Abstract
Four experiments studied the role of GABAA receptors in the temporal dynamics of memory retention. Memory for an active avoidance response was a nonmonotonic function of the retention interval. When rats were tested shortly (2 min) or some time (24 h) after training, retention was excellent, but when they were tested at intermediate intervals (1–4 h), retention was poor. Activity at GABAA receptors was critical for impairing memory retention at the intermediate intervals because injection of the GABAA receptor partial inverse agonist FG7142 prior to test significantly improved performance. These retention enhancing effects of FG7142 were dose-dependent and not due to any nonspecific effects of FG7142 on activity. Our results suggest that the temporal dynamics of memory retention may be caused by variations in neurotransmission through the GABAA receptor in the post-training period.
It is a common place observation that memories vary in strength as a function of the time that has elapsed since their formation. Generally, the longer the interval between encoding and retrieval, the greater the memory decays. In other words, memory is typically a monotonic function of retention interval. Nonetheless, there are circumstances under which the function that relates retention to time is nonmonotonic. Perhaps the best example is that described by Kamin (1957). In an avoidance conditioning experiment in rats, Kamin described a U-shaped function, so that memory was greatest shortly (i.e., minutes) or some time (>24 h) after training but was poor at intermediate intervals (i.e., hours).
The “Kamin effect” has been reported in a variety of species, including rats (Kamin 1957; Klein and Spear 1970), Aplysia (Sutton et al. 2001), and honey-bees (Gerber and Menzel 2000). It has long been studied using active and passive avoidance preparations in rats (for reviews, see Brush 1971; Spear 1978). More recently, it has been documented in other preparations. For example, McNally and Westbrook (2003) showed that for background contextual fear conditioning in rats, freezing was a nonmonotonic function of the retention interval, with levels highest 2 min or 24 h after conditioning and significantly lower 6 h after conditioning. Rudy and Wright-Hardesty (2007) reported that contextual memories in rats were highest immediately and 24 h after brief exposure to a context but were significantly lower 5 min, 1 h, or 6 h after such exposures. Carew and colleagues have demonstrated such effects at both behavioral and synaptic level in Aplysia. Tail-elicited siphon withdrawals (Sutton et al. 2001), synaptic facilitation (Mauelshagen et al. 1996), and protein kinase A activity (Muller and Carew 1998) all display a nonmonotonic function following sensitization training or 5-HT application so that each measure was initially high after training, decreased significantly within 3 h of training, and increased significantly 20–24 h after training.
Nonmonotonic retention functions have been subject to two different interpretations. The first has been to suppose that gaps in retention reflect differences in the temporal dynamics of storage of different memory traces. Specifically, the initial training is held to give rise to multiple memory traces. Information held by a short- or intermediate-term memory trace subserves performance in the minutes following training, whereas information held by a long-term trace subserves performance in the hours to days following training. Gaps in performance reflect temporal discontinuity between these memory traces. Analyses of retention of sensitization in Aplysia are especially instructive in this regard (for review, see Sutton and Carew 2002). Sensitization training or synaptic facilitation produced by applications of 5-HT initiates, among other processes, (1) a short-term process that does not require translation or transcription, (2) an intermediate-term process that requires translation but not transcription, and (3) a long-term process that requires transcription and translation. Any temporal discontinuity between the decay of one of these traces and the onset of the next will produce a nonmonotonic retention function, i.e., the Kamin effect (Sutton and Carew 2002). The second interpretation has supposed that memory gaps reflect variations in memory retrieval. According to this analysis, there is a discrepancy between the internal cues present during training and the internal cues present at intermediate retention intervals (Klein and Spear 1970; Klein 1972; Spear 1971, 1973). One source of such internal cues is endocrine changes that are initiated by the training event and that fluctuate across the hours immediately following training, rendering the internal state of the animal at intermediate retention intervals different to that at very short or long retention intervals. This discrepancy impairs memory retrieval at the intermediate interval. The fundamental difference between these two interpretations is that according to the former (variations in storage) memory gaps cannot be alleviated (the memory is in “transit” between different traces and not recoverable), whereas according to the latter (variations in retrieval) memory gaps can be alleviated.
A significant amount of experimental work has been dedicated to distinguishing between these interpretations, and in the case of aversive learning in mammals, it is clear that variations in performance under many circumstances are due to variations in memory retrieval (for review, see Spear 1978). However, there has been very little empirical attention directed toward understanding the neural mechanisms of the variations in performance. Specifically, what are the neuroanatomical, neuropharmacological, and cellular mechanisms for temporal variations in memory retrieval?
