were subtracted from the detected signals. Each individual sample was amplified by PCR at least three times.
In Situ Hybridization.
Hippocampal slices were fixed in 4% paraformaldehyde, dehydrated, paraffin embedded, cut serially in 10 μm sections, and collected on silane-coated slides. A 856-bp cDNA clone containing the full coding region of IL-1β and a 705-bp cDNA clone containing the 3′-coding region of the type I IL-1 receptor were obtained by RT-PCR from rat spleen RNA. The amplified DNA fragment was subcloned into a vector containing T7 and SP6 promotor. The sequence of the clones were confirmed by dideoxy sequencing. [35S]UTP-labeled antisense strand RNA probes and [35S]UTP-labeled sense strand RNAs used as control were synthesized by using T7 and SP6 polymerase respectively. All probes were hydrolyzed to a length of ≈150 nucleotides before use. In situ hybridization was performed as described by Hogan (20). In brief, after dewax, rehydratation, and denaturation in 0.2 M HCl, slices were treated with Pronase E (Sigma) and fixed in 4% paraformaldehyde. Sections were then acetylated and dehydrated.
Hybridization was carried out with 5 × 107 cpm/ml RNA probe in a volume of 20 μl hybridization buffer at 50°C overnight. Sections were than washed and treated with RNase A to reduce background. All of the washing solutions except RNase A buffer contained 20 mM 2-mercaptoethanol. Washed sections were dehydrated. Slides were coated with emulsion NTB-2 (Eastman Kodak) for autoradiography and exposed at 40°C for 2–4 wk. Developed sections were counterstained with hematoxylin, coversliped, and examined by dark-field microscopy.
Blockade of IL-1 Receptors in Vitro.
Ninety to 100 min after inducing LTP in hippocampal slices, IL-1ra (100 μg/ml, kindly provided by Dr. J. Relton, Upjohn, Boulder, Colorado) was perfused for 40 min. The same procedure was followed in control slices omitting the antagonist in the perfusion fluid.
Blockade of IL-1 Receptors in Vivo.
Physiological saline (0.9% NaCl) or IL-1ra (100 μg/μl) was administered intracerebroventricular (i.c.v.) in a total final volume of 5 μl (1 μl/min) to rats chronically implanted with recording and stimulating electrodes and an i.c.v. cannula, either 30 min before the induction of an unsaturated LTP, immediately after tetanus, or 90 min post-tetanus. The repetition of the weak tetanization with a 1-wk interval did not modify the time course of a second potentiation (21). Therefore, in these experiments, each animal served as its own control, i.e., in the first week the animals were subjected to the weak tetanization protocol under control conditions (saline injection). One week later the same procedure was followed, but IL-1ra was injected instead of saline.
RESULTS
IL-1β Gene Is Expressed in Hippocampal Slices During LTP.
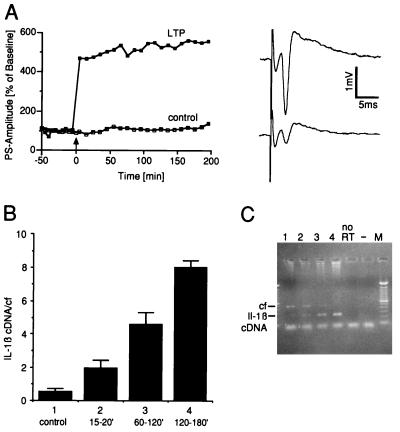
In a first set of experiments, rat hippocampal slices were used to explore whether changes in IL-1β gene expression occur during LTP. Following a preincubation period of at least 5 hr (see Material and Methods), field potentials were recorded from the pyramidal layer of the CA1 region. After stable baseline recordings were established, LTP was induced by a triple high frequency tetanic stimulation. At different times after tetanization, IL-1β expression was determined by a sensitive, semi-quantitative RT-PCR. Fifteen to 30 min after tetanic stimulation no significant changes in the levels of IL-1β transcripts were detected (Fig. 1). However, a clear increase in the accumulation of IL-1β mRNA was evident by 1 hr, as compared with control slices in which no LTP was induced. This increase in IL-1β expression persisted as long as LTP remained stable, as indicated by one slice that displayed a stable potentiation for 24 hr (data not shown). As an attempt to identify the cell origin of IL-1β transcripts, we conducted in situ hybridization studies in hippocampal slices. After a preincubation period of only 2 hr, a weak but reliable expression of the IL-1β gene was detected specially in the CA1 area. However, the expression of the IL-1β gene was transient because, after a preincubation period of 5 hr, it was no longer detectable by in situ hybridization neither in control slices nor in slices in which LTP was induced 3 hr before, i.e., at the time point when the highest IL-1β expression was detected by RT-PCR. Expression of IL-1 receptor Type I also was undetectable by in situ hybridization in these slices.
