Abstract
Age-related developmental differences in sensitivity to the acute effects of alcohol may play an important role in the development of alcoholism. The present study was designed to evaluate the acute effects of alcohol on cortical electroencephalogram (EEG) in adolescent (P36) and adult (P78) Wistar rats. Five minutes of EEG was recorded after administration of 0, 0.75 or 1.5 g/kg alcohol. The righting reflex was performed to measure the sedative effects of alcohol (3.5 g/kg) and total sleeping time for each rat. Our results showed that alcohol (1.5 g/kg) increased power in the 1–2 Hz band and decreased the power in the 32–50 Hz band in the parietal cortical region of adolescent rats. Alcohol (1.5 g/kg) also increased stability of the EEG power in the slow-wave frequency bands (2–4 Hz, 4–6 Hz, and 6–8 Hz) of adolescent rats. In the frontal cortex of adult rats, but not in adolescent rats, alcohol (1.5 or 0.75 g/kg) decreased the power in the 16–32 Hz frequency band. Alcohol (1.5 g/kg) differentially increased power in a multiple of slow-wave frequency bands (2–4 Hz and 4–6 Hz) in the parietal cortex of adult rats as compared to adolescent rats. Adolescent rats were shown significantly shorter sleeping time and higher blood alcohol levels after regaining reflex than adult rats. Our results provide additional evidence of age-related differences in the effects of acute alcohol on cortical EEG, sedation and tolerance.
Keywords: Adolescence, Alcohol, EEG, Alcoholism, Age, Gamma
1. Introduction
Age-related differences in sensitivity to alcohol have been strongly linked to the development of alcoholism (see Spear, 2000, for review and references). Ontogenetic differences in brain maturation and its differential responses to alcohol have been suggested in the regulation of mechanisms that could modulate the sensitivity and tolerance to alcohol (Spear, 2000; Witt, 1994). Studies have shown that adolescent rats are less sensitive than adult rats to acute alcohol-induced motor incoordination, sedation, and hypothermia (Ernst et al., 1976; Hollstedt et al., 1980; Little et al., 1996; Moy et al., 1998; Silveri and Spear, 1998, 2000; Varlinskaya and Spear, 2002). In addition to having higher resistance to the sedative effects of acute alcohol, adolescents have higher tolerance to acute administration of high dose of alcohol with higher blood alcohol levels and significant less sleeping time in regaining righting reflex compared to adult rats (Silveri and Spear, 1998). Adolescents are not only more resistant and tolerant to the sedative effects of acute alcohol, but also to withdrawal symptoms such as anxiety following alcohol challenge (Doremus et al., 2003). Due to these age-related differences in the sensitivity to the sedative effects of alcohol, adolescents may have higher neurological and physiological limits of alcohol intake compared to adults. This may lead to higher consumption and tolerance to alcohol than adults. The higher consumption and tolerance of alcohol have been proposed to enhance risk for alcohol dependence in adolescents (Ehlers et al., 2006; Spear, 2000; Witt, 1994).
The electroencephalogram (EEG) has been used to study age-related neurophysiologic alterations in neural organization of the brain (Roubicek, 1977). EEG power, a measure of the amplitude of the EEG as a function of frequency (microvolts squared per Hz), has been shown to decrease from childhood to late adulthood (Basar et al., 1997; Dustman et al., 1993; Katada et al., 1981). The EEG also has been proven useful in assessing the effects of alcohol following prenatal and adult exposure in humans (Ehlers et al., 1989; Kaneko et al., 1996; Scher et al., 1998). EEG recordings have also been useful assessing the acute and chronic effects of alcohol in rats (Ehlers and Chaplin, 1991; Robledo et al., 1993; Slawecki, 2002; Slawecki et al., 2001, 2006) and altered EEG power in specific frequency bands appears to be a sensitive marker of the acute and chronic effects of alcohol. The continuous rhythmic sinusoidal EEG activities are categorized into Delta (1–4 Hz), Theta (4–8 Hz), Alpha (8–16 Hz), Beta (16–32 Hz) and Gamma (32–50 Hz) frequencies and studies using spectral analysis of the EEG have contributed to our understanding of the effects of alcohol on CNS function (Cortese et al., 1997; Slawecki et al., 1999, 2001). In general, the slow-wave activities (1–4 Hz) are associated with sleep, less attentive conditions and drowsiness. On the other hand, the fast-wave activities (12–50 Hz) are associated with high arousal and attentive states. For example, acute alcohol increased the power of the 4 to 6 Hz EEG frequency range in adult rats, but not in adult rats that had been exposed to alcohol during adolescence (Slawecki, 2002). These data have been interpreted as suggesting that alcohol-induced sedative resistance can be established if adolescents are exposed to alcohol during adolescence. Alcohol-induced changes in the stability of the EEG have also been shown to be attenuated in adult rats previously exposed to alcohol vapor during adolescence (Slawecki, 2002). The coefficient of variance (CV) has been used as a measure of stability in the EEG and is also used as a sensitive indicator in the correlations of drug effects and drug-induced behavior (Ehlers and Havstad, 1982; Ehlers et al., 1989).