There is indirect evidence that that activity at GABAA receptors may contribute to these temporal dynamics. FG7142 is a partial inverse agonist at the benzodiazepine binding site on the GABAA receptor. Benzodiazepine binding sites are expressed on GABAA receptors containing α1, α2, α3, or α5 subunits (Sieghart 1995). FG7142 is nonselective for these α subunits, but as a negative modulator, it reduces channel operation and therefore reduces activation of the GABAA receptor. We have recently used FG7142 to document a role for GABAA receptors in regulating memory retrieval over long intervals. In those experiments, pretreatment with FG7142 promoted retrieval of fear memories that had been forgotten due to infantile amnesia (Kim et al. 2006). For example, 18-d-old rats subjected to auditory fear conditioning displayed excellent retention as indexed by freezing when tested 1 d after training but not 10 d after training. When the 10-d test was preceded by injections of FG7142, however, excellent retention was observed. Kim et al. (2006) also showed identical effects of FG7142 on infantile amnesia as measured by passive avoidance. Harris and Westbrook (1998) reported that retrieval of adult fear memories, which had been subject to inhibition by extinction training, could likewise be enhanced by injections of FG7142 prior to test. In each of these cases, the memory retrieval enhancing effects of FG1742 were independent of any effects on fear, anxiety, or activity.
The retrieval enhancing effects of GABAA receptor inverse agonism on fear memories forgotten due to the passage of time (infantile amnesia) or interference training (fear extinction) are consistent with the recently documented cognitive enhancing effects GABAA receptor inverse agonists (e.g., Collinson et al. 2002, 2006; Atack et al. 2006; Dawson et al. 2006). For example, targeted disruption of the GABAA receptor α5 subunit gene, causing global loss of α5 GABAA receptors (Collinson et al. 2002), or systemic administrations of the α5 selective inverse agonist L-655,708 (Atack et al. 2006) both significantly improve memory as assessed by the Morris water maze. Likewise, a histidine-arginine point mutation at position 105 of the mouse α5 subunit gene reduces hippocampal extra-synaptic (i.e., gephyrin-independent) α5GABAA receptor expression and facilitates hippocampal-dependent trace fear conditioning (Crestani et al. 2002).
These findings led us to hypothesize that the temporal dynamics of normal memory retention may be caused by variations in GABAergic neurotransmission through the GABAA receptor in the post-training period. The present experiments represent an examination of this hypothesis.
Results
Experiment 1: The temporal dynamics of memory retention
The aim of this experiment was to determine the temporal dynamics of memory following active avoidance training. The Kamin effect has been reported using different measures of aversive learning, including active avoidance (Kamin 1957), passive avoidance (Klein and Spear 1970), and freezing (McNally and Westbrook 2003). Extensive pilot studies in our laboratory showed that, in adult animals in our laboratory, active avoidance training was optimal for reliable detection of the Kamin effect. The nadir of memory retrieval following training is variable and depends on specific training parameters (for review, see Brush 1971). However, in aversive preparations, it is typically observed between 1 and 6 h after training. To this end, rats were trained using one-way active avoidance to a criterion of five consecutive avoidance responses. Upon reaching criterion, rats were returned to their home cages where they remained until testing. Different groups were tested for avoidance at different intervals following training: 2 min, 1 h, 4 h, and 24 h A control group that did not receive footshock during training but received equivalent exposure to the apparatus during training was also tested.
Active avoidance training proceeded uneventfully. There were no significant differences between groups in number of acquisition trials to criterion performance (group 2 min mean = 11.5, SEM = 1.3; group 1 h mean = 9.5, SEM = 0.5; group 4 h mean = 10.5, SEM = 0.8; group 24 h mean = 10.7, SEM = 0.5) (F(3,25) < 1, P > 0.05).
