Abstract
We present a cellular model of lipid biosynthesis in the plasma membrane that couples biochemical and biophysical features of the enzymatic network of the cell-wall-less Mycoplasma Acholeplasma laidlawii. In particular, we formulate how the stored elastic energy of the lipid bilayer can modify the activity of curvature-sensitive enzymes through the binding of amphipathic α-helices. As the binding depends on lipid composition, this results in a biophysical feedback mechanism for the regulation of the stored elastic energy. The model shows that the presence of feedback increases the robustness of the steady state of the system, in the sense that biologically inviable nonbilayer states are less likely. We also show that the biophysical and biochemical features of the network have implications as to which enzymes are most efficient at implementing the regulation. The network imposes restrictions on the steady-state balance between bilayer and nonbilayer lipids and on the concentrations of particular lipids. Finally, we consider the influence of the length of the amphipathic α-helix on the efficacy of the feedback and propose experimental measurements and extensions of the modeling framework.
INTRODUCTION
The primary function of the lipids in the plasma membrane is to form a bilayer that provides a permeability barrier between the cytoplasm and the environment. However, whereas lipids were once considered purely passive components, it is now clear that lipids play an active role in a variety of dynamic processes involving the membranes that compartmentalize the cell (1). To achieve this dual role of the membrane as a dynamic boundary and a continuous barrier, the cell must regulate the mechanical properties of the membrane and does so partly by controlling its lipid composition.
Membrane lipids are chemically diverse (2) but they can be classified into the broad categories of bilayer and nonbilayer lipids, depending on their (in)ability to self-assemble into bilayers. Bilayer formation is the result of a thermodynamic equilibrium in which the physicochemical properties of the lipids, such as the chemical structure of the headgroup and hydrocarbon chains, play a crucial role. The cell can therefore regulate the mechanical properties of the bilayer by modifying its lipid composition through lipid biosynthesis. The balance between bilayer and nonbilayer lipids in the plasma membrane has been the subject of many reviews (2–4). Experiments have shown that organisms change the lipid composition of their membranes in response to external variations in diet, pressure, and temperature (5–7; see also (18)). Moreover, many of the lipids found in biological membranes do not form bilayers under physiological conditions. Subsequent studies have confirmed that most organisms contain significant amounts of at least one nonbilayer lipid (8,9).
The underlying biophysical question is the relationship between the chemical diversity and variability of membrane lipid composition, the mechanical properties of the membrane, and the associated protein functions (10,11). A large experimental effort has been devoted to mapping lipid biosynthetic pathways by characterizing and mutating particular enzymes. There is also an increasing body of experiments that measure the relationship between the biophysical properties of lipids and enzyme activity (10,12). However, there have been few attempts (13) to consider theoretically the interdependence of these two phenomena by modeling the lipid biosynthetic network as an integrated system in which the biochemical and the biophysical descriptions of the metabolic network are fundamentally linked. The focus of the model presented here is to provide a set of tools to understand the interplay between the enzymes and lipids involved in lipid metabolism in relation to the biophysical properties of the bilayer.
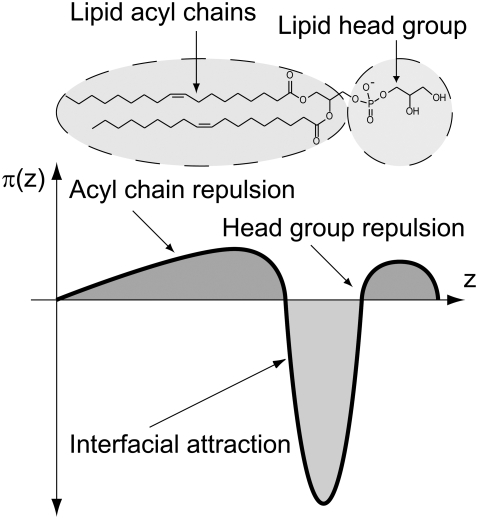
Fig. 1 depicts a simplified representation of the connection between the chemical structure of lipids and the mechanical properties of a lipid monolayer. A lipid monolayer consists of conformationally flexible lipids, whose amphiphilic nature leads to a nonuniform pressure distribution across the monolayer. The lateral pressure profile π(z) determines the average “molecular shape” that a lipid adopts and, more importantly, Js, the monolayer spontaneous curvature. Js is an intrinsic property of a lipid species that corresponds to the monolayer curvature in which a lipid in the monolayer is at the conformation with minimum free energy (14). The spontaneous curvature reflects the desire of a lipid monolayer to either curve away from, or curve toward, the membrane-water interface and whether a lipid is a bilayer or nonbilayer lipid.
FIGURE 1.
Forces that act between lipids at different depths include electrostatic and hydrogen bond interactions at the headgroup, interfacial tension at the hydrophilic-hydrophobic interface, and the packing of the hydrocarbon chains. The lateral pressure profile π(z) depends crucially on the chemical nature of the lipid head group and the length and saturation of the lipid hydrocarbon chains. The lateral pressure profile determines the spontaneous curvature Js of a lipid monolayer (14).
A lipid bilayer is formed by two monolayers back-to-back. This arrangement means that the monolayers may not be able to adopt their preferred curvature, Js, since the monolayers in the bilayer are held together by the hydrophobic effect. This leads to a difference between the actual curvature of a monolayer, as given by the principal curvatures c1 and c2, and its spontaneous curvature, Js (15). Based on this physical picture, Helfrich (16) formulated the stored elastic energy per unit area, g, of a lipid monolayer that is constrained to have principal curvatures c1 and c2:
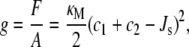 |
where F is the Helmholtz free energy, A is the area, and κM is the bending rigidity of the monolayer. The lipids are at the free energy minimum, when the total curvature, c1 + c2, is at the value of the spontaneous curvature Js. Because at equilibrium g is minimized, this means it is more difficult for lipids with large spontaneous curvatures to form a bilayer, which is a flat conformation with small c1 and c2. Indeed, it has been suggested that lipids with Js < −1/6 nm−1 (the negative sign is a convention to denote that the monolayer curves toward water in an interface) do not form bilayers. Instead, they form curved mesophases, such as the inverse hexagonal phase, which are porous (17).
From a biological perspective, porous mesophases would have severe consequences for cellular function and survival. Gruner (3) hypothesized that the average spontaneous curvature 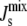 of the lipids in the plasma membrane must be tightly regulated to ensure that the membrane lipids form a (nonporous) bilayer and that the cell is able to control
of the lipids in the plasma membrane must be tightly regulated to ensure that the membrane lipids form a (nonporous) bilayer and that the cell is able to control 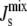 (15) by modifying its lipid composition through the biochemical networks of lipid metabolism.
(15) by modifying its lipid composition through the biochemical networks of lipid metabolism.
This insight has been confirmed experimentally. Lipid extracts from the cell-wall-less Mycoplasma Acholeplasma laidlawii grown under different conditions have average spontaneous curvatures 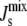 in the small range between −1/6.6 nm−1 and −1/8.1 nm−1 even though the membrane contains lipids with Js outside of this range (18). To achieve this robust regulation, A. laidlawii alters the ratio of its two main glucolipids in response to the length and saturation of exogenously fed fatty acids (5), thus maintaining
in the small range between −1/6.6 nm−1 and −1/8.1 nm−1 even though the membrane contains lipids with Js outside of this range (18). To achieve this robust regulation, A. laidlawii alters the ratio of its two main glucolipids in response to the length and saturation of exogenously fed fatty acids (5), thus maintaining 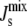 in a biologically viable “bilayer range” that ensures membrane integrity yet with enough stored elastic energy to allow for its dynamical behavior. Remarkably, although the average spontaneous curvature is controlled, the lipid concentrations exhibit wide variations. This suggests that the control of
in a biologically viable “bilayer range” that ensures membrane integrity yet with enough stored elastic energy to allow for its dynamical behavior. Remarkably, although the average spontaneous curvature is controlled, the lipid concentrations exhibit wide variations. This suggests that the control of 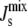 is not achieved by targeting specific lipid compositions. These observations also apply to Escherichia coli lipid extracts, which begin to form nonbilayer structures close to physiological conditions (7).
is not achieved by targeting specific lipid compositions. These observations also apply to Escherichia coli lipid extracts, which begin to form nonbilayer structures close to physiological conditions (7).
Biophysical control mechanisms integrated into lipid biosynthetic networks have been the subject of intense experimental study. An example is given by cytidine triphosphate/phosphocholine cytidyltransferase (CCT), an enzyme involved in the biosynthesis of the ubiquitous lipid phosphatidylcholine. CCT is inactive in the cytoplasm, but becomes active when membrane-bound. It has been shown that its activity is affected by the stored elastic energy in the membrane (12). The biophysical control mechanism arises from the presence of an amphipathic α-helix that affects enzyme activity by regulating the binding of CCT to lipid bilayers. In a broad sense, the amphipathic α-helix can be viewed as a “sensor” of the spontaneous curvature since it binds preferentially to lipid bilayers with large negative 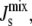 thus modulating the activity of the lipid biosynthetic enzyme. This biophysical control mechanism is chemically nonspecific, as it is based on a biophysical interaction between the enzyme and the membrane, and appears to be generic to a number of enzymes present in lipid biosynthetic pathways (12,19), including those present in A. laidlawii, which is the focus of this study.
thus modulating the activity of the lipid biosynthetic enzyme. This biophysical control mechanism is chemically nonspecific, as it is based on a biophysical interaction between the enzyme and the membrane, and appears to be generic to a number of enzymes present in lipid biosynthetic pathways (12,19), including those present in A. laidlawii, which is the focus of this study.
We have developed a modeling framework for the lipid biosynthetic pathways in A. laidlawii. Building upon the A. laidlawii biochemical network studied in detail by the groups of Lindblom, Rilfors, and Wieslander (5,6,19,20), we formulate a biophysical mechanism, based upon some of the conceptual foundations established in CCT (12), that couples the activity of lipid biosynthetic enzymes to the membrane composition. Our results show that the presence of feedback increases the robustness of the steady state of the system to parameter variations, in the sense that it decreases the probability of inviable values of 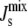 that would lead to porous phases. From a sensitivity analysis, we identify the enzymes that are most efficient in implementing the control of the network. We also study the restrictions that the network imposes on the steady-state concentrations of particular lipids and show that the system keeps a balance between bilayer and nonbilayer lipids. Finally, we consider the influence of the length of the amphipathic α-helix on the efficacy of the feedback.
that would lead to porous phases. From a sensitivity analysis, we identify the enzymes that are most efficient in implementing the control of the network. We also study the restrictions that the network imposes on the steady-state concentrations of particular lipids and show that the system keeps a balance between bilayer and nonbilayer lipids. Finally, we consider the influence of the length of the amphipathic α-helix on the efficacy of the feedback.
THE LIPID BIOSYNTHETIC NETWORK OF A. LAIDLAWII
We take the cell-wall-less Mycoplasma A. laidlawii as our system for the study of cellular models of lipid biosynthesis. This simple organism, which has been studied in great detail (5,6), has two features that make it ideal to showcase our modeling framework. First, virtually all the lipids in A. laidlawii are in the plasma membrane (21). This simplifies the model to a single lipid bilayer, avoiding the complexity of cell walls and intracellular compartments. Second, A. laidlawii cannot synthesize unsaturated fatty acids and is very limited in its synthesis of saturated fatty acids (5). Therefore, A. laidlawii exhibits a significantly reduced number of chemical species in the plasma membrane as it relies on exogenously fed fatty acids for lipid biosynthesis.
The limited fatty acid synthesis implies that the only response of A. laidlawii to variations in its fatty acid diet is to alter the composition of the headgroups of the lipids in the membrane through the network of enzymatic reactions represented in Fig. 2. Indeed, experiments show that the membrane lipid composition of A. laidlawii depends strongly on the length and saturation of exogenously fed fatty acids (5). When A. laidlawii is fed palmitic acid (a short, saturated fatty acid), monoglucosyldiacylglycerol (MGlcDAG) is the most abundant lipid; whereas when A. laidlawii is fed oleic acid (a long, unsaturated fatty acid), diglucosyldiacylglycerol (DGlcDAG) dominates. Central to our study is the observation that although the variation in the lipid composition can be large, the cell maintains the average monolayer spontaneous curvature of the plasma membrane 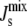 within a “window” in which the bilayer phase is stable (7) (Table 1). The lipid biosynthetic network is able to adjust the lipid composition to achieve a
within a “window” in which the bilayer phase is stable (7) (Table 1). The lipid biosynthetic network is able to adjust the lipid composition to achieve a 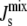 > −1/6 nm−1, thus maintaining a dynamic, yet impermeable plasma membrane.
