Abstract
The ability of secretory phospholipase A2 (sPLA2) to hydrolyze cell membranes is highly dependent on the physical properties of the membrane. The effects of cholesterol on these properties have been characterized in artificial bilayers and found to alter sPLA2 activity significantly. It is hypothesized that the natural difference in cholesterol content between erythrocytes and leukocytes is in part responsible for their differing susceptibility to hydrolysis by sPLA2. To test this hypothesis, defined amounts of cholesterol were removed from erythrocyte membranes using methyl-β-cyclodextrin. Treatment of cells with methyl-β-cyclodextrin increased the hydrolysis rate and total substrate hydrolyzed by sPLA2. In general, this effect of cholesterol removal was more pronounced at higher temperatures. Comparison of the level of membrane order (assessed with the fluorescent probe laurdan) with hydrolysis rate revealed that sPLA2 activity was greatly enhanced upon significant reductions in lipid order. Additional treatment of the cells with calcium ionophore further enhanced the hydrolysis rate and altered the relationship with membrane order. These data demonstrated that interactions with sPLA2 observed in artificial bilayers apply to biological membranes. It is also proposed that the high level of cholesterol in erythrocyte membranes is a protective mechanism to guard against hydrolytic enzymes.
INTRODUCTION
An important objective of membrane biophysics is to apply lessons learned with artificial bilayers to biological membranes. Oftentimes meeting this objective presents a significant challenge due to the complexity of biological membranes compared to the models commonly studied (e.g., Yethiraj and Weisshaar (1)). One possible candidate for making significant connections between artificial and physiological systems is the enzyme secretory phospholipase A2 (sPLA2). The prominent observation associated with this enzyme is its preference for substrate molecules in an aggregated state (as micelles, monolayer, or bilayer (reviewed in Gelb et al. (2) and Winget et al. (3)). Nevertheless, simple formation of a lipid aggregate is not the sole requirement for efficient catalysis, since the enzyme discriminates among potential substrates on the basis of the physical properties of the interface.
Many artificial membranes resist catalysis by sPLA2, especially those composed of saturated zwitterionic phospholipids such as phosphatidylcholine (4–8). However, specific physical perturbations to the membrane such as increased local curvature, incorporation of negative charges, or contamination with certain molecules such as fatty acid or lysophospholipid cause it to become susceptible to hydrolysis (4,8–14). A prominent feature common among many of these perturbations is a reduction in favorable interactions among neighboring phospholipids allowing easier access of substrate into the active site of enzyme adsorbed to the surface of the membrane (8,14,15). A second factor that influences the activity of sPLA2 is the membrane lipid phase expressed at the moment the perturbant and enzyme are added to the bilayer. In general, the enzyme is more active toward lipids in a liquid disordered phase (4,7,8,14,16,17). Several lines of evidence suggest that these two factors, the perturbant and the lipid phase, affect enzyme activity by separate mechanisms (5–8). Notwithstanding, the effectiveness of the perturbant may be influenced by the lipid phase present when the perturbant is introduced (7,8).
Initial attempts at applying these principles from artificial bilayers to biological membranes have been encouraging. In general, the membranes of healthy cells behave like unperturbed phosphatidylcholine bilayers in that they resist catalysis by the enzyme. Those of traumatized, necrotic, or apoptotic cells are more susceptible (reviewed in Brueseke and Bell (18)). Such behavior has been best established in human erythrocytes and various types of leukocytes (19–23). An important advantage of erythrocytes is the relative simplicity of their membrane compared to nucleated cells. Thus, they have been used as a model for investigations into the mechanisms governing the level of membrane hydrolysis by sPLA2. Cellular trauma is simulated in erythrocytes by using an ionophore (ionomycin) to load them with calcium. This treatment produces a significant enhancement in the ability of sPLA2 to hydrolyze membrane phospholipids and appears to mimic the behavior of the “perturbants” in artificial membranes (21–24). Moreover, it appears to do so by a mechanism analogous to that identified in artificial bilayers: facilitation of substrate entry into the enzyme active site (23,24).
Preliminary evidence suggests that the perturbation phenomenon is not the only parallel between biological and artificial membranes. Apparently, the second factor, lipid phase, is also relevant (22). Lipid phases cannot be defined rigorously for biological membranes, but temperature studies with erythrocytes suggest that increased lipid disorder induced by elevated temperature promotes the effectiveness of the perturbant (calcium loading) and the overall hydrolytic activity of sPLA2 (22,23). Nevertheless, these studies were limited by the range of membrane properties that could be explored by varying the experimental temperature.
Numerous studies in artificial bilayers suggest that removal of membrane cholesterol might be an effective means of better assessing the relationship between lipid order and sPLA2 activity in erythrocytes. In model membranes, the general effects of cholesterol on bilayer properties have been well characterized (25–32). Furthermore, the presence of cholesterol in the bilayer alters the activity of sPLA2 substantially in these model systems (33–37). Although the relationships among cholesterol, membrane order, and sPLA2 activity have not been studied in biological membranes, a few observations comparing erythrocytes to lymphocytes suggest that such investigations could be fruitful and physiologically relevant. First, the rate of hydrolysis of susceptible leukocytes is much greater than the rate in susceptible erythrocytes (19,21,23,24). Second, the cell membrane of erythrocytes contain 50–100% more cholesterol (38–40) than does that of other cells including leukocytes (40–42). Given the sensitivity of sPLA2 to membrane structure in general and cholesterol concentration specifically, it is reasonable to hypothesize that membrane cholesterol content might account, at least partially, for the difference in susceptibility of erythrocytes and lymphocytes to hydrolysis by the enzyme. Encouragement for this hypothesis can be found in a few older observations that phospholipase A2-catalyzed hydrolysis of mycoplasma and human platelet membranes is enhanced if these cells are deprived of cholesterol (43,44).
To test that hypothesis and better understand relationships between cell membrane properties and sPLA2 activity, we treated human erythrocytes with methyl-β-cyclodextrin (MBCD) to remove defined amounts of cholesterol from the membrane. Cyclodextrins have been used in pharmacology research for years to aid in the delivery of lipophilic drugs. Recently they have become important in membrane studies mainly because they have been shown to selectively extract membrane cholesterol in multiple cell types, including erythrocytes (45–48). The cell membrane effects mediated by cyclodextrins appear to be limited to their ability to extract lipids since they neither bind to nor incorporate into the membrane (46). MBCD acts at the membrane surface extracting cholesterol into a central, nonpolar cavity of cyclic oligomers of glucopyranoside (49). In this study, the effect of cholesterol removal on membrane hydrolysis by sPLA2 with or without calcium ionophore was assessed at various temperatures. The hydrolysis results were then compared to corresponding alterations in membrane structure identified using the fluorescent probe laurdan.
