Abstract
A large, negative ΔCp of DNA binding is a thermodynamic property of the majority of sequence-specific DNA-protein interactions, and a common, but not universal property of non-sequence-specific DNA binding. In a recent study of the binding of Taq polymerase to DNA, we showed that both the full-length polymerase and its “Klentaq” large fragment bind to primed-template DNA with significant negative heat capacities. Herein, we have extended this analysis by analyzing this data for temperature-variable heat capacity effects (ΔΔCp), and have similarly analyzed an additional 47 protein-DNA binding pairs from the scientific literature. Over half of the systems examined can be easily fit to a function that includes a ΔΔCp parameter. Of these, 90% display negative ΔΔCp values, with the result that the ΔCp of DNA binding will become more negative with rising temperature. The results of this collective analysis have potentially significant consequences for current quantitative theories relating ΔCp values to changes in accessible surface area, which rely on the assumption of temperature invariance of the ΔCp of binding. Solution structural data for Klentaq polymerase demonstrate that the observed heat capacity effects are not the result of a coupled folding event.
INTRODUCTION
Determination of ΔCp for a protein-DNA interaction involves measuring either the temperature dependence of ΔH directly (i.e., the definition of ΔCp), or measuring the temperature dependence of ΔG (the curvature of which defines the ΔCp). The ΔCp of a protein-DNA interaction is generally assumed to be invariant with temperature, particularly over restricted temperature ranges, and empirically the use of a temperature-invariant ΔCp often provides a good fit to experimental data. There is no a priori requirement that ΔCp be temperature-invariant for any molecular process (e.g., see (1,2)). The general assumption of temperature invariance of ΔCp is based both on empirical evidence that such variance is indeed small for solvent restructuring (2), and on calculations showing that for determination of many protein folding thermodynamic parameters, this assumption introduces no significant errors (3,4).
A few researchers, however, have extended analyses of their DNA-binding data to include a parameter for temperature variation of ΔCp (a ΔΔCp parameter). For example, Lundbäck et al. fit a non-sequence-specific protein-DNA interaction with a temperature-dependent ΔCp (5). Milev et al. describe a temperature-dependent heat capacity (ΔΔCp) and suggest it is caused by linked structural changes with temperature (6). Most recently, in a characteristically precise and thorough study, Kozlov and Lohman document a ΔΔCp for the binding of Escherichia coli SSB to single-stranded DNA that is also anion-dependent (7).
Determining whether ΔCp is temperature-dependent for an interaction can be elusive as it requires high precision data over a large temperature range, and involves quantifying small amounts of curvature in plots of ΔH versus temperature or subtle asymmetries in plots of ΔG versus temperature. In some of the very few studies of individual protein-DNA reactions where temperature dependence of ΔCp has been documented, there have been suggestions that this behavior might be a general phenomenon (e.g., (7)). In this short report, we show that a simultaneous comparative analysis of a large number of protein-DNA systems reveals a high prevalence of ΔΔCp values of similar magnitude, adding to the evidence that, indeed, temperature dependence of the heat capacity of protein-DNA interactions may be quite general.
MATERIALS AND METHODS
Determination of ΔΔCp: ΔΔCp in these analyses is defined as the linear temperature dependence of ΔCp,
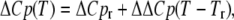 |
and can be obtained from ΔH versus T data using the equation
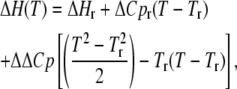 |
where ΔCp(T) is the heat capacity change at any temperature T, the ΔH(T) values are the binding enthalpies measured at different temperatures, and ΔCpr and ΔHr are the fitted heat capacity change and enthalpy values at any chosen reference temperature Tr. ΔH data for Taq/Klentaq are reproduced from Datta and LiCata (8). The enthalpy of binding of 63/70-mer primed-template DNA to Taq and Klentaq was determined as a function of temperature in a MicroCal VP-ITC in 10 mM Tris, 75 mM KCl, 5 mM MgCl2, pH 7.9. Additional experimental details can be found in Datta and LiCata (8).
For Gibbs-Helmholtz (ΔG versus T data), ΔΔCp is defined as above, and can be obtained from the equation
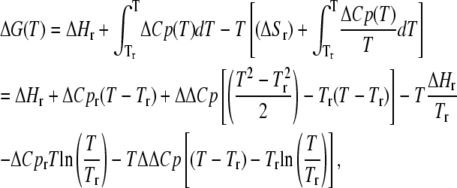 |
where ΔG(T) is the free energy change at each temperature T, and ΔCpr, ΔHr, and Tr are the fitted heat capacity change, enthalpy, and Tr values (Tr is at either temperature where ΔG = 0). ΔG versus T data for Taq/Klentaq are from Datta and LiCata (8) and are determined from fluorescence anisotropy-monitored binding of Taq and Klentaq to a 63/70-mer primed-template DNA in 10 mM Tris, 75 mM KCl, 5 mM MgCl2, pH 7.9 buffer at the indicated temperatures. Additional experimental details are in Datta and LiCata (8). All nonlinear fits were performed using KaleidaGraph (Synergy Software) and/or Origin 5.0 (Microcal Software).
Small angle x-ray scattering (SAXS) measurements of Rg were performed at the Stanford Synchrotron Radiation Research Laboratory on beamline 1–4 in 10 mM Tris, 75 mM KCl, 5 mM MgCl2, pH 7.9 at the indicated temperatures. The data were analyzed using Guinier plots where Rg values were determined from the linear portions of the plots (9,10), and/or using the full P(r) distance distribution function (11). Both approaches yield equivalent results. Rh measurements were conducted using a Zetasizer Nano DLS (dynamic light scattering) instrument in 10 mM KPO4, 250 mM KCl, 5 mM MgCl2, pH 7.5 at the indicated temperature. The data were analyzed using the manufacturer's software. Protein concentration in both sets of measurements was ∼5 mg/ml. The 25°C SAXS-determined Rg values have been published previously (12).
RESULTS AND DISCUSSION
ΔΔCp values for Taq and Klentaq polymerases
In a recent study of the thermodynamics of binding of Taq polymerase to DNA, we showed that both the full-length polymerase and its “Klentaq” large fragment domain bind to primed-template DNA with a heat capacity of −0.7 to −0.8 kcal/mole (8). A large, negative ΔCp of DNA binding is a property of the majority of sequence-specific DNA-protein interactions (13). The results for Taq and Klentaq are among those indicating that a smaller magnitude, but still relatively large ΔCp is a common, but not universal property of non-sequence-specific DNA binding (8,14). Herein, we extended this analysis of Taq and Klentaq by analyzing this data for temperature-variable heat capacity effects, or ΔΔCp. We find that both data sets return equivalent values of ΔΔCp.
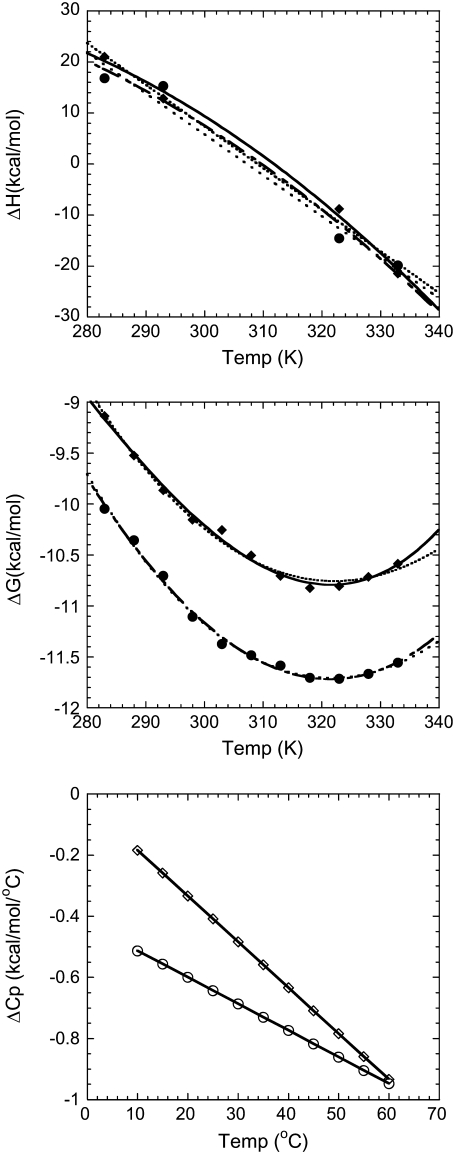
The top panel in Fig. 1 shows ΔH versus T data for full-length Taq and Klentaq polymerases, fit with and without inclusion of a ΔΔCp term. The middle panel shows a similar analysis for ΔG versus T data. By visual inspection, the fits appear nearly equivalent, but in both cases, including a ΔΔCp term improves the χ2 of the fit (see Table 1). ΔΔCp values determined for Taq and Klentaq range from −8 to −19 cal/mole K2. In general, however, the error envelopes for the ΔΔCp parameters for Taq or Klentaq are too large to establish them as statistically significant (see Table 1). What is intriguing, however, is:
The similarity of ΔΔCp values obtained from the calorimetric determinations of ΔH versus temperature and the equilibrium-binding determinations of ΔG versus temperature, because these are very different types of experiments, involving different potential for systematic or experimental errors.
The inability to obtain a better fit to the data with a zero ΔΔCp.
The fact that these seemingly minute ΔΔCp values result in relatively large excursions of ΔCp when propagated over a few decades of temperature.
The bottom panel of Fig. 1 shows the resultant ΔCp values for binding of Taq and Klentaq to DNA over the temperature range of 10–60°C. It is also notable that if we fit 4–5 of the highest temperature data points from Fig. 1, middle, to obtain a temperature-invariant ΔCp, that paralleling Fig. 1, bottom, we obtain a ΔCp value that is ∼0.5 kcal/mole K more negative than if we fit 4–5 of the lowest temperature data points. Despite all this circumstantial evidence, however, the presence of a ΔΔCp for Taq and Klentaq remains statistically unverified.
FIGURE 1.
Fitting Taq and Klentaq DNA polymerase binding data with and without a ΔΔCp parameter. (Top panel) The calorimetric ΔH for DNA binding by Taq (•) analyzed with (— −) and without (· · · ·) a ΔΔCp parameter; and Klentaq (▴) analyzed with (—) and without (······) a ΔΔCp parameter. Data are from Datta and LiCata (8). (Middle panel) Gibbs-Helmholtz plot for DNA binding by Taq (•) analyzed with (— −) and without (· · · ·) a ΔΔCp parameter; and Klentaq (▴) analyzed with (—) and without (······) a ΔΔCp parameter. Data are from Datta and LiCata (8). (Bottom panel) Calculated temperature dependence of the ΔCp for DNA binding by Taq (•) and Klentaq (▴). The ΔΔCp values used for this calculation are the means from the ΔH (top panel) and ΔG (middle panel) data sets (see Table 1).
TABLE 1.
Data sets with fitted ΔΔCp parameters
| Protein-DNA interaction | Data, Fig. 2 | ΔΔCp cal/mol K2 | Temp range °C | χ2 with ΔΔCp | χ2 without ΔΔCp | F† | Data ref. |
|---|---|---|---|---|---|---|---|
| PwTBP-hairpin loop | A | −35.0 ± 12.9 | 15–45 | 6.559 | 22.768 | 7.41 | 16 |
| PwTBP wt-20-mer | B | −16.4 ± 10.4 | 35–55 | 8.222 | 18.508 | 2.50 | 17 |
| PwTBP E128A-20-mer | C | −15.8 ± 3.8 | 30–55 | 4.525 | 29.934 | 16.8 | 17 |
| PwTBP E12AE128A-20-mer | D | −10.0 ± 9.9 | 25–45 | 7.473 | 11.279 | 1.02 | 17 |
| PwTBP Q103E-20-mer | E | −22.5 ± 9.1 | 35–55 | 6.353 | 25.799 | 6.12 | 17 |
| PwTBP Q103A-20-mer | F | −4.7 ± 10.5 | 30–50 | 8.437 | 13.212 | 4.73 | 17 |
| c-Myb R2R3*-MBS-I | G | −5.4 ± 5.3 | 12–30 | 0.089 | 0.135 | 1.03 | 18 |
| Sso 7d-poly(dGdC) | H | −4.5 ± 1.5‡ | 15–45 | 0.025 | 0.227 | 8.27 | 5 |
| Sso 7d-poly(dGdC) | I | −4.1 ± <0.01‡ | 16–35 | <0.001 | 0.022 | nd | 5 |
| Sox-5-10 bp | J | −20.9 ± 9.3 | 8–30 | 9.664 | 34.119 | 5.06 | 15 |
| vnd/NK-2 HD(wt)-18 bp | K | 12.0 ± 1.8 | 10–30 | 0.002 | 0.092 | 45.0 | 19 |
| GCN4-br-AP-1 | L | −2.9 ± 12.7 | 10–20 | 0.324 | 0.341 | 0.05 | 20 |
| GCN4-br-ATF/CREB | M | −3.6 ± 5.5 | 10–20 | 0.060 | 0.086 | 0.43 | 20 |
| MunI-SP | N | 23.3 ± 15.9 | 14–30 | 3.956 | 15.474 | 2.91 | 21 |
| MunI-SP | O | 6.6 ± 0.5 | 9–30 | 0.012 | 2.450 | 209.1 | 21 |
| MunI-SP | P | −8.1 ± 4.3 | 13–30 | 0.298 | 2.954 | 8.91 | 21 |
| Oct-1 POU-DNA | Q | −9.4 ± 8.4 | 12–35 | 4.916 | 5.606 | 1.26 | 22 |
| Trp repressor-18 bp | R | −19.8 ± 15.7 | 10–40 | 11.701 | 16.357 | 1.59 | 23 |
| PU.1 ETS-λB | S | −11.5 ± <0.01 | 0–37 | 0.001 | 0.004 | nd | 24 |
| INT-DBD-13 bp | T | −18.9 ± 6.2 | 4–30 | 123.8 | 190.8 | 9.20 | 6 |
| Zfl-3-15 bp | U | −1.7 ± 3.9 | 13–45 | 3.482 | 4.181 | 0.20 | 25 |
| MetJ-12 bp | V | −17.0 ± <0.01 | 11–36 | 0.290 | 2.407 | nd | 26 |
| GR DBD-pGRE | W | −13.1 ± 3.6 | 10–34 | 0.298 | 1.314 | 13.6 | 27 |
| Taq-63/70-mer DNA (ΔH) | X | −9.4 ± 23.7 | 10–60 | 22.609 | 26.200 | 0.16 | 8 |
| Taq-63/70-mer DNA (ΔG) | X | −6.9 ± 9.0 | 10–60 | 0.016 | 0.018 | 0.58 | 8 |
| Klentaq-63/70-mer DNA (ΔH) | Y | −11.1 ± 11.5 | 10–60 | 5.331 | 10.260 | 0.92 | 8 |
| Klentaq-63/70-mer DNA (ΔG) | Y | −19.1 ± 10.0 | 10–60 | 0.020 | 0.030 | 3.67 | 8 |
| PU.1 ETS-λB | a | −68.5 ± 25.7 | 0–60 | 0.011 | 0.092 | 7.36 | 24 |
| PU.1 ETS-λB | b | −81.7 ± 28.1 | 0–50 | 0.021 | 0.163 | 13.5 | 24 |
| PurR-30 bp | c | −108 ± 238 | 1–37 | 0.006 | 0.981 | 162.5 | 28 |
| PurR-30 bp | d | −186 ± 34 | 1–37 | 0.138 | 0.167 | 0.42 | 28 |
In the F-test, 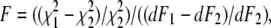 where
where 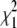 and
and 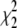 are the chi-squared values for the two different fits, and dF1 and dF2 are the degrees of freedom for each fit. F-values <1 indicate that the fit has not been improved by adding the new parameter beyond the statistical improvement expected from the reduction in degrees of freedom. nd, for some data sets F could not be reliably determined due to too few data points.
are the chi-squared values for the two different fits, and dF1 and dF2 are the degrees of freedom for each fit. F-values <1 indicate that the fit has not been improved by adding the new parameter beyond the statistical improvement expected from the reduction in degrees of freedom. nd, for some data sets F could not be reliably determined due to too few data points.
Lundbäck et al. previously reported a ΔΔCp of −5 cal/K mol for these data (5).
ΔΔCp in other protein-DNA interactions
To investigate this issue further, however, we similarly analyzed 47 additional protein-DNA interaction data sets from the scientific literature, from 21 different publications (5,6,15–33). Data sets where the protein clearly and identifiably begins unfolding at higher binding temperatures were not included (e.g., (34–37)). Data sets were included if the data extended across ∼20°C or more, and if the quantitative data were available in tabulated form. If data sets already included identification of significant linked processes with their own ΔCp values (e.g., 15), data were only used if “corrected” data were provided having had the effects of known linked processes subtracted. Most of the data sets used were ΔH versus temperature data (only a few were ΔG versus temperature). For most of these original data sets in isolation, especially where there are measurements at perhaps only a small number of temperatures, there would have been little justification for testing for inclusion of a ΔΔCp parameter. However, when examined in aggregate, some interesting patterns emerge.
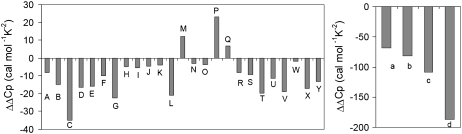
Fig. 2 graphically depicts the fitted ΔΔCp values found for 29 of the 49 data sets analyzed. Twenty-five data sets returned ΔΔCp values in the approximate range of ±30 cal/mol K2 (data sets A–Y), while four data sets returned somewhat larger ΔΔCp values (data sets a–d). Table 1 summarizes the fit parameters for each of these 29 data sets. Fifteen of the 49 data sets were not fit better with addition of a ΔΔCp parameter (these 15 data sets are not shown in Fig. 2 or in Table 1, but are listed in the legend to Fig. 2). In several cases, the same published study yielded some data sets that were fit better with a ΔΔCp parameter and some data sets that were not (5,15,17–19,23).
FIGURE 2.
ΔΔCp values for other protein-DNA interactions. ΔΔCp values were obtained from the equations described in the text. The left panel includes data sets that fit better with a ΔΔCp parameter in the range of ±30 cal/mol K2 while the right panel includes data sets with larger ΔΔCp values. These data sets are: (A) PwTBP-hairpin loop (16); (B–F) PwTBP wt-20-mer, PwTBP E128A-20-mer, PwTBP E12AE128A-20-mer, PwTBP Q103E-20-mer, PwTBP Q103A-20-mer, respectively (17); (G), c-Myb R2R3*-MBS-I (18); (H and I) Sso 7d-poly(dGdC) (5); (J) Sox-5-10 bp (15); (K) vnd/NK-2 HD(wt)-18 bp (19); (L and M) GCN4-br-AP-1, GCN4-br-ATF/CREB, respectively (20); (N–P) MunI-SP (21); (Q) Oct-1 POU-DNA (22); (R) Trp repressor-18 bp (23); (S) PU.1 ETS-λB (24); (T) INT-DBD-13 bp (6); (U) Zfl-3-15 bp (25); (V) MetJ-12 bp (26); (W) GR DBD-pGRE (27); (X) Taq-63/70-mer DNA (average of ΔH and ΔG data from Table 1) (8); (Y) Klentaq-63/70-mer DNA (average of ΔH and ΔG data from Table 1) (8); (a and b) PU.1 ETS-λB (24); (c and d) PurR-30 bp (28). Data sets which were not fit better with a ΔΔCp parameter are: one data set from Lundbäck et al. (5), two data sets from Privalov et al. (15), four data sets from Bergqvist et al. (17), one data set from Oda et al. (18), one data set from Gonzales et al. (19), one data set from Ladbury et al. (23), two data sets from Sieber and Allemann (29), one data set from Poon and Macgregor (30), and two data sets from Ha et al. (31) (total of 15 data sets that are not fit better with a ΔΔCp parameter). Presence or absence of a fitted ΔΔCp value could not be ascertained reliably for five of the data sets examined due to problematic fit diagnostics (such as indeterminate error envelopes for some parameters), these are: four data sets from Künne et al. (32) and one from Frank et al. (33).
Notable aspects of this analysis include: 1) the high prevalence of obtaining a better fit with addition of a ΔΔCp parameter (29 of 49, or 59% of data sets); 2) the fact that most (26 of 29, or 90%) of the returned ΔΔCp values are negative; and 3) the fact that the bulk of the ΔΔCp values are of similar magnitude. If addition of the extra parameter were simply fitting experimental noise, one would expect approximately equal/random distribution of positive and negative ΔΔCp values. If positive and negative ΔΔCp values were equally likely, a simple binomial probability distribution calculation would predict the probability (P(x)) of finding the distribution in Fig. 2 as being <0.0007%. I.e., if positive and negative ΔΔCp values were equally probable (p = 0.5), then 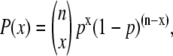 where n = number of trials and x = number of negative ΔΔCp values.
where n = number of trials and x = number of negative ΔΔCp values.
The fitted errors on ΔΔCp for 7 of the 29 data sets shown in Fig. 2 indicate that the fitted ΔΔCp values for those systems are statistically indistinguishable from zero (including, as mentioned above, our own data for Taq). The other data sets, however, return statistically significant ΔΔCp values (two others barely make the cut). The ΔΔCp values with large error envelopes are included here, however, because: 1) a comparably good fit for those data cannot be obtained by fixing the ΔΔCp value at zero; and 2) the best fit ΔΔCp value for those data sets match the pattern for the others. A distinguishing feature of meta-analysis, even in this simplified form, is the suggestion of patterns and correlations in large groups of data that are often not discernable and sometimes not statistically significant within the individual data sets. Even if these statistically borderline data sets are eliminated, the general conclusions of this analysis remain the same: a high percentage of the data sets analyzed are fit better with a negative ΔΔCp parameter of similar magnitude and sign. Either this striking pattern is communicating information about ΔCp behavior in protein-DNA interactions, or it is a highly improbable and coherent distribution of noise across a wide number of different experiments.
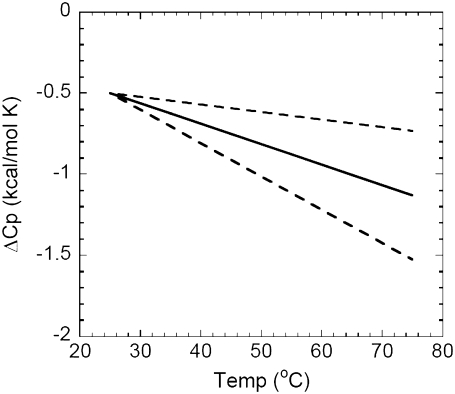
In Fig. 3, the mean ΔΔCp value from data sets A–Y is used to illustrate the resultant change in ΔCp versus temperature using an arbitrarily chosen starting ΔCp of −0.5 kcal/mol K at 25°C. The standard deviation on the mean ΔΔCp value from data sets A–Y was used to generate the dashed lines in the figure. The average net excursion of >−0.6 kcal/mole K over a 50°C range is a very large change of ΔCp—especially given that almost all ΔCp values measured for protein-DNA interactions fall within a 0 to −2.0 kcal/mole K range.
FIGURE 3.
Illustration of the average change in ΔCp for DNA binding that will occur as the temperature changes, given the ΔΔCp values from Fig. 2. An idealized reference ΔCp of −0.5 kcal/mol K at 25°C was chosen as a starting point. The ΔCp represented by the solid line is calculated utilizing the mean ΔΔCp values of data sets A–Y in Table 1 (mean ΔΔCp = −0.013 ± 0.008 kcal/mol K2). The dotted lines are ΔCp values calculated using the ± standard deviation range on ΔΔCp from data sets A–Y (i.e., lower line calculated with ΔΔCp = −0.021 kcal/mol K2, upper line calculated with ΔΔCp = −0.005 kcal/mol K2).
Temperature-induced compaction of Klentaq polymerase
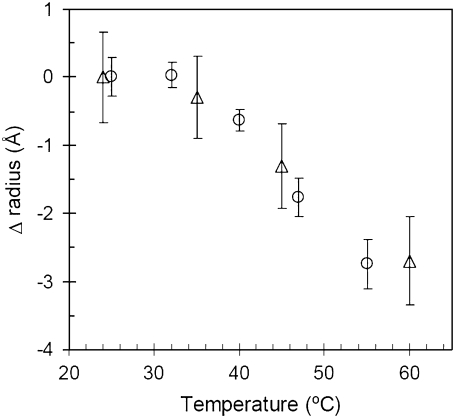
One of the most popular current molecular explanations for a negative heat capacity change in a biomolecular process is the burial of nonpolar surface area (31,38–42). Although Klentaq polymerase does not thermally unfold until >100°C (43), one can still imagine a scenario where elevated temperature might induce an effective expansion or increase in dynamic fluctuation of the native state. In such a scenario, a hypothetically expanded native state might then need to recompact upon binding, thus increasing the net surface area burial upon DNA binding as the temperature increases. This hypothesis is similar to the coupled binding-plus-folding hypothesis (38), but adds a temperature-dependent effect. Fig. 4 empirically assays for such a possibility by directly measuring the effective size of native Klentaq polymerase as a function of increasing temperature. Instead of an expansion, however, both small angle x-ray scattering (SAXS) and dynamic light scattering (DLS) show that Klentaq polymerase compacts in size upon heating. While SAXS and DLS are both scattering techniques, they are, in fact, different methodologies, relying on completely different types of experimental signals and analyses. SAXS measures the static scattered intensity versus the angle of scattering, while DLS measures time-based, diffusion-induced fluctuations in scattering intensity. The two techniques are subject to different potential sources of systematic error, thus it is significant that they return similar measurements of the temperature-induced compaction of Klentaq. A similar temperature-induced native state compaction effect has also recently been documented for plasminogen (44). This result is interesting in its own right, and investigations of the potential origins of this unusual phenomenon will be the subject of future studies. For the purposes of the present investigation, however, Fig. 4 serves to demonstrate that coupled folding and binding surface area changes cannot account for a temperature-dependent change in ΔCp for Klentaq (see also Potential Origins of ΔΔCp, below).
FIGURE 4.
The change in the radius of gyration (ΔRg, triangles) and the hydrodynamic radius (ΔRh, circles) for Klentaq polymerase as a function of temperature.
Implications of a ΔΔCp
As mentioned in the Introduction, we are not the first investigators to find a temperature-dependent ΔCp in their protein-DNA binding data. It is also likely that some of the investigators who do not mention such an effect in their studies could have fit for it and decided that the effect was too subtle to mention. What we have done, however, is analyze a large number of protein-DNA binding systems simultaneously and found that: 1), the majority of them (29 of 49) are fit better by including a temperature-dependent heat capacity; and 2), that the observed ΔΔCp values are clearly nonrandom, with the majority of them (26 of 29) being negative in the binding direction. This means that the ΔCp of binding for these 26 systems will become increasingly more negative as the temperature increases.
ΔCp, ΔΔCp, and ΔASA
The question of whether ΔCp is temperature-dependent is of interest because in many systems ΔCp has been proposed to be correlated with structural information: most commonly the change in accessible surface area upon binding (ΔASA) (3,31,38–42). At least five different quantitative relationships between ΔCp and the sum of buried nonpolar + polar surface areas have been proposed (3,39–42). All such relationships have the form: ΔCp = −(x * ΔASAnon-polar − y * ΔASApolar), where ΔASAnon-polar and ΔASApolar are the amounts of nonpolar and polar surface area buried in the interface, x and y are empirically determined constants, and ΔCp is assumed to be temperature invariant. While these quantitative relationships continue to work reasonably well for protein folding, the increasing number of protein-DNA systems that deviate from these relationships (e.g., (13,23,45–47)) have led to proposals such as simultaneous folding plus binding (38) to account for such deviations. Coupled folding plus binding can be a difficult hypothesis to experimentally test. Some authors have definitively ruled out such an explanation for high ΔCp values in some DNA-binding systems (5,6,23), while in other systems there is direct or crystal structure-based evidence for such an effect (22,33). Coupled binding and folding, however, does not account for either the value of ΔCp at 25°C for Taq/Klentaq (8), or for the ΔΔCp of binding. If burial of nonpolar surface area were the primary contributor to the negative ΔCp of Taq-DNA binding, the average fitted ΔΔCp value would correspond to >5000 Å2 of additional surface area burial that would need to be accounted for as the temperature increased by 50°C—and Fig. 4 predicts, conversely, that the ΔASA of binding will decrease with increasing temperature. Clearly the correlation of ΔASA and ΔCp is completely inapplicable to the binding of Taq/Klentaq to DNA. The collective analysis of Fig. 2 suggests that such inapplicability of any direct ΔASA-ΔCp correlation may also extend to more than half of all protein-DNA interactions.
It should be clarified that these analyses do not contradict the longstanding and well-established relationship between the sign of ΔCp and the burial of polar versus nonpolar surface area (the ΔCp-hydrophobic effect correlation). What these analyses do suggest, however, is that quantitative ΔCp-ΔASA relationships for protein-DNA interactions may be seriously perturbed by what may be a natural prevalence of temperature-dependent heat capacity changes. I.e., if the results of Figs. 2 and 3 are not merely statistical anomalies, then no current ΔASA-ΔCp correlation can be universally applied to all protein-DNA interactions. Kozlov and Lohman (7) have made a similar argument based on their documentation of both temperature and anion dependencies of ΔCp values for the E. coli SSB-DNA binding interaction. It may be possible, with adequate additional data, to add correction factors to these relationships, but this begs the question of how far one should stretch/adapt this correlation to attempt to fit all protein-DNA binding data. In our prior study of ΔCp effects for Taq/ Klentaq and Klenow polymerases, we suggested that DNA-binding interactions can be sorted into two bins: those with and those without a strong ΔCp-ΔASA correlation (14). For those systems where the correlation holds, the binding is likely dominated by the hydrophobic effect, while those systems for which the correlation does not hold must have other major molecular contributions to the binding and thus to their ΔCp values.
Potential origins of ΔΔCp
The analysis in this study cannot address the origins of the observed ΔΔCp values, but the main categories of potential sources can be discussed. It may be that ΔH versus temperature is inherently nonlinear for protein-DNA interactions. Linked molecular processes can also explain a temperature-dependent ΔCp. The molecular nature of an appropriately linked reaction could include any of a number of processes proposed to exhibit a ΔCp, including DNA distortion (46,48), restriction of vibrational freedom (23,35,49), linked protonation/deprotonation (50,51), multiple cooperative weak interactions (52), and, of course, additionally linked changes in surface area exposure (such as coupled folding-unfolding) (3,31,38–42).
Linked equilibria can only explain the observed ΔΔCp pattern if there exists a very specific combination of two partially overlapping enthalpic events. For two linked reactions to produce a concave-down curved ΔH versus temperature dependence (as found for 26 of the data sets examined herein) the following must be true: 1), the two processes must have differing ΔCp values; 2), the two processes must have different temperature ranges; and 3), both processes must have negative ΔCp values. If any one of these is not true, the observed curvature will not result: 1), if both processes have the same ΔCp, there is no change in slope of ΔH versus temperature; 2), if both processes have exactly overlapping temperature ranges, a cumulative ΔCp will be observed, but no curvature; and 3), if one process has as positive ΔCp or no ΔCp, the curve will be concave-up or will plateau.
Recent studies of heat capacity effects in protein-protein interactions have quantitatively accounted for some amount of similar concave-down curvature in plots of ΔHbinding versus temperature by including a term for the temperature-dependent fractional contribution of the unfolding enthalpy (53,54). While in the preceding section we briefly discussed potential contributions of coupled folding/unfolding to the magnitude of ΔCp, these recent studies explore the potential for contributions of folding/unfolding to the presence of a ΔΔCp. Even small amounts of unfolding (∼1%) in the experimental binding range can result in visible curvature of ΔHbinding versus T (53). The typically much larger magnitude of ΔHfolding versus ΔHbinding is what makes this possible. A similar analysis of our Taq/Klentaq data (Fig. 1, top, analyzed with Eq. 7 from (54)) indicate that these proteins would only need to unfold (and then refold upon binding) by 8% across the binding temperature range (10–60°C) to account for the experimental curvature in this data. However, previous thermal denaturation studies on Taq and Klentaq from our laboratory clearly show that neither protein even begins to unfold (≪1%) before 85°C (43). This reinforces the conclusion further that coupled folding-unfolding does not significantly contribute to ΔCp or ΔΔCp of DNA binding by Taq/Klentaq. It is certainly possible, however, that such coupled unfolding/refolding may account for some of the ΔΔCp values observed in other protein-DNA systems in Table 1. Given the significant consequences that even very small ΔΔCp values have on the determination of ΔCp, and for any quantitative predictive application of heat capacity information, continued investigation of this effect seems warranted.
Kausiki Datta's present address is Institute of Molecular Biology, University of Oregon, Eugene, OR 97403.
Editor: David P. Millar.
References
- 1.Dill, K. A., and S. Bromberg. 2003. Molecular Driving Forces. Garland Science, New York.
- 2.Gill, S. J., S. F. Dec, G. Olofsson, and I. Wadso. 1985. Anomalous heat capacity of hydrophobic solvation. J. Phys. Chem. 89:3758–3761. [Google Scholar]
- 3.Roberston, A. D., and K. P. Murphy. 1997. Protein structure and the energetics of protein stability. Chem. Rev. 97:1251–1267. [DOI] [PubMed] [Google Scholar]
- 4.Privalov, P. L., and S. J. Gill. 1988. Stability of protein structure and hydrophobic interaction. Adv. Protein Chem. 39:191–234. [DOI] [PubMed] [Google Scholar]
- 5.Lundbäck, T., H. Hansson, S. Knapp, R. Ladenstein, and T. Härd. 1998. Thermodynamics characterization of non-sequence-specific DNA-binding by the Sso7d protein from Sulfolobus solfataricus. J. Mol. Biol. 276:775–786. [DOI] [PubMed] [Google Scholar]
- 6.Milev, S., A. A. Gorfe, A. Karshikoff, R. T. Clubb, H. R. Bosshard, and I. Jalesarov. 2003. Energetics of sequence-specific protein-DNA association: binding of interase Tn916 to its target DNA. Biochemistry. 42:3481–3491. [DOI] [PubMed] [Google Scholar]
- 7.Kozlov, A. G., and T. M. Lohman. 2006. Effects of monovalent anions on a temperature-dependent heat capacity change for Escherichia coli SSB tetramer binding to single-stranded DNA. Biochemistry. 45:5190–5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datta, K., and V. J. LiCata. 2003. Thermodynamics of the binding of Thermus aquaticus DNA polymerase to primed-template DNA. Nucleic Acids Res. 31:5590–5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guinier, A., and G. Fournet. 1955. Small Angle Scattering of X-Rays. Wiley, NY.
- 10.Guinier, A. 1939. La diffraction des rayons X aux tres petits angles; application a l'etude de phenomenes ultramicroscopiques. Ann. Phys. 12:166–237. [Google Scholar]
- 11.Semenyuk, A. V., and D. I. Svergun. 1991. GNOM—a program package for small-angle scattering data-processing. J. Appl. Cryst. 24:537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joubert, A. M., A. S. Byrd, and V. J. LiCata. 2003. Global conformations, hydrodynamics, and x-ray scattering properties of Taq and Escherichia coli DNA polymerases in solution. J. Biol. Chem. 278:25341–25347. [DOI] [PubMed] [Google Scholar]
- 13.Jen-Jacobson, L., L. E. Engler, J. T. Amers, M. R. Kurpiewski, and A. Grigorescu. 2000. Thermodynamic parameters of specific and nonspecific protein-DNA binding. Supramol. Chem. 12:143–160. [Google Scholar]
- 14.Datta, K., A. J. Wowor, A. J. Richard, and V. J. LiCata. 2006. Temperature dependence and thermodynamics of Klenow polymerase binding to primed-template DNA. Biophys. J. 90:1739–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Privalov, P. L., I. Jelesarov, C. M. Read, A. I. Dragan, and C. Crane-Robinson. 1999. The energetics of HMG box interactions with DNA: thermodynamics of the DNA binding of the HMG box from mouse Sox-5. J. Mol. Biol. 294:775–786. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien, R., B. DeDecker, K. G. Fleming, P. B. Sigler, and J. E. Ladbury. 1998. The effect of salt on the TATA binding protein-DNA interaction from a hyperthermophilic archaeon. J. Mol. Biol. 279:117–125. [DOI] [PubMed] [Google Scholar]
- 17.Bergqvist, S., M. A. Williams, R. O'Brien, and J. E. Ladbury. 2004. Heat capacity effects of water molecules and ions at a protein-DNA interface. J. Mol. Biol. 336:829–842. [DOI] [PubMed] [Google Scholar]
- 18.Oda, M., K. Furukawa, K. Ogata, A. Sarai, and H. Nakamura. 1998. Thermodynamics of specific and non-specific DNA binding by the c-Myb DNA-binding domain. J. Mol. Biol. 276:571–590. [DOI] [PubMed] [Google Scholar]
- 19.Gonzales, M., S. Weiler, J. A. Ferretti, and A. Ginsburg. 2001. The vnd/NK-2 homeodomain: thermodynamics of reversible unfolding and DNA binding for wild-type and with residue replacements H52R and H52R/T56W in helix III. Biochemistry. 40:4923–4931. [DOI] [PubMed] [Google Scholar]
- 20.Wang, X., W. Cao, A. Cao, and L. Lai. 2003. Thermodynamics characterization of the folding coupled DNA binding by the monomeric transcription activator GCN4 peptide. Biophys. J. 84:1867–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haq, I,. R. O'Brien, A. Lagunavicius, V. Siksnys, and J. E. Ladbury. 2001. Specific DNA recognition by the type II restriction endonuclease MunI: the effect of pH. Biochemistry. 40:14960–14967. [DOI] [PubMed] [Google Scholar]
- 22.Lundbäck, T., J. Chang, K. Phillips, B. Luisi, and J. E. Ladbury. 2000. Characterization of sequences-specific DNA-binding by the transcription factor Oct-1. Biochemistry. 39:7570–7579. [DOI] [PubMed] [Google Scholar]
- 23.Ladbury, J. E., J. G. Wright, J. M. Sturtevant, and P. B. Sigler. 1994. A thermodynamics study of the trp repressor-operator interaction. J. Mol. Biol. 238:669–681. [DOI] [PubMed] [Google Scholar]
- 24.Poon, G. M. K., P. Gross, and R. B. Macgregor, Jr. 2002. The sequence-specific association of the ETS domain of Murine PU.1. with DNA exhibits unusual energetics. Biochemistry. 41:2361–2371. [DOI] [PubMed] [Google Scholar]
- 25.Liggins, J. R., and P. L. Privalov. 2000. Energetics of the specific binding interaction of the first three zinc fingers of the transcription factor TFIIIA with its cognate DNA sequence. Proteins. 4:50–62. [DOI] [PubMed] [Google Scholar]
- 26.Hyre, D. E., and L. D. Spicer. 1995. Thermodynamics evaluation of binding interactions in the methionine repressor system of Escherichia coli using isothermal titration calorimetry. Biochemistry. 34:3212–3221. [DOI] [PubMed] [Google Scholar]
- 27.Lundbäck, T., and T. Härd. 1996. Sequence-specific DNA-binding dominated by dehydration. Proc. Natl. Acad. Sci. USA. 93:4754–4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moraitis, M. I., H. Xu, and K. S. Matthews. 2001. Ion concentration and temperature dependence of DNA binding: comparison PurR and LacI repressor proteins. Biochemistry. 40:8109–8117. [DOI] [PubMed] [Google Scholar]
- 29.Sieber, M., and R. K. Allemann. 2000. Thermodynamics of DNA binding of MM17, a “single chain dimer” of transcription factor MASH-1. Nucleic Acids Res. 28:2122–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poon, G. M. K., and R. B. Macgregor, Jr. 2004. A thermodynamics basis of DNA sequence selectivity by the ETS domain of murine PU.1. J. Mol. Biol. 335:113–127. [DOI] [PubMed] [Google Scholar]
- 31.Ha, J., R. S. Spolar, and M. T. Record, Jr. 1989. Role of the hydrophobic effect in stability of site-specific protein-DNA complexes. J. Mol. Biol. 209:801–816. [DOI] [PubMed] [Google Scholar]
- 32.Künne, A. G. E., M. Sieber, D. Meierhans, and R. K. Allemann. 1998. Thermodynamics of the DNA binding reaction of transcription factor MASH-1. Biochemistry. 37:4217–4223. [DOI] [PubMed] [Google Scholar]
- 33.Frank, D. E., R. M. Saecker, J. P. Bond, M. W. Capp, O. V. Tsodikov, S. E. Melcher, M. M. Levandoski, and M. T. Record, Jr. 1997. Thermodynamics of the interactions of lac repressor with variants of the symmetric lac operator: effects of converting a consensus site to a non-specific site. J. Mol. Biol. 267:1186–1206. [DOI] [PubMed] [Google Scholar]
- 34.Takeda, Y., P. D. Ross, and C. P. Mudd. 1992. Thermodynamics of Cro protein-DNA interactions. Proc. Natl. Acad. Sci. USA. 89:8180–8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dragan, A. I., C. M. Read, E. N. Makeyeva, E. I. Milgotina, M. E. A. Churchill, C. Crane-Robinson, and P. L. Privalov. 2003. DNA binding of a non-sequence-specific HMG-D protein is entropy driven with a substantial nonelectrostatic contribution. J. Mol. Biol. 331:795–813. [DOI] [PubMed] [Google Scholar]
- 36.Dragan, A. I., J. Klass, C. Read, M. E. A. Churchill, C. Crane-Robinson, and P. L. Privalov. 2004. DNA binding and bending by HMG boxes: energetic determinants of specificity. J. Mol. Biol. 343:371–393. [DOI] [PubMed] [Google Scholar]
- 37.Peters, W. B., S. P. Edmondson, and J. W. Shriver. 2004. Thermodynamics of DNA binding and distortion by the hyperthermophile chromatin protein Sac7d. J. Mol. Biol. 343:339–360. [DOI] [PubMed] [Google Scholar]
- 38.Spolar, R. S., and M. T. Record, Jr. 1994. Coupling of local folding to site-specific binding of proteins to DNA. Science. 263:777–784. [DOI] [PubMed] [Google Scholar]
- 39.Spolar, R. S., J. R. Livingstone, and M. T. Record. 1992. Use of liquid hydrocarbon and amide transfer data to estimate contributions to thermodynamic functions of protein folding from the removal of nonpolar and polar surface from water. Biochemistry. 31:3947–3955. [DOI] [PubMed] [Google Scholar]
- 40.Murphy, K. P., and E. Freire. 1992. Thermodynamics of structural stability and cooperative folding behavior in proteins. Adv. Protein Chem. 43:313–361. [DOI] [PubMed] [Google Scholar]
- 41.Makhatadze, G. I., and P. L. Privalov. 1995. Energetics of protein structure. Adv. Protein Chem. 47:307–425. [DOI] [PubMed] [Google Scholar]
- 42.Myers, J. K., C. N. Pace, and J. M. Scholtz. 1995. Denaturant m values and heat capacity changes: relation to changes in accessible surface areas of protein unfolding. Protein Sci. 4:2138–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karantzeni, I., C. R. Ruiz, C.-C. Liu, and V. J. LiCata. 2003. Comparative thermal denaturation of Thermus aquaticus and Escherichia coli Pol I type DNA polymerases. Biochem. J. 374:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kornblatt, J. A., and P. Schuck. 2005. Influence of temperature on the conformation of canine plasminogen: an analytical ultracentrifugation and dynamic light scattering study. Biochemistry. 44:13122–13131. [DOI] [PubMed] [Google Scholar]
- 45.Merabet, E., and G. K. Ackers. 1995. Calorimetric analysis of λ cI repressor binding to DNA operator sites. Biochemistry. 34:8554–8563. [DOI] [PubMed] [Google Scholar]
- 46.Petri, V., M. Hsieh, and M. Brenowitz. 1995. Thermodynamic and kinetic characterization of the binding of the TATA binding protein to the adenovirus E4 promoter. Biochemistry. 34:9977–9984. [DOI] [PubMed] [Google Scholar]
- 47.Morton, C. J., and J. E. Ladbury. 1996. Water-mediated protein-DNA interactions: the relationship of thermodynamics to structural detail. Protein Sci. 5:2115–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kozlov, A. G., and T. M. Lohman. 1999. Adenine base unstacking dominates the observed enthalpy and heat capacity changes for the Escherichia coli SSB tetramer binding to single-stranded oligoadenylates. Biochemistry. 38:7388–7397. [DOI] [PubMed] [Google Scholar]
- 49.Sturtevant, J. M. 1977. Heat capacity and entropy changes in processes involving proteins. Proc. Natl. Acad. Sci. USA. 74:2236–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lundbäck, T., S. van Den Berg, and T. Härd. 2000. Sequence-specific DNA binding by the glucocorticoid receptor DNA-binding domain is linked to a salt-dependent histidine protonation. Biochemistry. 39:8909–8916. [DOI] [PubMed] [Google Scholar]
- 51.Kozlov, A. G., and T. M. Lohman. 2000. Large contributions of coupled protonation equilibria to the observed enthalpy and heat capacity changes for ssDNA binding to Escherichia coli SSB protein. Proteins. 4(Suppl.):8–22. [DOI] [PubMed] [Google Scholar]
- 52.Cooper, A. 2005. Heat capacity effects in protein folding and ligand binding: a re-evaluation of the role of water in biomolecular thermodynamics. Biophys. Chem. 115:89–97. [DOI] [PubMed] [Google Scholar]
- 53.Dogan, J., C. Lendel, and T. Härd. 2006. Thermodynamics of folding and binding in an affibody:affibody complex. J. Mol. Biol. 359:1305–1315. [DOI] [PubMed] [Google Scholar]
- 54.Cliff, M. J., M. A. Williams, J. Brooke-Smith, D. Barford, and J. E. Ladbury. 2006. Molecular recognition via coupled folding and binding in a TPR domain. J. Mol. Biol. 346:717–732. [DOI] [PubMed] [Google Scholar]