> −1/6 nm−1, thus maintaining a dynamic, yet impermeable plasma membrane.
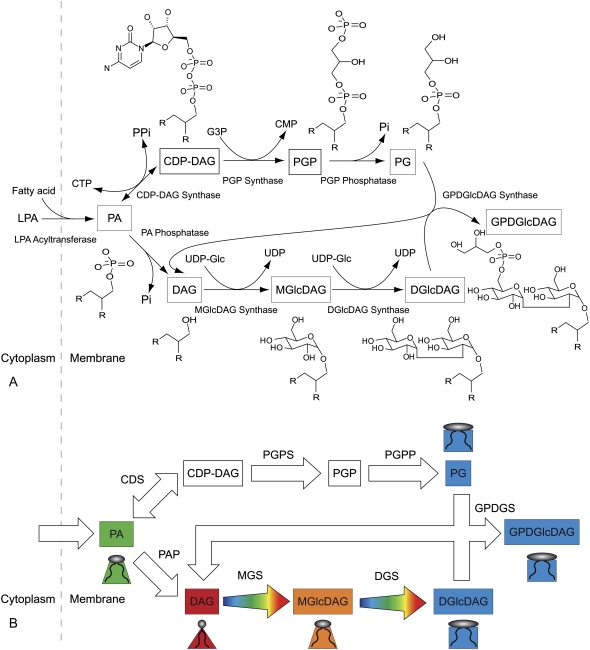
FIGURE 2.
A. laidlawii lipid biosynthetic network. (A) The biochemical network. The main lipids in the plasma membrane of A. laidlawii A-EF22 are (6): phosphatidylglycerol (PG), diacylglycerol (DAG), monoglucosyl-DAG (MGlcDAG), diglucosyl-DAG (DGlcDAG), and glycerophosphoryl-DGlcDAG (GPDGlcDAG). Phosphatidic acid (PA), the liponuleotide CDP-DAG, and PG-phosphate (PGP) are lipid intermediates. The top branch is the PG pathway and the bottom branch is the glucolipid pathway. The abbreviated soluble reactants are glucose (Glc) and UDP-Glc, glycerol-3-phosphate (G3P), the inorganic phosphate ions Pi and PPi, and the nucleotide CTP. R indicates an acyl chain. Six of the seven enzymes have irreversible rate equations. A. laidlawii also synthesizes three monoacyl derivatives of the glucolipids: monoacyl-MGlcDAG (MAMGlcDAG), monoacyl-DGlcDAG (MADGlcDAG), and monoacyl-bisglycerophosphoryl-DGlcDAG (MABGPDGlcDAG) (6). However, these lipids have been excluded from the model as they are not always synthesized (5) and their biosynthetic pathways have been postulated, but are not known (59). (B) A biophysical picture of the network. Lipids are color coded according to their Js, which is linked to their molecular shape as shown. The same color code is used to show that the activity of MGS and DGS increases when the plasma membrane has a large negative 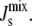 It can be seen, for instance, that in the case of the lower pathway the effect of MGS and DGS is to increase the effective size of the headgroup of the lipid upon which they are acting and therefore systematically increase the value of Js among DAG, MGlcDAG, and DGlcDAG. By controlling the rate of the steps between DAG/MGlcDAG and MGlcDAG/DGlcDAG, the system is capable of regulating
It can be seen, for instance, that in the case of the lower pathway the effect of MGS and DGS is to increase the effective size of the headgroup of the lipid upon which they are acting and therefore systematically increase the value of Js among DAG, MGlcDAG, and DGlcDAG. By controlling the rate of the steps between DAG/MGlcDAG and MGlcDAG/DGlcDAG, the system is capable of regulating 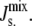 A. laidlawii also synthesizes three monoacyl-derivatives of the glucolipids: monoacyl-MGlcDAG (MAMGlcDAG), monoacyl-DGlcDAG (MADGlcDAG), and monoacyl-bisglycerophosphoryl-DGlcDAG (MABGPDGlcDAG) (6). However, these lipids have been excluded from the model as they are not always synthesized (5) and their biosynthetic pathways have been postulated, but are not known (59).
A. laidlawii also synthesizes three monoacyl-derivatives of the glucolipids: monoacyl-MGlcDAG (MAMGlcDAG), monoacyl-DGlcDAG (MADGlcDAG), and monoacyl-bisglycerophosphoryl-DGlcDAG (MABGPDGlcDAG) (6). However, these lipids have been excluded from the model as they are not always synthesized (5) and their biosynthetic pathways have been postulated, but are not known (59).
TABLE 1.
Spontaneous curvature and lipid composition of A. laidlawii grown in oleic acid
| Lipid | PA | CDP-DAG | PGP | PG | DAG | MGlcDAG | DGlcDAG | GPDGlcDAG |
|---|---|---|---|---|---|---|---|---|
| Js (nm−1) | −1/4.3 | 0 | 0 | −1/8.7 | −1/1.01 | −1/2.5 | −1/13.1 | −1/7.7 |
| Reference | (67) | * | * | (66) | (39) | (18) | (18) | * |
| Lexp (mol %) | 0.7† | 0.04† | 0.04† | 15.1 | 0.7† | 7.8 | 54.4 | 21.2 |
These Js values are estimated. See Appendix A for a discussion.
These lipid molar fractions were below the detection limit and are estimated. See Appendix A.
The lipid biosynthetic network: biochemical and biophysical descriptions
The biochemical description of the lipid biosynthetic network of A. laidlawii is presented in Fig. 2 A. The first step in the metabolic network is, as in other organisms, the acylation of soluble glycerol-3-phosphate (G3P) to form phosphatidic acid (PA) (22). The network then branches out into two pathways.
The upper branch is the phosphatidylglycerol (PG) pathway, well-studied in bacteria, in which PA is converted into PG through the intermediates cytidine diphosphate diacylglycerol (CDP-DAG) and phosphatidylglycerolphosphate (PGP). The corresponding enzymes CDP-DAG synthase (CDS), PGP synthase (PGPS), and PGP phosphatase (PGPP) have been characterized in E. coli (23,24) and in Clostridium perfringens (25,26).
The lower branch is a specific pathway in A. laidlawii, deduced from the discovery and purification of the PA phosphatase (PAP) (27) and the two consecutive glucosyltransferases, MGlcDAG synthase (MGS) (28) and DGlcDAG synthase (DGS) (29). The final enzymatic reaction is yet to be characterized since the glycerophosphoryl-DGlcDAG (GPDGlcDAG) synthase (GPDGS) that catalyzes the production of GPDGlcDAG has not been purified yet. However, the genetic similarity of MGS and DGS to the enzymes of Gram-positive bacteria (30) suggests that GPDGlcDAG could be synthesized by the transfer of G3P from PG to DGlcDAG, a reaction that occurs in the synthesis of lipoteichoic acids in the cell walls of Gram-positive bacteria (31).
Fig. 2 B presents a biophysical interpretation of the network, showing how the molecular shape of each lipid is reflected in its monolayer spontaneous curvature. This physical picture shows that the position of nonbilayer lipids (Js < −1/6 nm−1) and bilayer lipids (Js > −1/6 nm−1) within the network has an effect on which enzymes can exercise effective control of the 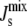 of the plasma membrane. By inspection, MGS and DGS are good candidates for the control of
of the plasma membrane. By inspection, MGS and DGS are good candidates for the control of 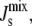 since MGS and DGS catalyze the reactions that lead from the lipid with the most negative Js (DAG) to the lipid with the least negative Js (DGlcDAG) (Fig. 2 B). This intuition is reinforced by a structural feature of these enzymes. MGS and DGS are both peripheral membrane proteins that translocate between the cytoplasm and the membrane. It is postulated that they are only active when they are inserted into the membrane, as suggested by the increased activity of both MGS (29) and DGS (32) in the presence of lipids with large negative Js. This picture leads to a biophysical, intrinsic mechanism for MGS and DGS to control lipid biosynthesis as a function of the average monolayer spontaneous curvature of the membrane. Our model is a mathematical formulation of these ideas.
since MGS and DGS catalyze the reactions that lead from the lipid with the most negative Js (DAG) to the lipid with the least negative Js (DGlcDAG) (Fig. 2 B). This intuition is reinforced by a structural feature of these enzymes. MGS and DGS are both peripheral membrane proteins that translocate between the cytoplasm and the membrane. It is postulated that they are only active when they are inserted into the membrane, as suggested by the increased activity of both MGS (29) and DGS (32) in the presence of lipids with large negative Js. This picture leads to a biophysical, intrinsic mechanism for MGS and DGS to control lipid biosynthesis as a function of the average monolayer spontaneous curvature of the membrane. Our model is a mathematical formulation of these ideas.
Cellular model of lipid biosynthesis
The biochemical constituents of our cellular model of lipid biosynthesis are the membrane lipids, the lipid biosynthetic enzymes, and the soluble cytoplasmic reactants. The membrane lipids are assumed to be homogenously distributed over both monolayers of the plasma membrane. Although labeling studies in E. coli show that lipid biosynthesis occurs mainly at the inner leaflet of the plasma membrane (33), we will assume that lipid transport from the inner to the outer leaflet of the membrane maintains a symmetric bilayer. Our assumption of spatial homogeneity for the lipids is based on the fast lateral diffusion of lipids in bilayers (34,35) and leads to a description in terms of ordinary differential equations. The soluble reactants (such as the nucleotide CTP or the inorganic phosphate ions Pi and PPi) are assumed to have constant, regulated cytoplasmic concentrations, due to their involvement in general cellular processes. Therefore, they are only parameters (not variables) of the model.
The A. laidlawii lipid biosynthetic network is modeled as a system of nonlinear differential equations for eight lipids with seven enzymatic reactions. The variables of the model are compiled into the vector of lipid surface concentrations expressed in molar fraction: LT = [{PA} {CDP-PAG} {PGP} {PG} {DAG} {MGlcDAG} {DGlcDAG} {GPDGlcDAG}]. The sum of the lipid molar fractions is 1 at all times: 1T L = 1.
Each enzymatic reaction has a nonlinear rate equation of the Michaelis-Menten type, modified using surface dilution kinetics, as explained below, to account for the fact that the reactions take place on the membrane. The enzyme rate equations are compiled into a vector vT = [vCDS vPGPS vPGPP vPAP vMGS vDGS vGPDGS]. The modulation of the enzyme activity due to the biophysical interaction with the mechanical properties of the membrane is introduced through a diagonal matrix Ka = diag([Ka,CDS Ka,PGPS Ka,PGPP Ka,PAP Ka,MGS Ka,DGS Ka,GPDGS]), which incorporates the possibility that some of the enzymatic rates, specifically those of MGS and DGS, could depend on 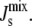 If the enzyme is curvature sensitive, its association constant Ka,Enzyme will depend on
If the enzyme is curvature sensitive, its association constant Ka,Enzyme will depend on 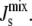 Otherwise, the corresponding Ka,Enzyme = 1. This is the basis of the biophysical feedback mechanism, which will be introduced in the following section.
Otherwise, the corresponding Ka,Enzyme = 1. This is the basis of the biophysical feedback mechanism, which will be introduced in the following section.
The topology of the reaction network is encoded in a stoichiometric matrix N, where Nij is the number of lipid species i consumed (negative) or produced (positive) in reaction j:
 |
The matrix N accounts for the enzymatic reactions and ensures mass conservation. However, our cellular model must also include both the lipid degradation into soluble products and the lipid insertion that enables a growing cell to double the number of lipids before cell division. These processes are incorporated through the transport vector t and the normalization vector n. The transport vector t encapsulates the balance of lipids inserted and extracted. In our model, only PA is inserted at a constant cellular rate V+,PA and lipid degradation is assumed not to play a significant role in A. laidlawii lipid metabolism (36). Therefore, tT = [ V+,PA 0 0 0 0 0 0 0].
The normalization vector n, given by
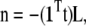 |
reduces the surface concentration of each lipid in proportion to its molar fraction while at the same time maintaining the sum of the molar fractions equal to 1.
Combining all the terms, the model can be written compactly as
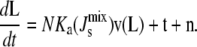 |
(1) |
This system has stationary points L*.
Finally, to close the system, we need to relate 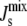 to the lipid concentration. Our underlying, linear assumption is that
to the lipid concentration. Our underlying, linear assumption is that 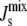 is well approximated by the weighted average of the spontaneous curvatures of the individual lipids:
is well approximated by the weighted average of the spontaneous curvatures of the individual lipids:
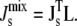 |
(2) |
This linear assumption has been shown experimentally to lead to an accurate approximation of the phase behavior of lipid mixtures (37). This linear assumption is also used in many of the experiments that measure the Js of neutral and anionic lipids (38–41). Using Eq. 2 with the Js values and experimental lipid composition Lexp in Table 1 leads to a calculated 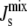 of −1/7.9 nm−1, which lies within the measured range from −1/6.6 nm−1 to −1/8.1 nm−1 of A. laidlawii lipid extracts.
of −1/7.9 nm−1, which lies within the measured range from −1/6.6 nm−1 to −1/8.1 nm−1 of A. laidlawii lipid extracts.