Figure 1.
IL-1β gene expression during LTP in hippocampal slices. (A Left) High frequency stimulation induced a robust LTP in comparison to controls. (Right) Analog traces of a representative recording obtained during baseline recording (Lower trace) and 10 min after tetanization (Upper trace). Arrow indicates time of tetanization. (B) IL-1β mRNA expression at different times after induction of LTP. Group 1 (control): slices without tetanic stimulation. The total time of incubation was comparable for all slices. Results of RT-PCR are expressed as mean ± SEM of the ratio IL-1β cDNA and the competitive fragment (cf). Group 1, n = 4; group 2, n = 8; group 3, n = 6; and group 4, n = 4. lL-1β gene expression was significantly increased in groups 3 and 4 as compared with the control group (P < 0.05; ANOVA followed by Fisher test for multiple comparisons). (C) Representative ethidium bromide-stained agarose gel showing RT-PCR amplified transcripts. Lane numbers correspond to the groups shown in B. M, 100-bp ladder molecular weight marker; no RT, RT-PCR without addition of reverse transcriptase; −, RT-PCR without addition of cDNA.
IL-1β Gene Is Expressed in the Hippocampus During LTP in Freely Moving Rats.
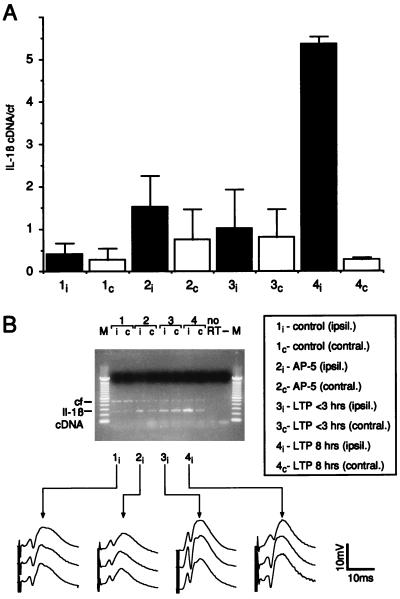
To ensure that the induction of IL-1β expression after tetanization represents a physiological response that is not confined to hippocampal slice preparations, we explored this phenomenon in vivo, i.e., in the hippocampus of freely moving animals. Rats killed 8 hr after the induction of a stable LTP displayed a strong expression of IL-1β mRNA in the ipsilateral hippocampus, i.e., the stimulated site (Fig. 2, 4i in A and B). In contrast, hippocampal IL-1β expression at the contralateral site remained at control levels (Fig. 2, 4c in A and B). Furthermore, in animals in which the induction of LTP was blocked by an i.c.v. intracerebroventricular injection of the NMDA-antagonist AP-5 (Fig. 2, 2i in A and B), or in rats that received the same tetanic stimulation but exhibited only a short-lasting potentiation of 2- to 3-hr duration (Fig. 2, 3i in A and B) no significant changes in IL-1β gene expression in the hippocampus were detected 8 hr after tetanic stimulation. The findings led us to speculate that activation of the IL-1β gene may be a key condition for LTP.
Figure 2.