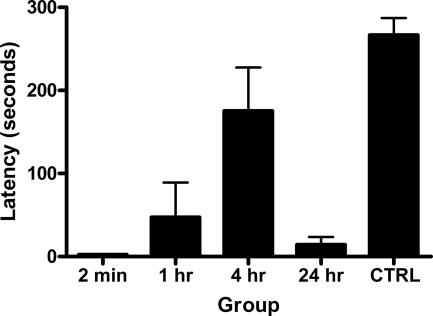
The data of primary interest are those from retention test. The mean and SEM avoidance latencies on retention test are shown in Figure 1. Inspection of the figure indicates that latencies showed a nonmonotonic function with performance best at 2 min and 24 h following training and poorest at 4 h following training. The statistical analysis showed that overall, trained rats had faster latencies than did control rats (F(1,32) = 40.8, P < 0.05). There was also a significant quadratic polynomial trend in latencies of trained rats (F(1,32) = 11.6, P < 0.05), indicating the presence of the Kamin effect: Avoidance latencies significantly increased then decreased across the retention interval. Casual observation of the trained rats indicated that rats tested at 4 h were not engaging in other fear-indicant behavior (e.g., freezing) but rather engaged in occasional bouts of exploratory activity and self-grooming prior to crossing into the white chamber.
Figure 1.
Mean and SEM latencies on test in Experiment 1. Groups were trained to criterion on one-way active avoidance prior to testing at 2 min, 1 h, 4 h, or 24 h after training. Group Control (CTRL) did not receive footshock during training. Retention was a nonmonotonic function of the interval between training and test.
Experiment 2: Effects of a GABAA receptor inverse agonist on memory retrieval
The results of Experiment 1 showed that memory retention was poorest at 4 h and excellent at 24 h after training. The aim of Experiment 2 was to determine the effects of FG7142 when administered prior to test at these two intervals. The experimental design was a 2 × 2 factorial. The first factor refers to the interval between training and test (4 h vs. 24 h). The second factor refers to the type of injection given 15 min prior to these tests (FG7142 vs. vehicle). FG7142 was injected at a dose of 10 mg/kg based on past research indicating that this was optimal for enhancing memory retrieval (Harris and Westbrook 1998; Kim et al. 2006).
Active avoidance training proceeded uneventfully. There were no significant differences between groups in number of acquisition trials to criterion performance (group 4 h: vehicle mean = 9.5, SEM = 1.2; FG1742 mean = 10.3, SEM = 0.5; group 24 h: vehicle mean = 10.1, SEM = 1; FG7142 mean = 10.1, SEM = 0.5; F(3,25) < 1, P > 0.05).
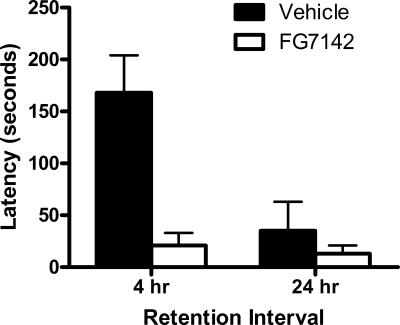
The mean and SEM avoidance latencies from retention test are shown in Figure 2. Inspection of the figure indicates that the vehicle groups showed the Kamin effect: Retention was poorer at 4 h than 24 h Strikingly, FG7142 administered prior to the 4-h retention test produced a pronounced improvement in retention. These observations were supported by the statistical analysis. There was a significant main effect of time, so that averaged across test interval, groups tested at 24 h had faster latencies than groups tested at 4 h (F(1,25) = 8.0, P < 0.05). There was also a main effect of pretest injection of FG7142 versus vehicle (F(1,25) = 11.4, P < 0.05). Importantly, there was a 2 × 2 interaction (F(1,25) = 6.2, P < 0.05), so that the effects of FG7142 on retention at 4 h were significantly greater than at 24 h. This shows that FG7142 promoted memory retrieval at 4 h following training.
Figure 2.
Mean and SEM latencies on test in Experiment 2. Groups were trained to criterion on one-way active avoidance prior to testing 4 h or 24 h later. Rats were injected with 10 mg/kg of the GABAA receptor inverse agonist FG7142 or vehicle 15 min prior to test.
Experiment 3: Dose response properties of FG7142 on retention
The aim of Experiment 3 was to characterize the dose-response properties of the effects of FG7142 on retention of the active avoidance memory. To this end, rats were trained to criterion in the active avoidance preparation. All rats were tested 4 h after training. Prior to retention test, rats were injected with 0, 0.1, 1, or 10 mg/kg FG7142.