While studies have shown that adolescent rats are less sensitive than adult rats to alcohol-mediated effects on behavioral sedation, the effects of acute alcohol on brain electrophysiological responses and its linkage to sedative effects of acute alcohol in adolescent rats are not well understood. The primary goal of this study was to characterize further the effects of acute alcohol on cortical EEG and behavioral sedation in adolescent and adult rats. The working hypothesis of this study is that the effects of alcohol on behavioral sedation are associated with an increase in cortical EEG power of slow-wave frequency bands in adult, but not adolescent rats.
2. Results
Behavioral State Assessment
Subjective visual inspection of adolescent and adult rats preceding alcohol administration showed normal exploratory and grooming behaviors in both groups. Acute administration of alcohol produced dose-dependent effects on rats’ behavior. Consistent with previous studies (e.g., (Slawecki, 2002)), administration of the 0.75 g/kg alcohol dose decreased motor activity and body tone during handling, whereas the 1.5 g/kg alcohol dose impaired gait with rats often falling to one side. Significant main effects of alcohol, and alcohol × age interactions observed on the EEG power and cortical variability in frontal and parietal cortices are described below.
EEG Assessment
2.1. Frontal and Parietal Cortical EEG Power
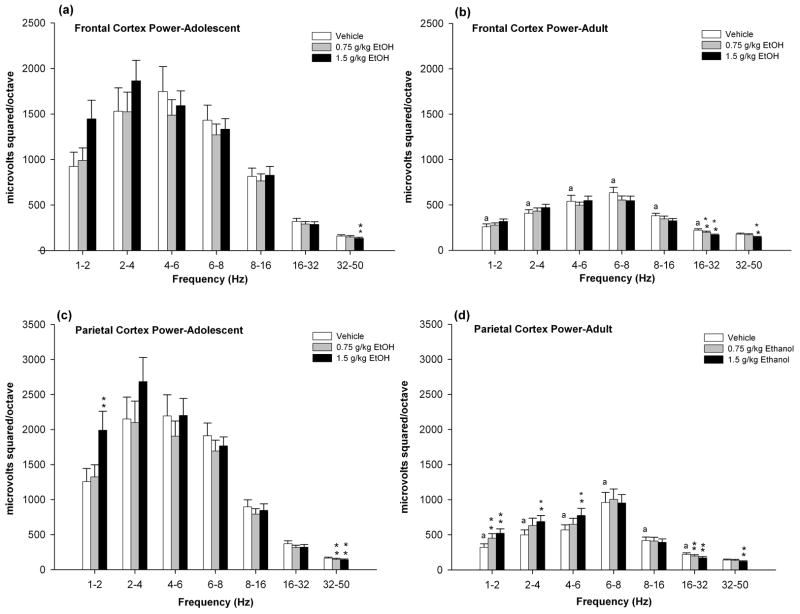
The frontal cortical power was found to be greater in adolescent rats than in adults [F’s (1,37) > 12.21, p’s < 0.01] in all bands except the 32–50 Hz bands (Fig. 1a & 1b). Further examination in the adolescent rats revealed significant alcohol effects [F’s (2,38) >17.48, p’s < 0.01] in the 32–50 Hz frequency band. Power in the 32–50 Hz was decreased by 1.5 g/kg alcohol (Fig. 1a). In adult rats, significant alcohol effects were reported in high frequency 16–32 and 32–50 bands [F’s (2,36) > 14.27, p’s < 0.01]. Power in the 16–32 Hz band was reduced by 0.75 and 1.5 g/kg alcohol, and in the 32–50 Hz band was reduced by 1.5 g/kg alcohol (Fig. 1b).
Figure 1.