IL-1β gene expression during LTP in vivo. (A) IL-1β mRNA expression 8 hr after tetanization. Thirty minutes before tetanization, animals received either physiological saline (groups 1, 3, and 4) or AP-5 (group 2) administered i.c.v. Black bars indicate measurements performed in ipsilateral (i) and white bars in contralateral (c) hippocampi. Group 1: hippocampi of animals recorded under baseline conditions without tetanic stimulation. Group 2: hippocampi of animals in which the expression of LTP after tetanization was blocked by AP-5. Group 3: hippocampi of animals showing a potentiation that returned to baseline within 3 hr. Group 4: hippocampi of animals with a robust LTP lasting for 8 hr. Results of RT-PCR are expressed as mean ± SEM of the ratio IL-1β cDNA and the competitive fragment (cf). n = 4 per group. Group 4i differs significantly from all other groups (P < 0.05; ANOVA followed by Fisher test for multiple comparisons). (B) Representative ethidium bromide-stained agarose gel showing the amplified transcripts of a RT-PCR obtained from ipsi- and contralateral hippocampi that were subjected to different experimental conditions (lane numbers correspond to the groups shown in A). The line graphs display representative analog traces recorded during baseline (Top), immediately after tetanization (Middle) and 8 hr after tetanus (Bottom). M, 100-bp ladder molecular weight marker; no RT, RT-PCR without addition of reverse transcriptase; −, RT-PCR without addition of cDNA.
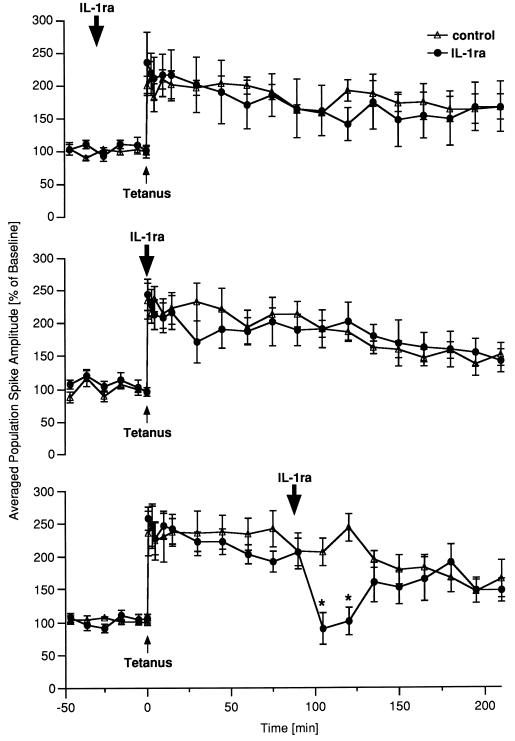
Figure 4.
Blockade of IL-1 receptors causes a reversible inhibition of LTP maintenance in vivo. Either physiological saline (control) or IL-1ra was administered i.c.v. Data are plotted as average change from baseline response (mean ± SEM). The times of tetanization and of drug application are indicated by an arrow. (Top) Application of IL-1ra 30 min before tetanization did not affect subsequent LTP (n = 6). (Middle) When IL-1ra was applied immediately after tetanus no statistically significant effects could be observed (n = 7). (Bottom) IL-ra infused 90 min post-tetanus caused a marked decrease of potentiation (n = 8, ∗, P < 0.05, ANOVA, followed by the Fisher test for multiple comparisons), which lasted for ≈40 min.
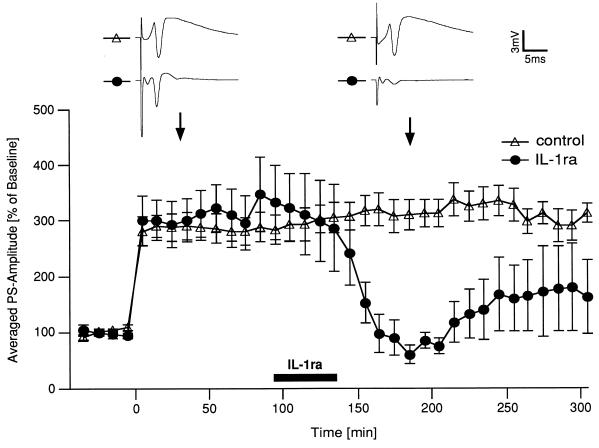
Figure 3.