MATERIALS AND METHODS
Reagents
Secretory PLA2 (monomeric aspartate 49 from the venom of Agkistrodon piscivorus piscivorus) was isolated according to published procedures (50) and dissolved in 50 mM KCl with 3 mM NaN3 as a preservative. Ionomycin was acquired from Calbiochem (La Jolla, CA) and dissolved in dimethylsulfoxide (DMSO). The probes laurdan (6-dodecanoyl-2-dimethylaminonapthalene) and acrylodan-labeled fatty acid-binding protein (ADIFAB) were purchased from Invitrogen (Carlsbad, CA). Laurdan was dissolved in DMSO and ADIFAB in 50 mM KCl with 3 mM NaN3. MBCD was obtained from Sigma-Aldrich (St. Louis, MO) and dissolved in modified basic salt solution (MBSS: NaCl = 134 mM, KCl = 6.2 mM, CaCl2 = 1.6 mM, MgCl2 = 1.2 mM, Hepes = 18.0 mM, and glucose = 13.6 mM, pH 7.4, 37°C). All other reagents were purchased from standard sources.
Preparation of erythrocytes
Blood samples were obtained from donors during physical exams at the Brigham Young University McDonald Health Center. The samples were fresh or stored up to 2 days at 4°C in EDTA vacutainers from which patient identification was removed. Control experiments comparing fresh blood with stored samples demonstrated that the storage conditions did not influence the results (21). Erythrocytes were isolated by centrifugation (plasma and buffy coat removed), washed, and resuspended in MBSS. This stock suspension was then diluted to 3% hematocrit.
To deplete membrane cholesterol, 50 μl samples of washed diluted erythrocytes were suspended in 1 ml MBSS containing 1.0 mM MBCD and incubated at 37°C for 30 min. After washing, cells were resuspended in MBSS and placed in a quartz cuvette. Control cells were treated identically with MBSS devoid of MBCD.
The cholesterol content of MBCD-treated erythrocytes was determined using the Amplex Red Cholesterol Assay Kit from Invitrogen. Cells were treated with MBSS or MBCD as described above, and isolated by centrifugation. Cholesterol was extracted from the supernatant by adding an equal volume of chloroform, vortexing, removing the organic layer, and drying under a nitrogen stream. Cell pellets were lysed by freezing in liquid nitrogen. Samples were quickly thawed, and lipids were extracted with chloroform and methanol as described (51). The organic layer was dried under a nitrogen stream. Samples were assayed for cholesterol according to instructions provided with the kit.
Fluorescence spectroscopy
Steady-state fluorescence (laurdan or ADIFAB) was detected using a photon-counting spectrofluorometer (Fluoromax-3 from Horiba Jobin Yvon, Edison, NJ). Sample homogeneity was assured by continuous stirring (magnetic stir bar), and temperature was maintained using a circulating water bath. Samples were equilibrated for at least 5 min at the experimental temperature before data acquisition. Simultaneous assessment of fluorescence intensity at multiple excitation and emission wavelengths was obtained by rapid sluing of monochromator mirrors using control software provided with the instrument. Monochromator bandpass was set at 4 nm.
For experiments designed to assess membrane physical properties, erythrocyte preparations were suspended in 2 ml of MBSS in a quartz fluorometer sample cell to a final density of ∼3–4 × 106 cells/ml (0.075% hematocrit). Samples were incubated at least 20 min at 40°C to equilibrate laurdan with cell membranes (final concentration = 0.25–2.5 μM, see legends to figures) before measuring the emission intensity at 435 and 500 nm (excitation, 350 nm). Results were quantified by calculating the generalized polarization (GP) from the two emission intensities as described by Parasassi et al. (52).
The fluorescent fatty acid binding protein ADIFAB (final concentration 65 nM) was used to assay the release of free fatty acids from erythrocytes (excitation, 390 nm; emission, 432 nm and 505 nm) (36,53). Erythrocyte preparations were suspended as in the laurdan experiments. After initiating data acquisition, ADIFAB was added at 80 s, followed by ionomycin (300 nM) or equivalent volume of control solvent (DMSO) at 100 s, and sPLA2 (1 μg/ml) at 700 s. In the absence of fatty acid, the acrylodan fluorophore in ADIFAB occupies the fatty acid binding site on the protein where it is mostly isolated from water molecules. Free fatty acids released during hydrolysis bind to ADIFAB displacing the acrylodan group and exposing it to solvent. The sensitivity of acrylodan to the solvent relaxation effect results in a proportional decrease in emission at 432 nm concurrent with an increase at 505 nm (36,54). To quantify the results, the GP was calculated from the intensities at 432 and 505 nm and then fit to a double exponential rate law by nonlinear regression as described previously (53). The initial rate (evaluated at the 20 s time point) and total amount of hydrolysis (estimated from the 500 s time point) were calculated directly using the regression parameter values.
Additional control experiments using MBCD preloaded with cholesterol were included to test the assumption that the effects of MBCD are confined to the removal of cholesterol from the membrane. Preloading was accomplished by incubating MBCD with erythrocytes at 0.75% hematocrit for 30 min at 37°C. Erythrocytes were then removed by centrifugation, and the supernatant (containing MBCD loaded with cholesterol) was added to an erythrocyte sample for extraction of cholesterol as described above.
Two-photon excitation scanning microscopy
Two-photon images were collected with an Axiovert 35 inverted microscope (Zeiss, Thornwood, NY) at the Laboratory for Fluorescence Dynamics (University of California, Irvine) as described previously (55). Instrument optics split the emission signal and directed it through interference filters (Ealing 490 and Ealing 440) to generate the data required for calculation of GP. Laser emission was set at 780 or 790 nm. Samples were prepared for laurdan fluorescence (250 nM laurdan) as described above and incubated in microscopy dishes thermostated at 37°C. Spatial maps of laurdan GP were created and analyzed with software provided by the laboratory. Histograms (number of pixels per GP value) and average GP values were obtained for individual cells and also for the central region (45.9 ± 4.9% of the total cell area) of those same cells. Histograms for the perimeter of each cell were then obtained by calculating the difference between the histograms for the whole cell and central region of the cell. The average GP value for the perimeter of each cell was calculated using the following equation with the following subscript designations: w = whole cell, c = central region, p = perimeter.
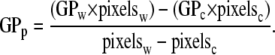 |
(1) |
RESULTS
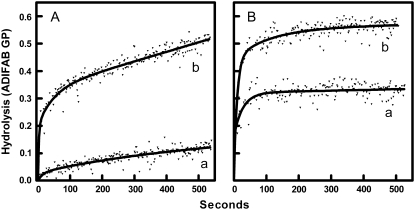
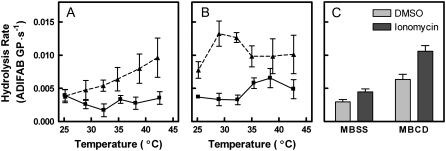
Fig. 1 shows time courses of membrane hydrolysis by sPLA2 at 37°C. In panel A, cells were treated with or without 1 mM MBCD, washed, and then mixed with sPLA2. This treatment procedure removed 73.4% of membrane cholesterol, thus reducing the cholesterol content from ∼47% of membrane lipids to ∼19%. Untreated erythrocytes were relatively resistant to hydrolysis by sPLA2, whereas MBCD-treated erythrocytes were more susceptible to hydrolysis by the enzyme. Panel B contains analogous data for cells also treated for 10 min with ionomycin. Ionomycin increased the initial rate of hydrolysis of both normal and MBCD-treated cells particularly at the shorter time intervals. The same set of experiments was repeated at various temperatures and the initial hydrolysis rate calculated (Fig. 2 A). As reported previously (22), the initial hydrolysis rate did not vary significantly with temperature in control samples (p = 0.54). Extraction of cholesterol by MBCD, however, produced an enhanced hydrolysis rate, especially at the upper end of the temperature range. Consequently, a significant trend with temperature was observed for MBCD-treated samples (p = 0.004 by linear regression). Addition of ionomycin (Fig. 2 B) to untreated cells generated an apparent trend with temperature effect as described previously (22), although regression analysis did not confirm statistical significance at the traditional level (p = 0.08). Cholesterol extraction further increased the hydrolysis rate of the ionomycin-treated cells at all temperatures. The general effect of cholesterol removal on hydrolysis was summarized by pooling the data at all temperatures and comparing the effects of ionomycin and MBCD by two-way analysis of variance (Fig. 2 C). Ten percent of the variation in the data was attributable to ionomycin with very high statistical significance (p < 0.0001). MBCD accounted for 26% of variation in initial rate (p < 0.0001). The interaction between MBCD and ionomycin accounted for 2.2% of variation in initial rate (p = 0.03).
FIGURE 1.
Effects of MBCD and ionomycin treatment on the time course of membrane hydrolysis by sPLA2. (A) Erythrocytes were treated with MBSS (curve a) or MBCD (curve b). Samples were then washed and incubated with ADIFAB and DMSO at 37°C as explained in Materials and Methods. The origin on the graph corresponds to the addition of sPLA2. Fatty acid release is expressed in GP units (i.e., representing the degree of change in ADIFAB fluorescence). Curves represent nonlinear regression fits of the data using an arbitrary function consisting of the sum of two exponential functions. (B) The experiments of panel A were repeated with ionomycin instead of DMSO.
FIGURE 2.
Effects of temperature on rate of hydrolysis by sPLA2. The experiments of Fig. 1 A (DMSO; shown in panel A) and B (ionomycin; shown in panel B) were repeated at the multiple temperatures indicated for control cells (squares, solid lines) and for cells treated with MBCD (triangles, dashed lines). The initial rate of hydrolysis was estimated by nonlinear regression as described in Materials and Methods. (C) Data from all temperatures were pooled and analyzed by two-way analysis of variance to assess the contributions of ionomycin (9.8% of variation, p < 0.0001), MBCD (26% of variation, p < 0.0001), and interaction between the two (2.2% of variation, p = 0.03), n = 5–7 per temperature.
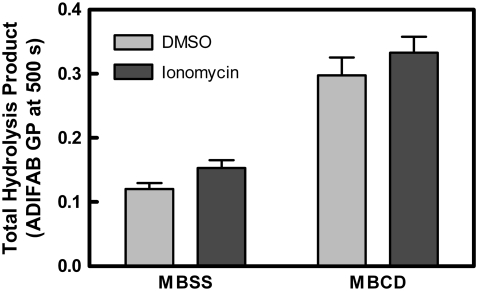
Fig. 3 illustrates the overall effect of cholesterol removal on total product hydrolyzed (analyzed as for Fig. 2 C). Cholesterol removal increased total product at all temperatures regardless of ionomycin. One percent of the variation was attributable to ionomycin (p = 0.09). MBCD accounted for 37% of variation (p < 0.0001). No significant interaction between MBCD and ionomycin was observed (p = 0.95).
FIGURE 3.
Effect of MBCD treatment on total hydrolysis by sPLA2. The total amount of hydrolysis was evaluated for the experiments of Fig. 2 at 500 s (represented by ADIFAB GP) as explained in Materials and Methods. Data were analyzed by two-way analysis of variance to calculate the contribution of ionomycin (1.4% of variation, p = 0.09), MBCD (37% of variation, p < 0.0001), and interaction between the two (0% of variation, p = 0.95), n = 32–35.
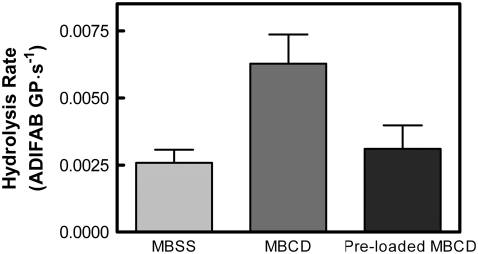
The assumption in all of these experiments is that the effect of MBCD is confined to the removal of cholesterol from the cell membrane. One way to test that assumption is to load MBCD with cholesterol before incubation with the erythrocytes. Presumably, preloading would interfere with the ability of the agent to remove cell cholesterol while retaining other potential artifactual effects. Fig. 4 demonstrates that preloading MBCD with cholesterol attenuated or removed its effect on the initial rate of membrane hydrolysis, suggesting that the results described above represent effects of cholesterol depletion rather than direct artifacts of MBCD.
FIGURE 4.
Preloading MBCD with cholesterol removes its effect on membrane hydrolysis. Erythrocytes were incubated with MBSS (as a control), MBCD, or preloaded MBCD according to Materials and Methods. The initial hydrolysis rate upon addition of sPLA2 was assessed as in Fig. 2. Data were analyzed by one-way analysis of variance (repeated measures) with a posttest (Bonferroni). The MBCD-treated samples were distinguishable from both the control and preloaded MBCD-treated groups (p < 0.01). There was no significant difference between the control and the preloaded MBCD treatment (p > 0.05), n = 7.
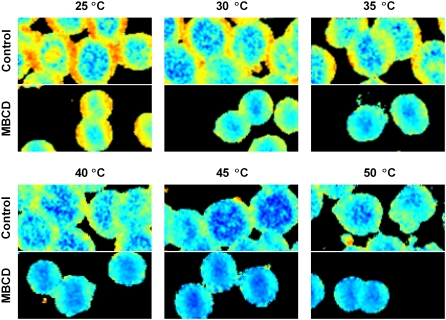
The consequences of cholesterol depletion on membrane structure were assessed by two-photon fluorescence microscopy using laurdan as a probe at multiple temperatures between 25°C and 50°C (Fig. 5). Laurdan is sensitive to the presence and mobility of water molecules trapped within the lipid bilayer (52) and has been shown to detect lipid order and phases in the membrane (52,56). Comparison of the top (control) and bottom (MBCD-treated) images of each panel in Fig. 5 reveals three observations associated with MBCD treatment. First, the average laurdan GP value was reduced after cholesterol depletion. Second, the change in GP was spread across the entire cell, although it appeared visually that the largest changes occurred where the highest GP values were previously observed. Third, the average cell diameter was reduced after MBCD treatment as expected (46). The experiments of Fig. 5 were repeated several times by fluorescence spectroscopy to obtain a more quantitative representation of the effect of MBCD. The influences of both temperature and MBCD were statistically significant based on a two-way analysis of variance. Temperature accounted for 8% of the variation (p < 0.0001) displaying a trend consistent with previous reports (22,29). MBCD treatment lowered the average GP value by 0.14 units. The effect of MBCD accounted for ∼33% of the variation in the data (p = 0.02; n = 5 (control) or 7 (MBCD)).
FIGURE 5.
Effects of MBCD treatment on laurdan GP at multiple temperatures. Two-photon images of erythrocytes were gathered at 25°C, 30°C, 35°C, 40°C, 45°C, and 50°C as explained in Materials and Methods. Control cells are shown on the top half of each panel with MBCD-treated cells below. Laurdan GP values range from −0.50 (blue) to 0.75 (red).
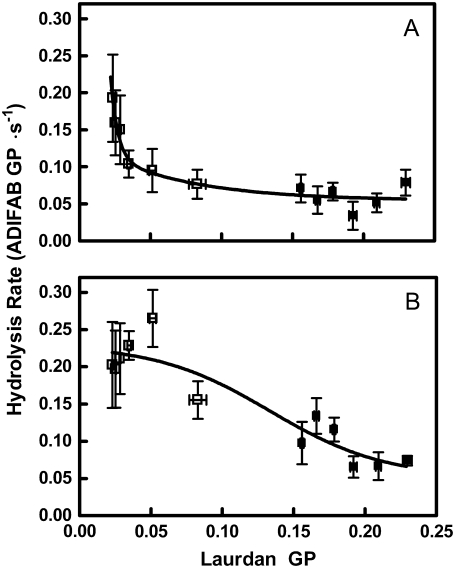
The relationship between laurdan GP and subsequent hydrolysis rates upon addition of sPLA2 are shown in Fig. 6. Panel A shows hydrolysis of cells that have not received ionomycin. The hydrolysis rate was relatively insensitive to lipid order except where the membrane was most disordered (i.e., at laurdan GP < 0.05). Upon ionophore addition, the relationship was altered substantially in that hydrolysis rates were sensitive to membrane order throughout the full range of laurdan GP values (panel B). In both cases, the overall trends were statistically significant (p < 0.0001).
FIGURE 6.
Relationship between laurdan GP and hydrolysis rate. The experiments of Fig. 5 were repeated with bulk samples by fluorescence spectroscopy (see Materials and Methods, 2.5 μM laurdan). The value of GP was calculated from the data and matched by temperature to the hydrolysis data from Fig. 2 for control samples (solid squares) and those treated with MBCD (open squares). (A) Hydrolysis data originated from DMSO samples in Fig. 2 A. (B) Hydrolysis data originated from ionomycin samples in Fig. 2 B.
Previous studies have suggested that the enhanced hydrolysis promoted by ionophore treatment relates to additional changes in membrane order created by the calcium influx (21,22,53,57). These additional changes were identified as local increases in membrane order focused in domains visible mostly in the central region of the erythrocyte disk. The boundaries around these nascent domains were hypothesized to be the regions of the membrane most vulnerable to attack by sPLA2 (22,23). If these changes in membrane order caused by calcium loading are truly the physical event responsible for enhanced susceptibility to hydrolysis, they should be exaggerated by MBCD treatment.
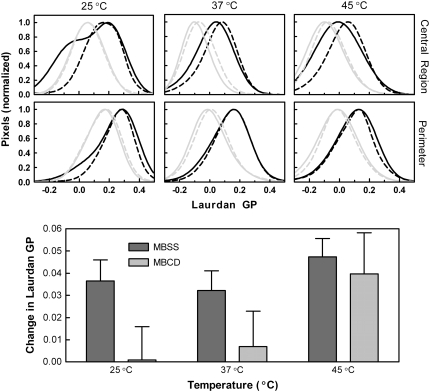
To test this prediction, two-photon images of the effect of ionomycin treatment on laurdan fluorescence were obtained without or with prior treatment with MBCD at 25°C, 37°C, and 45°C. The results were visualized by comparing histograms of GP values obtained by pooling data from several images (Fig. 7). Separate histograms are shown for the central (lower GP) region of the cells and for the perimeter. In control cells, ionomycin treatment caused an elevation of the average GP value and a narrowing of the distribution. The effect was generally more prominent in the central region of the cell; the perimeter was less affected. Cholesterol depletion as reported in Figs. 5 and 6 decreased the average GP value, and similar to ionomycin, narrowed the distribution of GP values. In cholesterol-depleted cells, ionomycin treatment had a minimal effect on laurdan fluorescence at 25°C (control and MBCD curves are essentially superimposable). At 37°C and 45°C, the observations were similar to those in control cells, but the effect of ionomycin was generally weaker. The bottom panel of Fig. 7 summarizes the average change in laurdan GP upon calcium loading observed in multiple samples (combining data from both microscopy and fluorescence spectroscopy). The overall effects observed in the two-photon experiments were reproducible. Two-way analysis of variance indicated that the effect of cholesterol depletion was more significant (p = 0.04) than the effect of temperature on the action of ionomycin (p = 0.11 interaction insignificant: p = 0.57, n = 12 in each group).
FIGURE 7.
Effect on ionomycin treatment on laurdan GP in control and MBCD-treated samples. (Upper two rows of panels) Laurdan GP was assessed from two-photon images as in Fig. 5 for control and MBCD-treated samples before and after addition of ionomycin. Histograms of the central region and perimeter of the cells at 25°C, 37°C, and 45°C were calculated from the images as described in Materials and Methods. These histograms were then fit by nonlinear regression (arbitrary function) and normalized. Control cells (MBSS-treated) are represented in black and MBCD-treated cells are represented in gray with the lighter, dashed lines corresponding to cells after ionomycin treatment. (Lower panel) The average change in laurdan GP after ionomycin treatment at 25°C, 37°C, and 45°C is shown for both control and MBCD-treated cells. Data from images (250 nM laurdan, n = 3 in each group) and bulk samples using fluorescence spectroscopy (250–460 nM laurdan; n = 9) have been pooled. Two-way analysis of variance showed the effects of cholesterol depletion (5.8% of variation, p = 0.04), temperature (5.9% of variation, p = 0.11), and interaction between the two (1.5% of variation, p = 0.57).
DISCUSSION
This study is, to our knowledge, the first attempt to relate sPLA2 activity, membrane cholesterol content, and membrane order in a living cell. Importantly, it has created a new opportunity to apply biophysical principles from artificial bilayers to cell membranes. Specifically, the data provide three insights. First, the summary in Fig. 6 demonstrates a strong relationship between apparent membrane order (laurdan GP) and the activity of sPLA2, especially for cells made susceptible by calcium influx. This relationship was implied previously in temperature studies (22) but is now greatly strengthened by the data from cells treated with MBCD. Second, the data support the hypothesis that cholesterol inhibits hydrolysis of biological membranes as it often does in artificial bilayers. Third, the data of Fig. 7 help resolve questions regarding the role of membrane order in the mechanism by which cells become susceptible to hydrolysis upon perturbation with elevated intracellular calcium.
Membrane order and sPLA2 activity
Several previous studies have addressed relationships between sPLA2 activity and the physical properties of artificial membranes (6–9,12–15,58–62). These relationships can be segregated into two categories. One category relates to membrane phase properties like lipid order and fluidity. In general, increases in membrane fluidity and disorder promote hydrolytic activity (4,5,7,15,63). The other category comprises specific perturbations to membrane structure that improve interactions of sPLA2 with the membrane surface and/or access of bilayer substrate to the enzyme's active site. Examples of these perturbations include increased surface curvature, negative surface charge, and the presence of bilayer contaminants that weaken phospholipid/neighbor interactions in the membrane (6–10,12–14,58–60). Even though these two categories are not entirely independent in terms of their mutual effects on membrane behavior and hydrolytic activity, we have classified them in this way because they appear to modulate the enzyme by separate means (7,8,14,64). For example, regardless of how fluid or disordered the membrane is, hydrolysis can still be greatly accelerated by inclusion of one of these perturbants (5–7). Moreover, the perturbants appear always to affect sPLA2 activity to a larger extent than do membrane order or fluidity (5–7).
The data in this report strongly support the conclusion that the action of sPLA2 on erythrocytes involves the same two categories of membrane behavior. Previous studies have provided ample evidence that calcium loading with ionomycin increases sPLA2 activity in a manner analogous to the perturbants described above for model membranes (21,23,53). An initial study in which temperature was varied suggested that the effects of lipid order and/or fluidity characterized in artificial bilayers also relate to erythrocyte membranes (22). However, that study was restricted by the fact that temperatures above 50°C were not reasonable to use, and membrane order could therefore not be reduced sufficiently to fully explore its impact in the biological membrane. Consequently, it was concluded that membrane order impacts the activity of sPLA2 only when the membrane has been made susceptible by ionophore treatment (22). As shown by the solid symbols in Fig. 6, the same conclusion would have been reached here if the data with MBCD treatment had not been included. In fact, similar to what has been observed in artificial membranes, the enzyme appeared exquisitely sensitive to lipid order when the membrane was highly disordered, and this dependence was different in the presence of an activating perturbation (i.e., ionomycin).
Protective effect of cholesterol
The presence of cholesterol in artificial membranes has been shown previously to modulate the activity of enzymes that act at the lipid-aqueous interface. Examples include sPLA2 and cholesterol oxidase (26,34). Presumably, such modulation reflects changes in membrane physical properties provoked by the sterol. In the case of sPLA2, membrane cholesterol often reduces the activity of the enzyme (33,34,36), although a few examples of stimulatory effects in artificial bilayers under specific experimental conditions have been reported (36,37). In part, the inhibition probably results from the ordering effect of cholesterol on bilayer phospholipids since sPLA2 is so sensitive to lipid dynamics (8,12,59,61–63). An excellent example of this phenomenon is the relationship among cholesterol superlattices, membrane structure, and sPLA2 function. At the critical sterol concentrations producing the superlattices, membrane order reaches a maximum, and the activity of sPLA2 reaches a minimum (34,65). Interestingly, not all interfacial enzymes are affected the same way as revealed by investigations with cholesterol oxidase, which is activated when superlattices are present (26).
The data of Figs. 1 and 2 reveal that cholesterol may also perform an inhibitory role in erythrocyte membranes. Interestingly, both the rate (Fig. 2) and the amount (Fig. 3) of hydrolysis were increased by removal of cholesterol. The impact on hydrolysis rate may well be explained by the membrane ordering effect of cholesterol (Fig. 6). On the other hand, the elevated amount of hydrolysis is not easily rationalized by appeal to studies on artificial bilayers. This unexpected result suggests that the pool of phospholipids accessible to the enzyme is less than the total population present in native membranes. Previously, it was shown that neither alteration of experimental temperature nor treatment with ionomycin altered the size of that pool (23), and our results here are generally consistent with that report. Apparently, cholesterol plays a role in limiting that pool in erythrocytes, perhaps by forming lipid domains resistant to hydrolysis. Regardless of mechanism, this behavior of the sterol would presumably perform a protective function for cells circulating among plasma sPLA2 molecules. This observation may also explain why the extent (in addition to the rate) of phospholipid hydrolysis by sPLA2 is so much greater in lymphocytes than it is in erythrocytes (using the same enzyme preparation (18,19,21)). Indeed, lymphocyte cell membranes contain much lower levels of cholesterol than do those of erythrocytes (∼30% as opposed to ∼50% (38,40–42)).
One important caveat to this and other studies with cyclodextrins is that their specificity for cholesterol is not absolute (reviewed in Zidovetzki and Levitan (66)). Additional lipids such as sphingomyelin, glycosphingolipids, and glycerophospholipids can also be extracted, especially with high MBCD concentrations and lengthy incubations. Based on previous measurements of relative phospholipid and cholesterol depletion from human erythrocytes, the mild conditions used here (1 mM MBCD, 30 min incubation) and the high efficiency with which cholesterol was removed suggest that no more than a few percent of phospholipids were extracted in our experiments (46,67). Furthermore, the control experiment displayed in Fig. 4 and our efforts to quantify the amount of cholesterol extracted support the idea that the effects studied here relate to cholesterol depletion. Nevertheless, we cannot exclude the possibility that additional lipids occupying the same site as cholesterol on the dextrin have been extracted and are relevant to the results reported here. This consideration should always temper interpretations using MBCD.
Does the perturbant act by changing membrane order?
One area that has been somewhat controversial in comparisons among artificial membranes, lymphocytes, and erythrocytes is the role of changes in membrane order induced by perturbants that cause enhanced hydrolysis by sPLA2. In artificial bilayers, the various perturbants that activate the enzyme exert a diversity of effects on membrane order: some increase the average order, some decrease it, and others have no effect (8,64). In lymphocytes, treatment of the cells with ionomycin to induce susceptibility to sPLA2 produces no change in membrane lipid order (24). Thus, the initial observations of an increase in the number of ordered domains in erythrocytes upon ionomycin treatment appeared unique to that cell type. Now, the data of Fig. 7 provide clarification by suggesting the absence of a correlation between effects of ionomycin on membrane order and on hydrolysis. For example, removal of cholesterol from the cell membrane below 30°C created a scenario in which the ability of ionomycin to induce susceptibility was great (Fig. 2) but the change in laurdan GP associated with the ionophore was absent (Fig. 7). Thus, we conclude that perturbants do not induce membrane susceptibility to sPLA2 by changing membrane order. This interpretation appears to apply broadly to artificial bilayers and to all cell types tested thus far. Instead, the hypothesis proposed from model membranes that perturbants promote hydrolysis by increasing negative surface charge and/or by reducing the strength of interactions among neighboring phospholipids appears to be a superior explanation (8,23,24).
CONCLUSIONS
In summary, two conclusions can be drawn from these studies. First, biophysical observations of the behavior of sPLA2 in model membranes apply also to biological membranes, notwithstanding the complexity of that behavior. This conclusion appears to be true both in terms of the effects of lipid order/fluidity on the activity of the enzyme as well as on the ability of specific bilayer perturbations (e.g., fatty acid or lysophospholipid in model membranes, ionophore treatment on cell membranes) to further render the membrane susceptible to hydrolytic attack. Second, the high concentration of cholesterol in erythrocyte membranes has been suggested to play an important protective role for the cells by conferring mechanical stability to the cell membrane (68). We now show that the same may be true physiologically for protecting cells from attack by hydrolytic enzymes present in the blood. This idea makes sense because nucleated cells possess other mechanisms such as endocytosis that appear to confer protection against hydrolytic enzymes by sequestering and clearing them from the cell surface (69). Erythrocytes, however, are incapable of such processes and therefore must rely on the biophysical properties of their membranes as their sole protection from these enzymes.
Acknowledgments
Two-photon experiments were performed at the Laboratory for Fluorescence Dynamics (LFD) at the University of California, Irvine. We gratefully acknowledge the assistance of LFD personnel, especially Enrico Gratton (principal investigator), Susana Sanchez (user coordinator), Oliver Holub, and Theodore Hazlett (director).
This work was supported by the National Institutes of Health (GM073997).
Editor: Lukas K. Tamm.
References
- 1.Yethiraj, A., and J. C. Weisshaar. 2007. Why are lipid rafts not observed in vivo? Biophys. J. 93:3113–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gelb, M. H., M. K. Jain, A. M. Hanel, and O. G. Berg. 1995. Interfacial enzymology of glycerolipid hydrolases: lessons from secreted phospholipases A2. Annu. Rev. Biochem. 64:653–688. [DOI] [PubMed] [Google Scholar]
- 3.Winget, J. M., Y. H. Pan, and B. J. Bahnson. 2006. The interfacial binding surface of phospholipase A2s. Biochim. Biophys. Acta. 1761:1260–1269. [DOI] [PubMed] [Google Scholar]
- 4.Lichtenberg, D., G. Romero, M. Menashe, and R. L. Biltonen. 1986. Hydrolysis of dipalmitoylphosphatidylcholine large unilamellar vesicles by porcine pancreatic phospholipase A2. J. Biol. Chem. 261:5334–5340. [PubMed] [Google Scholar]
- 5.Bell, J. D., and R. L. Biltonen. 1989. The temporal sequence of events in the activation of phospholipase A2 by lipid vesicles. Studies with the monomeric enzyme from Agkistrodon piscivorus piscivorus. J. Biol. Chem. 264:12194–12200. [PubMed] [Google Scholar]
- 6.Bell, J. D., and R. L. Biltonen. 1992. Molecular details of the activation of soluble phospholipase A2 on lipid bilayers. Comparison of computer simulations with experimental results. J. Biol. Chem. 267:11046–11056. [PubMed] [Google Scholar]
- 7.Bell, J. D., M. L. Baker, E. D. Bent, R. W. Ashton, D. J. Hemming, and L. D. Hansen. 1995. Effects of temperature and glycerides on the enhancement of Agkistrodon piscivorus piscivorus phospholipase A2 activity by lysolecithin and palmitic acid. Biochemistry. 34:11551–11560. [DOI] [PubMed] [Google Scholar]
- 8.Henshaw, J. B., C. A. Olsen, A. R. Farnbach, K. H. Nielson, and J. D. Bell. 1998. Definition of the specific roles of lysolecithin and palmitic acid in altering the susceptibility of dipalmitoylphosphatidylcholine bilayers to phospholipase A2. Biochemistry. 37:10709–10721. [DOI] [PubMed] [Google Scholar]
- 9.Jain, M. K., and G. H. de Haas. 1983. Activation of phospholipase A2 by freshly added lysophospholipids. Biochim. Biophys. Acta. 736:157–162. [DOI] [PubMed] [Google Scholar]
- 10.Menashe, M., G. Romero, R. L. Biltonen, and D. Lichtenberg. 1986. Hydrolysis of dipalmitoylphosphatidylcholine small unilamellar vesicles by porcine pancreatic phospholipase A2. J. Biol. Chem. 261:5328–5333. [PubMed] [Google Scholar]
- 11.Gheriani-Gruszka, N., S. Almog, R. L. Biltonen, and D. Lichtenberg. 1988. Hydrolysis of phosphatidylcholine in phosphatidylcholine-cholate mixtures by porcine pancreatic phospholipase A2. J. Biol. Chem. 263:11808–11813. [PubMed] [Google Scholar]
- 12.Jain, M. K., B. Z. Yu, and A. Kozubek. 1989. Binding of phospholipase A2 to zwitterionic bilayers is promoted by lateral segregation of anionic amphiphiles. Biochim. Biophys. Acta. 980:23–32. [DOI] [PubMed] [Google Scholar]
- 13.Bent, E. D., and J. D. Bell. 1995. Quantification of the interactions among fatty acid, lysophosphatidylcholine, calcium, dimyristoylphosphatidylcholine vesicles, and phospholipase A2. Biochim. Biophys. Acta. 1254:349–360. [DOI] [PubMed] [Google Scholar]
- 14.Bell, J. D., M. Burnside, J. A. Owen, M. L. Royall, and M. L. Baker. 1996. Relationships between bilayer structure and phospholipase A2 activity: interactions among temperature, diacylglycerol, lysolecithin, palmitic acid, and dipalmitoylphosphatidylcholine. Biochemistry. 35:4945–4955. [DOI] [PubMed] [Google Scholar]
- 15.Hoyrup, P., T. H. Callisen, M. O. Jensen, A. Halperin, and O. G. Mouritsen. 2004. Lipid protrusions, membrane softness, and enzymatic activity. Phys. Chem. Chem. Phys. 6:1608–1615. [Google Scholar]
- 16.Honger, T., K. Jorgensen, R. L. Biltonen, and O. G. Mouritsen. 1996. Systematic relationship between phospholipase A2 activity and dynamic lipid bilayer microheterogeneity. Biochemistry. 35:9003–9006. [DOI] [PubMed] [Google Scholar]
- 17.Salomon, Y., C. Londos, and M. Rodbell. 1974. A highly sensitive adenylate cyclase assay. Anal. Biochem. 58:541–548. [DOI] [PubMed] [Google Scholar]
- 18.Brueseke, T. J., and J. D. Bell. 2006. A new hat for an old enzyme: Waste management. Biochim. Biophys. Acta. 1761:1270–1279. [DOI] [PubMed] [Google Scholar]
- 19.Wilson, H. A., J. B. Waldrip, K. H. Nielson, A. M. Judd, S. K. Han, W. Cho, P. J. Sims, and J. D. Bell. 1999. Mechanisms by which elevated intracellular calcium induces S49 cell membranes to become susceptible to the action of secretory phospholipase A2. J. Biol. Chem. 274:11494–11504. [DOI] [PubMed] [Google Scholar]
- 20.Nielson, K. H., C. A. Olsen, D. V. Allred, K. L. O'Neill, G. F. Burton, and J. D. Bell. 2000. Susceptibility of S49 lymphoma cell membranes to hydrolysis by secretory phospholipase A(2) during early phase of apoptosis. Biochim. Biophys. Acta. 1484:163–174. [DOI] [PubMed] [Google Scholar]
- 21.Smith, S. K., A. R. Farnbach, F. M. Harris, A. C. Hawes, L. R. Jackson, A. M. Judd, R. S. Vest, S. Sanchez, and J. D. Bell. 2001. Mechanisms by which intracellular calcium induces susceptibility to secretory phospholipase A2 in human erythrocytes. J. Biol. Chem. 276:22732–22741. [DOI] [PubMed] [Google Scholar]
- 22.Best, K. B., A. J. Ohran, A. C. Hawes, T. L. Hazlett, E. Gratton, A. M. Judd, and J. D. Bell. 2002. Relationship between erythrocyte membrane phase properties and susceptibility to secretory phospholipase A2. Biochemistry. 41:13982–13988. [DOI] [PubMed] [Google Scholar]
- 23.Jensen, L. B., N. K. Burgess, D. D. Gonda, E. Spencer, H. A. Wilson-Ashworth, E. Driscoll, M. P. Vu, J. L. Fairbourn, A. M. Judd, and J. D. Bell. 2005. Mechanisms governing the level of susceptibility of erythrocyte membranes to secretory phospholipase A2. Biophys. J. 88:2692–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey, R. W., E. D. Olson, M. P. Vu, T. J. Brueseke, L. Robertson, R. E. Christensen, K. H. Parker, A. M. Judd, and J. D. Bell. 2007. Relationship between membrane physical properties and secretory phospholipase A2 hydrolysis kinetics in S49 cells during ionophore-induced apoptosis. Biophys. J. 93:2350–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parasassi, T., M. Di Stefano, M. Loiero, G. Ravagnan, and E. Gratton. 1994. Cholesterol modifies water concentration and dynamics in phospholipid bilayers: a fluorescence study using Laurdan probe. Biophys. J. 66:763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, M. M., M. Olsher, I. P. Sugar, and P. L. Chong. 2004. Cholesterol superlattice modulates the activity of cholesterol oxidase in lipid membranes. Biochemistry. 43:2159–2166. [DOI] [PubMed] [Google Scholar]
- 27.Veatch, S. L., and S. L. Keller. 2005. Seeing spots: complex phase behavior in simple membranes. Biochim. Biophys. Acta. 1746:172–185. [DOI] [PubMed] [Google Scholar]
- 28.Barenholz, Y. 2004. Sphingomyelin and cholesterol: from membrane biophysics and rafts to potential medical applications. Subcell. Biochem. 37:167–215. [DOI] [PubMed] [Google Scholar]
- 29.Wilson-Ashworth, H. A., Q. Bahm, J. Erickson, A. Shinkle, M. P. Vu, D. Woodbury, and J. D. Bell. 2006. Differential detection of phospholipid fluidity, order, and spacing by fluorescence spectroscopy of bis-pyrene, prodan, nystatin, and merocyanine 540. Biophys. J. 91:4091–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McConnell, H., and A. Radhakrishnan. 2006. Theory of the deuterium NMR of sterol-phospholipid membranes. Proc. Natl. Acad. Sci. USA. 103:1184–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mannock, D. A., R. N. Lewis, and R. N. McElhaney. 2006. Comparative calorimetric and spectroscopic studies of the effects of lanosterol and cholesterol on the thermotropic phase behavior and organization of dipalmitoylphosphatidylcholine bilayer membranes. Biophys. J. 91:3327–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandit, S. A., G. Khelashvili, E. Jakobsson, A. Grama, and H. L. Scott. 2007. Lateral organization in lipid-cholesterol mixed bilayers. Biophys. J. 92:440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Op den Kamp, J. A., M. T. Kauerz, and L. L. Van Deenen. 1975. Action of pancreatic phospholipase A2 on phosphatidylcholine bilayers in different physical states. Biochim. Biophys. Acta. 406:169–177. [DOI] [PubMed] [Google Scholar]
- 34.Liu, F., and P. L. Chong. 1999. Evidence for a regulatory role of cholesterol superlattices in the hydrolytic activity of secretory phospholipase A2 in lipid membranes. Biochemistry. 38:3867–3873. [DOI] [PubMed] [Google Scholar]
- 35.Kannagi, R., and K. Koizumi. 1979. Effect of different physical states of phospholipid substrates on partially purified platelet phospholipase A2 activity. Biochim. Biophys. Acta. 556:423–433. [DOI] [PubMed] [Google Scholar]
- 36.Richieri, G. V., and A. M. Kleinfeld. 1995. Continuous measurement of phospholipase A2 activity using the fluorescent probe ADIFAB. Anal. Biochem. 229:256–263. [DOI] [PubMed] [Google Scholar]
- 37.Koumanov, K. S., P. J. Quinn, G. Bereziat, and C. Wolf. 1998. Cholesterol relieves the inhibitory effect of sphingomyelin on type II secretory phospholipase A2. Biochem. J. 336:625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gottfried, E. L. 1967. Lipids of human leukocytes: relation to cell type. J. Lipid Res. 8:321–327. [PubMed] [Google Scholar]
- 39.Parmahamsa, M., K. R. Reddy, and N. Varadacharyulu. 2004. Changes in composition and properties of erythrocyte membrane in chronic alcoholics. Alcohol Alcohol. 39:110–112. [DOI] [PubMed] [Google Scholar]
- 40.Ashworth, L. A., and C. Green. 1966. Plasma membranes: phospholipid and sterol content. Science. 151:210–211. [DOI] [PubMed] [Google Scholar]
- 41.Marcus, A. J., H. L. Ullman, and L. B. Safier. 1969. Lipid composition of subcellular particles of human blood platelets. J. Lipid Res. 10:108–114. [PubMed] [Google Scholar]
- 42.Johnson, S. M., and R. Robinson. 1979. The composition and fluidity of normal and leukaemic or lymphomatous lymphocyte plasma membranes in mouse and man. Biochim. Biophys. Acta. 558:282–295. [DOI] [PubMed] [Google Scholar]
- 43.Rigaud, J. L., and G. Leblanc. 1980. Effect of membrane cholesterol on action of phospholipase A2 in Mycoplasma mycoides var. Capri. Evidence for lysophospholipase activity. Eur. J. Biochem. 110:77–84. [DOI] [PubMed] [Google Scholar]
- 44.Kochhar, N., and D. Kaul. 1992. Molecular link between membrane cholesterol and Na+/H+ exchange within human platelets. FEBS Lett. 299:19–22. [DOI] [PubMed] [Google Scholar]
- 45.Klein, U., G. Gimpl, and F. Fahrenholz. 1995. Alteration of the myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry. 34:13784–13793. [DOI] [PubMed] [Google Scholar]
- 46.Ohtani, Y., T. Irie, K. Uekama, K. Fukunaga, and J. Pitha. 1989. Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur. J. Biochem. 186:17–22. [DOI] [PubMed] [Google Scholar]
- 47.Wolkers, W. F., L. M. Crowe, N. M. Tsvetkova, F. Tablin, and J. H. Crowe. 2002. In situ assessment of erythrocyte membrane properties during cold storage. Mol. Membr. Biol. 19:59–65. [DOI] [PubMed] [Google Scholar]
- 48.Yancey, P. G., W. V. Rodrigueza, E. P. Kilsdonk, G. W. Stoudt, W. J. Johnson, M. C. Phillips, and G. H. Rothblat. 1996. Cellular cholesterol efflux mediated by cyclodextrins. Demonstration of kinetic pools and mechanism of efflux. J. Biol. Chem. 271:16026–16034. [DOI] [PubMed] [Google Scholar]
- 49.Pitha, J., T. Irie, P. B. Sklar, and J. S. Nye. 1988. Drug solubilizers to aid pharmacologists: amorphous cyclodextrin derivatives. Life Sci. 43:493–502. [DOI] [PubMed] [Google Scholar]
- 50.Maraganore, J. M., G. Merutka, W. Cho, W. Welches, F. J. Kezdy, and R. L. Heinrikson. 1984. A new class of phospholipases A2 with lysine in place of aspartate 49. Functional consequences for calcium and substrate binding. J. Biol. Chem. 259:13839–13843. [PubMed] [Google Scholar]
- 51.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911–917. [DOI] [PubMed] [Google Scholar]
- 52.Parasassi, T., G. De Stasio, G. Ravagnan, R. M. Rusch, and E. Gratton. 1991. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys. J. 60:179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harris, F. M., S. K. Smith, and J. D. Bell. 2001. Physical properties of erythrocyte ghosts that determine susceptibility to secretory phospholipase A2. J. Biol. Chem. 276:22722–22731. [DOI] [PubMed] [Google Scholar]
- 54.Richieri, G. V., R. T. Ogata, and A. M. Kleinfeld. 1992. A fluorescently labeled intestinal fatty acid binding protein. Interactions with fatty acids and its use in monitoring free fatty acids. J. Biol. Chem. 267:23495–23501. [PubMed] [Google Scholar]
- 55.Yu, W., P. T. So, T. French, and E. Gratton. 1996. Fluorescence generalized polarization of cell membranes: a two-photon scanning microscopy approach. Biophys. J. 70:626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harris, F. M., K. B. Best, and J. D. Bell. 2002. Use of laurdan fluorescence intensity and polarization to distinguish between changes in membrane fluidity and phospholipid order. Biochim. Biophys. Acta. 1565:123–128. [DOI] [PubMed] [Google Scholar]
- 57.Vest, R. S., L. J. Gonzales, S. A. Permann, E. Spencer, L. D. Hansen, A. M. Judd, and J. D. Bell. 2004. Divalent cations increase lipid order in erythrocytes and susceptibility to secretory phospholipase A2. Biophys. J. 86:2251–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jain, M. K., and D. V. Jahagirdar. 1985. Action of phospholipase A2 on bilayers. Effect of fatty acid and lysophospholipid additives on the kinetic parameters. Biochim. Biophys. Acta. 814:313–318. [DOI] [PubMed] [Google Scholar]
- 59.Burack, W. R., Q. Yuan, and R. L. Biltonen. 1993. Role of lateral phase separation in the modulation of phospholipase A2 activity. Biochemistry. 32:583–589. [DOI] [PubMed] [Google Scholar]
- 60.Brown, S. D., B. L. Baker, and J. D. Bell. 1993. Quantification of the interaction of lysolecithin with phosphatidylcholine vesicles using bovine serum albumin: relevance to the activation of phospholipase A2. Biochim. Biophys. Acta. 1168:13–22. [DOI] [PubMed] [Google Scholar]
- 61.Honger, T., K. Jorgensen, D. Stokes, R. L. Biltonen, and O. G. Mouritsen. 1997. Phospholipase A2 activity and physical properties of lipid-bilayer substrates. Methods Enzymol. 286:168–190. [DOI] [PubMed] [Google Scholar]
- 62.Burack, W. R., A. R. Dibble, M. M. Allietta, and R. L. Biltonen. 1997. Changes in vesicle morphology induced by lateral phase separation modulate phospholipase A2 activity. Biochemistry. 36:10551–10557. [DOI] [PubMed] [Google Scholar]
- 63.Menashe, M., D. Lichtenberg, C. Gutierrez-Merino, and R. L. Biltonen. 1981. Relationship between the activity of pancreatic phospholipase A2 and the physical state of the phospholipid substrate. J. Biol. Chem. 256:4541–4543. [PubMed] [Google Scholar]
- 64.Sheffield, M. J., B. L. Baker, D. Li, N. L. Owen, M. L. Baker, and J. D. Bell. 1995. Enhancement of Agkistrodon piscivorus piscivorus venom phospholipase A2 activity toward phosphatidylcholine vesicles by lysolecithin and palmitic acid: studies with fluorescent probes of membrane structure. Biochemistry. 34:7796–7806. [DOI] [PubMed] [Google Scholar]
- 65.Parasassi, T., A. M. Giusti, M. Raimondi, and E. Gratton. 1995. Abrupt modifications of phospholipid bilayer properties at critical cholesterol concentrations. Biophys. J. 68:1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zidovetzki, R., and I. Levitan. 2007. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim. Biophys. Acta. 1768:1311–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kilsdonk, E. P., P. G. Yancey, G. W. Stoudt, F. W. Bangerter, W. J. Johnson, M. C. Phillips, and G. H. Rothblat. 1995. Cellular cholesterol efflux mediated by cyclodextrins. J. Biol. Chem. 270:17250–17256. [DOI] [PubMed] [Google Scholar]
- 68.Lange, Y., H. B. Cutler, and T. L. Steck. 1980. The effect of cholesterol and other intercalated amphipaths on the contour and stability of the isolated red cell membrane. J. Biol. Chem. 255:9331–9337. [PubMed] [Google Scholar]
- 69.Kim, K. P., J. D. Rafter, L. Bittova, S. K. Han, Y. Snitko, N. M. Munoz, A. R. Leff, and W. Cho. 2001. Mechanism of human group V phospholipase A2 (PLA2)-induced leukotriene biosynthesis in human neutrophils. A potential role of heparan sulfate binding in PLA2 internalization and degradation. J. Biol. Chem. 276:11126–11134. [DOI] [PubMed] [Google Scholar]