Mean EEG power in frontal and parietal cortices of adolescent and adult rats after injection of 0, 0.75, or 1.5 g/kg of alcohol. Data are the mean ± standard error of the mean. 0 g/kg alcohol (white bars). 0.75 g/kg alcohol (grey bars). 1.5 g/kg alcohol (black bars). a Statistically significant differences from adolescent baseline. **p<0.01 indicates significant different from vehicle.
Similar to the frontal cortex, the parietal cortical power was found to be greater in adolescent rats as compared to adults in all bands except the 32–50 Hz band (Fig. 1c & 1d). Further examination in the adolescent rats revealed significant alcohol effects [F’s (2,38) > 5.51, p’s < 0.01] in the 1–2 and 32–50 Hz frequency bands. Power in the 1–2 Hz band was increased by 1.5 g/kg alcohol, the 32–50 Hz band was decreased by 0.75 and 1.5 g/kg alcohol (Fig. 1c). In adult rats, alcohol produced significant increases in mean power in multiple bands (i.e, 1–2, 2–4, 4–6 Hz bands) [F’s (2,36) > 3.86, p’s < 0.01] and decreases in the 16–32 and 32–50 Hz bands [F’s (2,36) > 10.36, p’s < 0.01] (Fig. 1d). Power in the 16–32 Hz band was reduced by 0.75 and 1.5 g/kg alcohol, and the 32–50 Hz band was reduced by 1.5 g/kg alcohol.
2.2. Frontal and Parietal Cortical EEG Variability
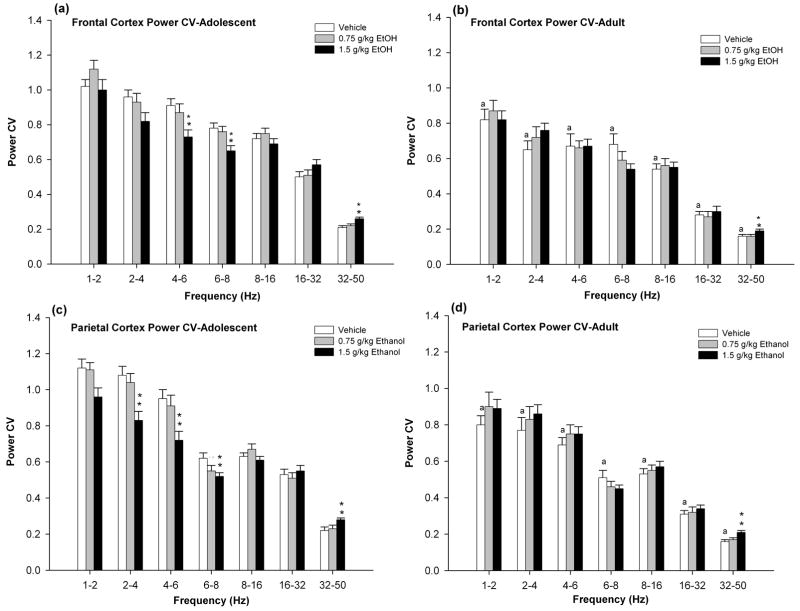
Analysis of the power CV (cortical EEG variability) in the frontal cortical EEG demonstrated significant age effects [F’s (1,37) > 10.28, p’s < 0.01] in all bands (Fig. 2a and 2b). Further examination in adolescent rats, demonstrated that alcohol produced decreases in power CV across two frequency bands (4–6 and 6–8 Hz frequency bands) [F’s (2,38) > 7.74, p’s < 0.01] and increases in the 32–50 Hz band [F’s (2,38) = 16.74, p’s < 0.01] (Fig. 2a). These effects were observed primarily after the administration of 1.5 g/kg alcohol. In contrast to adolescent rats, 1.5 g/kg alcohol only produced increases in power CV in the 32–50 Hz band [F’s (2,36) = 11.24, p’s < 0.01] (Fig. 2b).
Figure 2.
EEG power CV in frontal and parietal cortices of adolescent and adult rats after injection of 0, 0.75, or 1.5 g/kg of alcohol. Data are the mean ± standard error of the mean. 0 g/kg alcohol (white bars). 0.75 g/kg alcohol (grey bars). 1.5 g/kg alcohol (black bars). a Statistically significant differences from adolescent baseline. **p<0.01 indicates significant different from vehicle.