Active avoidance training proceeded uneventfully. There were no significant differences between groups in number of acquisition trials to criterion performance (group 0 mg/kg mean = 10.7, SEM = 0.8; group 0.1 mg/kg mean = 10.2, SEM = 0.9; group 1 mg/kg mean = 8.5, SEM = 0.6; group 10 mg/kg mean = 9.3, SEM = 0.7; F(3,25) = 1.9, P > 0.05).
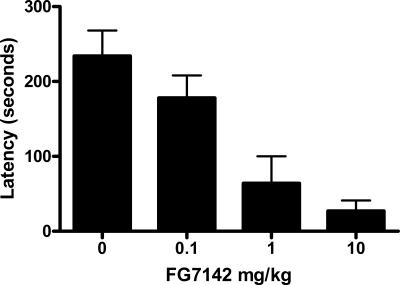
The mean and SEM avoidance latencies from retention test are shown in Figure 3. Inspection of the figure indicates that avoidance latencies were a function of dose of FG7142. The statistical analysis showed that rats injected with FG7142 had significantly faster latencies than rats injected with vehicle (0 mg/kg; F(1,20) = 17.6, P < 0.05). Among rats injected with FG7142 (0.1–10 mg/kg), latencies decreased significantly as dose of FG7142 increased (F(1,20) = 12.8, P < 0.05). These results show that the effects of FG7142 on retention of an active avoidance memory are dose-dependent and that even low doses of FG7142 (1 mg/kg) are effective in enhancing retention of active avoidance.
Figure 3.
Mean and SEM latencies on test in Experiment 3. Groups were trained to criterion on one-way active avoidance prior to testing 4 h later. Rats were injected with 0, 0.1, 1, or 10 mg/kg of the GABAA receptor inverse agonist FG7142 15 min prior to test.
Experiment 4: Effects of FG7142 in nontrained animals
The experiments described thus far show that FG7142 changes the temporal dynamics of memory retention following active avoidance training. One interpretation of these effects is that FG7142 facilitates memory retrieval under circumstances when that retrieval is otherwise not observed (i.e., at the nadir of the retention function). However, another possibility is that FG7142 simply decreased avoidance latencies because of nonspecific effects on anxiety or activity. For example, if FG7142 increased locomotor activity or induced anxiety, it might decrease the latency with which rats crossed from the black side to the white side of the avoidance apparatus. It is worth emphasizing that the Kamin effect itself (poor retention at intermediate intervals) is not due to training-induced changes in activity (for review, see Spear 1978). Regardless, the designs of the previous experiments do not permit the conclusion that the effects of FG7142 depended on prior active avoidance training. It was the intention of this experiment to provide such evidence.
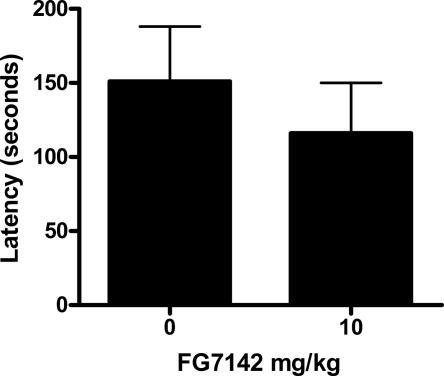
Two groups of rats were confined to the black side of the avoidance chambers for 50 sec. No shocks were presented, and the entrance to the white chamber was closed. This time period was chosen because it was the average amount of time that rats spent in the black chamber during Experiment 1. It ensured that rats in this experiment were equated with those in previous experiments on exposure to the black chamber. Four hours later, rats were tested for latency to enter the white chamber after placement in the black chamber. Prior to this test, rats were injected with 10 mg/kg FG7142 or vehicle. If FG7142 had decreased avoidance latencies in previous experiments because of nonspecific effects on locomotor activity or anxiety, then group FG7142 should show faster latencies to enter the white chamber than group vehicle. The results are shown in Figure 4. There was no significant difference between groups in latency to enter the white chamber (F(1,10) < 1, P > 0.05).
Figure 4.
Mean and SEM latencies on test in Experiment 4. Effects of 0 versus 10 mg/kg of the GABAA receptor inverse agonist FG7142 on latencies to cross from the black to the white side of the avoidance chamber in nontrained rats.