Blockade of IL-1 receptors inhibits LTP maintenance in hippocampal slices. Ninety to 100 min after inducing LTP in hippocampal slices, IL-1ra was perfused for 40 min (n = 5). The same procedure was followed in control slices omitting the antagonist in the perfusion fluid (n = 6). Results (mean ± SEM) are expressed as percentage of the pretetanic PS-amplitude of each preparation. Each point in the curves shows the average of two consecutive recordings. Application of IL-1ra resulted in a complete elimination of potentiation 1–2 hr after starting IL-1ra perfusion. (P < 0.05, ANOVA, followed by the Fisher test for multiple comparisons). Representative analog traces corresponding to the points indicated by the arrows are shown in the upper part.
Blockade of IL-1 Receptors Interferes with LTP in Hippocampal Slices.
To study possible effects of endogenously produced IL-1β, we conducted experiments in which the action of the cytokine was blocked by application of IL-1ra, which binds with high affinity to IL-1 receptors (1), but completely lacks agonist activity (22, 23). First, we investigated the effect of IL-1ra in hippocampal slices during LTP. IL-1ra, perfused 90–100 min after tetanic stimulation for 40 min, caused a 70–80% decrease in PS amplitude that became discernible 1–2 hr after starting the perfusion (Fig. 3). Thereafter, the PS-amplitude increased and tended to stabilize between pre- and post-tetanic levels. Application of IL-1ra before tetanization did not affect LTP (data not shown).
Blockade of IL-1 Receptors Interferes with LTP Maintenance in the Hippocampus of Freely Moving Rats.
The previous results prompted us to perform comparable studies in freely moving rats in which LTP was induced in the dentate gyrus. For these experiments, we used a stimulation protocol that induced a weak, “unsaturated” LTP decaying to pretetanus values within 5–7 hr (21). This “weak” LTP has the advantage that both reinforcing and inhibitory effects on potentiation can be detected readily. IL-1ra injected into the lateral cerebral ventricle 30 min before tetanic stimulation had virtually no effect on the induction and subsequent maintenance of the potentiation (Fig. 4 Top). Similarly, application of the antagonist immediately after tetanization had no significant effect on potentiation, although the PS-amplitude tended to decline transiently 30 min after tetanization (Fig. 4 Middle). In contrast, IL-1ra administered 90 min after tetanization induced in all rats a marked decrease of PS-amplitude, which reached pretetanic values (Fig. 4 Bottom). This effect was reversible because the PS amplitude returned to values comparable to those of control rats after 40 min. Thus, the effect of blocking IL-1 receptors on LTP clearly depended on the time elapsed after tetanization and was manifested when IL-1β production was expected.
DISCUSSION
We have investigated the possibility that IL-1β plays a physiologic function in the “healthy” brain. We chose to study whether a sustained increase in the activity of a discrete population of hippocampal neurons leads to the stimulation of IL-1β gene expression and whether, in turn, the induced cytokine affects the activity of these neurons. To approach this issue experimentally, we took advantage of LTP in the hippocampus. This intensively investigated cellular correlate of learning and memory was the model of choice because, during this process, neurons are switched to a higher level of activity that lasts for a sufficient time to allow an eventual de novo synthesis and subsequent release of IL-1β. Because several effects of IL-1 are known to be exerted in an autocrine/paracrine fashion by very low quantities of the cytokine, we have anticipated that low, but physiologically relevant amounts of IL-1 that are below quantities that may cause inflammatory effects, would be produced in the hippocampus during LTP. In fact, it has been shown that biological significant effects of IL-1 can be detected following the occupancy of <10 receptors per cell (24, 25). Low basal levels of IL-1β expression in the hippocampus can be detected by using RT-PCR (19). However, in agreement with a recent report (26), we were not able to detect basal IL-1β expression in this brain region by using in situ hybridization. We also could not demonstrate expression of IL-1β gene during LTP by using this technique. Regarding receptors for the cytokine, expression of the IL-1 type I receptor in the hippocampus has been shown repeatedly by in situ hybridization in the mouse (for review, see ref. 4). In contrast, by using this technique, we could not detect gene expression of these receptors in the hippocampus of the rat, as reported by other authors (27). However, negative findings by using in situ hybridization do not exclude the presence of IL-1 receptors in the rat hippocampus because specific binding sites for the cytokine have been shown in this brain region by using other methodologies (28, 29). When a sensitive semi-quantitative RT-PCR was used (19), a clear increase in IL-1β transcripts in the hippoccampus was observed during LTP. Unfortunately, this technique does not allow to identify which type of brain cell(s) produces IL-1β during LTP because both neurons and glial cells are potential sources of the cytokine. However, our results indicate that IL-1β is a relevant gene product expressed during LTP and triggered by mechanisms intrinsic to the potentiation. An increased gene expression was observed in rats that displayed a stable LTP until the hippocampus was dissected 8 hr post-tetanization. In contrast, low levels of IL-1β transcripts were detected in animals in which, although receiving the same type of stimuli, hippocampal neuronal activity returned to pretetanic levels several hours before. Furthermore, no differences in the levels of IL-1β transcripts were noticed between the ipsilateral and contralateral side of the hippocampus in control animals, and the increased accumulation of IL-1β transcripts during LTP was detected only in the ipsilateral side. This rules out cytokine expression caused by nonspecific surgical stress and possible sub-clinical infections and effects caused by the implanted electrode. Importantly, tetanic stimulation per se cannot account for the activation of the IL-1β gene as proven by the basal levels of the cytokine transcripts detected in rats in which the potentiation was prevented by application of the NMDA-antagonist AP-5 and in animals with decremental LTP.