Analysis of the power CV in the parietal cortical EEG also showed significant age effect [F’s (1,37) > 8.15, p’s < 0.01] in all bands (Fig. 2c and 2d). Further examination in adolescent rats, also demonstrated that alcohol significantly altered power CV across a wide range of slow-wave frequencies (i.e, 2–4, 4–6 and 6–8 Hz frequency bands) [F’s (2,38) > 8.67, p’s < 0.01] and increase in the 32–50 Hz frequency band [F’s(2,38) = 6.80, p’s < 0.01] (Fig 2c). These effects were observed primarily after the administration of 1.5 g/kg alcohol. Similar to frontal cortex, in adult rats, 1.5 g/kg alcohol only produced increases in power CV in the 32–50 Hz band [F’s (2,36) = 12.98, p’s < 0.01] (Fig. 2d). Statistically significant age × alcohol interactions were observed on EEG power CV in the 2–4 and 4–6 Hz frequency bands [F’s (2,74) > 5.87, p’s <0.01]. 1.5 g/kg alcohol significantly decreased CV in the 2–4 and 4–6 Hz bands in adolescent rats (Fig. 2c). In contrast, power CV increased in the same frequency bands in adult rats after administration of 1.5 g/kg alcohol (Fig. 2d).
2.3. Loss of Righting Reflex
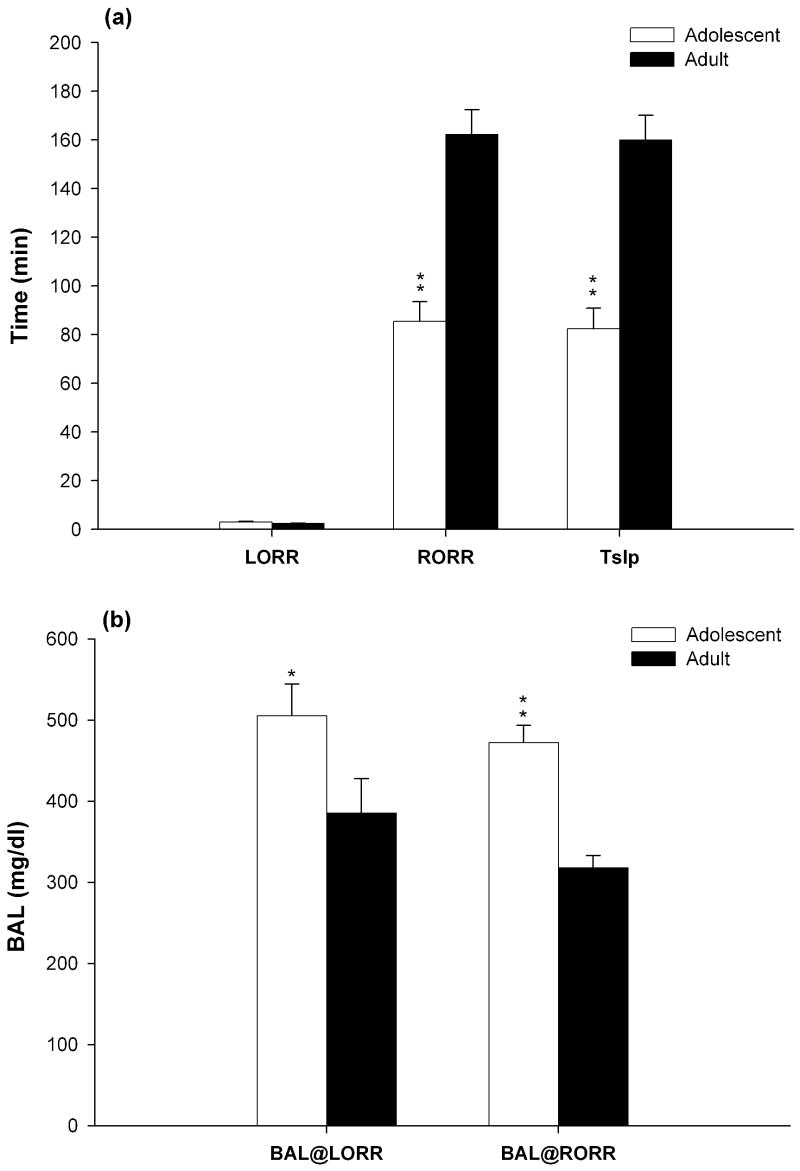
Though there was no significant difference in the latency of the onset of the loss of righting reflex (LORR) between adolescent and adult rats, there were significant age differences in the duration of the LORR or total sleeping time (Tslp) [t(25)= −5.692, p< 0.01] (Fig. 3a). After 3.5 g/kg alcohol i.p. injection, adolescent rats regained their righting reflex (RORR) significantly earlier than adult rats [t(25)= −5.703, p< 0.01] (Fig. 3a). Furthermore, blood alcohol levels (BALs) of adolescent rats at the time to the regain of righting reflex (RORR) were significantly higher than the BALs of adult rats [t(25)= −6.04, p< 0.01] (Fig. 3b).
