Abstract
Planning and directing thought and behavior require the working memory (WM) functions of prefrontal cortex. WM is compromised by stress, which activates phosphatidylinositol (PI)-mediated IP3-PKC intracellular signaling. PKC overactivation impairs WM operations and in vitro studies indicate that IP3 receptor (IP3R)-evoked calcium release results in SK channel-dependent hyperpolarization of prefrontal neurons. However, the effects of IP3R signaling on prefrontal function have not been investigated. The present findings demonstrate that blockade of IP3R or SK channels in the prefrontal cortex enhances WM performance in rats, suggesting that both arms of the PI cascade influence prefrontal cognitive function.
The prefrontal cortex coordinates and controls cognitive and emotional processes utilizing appropriate judgment, flexibility, and attention (Goldman-Rakic 1995a, b; Fuster 2000). A hallmark operation of the prefrontal cortex is working memory (WM)—the ability to internally maintain information in the absence of external representation. Debilitating deficits in WM and prefrontal function are associated with numerous conditions including schizophrenia, attention deficit hyperactivity disorder, aging, and stress (Goldman-Rakic 1992; Arnsten 1993; Goldman-Rakic and Selemon 1997; Ramos et al. 2003; Birnbaum et al. 2004). Elucidating mechanisms of cognitive impairment will contribute to identification and development of novel therapeutic targets for treatment of prefrontal decline.
Local excitatory circuits are believed to underlie WM operations (Goldman-Rakic 1995a, b) and disruption in prefrontal neuronal activity is associated with WM impairment (Goldman-Rakic 1995a, b). Disruptions can arise from altered catecholamine release within the prefrontal cortex. High levels of norepinephrine release, which typically follow a stressor (Rossetti et al. 1990; Finlay et al. 1995), impair WM performance by binding Gq-coupled α1-adrenoceptors, leading to activation of the phosphatidylinositol (PI) cascade (Garcia-Sainz 1993; Birnbaum et al. 1999, 2004). In the PI pathway, phospholipase C cleaves the phospholipid phosphatidylinositol bisphosphate (PIP2) to generate membrane-bound diacylglycerol (DAG) and diffusible inositol 1, 4, 5-trisphosphate (IP3). IP3 interacts with receptors (IP3R) on the endoplasmic reticulum to mediate the release of calcium (Ca2+) from intracellular stores. Rises in intracellular Ca2+ serve a variety of regulatory and signaling functions and may activate kinases and open ion channels to influence processes relevant to prefrontal network integrity, including synaptic plasticity and neuronal excitability (for review, see Berridge 1993).
Recent studies have established that Ca2+-dependent kinases negatively influence cognitive operations of the prefrontal cortex. Activation of Ca2+-calmodulin-dependent kinase II (CaMKII) and inhibition of the Ca2+-activated phosphatase, calcineurin, impair WM performance in the rat (Runyan et al. 2005; Dash et al. 2007). Protein kinase C (PKC), which is cooperatively activated by Ca2+ and DAG following PI signaling, suppresses delay-related firing of prefrontal neurons in monkeys performing a WM task (Birnbaum et al. 2004) and impairs spatial WM in rats (Birnbaum et al. 2004; Runyan et al. 2005). Another candidate target for the impairing effects of intracellular Ca2+ is the Ca2+-activated small conductance potassium (SK) channel (Kohler et al. 1996). SK channels influence firing frequency by modulating after-hyperpolarization (Pedarzani et al. 2005; Sah 1996) and are therefore positioned to impact the network functions of the prefrontal cortex, which rely on highly synchronized sequences of firing. A recent study by Hagenston et al. (2007) indicates that IP3R-SK channel signaling regulates prefrontal neuron firing. IP3-mediated release of Ca2+ resulted in hyperpolarization of layer V pyramidal neurons in rat medial prefrontal slices (Hagenston et al. 2007). This hyperpolarization was reversed by application of the SK channel blocker, apamin (Hagenston et al. 2007), suggesting a mechanistic role of SK channels in prefrontal dysfunction.
This study sought to clarify whether IP3R signaling influences prefrontal cognitive function, and whether SK channel activation may underlie these effects. We identified doses of IP3R and SK channel pharmacological blockers capable of enhancing basal WM performance in rats when delivered directly to the medial prefrontal cortex. Our results indicate that both arms of the PI pathway (IP3, PKC) regulate prefrontal cognitive function and IP3R-mediated signaling is likely central to previous reports of Ca2+-activated enzymes impairing WM (Birnbaum et al. 2004; Runyan et al. 2005). These findings also suggest that suppression of IP3R signaling may be useful in treating prefrontal cognitive impairment.