Functional form of the lipid biosynthetic enzyme kinetic rates, v(L)
Before considering the biophysical mechanism that couples the biochemical reactions to the mechanical properties of the membrane, we state first some specific features of the enzyme kinetic rate equations of the membrane lipid network. The functional form of the rate equations v(L) in the model differs from standard enzyme kinetics (42) in two respects. First, our cellular model must take into account the number of copies of the enzyme in the cell. Second, we must account for the fact that lipid biosynthetic enzymes have soluble, cytoplasmic reactants that diffuse in three dimensions, whereas their lipid reactants diffuse within the two-dimensional membrane.
Kinetic studies (27–29) have fitted the rates of A. laidlawii lipid biosynthetic enzymes to surface-dilution kinetics, in which soluble reactants have a bulk concentration in units of molarity and membrane reactants have a surface concentration in (dimensionless) molar fraction (43). All of the enzymatic reactions in the network, except for the reaction catalyzed by CDS, can be assumed to be irreversible. There is experimental evidence that supports this assumption, e.g., the hydrolyses of the phosphoanhydride bonds in PA and PGP are irreversible (44). Therefore, the rate equations for vPGPS, vPGPP, vPAP, vMGS, and vDGS are of the form
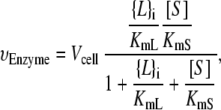 |
(3) |
where {L}i is the surface concentration of the lipid substrate (in molar fraction) and [S] is the bulk concentration of the soluble substrate (in units of molarity). Similarly, KmL is the Michaelis constant of {L}i (in molar fraction) and KmS is the Michaelis constant of [S] (in units of molarity). Experimental values of the enzyme kinetic constants are listed in Appendix B.
Note that Vcell is the rate for all copies of the enzyme in the cell (in units of molar fraction/min):
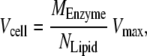 |
(4) |
where MEnzyme is the total mass of each enzyme in the cell and NLipid is the number of moles of lipid in the cell. Vmax is the standard Michaelis-Menten limiting rate, which typically has units of moles of product synthesized per milligrams of enzyme per minute (42). It is assumed that the ratio MEnzyme/NLipid is kept constant in a growing cell over the cell cycle. In Appendix B we show how we have estimated these parameters.
Two of the enzymatic reactions have slightly different functional forms. The final reaction of the lower path, catalyzed by GPDGS, although irreversible, involves two lipid substrates. As mentioned above, the CDS reaction is modeled reversibly since the equilibrium constant is much less than 1 (23). The rate equations of these reactions are listed in Appendix B.
Spontaneous-curvature-sensitive enzymes
We now introduce the terms in the model that describe how the activity of an enzyme with an amphipathic α-helix is modulated as a function of spontaneous curvature, which is in turn a function of the lipid composition. As mentioned above, there is extensive evidence that supports the theory that the activity and function of many proteins, both integral and peripheral, are regulated by the biophysical properties of biological membranes (10,35). Such phenomena differ markedly from specific protein-lipid interactions. Although our model deals with the binding of an amphipathic α-helix to the membrane, the mechanism could be extended to describe the binding of other amphipathic motifs to the membrane.
Enzyme kinetic studies have shown that lipids with large negative Js increase the activity of both MGS (29) and DGS (32). MGS has an amphipathic α-helix between residues 67 and 85 (30,45), that shares 5 of its first 8 residues with an α-helix of the E. coli division-site-selection protein MinD (46) that targets heterologous proteins to the membrane (47). Since it has been shown that MGS (19,29), the MGS amphipathic α-helix (20), and the MinD amphipathic α-helix (48) all preferentially bind to membranes with large negative 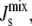 we hypothesize that the curvature-sensitive activity of MGS is a result of the membrane binding of this α-helix.
we hypothesize that the curvature-sensitive activity of MGS is a result of the membrane binding of this α-helix.
Through surface plasmon resonance (SPR) experiments, it has been concluded that liposomes bind to MGS through a two-step process (19). The first binding step is independent of lipid composition and has a dissociation constant of ∼10 nM. The second binding step has a large dependence on lipid composition, as its dissociation constant decreases from 10 mM to 100 nM when the liposomes have large negative 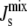 (19). Since liposomes with large
(19). Since liposomes with large 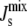 increase both the activity of MGS and the strength of the second binding step, it follows that MGS is only active after the second binding step.
increase both the activity of MGS and the strength of the second binding step, it follows that MGS is only active after the second binding step.
Amphipathic peptides form random coils in solution. The first binding step corresponds to surface adhesion induced by the electrostatic attraction of exposed basic residues to acidic membrane lipids. The second, subsequent step is the insertion of the hydrophobic residues into the membrane coupled with the emergence of the α-helix, which is entropically favored by the hydrophobic membrane environment. This two-step membrane binding (49) can be summarized through a simple kinetic mechanism,
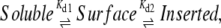 |
where Kd1 and Kd2 are the dissociation constants of the binding steps. At steady state, the fraction of membrane-inserted amphipathic α-helices that result in active enzymes is given by the association constant
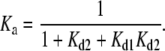 |
(5) |
We now derive expressions for Kd1 and Kd2 from biophysical considerations.
First binding step, Kd1
From SPR studies, Kd1 is measured to be ∼10 nM (19). It is proposed that this first (irreversible) binding is a result of electrostatic attraction. Structurally, the presence of eight positively charged residues on the 19-residue amphipathic α-helix (see Fig. 4 B) will produce a strong electrostatic attraction. Indeed, there is ample evidence that negatively charged anionic lipids are essential for the binding and activity of MGS. For instance, it is known that shielding the anionic lipids with 0.75 M NaCl prevents the binding of MGS (19).
FIGURE 4.
(A) Histogram of the distribution PMGS(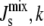 ) of the steady-state
) of the steady-state 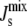 for a sampling of 106 random parameter sets, where Vcell,MGS is fixed and the other six Vcell,Enzyme values are varied. k is defined in Eq. 10 and measures the logarithmic variation of the parameter set. The individual Vcell,Enzyme distribution used (solid, top inset) ensures that the logarithmic variation k is sampled uniformly (solid, bottom inset). If uniform individual Vcell,Enzyme distributions were used (dashed, top inset), then k would approach a Gaussian distribution (dashed, bottom inset). (B) Marginal probability distributions PEnzyme(
for a sampling of 106 random parameter sets, where Vcell,MGS is fixed and the other six Vcell,Enzyme values are varied. k is defined in Eq. 10 and measures the logarithmic variation of the parameter set. The individual Vcell,Enzyme distribution used (solid, top inset) ensures that the logarithmic variation k is sampled uniformly (solid, bottom inset). If uniform individual Vcell,Enzyme distributions were used (dashed, top inset), then k would approach a Gaussian distribution (dashed, bottom inset). (B) Marginal probability distributions PEnzyme(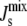 ) of all seven enzymes, where one Vcell,Enzyme is fixed, whereas the other six Vcell,Enzyme are varied. The dashed vertical line indicates the critical value
) of all seven enzymes, where one Vcell,Enzyme is fixed, whereas the other six Vcell,Enzyme are varied. The dashed vertical line indicates the critical value 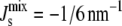 below which bilayers do not form. (C) Cumulative probability that the steady state will be viable (
below which bilayers do not form. (C) Cumulative probability that the steady state will be viable (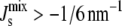 ) against the logarithmic variation k of the state.
) against the logarithmic variation k of the state.
From simple electrostatic considerations, Kd1 is given by the Boltzmann relation,
 |
(6) |
where zpe= +8e is the net charge of the amphipathic α-helix and ψ0 is the membrane surface potential. A dissociation constant of 10 nM would imply ψ0 ≅ −60 mV at 40°C, which is comparable to the measured membrane surface potentials of bacterial lipid bilayers (50). This simple estimate reinforces the plausibility of the interpretation of the first binding step in terms of electrostatic interactions. Clearly, the biophysical picture will be complex, including the shielding of charges on the peptide to give an effective valence (51) and the likely involvement of other positively charged enzyme domains.
Second binding step, Kd2
The second binding step involves at least three energetic processes: membrane insertion of the hydrophobic residues; peptide folding to form the α-helix; and lipids bending to accommodate the inserted α-helix. It has been observed that Kd2 decreases dramatically along the lipid sequence dioleoylphosphatidylglycerol (DOPG) > cardiolipin (CL) > dioleoylphosphatidylethanolamine (DOPE) > dioleoylglycerol (DOG) (19), i.e., as Js becomes more negative (39,52). This is the basis for our assumption that the second binding step is dominated by the energy of lipids bending to accommodate the helix.
We can understand this process through the following simplified biophysical picture. Consider a locally flat bilayer with average monolayer spontaneous curvature 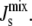 The diameter of the α-helix is comparable to that of a lipid. Consequently, the insertion of an amphipathic α-helix into a flat membrane does not result in a change of the monolayer curvature, yet it leads to a change in the molecular shape of the lipids alongside the α-helix (12) (Fig. 3 A). This would translate into a monolayer curvature, cbound ≠ 0, for a monolayer formed entirely by lipids like those surrounding the amphipathic α-helix.
The diameter of the α-helix is comparable to that of a lipid. Consequently, the insertion of an amphipathic α-helix into a flat membrane does not result in a change of the monolayer curvature, yet it leads to a change in the molecular shape of the lipids alongside the α-helix (12) (Fig. 3 A). This would translate into a monolayer curvature, cbound ≠ 0, for a monolayer formed entirely by lipids like those surrounding the amphipathic α-helix.
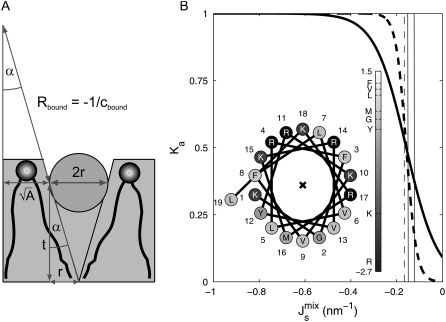
FIGURE 3.
(A) Geometric argument used to calculate cbound, the curvature of a lipid monolayer consisting entirely of lipids that lie alongside an amphipathic α-helix. (B) Association constant Ka as a function of 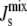 for a 19-residue α-helix (dark solid line) and, for a 58-residue α-helix, such as that of CCT, plotted for comparison (dark dashed line). The dashed vertical line is Js = −1/6 nm−1 and the solid vertical lines give the measured range of
for a 19-residue α-helix (dark solid line) and, for a 58-residue α-helix, such as that of CCT, plotted for comparison (dark dashed line). The dashed vertical line is Js = −1/6 nm−1 and the solid vertical lines give the measured range of 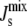 of lipid extracts (5). (Inset) Helical-wheel projection of residues 67–85 of MGS. The bar gives the Eisenberg consensus normalized hydrophobicity scale (76).
of lipid extracts (5). (Inset) Helical-wheel projection of residues 67–85 of MGS. The bar gives the Eisenberg consensus normalized hydrophobicity scale (76).
Fig. 3 A sketches a very simple geometrical argument to obtain a first-order estimate of cbound:
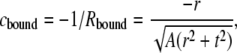 |
(7) |
where r = 0.45 nm is the radius of the α-helix (53); t is the monolayer thickness (the distance between the middle of the α-helix and the bilayer midpoint), which is measured to be 1.71 nm (53); and A is the average interfacial surface area of the A. laidlawii lipids, which is measured to be 0.65 nm2 (5). A is assumed to be square and the pivotal plane is assumed to coincide with the middle of the α-helix. Equation 7 gives an estimated cbound ≈ −1/3.2 nm−1, which is significantly nonflat.
The cylindrical deformation of the α-helix ensures that one of the principal curvatures is zero, c2 = 0. Therefore, the change in the stored elastic energy in Eq. 1 due to the bending of the lipids alongside the amphipathic α-helix is
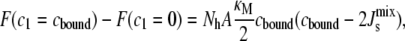 |
(8) |
where κM is the bending rigidity of lipids, which we take to be 10 kBT (38), and Nh = 7.1 is the number of lipids that adopt curvature cbound along both sides of the amphipathic α-helix. Nh is calculated for a 19-residue α-helix of length 2.85 nm with 3.5 lipids of length 0.651/2 nm along each side of its long axis. For the range of Js values in Table 1, the free energy is between −1.7 kBT/lipid and 0.2 kBT/lipid. These energies are not large enough to cause the lateral sequestration of lipids around the α-helix (54), thus justifying the use of 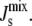
Equation 9 provides an estimate for the binding energy if we assume that the main energetic contribution to this process comes from lipid bending. The dissociation constant of the second binding step would then be given by the Boltzmann relation:
 |
(9) |
Note that for the range of Js values in Table 1, the modeled KD2 ranges between 4 M and 5 μM. The difference with the experimental values of KD2 may be explained by the enhanced electrostatic attraction due to the absence of divalent cations and the use of zwitterionic lipids in the SPR experiments (19,20). Note that when 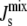 = cbound/2 = −1/6.3 nm−1, the binding energy is zero and MGS is equally likely to be bound or unbound. Reassuringly, this bound-to-unbound transition is centered at a value of
= cbound/2 = −1/6.3 nm−1, the binding energy is zero and MGS is equally likely to be bound or unbound. Reassuringly, this bound-to-unbound transition is centered at a value of 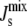 that lies between the formation of nonbilayer structures and the lower bound of the experimental curvature of lipid extracts in A. laidlawii: −1/6 < cbound/2 < −1/6.6 (Table 1).
that lies between the formation of nonbilayer structures and the lower bound of the experimental curvature of lipid extracts in A. laidlawii: −1/6 < cbound/2 < −1/6.6 (Table 1).
Equations 6 and 9 provide the biophysical feedback for the system in Eq. 1, as the association constant Ka,MGS multiplies the rate vMGS. Given the individual lipid spontaneous curvatures Js in the system (Table 1), and assuming a constant Kd1 = 10 nM, the association constant is constrained to be in the range 1 ≥ Ka,MGS ≥ 0.23 (Fig. 3 B), and the 19-residue α-helix provides a fourfold regulation of MGS activity. At large negative 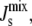 almost all MGS is active and the synthesis of MGlcDAG increases
almost all MGS is active and the synthesis of MGlcDAG increases  The opposite effect is produced when
The opposite effect is produced when 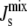 is less negative. Clearly, a longer amphipathic α-helix would produce significantly stronger regulation of activity (Fig. 3 B).
is less negative. Clearly, a longer amphipathic α-helix would produce significantly stronger regulation of activity (Fig. 3 B).
Parameter estimation for the model
The time evolution of the system in Eq. 1 and its corresponding stationary point depend on the model parameters. Most of these parameters have been collected from an extensive survey of the literature, or have been estimated or measured directly. As is usual in the literature, some of the parameters carry substantial uncertainty. In addition, there is an absence of kinetic parameters for some of the enzymes of the A. laidlawii lipid biosynthetic network.
To complete our parametric description, we carry out a constrained nonlinear parameter estimation in which we search for the positive parameter set p that reproduces the experimentally observed lipid concentrations Lexp as close as possible while minimizing the distance to the reliable literature values. Our method of choice to solve this constrained optimization is the Stochastic Ranking Evolutionary Strategy (SRES) (55), an evolutionary strategy with stochastic ranking, which has been shown in a recent survey (56) to be successful in finding feasible parameters in nonlinear biochemical pathways.
For such an underdetermined system, a multiobjective optimization is pursued. The primary objective is to minimize the difference between L*(p), the stationary point of the model in Eq. 1 for the parameter set p, and the experimentally observed lipid composition Lexp given in Table 1,
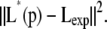 |
The secondary objective is to minimize the difference between the estimated parameter set p and the literature parameters plit. Instead of minimizing the Euclidean norm ‖p − plit‖, in our case we minimize a more appropriate measure of the relative distance between the parameter sets, previously introduced to quantify the robustness of dynamical systems (57),
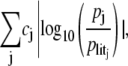 |
i.e., the sum of the absolute logarithmic errors between p and plit weighted by cj, the confidence in the jth literature parameter. In particular, parameters obtained from A. laidlawii experiments have been assigned cj = 1, whereas cj = 1/2 for parameters taken from experiments on other organisms, such as E. coli. As a check that the combination of our model and this multiobjective estimation procedure produces plausible parameter sets, we have verified that we can obtain parameters for which the model reproduces all the different lipid compositions that have been observed experimentally (5).
There are a total of 25 kinetic parameters in the model, of which only 18 have literature values. We run our multiobjective optimization by varying 15 parameters (the 7 unknown and 8 parameters with uncertain literature values) and obtain the estimated parameter set p0 presented in Table 2. The distance between each estimated parameter and its literature value has been constrained to be at most two orders of magnitude. The estimated parameter set reflects the sensitivity of the steady state to particular parameters, specifically those appearing in the numerator of the rate equations: Vcell,Enzyme and the soluble substrate concentrations. This emphasizes the importance of measuring intracellular metabolite concentrations.
TABLE 2.
Model parameters for the reference system
| Parameter | Literature (plit) | Estimated (p0) |
|---|---|---|
| V+,PA, min−1 | 8.2 × 10−3 | |
| Vcell,CDS, min−1† | 9.6 × 10−3 | |
| Vcell,PGPS, min−1† | 4.7 × 10−3 | |
| Vcell,PGPP, min−1 | * | 3.2 |
| Vcell,PAP, min−1 | 4.1 × 10−2 | 7.7 × 10−2 |
| Vcell,MGS, min−1 | 1.8 × 10−3 | 1.4 × 10−2 |
| Vcell,DGS, min−1 | 3.5 × 10−4 | 1.2 × 10−3 |
| Vcell,GPDGS, min−1 | * | 1.5 × 10−2 |
| CDS KM,PA, mol % | * | 9.3 × 10−2 |
| PGPS KM,CDPDAG, mol % | * | 1.0 × 10−2 |
| PGPP KM,PGP, mol % | * | 38.2 |
| PAP KM,PGP, mol % | 10 | |
| MGS KM,DAG, mol % | 6 | 5.2 |
| DGS KM,MGlcDAG, mol % | 1 | |
| GPDGS KM,PG, mol % | * | 25 |
| GPDGS KM,DGlcDAG, mol % | * | 85 |
| CDS KM,CTP, mM† | 0.58 | |
| PGPS KM,G3P, mM† | 0.32 | |
| MGS KM,UDP-Glc, mM | 0.4 | |
| DGS KM,UDP-Glc, mM | 0.14 | |
| CDS Keqm | 0.22 | |
| CTP, mM | 0.5 | |
| UDP-Glc, mM† | 0.4 | 2.5 |
| PPi, mM† | 1 | |
| G3P, mM† | 0.2 | 0.32 |
Parameters are obtained through a multiobjective optimization using the evolutionary algorithm SRES (55). Parameters marked * have not been measured. Parameters marked † correspond to E. coli and are given less weight in the optimization. See Appendix B for references and details of the literature parameters.
Our model in Eq. 1 with the estimated parameter set p0 in Table 2 will be our reference system henceforth. This system has a stationary point at the experimental lipid composition shown in Table 1, with 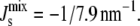 In the next section, we explore the effect of the biophysical regulation mechanism introduced above.
In the next section, we explore the effect of the biophysical regulation mechanism introduced above.
RESULTS
We now investigate the behavior of the cellular model of lipid biosynthesis through the numerical integration of Eq. 1 under a variety of conditions. The first robust feature of the model is that it evolves to a steady state that is independent of the initial condition. Although we have not proved global stability explicitly, numerical integrations from more than 105 randomly generated initial conditions all converge to the same stationary lipid composition. This is strong evidence that the steady state is globally attracting. Our numerical investigation of the model therefore translates into an evaluation of how the fixed point L*(p) changes in response to variations in the parameters or in the presence of feedback.
Which enzyme rates most affect 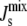 ?
?
Experiments indicating that the activities of both MGS and DGS are curvature-sensitive (28,29) have led to the hypothesis that these two enzymes are responsible for the control of 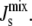 If this is true, the rates of MGS and DGS must have a large effect on the steady-state
If this is true, the rates of MGS and DGS must have a large effect on the steady-state 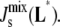 In this section, we use our model to determine which enzymes of the A. laidlawii lipid biosynthetic network have the largest effect on
In this section, we use our model to determine which enzymes of the A. laidlawii lipid biosynthetic network have the largest effect on 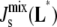 in the absence of feedback. This is an initial step before we introduce the biophysical feedback explicitly in the next section, i.e., in this section the matrix Ka does not depend on the curvature
in the absence of feedback. This is an initial step before we introduce the biophysical feedback explicitly in the next section, i.e., in this section the matrix Ka does not depend on the curvature 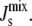
This question can be posed in the well-known framework of sensitivity analysis, which has been used to characterize, e.g., the robustness of bacterial chemotaxis networks (57). For the reference parameters p0 (Table 2), the model evolves to the experimental lipid composition Lexp (Table 1), i.e., L*(p0) = Lexp. However, the parameters p are inherently noisy. Specifically, the seven Vcell,Enzyme have the most influence on the steady state while at the same time having large variability. Therefore, our sensitivity analysis investigates how changes in the different Vcell,Enzyme translate into variations of the steady-state 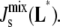
The sensitivity analysis is performed through Monte Carlo sampling, one enzyme at a time. The Vcell,Enzyme of the enzyme under study is fixed at the reference value in Table 2. We then produce 106 parameter sets where the other six Vcell,Enzyme are drawn from a random distribution conditioned to produce uniform sampling (over the interval [0,6]) of the logarithmic variation of the parameter set, k:
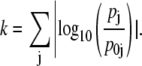 |
(10) |
Clearly, this implies that the individual Vcell,Enzyme parameters are not sampled uniformly (see the inset of Fig. 4 A). Effectively, our sensitivity analysis considers variations of up to almost two orders of magnitude in each of the six Vcell,Enzyme, and an overall uniform variation of six orders of magnitude for the complete parameter set. The fixed point for each of the 106 parameter sets is obtained and the corresponding 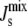 is calculated.
is calculated.
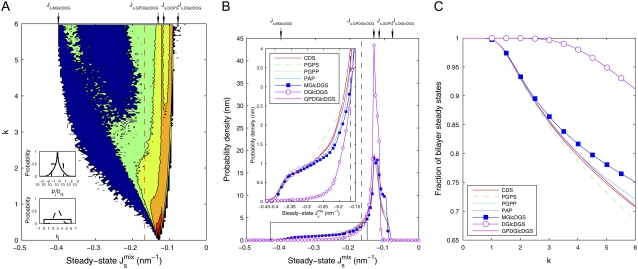
Fig. 4 A shows the results for the enzyme MGS as a two-dimensional histogram of PMGS(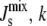 ), the distribution of the 106 random parameter sets. As expected, the distribution is centered on the reference value of
), the distribution of the 106 random parameter sets. As expected, the distribution is centered on the reference value of 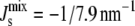 and becomes broader as the variation of the parameter set, k, grows. Since biophysical experiments show that membranes with
and becomes broader as the variation of the parameter set, k, grows. Since biophysical experiments show that membranes with 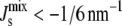 do not form bilayers, we can consider such compositions as biologically nonviable (17). This is marked as a dashed line in Fig. 4 A. Therefore, the distribution P(
do not form bilayers, we can consider such compositions as biologically nonviable (17). This is marked as a dashed line in Fig. 4 A. Therefore, the distribution P(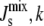 ), or its marginal P(
), or its marginal P(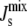 ), quantifies how likely it is for the system to evolve to a nonbilayer state when a particular enzyme is kept fixed at its reference value and all other enzymes have uncertain Vcell,Enzyme values. Essentially, this is also a measure of the relevance of the particular enzyme for the controllability of the system, as it quantifies the variability of the output when a given parameter is kept tightly controlled.
), quantifies how likely it is for the system to evolve to a nonbilayer state when a particular enzyme is kept fixed at its reference value and all other enzymes have uncertain Vcell,Enzyme values. Essentially, this is also a measure of the relevance of the particular enzyme for the controllability of the system, as it quantifies the variability of the output when a given parameter is kept tightly controlled.
The complete results for the system are summarized in Fig. 4 B, where we plot the marginal distributions PEnzyme(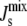 ) for the seven enzymes. All the distributions are centered on the reference
) for the seven enzymes. All the distributions are centered on the reference 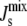 value of −1/7.9 nm−1; however, the variance and the tails of the distributions differ. Specifically, DGS and MGS (marked with symbols in Fig. 4 B) have much smaller variances and sharper decay tails than the other five enzymes. The left tail of the distribution is relevant as it gives the proportion of biologically inviable stationary states that do not form bilayers. Fig. 4 C shows the fraction of viable steady-state lipid compositions as a function of the variability k. The results clearly show that keeping the rate of DGS fixed to its reference value is the most efficient way of guaranteeing a viable system.
value of −1/7.9 nm−1; however, the variance and the tails of the distributions differ. Specifically, DGS and MGS (marked with symbols in Fig. 4 B) have much smaller variances and sharper decay tails than the other five enzymes. The left tail of the distribution is relevant as it gives the proportion of biologically inviable stationary states that do not form bilayers. Fig. 4 C shows the fraction of viable steady-state lipid compositions as a function of the variability k. The results clearly show that keeping the rate of DGS fixed to its reference value is the most efficient way of guaranteeing a viable system.
The reason for this sensitivity is clear if we examine the network in Fig. 2 B. The enzyme DGS catalyzes the synthesis of DGlcDAG, with a small negative Js, from MGlcDAG, with a large negative Js. Since the membrane binding of amphipathic α-helices is increased by lipids with large negative Js, this provides a direct link with the biophysical feedback mechanism described above. The implication is that enzymes that catalyze reactions that result in a large positive change in 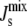 (i.e., DGS and to a lesser extent MGS) are strong candidates to exert the biophysical feedback control of membrane curvature, in accordance with kinetic data. In the next section, the effect of the biophysical feedback provided by the amphipathic motifs is investigated in detail.
(i.e., DGS and to a lesser extent MGS) are strong candidates to exert the biophysical feedback control of membrane curvature, in accordance with kinetic data. In the next section, the effect of the biophysical feedback provided by the amphipathic motifs is investigated in detail.
Effect of the biophysical feedback on 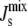
We now study the effect of the biophysical feedback, mediated by amphipathic α-helices, on the control of the steady-state 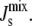 Our model encodes this mechanism through the association constants Ka,MGS and Ka,DGS collected in the matrix
Our model encodes this mechanism through the association constants Ka,MGS and Ka,DGS collected in the matrix 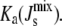
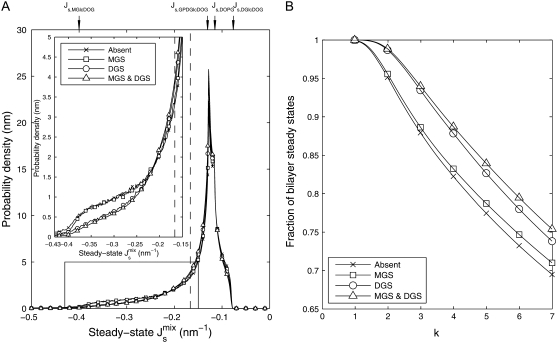
A sensitivity analysis similar to that performed in the preceding section is carried out to measure how much the variability of the system is reduced in the presence of feedback. We draw 106 parameter sets from a random distribution of Vcell,Enzyme such that the logarithmic variation k, defined in Eq. 10, is uniform over the interval [0,7]. Fig. 5 A shows four marginal distributions of the steady-state 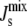 of the system without feedback and with different combinations of feedback on MGS and DGS. In particular, we model MGS to have a 19-residue amphipathic α-helix (see Fig. 3 B) and we hypothesize that a similar α-helix is responsible for the curvature-sensitive activity of DGS, as suggested by secondary-structure predictions (30). The numerics show that the effect of feedback is noticeable in the reduction of the left tail of the distribution. This means that inviable, nonbilayer steady states are less probable when feedback is present. This is especially prominent for DGS, although MGS also contributes to the control of
of the system without feedback and with different combinations of feedback on MGS and DGS. In particular, we model MGS to have a 19-residue amphipathic α-helix (see Fig. 3 B) and we hypothesize that a similar α-helix is responsible for the curvature-sensitive activity of DGS, as suggested by secondary-structure predictions (30). The numerics show that the effect of feedback is noticeable in the reduction of the left tail of the distribution. This means that inviable, nonbilayer steady states are less probable when feedback is present. This is especially prominent for DGS, although MGS also contributes to the control of 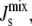 as shown in Fig. 5 B. The combined feedback of MGS and DGS reduces the fraction of inviable oleoyl acyl lipid compositions by 19%.
as shown in Fig. 5 B. The combined feedback of MGS and DGS reduces the fraction of inviable oleoyl acyl lipid compositions by 19%.
FIGURE 5.
(A) Effect of the biophysical feedback on the control of the steady-state 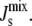 The four marginal distributions P(
The four marginal distributions P(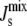 ), obtained from 106 random parameter sets in which all seven enzyme rates Vcell,Enzyme are varied, show a reduction of the probability of inviable states with
), obtained from 106 random parameter sets in which all seven enzyme rates Vcell,Enzyme are varied, show a reduction of the probability of inviable states with 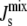 < −1/6 nm−1. In the absence of feedback, we fix Ka,MGS at the reference value of Ka,MGS (
< −1/6 nm−1. In the absence of feedback, we fix Ka,MGS at the reference value of Ka,MGS (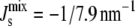 ) shown in Fig. 3 B (×). MGS is modeled to have a 19-residue α-helix (□), DGS is modeled with a 19-residue α-helix (○), both MGS and DGS are modeled with 19-residue α-helices (▵). (B) The cumulative probability that the steady state forms a bilayer (
) shown in Fig. 3 B (×). MGS is modeled to have a 19-residue α-helix (□), DGS is modeled with a 19-residue α-helix (○), both MGS and DGS are modeled with 19-residue α-helices (▵). (B) The cumulative probability that the steady state forms a bilayer (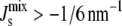 ) as a function of the logarithmic variation k.
) as a function of the logarithmic variation k.
The robustness of the lipid compositions
A central feature of the biophysical feedback mechanism is the fact that the enzymatic network controls the physical property 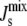 and not the steady-state lipid concentrations L*. However,
and not the steady-state lipid concentrations L*. However, 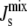 is a function of the lipid concentrations, and it is important to study the underlying variability of L*, with respect to the reference lipid composition Lexp, when the parameters of the model are uncertain. This point can be illustrated with the data obtained in the preceding section through our sensitivity analysis. Fig. 6 shows the probability distribution of steady-state compositions L* as a function of
is a function of the lipid concentrations, and it is important to study the underlying variability of L*, with respect to the reference lipid composition Lexp, when the parameters of the model are uncertain. This point can be illustrated with the data obtained in the preceding section through our sensitivity analysis. Fig. 6 shows the probability distribution of steady-state compositions L* as a function of 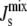 and ‖L* − Lexp‖1, the distance to the experimental lipid composition, in the absence and in the presence of feedback.
and ‖L* − Lexp‖1, the distance to the experimental lipid composition, in the absence and in the presence of feedback.
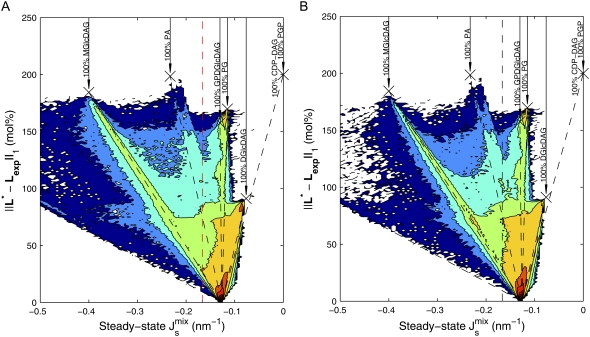
FIGURE 6.
Histogram of the distribution of steady-states L*, showing the probability distribution of 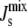 and the distance of L* to the experimental concentration Lexp. The data are obtained by sampling 106 random parameter sets as in Fig. 5. (A) Distribution in the absence of feedback. The vertical dashed line at
and the distance of L* to the experimental concentration Lexp. The data are obtained by sampling 106 random parameter sets as in Fig. 5. (A) Distribution in the absence of feedback. The vertical dashed line at 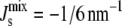 separates the nonbilayer and bilayer fractions. The dashed lines correspond to the steady-state
separates the nonbilayer and bilayer fractions. The dashed lines correspond to the steady-state 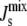 that results from adding a particular lipid to Lexp until one reaches a monocomponent state, marked by crosses. (B) Distribution when both MGS and DGS exert biophysical feedback through a 19-residue amphipathic α-helix.
that results from adding a particular lipid to Lexp until one reaches a monocomponent state, marked by crosses. (B) Distribution when both MGS and DGS exert biophysical feedback through a 19-residue amphipathic α-helix.
In the absence of feedback (Fig. 6 A), the data show that a majority of L* are close to Lexp, but a range of lipid mixtures is allowed by the system. Note that the system does not by default evolve toward pure, monocomponent compositions. In addition, the L* in the biologically viable region (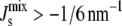 ) consist mostly of mixtures of PG, DGlcDAG, and GPDGlcDAG, and not of the intermediates PA, CDP-DAG, or PGP. On the other hand, the inviable L*, with
) consist mostly of mixtures of PG, DGlcDAG, and GPDGlcDAG, and not of the intermediates PA, CDP-DAG, or PGP. On the other hand, the inviable L*, with 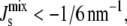 appear through the increase in MGlcDAG, and not of DAG. These trends stem from the constraints that the network structure imposes on the control mechanism and highlight how the steady-state
appear through the increase in MGlcDAG, and not of DAG. These trends stem from the constraints that the network structure imposes on the control mechanism and highlight how the steady-state 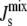 is bounded by the simplex of the Js values of the individual lipids.
is bounded by the simplex of the Js values of the individual lipids.
Fig. 6 B shows that the most notable effect of the feedback is to reduce the likelihood of inviable states with values of 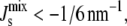 specifically those with high concentrations of MGlcDAG. In addition, our numerical results clearly indicate that the control is not achieved by targeting specific (possibly monocomponent) compositions, as the overall shape of the probability distribution remains broadly unchanged and mixed states are the norm. In fact, the proportion of monocomponent states is reduced when the feedback is on. Another effect of the feedback is the increase of the probability of states with
specifically those with high concentrations of MGlcDAG. In addition, our numerical results clearly indicate that the control is not achieved by targeting specific (possibly monocomponent) compositions, as the overall shape of the probability distribution remains broadly unchanged and mixed states are the norm. In fact, the proportion of monocomponent states is reduced when the feedback is on. Another effect of the feedback is the increase of the probability of states with 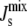 close to the boundary between bilayer and nonbilayer states. This follows from the functional form of the feedback that is centered on the value cbound/2 = −1/6.3 nm−1. This is clearly observable in Fig. 6. The implication is that lipid compositions with large negative
close to the boundary between bilayer and nonbilayer states. This follows from the functional form of the feedback that is centered on the value cbound/2 = −1/6.3 nm−1. This is clearly observable in Fig. 6. The implication is that lipid compositions with large negative 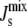 are more improbable, but at the same time there is an increase in lipid compositions with
are more improbable, but at the same time there is an increase in lipid compositions with 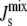 close to the bilayer to nonbilayer transition.
close to the bilayer to nonbilayer transition.
The effect of the length of the amphipathic α-helices
As seen in Fig. 3 B, the proposed biophysical feedback exerted by a 19-residue amphipathic α-helix gives rise to a fourfold regulation, which results in a modest reduction of the likelihood of inviable states. The functional form of our feedback implies that the magnitude of the feedback depends strongly on the length of the amphipathic α-helix. We now investigate how the length and number of amphipathic α-helices on MGS and DGS affect 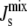 regulation.
regulation.
Biological evidence indicates that the size of amphipathic motifs should be studied parametrically as it is an important feature in protein-membrane interactions. Secondary-structure predictions suggest that both MGS and DGS have multiple α-helices that may insert into the membrane (20,30). However, it is difficult to identify amphipathic α-helices and determine their length from genetic sequences. It is also important to mention that some enzymes with amphipathic α-helices can act in concert. For instance, there is evidence that CCT acts as a dimer that is only active when the 58-residue amphipathic α-helices of both monomers are bound to the membrane (58). In a simplified picture, this dimer would be viewed as having an effective amphipathic α-helix of 116 residues.
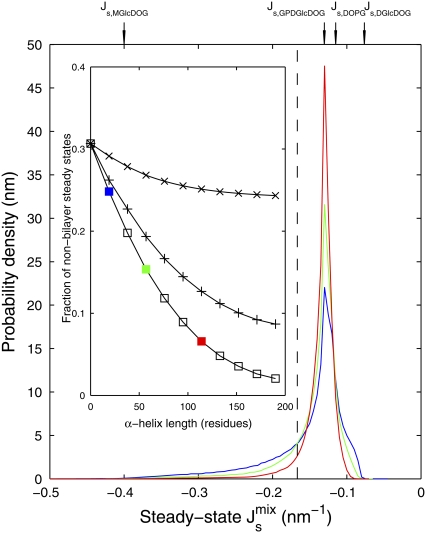
Fig. 7 plots the dependence on the length of the amphipathic α-helices of the marginal distribution P(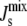 ) in Fig. 5 A, where both MGS and DGS are curvature sensitive. Clearly, as the length of the α-helices is increased, the distribution becomes sharper and the likelihood of getting inviable states is reduced. As the inset of Fig. 7 shows, the reduction in the proportion of nonbilayer states increases to more than 50% when both MGS and DGS have 60-residue α-helices.
) in Fig. 5 A, where both MGS and DGS are curvature sensitive. Clearly, as the length of the α-helices is increased, the distribution becomes sharper and the likelihood of getting inviable states is reduced. As the inset of Fig. 7 shows, the reduction in the proportion of nonbilayer states increases to more than 50% when both MGS and DGS have 60-residue α-helices.
FIGURE 7.
Effect of amphipathic α-helix length on steady-state 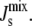 Shown are three marginal distributions P(
Shown are three marginal distributions P(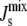 ) of the steady-state
) of the steady-state 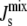 under uncertain parameters where both MGS and DGS are curvature sensitive with amphipathic α-helices of different lengths: 19-residue (blue, same as Fig. 5 A), 57-residue (green), and 114-residue (red). Vertical dashed line at
under uncertain parameters where both MGS and DGS are curvature sensitive with amphipathic α-helices of different lengths: 19-residue (blue, same as Fig. 5 A), 57-residue (green), and 114-residue (red). Vertical dashed line at 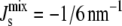 indicates the nonbilayer region. (Inset) Probability that the steady state does not form a bilayer against α-helix length, when MGS has an α-helix (×); DGS has an α-helix (+); and MGS and DGS both have α-helices (□). Colored squares correspond to the three plotted P(
indicates the nonbilayer region. (Inset) Probability that the steady state does not form a bilayer against α-helix length, when MGS has an α-helix (×); DGS has an α-helix (+); and MGS and DGS both have α-helices (□). Colored squares correspond to the three plotted P(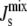 ) in the main figure.
) in the main figure.
DISCUSSION
This article outlines a bottom-up modeling framework that couples a biochemical network with an intrinsic biophysical feedback mechanism. The central idea behind the biophysical regulation is that the activity of certain enzymes involved in lipid biosynthesis is dependent on the spontaneous curvature of the lipids, which is itself a function of the lipid composition. This introduces a feedback loop that can regulate 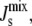 a property that must be kept within a narrow window to allow for cellular activity and survival (3,7).
a property that must be kept within a narrow window to allow for cellular activity and survival (3,7).
The numerical results of our model show that the system evolves toward biologically plausible mixtures of lipids. When the biophysical regulation is present, it decreases the likelihood of inviable steady-state lipid compositions that would not be expected to form a lamellar bilayer. Moreover, our sensitivity analysis indicates that two enzymes in the network (DGS and MGS) have the largest effect on the steady-state 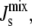 in agreement with kinetic data. This fact follows from both the intrinsic properties of the enzymatic reactions (the corresponding substrates have large negative Js and the reactions result in large positive changes in Js), and from their position in the lipid biosynthetic network. Therefore, the model underscores the possibility that a chemically nonspecific, biophysical mechanism can participate effectively in the regulation of
in agreement with kinetic data. This fact follows from both the intrinsic properties of the enzymatic reactions (the corresponding substrates have large negative Js and the reactions result in large positive changes in Js), and from their position in the lipid biosynthetic network. Therefore, the model underscores the possibility that a chemically nonspecific, biophysical mechanism can participate effectively in the regulation of 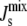 in the plasma membrane. Such a mechanism implies that the lipid biosynthetic enzymes regulate the concentration of individual lipids not only as a function of their own concentration but also as a result of larger scale, mechanical properties of the membrane.
in the plasma membrane. Such a mechanism implies that the lipid biosynthetic enzymes regulate the concentration of individual lipids not only as a function of their own concentration but also as a result of larger scale, mechanical properties of the membrane.
The sensitivity analysis used here brings to the fore the importance of characterizing parameter variability in biological systems. Indeed, the sensitivity of 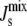 is dependent on how the parameters are varied. In the absence of additional knowledge, our approach has been to sample the Vcell,Enzyme parameter sets uniformly according to their relative logarithmic distance to the reference set, k, with a maximum variation for each individual parameter of ∼2 orders of magnitude. This leads to mixed steady-state lipid compositions localized mostly in the biologically viable region (Fig. 6). However, if the parameter variation is larger, our numerics indicate that monocomponent steady-state lipid compositions begin to appear. At present, it is difficult to infer from experimental results the range of Vcell,Enzyme a cell is likely to experience and is likely to be robust to. For example, cells that overexpress enzymes 100 times are often still viable; however, this is unlikely to result in a 100-fold increase in enzyme activity. Clearly, the shape and width of the parameter distributions are an essential part of a meaningful sensitivity analysis of biological systems, and further experimental characterization in this area is needed.
is dependent on how the parameters are varied. In the absence of additional knowledge, our approach has been to sample the Vcell,Enzyme parameter sets uniformly according to their relative logarithmic distance to the reference set, k, with a maximum variation for each individual parameter of ∼2 orders of magnitude. This leads to mixed steady-state lipid compositions localized mostly in the biologically viable region (Fig. 6). However, if the parameter variation is larger, our numerics indicate that monocomponent steady-state lipid compositions begin to appear. At present, it is difficult to infer from experimental results the range of Vcell,Enzyme a cell is likely to experience and is likely to be robust to. For example, cells that overexpress enzymes 100 times are often still viable; however, this is unlikely to result in a 100-fold increase in enzyme activity. Clearly, the shape and width of the parameter distributions are an essential part of a meaningful sensitivity analysis of biological systems, and further experimental characterization in this area is needed.
Our study also highlights the importance of the length of the amphipathic α-helix as it is related (linearly in our simple model) to the energy released when it is inserted into the membrane. This suggests that the membrane binding of short amphipathic α-helices, such as the eight-residue α-helix on E. coli MinD (46), is less sensitive to 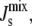 whereas the binding of long amphipathic α-helices is much more sensitive to
whereas the binding of long amphipathic α-helices is much more sensitive to 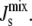 In addition, enzymes that act as oligomers provide more effective regulation of
In addition, enzymes that act as oligomers provide more effective regulation of 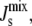 as seems to be the case with CCT. This aspect of the influence of α-helix length on the regulation of
as seems to be the case with CCT. This aspect of the influence of α-helix length on the regulation of 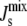 could be investigated experimentally by site-directed mutagenesis.
could be investigated experimentally by site-directed mutagenesis.
The proposed modeling framework could be extended in several directions. First, our model has been constructed considering amphipathic α-helices at its core, with an estimate for the binding energy based on a simple geometric calculation of the curvature around an α-helix. This very simplified picture could be generalized to include entropic contributions and the possibility of more general amphipathic motifs (10). Such an extension would be incorporated into the association constant that encodes the regulation. Second, our model excludes the monoacyl (MA)-derivatives of the glucolipids: MAMGlcDAG, which is rarely present, and MADGlcDAG and MABGPDGlcDAG, which are each only present at ∼10 mol % when the lipids have a palmitoleoyl fraction >30 mol % (5). The model could be extended to include the MA-derivatives, although this would require a more detailed understanding of the postulated biosynthetic pathways (59). However, based on the insight provided by the current model, it is unlikely that the enzymes involved in the synthesis of MA-derivatives would have their activity modulated by amphipathic α-helices, since synthesis of these lipids would lead to a more negative Js,mix.
Another extension would be to model the effect of lipid acyl chain length, which directly affects the Js of a lipid, on the lipid composition (5,18,60). In its present form, the model only considers lipids with oleoyl acyl chains. However, Fig. 6 illustrates how important the individual lipid spontaneous curvatures are in determining the steady-state 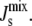 Experimentally, it is known that if A. laidlawii is fed shorter and saturated fatty acids (making the Js values in Table 1 more positive), the organism reacts by changing the proportion of lipid headgroups (5). The length and saturation of the lipid acyl chains have a nonlinear, but as yet unquantified, effect on the spontaneous curvature (39). The challenge is to model how the length and degree of unsaturation of the lipid acyl chains affect the lipid Js values and to relate this to lipid biosynthesis. Based on our results, the acyl chain length will undoubtedly affect the regulatory role of MGS and DGS. When the lipids have shorter, saturated acyl chains, MGlcDAG and DAG are present at a significantly higher molar fraction. Since MGS catalyzes the reaction between these two lipids, it is hypothesized that when the lipids have shorter, saturated acyl chains, MGS will play a larger regulatory role.
Experimentally, it is known that if A. laidlawii is fed shorter and saturated fatty acids (making the Js values in Table 1 more positive), the organism reacts by changing the proportion of lipid headgroups (5). The length and saturation of the lipid acyl chains have a nonlinear, but as yet unquantified, effect on the spontaneous curvature (39). The challenge is to model how the length and degree of unsaturation of the lipid acyl chains affect the lipid Js values and to relate this to lipid biosynthesis. Based on our results, the acyl chain length will undoubtedly affect the regulatory role of MGS and DGS. When the lipids have shorter, saturated acyl chains, MGlcDAG and DAG are present at a significantly higher molar fraction. Since MGS catalyzes the reaction between these two lipids, it is hypothesized that when the lipids have shorter, saturated acyl chains, MGS will play a larger regulatory role.
The model can be used to study lipid biosynthesis in other organisms, but it would have to be extended to deal with the particular biochemical and biophysical characteristics of each network. In particular, it is likely that more lipid species will be present, thus increasing the dimensionality of the model and leading to diverse control strategies in different organisms. For instance, E. coli is known to regulate 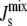 by changing the unsaturation of the lipid acyl chains (7). This results in a combinatorial increase in the number of chemical species and to a lesser extent the number of enzymes. In addition, a detailed understanding of the lipid-dependent activity of the enzymes that control the metabolism of lipid acyl chains would also be necessary. This does, however, highlight the importance of characterizing the biophysical properties of individual lipid types including the values of their monolayer spontaneous curvature. At present this is a relatively neglected area of research and this is a damaging oversight given the role of lipids in regulating key biological processes.
by changing the unsaturation of the lipid acyl chains (7). This results in a combinatorial increase in the number of chemical species and to a lesser extent the number of enzymes. In addition, a detailed understanding of the lipid-dependent activity of the enzymes that control the metabolism of lipid acyl chains would also be necessary. This does, however, highlight the importance of characterizing the biophysical properties of individual lipid types including the values of their monolayer spontaneous curvature. At present this is a relatively neglected area of research and this is a damaging oversight given the role of lipids in regulating key biological processes.
Finally, the connection of lipid biosynthesis to the cell cycle and the spatial inhomogeneity of lipids are two closely interconnected areas in which the modeling framework could be extended. Lipid biosynthesis is linked to the cell cycle by the need to double the lipid mass before cell division (61). Furthermore, experiments show that the anionic lipid cardiolipin (62) and peptides that contain amphipathic α-helices, such as MinD (63) and MGS (45), localize at the bacterial poles. This localization may act as a trigger for lipid biosynthesis and cell division. Experimental evidence also shows that bacterial membranes may exhibit transbilayer asymmetry (64) and may be divided into domains, for instance a septal and a polar region (62), which have vastly different lipid compositions and enzyme concentrations. In these cases, the modeling framework could be extended to model the lipid compositions in the different lipid domains and lipid monolayers separately.
Acknowledgments
The authors thank Åke Wieslander for providing invaluable expertise of the lipid biosynthesis of A. laidlawii. We also thank Robin Leatherbarrow for insightful discussion. We are grateful to Thomas P. Runarsson and Xin Yao for their SRES algorithm, which is available online.
APPENDIX A: LIPID COMPOSITIONS AND JS VALUES (TABLE 1)
The lipid composition in Table 1 is Extract 13 from Andersson et al. (5). This lipid composition also contains 3.2 mol % of the monoacyl-DGlcDAG (MADGlcDAG), which we model as MGlcDAG in Table 1. The assumption is that the Js of MADGlcDAG (2 glucose: 3 acyl chains) is close to that of MGlcDAG (1 glucose: 2 acyl chains). The molar fractions of DAG and the lipid intermediates, PA, CDP-DAG, and PGP, are often below the experimental detection limit. In our model, any unaccounted molar fraction is distributed among these lipids and we assume that PA and DAG are present at 20 times the level of CDP-DAG and PGP, based on E. coli data (65).