Discussion
These experiments studied the role of GABAA receptors in the temporal dynamics of memory retention. The results can be summarized succinctly. In an active avoidance preparation, memory was a nonmonotonic function of the retention interval. Retention was best shortly (2 min) or some time (24 h) after training and was poor at intermediate intervals (1–4 h). Injection of the GABAA receptor inverse agonist FG7142 improved retention at the 4-h interval. These retrieval enhancing effects of FG7142 were dose-dependent, being observed even at the relatively low dose of 1 mg/kg. Although it is difficult to make cross-experiment comparisons, this is interesting for two reasons. First, it stands in contrast to the failure of such low doses to facilitate recovery from infantile amnesia (Kim et al. 2006) and fear extinction (Harris and Westbrook 1998), suggesting that the Kamin effect may be especially sensitive to GABAA receptor function. Second, this dose is well below that reported to be anxiogenic, which is consistent with previous reports that the memory retrieval enhancing effects of GABAA receptor inverse agonists can be dissociated from their potential anxiogenic effects (Harris and Westbrook 1998; Collinson et al. 2002, 2006; Atack et al. 2006; Dawson et al. 2006; Kim et al. 2006). There was no evidence here that these retrieval enhancing effects of FG7142 were due to nonspecific actions on anxiety or activity.
Taken together, these results suggest that the temporal dynamics of memory retention may be caused by variations in neurotransmission through the GABAA receptor in the post-training period. However, it is worth noting the possibility that even though FG7142 alleviated the Kamin effect, showing an important role for GABAA receptors in regulating memory retrieval, activity at GABAA receptors may not be causal to the Kamin effect. That is, even though FG7142 can reverse a deficit in memory, it does not necessarily mean that it is acting at the substrate producing the deficit. It is possible that FG7142 simply enhances memory. We consider this possibility unlikely because we have shown that FG7142 does not increase retention in the absence of spontaneous forgetting or interference training (Kim et al. 2006). Moreover, FG7142 does not always enhance retrieval or improve memory retention even under conditions of significant forgetting (Kim et al. 2006). Rather, the available evidence suggests that GABAA receptor activity may be a key mechanism for suppressing memory retrieval.
These findings add to a large literature indicating that nonmonotonic retention functions can be due to memory retrieval failures. They are inconsistent with storage-based interpretations of the Kamin effect. Such interpretations typically have two premises. The first is that a training episode initiates multiple memory traces with different temporal and biological properties. The second is that temporal discontinuity between the dissipation of one trace and the onset of the next causes nonmonotonic retention, i.e., the Kamin effect. So, according to this line of reasoning, poor memory at intermediate retention intervals should not have been alleviated by FG7142 in these experiments because there is no biological basis for performance, the short- or intermediate-term trace having decayed and the long-term trace yet to be established (for a recent review, see Rudy and Wright-Hardesty 2007). Demonstrations such as those provided here argue strongly against the second of these premises, at least as applied to retention of aversive memories in adult mammals. These results provide broad support for a retrieval-based interpretation of the Kamin effect because they indicate the original training memory can be recovered or retrieved at intermediate retention intervals—a time when the subject is otherwise not displaying evidence of retention. Regardless, it is not immediately clear whether this dichotomy between storage and retrieval is a useful way of advancing understanding the Kamin effect and of memory failures more generally. For example, the effects of FG7142 reported here do not speak to the first premise of the multiple memory trace account, nor do they require that only a single memory trace be formed by active avoidance training. The available evidence suggests that both storage and retrieval processes can contribute to nonmonotonic retention functions. The issue may therefore be more profitably viewed as understanding the circumstances that favor contribution of storage versus retrieval mechanisms as well as defining the characteristics of each.
The neuroanatomical locus and mechanisms through which GABAA receptors influence the temporal dynamics of aversive memory retention remain unclear, but it is incumbent on any retrieval-based explanation of the Kamin effect, and more generally accounts of memory retrieval failure, to specify them. Recent findings raise an interesting possibility. Stevenson et al. (2007) studied the effects of systemic administrations of FG7142 on corticolimbic interactions in rats. Systemic administrations of FG7142 decreased burst firing in units recorded from the medial prefrontal cortex as well as the basolateral amygdala, and importantly, reduced the synchronized firing typically observed between these regions. These findings show clearly that FG7142 can disrupt prefrontal–amygdala interactions. They are important because it is precisely such structures, and their interactions, which have been implicated in regulating memory retrieval. For example, in human subjects, prefrontal cortical activation during encoding (Wagner et al. 1998) or testing (Anderson et al. 2004) predicts the magnitude of forgetting on a word memory task. Moreover, in rats, burst firing in prefrontal units (Burgos-Robles et al. 2007) and prefrontal–amygdala interactions are important for inhibiting fear memory retrieval after extinction training (Quirk and Maren 2004). It is possible then that the temporal dynamics of aversive memory retention are caused by prefrontal–amygdala interactions in the post-training period so that poor memory at intermediate retention intervals is due to increased activity in prefrontal cortex. Current research in our laboratory is investigating this possibility.