The present study used adult (4–8 mo), male Sprague-Dawley rats (Taconic). All procedures were approved by the Yale Animal Care and Use Committee and were in accordance with the National Institutes of Health’s Guide for Care and Use of Laboratory Animals. Rats were single-housed in filter frame cages, maintained on a 12 h light/dark cycle, and fed a restricted diet of Purina rat chow (∼12 g/d per rat) following behavioral testing. Food rewards during cognitive testing were miniature chocolate chips. Water was available ad libitum.
WM was evaluated using the medial prefrontal cortex-dependent spatial delayed alternation task (Wortwein et al. 1994). Training, testing, surgery, and drug administration have been described previously (Birnbaum et al. 1999). Briefly, testing was conducted within the same time period (e.g., 9:00 a.m.–11:00 a.m) 5 d/week by a single experimenter who remained blind to treatment conditions throughout the study. Rats were habituated to a T maze (dimensions, 90 × 65 cm) until taking rewards from the hand of an experimenter and then trained to alternate. On the first trial, animals were rewarded for entering either arm. Thereafter, for a total of 10 trials per session, rats were rewarded only if they entered the maze arm that was not previously chosen. Between trials, the choice point was wiped with alcohol to remove any olfactory clues.
Upon achievement of a 2-d average of at least 70% correct at a 0-sec delay, animals underwent surgery to implant indwelling, guide cannulae (9.0 mm, 23 gauge, Plastics One). Cannulae were directed to deliver infusions into the prelimbic and infralimbic cortices (stereotaxic coordinates: anterioposterior, +3.2 mm; mediolateral, ±0.75 mm; dorsoventral, −3.0 mm). Surgery was performed under ketamine (80 mg/kg) and xylazine (10 mg/kg) anesthesia using aseptic methods. Sterile stylets were inserted to prevent occlusion. Testing recommenced 10 d after surgery. Inter-trial delays were raised in 5-sec intervals to maintain performance between 65% and 75% correct, allowing for detection of improvement or impairment following drug administration. If inter-trial delays exceeded 20 sec, rats were tested on a 12-trial task with four quasi-randomly distributed delay lengths (e.g., 0, 10, 20, and 30 sec; mean delay = 15 sec/trial). Variable delay testing enabled maintenance of the desired baseline without requiring mean delays in excess of 40 sec/trial. Two animals received treatment while being tested with the variable delay paradigm. As expected, ceiling effects were observed for the two shortest delays. Drug-related improvements were observed in the two longer delays. However, analysis by delay was not performed due to the small subject number.
Animals were adapted to the infusion procedure, and infusions of drug or vehicle were administered only when performance was stable (≥65% correct) following a “mock” infusion. For infusions and mocks, 30-gauge sterile infusion probes extending 4.5 mm ventral to the skull were inserted. Bilateral infusions (0.5 μL/hemisphere) were delivered by a syringe pump (Harvard Apparatus) at a rate of 0.25 μL/min. Testing commenced 15 or 20 min following the infusion, depending on the study. Run times were calculated (total delay time minus the total time required for the animals to complete the task) for test sessions following a drug or vehicle treatment. Infusions that failed to deliver at least 75% of the intended volume for each hemisphere were excluded from the analyses (Birnbaum et al. 1999, 2004; Ramos et al. 2006).
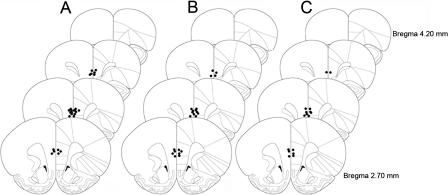
A range for drug dose and latency from infusion to cognitive testing were informed by previous infusion studies of channel blockers and cell permeable agents (Birnbaum et al. 1999, 2004; Ramos et al. 2006; Wang et al. 2007). For the present study, optimal doses and latencies were determined by pilot studies. Rats in Experiment 1 (n = 13) received 0.01 μg/0.5 μL 2-aminoethoxydiphenyl borate (2-APB, Tocris) in sterile PBS or an equal volume of vehicle 15 min prior to cognitive assessment. For Experiment 2 (n = 11), Xestospongin C (XeC, Sigma-Aldrich) was dissolved at a concentration of 1 μg/1 μL in dimethyl sulfoxide (DMSO) and diluted in sterile PBS to a concentration of 0.1 ng/0.5 μL. XeC and vehicle treatments were infused 20 min prior to cognitive assessment. In Experiment 3, rats (n = 8) received 0.1 μg/0.5 μL apamin (Alexis) dissolved in PBS, or vehicle, 15 min prior to cognitive assessment. Doses of apamin exceeding 0.1 μg/0.5 μL resulted in seizures in three animals; thus, these doses were avoided in all subsequent experiments. Doses of apamin lower than 0.1 μg/0.5 μL were found to have no effect on WM performance. At the completion of each experiment, rats were anesthetized with isoflurane and rapidly decapitated. Brains were stored in formalin, sectioned, and analyzed for histological verification of drug delivery. The ventral extent of all infusion probes reached the prelimbic or dorsal infralimbic corticies, indicating that all infusions were delivered to the medial prefrontal cortex (Fig. 1).