The Js values of DOPG (66), dioleoylphosphatidic acid (DOPA) (67), and DOG (39) have been measured experimentally. The Js values of DOPG and dioleoylphosphatidic acid are both taken from experiments with a divalent cation concentration above 20 mM. This reproduces the A. laidlawii cytoplasmic environment where a divalent cation is bound to 1 in 10 anionic lipids (68). The Js values of MGlcDOG, DGlcDOG, and the A. laidlawii lipid extracts are estimated from the measured hexagonal phase cylinder diameter d (18), using the equation Js = 1/(d/2 − 0.9 nm). CDP-DAG and PGP are present at negligible amounts and their Js values are estimated to be 0 nm−1. The estimated spontaneous curvature of GPDGlcDOG follows from using the linear assumption in Eq. 2 and the experimental range of lipid extract 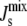 values of between −1/6.6 nm−1 and −1/8.1 nm−1 to give a value of Js = −1/7.7 nm−1. This estimated value suggests GPDGlcDAG forms a lipid bilayer and is consistent with observations that GPDGlcDAG, with a mixture of palmitoyl and oleoyl acyl chains, forms a mixture of micellar and lamellar aggregates (69). The Js value of the anionic lipid GPDGlcDAG is also likely to depend on the concentration of free and bound cations (66). The lipid Js values in Table 1 give a
values of between −1/6.6 nm−1 and −1/8.1 nm−1 to give a value of Js = −1/7.7 nm−1. This estimated value suggests GPDGlcDAG forms a lipid bilayer and is consistent with observations that GPDGlcDAG, with a mixture of palmitoyl and oleoyl acyl chains, forms a mixture of micellar and lamellar aggregates (69). The Js value of the anionic lipid GPDGlcDAG is also likely to depend on the concentration of free and bound cations (66). The lipid Js values in Table 1 give a 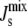 in the experimental range for all other measured oleoyl acyl lipid compositions (5,18).
in the experimental range for all other measured oleoyl acyl lipid compositions (5,18).
APPENDIX B: LITERATURE MODEL PARAMETERS (TABLE 2)
Functional forms for vGPDGS and vCDS
Two reactions of the lipid biosynthetic network are not described by Eq. 3, which is an irreversible Michaelis-Menten kinetic rate equation with one lipid substrate and one soluble substrate. GPDGS catalyzes an irreversible reaction, but the reaction involves two lipid substrates. This gives a rate equation with the functional form,
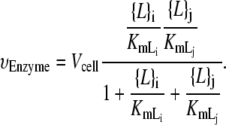 |
(B1) |
The reaction catalyzed by CDS is reversible and is modeled with a reversible rate equation,
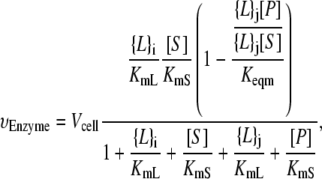 |
(B2) |
where [P] is the soluble product bulk concentration; the lipid product and soluble product Michaelis constants are assumed to be equal to the substrate Michaelis constants KmL and KmS, respectively; and Keqm is the equilibrium constant.
Enzyme kinetic constants
Three of the seven A. laidlawii lipid biosynthetic enzymes have been studied in detail. In addition, we use experimental data for two other reactions from E. coli. Although E. coli and A. laidlawii are very different organisms, most sequenced bacterial genomes are found to encode a protein that has a strong homology to CDP-DAG synthase of E. coli (22). Table 3 summarizes the corresponding Vmax, KmL, KmS, and Keqm values.
TABLE 3.
Enzyme kinetic constants
| Enzyme | Vmax (μmol mg−1 min−1) | KmL (molar fraction) | KmS (mM) | Keqm | Reference |
|---|---|---|---|---|---|
| CDP-DAG synthase* | 55 | † | 0.58 | 0.22 | (23) |
| PGP synthase* | 20 | † | 0.32 | N/A | (24) |
| PA phosphatase | 12.8 | 0.1 | N/A | N/A | (27) |
| MGlcDAG synthase | 12 | 0.08 | 0.4 | N/A | (28) |
| DGlcDAG synthase | 1.3 | 0.01 | 0.14 | N/A | (29) |
Vmax and Km kinetic constants of these enzymes are taken from E. coli.
These enzymes were not studied with the surface dilution mechanism.
The Vcell values are obtained from Eq. 4 using the Vmax in Table 3 and the ratio MEnzyme/NLipid (protein mass to moles of lipid per cell), which is estimated as follows. A. laidlawii has 1.35 μmol of polar lipid for each milligram of membrane protein (70). Polar lipids constitute ∼40% of the A. laidlawii membrane. Membrane proteins are measured to constitute 21.2% of the overall protein mass (71). This gives an MProtein/NLipid ratio of 1.4 mg μmol−1. The enzyme yield MEnzyme/MProtein is taken from the purification of each enzyme and is given in Table 4, which also presents the resulting estimate of Vcell.
TABLE 4.
Vcell
| Enzyme | Enzyme yield | Vcell, Enzyme (min−1) | Reference |
|---|---|---|---|
| CDP-DAG synthase | 1/8000* | 9.6 × 10−3 | (23) |
| PGP synthase | 1/6000* | 4.7 × 10−3 | (24) |
| PA phosphatase | 1/440 | 4.1 × 10−2 | (27) |
| MGlcDAG synthase | 1/9100 | 1.8 × 10−3 | (28) |
| DGlcDAG synthase | 1/5250 | 3.5 × 10−4 | (29) |
Enzyme yield of these enzymes is taken from E. coli.
Soluble reactant concentrations
The rate equations for the enzymatic reactions feature the soluble substrates G3P and uridine diphosphate (UDP)-glucose, CTP, and PPi. In our model, we assume that the soluble metabolites are not dynamic variables and have the constant, regulated cytoplasmic concentrations in Table 5. This assumption is motivated by their involvement in many cellular processes other than lipid biosynthesis.
TABLE 5.
Soluble reactant concentrations
These intracellular concentrations are not from A. laidlawii.
Lipid insertion
The enzyme that synthesizes PA in A. laidlawii is not well characterized. Therefore, V+,PA is estimated from the doubling time of A. laidlawii. In an exponentially growing membrane, the number of lipids is given by
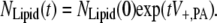 |
(B3) |
Taking the doubling time of A. laidlawii in the exponential growth phase to be between 80 and 90 min (Å. Wieslander, University of Stockholm personal communication, 2003, 2004), V+,PA is 8.2 × 10−3 min−1.
Editor: Thomas J. McIntosh.
References
- 1.Ces, O., and X. Mulet. 2006. Physical coupling between lipids and proteins: a paradigm for cellular control. Signal Transduct. 6:112–132. [Google Scholar]
- 2.Dowhan, W. 1997. Molecular basis for membrane phospholipid diversity: why are there so many lipids? Annu. Rev. Biochem. 66:199–232. [DOI] [PubMed] [Google Scholar]
- 3.Gruner, S. M. 1985. Intrinsic curvature hypothesis for biomembrane lipid composition: a role for nonbilayer lipids. Proc. Natl. Acad. Sci. USA. 82:3665–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cronan, J. E. 2003. Bacterial membrane lipids: where do we stand? Annu. Rev. Microbiol. 57:203–224. [DOI] [PubMed] [Google Scholar]
- 5.Andersson, A. S., R. A. Demel, L. Rilfors, and G. Lindblom. 1998. Lipids in total extracts from Acholeplasma laidlawii A pack more closely than the individual lipids. Monolayers studied at the air-water interface. Biochim. Biophys. Acta. 1369:94–102. [DOI] [PubMed] [Google Scholar]
- 6.Andersson, A. S., L. Rilfors, M. Bergqvist, S. Persson, and G. Lindblom. 1996. New aspects on membrane lipid regulation in Acholeplasma laidlawii A and phase equilibria of monoacyldiglucosyldiacylglycerol. Biochemistry. 35:11119–11130. [DOI] [PubMed] [Google Scholar]
- 7.Morein, S., A. Andersson, L. Rilfors, and G. Lindblom. 1996. Wild-type Escherichia coli cells regulate the membrane lipid composition in a “window” between gel and non-lamellar structures. J. Biol. Chem. 271:6801–6809. [DOI] [PubMed] [Google Scholar]
- 8.Goldfine, H. 1982. Lipids of prokaryotes; structure and distribution. In Current Topics in Membrane Transport, Vol. 17. S. Razin and S. Rottem, editors. Academic Press. New York. 1–43.
- 9.Ansell, G., J. N. Hawthorne, and R. M. C. Dawson. 1973. Form and Function of Phospholipids. Elsevier Science Publishers, Amsterdam.
- 10.McIntosh, T. J., and S. A. Simon. 2006. Roles of bilayer material properties in function and distribution of membrane proteins. Annu. Rev. Biophys. Biomol. Struct. 35:177–198. [DOI] [PubMed] [Google Scholar]
- 11.Rilfors, L., and G. Lindblom. 2002. Regulation of lipid composition in biological membranes—biophysical studies of lipids and lipid synthesizing enzymes. Colloids Surf. B Biointerfaces. 26:112–124. [Google Scholar]
- 12.Attard, G. S., R. H. Templer, W. S. Smith, A. N. Hunt, and S. Jackowski. 2000. Modulation of CTP:phosphocholine cytidylyltransferase by membrane curvature elastic stress. Proc. Natl. Acad. Sci. USA. 97:9032–9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnan, J., and P. A. Iglesias. 2004. A modeling framework describing the enzyme regulation of membrane lipids underlying gradient perception in Dictyostelium cells. J. Theor. Biol. 229:85–99. [DOI] [PubMed] [Google Scholar]
- 14.Hamm, M., and M. M. Kozlov. 2000. Elastic energy of tilt and bending of fluid membranes. Eur. Phys. J. E. 3:323–335. [Google Scholar]
- 15.Zimmerberg, J., and M. M. Kozlov. 2006. How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 7:9–19. [DOI] [PubMed] [Google Scholar]
- 16.Helfrich, W. 1973. Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch. 28(C):693–703. [DOI] [PubMed] [Google Scholar]
- 17.May, S., and A. Ben-Shaul. 1999. Molecular theory of lipid-protein interaction and the L-α- H-II transition. Biophys. J. 76:751–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osterberg, F., L. Rilfors, A. Wieslander, G. Lindblom, and S. M. Gruner. 1995. Lipid extracts from membranes of Acholeplasma laidlawii A grown with different fatty acids have a nearly constant spontaneous curvature. Biochim. Biophys. Acta. 1257:18–24. [DOI] [PubMed] [Google Scholar]
- 19.Li, L., P. Storm, O. P. Karlsson, S. Berg, and A. Wieslander. 2003. Irreversible binding and activity control of the 1,2-diacylglycerol 3-glucosyltransferase from Acholeplasma laidlawii at an anionic lipid bilayer surface. Biochemistry. 42:9677–9686. [DOI] [PubMed] [Google Scholar]
- 20.Lind, J., T. Ramo, M. L. Klement, E. Barany-Wallje, R. M. Epand, R. F. Epand, L. Maler, and A. Wieslander. 2007. High cationic charge and bilayer interface-binding helices in a regulatory lipid glycosyltransferase. Biochemistry. 46:5664–5677. [DOI] [PubMed] [Google Scholar]
- 21.Razin, S., D. Yogev, and Y. Naot. 1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 62:1094–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dowhan, W. 1997. CDP-diacylglycerol synthase of microorganisms. Biochim. Biophys. Acta. 1348:157–165. [DOI] [PubMed] [Google Scholar]
- 23.Sparrow, C. P., and C. R. Raetz. 1985. Purification and properties of the membrane-bound CDP-diglyceride synthetase from Escherichia coli. J. Biol. Chem. 260:12084–12091. [PubMed] [Google Scholar]
- 24.Hirabayashi, T., T. J. Larson, and W. Dowhan. 1976. Membrane-associated phosphatidylglycerophosphate synthetase from Escherichia coli: purification by substrate affinity chromatography on cytidine 5′-diphospho-1,2-diacyl-sn-glycerol sepharose. Biochemistry. 15:5205–5211. [DOI] [PubMed] [Google Scholar]
- 25.Carman, G. M., and D. S. Wieczorek. 1980. Phosphatidylglycerophosphate synthase and phosphatidylserine synthase activites in Clostridium perfringens. J. Bacteriol. 142:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carman, G. M., R. L. Zaniewski, and J. J. Cousminer. 1982. CDP-diacylglycerol synthease activity in Clostridium perfringens. Appl. Environ. Microbiol. 43:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berg, S., and A. Wieslander. 1997. Purification of a phosphatase which hydrolyzes phosphatidic acid, a key intermediate in glucolipid synthesis in Acholeplasma laidlawii A membranes. Biochim. Biophys. Acta. 1330:225–232. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson, O. P., A. Dahlqvist, S. Vikstrom, and A. Wieslander. 1997. Lipid dependence and basic kinetics of the purified 1,2-diacylglycerol 3-glucosyltransferase from membranes of Acholeplasma laidlawii. J. Biol. Chem. 272:929–936. [DOI] [PubMed] [Google Scholar]
- 29.Vikstrom, S., L. Li, O. P. Karlsson, and A. Wieslander. 1999. Key role of the diglucosyldiacylglycerol synthase for the nonbilayer-bilayer lipid balance of Acholeplasma laidlawii membranes. Biochemistry. 38:5511–5520. [DOI] [PubMed] [Google Scholar]
- 30.Edman, M., S. Berg, P. Storm, M. Wikstrom, S. Vikstrom, A. Ohman, and A. Wieslander. 2003. Structural features of glycosyltransferases synthesizing major bilayer and nonbilayer-prone membrane lipids in Acholeplasma laidlawii and Streptococcus pneumoniae. J. Biol. Chem. 278:8420–8428. [DOI] [PubMed] [Google Scholar]
- 31.Brissette, J. L., E. A. Cabacungan, and R. A. Pieringer. 1986. Studies on the antibacterial activity of dodecylglycerol. Its limited metabolism and inhibition of glycerolipid and lipoteichoic acid biosynthesis in Streptococcus mutans BHT. J. Biol. Chem. 261:6338–6345. [PubMed] [Google Scholar]
- 32.Berg, S., M. Edman, L. Li, M. Wikstrom, and A. Wieslander. 2001. Sequence properties of the 1,2-diacylglycerol 3-glucosyltransferase from Acholeplasma laidlawii membranes. Recognition of a large group of lipid glycosyltransferases in eubacteria and archaea. J. Biol. Chem. 276:22056–22063. [DOI] [PubMed] [Google Scholar]
- 33.Huijbregts, R. P., A. I. de Kroon, and B. de Kruijff. 1996. Rapid transmembrane movement of C6-NBD-labeled phospholipids across the inner membrane of Escherichia coli. Biochim. Biophys. Acta. 1280:41–50. [DOI] [PubMed] [Google Scholar]
- 34.Almeida, P. F., W. L. Vaz, and T. E. Thompson. 2005. Lipid diffusion, free area, and molecular dynamics simulations. Biophys. J. 88:4434–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindblom, G., G. Oradd, L. Rilfors, and S. Morein. 2002. Regulation of lipid composition in Acholeplasma laidlawii and Escherichia coli membranes: NMR studies of lipid lateral diffusion at different growth temperatures. Biochemistry. 41:11512–11515. [DOI] [PubMed] [Google Scholar]
- 36.McElhaney, R. N., and M. E. Tourtellotte. 1970. Metabolic turnover of the polar lipids of Mycoplasma laidlawii strain B. J. Bacteriol. 101:72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keller, S. L., S. M. Bezrukov, S. M. Gruner, M. W. Tate, I. Vodyanoy, and V. A. Parsegian. 1993. Probability of alamethicin conductance states varies with nonlamellar tendency of bilayer phospholipids. Biophys. J. 65:23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuller, N., and R. P. Rand. 2001. The influence of lysolipids on the spontaneous curvature and bending elasticity of phospholipid membranes. Biophys. J. 81:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szule, J. A., N. L. Fuller, and R. P. Rand. 2002. The effects of acyl chain length and saturation of diacylglycerols and phosphatidylcholines on membrane monolayer curvature. Biophys. J. 83:977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leikin, S., M. M. Kozlov, N. L. Fuller, and R. P. Rand. 1996. Measured effects of diacylglycerol on structural and elastic properties of phospholipid membranes. Biophys. J. 71:2623–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuller, N., C. R. Benatti, and R. P. Rand. 2003. Curvature and bending constants for phosphatidylserine-containing membranes. Biophys. J. 85:1667–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cornish-Bowden, A. 1995. Fundamentals of Enzyme Kinetics. Portland Press, London.