A second line of support for a physiological role of IL-1β in the brain was provided by experiments in which actions of IL-1β were blocked by the specific receptor antagonist IL-1ra. We found a salient impairment of LTP after application of IL-1ra that was partially (in vitro) or totally (in vivo) reversible after cessation of treatment with the natural antagonist and, more remarkable, that was confined to the time after tetanization for which an increase accumulation of cytokine transcripts was detected. Two conclusions can be drawn from these experiments: first, the LTP-induced IL-1β mRNA is translated into biologically significant amounts of the cytokine; and second, increased levels of IL-1β rather than basal concentrations contribute to LTP maintenance. These results suggest that IL-1β plays a regulatory role during this process because potentiated neurons trigger IL-1β production which in turn contributes to LTP maintenance.
Collectively, our in vivo and in vitro data indicate that increased local production of IL-1β in the hippocampus during LTP is a critical factor for the maintenance of this process and thus constitutes an example of a physiological role for this endogenous cytokine beyond pathophysiological conditions. Our results are in line with other findings demonstrating that LTP and memory require the specific and sequential activation of distinct genes to become consolidated (30–36). In contrast to its effect on LTP maintenance, IL-1 has apparently no function in LTP induction, which is in contradiction to published data reporting that recombinant IL-1β inhibits the induction of LTP in vitro (37–39). However, the amount of cytokine added to slices in these studies might be comparable to concentrations attained in the brain during infective or inflammatory processes, thereby not allowing physiological conclusions. Furthermore, exogenous administration of IL-1β is unlikely to resemble the temporal and spacial patterns of endogenously produced IL-1β during LTP.
The temporary inhibition of an established potentiation without affecting the induction of LTP is an intriguing finding and, to the best of our knowledge, not previously described. Even agents, such as inhibitors of haem-oxygenase, that affect LTP maintenance, also impair its induction (40). The rapid effects of IL-1ra in vivo indicate that IL-1β operates directly on processes underlying LTP. One mode of action of IL-1 could be to provide metabolic support to potentiated neurons. Neurons metabolize glucose as their main source of energy, and IL-1β can stimulate glucose uptake in different cell types in an insulin-independent manner (41–44). Other possible routes by which IL-1 could act on potentiation include the release of substances such as neurotransmitters (norepinephrine and dopamine) (45) or retrograde messengers [e.g. arachidonic acid, nitric oxide, and platelet-activating factor (17, 46–48)], which are known to be involved in LTP.
Evaluated together, our results strongly suggest that IL-1β is a key mediator for the maintenance of LTP, which acts downstream of the initial events of LTP induction. These results indicate a physiological role of IL-1β in synaptic plasticity and implicates this cytokine in the cellular mechanisms underlying learning and memory.