Figure 1.
Brains were histologically examined for verification of cannulae placement. All rats had correctly placed cannulae. The ventral-most extent of tracks from probes delivering drug to the prelimbic and infralimbic cortex are illustrated. (A) 2-APB; (B) XeC; (C) apamin. Reprinted with permission from Elsevier © 1998, Paxinos and Watson 1998.
This study used a within-subjects design. All rats received one of the three drug treatments (2-APB, XeC, or apamin) and the corresponding vehicle. Vehicle and drug administration were counterbalanced with at least 1 wk washout between each treatment. Scores following a specific treatment were averaged. Mean scores reflect, on average, two treatments (each, drug and vehicle) per animal per experiment. For each study, animals received an average of four infusions (minimum, two; maximum, nine). Higher numbers of infusions were required when a previous infusion failed to deliver an adequate volume of drug. For each experiment, group comparisons were evaluated using paired two tailed t-tests, and values were represented as the means ± SEM. An α level below 0.05 was considered statistically significant.
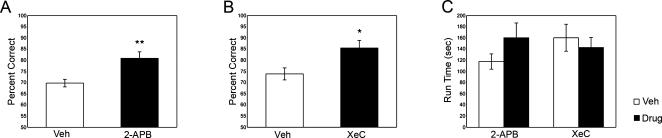
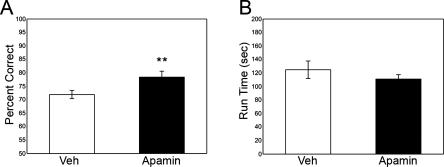
Infusions of IP3R and SK channel blockers directly into the medial prefrontal cortex improved spatial WM performance. IP3R blockade with 2-APB or XeC significantly improved performance relative to vehicle (2-APB = 80.9 ± 2.9% correct; vehicle2-APB = 69.8 ± 1.7% correct; t12 = 3.317, P = 0.006; XeC = 85.5 ± 3.4% correct; vehicleXeC = 73.9 ± 2.7% correct; t10 = 3.082, P = 0.012; Fig. 2A,B). Run-time was determined by subtracting the total delay time from the total time to completion. Run-time was not significantly affected by IP3R blockade (2-APB = 135.6 ± 26.4 sec; vehicle2-APB = 117.8 ± 13.8 sec; t12 = −0.966, P = 0.353; XeC = 142.9 ± 18.0 sec; vehicleXeC = 160.4 ± 24.2 sec; t9 = 0.586, P = 0.572; Fig. 2C), indicating that performance enhancement was unlikely attributed to nonspecific sensorimotor or motivational effects. SK channel blockade also significantly improved performance (apamin = 78.4 ± 2.2% correct; vehicleapamin = 71.9 ± 1.5% correct; t7 = 3.945; P = 0.006; Fig. 3A), and no significant effects of apamin on run-time were observed (apamin = 111.1 ± 6.5 sec; vehicleapamin = 124.9 ± 13.0 sec; t9 = 1.172, P = 0.280; Fig. 3B).
Figure 2.
Experiments 1 and 2 examined WM performance following intra-prefrontal cortical infusions of the IP3R blockers, 2-APB and XeC. (A) A total of 0.01 μg/0.5 μL 2-APB significantly improved performance relative to vehicle; (B) 0.1 ng/0.05 μL XeC also significantly improved performance. (C) Run-time following treatment with 2-APB and XeC did not significantly differ from the respective vehicle treatments, indicating that enhanced WM performance was not due to nonspecific motor or motivational effects. Means ± SEM are shown. (**) P < 0.01; (*) P < 0.05.
Figure 3.