Implicit in this line of reasoning is the possibility that nonmonotonic retention is simply one manifestation of a common neural mechanism for inhibiting retrieval of aversive memories which is shared across otherwise different experimental preparations. The sensitivity of the Kamin effect, fear extinction, and infantile amnesia to administrations of FG7142 as well as other retrieval enhancing treatments (e.g., memory reactivation) are consistent with this possibility. However, some recent data from our laboratory place constraints on this possibility. Tang et al. (2007) studied the effects of FG7142 on the Kamin effect in infant rats. The rats were trained to fear a discrete conditioned stimulus (CS) and were then assessed for fear of that CS at varying intervals after training. Freezing responses to the CS showed a nonmonotonic retention function which was unaffected by FG7142. The reason for the discrepancy between the present results and those reported by Tang et al. (2007) are unclear. They could be related to different ages of subjects (infant vs. adult) or different approaches to studying fear (active avoidance vs. freezing). Regardless, the available data raise the interesting possibility that analyses and understanding of fear extinction, the Kamin effect, and infantile amnesia may well profit from consideration of the others.
In conclusion, these experiments have confirmed that retention of an avoidance response is a nonmonotonic function of the interval since training and have shown, for the first time, that reducing activity at GABAA receptors can significantly improve retention at intermediate intervals. Our results suggest that these temporal dynamics of memory retention may be caused by variations in neurotransmission through the GABAA receptor in the post-training period.
Materials and Methods
Subjects
The subjects were experimentally naive, adult, male Wistar rats (280–450 g) obtained from a commercial supplier (Gore Hill Research Laboratories, Sydney, Australia). After arrival, rats were housed in groups of eight in plastic cages and maintained on a 12-h light-dark cycle (lights on at 0700 h) with free access to food and water. The rats were handled (1–2 min per rat per day) for 3 d to habituate them to the experimenter. The procedures used were approved by the Animal Ethics Committee at the University of New South Wales and were conducted in accordance with the National Institutes of Health’s (1986) Guide for the Care and Use of Laboratory Animals.
Apparatus
The apparatus was a two-chamber, black and white avoidance apparatus. Each chamber was 23 × 23 × 23 cm (l × w × h) and was constructed of Perspex. The walls were covered in black or white cardboard. The floor of the black chamber consisted of stainless steel grid bars, 2-mm diameter, spaced 5-mm center-to-center through which the 1-sec, 0.8-mA unscrambled footshock was delivered. The floor of the white chamber consisted of Perspex. The two chambers were separated by a stainless steel insert with a 11 × 5 cm (w × h) opening, allowing the rat to move between the chambers.
Procedure
Experiment 1
Subjects received one-way active avoidance training to a criterion of five successful avoidances (following at least one failure to avoid). In order to avoid shock, the rat had to cross from the black chamber to the white chamber within 5 sec of placement in the black chamber, otherwise a footshock was delivered every 5 sec until the rat crossed. The rat remained in the white chamber for 15 sec prior to being removed and placed again in the black chamber. Any rat that failed to cross to the white chamber within 50 sec on any trial during training (i.e., had received 10 footshocks on a trial) was excluded from further training and was not tested. This was done so as to reduce exposure to footshock stress. After reaching criterion, rats were returned to their home cages, where they remained for 2 min, 1 h, 4 h, or 24 h prior to testing. For testing, rats were placed in the black chamber and latency to enter the white chamber was recorded. If no response was made within 300 sec, rats were returned to their home cages and a latency of 300 sec was recorded. A control group that did not receive footshock during training but instead received 50 sec exposure to the black chamber (the average amount of time spent in the black chamber by shocked rats during training) was included. Final group sizes were as follows: 2 min, n = 6 (two rats excluded); 1 h, n = 8; 4 h, n = 8; 24 h, n = 7 (one rat excluded); and group control, n = 8. All subjects in this and remaining experiments were tested once only.