Figure 3.
(a) Mean (S.E.M.) time to loss of righting reflex (LORR), to regain righting reflex (RORR) and total sleeping time (min: following 3.5 g/kg alcohol injection) for adolescent rats (white bars) and adult rats (black bars). (b) Blood alcohol level (BAL in mg/dl) at the times of LORR and RORR in adolescent rats (white bars) and adult rats (black bars). *p<0.05 or **p<0.01 indicates significant different from adult rats.
3. Discussion
Previous studies from our laboratory have shown that alcohol exposure during adolescence produces long-term neurobehavioral consequences that could be related to an increased risk for developing alcoholism in adulthood (Slawecki, 2002; Slawecki et al., 2001, 2006). Studies in human subjects have indicated that the response to alcohol may be one of the factors that contribute to alcoholism risk (Schuckit, 1999; Schuckit and Smith, 1996). However, the effect of acute alcohol on EEG responses in adolescent rats has not been examined. Findings from the present study provide new evidence of differential effects of acute alcohol on the power and stability of cortical EEG in adolescent and adult rats. These effects selectively affected specific frequency bands and were consistent with the behavioral effects of acute alcohol on sleeping time and righting reflex.
We used righting reflex to index behavioral sedation following acute alcohol administration in adolescent and adult rats. Consistent with previous behavioral studies (Cha et al., 2006; Little et al., 1996; York and Chan, 1993), we found that adolescent rats regained their voluntary motor activity faster than adult rats. These findings in adolescent rats were observed at significantly higher BALs than adult rats at the times of regaining their righting reflexes. Little et al. (1996) also observed that sensitivity to the sedative effects of acute alcohol in rats was increased with increasing age, and adolescent rats developed acute tolerance to alcohol at a more rapid rate and at a higher level than adult rats (Grieve and Littleton, 1979; Silveri and Spear, 1999; Swartzwelder et al., 1998). These results suggest that adolescent rats are more resistant to the sedative effects of alcohol than adult rats. However, whether the acute effects of alcohol on cortical EEG power and stability are also differentially affected in adolescent and adult rats remains unclear. Moreover, the relationship between the differential effects of acute alcohol on the righting reflex and cortical EEG activity are also not well understood. To address this question, we characterized the acute effects of alcohol on cortical EEG power and stability in adolescent and adult rats.
The present study found that acute administration of alcohol increased the power in the slow-wave frequency bands in the parietal cortex of adult rats more than in adolescent rats. While alcohol increased the parietal power in all slow-wave bands (1–6 Hz) of adult rats, it only increased the 1 to 2 Hz power in the parietal cortex of adolescent rats, but had no effect on the power of the 2–4 and 4–6 Hz bands. These data suggest that alcohol induces increases in slow-wave power in the parietal cortex were significantly attenuated in the 2–6 Hz frequency bands in adolescent rats. Taking into consideration our behavioral results, these findings support the assertion that the increase in EEG power seen in the 4 to 6 Hz frequency range produced by acute intoxicating doses of alcohol is related to alcohol’s sedative actions (Slawecki, 2002).
The mechanism(s) mediating the differential effects of alcohol on cortical EEG in adolescent and adult rats remain unclear. Two potential candidate mechanisms that have been implicated are the γ-aminobutyric acid (GABA) and the N-methyl-D-aspartate (NMDA) receptor systems. Some studies have shown that the activity of GABA and its receptors could be enhanced by alcohol (Grobin et al., 1998; Mihic, 1999; Proctor et al., 1992; Weiner et al., 1994, 1997). Additionally, it has been shown that the function and maturity of the GABAergic system is lower in adolescents compared to adults (Moy et al., 1998; Silveri and Spear, 2002). Since GABA is the major known inhibitory neurotransmitter in the brain, the lower response to sedative effects of alcohol in adolescents could be due to lower levels of GABA in the cortex and/or a developmental immaturity of its receptors.
The overexpression of NMDA receptors in adolescent brains may be critical for synaptic plasticity and could also make the brain more vulnerable to NMDA neurotoxicity (McDonald et al., 1989; Silveri and Spear, 2002). Silveri and Spear (1998) indicated that age-related developmental overexpression of the NMDA system may contribute to lower sensitivity to alcohol in younger animals in comparison to older animals. The attenuated slow-wave frequency power in adolescents observed in this study may due to the combination of age-related differential sensitivities of a number of neural transmission systems to alcohol.
The present study also showed that while the high dose of alcohol (1.5 g/kg) reduced the γ (32–50 Hz) power in both frontal and parietal cortices of adolescent and adult rats, the low dose of alcohol (0.75 g/kg) reduced the γ power only in the parietal cortex of adolescent rats. These results suggest that adolescent rats are more vulnerable than adult rats to the acute effects of alcohol on γ frequency band. The γ frequency band (32–50 Hz) has been associated with the integration of sensory and cognitive processes (Engel et al., 1992; Herrmann and Demiralp, 2005). While previous studies have shown that lower evoked γ band activity could be a marker in the development of alcoholism (Padmanabhapillai et al., 2006), these findings suggest that the effects of alcohol on γ power in parietal cortex is independent to alcohol’s sedative effects observed in the righting reflex experiment.
In contrast to our finding with the γ band, we found that alcohol had no effect on frontal cortical power in the β frequency range (16–32 Hz) in adolescent rats. However, alcohol produced significant inhibition on frontal β frequency range cortical power in adult rats. Power in the β frequency range has been positively correlated with arousal level (Mercia and Gaillard, 1992; Mercia and Fortune, 2004). Similar to frontal cortex, alcohol had no effect in β frequency band on parietal cortical power in adolescent rats. In contrast to adolescent rats, in adult rats, either 0.75 or 1.5 g/kg alcohol produced significant inhibition on parietal cortical power in β frequency band. The increased sensitivity of β frequency in adult rats is consistent with previous behavioral studies (Ernst et al., 1976; Hollstedt et al., 1980; Little et al., 1996; Moy et al, 1998; Silveri and Spear, 1998, 2000; Varlinskaya and Spear, 2002) showing that adolescent rats are less sensitive to the sedative effects of alcohol than adult rats and maintain higher arousal levels than adult rats following acute administration of alcohol.
Although the exact mechanisms underlying the differential responses between adolescents and adults in γ and β bands are not clear, there are some studies indicated that sensitivity of some neural transmission systems to alcohol is increasing with age (Li et al., 2006; Silveri and Spear, 2002). For example, it has been shown that the alcohol sensitivity of evoked GABA receptor-mediated inhibitory postsynaptic currents (eIPSCs) increases steadily during adolescence (Li et al., 2003). Furthermore, it is also has been shown that spontaneous GABA receptor-mediated inhibitory postsynaptic currents (sIPSCs) is enhanced by alcohol in adults, but not in adolescents (Li et al., 2006). Because EEG γ (32–50 Hz) and β (16–32 Hz) bands are markers of cognitive activities and arousal, the different alcohol responses in γ and β bands between adolescents and adults could be due to age-related developmental differences in inhibitory neural transmission systems.
Consistent with our previous findings (Slawecki et al., 2006), in the present study we observed significantly higher frontal and parietal cortical power in a wide range of EEG frequencies in adolescent rats as compared to adult rats. Higher EEG power in adolescents could theoretically be due to the age-related synaptic pruning process that occurs from adolescence to early adulthood (Feinberg, 1982; Huttenlocher, 1979; Purves and Lichtman, 1980; Whitford et al., 2007). In fact, the marked age-related changes in the frontal and parietal cortices indicate that these regions undergo an age-dependent reduction in the number of active synapses (Huttenlocher and Dabholkar, 1997; Rakic et al., 1986). Age has been shown to be one of the most important factors in modulation of EEG power during brain development (Basar et al., 1997; Dustman et al., 1993; Katada et al., 1981). Adolescent rats have more active cortical synapses than adult rats, which may be responsible for their higher cortical mean EEG power compared with adult rats. Consistent with our observations, higher cortical power in the EEG has also been reported in human adolescents relative to adults (Dustman et al., 1985, 1999; Ehlers et al., 2001).
Our findings of increased power in the slow-wave frequency bands in adolescent rats are also consistent with human studies (Gasser et al., 1988; Matsuura et al., 1985). Theoretically, active synapses responsible for the slow-wave frequency bands may be under more extensive “rewiring” and synaptic pruning processes than active synapses responsible for high frequency bands during adolescence. The slow-wave frequency bands are thought to arise primarily from highly synchronous local neural activity (Whitford et al., 2007). This synchrony is responsible for the large amplitude (increased power) associated with slow-wave activity (Steriade et al., 1990). Thus, loss in number of synapses involved in slow-wave activity will lead to loss of EEG power (Whitford et al., 2007). On the other hand, the high frequency is thought to arise primarily from asynchronous activity and is associated with low EEG power compared to the slow-wave frequency (Whitford et al., 2007). As a consequence, when animals mature into adulthood, the power in the slow-wave frequencies is dramatically reduced as suggested by the findings in adult rats from the present study.
Our data also demonstrate that cortical EEG variability is higher in adolescent rats than in adult rats across the slow-wave frequency bands in both frontal and parietal cortices. Since EEG waves recorded from the electrodes are produced by slow voltage changes (inhibitory and excitatory postsynaptic potentials) occurring synchronously in large groups of neurons (Elul, 1971), its variability could be determined by the balance of inhibitory and excitatory neural systems. We found that following acute alcohol administration cortical EEG variability was reduced in the 4–8 Hz bands in the frontal and 2–8 Hz bands in parietal cortices of adolescent rats, but not in adult rats. Alcohol-induced reduction of cortical EEG variability in adolescent rats could be mediated by the immaturity of regulatory mechanisms in postsynaptic excitatory and inhibitory neural systems. Additionally, the receptors of these neurotransmitter systems may be overproduced and are still undergoing pruning during adolescence (Lidow et al., 1991; Spear, 2000). Moreover, these neurotransmitter systems have also been identified in the brain as major sites of action for alcohol (Lovinger, 1999; Mehta and Ticku, 1999; Wright et al., 1996). The synergy of these immature neural transmission systems in adolescent rats could result in a reduction in the effects of alcohol on cortical EEG variability.
Cortical EEG variability provides a good measurement for behavioral state of the animal (Ehlers and Havstad, 1982; Ehlers and Foote, 1984; Killam et al., 1976) and it was shown that the decreased cortical EEG variability could serve as an index of decreased sedation after administration of acute alcohol (Slawecki, 2002; Slawecki et al., 2000). Some studies have shown that chronic alcohol exposure caused long-term disruption of spatial memory and paradoxical sleep (Gitlow et al., 1973; White et al., 2000). Slawecki (2002) also found that alcohol produced greater increases in cortical variability in alcohol-exposed rats during adolescence as compared with non-alcohol exposed rats. Furthermore, Slawecki (2002) also reported that enhanced intoxication scores were not observed in alcohol-exposed rats after acute moderate dose of alcohol exposure. As the results, the EEG variability could be not just an index of decreased sedation, it is also could be a marker for alcohol’s effects on cognition (Slawecki, 2002). It is possible that higher cortical EEG variability is an age-determined phenomenon that is related to transitional process in rapid changing learning and memory functions under extensive synaptic pruning and neural reorganization during adolescence, and also could be more sensitive to the effects of acute alcohol.
4. Conclusion
Our studies suggest that adolescent rats have higher cortical EEG power and are more resistant than adult rats to the sedative effects of acute alcohol administration. Findings from these studies support our working hypothesis that alcohol’s sedative actions are associated with an increase in parietal EEG power in the slow-wave frequency bands in adult, but not adolescent rats. Additionally, we found that the inhibitory effects of acute alcohol on cortical EEG stability are greater in adolescent rats than in adult rats. While the significance of these findings remains unclear, these data suggests that adolescent rats could be more vulnerable to detrimental effects of acute alcohol administration on integrative sensory processes or cognitive functions. These differential responses on the EEG spectral profile could be a tool to identify the underlying neurobiological substrates of the decreased or increased sensitivity to acute alcohol administration. Our results may provide an additional marker in differential responses of adolescents and adults to acute effects of alcohol.
5. Experimental Procedure
5.1. Subjects
Postnatal 36 days (P36) male adolescent Wistar rats (n=25) and postnatal 78 days (P78) male adult Wistar rats (n=25) were used in this study. Upon receipt, adolescent rats (P24) averaged 64±2 g and adult rats (P71) averaged 304±6 g. Rats were housed two/cage in standard plastic cages [25 (w) × 20 (h) × 45 cm (l)] during the experiment. For the duration of the experiment, a 12 h light/dark cycle (lights on at 6 am) was in effect and ad libitum food/water access was maintained. Temperature of the colony and experimental rooms were constantly maintained at 71 F. Animal care was in accordance with NIH and institutional guidelines.