Experiment 3 examined WM performance following intra-prefrontal cortical infusion of the SK2 channel blocker, apamin. (A) Performance following 0.1 μg/0.5 μL apamin was significantly improved relative to vehicle. (B) Run-time was not significantly influenced by drug treatment, indicating that enhanced WM performance was not due to nonspecific motor or motivational effects. Means ± SEM are shown. (**) P < 0.01.
The present study is the first to demonstrate that IP3R activation negatively regulates prefrontal cognitive function. More specifically, blocking IP3-mediated intracellular Ca2+ release within the medial prefrontal cortex enhanced WM performance by ∼10% relative to vehicle. This is consistent with previous reports of Ca2+-dependent enzymes impairing WM performance in rats (Birnbaum et al. 2004; Runyan et al. 2005). We found a similar magnitude of WM enhancement following treatment with apamin, suggesting that IP3R effects may be mediated, in part, by activation of Ca2+-dependent SK channels. Pharmacological blockade was most effective in very low doses. This may be explained by the regulatory functions served by Ca2+ and SK channels. For instance, high doses of blockers may interfere with Ca2+-regulated cellular homeostatic mechanisms (e.g., Berridge 1993) or activation of calcineurin, a phosphatase activated by small rises in intracellular Ca2+ concentration that is required for optimal WM performance (Runyan et al. 2005; Dash et al. 2007).
IP3R signaling research has been greatly limited by the dearth of reliable IP3R agonists and antagonists. Furthermore, the specificity and mechanism of action of available IP3R antagonists have not been fully elucidated, and there are no previous reports of IP3R blockers being used in vivo. In an effort to avoid misinterpretation of our results due to a nonspecific effect of a membrane permeant IP3R blocker, we tested two compounds, 2-APB and XeC, which are known to block IP3R-mediated intracellular Ca2+ release, but have unrelated nonspecific effects (Gafni et al. 1997; Bootman et al. 2002). For example, in addition to blocking IP3Rs, 2-APB has been reported to block store-operated channels in non-neuronal cells, thereby preventing replenishment of the endoplasmic reticulum Ca2+ (Bootman et al. 2002). Little is known, however, about whether store-operated channels play a role, or even exist, in neurons. The other IP3R blocker that we tested, XeC, is a bis-1-oxaquinolizodine isolated from the marine sponge Xestospongia. XeC has been reported to block IP3R more specifically than 2-APB (Gafni et al. 1997; Bootman et al. 2002). The slower action of XeC was not an impediment in the present in vivo experiments, as cognitive function was measured 20 min following drug infusion. Our observation of similar effects of XeC and 2-APB indicate that effects of these drugs are due to their common mechanism of reduced intracellular Ca2+ release.
The beneficial effects of IP3R blockade likely arise, at least in part, from reduced activation of SK channels, which are capable of transducing fluctuations in intracellular Ca2+ concentration into changes in membrane potential (Xia et al. 1998). We observed that SK channels play a similar role to XeC and 2-APB in WM function. Since SK channels require a rise in cytosolic Ca2+ (and are unlikely to be effected by store-operated channels), this is further support that XeC and 2-APB acted by inhibiting IP3R-mediated internal Ca2+ release. Previous research indicates a role for apamin-sensitive SK channels in behaviorally relevant neuronal activity in the hippocampus, amygdala, and sensory neurons (Stackman et al. 2002; Faber et al. 2005; Hammond et al. 2006; Ellis et al. 2007). Cortical pyramidal neurons also express SK channels (Stocker and Pedarzani 2000; Hagenston et al. 2007). Since these channels play a role in various aspects of neuronal dynamics including synaptic excitability (Stackman et al. 2002) and spike frequency adaptation (Pedarzani et al. 2005), it is likely that they influence the reverberating, excitatory prefrontal networks that maintain information over the delay period of a WM task (Goldman-Rakic 1995a, b). Consistent with in vitro studies showing SK channel activation underlies IP3-mediated prefrontal cortical pyramidal cell hyperpolarization (Hagenston et al. 2007), we found that blockade of SK channels within the medial prefrontal cortex improved WM performance. It is unlikely that these enhancements reflect nonspecific motor effects, as run-times were similar following drug and vehicle treatments.
The beneficial effects of IP3R blockade may arise from inhibition of Ca2+-dependent enzymes such as PKC and CamKII, which impair basal WM performance (Birnbaum et al. 2004; Runyan et al. 2005). This is consistent with the Ca2+ phosphatase calcineurin improving WM performance (Runyan et al. 2005; Dash et al. 2007). The mechanism through which Ca2+-activated enzymes effect WM performance may include SK channel activation; however, it is important to note that they can also influence the kinetics of a variety of other channels including Kv 1.4, Kv 2.1, and L-type Ca2+ channels (Roeper et al. 1997; Norris et al. 2002; Misonou et al. 2004).