Experiment 2
Subjects received one-way active avoidance training to a criterion of five successful avoidances (following at least one failure to avoid) as described above. Rats were tested 4 h or 24 h after training. Fifteen minutes prior to test rats were injected subcutaneously (s.c.) in the dorsal neck region with 10 mg/kg FG7142 or vehicle. FG-7142 (N-methyl-β-carboline-3-carboxymide; Sigma-Aldrich) was suspended at a concentration of 10 mg/mL in saline (0.9% w/v) using 1 drop of Tween 80 per 5 mL saline. This suspension, or the vehicle (saline plus Tween 80), was administered in a volume of 1 mL/kg. Final group sizes were as follows: 4 h—vehicle, n = 6 (two rats excluded), and FG7142, n = 7 (one rat excluded); 24 h—vehicle, n = 8, and FG7142, n = 8.
Experiment 3
Subjects received one-way active avoidance training to a criterion of five successful avoidances (following at least one failure to avoid) as described above. Rats were tested 4 h after training. Fifteen minutes prior to test rats were injected s.c. in the dorsal neck region with 0.1, 1, or 10 mg/kg FG7142 or vehicle (n = 6 per group). FG-7142 was suspended in saline (0.9% w/v) using 1 drop of Tween 80 per 5 mL saline. This suspension, or the vehicle (saline plus Tween 80), was administered in a volume of 1 mL/kg.
Experiment 4
Subjects were exposed to the black side of the active avoidance apparatus for 50 sec, and no footshock was administered. Rats were tested 4 h later. Fifteen minutes prior to testing, rats were injected s.c. in the dorsal neck region with 10 mg/kg FG7142 (n = 6) or vehicle (n = 6), which was prepared as described above.
Data analysis
The number of trials to criterion performance during training were analyzed using one-way ANOVA. Latencies from test were analyzed by means of ANOVA testing planned orthogonal contrasts. The decision-wise error rate (α) was controlled at the 0.05 level using the procedures described by Hays (1972). None of the conclusions from the analyses reported here would be altered by use of nonparametric statistics.
Acknowledgments
We thank Peregrine Osborne for helpful discussions of these experiments. These experiments were supported by a Discovery Project grant from the Australian Research Council to G.P.M. and R.R. (DP0559967).
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.806008
References
- Anderson M.C., Ochsner K.N., Kuhl B., Cooper J., Robertson E., Gabrieli S.W., Glover G.H., Gabrielli J.D.E. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Atack J.R., Bayley P.J., Seabrook G.R., Wafford K.A., McKernan R.M., Dawson G.R. L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for α5-containing GABAA receptors. Neuropharmacology. 2006;51:1023–1029. doi: 10.1016/j.neuropharm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Brush F.R. Retention of aversively motivated behavior. In: Brush F.R., editor. Aversive conditioning and learning. Academic Press; New York: 1971. pp. 401–465. [Google Scholar]
- Burgos-Robles A., Vidal-Gonzalez I., Santini E., Quirk G.J. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Collinson N., Kuenzi F.M., Jarolimek W., Maubach K.A., Cothliff R., Sur C., Smith A., Out F.M., Howell O., Atack J.R., et al. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the α5 subunit of the GABAA receptor. J. Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson N., Atack J.R., Laughton P., Dawson G.R., Stephens D.N. An inverse agonist selective for α5 subunit-containing GABAA receptors improves encoding and recall but not consolidation in the Morris water maze. Psychopharmacology. 2006;188:619–628. doi: 10.1007/s00213-006-0361-z. [DOI] [PubMed] [Google Scholar]
- Crestani F., Keist R., Fritschy J.M., Benke D., Vogt K., Prut L., Bluthmann H., Mohler H., Rudolph U. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc. Natl. Acad. Sci. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G.R., Maubach K.A., Collinson N., Cobain M., Everitt B.J., MacLeod A.M., Choudhury H.I., McDonald L.M., Pillai G., Rycroft W., et al. An inverse agonist selective for α5 subunit-containing GABAA receptors enhances cognition. J. Pharmacol. Exp. Ther. 2006;316:1335–1345. doi: 10.1124/jpet.105.092320. [DOI] [PubMed] [Google Scholar]
- Gerber B., Menzel R. Contextual modulation of memory consolidation. Learn. Mem. 2000;7:151–158. doi: 10.1101/lm.7.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J.A., Westbrook R.F. Evidence that GABA transmission mediates context-specific extinction of learned fear. Psychopharmacology. 1998;140:105–115. doi: 10.1007/s002130050745. [DOI] [PubMed] [Google Scholar]
- Hays W.L. Statistics for the social sciences. Holt, Rinehart and Winston; New York: 1972. [Google Scholar]
- Kamin L.J. Retention of an incompletely learned avoidance response. J. Comp. Physiol. Psychol. 1957;50:457–460. doi: 10.1037/h0044226. [DOI] [PubMed] [Google Scholar]
- Kim J., McNally G.P., Richardson R. Recovery of fear memories in rats: role of γ-amino butyric acid (GABA) in infantile amnesia. Behav. Neurosci. 2006;120:40–48. doi: 10.1037/0735-7044.120.1.40. [DOI] [PubMed] [Google Scholar]
- Klein S.B. Adrenal-pituitary influence in reactivation of avoidance-learning memory in the rat after intermediate intervals. J. Comp. Physiol. Psychol. 1972;79:341–359. doi: 10.1037/h0032809. [DOI] [PubMed] [Google Scholar]
- Klein S.B., Spear N.E. Forgetting by the rat after intermediate intervals (“Kamin effect”) as retrieval failure. J. Comp. Physiol. Psychol. 1970;71:165–170. [Google Scholar]
- Mauelshagen J., Parker G.R., Carew T.J. Dynamics of induction and expression of long-term synaptic facilitation in Aplysia. J. Neurosci. 1996;16:7099–7108. doi: 10.1523/JNEUROSCI.16-22-07099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally G.P., Westbrook R.F. Anterograde amnesia for Pavlovian fear conditioning and the role of one-trial overshadowing: Effects of preconditioning exposures to morphine in the rat. J. Exp. Psychol. Anim. Behav. Process. 2003;29:222–232. doi: 10.1037/0097-7403.29.3.222. [DOI] [PubMed] [Google Scholar]
- Muller U., Carew T.J. Serotonin induces temporally and mechanistically distinct phases of persistent PKA activity in Aplysia sensory neurons. Neuron. 1998;21:1423–1434. doi: 10.1016/s0896-6273(00)80660-1. [DOI] [PubMed] [Google Scholar]
- Quirk G.J., Maren S. Neuronal signalling of fear memory. Nat. Rev. Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Rudy J.W., Wright-Hardesty K. The temporal dynamics of retention of a context memory: Something is missing. Learn. Mem. 2007;12:171–177. doi: 10.1101/lm.84005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of γ-aminobutyric acid A receptor subtypes. Pharmacol. Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Spear N.E. Forgetting as retrieval failure. In: Honig W.K., James P.H.R., editors. Animal memory. Academic Press; New York: 1971. pp. 45–109. [Google Scholar]
- Spear N.E. Retrieval of memory in animals. Psychol. Rev. 1973;80:163–194. [Google Scholar]
- Spear N.E. The processing of memories: Forgetting and retention. Lawrence Erlbaum Associates; Mahwah, NJ: 1978. [Google Scholar]
- Stevenson C.W., Halliday D.M., Marsden C.A., Mason R. Systemic administration of the benzodiazepine receptor partial inverse agonist FG7142 disrupts corticolimbic network interactions. Synapse. 2007;61:646–663. doi: 10.1002/syn.20414. [DOI] [PubMed] [Google Scholar]
- Sutton M.A., Carew T.J. Behavioral, cellular, and molecular analysis of memory in Aplysia I: Intermediate-term memory. Integr. Comp. Biol. 2002;42:725–735. doi: 10.1093/icb/42.4.725. [DOI] [PubMed] [Google Scholar]
- Sutton M.A., Masters S.E., Bagnall M.W., Carew T.J. Molecular mechanisms underlying a unique intermediate phase of memory in aplysia. Neuron. 2001;31:143–154. doi: 10.1016/s0896-6273(01)00342-7. [DOI] [PubMed] [Google Scholar]
- Tang H.H.Y., McNally G.P., Richardson R. The effects of FG7142 on two types of forgetting in 18-day old rats. Behav. Neurosci. 2007;121:1421–1425. doi: 10.1037/0735-7044.121.6.1421. [DOI] [PubMed] [Google Scholar]
- Wagner A.D., Schachter D.L., Rotte M., Koustall W., Maril A., Dale A.M., Rosen B.R., Buckner R.L. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]