- 43.Carman, G. M., R. A. Deems, and E. A. Dennis. 1995. Lipid signaling enzymes and surface dilution kinetics. J. Biol. Chem. 270:18711–18714. [DOI] [PubMed] [Google Scholar]
- 44.Romero, P. J., and L. de Meis. 1989. Role of water in the energy of hydrolysis of phosphoanhydride and phosphoester bonds. J. Biol. Chem. 264:7869–7873. [PubMed] [Google Scholar]
- 45.Wikstrom, M., J. Xie, M. Bogdanov, E. Mileykovskaya, P. Heacock, A. Wieslander, and W. Dowhan. 2004. Monoglucosyldiacylglycerol, a foreign lipid, can substitute for phosphatidylethanolamine in essential membrane-associated functions in Escherichia coli. J. Biol. Chem. 279:10484–10493. [DOI] [PubMed] [Google Scholar]
- 46.Szeto, T. H., S. L. Rowland, L. I. Rothfield, and G. F. King. 2002. Membrane localization of MinD is mediated by a C-terminal motif that is conserved across eubacteria, archaea, and chloroplasts. Proc. Natl. Acad. Sci. USA. 99:15693–15698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szeto, T. H., S. L. Rowland, C. L. Habrukowich, and G. F. King. 2003. The MinD membrane targeting sequence is a transplantable lipid-binding helix. J. Biol. Chem. 278:40050–40056. [DOI] [PubMed] [Google Scholar]
- 48.Mileykovskaya, E., I. Fishov, X. Fu, B. D. Corbin, W. Margolin, and W. Dowhan. 2003. Effects of phospholipid composition on MinD-membrane interactions in vitro and in vivo. J. Biol. Chem. 278:22193–22198. [DOI] [PubMed] [Google Scholar]
- 49.Wieprecht, T., O. Apostolov, M. Beyermann, and J. Seelig. 1999. Thermodynamics of the α-helix-coil transition of amphipathic peptides in a membrane environment: implications for the peptide-membrane binding equilibrium. J. Mol. Biol. 294:785–794. [DOI] [PubMed] [Google Scholar]
- 50.Christiansson, A., L. E. Eriksson, J. Westman, R. Demel, and A. Wieslander. 1985. Involvement of surface potential in regulation of polar membrane lipids in Acholeplasma laidlawii. J. Biol. Chem. 260:3984–3990. [PubMed] [Google Scholar]
- 51.Mosior, M., and S. McLaughlin. 1992. Binding of basic peptides to acidic lipids in membranes: effects of inserting alanine(s) between the basic residues. Biochemistry. 31:1767–1773. [DOI] [PubMed] [Google Scholar]
- 52.Lewis, R. N., and R. N. McElhaney. 2000. Surface charge markedly attenuates the nonlamellar phase-forming propensities of lipid bilayer membranes: calorimetric and (31)P-nuclear magnetic resonance studies of mixtures of cationic, anionic, and zwitterionic lipids. Biophys. J. 79:1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hristova, K., W. C. Wimley, V. K. Mishra, G. M. Anantharamiah, J. P. Segrest, and S. H. White. 1999. An amphipathic α-helix at a membrane interface: A structural study using a novel X-ray diffraction method. J. Mol. Biol. 290:99–117. [DOI] [PubMed] [Google Scholar]
- 54.Wang, J., A. Gambhir, S. McLaughlin, and D. Murray. 2004. A computational model for the electrostatic sequestration of PI(4,5)P2 by membrane-adsorbed basic peptides. Biophys. J. 86:1969–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Runarsson, T. P., and X. Yao. 2000. Stochastic ranking for constrained evolutionary optimization. IEEE Trans. Evol. Comput. 4:284–294. [Google Scholar]
- 56.Moles, C. G., P. Mendes, and J. R. Banga. 2003. Parameter estimation in biochemical pathways: A comparison of global optimization methods. Genome Res. 13:2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barkai, N., and S. Leibler. 1997. Robustness in simple biochemical networks. Nature. 387:913–917. [DOI] [PubMed] [Google Scholar]
- 58.Cornell, R. B., and I. C. Northwood. 2000. Regulation of CTP:phosphocholine cytidylyltransferase by amphitropism and relocalization. Trends Biochem. Sci. 25:441–447. [DOI] [PubMed] [Google Scholar]
- 59.Hauksson, J. B., L. Rilfors, G. Lindblom, and G. Arvidson. 1995. Structures of glucolipids from the membrane of Acholeplasma laidlawii strain A-EF22. III. Monoglucosyldiacylglycerol, diglucosyldiacylglycerol, and monoacyldiglucosyldiacylglycerol. Biochim. Biophys. Acta. 1258:1–9. [DOI] [PubMed] [Google Scholar]
- 60.Andersson, A. S., L. Rilfors, G. Oradd, and G. Lindblom. 1998. Total lipids with short and long acyl chains from Acholeplasma form nonlamellar phases. Biophys. J. 75:2877–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jackowski, S. 1996. Cell cycle regulation of membrane phospholipid metabolism. J. Biol. Chem. 271:20219–20222. [DOI] [PubMed] [Google Scholar]
- 62.Mileykovskaya, E., and W. Dowhan. 2005. Role of membrane lipids in bacterial division-site selection. Curr. Opin. Microbiol. 8:135–142. [DOI] [PubMed] [Google Scholar]
- 63.Rothfield, L. I., Y. L. Shih, and G. King. 2001. Polar explorers: membrane proteins that determine division site placement. Cell. 106:13–16. [DOI] [PubMed] [Google Scholar]
- 64.Devaux, P. F. 1991. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 30:1163–1173. [DOI] [PubMed] [Google Scholar]
- 65.Raetz, C. R., and E. P. Kennedy. 1973. Function of cytidine diphosphate-diglyceride and deoxycytidine diphosphate-diglyceride in the biogenesis of membrane lipids in Escherichia coli. J. Biol. Chem. 248:1098–1105. [PubMed] [Google Scholar]
- 66.Alley, S. H., O. Ces, M. Barahona, and R. H. Templer. X-ray diffraction measurement of the monolayer spontaneous curvature of dioleoylphosphatidylglycerol. Chem. Phys. Lipids. In press. [DOI] [PubMed]
- 67.Kooijman, E. E., V. Chupin, N. L. Fuller, M. M. Kozlov, B. de Kruijff, K. N. Burger, and P. R. Rand. 2005. Spontaneous curvature of phosphatidic acid and lysophosphatidic acid. Biochemistry. 44:2097–2102. [DOI] [PubMed] [Google Scholar]
- 68.Niemi, A. E., A. Andersson, L. Rilfors, G. Lindblom, and G. Arvidson. 1997. The effects of hydration and divalent cations on lamellar-nonlamellar phase transitions in membranes and total lipid extracts from Acholeplasma laidlawii A-EF22 - a 2H NMR study. Eur. Biophys. J. 26:485–493. [Google Scholar]
- 69.Danino, D., A. Kaplun, G. Lindblom, L. Rilfors, G. Oradd, J. B. Hauksson, and Y. Talmon. 1997. Cryo-TEM and NMR studies of a micelle-forming phosphoglucolipid from membranes of Acholeplasma laidlawii A and B. Chem. Phys. Lipids. 85:75–89. [DOI] [PubMed] [Google Scholar]
- 70.Wieslander, A., and L. Rilfors. 1977. Qualitative and quantitative variations of membrane lipid species in Acholeplasma laidlawii A. Biochim. Biophys. Acta. 466:336–346. [DOI] [PubMed] [Google Scholar]
- 71.Archer, D. B., A. W. Rodwell, and E. S. Rodwell. 1978. The nature and location of Acholeplasma laidlawii membrane proteins investigated by two-dimensional gel electrophoresis. Biochim. Biophys. Acta. 513:268–283. [DOI] [PubMed] [Google Scholar]
- 72.Vikstrom, S., L. Li, and A. Wieslander. 2000. The nonbilayer/bilayer lipid balance in membranes. Regulatory enzyme in Acholeplasma laidlawii is stimulated by metabolic phosphates, activator phospholipids, and double-stranded DNA. J. Biol. Chem. 275:9296–9302. [DOI] [PubMed] [Google Scholar]
- 73.Mijakovic, I., S. Poncet, A. Galinier, V. Monedero, S. Fieulaine, J. Janin, S. Nessler, J. A. Marquez, K. Scheffzek, S. Hasenbein, W. Hengstenberg, and J. Deutscher. 2002. Pyrophosphate-producing protein dephosphorylation by HPr kinase/phosphorylase: a relic of early life? Proc. Natl. Acad. Sci. USA. 99:13442–13447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Even, S., N. D. Lindley, and M. Cocaign-Bousquet. 2001. Molecular physiology of sugar catabolism in Lactococcus lactis IL1403. J. Bacteriol. 183:3817–3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aiello, D. P., L. Fu, A. Miseta, and D. M. Bedwell. 2002. Intracellular glucose 1-phosphate and glucose 6-phosphate levels modulate Ca2+ homeostasis in Saccharomyces cerevisiae. J. Biol. Chem. 277:45751–45758. [DOI] [PubMed] [Google Scholar]
- 76.Eisenberg, D. 1984. Three-dimensional structure of membrane and surface proteins. Annu. Rev. Biochem. 53:595–623. [DOI] [PubMed] [Google Scholar]