Acknowledgments
The skillful technical assistance of S. Petzoldt, U. Stolze, and S. Vieweg is gratefully acknowledged. We are very thankful to N. Federov for his helpful advice. The recombinant human IL-1ra used in these studies was kindly provided by Dr. J. Relton, Synergen, Boulder, CO. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 297) and the Volkswagen Stiftung.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: AP-5, (±)-2-amino-5-phosphonopentanoic acid; IL, interleukin; IL-1β; interleukin-1β, IL-1β; IL-1ra, interleukin-1 receptor antagonist; LTP, long-term potentiation; NMDA, N-methyl-d-aspartate; RT-PCR, reverse transcriptase–PCR; PS, population spike; i.c.v., intracerebroventricular.
References
- 1.Dinarello C A. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 2.Besedovsky H O, del Rey A. Endocr Rev. 1996;17:64–102. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- 3.Haas H S, Schauenstein K. Prog Neurobiol. 1997;51:195–222. doi: 10.1016/s0301-0082(96)00055-x. [DOI] [PubMed] [Google Scholar]
- 4.Rothwell N J, Hopkins S J. Trends Neurosci. 1995;18:130–136. doi: 10.1016/0166-2236(95)93890-a. [DOI] [PubMed] [Google Scholar]
- 5.Kabiersch A, del Rey A, Honegger C G, Besedovsky H O. Brain Behav Immun. 1988;2:267–274. doi: 10.1016/0889-1591(88)90028-1. [DOI] [PubMed] [Google Scholar]
- 6.Dunn A J. Life Sci. 1988;43:429–435. doi: 10.1016/0024-3205(88)90522-x. [DOI] [PubMed] [Google Scholar]
- 7.Miller L G, Galpern W R, Dunlap K, Dinarello C A, Turner T J. Mol Pharmacol. 1991;39:105–108. [PubMed] [Google Scholar]
- 8.Rettori V, Belova N, Gimeno M, McCann S M. NeuroImmunoModulation. 1994;1:116–120. doi: 10.1159/000097144. [DOI] [PubMed] [Google Scholar]
- 9.Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H O. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- 10.Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Science. 1987;238:522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- 11.Uehara A, Gottschall P E, Dahl R R, Arimura A. Endocrinology. 1987;121:1580–1582. doi: 10.1210/endo-121-4-1580. [DOI] [PubMed] [Google Scholar]
- 12.Kent S, Bluthé R M, Kelley K W, Dantzer R. Trends Pharmacol Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- 13.Gibertini M, Newton C, Friedman H, Klein T W. Brain Behav Immun. 1995;9:113–128. doi: 10.1006/brbi.1995.1012. [DOI] [PubMed] [Google Scholar]
- 14.Oitzl M S. Brain Res. 1993;613:160–163. doi: 10.1016/0006-8993(93)90468-3. [DOI] [PubMed] [Google Scholar]
- 15.Bliss T V P, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 16.Tsien J Z, Chen D F, Gerber D, Tom C, Mercer E H, Anderson D J, Mayford M, Kandel E R, Tonegawa S. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- 17.Malenka R C. Cell. 1994;78:535–538. doi: 10.1016/0092-8674(94)90517-7. [DOI] [PubMed] [Google Scholar]
- 18.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd Ed. New York: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 19.Pitossi F, del Rey A, Kabiersch A, Besedovsky H O. J Neurosci Res. 1997;48:1–12. [Google Scholar]
- 20.Hogan B L M, Constantin F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. NY: Cold Spring Harbor Lab. Press; Plainview; 1986. pp. 228–242. [Google Scholar]
- 21.Seidenbecher T, Reymann K G, Balschun D. Proc Natl Acad Sci USA. 1997;94:1494–1499. doi: 10.1073/pnas.94.4.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenfeder S A, Nunes P, Kwee L, Labow M, Chizzonite R A, Ju G. J Biol Chem. 1995;270:13757–13765. doi: 10.1074/jbc.270.23.13757. [DOI] [PubMed] [Google Scholar]