Increased intracellular Ca2+ may also stimulate adenylyl cyclase (Litvin et al. 2003), resulting in increased cAMP-hyperpolarization-activated cyclic nucleotide-gated cation (HCN) channel signaling (for review, see Dash et al. 2007; Wang et al. 2007). Our lab has recently shown that HCN channels are in close conjunction with both glutamatergic and adrenergic synapses and act to shunt local current. cAMP-HCN channel signaling profoundly impacts prefrontal network operations, and ultimately, impairs WM performance (Wang et al. 2007). Similar studies examining how the spatial and temporal properties of IP3R and SK channels might contribute to prefrontal cortex network function underlying WM have yet to be performed.
The work presented here indicates that WM is impaired by IP3R-mediated signaling within the prefrontal cortex. Inhibiting this arm of the PI cascade may enhance or protect prefrontal cognitive function by (1) blocking Ca2+-activated enzymes that have been previously shown to impair WM, (2) inhibiting the activation of SK channels, and thereby protecting the excitatory networks that underlie WM, or (3) some combination thereof. Research delineating the contributions of specific ion channels and kinases to prefrontal neuronal and cognitive function will provide important information about the therapeutic potential of the agents investigated in this study. Such studies will benefit from in vivo reversal studies requiring IP3R activation. Until a reliable IP3R agonist becomes available, the evidence for a link between IP3R and SK channel activity in prefrontal cognitive function will remain indirect.
Acknowledgments
This research was supported by NIH Conte Center grant P50MH068789 to A.F.T.A and M.Y. and The National Science Foundation Graduate Research Fellowship to A.R.B.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.767408
References
- Arnsten A.F. Catecholamine mechanisms in age-related cognitive decline. Neurobiol. Aging. 1993;14:639–641. doi: 10.1016/0197-4580(93)90054-f. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Birnbaum S., Gobeske K.T., Auerbach J., Taylor J.R., Arnsten A.F. A role for norepinephrine in stress-induced cognitive deficits: α-1-adrenoceptor mediation in the prefrontal cortex. Biol. Psychiatry. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- Birnbaum S.G., Yuan P.X., Wang M., Vijayraghavan S., Bloom A.K., Davis D.J., Gobeske K.T., Sweatt J.D., Manji H.K., Arnsten A.F. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- Bootman M.D., Collins T.J., Mackenzie L., Roderick H.L., Berridge M.J., Peppiatt C.M. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- Dash P.K., Moore A.N., Kobori N., Runyan J.D. Molecular activity underlying working memory. Learn. Mem. 2007;14:554–563. doi: 10.1101/lm.558707. [DOI] [PubMed] [Google Scholar]
- Ellis L.D., Mehaffey W.H., Harvey-Girard E., Turner R.W., Maler L., Dunn R.J. SK channels provide a novel mechanism for the control of frequency tuning in electrosensory neurons. J. Neurosci. 2007;27:9491–9502. doi: 10.1523/JNEUROSCI.1106-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber E.S., Delanet A.J., Sah P. SK channels regulate excitatory synaptic transmission and plasticity on the lateral amygdala. Nat. Neurosci. 2005;24:635–641. doi: 10.1038/nn1450. [DOI] [PubMed] [Google Scholar]
- Finlay J.M., Zigmond M.J., Abercrombie E.D. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: Effects of diazepam. Neuroscience. 1995;64:619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- Fuster J.M. Memory networks in the prefrontal cortex. Prog. Brain Res. 2000;122:309–316. doi: 10.1016/s0079-6123(08)62147-0. [DOI] [PubMed] [Google Scholar]
- Gafni J., Munsch J.A., Lam T.H., Catlin M.C., Costa L.G., Molinski T.F., Pessah I.N. Xestospongins: Potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- Garcia-Sainz J.A. α 1-adrenergic action: Receptor subtypes, signal transduction and regulation. Cell. Signal. 1993;5:539–547. doi: 10.1016/0898-6568(93)90049-r. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P.S. Working memory and the mind. Sci. Am. 1992;267:110–117. doi: 10.1038/scientificamerican0992-110. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P.S. Architecture of the prefrontal cortex and the central executive. Ann. N. Y. Acad. Sci. 1995a;769:71–83. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P.S. Cellular basis of working memory. Neuron. 1995b;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P.S., Selemon L.D. Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophr. Bull. 1997;23:437–458. doi: 10.1093/schbul/23.3.437. [DOI] [PubMed] [Google Scholar]
- Hagenston A.M., Fitzpatrick J.S., Yeckel M.F. MGluR-mediated calcium waves that invade the soma regulate firing in layer V medial prefrontal cortical pyramidal neurons. Cereb. Cortex. 2007 doi: 10.1093/cercor/bhm075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R.S., Bond C.T., Strassmaier T., Ngo-Anh T.J., Adelman J.P., Maylie J., Stackman R.W. Small conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory and synaptic plasticity. J. Neurosci. 2006;26:1844–1853. doi: 10.1523/JNEUROSCI.4106-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M., Hirschberg B., Bond C.T., Kinzie J.M., Marrion N.V., Maylie J., Adelman J.P. Small-conductance, calcium activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- Litvin T.N., Kamenetsky M., Zarifyan A., Buck J., Levin L.R. Kinetic properties of “soluble” adenylyl cyclase. J. Biol. Chem. 2003;278:15922–15926. doi: 10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- Misonou H., Mohapatra D.P., Park E.W., Leung V., Zhen D., Misonou K., Anderson A.E., Trimmer J.S. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat. Neurosci. 2004;7:711–718. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- Norris C.M., Blalock E.M., Chen K.C., Porter N.M., Landfield P.W. Calcineurin enhances L-type Ca2+ channel activity in hippocampal neurons: Increased effect with age in culture. Neuroscience. 2002;110:213–225. doi: 10.1016/s0306-4522(01)00574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Pedarzani P., McCutcheon J.E., Rogge G., Christophersen P., Hougaard C., Strobaek D., Stocker M. Specific enhancement of SK channel activity selectively potentiates the afterhyperpolarizing current IAHP and modulates the firing properties of hippocampal pyramidal neurons. J. Biol. Chem. 2005;50:41404–41411. doi: 10.1074/jbc.M509610200. [DOI] [PubMed] [Google Scholar]
- Ramos B.P., Birnbaum S.G., Lindenmayer I., Newton S.S., Duman R.S., Arnsten A.F. Dysregulation of protein kinase a signaling in the aged prefrontal cortex: New strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- Ramos B.P., Stark D., Verduzco L., Van Dyck C.H., Arnsten A.F. α2A-adrenoceptor stimulation improves prefrontal cortical regulation of behavior through cAMP signaling in aged animals. Learn. Mem. 2006;13:770–776. doi: 10.1101/lm.298006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeper J., Lorra C., Pongs O. Frequency-dependent inactivation of mammalian A-type K+ channel KV1.4 regulated by Ca2+/calmodulin-dependent protein kinase. J. Neurosci. 1997;17:3379–3391. doi: 10.1523/JNEUROSCI.17-10-03379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti Z.L., Portas C., Pani L., Carboni S., Gessa G. Stress increases norepinephrine release in the rat frontal cortex: Prevention by diazepam. Eur. J. Pharmacol. 1990;176:229–231. doi: 10.1016/0014-2999(90)90533-c. [DOI] [PubMed] [Google Scholar]
- Runyan J.D., Moore A.N., Dash P.K. A role for prefrontal calcium-sensitive protein phosphatase and kinase activities in working memory. Learn. Mem. 2005;12:103–110. doi: 10.1101/lm.89405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P. Ca2+-activated K+ currents in neurones: Types, physiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- Stackman R.W., Hammond R.S., Linardatos E., Gerlach A., Maylie J., Adelman J.P., Tzounopoulos T. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J. Neurosci. 2002;22:10163–10171. doi: 10.1523/JNEUROSCI.22-23-10163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M., Pedarzani P. Differential distribution of three Ca2+-activated K+ channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol. Cell. Neurosci. 2000;15:476–493. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- Wang M., Ramos B.P., Paspalas C.D., Shu Y., Simen A., Duque A., Vijayraghavan S., Brennan A., Dudley A., Nou E., et al. α2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Wortwein G., Mogensen J., Divac I. Retention and relearning of spatial delayed alternation in rats after ablation of the prefrontal or total non-prefrontal isocortex. Behav. Brain Res. 1994;63:127–131. doi: 10.1016/0166-4328(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Xia X.M., Fakler B., Rivard A., Wayman G., Johnson-Pais T., Keen J.E., Ishii T., Hirschberg B., Bond C.T., Lutsenko S., et al. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]