5.2. Surgical Procedure
Rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneally). Atropine (24 μg, subcutaneously) coadministration minimized respiratory suppression. Surgical coordinates were obtained from the Paxinos and Watson (1986) atlas. For the purpose of comparison with our previous studies in cortical EEG power (Slawecki, 2002; Slawecki et al., 2001,2006), screw electrodes were placed in the skull overlying the frontal cortex (AP: +1.5 mm, ML: ±3.0 mm) and parietal cortex (AP: −4.5 mm, ML: ±4.5 mm) in adult rats and the frontal cortex (AP: +1.5mm, ML: ±2.0mm) and parietal cortex (AP: −4.0mm, ML: ±3.5mm) in adolescent rats. The stereotaxic coordinates for adolescent rats were based on our previous pilot studies, which resulted in placement overlaying the same cortical areas, i.e, FR1/FR2 and PAR1 (Slawecki et al., 2006). A midline screw electrode was placed posterior to lambda in the skull overlying the cerebellum. The tooth bar was set at −3.3 mm. Electrode connections were made to an Amphenol five-pin connector, and the assembly was anchored to the skull with dental acrylic and anchor screws. One-week recovery period was provided before the effects of alcohol were assessed.
5.3. Electrophysiological Recording Procedures
After 1–2 weeks period of recovery from surgery (the adolescent and adult rats were received on P 25 and P 67 respectively). The acute effects of alcohol on cortical EEG were tested on P36, P38 and P40 in adolescent rats (Average adolescence period in rat is P28–P60 and after P60 is considered as adulthood in rat) (Spear, 2000) and P78, P80 and P82 in adult rats. Each rat was tested with only one dose each day. Five minutes after alcohol administration, the rat was transferred to the recording chamber from the home cage.
Recordings were conducted in SR LAB chambers (San Diego Instrument: San Diego, CA). Each chamber contained a 9 cm (diameter) × 16 cm (length) Plexiglas tube, which was equipped with moving sensor (The chamber was designed for EEG, ERP (event-related potentials) recordings and motor response). EEG recordings were collected between 8 AM and 4 PM.
EEGs were recorded from two monopolar leads referenced to cerebellum ground (i.e., frontal cortex and parietal cortex). Five minutes of EEG were recorded on a preamplifier/amplifier unit (Sensorium Inc., Shelburne, VT) with a band pass of 0.53 to 70 Hz. Data were digitized at a rate of 256 Hz and then transferred to an IBM compatible PC. Consecutive 4-sec epochs of EEG were Fourier-transformed over a spectra of 1 to 64 Hz. EEG spectra were identified as containing artifact when average cortical power was >2000 μV2/octave. Artifact epochs were excluded only after visual analysis of the raw EEG and spectral distributions. Spectra from each EEG epoch then were averaged and compressed into broader frequency bands. Artifact epochs were excluded only after being verified by visual analysis of the raw EEG and spectral distributions. Individual spectra from each 4 s epochs were then averaged. These data were then compressed into seven frequency bands: 1–2, 2–4, 4–6, 6–8, 8–16, 16–32, and 32–50 Hz. Mean spectral power (i.e., a measure of the amplitude of the EEG) and variability (i.e., a measure of stability in EEG power, coefficient of variation (CV) = standard deviation power/mean power) in each band of the EEG were calculated. These analysis procedures have been described previously (Ehlers and Havstad, 1982). For example, the CV will be increased if there are many large variations in power with respect to the mean power. Subjects were omitted from assessment of the EEG for reasons that included not recovering from surgery, damage to the headstage prior to or during recording, or poor recording quality. As a result, the total number of subjects assessed electrophysiologically in each group was as follows: twenty adolescent rats (n=20) and nineteen adult rats (n=19).
5.4. Behavioral Procedures: Righting Reflex
Alcohol (3.5 g/kg, 25% v/v) was injected intraperitoneally (i.p.) into adolescent (n=12) and adult (n=15) rats. After the injection, rats were placed on double cotton pads for maintenance of body temperature, and the time to loss of the righting reflex (LORR) was recorded. The duration of LORR was also recorded. Time to LORR was defined as the time post-injection when the rat could no longer right itself onto all 4 paws within 60 seconds. