Abstract
Mechanosensitive channels rescue bacterial cells from a fate of lysis when they transfer from a high- to low-osmolarity environment. Of three Escherichia coli mechanosensitive proteins studied to date, only MscS-Ec demonstrates a small anionic preference and a desensitized, nonconducting state under sustained pressure. Little is known about the mechanisms generating these distinctive properties. Eliminating the sole positive charge in the MscS-Ec pore region (Arg88) did not alter anionic preference. Adding positive charges at either end of the pore did not augment anionic preference, and placing negative charges within the pore did not diminish it. Thus, pore charges do not control this characteristic. However, from this analysis we identified mutations in the hinge region of the MscS-Ec pore helix (at Gly113) that profoundly affected ability of the channel to desensitize. Substitution with nonpolar (Ala, Pro) or polar (Asp, Arg, Ser) residues inhibited transition to the desensitized state. Interestingly, Gly113 replaced with Met did not impede desensitization. Thus, although Gly is not specifically required at position 113, MscS desensitization is strongly influenced by the residue situated here. Mutations at residues further into the pore also regulated desensitization. Transition to this unique mechanosensitive channel state is discussed in terms of existing data.
INTRODUCTION
MS channels respond to increases in transmembrane pressure transduced directly through the bilayer as increased tension (1–3). They represent a significant paradigm for the interactions between membrane proteins and the lipids of the bilayer. Changes in lipid packing that lead to modifications of tension within the plane of the bilayer are transduced into gating signals. In Escherichia coli, three genes for MS channels have been cloned: mscL, mscS and mscK (formerly known as kefA), and pressure activation of their protein products has been established using physiological and electrophysiological protocols (3–7). A fourth pressure-induced activity has also been detected in patch-clamp analysis, MscM (8), but no gene product has yet been assigned. Electrophysiological findings directed the nomenclature of the proteins: MscL, MS channel of large conductance; MscS, MS channel of small conductance; MscK, MS channel requiring extracellular K+ for activation; and MscM, MS channel of miniconductance, and highlighted the pressure threshold for gating as being a gradient from MscM, opening at the lowest pressures, to MscL, being activated by the greatest pressures—those just below lytic levels for the cell (6).
Crystal structures have been resolved for MscL from Mycobacterium tuberculosis (9) and for MscS from E. coli (10), and numerous studies have been conducted on their structure-function organization and mechanisms of gating (reviewed (11–13)). Biochemical analysis (14) and the structure of the E. coli MscS (10) revealed that this protein is a homoheptamer with each subunit containing a membrane domain with three TM spans followed by a large cytoplasmic carboxy-terminal domain (Fig. 1). The third TM helix (TM3) lines the channel lumen and then bends sharply at Gly113 to continue along the plane of the membrane at the membrane/cytoplasmic interface, connecting to the carboxy-terminal domain. Two Leu residues in the pore-lining section of TM3 (at positions 105 and 109) seal the channel securely against the passage of ions. The MscS channel pore attains a diameter calculated to be ∼14–16 Å in the open conformation (15). During gating it has been proposed that the TM helices undergo tilt and rotation movements (13) but that the cytoplasmic vestibule also realizes significant rearrangements (14,16). The internal diameter of the sphere-shaped cytoplasmic vestibule is ∼50 Å, and thus, for the bulk ion movement achieved during channel operation, a direct pathway from the cytoplasm to the pore is required. This is affected through lateral portals formed by the boundaries of the seven subunits at the interfaces between the two major domains that form the vestibule, the upper β and lower αβ domains (10). One characteristic of E. coli MscS (MscS-Ec) not shared by MscS from other organisms or by MscL channels is a slight preference for anions (3,7,17), and it has been suggested that the portals may influence this by acting as selectivity filters (10).
FIGURE 1.
Location of pore mutations in the MscS-Ec crystal structure. The heptameric structure of MscS-Ec (30) is depicted in full length as a side view (left panel) and as viewed from the top (bottom right), and a zoomed-in view of the pore region for two subunits is also shown (residues 80–127, chains B and E; top right). Each subunit backbone is drawn with residues mutated in this study highlighted by space filling. (Images created in Chime (32).)
A second unique characteristic of MscS-Ec channels is their ability to desensitize: on extended applications of subsaturating pressures, channels progress into an inactivated, nonconducting state from which they must attain the closed state before reactivation can be induced (6,16,18). Thus, desensitization/inactivation, with regard to MscS-Ec, is principally reversible. The mean open dwell time for MscS-Ec channels is ∼150 ms (13) at low activating pressures in membrane patches, i.e., pressures required to open only one or two channels in each membrane patch tested. It has been proposed that the desensitization at higher, but submaximal, pressures could provide a mechanism of protecting cells against perturbation of homeostasis after gating. In cells, MS channels gate in response to excessive turgor pressure that modifies the tension in the plane of the membrane bilayer. The opening of relatively nonspecific channels with a high capacity for hydrated solute movement can relieve the pressure by allowing bulk flow of solutes from cytoplasm to environment but also has the potential to equilibrate ion gradients, including those for H+ and Na+, ions that are normally excluded from the cells. Therefore, returning of the channels to a nonconducting state is critical to cell function, and mechanisms that ensure rapid closure after gating are important.
We have previously shown that deletion of the last 21 amino acids from each subunit (which removes a β-barrel structure) interferes with the capacity of the channels to transform from the desensitized state back to a configuration that can be reopened (19), but the molecular basis of desensitization is unknown. In this study, we have probed the mechanisms of MscS anionic preference and desensitization by examining mutations placed within and around the pore-lining helix TM3. We find that addition or removal of rings of positive or negative charge at either end of the pore does not alter ionic preference. However, the mutations assayed generally affected pressure sensitivity of the channel and exhibited an unexpected attribute: lack of desensitization. Further mutations, engineered to explore the importance of Gly113 for the desensitization state, revealed that the particular amino acid at position 113 is key, but not restricted to a Gly residue. Our findings that residues throughout the length of TM3 regulate transformation to the desensitized conformation are discussed in relation to the current models of MscS protein transitions.
