Abstract
Evidence suggests that plasticity of the amygdalar and hippocampal GABAergic system is critical for fear memory formation. In this study we investigated in wild-type and genetically manipulated mice the role of the activity-dependent 65-kDa isozyme of glutamic acid decarboxylase (GAD65) in the consolidation and generalization of conditioned fear. First, we demonstrate a transient reduction of GAD65 gene expression in the dorsal hippocampus (6 h post training) and in the basolateral complex of the amygdala (24 h post training) during distinct phases of fear memory consolidation. Second, we show that targeted ablation of the GAD65 gene in Gad65−/− mice results in a pronounced context-independent, intramodal generalization of auditory fear memory during long-term (24 h or 14 d) but not short-term (30 min) memory retrieval. The temporal specificity of both gene regulation and memory deficits in Gad65 mutant mice suggests that GAD65-mediated GABA synthesis is critical for the consolidation of stimulus-specific fear memory. This function appears to involve a modulation of neural activity patterns in the amygdalo-hippocampal pathway as indicated by a reduction in theta frequency synchronization between the amygdala and hippocampus of Gad65−/− mice during the expression of generalized fear memory.
In this study, we investigated the role of γ-aminobutyric acid (GABA), the major inhibitory transmitter of the brain, in the consolidation and generalization of conditioned fear and in associated network activities in the amygdala and hippocampus. GABA is known to be critically involved in the control of fear and anxiety through the amygdala, and changes in GABAergic function have been observed in the amygdala and other areas of the limbic system following stressful experiences (Anagnostaras et al. 1999; Fendt and Fanselow 1999; Pape and Stork 2003; Zwanzger and Rupprecht 2005). These processes, at least in part, are mediated by the experience-dependent regulation of GABA synthesis through the 65-kDa isoform of glutamic acid decarboxylase, GAD65. GAD65 is co-expressed with a second isozyme, GAD67, in most GABAergic neurons, and together they account for more than 99% of the GABA synthesis in the brain (Asada et al. 1996; Soghomonian and Martin 1998). However, these two isozymes differ in their expression level, subcellular localization, and cofactor-dependent activation (Bowers et al. 1998; Kanaani et al. 1999; Makinae et al. 2000; Newton and Lu 2006). With the generation of specific mouse mutants, it has become possible to address the role of each isoform in brain function; while Gad67 mutant mice die shortly after birth, Gad65 null mutant mice are viable (Asada et al. 1996, 1999). In previous studies, these mutants have shown a delayed maturation of the GABAergic system, increased anxiety-like behavior, insensitivity to diazepam, paroxysmal activity, and seizures (Kash et al. 1999; Stork et al. 2000). Importantly, they also display heightened behavioral responses during the retrieval of auditory cued and contextual fear memory (Stork et al. 2003).
Classical fear conditioning is a well-established learning model in which the animal is exposed to an innocuous conditional stimulus which is either associated (CS+), or not (CS−), with an unconditioned stimulus (US), in the form of an electric shock to the paws (LeDoux 2000). It has proven a useful model for understanding the psychological and pathological bases of emotional disorders (Maren 2005; Raua et al. 2005). Fear conditioning is critically dependent on the structural and functional integrity of the amygdala, which is considered to be a convergence site for the sensory and affective information during fear memory formation. In addition, the hippocampal formation processes multimodal information concerning contexts that define the time and place of aversive experiences during fear conditioning (Schafe and LeDoux 2000; Schafe et al. 2000). Cellular and molecular correlates of synaptic plasticity as well as network activity patterns in these structures have been identified that relate to the formation of short-term and long-term fear memories (Adamec 2000; Buzsáki 2002; Paré et al. 2002; Pelletier and Paré 2004; Santini et al. 2004; Stork et al. 2004). Specifically, increased theta synchronization between the amygdala and hippocampus has been postulated as a neural correlate of long-term cued fear memory (Seidenbecher et al. 2003; Narayanan et al. 2007).
In the present study we demonstrate transient changes of GAD65 gene expression in the mouse amygdala and hippocampus at different time points following fear-conditioning training. Moreover, we provide evidence for a context-independent generalization and reduction of long-term, but not short-term, cue-specific fear memory in mutant mice deficient in GAD65. Finally, we describe changes in amygdalo-hippocampal theta synchronization that are associated with generalized fear in Gad65−/− mice. Our data suggest that GAD65-mediated GABA synthesis is involved in the consolidation and specificity of long-term fear memory and identify network activities potentially involved in these processes.
Results
GAD gene expression
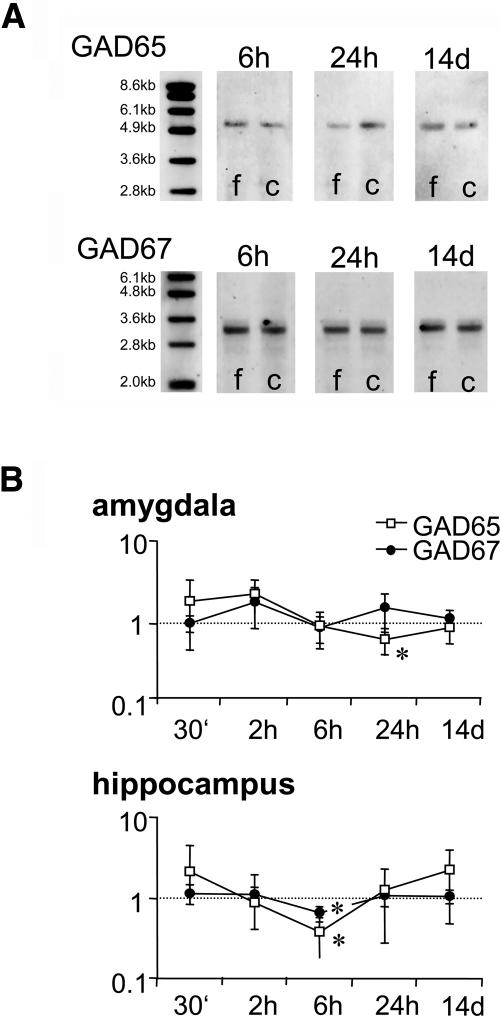
Gene expression analysis on pooled amygdala samples revealed a transient and isoform-specific regulation of GAD following fear-conditioning training (Fig. 1), confirming our previous finding (Pape and Stork 2003). cDNA probes targeting GAD65 and GAD67 revealed single bands on virtual Northern blots with the expected sizes of 5.7 kb for GAD65 and 3.7 kb for GAD67. GAD65 and GAD67 gene expression was quantified at three different time points, 6 h, 24 h, and 14 d, after fear conditioning, using the housekeeping gene GAPDH as a standard. In all experiments, we observed a reduction of the GAD65 signal at the 24-h time point (to the 0.38 ± 0.11-fold level of naïve controls, P < 0.01), but no consistent effect could be found at the 6-h (1.58 ± 1.02) or 14-d (1.21 ± 0.13) time points. The expression in fear-conditioned animals was also significantly lower than in unpaired controls 24 h after training (0.94 ± 0.25, P < 0.01). On the contrary, no significant change in GAD67 gene expression was observed at any of the time points investigated.
Figure 1.
Expression of GAD65 mRNA following fear-conditioning training. (A) Representative cDNA blots showing expression of GAD65 and GAD67 in fear-conditioned mice (f) and unpaired controls (c). Selectively reduced expression of GAD65 is evident 24 h after training, whereas levels at 6 h or 14 d do not differ between fear-conditioned and control groups. (B) An independent group of animals (N = 6 per group) was investigated with quantitative PCR for GAD65 and GAD67 expression in the amygdala and hippocampus at different time points (30 min, 2 h, 6 h, 24 h, and 14 d) after conditioning (mean ± SEM). Reduction of GAD65 mRNA is confirmed in the amygdala, 24 h after training, and furthermore observed for both GAD65 and GAD67 in the hippocampus, 6 h after training. Highly variable increases of GAD65 and partly GAD67 mRNA at earlier time points failed to reach significance level. *P < 0.05 compared to unpaired controls.
Next we employed quantitative PCR to determine GAD65 expression in the basolateral complex of the amygdala and dorsal hippocampus of individual animals. In this experiment, we increased the number of time points investigated to include potential short-term regulation (30 min and 2 h post training). However, we used only explicitly unpaired conditioned animals as controls, as those had not differed from naïves in our first experiment, and confirmed the specificity of regulation through a second housekeeping gene, phosphoglycerate kinase (PGK). First, our measurements confirmed that GAPDH was not regulated in expression at any time point (data not shown). Second, we confirmed a significant reduction in the amygdala 24 h after fear conditioning of GAD65 (to the 0.62 ± 0.22-fold level of explicitly unpaired controls, P < 0.05) but not GAD67 expression (1.52 ± 0.77). No significant difference was observed at any other time point investigated (30 min, 1.84 ± 1.39 for GAD65, 0.98 ± 0.22 for GAD67; 2 h, 2.30 ± 1.00 for GAD65, 1.80 ± 0.95 for GAD67; 6 h, 0.91 ± 0.43 for GAD65, 0.88 ± 0.31 for GAD67; 14 d, 0.86 ± 0.29 for GAD65, 1.12 ± 0.31 for GAD67), although a trend toward increased GAD expression levels was evident in the early post-training period (Fig. 1B). Moreover, expression monitoring revealed a significant reduction of both GAD65 (to 0.38 ± 0.20-fold levels of unpaired controls, P < 0.02) and GAD67 mRNA (0.65 ± 0.14, P < 0.05) in the hippocampus 6 h after conditioning. Other time points produced only small or highly variable changes and thus produced no significant change (30 min, 2.11 ± 2.30 for GAD65, 1.14 ± 0.32 for GAD67; 2 h, 0.88 ± 0.47 for GAD65, 1.11 ± 0.26 for GAD67; 24 h, 1.26 ± 0.98 for GAD65, 1.05 ± 0.27 for GAD67; 14 d, 2.21 ± 1.73 for GAD65, 1.05 ± 0.21 for GAD67).
Behavior of Gad65 mutant mice
Next we investigated, in a differential fear-conditioning paradigm, memory formation and its specificity in Gad65 mutants. Two acoustic stimuli (CS− and CS+) were employed, and memory retrieval was tested at different time points after training (30 min, 24 h, and 14 d) and in different (neutral and training) contexts. To minimize a potential role of the CS− as an explicit safety signal in our differential conditioning paradigm, we presented this test stimulus in conjunction with the training context repetitively prior to but not during the training session. We have previously employed this procedure to demonstrate overtraining-induced generalization of fear memory and observed that inhibitory conditioning to the CS− is only observed when the CS+ itself is presented in explicitly unpaired fashion (Laxmi et al. 2003).
Neutral context
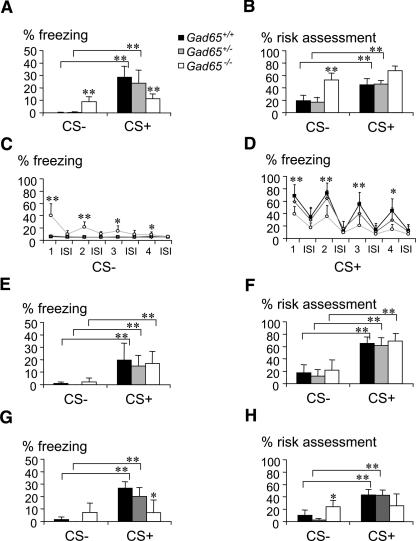
Testing the specificity of long-term fear memory in Gad65 mutant mice, we observed a significant genotype × stimulus interaction for freezing behavior (F(2,61) = 13.806; P < 0.0001). Our results confirm previous observations (Stork et al. 2003) of reduced freezing behavior of Gad65−/− mice during the CS+ test period (Gad65+/+, 28.5% ± 13.2%; Gad65+/−, 23.8% ± 13.2%; and Gad65−/−, 11.3% ± 5.3%; all values are mean ± SD, F(2,31) = 6.274; P < 0.006) (Fig. 2A). Risk-assessment behavior in contrast was not different between groups (Fig. 2B). Reduced freezing of Gad65−/− mice was evident specifically during CS+ tone presentations (repeated-measures ANOVA, genotype effects: F(2,29) = 12.472, P < 0.0001) and not significant during interstimulus intervals (ISIs). All groups reduced their freezing response gradually upon repetitive stimulation (within-subject comparison: F(3,87) = 6.509, P < 0.001) (Fig. 2D).
Figure 2.
Generalization of long-term fear memory in Gad65−/− mice. (A) Gad65−/− mice displayed increased freezing and (B) elevated risk-assessment behavior toward the CS- during a retrieval test 24 h after training. (C) Generalized freezing in Gad65−/− mice was specifically associated with tone presentation during the CS− session, but not seen during interstimulus intervals (ISIs). (D) Similarly, reduced freezing of Gad65−/− mice to the CS+ was only observed during CS+ tone presentation. (E) Short-term memory retrieval 30 min after training, in contrast, produced a highly specific freezing response toward the CS+ in all groups without evidence for generalization to the CS− in Gad65−/− mice. (F) Similarly, no difference between genotypes was observed in their risk-assessment behavior during the analysis of short-term fear memory. (G) Long-term memory (LTM) retrieval after 14 d produced a similar result as 24 h LTM, with a reduced freezing of Gad65−/− mice to the CS+ and a failure to discriminate the CS−. (H) Risk-assessment behavior was increased in Gad65−/− mice during14 d LTM, when compared to their wild-type and heterozygous littermates. **P < 0.01, *P < 0.05, when compared to Gad65+/+. (1–4) Stimulus number. Values are mean + SEM.
Strikingly, Gad65−/− mice also showed a significantly increased freezing response during the CS− test period (8.8% ± 6.2%), in contrast to their Gad65+/+ (0.1% ± 0.2%) or Gad65+/− (0.2% ± 0.2%) littermates (P < 0.0001). Thus, while wild types and heterozygotes both clearly differentiated between the acoustic stimuli (P < 0.0001), homozygous mutants displayed similar levels of freezing during both CS+ and CS− test periods (P > 0.24). Similar to freezing, risk-assessment behavior (Fig. 2B) was significantly affected by genotype during the CS− test period (F(2,31) = 29.025; P < 0.0001), showing a selective increase in Gad65−/− mice as compared to their Gad65+/+ (P < 0.0001) and Gad65+/− (P < 0.0001) littermates. Further analysis revealed that generalized freezing behavior of Gad65−/− mice was specific to the CS− tone presentation and not seen in the ISIs (Fig. 2C). Repeated-measures ANOVA showed a significant effect of genotype across the CS− presentations (F(2,29) = 23.150, P < 0.0001) and a significant stimulus × genotype interaction (F(6,87) = 6.538, P < 0.0001), due to efficient habituation of the generalized response in Gad65−/− mice (within-subject comparison: F(3,33) = 8.537, P < 0.0001). Post-hoc analysis confirmed increased freezing behavior of Gad65−/− mice in response to the first and second (P < 0.001) as well as the third CS− tone (P < 0.05), when compared to Gad65+/+ mice. No such increase was observed in Gad65+/− mice.
Sensory and baseline behavior controls
Control measures of fear-related behavior before (freezing and risk assessment undetectable) and immediately after fear conditioning (freezing, 10.1%–14.9%, P > 0.09; risk assessment, 56.5%–61.7%) did not show any difference between genotypes. Moreover, no indication was found for an altered pain sensitivity in Gad65−/− mice, as genotypes did not differ in their behavioral response to increasing foot-shock currents (first immobilization/vocalization at 0.14/0.26 mA in Gad65+/+ [N = 7], 0.12/0.3 mA in Gad65+/− [N = 5], and at 0.11/0.29 mA in Gad65−/− mice [N = 5], respectively).
Memory time course
To investigate whether the observed deficits of Gad65−/− mice may be related to a function of GAD65 in fear memory consolidation, we next analyzed the mutants’ performance at different time points after training. Considering the observed changes of GAD65 gene expression in wild types, we decided to investigate fear memory 30 min and 14 d after conditioning; hence, we tested mutants and wild types both prior to and after the time window of GAD65 regulation.
First, during short-term memory (STM) retrieval, 30 min after training, no change in performance was observed in Gad65−/− mice that would be comparable to their long-term memory (LTM) deficit. Significant genotype effects could neither be observed during the CS− test period (ANOVA, F(2,23) = 0.59, P > 0.6 for risk assessment, P > 0.3 for freezing) nor the CS+ test period (P > 0.6 for risk assessment and P > 0.7 for freezing). Gad65−/− mice did not show reduced freezing during the CS+ test period (16.9% ± 9.6%) or increased freezing during the CS− test period (2.1% ± 2.7%), when compared to their littermates (Fig. 2E,F). Comparison between LTM and STM tests showed that Gad65−/− mice failed to increase their freezing response during the CS+ test period upon fear memory consolidation, as opposed to Gad65+/+ mice (P < 0.015) (Fig. 2A,E). Risk assessment in contrast was different (F(1,54) = 13,671, P < 0.001) between STM and LTM tests in general; genotype also generally affected performance (F(2,54) = 7.895, P < 0.001). The differences resulted from the increased risk-assessment behavior in Gad65+/+ and Gad65+/− mice during STM as compared to LTM testing (Fig. 2B,F). Moreover, a significant test × genotype interaction was found for freezing behavior during the CS− test period (F(2,54) = 6.634, P > 0.003), due to the selective increase of freezing by Gad65−/− mice in long-term memory retrieval (P < 0.001). Comparison of STM and LTM showed analogous differences for risk assessment (F(1,54) = 9.348, P < 0.004), as the generalization of cued fear memory was not observed in Gad65−/− during STM retrieval.
Second, a group of animals was tested 14 d after fear-conditioning training, i.e., at a time point when GAD65 mRNA expression in wild types had returned to control levels (Fig. 2G,H). At this time point, we observed a stimulus × genotype interaction for both freezing (F(2,45) = 9.468, P < 0.0001) and risk-assessment behavior (F(2,45) = 9.298, P < 0.0001) that was similar to the effects in the 24-h LTM group. Freezing to the CS+ was dependent on genotype (F(2,22) = 7.728, P < 0.003), with Gad65−/− mice showing reduced levels (7.1% ± 9.8%) compared to both Gad65+/+ (26.8% ± 5.0%, P < 0.001) and Gad65+/− mice (20.2% ± 6.9%, P < 0.032). Risk-assessment behavior (25.4%–42.9%) appeared to be somewhat lower in the mutants, yet the difference between genotypes failed to reach significance. During the CS−, a significant effect of genotype was not observed toward freezing behavior, although Gad65−/− mice (7.3% ± 7.3%) showed a somewhat increased response. However, risk assessment proved to be dependent on genotype (F(2,22) = 7.950, P < 0.003), due to an increase in Gad65−/− mice (23.8% ± 8.1%), compared to Gad65+/+ (10.2% ± 8.1%, P < 0.012) and Gad65+/− mice (2.3% ± 1.9%, P < 0.001). By comparing responses within each genotype, it became evident that both Gad65+/+ and Gad65+/− mice distinguished well between CS+ and CS− (P < 0.001 for both freezing and risk assessment), whereas Gad65−/− mice did not. Finally, when comparing the different stages (24 h and 14 d) of LTM, we found no interaction of test and genotype concerning freezing behavior (CS−, F(1,53) = 0.380, P > 0.686; or CS+, F(1,53) = 0.070, P > 0.932), but an interaction was evident for risk-assessment behavior during both CS− (F(1,53) = 3.588, P < 0.035) and CS+ (F(1,53) = 15,237, P < 0.0001), which is explained in the reduction of this behavior in the Gad65−/− after 14 d (CS−, P < 0.0001; CS+, P < 0.0001) compared to the 24-h time point.
Training context
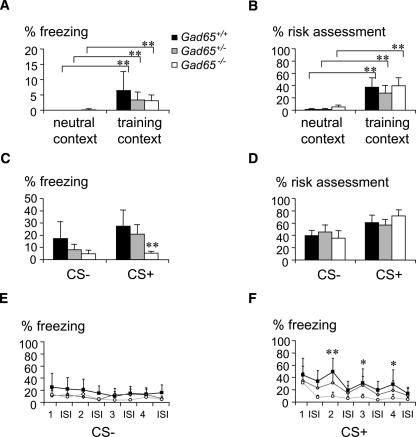
Next, 24-h long-term fear memory was tested in the training context, to address the potential importance of contextual background for generalization of auditory-cued fear memory in Gad65 mutant mice (Fig. 3A,B). Background context exposure indeed induced a moderate fear response (freezing, 3.1%–6.4%, and risk assessment, 27.7%–39.4%) in all genotypes compared to the neutral context (P < 0.0001), comparable to the response observed previously in this paradigm (Laxmi et al. 2003). No significant difference of background contextual conditioning was observed between the genotypes (P > 0.3). However, upon confrontation with the CS+ in the conditioning context, a genotype effect (F(2,23) = 7.447, P < 0.004) similar to that seen in the neutral context emerged; i.e., Gad65−/− mice showed reduced freezing (Fig. 3C) compared to Gad65+/+ (P < 0.001) and Gad65+/− mice (P < 0.017). The statistical analysis of context effects (neutral context vs. shock context) revealed a significant genotype × test interaction during the CS− test period (ANOVA, F(2,54) = 7.662, P < 0.001 for freezing and F(2,54) = 16.689, P < 0.0001 for risk assessment). While both Gad65+/+ and Gad65+/− mice showed a context-dependent increase of freezing and risk assessment during the CS− test period in the shock context (P < 0.003), the behavioral response of Gad65−/− was similar in both tests (freezing, 8.8% ± 6.2% neutral context and 4.6% ± 3.1% shock context; risk assessment, 52.5% ± 13.4% neutral context and 35.7% ± 13.8% shock context). Stimulus-by-stimulus analysis, again, revealed a reduced freezing of Gad65−/− mice during CS+ tone presentation, similar to that observed in the neutral context (cf. Fig. 2D). A significant genotype effect (F(2,29) = 5.384, P < 0.01) was observed, with reduced freezing of Gad65−/− mice to the second (P < 0.001), third, and fourth (P < 0.05) presentations of the CS+ compared to Gad65+/+ mice. Again, no significant change was observed in Gad65+/− mice.
Figure 3.
Lack of contextual priming for fear memory generalization in Gad65−/− mice. During retrieval of background contextual long-term fear memory, freezing (A) and risk-assessment (B) responses did not differ between genotypes. (C) Gad65+/+ and Gad65+/− mice showed significant freezing to both CS− and CS+ when presented in the training context, indicative of context-dependent generalization. Gad65−/− mice displayed low levels of freezing to the CS−, which did not differ from their response to the CS+ or to the CS− when presented in the neutral context (see Fig. 2). This indicates that the generalization of fear memory in Gad65−/− mice occurred independently of the retrieval context, in contrast to generalization in Gad65+/+ and Gad65+/− mice. (D) Risk-assessment behavior was not different between genotypes. (E) Detailed analysis of freezing responses demonstrates the evenly enhanced freezing response of Gad65+/+ and Gad65+/− mice across CS− stimuli and interstimulus intervals. (F) As in the neutral context, the freezing response of Gad65−/− mice was specifically reduced during CS+ tone presentation, compared to Gad65+/+ and Gad65+/− mice. **P < 0.01, *P < 0.05, when compared to Gad65+/+. (1–4) Stimulus number. Values are mean + SEM.
Network activity during fear memory retrieval
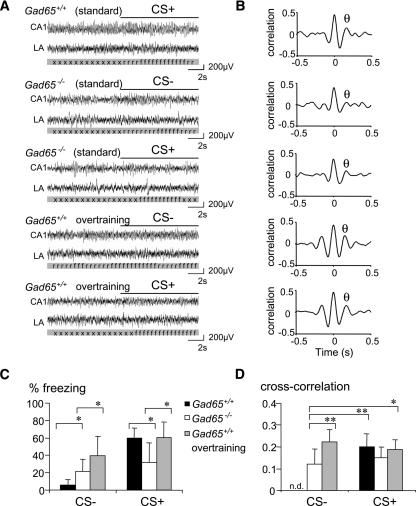
To begin to investigate network activity related to fear memory generalization, we next studied neural activities in the lateral amygdala and CA1 region of the hippocampus of fear-conditioned Gad65 mutants (representative original recordings shown in Fig. 4A). By calculating the cross-correlation of activities between these structures, amygdalo-hippocampal theta synchronization was determined before and during exposure to CS− and CS+ and during defined freezing episodes (Seidenbecher et al. 2003). We also included a group of overtrained Gad65+/+ mice in this analysis in order to compare the network activity patterns between genotypes during fear memory generalization. All groups displayed theta synchronization with a peak between 5.9 and 6.5 Hz (i.e., type-2 theta) during freezing, as described previously (Fig. 4B; Seidenbecher et al. 2003; Narayanan et al. 2007).
Figure 4.
Network activity during fear memory retrieval and generalization. (A) Representative original traces from the hippocampus and amygdala of a standard conditioned Gad65+/+, a standard conditioned Gad65−/−, and an overtrained Gad65+/+ animal during pre-stimulus and (CS+, CS−) stimulus phases with freezing behavior; (x) exploration; (r) risk-assessment; (f) freezing. (B) Averaged cross-correlograms demonstrate that theta frequency synchronization (θ, peak theta synchronization) occurs in all groups during freezing behavior, albeit at different levels. Note, in particular, the low theta peak in a Gad65−/− mice. (C) Behavioral analysis of the recorded animals confirmed the increased freezing response of Gad65−/− mice to the CS− and their reduced freezing to the CS+. Overtraining of Gad65+/+ mice also produced a strongly generalized fear response, whereas standard training failed to induce generalized freezing in the Gad65+/+ mice. (D) Theta correlation during episodes of freezing was significantly lower in generalizing Gad65−/− mice than in overtrained Gad65+/+ mice (CS−). Levels were also lower than those in standard-conditioned Gad65+/+ mice during memory retrieval (CS+). Due to the low level of freezing behavior, theta synchronization could not be determined in standard-conditioned Gad65+/+ mice during exposure to the CS−. **P < 0.01, *P < 0.05, in the indicated comparisons. Values are mean + SEM.
Behavioral analysis of the recorded animals confirmed our findings of reduced freezing to the CS+ tone presentation (31.9% ± 27.5% in Gad65−/− vs. 60.0% ± 13.5% in Gad65+/+; F(1,15) = 5.259, P < 0.038) and increased generalization to the CS− tone presentation (21.5 ± 16.8% Gad65−/− vs. 3.3 ± 4.8% Gad65+/+; F(1,15) = 5.419, P < 0.037) in Gad65−/− mice when compared to their Gad65+/+ littermates (Fig. 4C). Overtrained Gad65+/+ mice also showed significant generalization to the CS− (39.5% ± 27.6%; F(1,11) = 8.21, P < 0.017 compared to Gad65+/+ with standard training) as well as pronounced freezing to the CS+ tone presentation (60.3% ± 19.2%). When comparing these three groups of mice, we observed significant effects on amygdalo-hippocampal synchronization during both CS− (F(2.21) = 7.699, P < 0.004) and CS+ tone presentations (F(2.21) = 16.396, P < 0.0001). During CS−, overtrained animals showed a significant increase in correlation (0.21 ± 0.07), compared with Gad65+/+ (0.10 ± 0.06, P < 0.019) and Gad65−/− mice (0.06 ± 0.04, P < 0.001). This increased synchronization was comparable to the response of both overtrained (0.24 ± 0.05) and standard protocol-trained Gad65+/+ mice (0.22 ± 0.05) to the CS+. In contrast, Gad65−/− mutant mice failed to increase amygdalo-hippocampal theta correlation during the CS+ tone presentation (0.05 ± 0.05; P < 0.0001 compared to both other groups; Fig. 4D).
We considered that this result may have been confounded by the reduced freezing behavior of Gad65−/− compared to Gad65+/+ and overtrained animals. We therefore focused our attention further on the analysis of network activities during freezing episodes only, which are shown by Gad65+/+ mice during the CS+ tone presentation and by Gad65−/− mice and overtrained Gad65+/+ mice during both CS+ and CS− tone presentations. During these freezing periods, amygdalo-hippocampal theta synchronization was different between groups (F(4,37) = 3.715, P < 0.01), as Gad65−/− animals showed generally lower cross-correlation levels (0.12 ± 0.06 during CS− and 0.15 ± 0.04 during CS+) than the other groups (Fig. 4B). In particular, theta synchronization in Gad65−/− mice during generalized freezing to the CS− tone presentation was significantly lower than that of overtrained Gad65+/+ mice freezing to the CS− (0.22 ± 0.05, P < 0.002) or CS+ (0.18 ± 0.04, P < 0.035). They were also lower than those of standard-conditioned Gad65+/+ mice during CS+ freezing (0.19 ± 0.05, P < 0.009). The reduction of theta synchronization of Gad65−/− mice during the CS+ tone presentation, in contrast, failed to reach significance level.
Discussion
In this study we present evidence for an involvement of the 65-kDa isoform of GABA-synthesizing enzyme glutamic acid decarboxylase, GAD65, in consolidation and generalization of conditioned fear. First, we describe a transient, temporal, and regional-specific regulation of GAD65 mRNA expression in the amygdala and hippocampus following fear-conditioning training. Second, we demonstrate that a deficiency in GAD65 results in a disturbance of stimulus-specificity during long-term fear memory consolidation, and, third, we describe network activity patterns that are associated with fear memory generalization in Gad65 mutant and wild-type mice.
As the key enzyme for GABA synthesis, GAD is expressed in the majority if not all GABAergic neurons in the amygdala and hippocampus, and modulation of GAD65/67 expression may serve as a general regulator of inhibitory control in these brain areas. In fact, modulation of GAD65 and GAD67 expression by acute and chronic stress has been reported previously (Bowers et al. 1998). Differential regulation of the GAD isoforms is of relevance, as GAD65 is considered to be the activity-dependent and synaptically localized isoform providing GABA for phasic inhibition, whereas GAD67, as the cytosolic isoform, is critical for metabolic GABA synthesis and tonic inhibition. Our observation of reduced GAD65 mRNA after fear conditioning (Fig. 1) hence lends itself to two interpretations: On the one hand, reduced GAD65 expression may be taken as an indicator of transiently reduced GABAergic neuronal activity during fear memory consolidation or retention. In fact, different forms of synaptic plasticity have been demonstrated in GABAergic neurons of the BLA and hippocampus using electrophysiological tools (Mahanty and Sah 1998; Bauer and LeDoux 2004; Kullmann and Lamsa 2007). On the other hand, the transient GAD gene regulation itself may result in an altered inhibition within the lateral and basolateral amygdala nuclei as well as the hippocampus (Shimizu et al. 2005; Szinyei et al. 2007). Indeed, transient changes of gephyrin expression and GABAA receptor binding are evident in the amygdala 2–6 h after fear conditioning (Chhatwal et al. 2005), indicating a more general change of the GABAergic system during fear memory consolidation. The time point of GAD65 mRNA regulation in the amygdala at 24 h after fear conditioning strikingly coincides with a LTM phase that is dependent on the functional integrity of the amygdala (Suzuki et al. 2004) and involves the integration of amygdalar neurons into extended network activities (Narayanan et al. 2007). In light of the differential information processing through subsets of GABA neurons, reduced GABAergic function might thus be involved in the fine tuning of long-term information storage and may contribute to pronounced and lasting reduction of extracellular GABA levels that can be observed in the amygdala during fear memory retrieval (Stork et al. 2002).
Therefore, we addressed the question whether ablation of the GAD65 gene would affect the consolidation of stimulus-specific long-term fear memory. Our data confirm previous observations of a reduced freezing response in Gad65−/− mice to an auditory fear cue and extend it by showing a pronounced, context-independent intramodal generalization of their fear memory. Strikingly, this phenotype was only observed in long-term (24 h and 14 d) but not in short-term (30 min) memory retrieval, indicating that it may result from a disturbance in the fear memory consolidation process. In fact, while wild-type littermates increased their stimulus-specific response during consolidation by increasing freezing to the CS+ from STM to LTM, Gad65 null mutants not only failed to increase their response to the CS+ but instead increased freezing to the CS−. Moreover, while Gad65+/+ mice showed a context-specific generalization to the CS−, the response of Gad65−/− mice was indistinguishable in the neutral and shock contexts. In fact, all genotypes showed an ambiguous fear-related behavior with low freezing and high risk-assessment scores in the shock context, indicating a normal behavioral response of Gad65 mutants to situational reminders after cued conditioning. This is in agreement with our previous observations in wild types (Laxmi et al. 2003) and with the normal performance of Gad65−/− mice in two hippocampus-dependent tasks: passive avoidance and water maze navigation (Asada et al. 1996).
The question arises then of how reduced expression of GAD65 may be involved in consolidation of stimulus-specific fear memory in wild types while its absence in null mutants leads to a reduced and generalized long-term fear memory. First, it should be considered that different levels of GAD65 expression during consolidation or reduction in specific cell types may be important for stimulus specificity. A complete loss of GAD65 activity in the mutant mice may thus exaggerate the effects of endogenous regulation in a gene-dosage–dependent manner. The lack of generalization in Gad65+/− mice appears to argue against this, but it should be considered that adult heterozygotes display normal GABA content in the amygdala and hippocampus (Stork et al. 2000). Second, GAD65 regulation and function may differ between subpopulations of GABA interneurons, which are characterized by particular morphology, physiology, and expression of neurochemical factors (Freund and Buzsáki 1996; Frenois et al. 2005; Mascagni and McDonald 2007; Muller et al. 2007) and are thought to mediate specific aspects of fear memory-related information processing within the amygdala (e.g., Quirk et al. 2003; Azad et al. 2004; Marowsky et al. 2005) and hippocampus (Crestani et al. 1999; Gafford et al. 2005). Third, if compensatory mechanisms are active in the Gad65 null mutants, then the inability of these animals to change GABAergic function via regulation of GAD65 expression may be important. Finally, it must be considered that Gad65 mutation may affect network properties in the amygdala and hippocampus even before training, thereby interfering with cellular processes that are triggered during the memory-acquisition phase or nonspecifically increasing the responsiveness to aversive stimuli by enhancing general anxiety level (Stork et al. 2000). However, the latter explanation appears unlikely since immediate post-training freezing, which is a function of training intensity and aversiveness (Laxmi et al. 2003), was not altered in the Gad65 mutant mice.
Recently it has been suggested that two fundamentally different components, a stimulus-specific memory component and a nonassociative sensitization component, exist in fear memory (Siegmund and Wotjak 2007), the interplay of which may determine strength, persistence, and specificity of the memory. Changes in GABAergic inhibition in the amygdala and hippocampus are likely to contribute to this balance. Although GABAergic local circuit neurons comprise only 10%–20% of the neuronal population in the lateral and basolateral nuclei of the amygdala, they tightly control the spontaneous and evoked activity of projection neurons in this region (Rainnie et al. 1991) and govern the pattern of sensory-evoked responses and the activity of the synaptic network (Lang and Pare 1997a, b; Szinyei et al. 2000). On the one hand, reduced inhibitory control in the amygdala is typically expected to enhance long-term potentiation (LTP) and to facilitate Pavlovian fear conditioning, whereas enhanced inhibition has opposite effects (Muller et al. 1997; Wilensky et al. 1999). Recent studies suggest that GABAergic mechanisms in the amygdala are indeed involved in the facilitation of plasticity and enhancement of fear conditioning following, e.g., benzodiazepine withdrawal (Isoardi et al. 2004) and acute stress (Rodriguez-Manzanares et al. 2005). On the other hand, pharmacological and genetic reduction of presynaptic inhibition at glutamatergic terminals unmasks nonassociative, homosynaptic LTP in the basolateral amygdala and evokes generalization of auditory cued fear memory (Shaban et al. 2006). In light of these findings, it is also interesting that spike-timing–dependent LTP in the amygdala is tightly controlled by the level of GABAergic inhibition (Shin et al. 2006). Concerning the hippocampus, it has been shown that deficits in GABAA receptor clustering increase anxiety in mutant mice heterozygous for the γ2 subunit of the GABAA receptor (Crestani et al. 1999) and that post-training injection of the benzodiazepine midazolam interferes with context memory consolidation (Gafford et al. 2005). Gad65−/− mice display deficits in the post-tetanic increase of inhibition following afferent stimulation in slice preparations of the amygdala or hippocampus, with spontaneous activity remaining unaffected (Tian et al. 1999; K. Kaneko and K. Obata, unpubl.). Thus GAD65-mediated GABA synthesis may well be involved in both associative and nonassociative aspects of plasticity in the amygdala and hippocampus during fear memory formation.
We therefore began to address the network activities that may be involved in these functions by analyzing activity patterns in the amygdala of Gad65−/− mice during fear memory retrieval and their incorporation into larger ensembles known to act within the amygdalo-hippocampal system (Seidenbecher et al. 2003). To investigate whether the observed network activities also translate to experience-dependent generalization of fear memory and to be able directly compare network activities of generalized fear memory during the CS− between Gad65+/+ and Gad65−/− mice, we included a group of overtrained wild-type mice in this analysis. In agreement with previous observations (Seidenbecher et al. 2003; Narayanan et al. 2007), we observed a pronounced expression of 4- to 8-Hz type-2 theta activity in the amygdala and hippocampus of standard-conditioned Gad65+/+, standard-conditioned Gad65−/−, and overtrained Gad65+/+ mice. Moreover, we could observe considerable synchronization of theta activities between amygdala and hippocampus during both CS- and CS+-induced freezing episodes (Fig. 4). However, reduced levels of theta synchronization were observed in generalizing Gad65−/− mice compared to overtrained Gad65+/+ mice during the CS−. It is important to point out that, in wild-type mice, increased theta synchronization appears to be specific for a LTM stage around 24 h after training (Narayanan et al. 2007), which is coincident with the reduction of GAD65 gene expression in the amygdala. Thus, theta synchronization clearly is not required for the retrieval or expression of conditioned fear per se but likely represents a correlate of amygdalo-hippocampal information processing during this late stage of fear memory consolidation. Our data now suggest that substantial differences exist in information processing in the amygdalo-hippocampal pathway during the (context-independent) expression of generalized fear memory in Gad65−/− mice and its (context-dependent) expression in overtrained Gad65+/+ mice.
Conclusion
Memory generalization is thought to be a “cognitive act that occurs when the judgment of two different situations are likely to belong to a set of situations having the same consequence” (Shepard 1987). While critically important for categorization of memories, the flip side of this phenomenon is apparent when individuals generalize as a result of traumatic experience, e.g., in anxiety and mood disorders (Charney 2004; Ryngala et al. 2005). Our observations demonstrate that, in mice, the GABA-synthetic enzyme, GAD65, is critically involved in the consolidation and generalization of fear memory and its associated network activities. Recent evidence suggests that GABAergic cell assemblies exist in the amygdala that are capable of producing rhythmic network activities in the theta and gamma frequency ranges (Driesang and Pape 2000; Collins et al. 2001), as has been extensively described in the hippocampus (Freund 2003). Therefore, future studies will have to address whether specific subpopulations of GABA neurons in the amygdalo-hippocampal system are functionally modulated during fear memory consolidation and generalization and how GABAergic transmission may be involved in theta resonance of the amygdalo-hippocampal pathway.
Materials and Methods
Animals
Eight- to 10-wk-old male C57BI/6 (M&B Taconic) and mutant mice lacking the gene coding for the GABA-synthesizing enzyme GAD65 (Asada et al. 1996) were used in these experiments. Homozygous (Gad65−/−) and heterozygous (Gad65+/−) Gad65 mutants and their wild-type littermates (Gad65+/+) were on a C57Bl/6 genetic background (>10 generations of backcross) and obtained from Gad65+/− × Gad65+/− breeding. Animals were kept in groups of 3–6 under a 12:12 light/dark cycle with food and water ad libitum. Genotypes were determined with allele-specific PCR at the time of weaning, and animals selected for the experiments were housed individually for 1 wk before the beginning of experiments. All procedures were performed under strict observance of German regulations for the use of laboratory animals (permission NR 42502/2–441 UNI MD).
Fear conditioning
The training apparatus (TSE Systems) was composed of a 36 cm × 21 cm × 21 cm light-blue acrylic glass arena with a grid floor for delivery of electric foot shocks. It was enclosed in an isolation cubicle containing a speaker, a ventilation fan providing fresh air, and background noise of 70-dB sound-pressure level (SPL). Animals were fear conditioned with auditory-cued conditioning protocols, as described previously (Laxmi et al. 2003). Briefly, on the first day of the experiment, the animals were exposed twice to the training apparatus and a set of six acoustic stimuli (2.5-kHz sinus tone, 85-dB SPL, 10 sec with 20-sec ISIs) serving as the CS−. On the following day, after 2 min of habituation to the apparatus, animals were fear conditioned with three conditional stimuli (CS+; 10-kHz sinus tone, 85-dB SPL, 10 sec with 20-sec ISIs) that each coterminated with an unconditional stimulus (US; 1-sec scrambled foot shock, 0.4 mA). These training conditions have previously been shown to produce a robust and highly specific freezing response in C57Bl/6 mice (Laxmi et al. 2003). Fear memory retrieval was tested either at short-term (30 min post training) or long-term (24 h or 14 d post training) time points in a neutral context, in a white, acrylic 26 cm × 20 cm × 14 cm “standard cage” with sawdust filling, placed in a different room without an isolation cubicle. A fourth group of animals was tested for long-term fear memory (24 h post training) in the conditioning context, to address the potential context dependency of generalization. In all retrieval tests, after 2 min of habituation, animals were confronted with a set of four CS− and subsequently with four CS+ with 20-sec ISIs. As measures of fear memory, freezing (complete immobilization except for respiratory movements) and risk-assessment behavior (overt watching, stretched attending) were evaluated off-line using a time-line version of the public domain program WinTrack generously provided by D. Wolfer (University of Zurich, Switzerland).
Statistical analyses of the behavioral data were done with multivariate ANOVA, comparing the three genotypes (Gad65+/+, Gad65+/−, and Gad65−/−) over 2-min periods of context exposure, all four CS− including ISIs, and all four CS+ including ISIs. Post-hoc comparisons were done between genotypes as well as between tests (STM vs. LTM 24 h; LTM 24 h vs. LTM 14 d; and LTM in the neutral context vs. LTM in the shock context) for each genotype using Fisher’s protected least-significant difference (PLSD) test. To control for general changes in fear- and anxiety-related behaviors, we determined freezing and risk assessment before (last 2 min of the second adaptation period) and 2 min immediately after training. Moreover, in one group of naïve Gad65 mutant mice, foot-shock sensitivity was analyzed in the fear-conditioning apparatus. Electrical shocks of 1-sec duration were applied at increasing intensity from 0.05 to 0.4 mA in incremental steps of 0.05 mA, with 1- to 2-min ISI. Vocalization, immobilization (freezing), or jumping/escape behavior were recorded as measures of pain and compared between genotypes.
Gene expression analysis
Fear-conditioned C57Bl/6 mice receiving three explicit CS+/US pairings, and control mice receiving either three CS+ and three US without temporal coincidence (unpaired control) or three CS+ only (shock-naïve control) were killed by cervical dislocation 6 h, 24 h, or 14 d after the last stimulus. Brains were quickly removed from the skull and frozen with dry ice-cooled isopentane, and gene expression analyses were done as described previously (Stork et al. 2001). Under binocular vision, the basolateral complex of the amygdala was punched bilaterally from 180-μm-thick coronal sections.
cDNA blot analysis
Micropunches for each of four animals per group at each time point were pooled and homogenized, and total RNA was isolated from the homogenate with RNA spin columns according to the manufacturer’s protocol (Qiagen). First-strand synthesis was done with MMLV reverse transcriptase (SuperScript II, Promega) in the presence of modified oligo-dT cDNA synthesis primer and 5′ extension oligonucleotides (SMART II oligonucleotide; Clontech). For virtual Northern blot analysis, first-strand cDNA of fear-conditioned and pseudoconditioned animals was amplified with 19 cycles of long-distance PCR. Each 1 μg of the product was loaded on an agarose gel, separated by gel-electrophoresis, and blotted onto nylon membranes (Hybond N). Digoxigenin-labeled probes were generated from full-length GAD65, GAD67, and GAPDH clones through amplification with standard T3 and T7 primers in the presence of digoxigenin-coupled dUTP. Hybridization, stringency washing, and signal detection with CDPStar chemiluminescence substrate were done as described elsewhere, followed by a quantitative analysis of signal intensity with a chemiluminescence detection system (ChemiDoc, Bio-Rad) using GAPDH as a standard. Based on three independent behavioral experiments (with N = 4 for each of the behavioral groups), three virtual Northern blot analyses were performed. The results of these three experiments were evaluated statistically with ANOVA and compared post-hoc with Newman–Keul’s test.
qPCR analysis
Micropunches of individual animals (N = 6 per training group and time point) were collected from the basolateral complex of the amygdala and the dorsal hippocampus (centered to include major parts of CA1, CA3, and dentate gyrus) and were immediately homogenized with cells-to-cDNA lysis buffer (Ambion) for 10 min at 75°C. Homogenates were treated with DNase I for 30 min at 37°C, followed by a DNase inactivation for 5 min at 75°C. First-strand synthesis was done with M-MLV reverse transcriptase in the presence of 2.5 mM dNTPs, 50 μM random decamer oligonucleotides, and RNase inhibitor for 60 min at 42°C. First-strand cDNA was used for detection of GAD65, GAD67, GAPDH, and phosphoglycerate-kinase (PGK) with commercial FAM and VIC-labeled RNA detection assays (TaqMan, Applied Biosystems) in triplicate on a StepOnePlus thermocycler (Applied Biosystems). All runs, consisting of 50 cycles of 15 sec at 95°C and 1 min at 60°C, were preceded by a 2-min decontamination step at 50°C with uracil-N-glycosidase. Mean cycle threshold (CT) values were determined for each triplicate assay and used for sample comparison according to the ΔΔCT method (Livak and Schmittgen 2001). For each time point, samples from fear-conditioned animals (N = 6) and matched unpaired controls (N = 6) were processed and analyzed entirely in parallel. Differences in ΔCT values (relative to PGK) were statistically analyzed with Student’s paired t-test. For illustration, transformation to relative expression value was done according to RQ = 2−ΔΔCT with RQ(unpaired) = 1.
Electrophysiology
Gad65+/+ mice and their Gad65−/− littermates were implanted with electrodes under phenobarbital anesthesia (50 mg/kg i.p.) using a small animal stereotactic device (World Precision Instruments). Reference and ground silver electrodes were implanted close to the midline over the nasal and cerebellar regions, respectively. Stainless steel electrodes were positioned unilaterally into the left hemisphere at the coordinates AP −1.94 mm, ML 1 mm, DV 1.25 mm from bregma and AP −2.06 mm, ML 3.25 mm, DV 4.2 mm from bregma, aiming at the CA1 hippocampal region and the lateral amygdala, respectively (Seidenbecher et al. 2003; Narayanan et al. 2007). The electrode ensemble was fed through a rubber socket and fixed on the skull with dental cement. The prepared animals were allowed to recover for 3–4 d before behavioral training commenced.
Fear conditioning of implanted animals was conducted as described above, except that training was repeated on the following day for more stable results (i.e., there were two training sessions separated by 1 d). An additional group of Gad65+/+ mice was overtrained with two training sessions each containing 10 CS+/US pairings at an increased US intensity (0.6 mA), a protocol known to induce significant generalization to the CS− (Laxmi et al. 2003). One day after training, retrieval of conditioned fear and associated network activities were assessed in a neutral context. Before testing, a plug was connected to the implanted socket under a low and brief dose of an inhalable anesthetic (isoflurane) to allow simultaneous recording of the amygdalar and hippocampal field potentials. Animals were tested with four CS− and four CS+, as described above. The amplified signals were band-pass–filtered at 0.3 and 30 Hz with a 1-kHz sampling rate, fed to an A/D converter (Cambridge Electronic Devices Ltd.), and digitally stored together with markers of freezing and risk-assessment behavior on a PC for off-line analysis using Spike 2 software. After completion of the experiments, animals were sacrificed with an overdose of pentobarbital (200 mg/kg i.p.), and the locations of the electrode tips were verified histologically in accordance with a mouse brain atlas (Franklin and Paxinos 1997).
Data analysis was done for periods of 10 sec before commencement of acoustic stimuli (pre-stimulus), during CS− and CS+ presentation, as well as for defined freezing episodes of 5- to 8-sec duration during CS− and CS+. To determine amygdalo-hippocampal theta synchronization, cross-correlograms between the amygdala and hippocampus were calculated using low-pass (17.5 Hz; transition gap, 11.0) filtered waveforms with (2000 bit; 1-sec offset) phase shifts aligned (x = 0) to the maximal positive peak. For statistical comparison with ANOVA and post-hoc PLSD, the cross-correlation maxima in the theta frequency range were determined for each recorded episode and compared between stimuli and animal groups.
Acknowledgments
We thank Dr. T. Seidenbecher for helpful discussions and support in electrophysiological experiments, M. Schmidt for expert technical assistance, and E. Friedel for excellent animal care. This work was supported by a grant from the German Research Foundation (SFB 426, TP7 to H.C.P. and O.S.; Pa 337/15-1 to H.C.P.). S.S. is supported by the International Human Frontier Science Program Organization.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.705408.
References
- Adamec R.E. Evidence that long-lasting potentiation of amygdala efferents in the right hemisphere underlies pharmacological stressor (FG-7142) induced lasting increases in anxiety-like behavioural changes. J. Psychopharmacol. 2000;14:323–339. doi: 10.1177/026988110001400418. [DOI] [PubMed] [Google Scholar]
- Anagnostaras S.G., Craske M.G., Fanselow M.S. Anxiety: At the intersection of genes and experience. Nat. Neurosci. 1999;2:780–782. doi: 10.1038/12146. [DOI] [PubMed] [Google Scholar]
- Asada H., Kawamura Y., Maruyama K., Kume H., Ding R., Ji F.Y., Kanbara N., Kuzume H., Sanbo M., Yagi T., et al. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem. Biophys. Res. Commun. 1996;229:891–895. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- Asada H., Kawamura Y., Maruyama K., Kume H., Ding R.G., Kanbara N., Kuzume H., Sanbo M., Yagi T., Obata K. Cleft palate and decreased brain γ-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc. Natl. Acad. Sci. 1999;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad S.C., Eder M., Marsicano G., Lutz B., Zieglgansberger W., Rammes G. Circuitry for associative plasticity in the amygdala involves endocannabinoid signalling. J. Neurosci. 2004;24:9953–9961. doi: 10.1523/JNEUROSCI.2134-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer E.P., LeDoux J.E. Heterosynaptic long-term potentiation of inhibitory interneurons in the lateral amygdala. J. Neurosci. 2004;24:9507–9512. doi: 10.1523/JNEUROSCI.3567-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers G., Cullinan W.E., Herman J.P. Region-specific regulation of glutamic acid decarboxylase (GAD) mRNA expression in central stress circuits. J. Neurosci. 1998;18:5938–5947. doi: 10.1523/JNEUROSCI.18-15-05938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Charney D.S. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am. J. Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Chhatwal J.P., Myers K.M., Ressler K.J., Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J. Neurosci. 2005;25:502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D.R., Pelletier J.G., Pare D. Slow and fast (gamma) neuronal oscillations in the perirhinal cortex and lateral amygdala. J. Neurophysiol. 2001;85:1661–1672. doi: 10.1152/jn.2001.85.4.1661. [DOI] [PubMed] [Google Scholar]
- Crestani F., Lorez M., Baer K., Essrich C., Benke D., Laurent J.P., Belzung C., Fritschy J.M., Lüscher B., Mohler H. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat. Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- Driesang R.B., Pape H.-C. Spike doublets in neurons of the lateral amygdala: Mechanisms and contribution to rhythmic activity. NeuroReport. 2000;11:1703–1708. doi: 10.1097/00001756-200006050-00022. [DOI] [PubMed] [Google Scholar]
- Fendt M., Fanselow M.S. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci. Biobehav. Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Franklin K.B.J., Paxinos G. The mouse in stereotaxic coordinates. Academic Press; Orlando, FL: 1997. [Google Scholar]
- Frenois F., Stinus L., Di Blasi F., Cador M., Le Moine C. A specific limbic circuit underlies opiate withdrawal memories. J. Neurosci. 2005;25:1366–1374. doi: 10.1523/JNEUROSCI.3090-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund T.F. Interneuron diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Freund T.F., Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gafford G.M., Parsons R.G., Helmstetter F.J. Effects of post-training hippocampal injections of midazolam on fear conditioning. Learn. Mem. 2005;12:573–578. doi: 10.1101/lm.51305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoardi N.A., Martijena I.D., Carrer H.F., Molina V.A. Increased fear learning coincides with neuronal dysinhibition and facilitated LTP in the basolateral amygdala following benzodiazepine withdrawal in rats. Neuropsychopharmacology. 2004;29:1852–1864. doi: 10.1038/sj.npp.1300478. [DOI] [PubMed] [Google Scholar]
- Kanaani J., Lissin D., Kash S.F., Baekkeskov S. The hydrophilic isoform of glutamate decarboxylase, GAD67, is targeted to membranes and nerve terminals independent of dimerization with the hydrophobic membrane-anchored isoform, GAD65. J. Biol. Chem. 1999;274:37200–37209. doi: 10.1074/jbc.274.52.37200. [DOI] [PubMed] [Google Scholar]
- Kash S.F., Tecott L.H., Hodge C., Baekkeskov S. Increased anxiety and altered responses to anxiolytics in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc. Natl. Acad. Sci. 1999;96:1698–1703. doi: 10.1073/pnas.96.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann D.M., Lamsa K.P. Long-term synaptic plasticity in hippocampal interneurons. Nat. Rev. Neurosci. 2007;8:687–699. doi: 10.1038/nrn2207. [DOI] [PubMed] [Google Scholar]
- Lang E.J., Pare D. Similar inhibitory processes dominate the responses of cat lateral amygdaloid projection neurons to their various afferents. J. Neurophysiol. 1997a;77:341–352. doi: 10.1152/jn.1997.77.1.341. [DOI] [PubMed] [Google Scholar]
- Lang E.J., Pare D. Synaptic and synaptically activated intrinsic conductances underlie inhibitory potentials in cat lateral amygdaloid projection neurons in vivo. J. Neurophysiol. 1997b;77:353–363. doi: 10.1152/jn.1997.77.1.353. [DOI] [PubMed] [Google Scholar]
- Laxmi T.R., Stork O., Pape H.-C. Generalisation of conditioned fear and its behavioural expression in mice. Behav. Brain Res. 2003;145:89–98. doi: 10.1016/s0166-4328(03)00101-3. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mahanty N.K., Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- Makinae K., Kobayashi T., Kobayashi T., Shinkawa H., Sakagami H., Kondo H., Tashiro F., Miyazaki J., Obata K., Tamura S., et al. Structure of the mouse glutamate decarboxylase 65 gene and its promoter: Preferential expression of its promoter in the GABAergic neurons of transgenic mice. J. Neurochem. 2000;75:1429–1437. doi: 10.1046/j.1471-4159.2000.0751429.x. [DOI] [PubMed] [Google Scholar]
- Maren S. Building and burying fear memories in the brain. Neuroscientist. 2005;11:89–99. doi: 10.1177/1073858404269232. [DOI] [PubMed] [Google Scholar]
- Marowsky A., Yanagawa Y., Obata K., Vogt K.E. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Mascagni F., McDonald A.J. A novel subpopulation of 5-HT type 3A receptor subunit immunoreactive interneurons in the rat basolateral amygdala. Neuroscience. 2007;144:1015–1024. doi: 10.1016/j.neuroscience.2006.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J., Corodimas K.P., Fridel Z., LeDoux J.E. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav. Neurosci. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- Muller J.F., Mascagni F., McDonald A.J. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J. Comp. Neurol. 2007;500:513–529. doi: 10.1002/cne.21185. [DOI] [PubMed] [Google Scholar]
- Narayanan R.T., Seidenbecher T., Kluge C., Bergado J., Stork O., Pape H.C. Dissociated theta phase synchronization in amygdalo-hippocampal circuits during various stages of fear memory. Eur. J. Neurosci. 2007;25:1823–1831. doi: 10.1111/j.1460-9568.2007.05437.x. [DOI] [PubMed] [Google Scholar]
- Newton H., Lu B. Regulation of cortical interneurons by neurotrophins: From development to cognitive disorders. Neuroscientist. 2006;12:43–56. doi: 10.1177/1073858405284360. [DOI] [PubMed] [Google Scholar]
- Pape H.-C., Stork O. Genes and mechanisms in the amygdala involved in the formation of fear memory. Ann. N.Y. Acad. Sci. 2003;985:92–105. doi: 10.1111/j.1749-6632.2003.tb07074.x. [DOI] [PubMed] [Google Scholar]
- Paré D., Collins D.R., Pelletier J.G. Amygdala oscillations and the consolidation of emotional memories. Trends Cogn. Sci. 2002;6:306–314. doi: 10.1016/s1364-6613(02)01924-1. [DOI] [PubMed] [Google Scholar]
- Pelletier J.G., Paré D. Role of amygdala oscillations in the consolidation of emotional memories. Biol. Psychiatry. 2004;55:559–562. doi: 10.1016/j.biopsych.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Quirk G.J., Likhtik E., Pelletier J.G., Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J. Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie D.G., Asprodini E.K., Shinnick-Gallagher P. Inhibitory transmission in the basolateral amygdala. J. Neurophysiol. 1991;66:999–1009. doi: 10.1152/jn.1991.66.3.999. [DOI] [PubMed] [Google Scholar]
- Raua V., DeColab J.P., Fanselow M.S. Stress-induced enhancement of fear learning: An animal model of posttraumatic stress disorder. Neurosci. Biobehav. Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzanares P.A., Isoardi N.A., Carter H.F., Molina V.A. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J. Neurosci. 2005;25:8725–8734. doi: 10.1523/JNEUROSCI.2260-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryngala D.J., Shields A.L., Caruso J.C. Reliability generalization of the Revised Children’s Manifest Anxiety Scale. Educ. Psychol. Meas. 2005;65:259–271. [Google Scholar]
- Santini E., Ge H., Ren K., de Peña Ortiz S., Quirk G.J. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J. Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe G.E., LeDoux J.E. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J. Neurosci. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe G.E., Atkins C.M., Swank M.W., Bauer E.P., Sweatt J.D., LeDoux J.E. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J. Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenbecher T., Laxmi T.R., Stork O., Pape H.C. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;30:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- Shaban H., Humeau Y., Herry C., Cassasus G., Bettler B., Luthi A. Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nat. Neurosci. 2006;9:1028–1035. doi: 10.1038/nn1732. [DOI] [PubMed] [Google Scholar]
- Shepard R.N. Toward a universal law of generalization for psychological science. Science. 1987;237:1317–1323. doi: 10.1126/science.3629243. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Hayashi Y., Yamasaki R., Yamada J., Zhang J., Ukai K., Koike M., Mine K., von Figura K., Peters C., et al. Proteolytic degradation of glutamate decarboxylase mediates disinhibition of hippocampal CA3 pyramidal cells in cathepsin D-deficient mice. J. Neurochem. 2005;94:680–690. doi: 10.1111/j.1471-4159.2005.03250.x. [DOI] [PubMed] [Google Scholar]
- Shin R.M., Tsvetkov E., Bolshakov V.Y. Spatiotemporal asymmetry of associative synaptic plasticity in fear conditioning pathways. Neuron. 2006;52:883–896. doi: 10.1016/j.neuron.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund A., Wotjak C.T. A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J. Psychiatr. Res. 2007;41:848–860. doi: 10.1016/j.jpsychires.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Soghomonian J.-J., Martin D.L. Two isoforms of glutamate decarboxylase: Why? Trends Pharmacol. Sci. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- Stork O., Ji F.Y., Kaneko K., Stork S., Yoshinobu Y., Moriya T., Shibata S., Obata K. Postnatal development of a GABA deficit and disturbance of neural functions in mice GAD65. Brain Res. 2000;865:45–58. doi: 10.1016/s0006-8993(00)02206-x. [DOI] [PubMed] [Google Scholar]
- Stork O., Stork S., Pape H.-C., Obata K. Identification of genes expressed in the amygdala during the formation of fear memory. Learn. Mem. 2001;8:209–219. doi: 10.1101/lm.39401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork O., Ji F.Y., Obata K. Reduction of extracellular GABA in the mouse amygdala during and following confrontation with a conditioned fear stimulus. Neurosci. Lett. 2002;327:138–142. doi: 10.1016/s0304-3940(02)00387-7. [DOI] [PubMed] [Google Scholar]
- Stork O., Yamanaka H., Stork S., Kume N., Obata K. Altered conditioned fear behavior in glutamate decarboxylase 65 null mutant mice. Genes Brain Behav. 2003;2:65–67. doi: 10.1034/j.1601-183x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- Stork O., Zhdanov A., Kudersky A., Yoshikawa T., Obata K., Pape H.-C. Neuronal functions of the novel serine/threonine kinase Ndr2. J. Biol. Chem. 2004;279:45773–45781. doi: 10.1074/jbc.M403552200. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Josselyn S.A., Frankland P.W., Masushige S., Silva A.J., Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szinyei C., Heinbockel T., Montagne J., Pape H.-C. Putative cortical and thalamic inputs elicit convergent excitation in a population of GABAergic interneurons of the lateral amygdala. J. Neurosci. 2000;20:8909–8915. doi: 10.1523/JNEUROSCI.20-23-08909.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szinyei C., Narayanan R.T., Pape H.-C. Plasticity of inhibitory synaptic network interactions in the lateral amygdala upon fear conditioning in mice. Eur. J. Neurosci. 2007;25:1205–1211. doi: 10.1111/j.1460-9568.2007.05349.x. [DOI] [PubMed] [Google Scholar]
- Tian N., Petersen C., Kash S., Baekkeskov S., Copenhagen D., Nicoll R. The role of the synthetic enzyme GAD65 in the control of neuronal γ-aminobutyric acid release. Proc. Natl. Acad. Sci. 1999;96:12911–12916. doi: 10.1073/pnas.96.22.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky A.E., Schafe G.E., LeDoux J.E. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J. Neurosci. 1999;19:RC48. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwanzger P., Rupprecht R. Selective GABAergic treatment for panic? Investigations in experimental panic induction and panic disorder. J. Psychiatry Neurosci. 2005;30:167–175. [PMC free article] [PubMed] [Google Scholar]