- 23.Greenfeder S A, Varnell T, Powers G, Lombard-Gillooly K, Shuster D, McIntyre K W, Ryan D E, Levin W, Madison V, Ju G. J Biol Chem. 1995;270:22460–22466. doi: 10.1074/jbc.270.38.22460. [DOI] [PubMed] [Google Scholar]
- 24.Orencole S F, Dinarello C A. Cytokine. 1989;1:14–22. doi: 10.1016/1043-4666(89)91044-2. [DOI] [PubMed] [Google Scholar]
- 25.Stylianou E, O’Neill L A, Rawlinson L, Edbrooke M R, Woo P, Saklatvala J. J Biol Chem. 1992;267:15836–15841. [PubMed] [Google Scholar]
- 26.Wong M-L, Bongiorno P B, Rettori V, McCann S M, Licinio J. Proc Natl Acad Sci USA. 1997;94:227–232. doi: 10.1073/pnas.94.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erikson A, Liu C, Hart R P, Sawchenko P E. J Comp Neurol. 1995;361:681–698. doi: 10.1002/cne.903610410. [DOI] [PubMed] [Google Scholar]
- 28.Farrar W L, Kilian P L, Ruff M R, Hill J M, Pert C B. J Immunol. 1987;139:459–463. [PubMed] [Google Scholar]
- 29.Katsuura G, Gottschall P E, Arimura A. Biochem Biophys Res Commun. 1988;156:61–67. doi: 10.1016/s0006-291x(88)80805-2. [DOI] [PubMed] [Google Scholar]
- 30.Stanton P K, Sarvey J M. J Neurosci. 1984;4:3080–3088. doi: 10.1523/JNEUROSCI.04-12-03080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krug M, Loessner B, Ott T. Brain Res Bull. 1984;13:39–42. doi: 10.1016/0361-9230(84)90005-4. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen P V, Abel T, Kandel E R. Trends Pharmacol Sci. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- 33.Rosenzweig M R. Annu Rev Psychol. 1996;47:1–32. doi: 10.1146/annurev.psych.47.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Frey U, Morris R G M. Nature (London) 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 35.Chen C, Tonegawa S. Annu Rev Neurosci. 1997;20:157. doi: 10.1146/annurev.neuro.20.1.157. [DOI] [PubMed] [Google Scholar]
- 36.Rotenberg A, Mayford M, Hawkins R D, Kandel E R, Muller R U. Cell. 1996;87:1351–1361. doi: 10.1016/s0092-8674(00)81829-2. [DOI] [PubMed] [Google Scholar]
- 37.Katsuki H, Nakai Y, Akaji K, Satoh M. Eur J Pharmacol. 1990;181:323–326. doi: 10.1016/0014-2999(90)90099-r. [DOI] [PubMed] [Google Scholar]
- 38.Bellinger F P, Madamba S, Siggins G R. Brain Res. 1993;628:227–234. doi: 10.1016/0006-8993(93)90959-q. [DOI] [PubMed] [Google Scholar]
- 39.Cunningham A J, Murray C A, O’Neill L A, Lynch M A, O’Connor J J. Neurosci Lett. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- 40.Stevens C F, Wang Y. Nature (London) 1993;364:47–149. doi: 10.1038/364147a0. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Welsh A, Schneiderman J S, Baly D L. FEBS Lett. 1990;269:421–424. doi: 10.1016/0014-5793(90)81207-5. [DOI] [PubMed] [Google Scholar]
- 42.Taylor D J, Faragher E B, Evanson J M. Circ Shock. 1992;37:105–110. [PubMed] [Google Scholar]
- 43.del Rey A, Besedovsky H O. Am J Physiol. 1987;253:R794–R798. doi: 10.1152/ajpregu.1987.253.5.R794. [DOI] [PubMed] [Google Scholar]
- 44.del Rey A, Besedovsky H O. Proc Natl Acad Sci USA. 1989;86:5943–5947. doi: 10.1073/pnas.86.15.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shintani F, Kanba S, Nakaki T, Nibuya M, Kinoshita N, Suzuki E, Yagi G, Kato R, Asai M. J Neurosci. 1993;13:3574–3581. doi: 10.1523/JNEUROSCI.13-08-03574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braquet P, Rola-Pleszczynski M. Immunol Today. 1987;8:345–352. doi: 10.1016/0167-5699(87)90010-7. [DOI] [PubMed] [Google Scholar]
- 47.Stanton P K, Sarvey J M. Brain Res. 1985;361:276–283. doi: 10.1016/0006-8993(85)91299-5. [DOI] [PubMed] [Google Scholar]
- 48.Huang Y-Y, Kandel E R. Proc Natl Acad Sci USA. 1995;92:2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]