MATERIALS AND METHODS
Bacterial strains
Strains MJF465 (mscL∷Cm, mscS−, mscK∷Kan) and MJF429 (mscS−, mscK∷Kan) were used to express the MscS plasmids for both biochemical and physiological analyses and have been described previously (6). Strain PB114 (recA∷Tn10, mscS−, mscK∷Kan, yjeP−), a kind gift from Paul Blount (UT Southwestern Medical Center at Dallas, TX), was used in some initial electrophysiology experiments. Cells were grown at 37°C in LB medium containing (per liter): 10 g tryptone, 5 g yeast extract, and 5 g NaCl, and strains were stored at 4°C on LB plates containing ampicillin (25 μg/ml). Ampicillin (25 μg/ml) was also added to overnight cultures to ensure that plasmids were maintained. Solid medium contained 14 g/liter agar. Chloramphenicol and kanamycin were used at 12.5 μg/ml and 50 μg/ml, respectively, when required.
DNA manipulations
The plasmids expressing wild-type MscS—pMscS, pMscSH6, and pETMscSH6—have been described previously (6,20). Site-directed mutagenesis (Stratagene Quickchange protocol) was used to introduce point mutations into the cloned wild-type gene, with successful substitutions being identified by engineered restriction sites and confirmed by full sequencing (twice on each strand) (20). Primers were manufactured by Sigma-Genosys (Dorset, UK), sequencing was carried out by the Dundee Sequencing Service (University of Dundee, Dundee, UK), and gene sequences were analyzed using the DNASTAR suite of programs (DNASTAR, Madison, WI).
Membrane preparations and Western blotting
Plasmids were transformed into MJF465. Cells were grown to OD650 ≈ 0.4 and induced with 0.3 mM IPTG for 30 min before being harvested and lysed by French press (18,000 psi), as described previously (20). Membrane fractions were isolated by differential centrifugation (20) and resuspended in phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 10 mM NaH2PO4, 1.4 mM K2HPO4, pH 7.4). Protein concentration was assayed by the Folin-Ciocalteau method (21), and Western blots were generated as described (20). The primary antibody used was peptide-specific, anti-YggB, and the secondary antibody was peroxidase-conjugated, antirabbit IgG (20).
Physiological assays
To examine the phenotype induced by mutations, plasmids were transformed into MJF465 cells. To assess if mutations caused a GOF phenotype, cells were serially diluted immediately after recovery from transformation, and 5-μl spots of each dilution were pipetted onto an agar plate containing ampicillin and in the presence or absence of 1 mM IPTG. Extent of colony growth was recorded after incubation at 37°C overnight, a GOF phenotype being signaled by reduced growth and/or unhealthy, translucent colonies. At least two replicates were analyzed for each mutant.
Channel function was assessed by investigating the survival of MJF465 cells expressing the mutant proteins after downshock from LB medium containing 0.3 M NaCl to LB, as previously described (20). Experiments have been replicated a minimum of three times.
Electrophysiology
MscS plasmids were introduced into strain MJF429 (6), and patch-clamp recordings (22) were conducted on inside-out excised patches from giant protoplasts as described previously (13). Protein expression was sometimes induced with 1 mM IPTG (15–45 min) to improve the probability of locating channels within each membrane patch. Recordings were made at room temperature in either symmetrical (200 mM KCl in both pipette and bath) or asymmetric (200 mM KCl in the pipette and 600 mM in the bath) solutions containing 90 mM MgCl2, 10 mM CaCl2, and 5 mM HEPES buffer at pH 7.1 ± 0.1. Asymmetric solutions were used to examine ionic preference by compiling current-voltage plots and determining the voltage at which zero net current passed through the channel on pressure application. Data were acquired at a membrane potential of −20 mV or at 10 mV intervals between −50 mV and +50 mV for the current-voltage experiments, using an AXOPATCH 200B amplifier and pCLAMP9 software (Molecular Devices, Union City, CA), at a sampling rate of 50 kHz with 5-kHz filtration. To assess the pressure threshold for activation of the MscS channels, a pressure ratio (PL:PS) was determined for each mutant by comparing the pressure required to gate MscL with that required to open the MscS channels in the same patch, as employed previously (13). Dwell times of the open state and measurements of ion current for calculation of conductance were undertaken using all-points histograms within the pCLAMP software. Data have been obtained from at least two protoplast preparations for each MscS mutant channel, and statistical comparisons were carried out using the Student's t-test.
Materials
Media components were purchased from Oxoid (Basingstoke, UK), and all salts were obtained from Sigma (Dorset, UK) or VWR (Lutterworth, UK). Restriction enzymes were obtained from Roche Diagnostics Ltd (Burgess Hill, UK) or Promega (Southampton, UK), and precast NOVEX SDS-polyacrylamide gels were from Invitrogen-Life Technologies (Paisley, UK). The SUPERSIGNAL Dura substrate for Western blots came from Perbio (Northumberland, UK).
RESULTS
Expression and physiology of charge-change mutants
The MscS-Ec pore created by the packing of the TM3A helices is predominantly hydrophobic in character with only Arg88 at the external mouth potentially contributing a selectivity filter. To assess the significance of charged residues in the pore for ionic preference, single point mutations were created at residues Arg88, Gly101, and Gly113 in the cloned mscS gene. These mutation sites represent opportunities to remove or to introduce charged residues into the outer, middle, and inner regions of the pore (Fig. 1). Substitution of Gly at position 113 with either a negative amino acid (G113D) or a positive amino acid (G113R) or removal of the positively charged residue at position 88 (R88S) produced MscS mutant proteins that expressed wild-type levels in the membrane after induction with IPTG (0.3 mM for 30 min; Fig. 2). Mutation of the Gly at position 101 to Asp (G101D) also allowed expression in the membrane (Fig. 2); however, we were unable to create G101R within our standard vector system. Our pMscSH6 plasmid contains the pTrc promoter that allows basal levels of gene expression in the absence of induction, and the lack of successful G101R creation suggests that this substitution likely produces a protein that reduces cell viability. We have previously described a GOF mutation, T93R, that introduces a ring of positive charge at the mouth of the pore (20). This mutant protein also expresses similar levels as the wild-type protein. Assessment of growth of MJF465 cells expressing either G113D, G113R, or R88S showed no effect, even in the presence of IPTG; however, G101D impaired colony formation when overexpressed (data not shown), and the GOF phenotype of T93R has previously been reported (20).
FIGURE 2.
Membrane expression of mutant MscS proteins. Mutant channels were overexpressed in MJF465 cells (30 min 0.3 mM IPTG), and membrane protein samples were extracted, separated by SDS-PAGE, transferred to nitrocellulose membrane, and probed with anti-YggB antibody. (Left-hand blot) 1, markers; 2, MJF465 cells containing no plasmid; 3, pMscSH6 (His-tagged wild-type MscS); 4, R88S pMscSH6; 5, T93R pMscSH6; 6, G101D pMscSH6. (Right-hand blot) 1, markers; 2, MJF465 cells containing no plasmid; 3, pMscSH6 (His-tagged wild-type MscS); 4, G113A pMscS; 5, G113D pMscSH6; 6, G113M pMscS; 7, G113P pMscS; 8, G113R pMscSH6; 9, G113S pMscS. Note that some of the G113 mutants were constructed in a non-His plasmid, and accordingly, these mutant proteins run slightly faster on the gel (lanes 4, 6, 7, and 9).
A more direct assay of channel function can be ascertained from their ability to provide resistance to hypoosmotic shock. Mutant channels that have reduced function confer impaired protection when expressed at low levels but can provide some protection when overexpressed. In contrast, GOF mutants generally protect when expressed at low levels but are inhibitory when expression is induced (23). Although G113D and G113R MscS channels exhibited low protection without induction, complete protection was observed for G113D, G113R, and R88S MscS channels with overexpression, whereas G101D-mutated channels provided only minimal protection to down-shocked cells (not shown). Thus, of the four charge-change mutants studied here, only one, G101D, significantly affected physiological activity of MscS.
Patch-clamp analysis of pressure sensitivity of charge-change mutants
For electrophysiological analysis of the charge-change mutants, the wild-type and mutant plasmids were expressed in MJF429, an E. coli strain deleted for the mscS and mscK genes but that retains MscL expression (6). This allowed comparison of the gating pressure for MscS channels with the pressure required to open MscL within the same patch (PL:PS ratio (4)). The mean PL:PS ratios for G113D and G113R MscS were 1.31 ± 0.03 (n = 7) and 1.43 ± 0.03 (n = 8), respectively, compared with 1.62 ± 0.03 (n = 15) for the wild-type MscS. Thus, these channels required greater pressure than wild-type to open (p < 0.05 for both G113D and G113R versus wild-type), consistent with the levels of protection these mutant channels provided during hypoosmotic shock. R88S channels also exhibited a significantly increased threshold for gating with an average PL:PS ratio of 1.39 ± 0.03 (n = 5; p < 0.05). In contrast, the T93R mutation decreased the threshold for opening (PL:PS ratio 1.8 ± 0.07, n = 3; p < 0.05), which is in agreement with the GOF nature of this mutant in physiological analysis (20). G101D channels appeared to open at variable pressure levels (PL:PS range = 1.33–1.83, n = 6), with a median value of 1.62. Channels with short open dwell times may give misleading PL:PS ratios; if channels stay open for less time, the pressure reading recorded for the simultaneous opening of two MscS channels may be erroneously high, thus producing a lower PL:PS value and masking increased pressure sensitivity. The open state of the G101D mutant protein was less stable than the wild-type, with open dwell times ∼10-fold shorter for G101D channels (data not shown) compared with wild-type activity (13). Thus, this characteristic may cause the variability seen for the G101D channel gating pressures. All other mutants, apart from G101D, exhibited wild-type-like openings and closures (data not shown). The conductance of each mutant tested here was similar to the mean of 1.25 nS reported previously for wild-type MscS (13), although G113D MscS channels tended to exhibit a ∼10% decrease in conductance (p < 0.05) (data not shown).
Current-voltage relation and charged residues in the pore region
Arg88 lies at the top of TM2 and probably forms part of the upper rim of the MscS pore (10); no other charged amino acids appear within the sequence lining the pore lumen. To assess whether the anionic preference of MscS is influenced by the mutations introduced into the channel, the current-voltage relation was measured for the mutant channels and compared with that of the wild-type. The reversal potentials derived from the plotted data show that removal of the positive charge in the outer region of the MscS pore (R88S) does not alter ionic preference in asymmetric solutions (Fig. 3 A and Table 1). Similarly, introduction of a second ring of positive charges in that region, by mutation T93R, did not significantly change the reversal potential for the channel (Fig. 3 A and Table 1). Furthermore, placing a ring of Arg at the cytoplasmic end of the pore (G113R) also had no effect on the reversal potential (Fig. 3 A and Table 1). Introduction of negatively charged amino acids into the pore was also without effect on the reversal potential: neither G101D (toward the periplasmic end) nor G113D (at the cytoplasmic end) modified the anion preference (Fig. 3 B and Table 1).
FIGURE 3.
Current-voltage properties of mutant MscS channels. Protoplasts containing either wild-type or mutant MscS channels were prepared. Excised patches were bathed in symmetrical solutions (200 mM KCl), and channel activity was recorded from holding potentials of −50 mV to +50 mV (10-mV steps). The bath solution was then replaced with buffer containing 600 mM KCl, and channel activity was recorded again at the same potentials. Single-channel current is plotted against membrane voltage for both sets of recording conditions (mean ± SE). (A) Plots for wild-type (WT) MscS and single-point mutants with a positively charged Arg either removed or inserted. (B) Plots for single-point mutants where a negatively charged Asp has been added to the pore region. (C) The mean current (+ SE) measured at +50 mV in symmetrical solutions is compared for each channel. Only G113R channels exhibited significantly lower levels of current compared with wild-type channels (p < 0.05, Student's t-test). (D) The deletion mutant Δ266–286, where the last 21 amino acids are absent from each subunit, shows no significant change in reversal potential in asymmetric solutions; however, this mutant channel exhibits a much larger conductance than wild-type channels at positive membrane potentials in both symmetrical and asymmetric solutions.
TABLE 1.
Reversal potentials for MscS mutant channels
| Channel | Erev (mV) |
|---|---|
| Wild-type | −9.5 |
| R88S | −11.3 |
| T93R | −11.0 |
| G101D | −8.0 |
| G113A | −10.0 |
| G113D | −12.4 |
| G113M | −10.0 |
| G113R | −9.3 |
The mean reversal potential (Erev) was determined from the current-voltage plots recorded for each mutant. None of the mutant reversal potentials were significantly different from that of wild-type MscS channels (p > 0.05 for each mutant channel reversal potential versus wild-type).
The current-voltage plot for wild-type MscS in symmetrical solutions displays rectification at positive membrane potentials (pipette negative) (Fig. 3 A). Sukharev and colleagues (3,7) showed that the change in current did not parallel the increase in applied voltage across the patch. This rectification is also experienced in asymmetric solutions (Fig. 3 A). We found that each mutant channel demonstrated this phenomenon but that the substitution G113R produced severe rectification in the presence of both balanced and unbalanced KCl (Fig. 3). Comparison of peak current amplitudes in symmetrical solutions at +50 mV for each mutant showed that only G113R differed significantly from wild-type amplitudes at this membrane potential (Fig. 3 C; p < 0.05 for G113R versus wild-type); in fact, very little total increase in current was measurable between membrane potentials of +10 and +50 mV for G113R. As previously noted (24), we observed that channels often opened to substate levels at higher depolarizing potentials (pipette negative) and that openings tended to “flicker” rapidly between the substate and fully open conductance, resulting in a decrease in the time that channels spent in the fully open state. For wild-type channels, this occurred from +40 mV. This behavior was greatly augmented in G113R channels, which flickered in and out of substate(s) at much lower potentials (from +20 mV) (Fig. 4). The “flickery” activity persisted and intensified at higher voltages, which made accurate measurement of the fully open current amplitude difficult. This effect, therefore, is likely responsible for the apparent strong rectification seen in G113R current-voltage plots.
FIGURE 4.
Mutation G113R induces visits to substates at lower membrane potentials. Zoomed-in sections of traces illustrating single channel openings for (A) wild-type (WT) and (B) G113R MscS channels at +20 mV membrane potential. Wild-type channels tend to open or close at this voltage and rarely visit subconducting states; however, G113R channels flicker in and out of substates. Usually this occurs rapidly (see examples i and ii), but sometimes the protein will remain in the subconducting conformation for tens of milliseconds (see example ii, arrow).
The current-voltage relation of two further mutants (created to assess desensitization; see below) were also studied as controls for amino acid changes at position 113 that did not involve addition of charge. The reversal potentials seen for G113A and G113M mutant MscS channels were not significantly different from those of wild-type channels (Table 1).
We have previously described an MscS-Ec mutation in which the residues forming the immediate carboxy-terminal sequence have been deleted: Δ266–286 (19). This mutant channel assembles in the membrane, gates with applied pressure, and protects against hypoosmotic shock when overexpressed. The deletion removes the sequence that forms the base of the vestibule and the carboxy-terminal β-barrel and potentially creates a large (∼25–30 Å diameter) opening at the base of the vestibule. Current-voltage measurements of Δ266–286 MscS exhibited a higher conductance than the parent channel, and this effect was most marked at positive membrane potentials, suggesting that a kinetic constraint on ion permeation had been relieved (Fig. 3 D). However, the ionic preference of the channel remained unchanged: in asymmetric solutions the zero current was observed around −14 mV. Thus, although the deletion increased ion conduction, it did not modify the level of selectivity.
Mutations at position 113 eliminate desensitization of MscS
Desensitization is a unique characteristic of MscS-Ec (6,7,18) and has not been observed in other members of the family that have been analyzed using electrophysiology. The basis of this phenomenon is poorly understood. While examining the above point mutations, we noticed that channels with substitutions at Gly113 showed unusual characteristics on extended pressure applications. A defined protocol was employed to test the propensity of mutant channels to desensitize: after an excised membrane patch was obtained, the number of MscS channels present in the patch was determined by transient (<5 s) pressure applications, and then the pressure required to activate all the MscS channels in the patch, but no MscL channels, was applied and clamped. The activity of the MscS channels was recorded either until full desensitization was observed (ensured by continuing to hold pressure for 1 min after the last channel closure) or for 4–5 min (or until the patch burst, if sooner). We found that all the wild-type MscS channels within an excised patch typically inactivated within 2 min (>80% of patches; n = 14/17 patches tested) (see Fig. 5 for representative example (19)); patches in these experiments contained between 3 and 30 channels each. In contrast, MscS channels incorporating either G113D or G113R mutations (between 2 and 40 channels per patch) continued to close and reopen and/or remained open over minutes of sustained pressure (n = 6 for G113D and n = 5 for G113R, pressure-clamped for at least 2 min; Fig. 5). Thus, insertion of a bulky charged residue at position 113 lead to loss of the ability of the channel to attain the desensitized state.
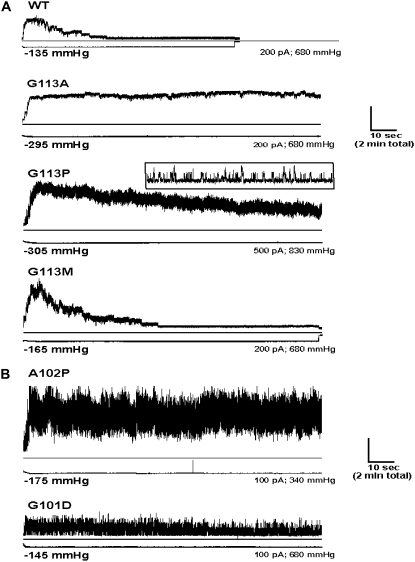
FIGURE 5.
Bulky, polar substitutions at residue 113 inhibit desensitization of MscS-Ec. Proteins containing a substitution at position 113 to Asp, Arg, or Ser were expressed in MJF429 cells, and protoplasts were prepared. Pressure was applied to excised membrane patches and clamped once all MscS channels had opened. Channel activity was followed under the maintained stimulus for up to 4 min, and 2 min of representative example recordings are depicted. For each example, traces show total current (channel activity) in upper panel and pressure level in lower panel (the actual pressure applied for each example is indicated under the pressure trace). Wild-type channels desensitize fully within this time, but G113D, G113R, and G113S MscS channels continue to close and reopen for as long as pressure is held.
We have previously analyzed a G113S mutant MscS channel that was essentially wild-type in physiological and electrophysiological assays (13). Desensitization of G113S MscS was tested here under the same conditions as above and showed that insertion of a Ser at this position caused a reduced capacity of the channel to inactivate under the above regime: over a 2-min period, on average 60% of the G113S channels closed at any one time under maintained pressure, but channels present continued to reopen and did not show desensitization (n = 5; Fig. 5). In light of the above findings, we engineered three further mutations at G113 to encompass amino acids of varying nature and analyzed their effects on desensitization. We found that MscS mutants G113A, G113P, and G113M all expressed in the membrane, although levels of G113P were lower than that of wild-type MscS (Fig. 2). Substitution with Ala significantly decreased the pressure sensitivity of MscS (PL:PS ratio 1.45 ± 0.04, n = 8; p < 0.05) and inhibited desensitization (n = 4; Fig. 6 A). Thus, even a small, nonpolar side chain at this “hinge” position disrupted attainment of the desensitized state. Both Pro and Gly residues are known to induce kinks in TM helices (25,26). Assessment of G113P MscS showed that this exchange appeared to decrease the protein's sensitivity to pressure (PL:PS ratio 1.33 ± 0.03, n = 5; p < 0.05) but also decreased stability of the channel in the open conformation, resulting in a flickery appearance of patch-clamp recordings as the channels rapidly alternated between the closed and open states (Fig. 6 A, inset). Further, these mutant channels commonly opened to a single-channel conductance level of only ∼750 pS and appeared unable to achieve the fully open state because open dwell times were not sufficiently long to capture measurable larger conductance levels with our recording system. On application of continuous pressures, similar to G113S MscS, a proportion of G113P channels closed at any one time, but openings and closings continued throughout (n = 3; Fig. 6 A). Thus, again, the ability to transform into the desensitized state had been diminished, this time by the presence of a bulky nonpolar group. In contrast, placement of Met at position 113 produced an MscS protein with wild-type-like characteristics. Channels containing G113M gated with a PL:PS ratio of 1.54 ± 0.04 (n = 7; p > 0.05 versus wild-type) and reversibly desensitized when pressure was clamped (n = 5; Fig. 6 A).
FIGURE 6.
Mutations at a number of TM3 sites block MscS-Ec desensitization. Experiments were carried out as described for Fig. 5, and representative current and pressure traces are similarly depicted. (A) Gly113 is not essential for desensitization. Although substitutions G113A and G113P removed the ability of the protein to enter the desensitized state, mutation G113M had no such effect. Placement of Pro at 113 shortened channel open dwell times, producing a flickery channel activity. Inset illustrates these single channel openings at an increased time scale. (B) Mutations at Ala102 and Gly101 also eliminated the capacity for desensitization. As detailed in the main text, A102P and G101D channels gated with shorter open dwell times, thus producing the flickery activity observed in their current traces. Also, A102P channels exhibited smaller conductance—about half of wild-type MscS—and thus the figure depicts a patch where ∼10–20 channels are open at any one time.
Mutation of residues in the pore can also impair desensitization
We have previously described an extreme GOF mutation, A102P MscS (20), that reduced growth of cells at low levels of expression and completely blocked growth when overexpressed. Here, electrophysiological characterization revealed that channels with a Pro inserted in this position behaved in a similar manner to Pro substitution at position 113: unstable in the open state, displaying extremely short open dwell times. A102P channel openings were so rapid as to preclude accurate measurement of the open-state conductance with the temporal resolution of our equipment, but a common level of current could be observed from a “stretch” of recording that suggested that the maximum single-channel open-state conductance was about half that of wild-type channels, ∼625 pS (data not shown). These mutant channels, as expected, opened at lower pressures than wild-type MscS (PL:PS ratio 1.71 ± 0.08, n = 3), and assessment of A102P channels under sustained pressure showed that as well as this mutant being unable to gate properly, it was also inhibited in attaining the desensitized conformation (n = 3; Fig. 6 B). Interestingly, G101D MscS channels also lost the ability to desensitize (n = 3; Fig. 6 B). Our data illustrate how residues throughout the pore-lining helix can influence the conformational changes of MscS into the desensitized state.
DISCUSSION
This study presents analysis of two important defining characteristics of the MscS-Ec channel through mutagenesis of residues in the pore region. First, we investigated the effect of adding or removing charged amino acids on the slight anionic preference of MscS conductance. Although only a weak preference for anionic species, this property of MscS has always been reproducibly reported, irrespective of the specific recording conditions (1,3,7,17). Substituting positive or negative amino acids into the MscS pore region creates a ring of charge because of the heptameric structure of the protein. In contrast, deleting a charged residue will eliminate a band of charge. A caveat to all genetic studies is that the analysis is, by definition, of a mutated form of the protein and must be interpreted in the light of other changes, such as expression and intrinsic activity. For MscS these parameters are expression level (representing assembly and stability of the heptamer), gating threshold (representing the ease of the structural transition to the open state), conductance (representing the diameter of the open pore), and open dwell time (representing the relative stabilities of the open and closed forms of the channel) (23). With respect to ionic preference, it is reasonable to expect that despite changes in gating threshold pressure, the permeation pathway is not significantly affected if the other parameters remain essentially constant. Of the mutants analyzed here, only G113D showed a significant reduction in conductance, although several mutants exhibited modified pressure sensitivity. In no example did introduction or removal of charged residues significantly modify the anionic preference.
The absence of effects arising from introduction of charged residues into the pore on anionic preference suggests that the intrinsic character of the open pore and/or the structure of the cytoplasmic lateral portals determine the anionic inclination. Deletion of the base of the vestibule, which potentially creates a very large portal, 25–30 Å in diameter, did not change the anionic preference of the MscS-Ec channel but did increase its conductance. Thus, it seems that the portals to some extent restrict flow to the cytoplasmic end of the pore. The residues surrounding the lateral portals include many Arg residues that impart a strong electropositive character, which could attract anions and may be sufficient to produce the preference observed. Alignment of the MscS-Ec protein sequence with homologs from Methanococcus jannaschii, archaeon MS channels that exhibit cation preference (27), shows that there are fewer positively charged and polar residues in the sequences potentially bordering the M. jannaschii portals (Supplementary Material, Fig. S1). This would be consistent with a role for the MscS-Ec portals in “crowding” negative ions. Given the requirement for large-scale movement of ions from the cytoplasm through the portals to the pore, the data suggest that selectivity may be mediated through creation of ion clouds around the portals. The overall preference for anions is quite small when compared with the selectivity of K+- or Cl−-specific channels (28). The interior of the vestibule is coated with polar groups that might also form the basis for an enrichment of Cl− ions. It is notable that Cl− binding in ClC-type transporters and channels is mediated via amide groups from the peptide backbone (29), and this interaction might be augmented in MscS channels by the Asn and Gln residues within the vestibule.
MscS channels have been demonstrated to occupy at least three distinct states: closed, open, and desensitized (6,19,24). Although closed and open are relatively self-explanatory terms (despite the lack of clear data on the structures that they represent), our understanding of the desensitized state is poor. In this study we have identified a number of amino acid substitutions that modify the kinetics of desensitization. The exact amino acid residue at position 113 has been shown to play a significant role in determining whether the channel desensitizes. Gly (the native residue) and Met allowed the channel to enter the desensitized state with similar kinetics, whereas the presence of Asp, Arg, Ala, Ser, or Pro inhibited attainment of the desensitized state. In the crystal structure, the α-carbon of Gly113 is exposed to the vestibule (30), and consequently, there are no obvious packing constraints that would prevent substitution with a range of residues of different sizes and characters. This is confirmed here by the incorporation of the Gly113 mutant proteins into the membrane and their pressure-induced activity in patch-clamp analysis. The failure to see desensitization does not appear to be related to a requirement for higher pressures to open the channels because although the pressure thresholds for many of the Gly113 mutant channels are higher than for the wild-type, there is little correlation evident between the gating pressure ratio and the tendency to desensitize (Table 2). A surprising finding was that G101D and A102P mutations also led to inhibition of desensitization (additionally, we have identified other mutations throughout the sequence lining the pore, created as part of other projects, that do not desensitize, for example, L109A and A102G/A103G; S. S. Black, M. D. Edwards, W. Bartlett, S. Miller, and I. R. Booth, unpublished data). These intriguing results not only highlight the complexity of the transition to the desensitized state but also emphasize the importance of a number of different positions within TM3 that impact on this transition.
TABLE 2.
Pressure ratios and desensitization characteristics of MscS mutant channels
| Channel | PL:PS* | Desensitize under Pr-clamp§ |
|---|---|---|
| Wild-type | 1.62 ± 0.03 | Yes |
| G113D | 1.31 ± 0.03 | No |
| G113R | 1.43 ± 0.03 | No |
| G113S | 1.53 ± 0.04† | No |
| G113A | 1.45 ± 0.04 | No |
| G113P | 1.33 ± 0.03 | No |
| G113M | 1.54 ± 0.04 | Yes |
| G101D | [1.62]‡ | No |
| A102P | 1.71 ± 0.08 | No |
The pressure ratio (PL:PS ratio) is shown for wild-type MscS and each mutant; mean ± SE.
PL:PS ratio given is the published value (13).
G101D channels exhibited variable pressure ratios (ranging from 1.33 to 1.83), and thus the median value is given.
Channels were assessed for their ability to desensitize in a wild-type-like manner (“Yes”): sufficient pressure was applied to the membrane patch to open all MscS channels present, and then the pressure was clamped and channel activity followed. Failure to desensitize is indicated as “No”.
The introduction of an Arg residue at position 113 (G113R) has significant effects on the channel properties. Not only do the channels fail to desensitize, but they also exhibit an increased tendency to visit subconducting states when small negative voltages are applied to the periplasmic side of the channel (in the pipette); this does not occur in the wild-type channel until greater negative holding potentials are experienced. Under such polarized conditions, positive ions will flow from the bath into the pipette when channels open, and negative ions will flow in the opposite direction. In wild-type MscS at high negative pipette voltages, the increased probability of conducting to substate levels has been suggested to result from detachment of some TM1-TM2 pairs from their interaction with the pore helices and that the substates are intermediates on the pathway to the inactivated state (24). However, given the tendency for channels to flicker rapidly between sub- and full conductance states (before/if they inactivate at all), it seems likely that this is not the full explanation. The functional significance of subconducting states in MscS channels is not obvious and is complicated by the fact that they tend to occur at the higher membrane potentials for wild-type channels. In cells, MscS opens after MscM and MscK and is, therefore, likely to be most frequently open in cells in which the membrane potential is close to zero. It is possible that Arg placed at residue 113 exaggerates the substate phenomenon because of its size and positive charge. The side chain of Arg is relatively long, and, depending on its orientation as the channel visits the open state under positive membrane potential conditions, it could act as a semiseal at the bottom of the pore. Thus, Arg at position 113 may both reduce conductance and intermittently repel a quantity of positive species as they attempt to enter the channel lumen, even at smaller depolarizing potentials, leading to the transient subconductance levels observed.
In wild-type MscS, the rate of desensitization is essentially inversely proportional to the magnitude of pressure (16). Further, if saturating pressures are applied (defined for MscS channels in patch clamp as a level where all MscS channels within a patch are open and are at equilibrium, no longer opening and closing), no inactivation occurs (7). Thus, the desensitized state appears to be strongly linked with the tension-sensing mechanism of the channel. Our initial observations that Gly113 mutations did not respond to clamped pressures as wild-type channels do were surprising, but by using a specific protocol to open channels yet not “lock” them in the open state, we were able to strictly characterize the desensitization properties of the mutants. The first data set for substitutions at position 113 (G113D, G113R, G113S) indicated that a Gly residue at this bend in the TM3 helix could be necessary for the protein to transform into the desensitized conformation and that replacing it with a large polar amino acid blocked the required helix movement to enter that state. Subsequent mutations to small or bulky nonpolar residues (G113A, G113P) also created proteins inhibited in achieving the desensitized state, even though Pro residues are similar to Gly in terms of their helix-breaking property. We found no correlation between size or charge of amino acid at position 113 and the capacity of the channel to desensitize. Unexpectedly, mutation G113M (Met being an amino acid proposed to support α-helix formation, not bend it) allowed MscS to respond as wild-type channels do to continuous pressure, raising questions as to what the exact requirements of the residue at this position are, for the desensitized conformation, and why substitutions with such different characters can interfere?
The precise conformation of MscS-Ec from which the desensitized state can be entered is at present unclear. A recent study showed that the inactivation process is voltage dependent, where although MscS has a natural tendency to desensitize, the rate is markedly faster at higher positive membrane potentials (pipette negative) (24). The authors also describe the decrease seen in the total number of channels that open within a patch when pressures are applied more slowly, and they propose that this occurs because some of the channels have “flipped” directly into the inactivated state, bypassing the open conformation. Thus, they suggest that MscS may enter the desensitized state from at least two conformations: open and closed. An alternative interpretation is that at high pressures, when the open probability is very high (∼1), MscS is unable to visit the desensitized conformation from the fully open state. Hence, it may be that desensitization can be achieved only from closing conformations. The MscS open state is proposed to involve expansion of the pore diameter, which must include a degree of unpacking via movement of the TM3 helices (13,15). The general consensus (10,11,31) is that the TM3 helices in the current crystal structure are packed as closely as theoretically possible. It follows that opening to a wider pore diameter must involve helix separation. One can hypothesize that the pathway from the “less packed” open state to the closed conformation is influenced by the residues of the pore-lining TM3 helices. Introduction of mutations may predispose the open-to-closed transition to follow a path that avoids an alternative desensitized state. Continued mutational analysis using both electrophysiological and biochemical techniques will allow us to further probe the complex and intriguing mechanism of desensitization in MscS-Ec.
SUPPLEMENTARY MATERIAL
To view all of the supplemental files associated with this article, visit www.biophysj.org.
Acknowledgments
The authors thank Samantha Miller, Susan Black, Ulrike Schumann, Tim Rasmussen, Paul Blount, and Yuezhou Li for their helpful discussions during this work and Sally Dennison for technical assistance with mutant creation.
This research was supported by The Wellcome Trust (grant No. 040174).
Abbreviations used: MS, mechanosensitive; GOF, gain-of-function; TM, transmembrane.
Editor: Richard W. Aldrich.
References
- 1.Martinac, B., M. Buehner, A. H. Delcour, J. Adler, and C. Kung. 1987. Pressure-sensitive ion channel in Escherichia coli. Proc. Natl. Acad. Sci. USA. 84:2297–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berrier, C., A. Coulombe, C. Houssin, and A. Ghazi. 1989. A patch-clamp study of ion channels of inner and outer membranes and of contact zones of E. coli, fused into giant liposomes. FEBS Lett. 259:27–32. [DOI] [PubMed] [Google Scholar]
- 3.Sukharev, S. I., B. Martinac, V. Y. Arshavsky, and C. Kung. 1993. Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys. J. 65:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blount, P., S. I. Sukharev, P. C. Moe, S. K. Nagle, and C. Kung. 1996. Towards an understanding of the structural and functional properties of MscL, a mechanosensitive channel in bacteria. Biol. Cell. 87:1–8. [PubMed] [Google Scholar]
- 5.Moe, P. C., P. Blount, and C. Kung. 1998. Functional and structural conservation in the mechanosensitive channel MscL implicates elements crucial for mechanosensation. Mol. Microbiol. 28:583–592. [DOI] [PubMed] [Google Scholar]
- 6.Levina, N., S. Totemeyer, N. R. Stokes, P. Louis, M. A. Jones, and I. R. Booth. 1999. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 18:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sukharev, S. 2002. Purification of the small mechanosensitive channel of Escherichia coli (MscS): the subunit structure, conduction, and gating characteristics in liposomes. Biophys. J. 83:290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berrier, C., M. Besnard, B. Ajouz, A. Coulombe, and A. Ghazi. 1996. Multiple mechanosensitive ion channels from Escherichia coli, activated at different thresholds of applied pressure. J. Membr. Biol. 151:175–187. [DOI] [PubMed] [Google Scholar]
- 9.Chang, G., R. H. Spencer, A. T. Lee, M. T. Barclay, and D. C. Rees. 1998. Structure of the MscL homolog from Mycobacterium tuberculosis: A gated mechanosensitive ion channel. Science. 282:2220–2226. [DOI] [PubMed] [Google Scholar]
- 10.Bass, R. B., P. Strop, M. Barclay, and D. C. Rees. 2002. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 298:1582–1587. [DOI] [PubMed] [Google Scholar]
- 11.Perozo, E., and D. C. Rees. 2003. Structure and mechanism in prokaryotic mechanosensitive channels. Curr. Opin. Struct. Biol. 13:432–442. [DOI] [PubMed] [Google Scholar]
- 12.Martinac, B. 2004. Mechanosensitive ion channels: molecules of mechanotransduction. J. Cell Sci. 117:2449–2460. [DOI] [PubMed] [Google Scholar]
- 13.Edwards, M. D., Y. Li, S. Kim, S. Miller, W. Bartlett, S. Black, S. Dennison, I. Iscla, P. Blount, J. U. Bowie, and I. R. Booth. 2005. Pivotal role of the glycine-rich TM3 helix in gating the MscS mechanosensitive channel. Nat. Struct. Mol. Biol. 12:113–119. [DOI] [PubMed] [Google Scholar]
- 14.Miller, S., M. D. Edwards, C. Ozdemir, and I. R. Booth. 2003. The closed structure of the MscS mechanosensitive channel - Cross-linking of single cysteine mutants. J. Biol. Chem. 278:32246–32250. [DOI] [PubMed] [Google Scholar]
- 15.Anishkin, A., and S. Sukharev. 2004. Water dynamics and dewetting transitions in the small mechanosensitive channel MscS. Biophys. J. 86:2883–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koprowski, P., and A. Kubalski. 2003. C-termini of the Escherichia coli mechanosensitive ion channel (MscS) move apart upon the channel opening. J. Biol. Chem. 278:11237–11245. [DOI] [PubMed] [Google Scholar]
- 17.Li, Y., P. C. Moe, S. Chandrasekaran, I. R. Booth, and P. Blount. 2002. Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli. EMBO J. 21:5323–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koprowski, P., and A. Kubalski. 1998. Voltage-independent adaptation of mechanosensitive channels in Escherichia coli protoplasts. J. Membr. Biol. 164:253–262. [DOI] [PubMed] [Google Scholar]
- 19.Schumann, U., M. D. Edwards, C. Li, and I. R. Booth. 2004. The conserved carboxy-terminus of the MscS mechanosensitive channel is not essential but increases stability and activity. FEBS Lett. 572:233–237. [DOI] [PubMed] [Google Scholar]
- 20.Miller, S., W. Bartlett, S. Chandrasekaran, S. Simpson, M. Edwards, and I. R. Booth. 2003. Domain organization of the MscS mechanosensitive channel of Escherichia coli. EMBO J. 22:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowry, O., N. Rosebrough, A. Farr, and R. Randall. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193:265–275. [PubMed] [Google Scholar]
- 22.Hamill, O. P., A. Marty, E. Neher, B. Sakmann, and F. J. Sigworth. 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391:85–100. [DOI] [PubMed] [Google Scholar]
- 23.Booth, I. R., M. D. Edwards, S. Black, U. Schumann, W. Bartlett, T. Rasmussen, A. Rasmussen, and S. Miller. 2007. Physiological analysis of bacterial mechanosensitive channels. Methods Enzymol. 428:47–61. [DOI] [PubMed] [Google Scholar]
- 24.Akitake, B., A. Anishkin, and S. Sukharev. 2005. The “dashpot” mechanism of stretch-dependent gating in MscS. J. Gen. Physiol. 125:143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barlow, D. J., and J. M. Thornton. 1988. Helix geometry in proteins. J. Mol. Biol. 201:601–619. [DOI] [PubMed] [Google Scholar]
- 26.Gunasekaran, K., H. A. Nagarajaram, C. Ramakrishnan, and P. Balaram. 1998. Stereochemical punctuation marks in protein structures: glycine and proline containing helix stop signals. J. Mol. Biol. 275:917–932. [DOI] [PubMed] [Google Scholar]
- 27.Kloda, A., and B. Martinac. 2001. Structural and functional differences between two homologous mechanosensitive channels of Methanococcus jannaschii. EMBO J. 20:1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Booth, I. R., M. D. Edwards, and S. Miller. 2003. Bacterial ion channels. Biochemistry. 42:10045–10053. [DOI] [PubMed] [Google Scholar]
- 29.Dutzler, R., E. B. Campbell, M. Cadene, B. T. Chait, and R. MacKinnon. 2002. X-ray structure of a ClC chloride channel at 3.0Å reveals the molecular basis of anion selectivity. Nature. 415:287–294. [DOI] [PubMed] [Google Scholar]
- 30.Steinbacher, S., R. Bass, P. Strop, and D. C. Rees. 2007. Structures of the prokaryotic mechanosensitive channels MscL and MscS. In Mechanosensitive Ion Channels, Part A, Current Topics in Membranes, Vol. 58. Owen P. Hamill, editor. Elsevier, Academic Press, San Diego, CA. 1–24.
- 31.Kim, S., A. K. Chamberlain, and J. U. Bowie. 2004. Membrane channel structure of Helicobacter pylori vacuolating toxin: Role of multiple GXXXG motifs in cylindrical channels. Proc. Natl. Acad. Sci. USA. 101:5988–5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martz, E. 2002. Protein explorer: easy yet powerful macromolecular visualization. Trends Biochem. Sci. 27:107–109. [DOI] [PubMed] [Google Scholar]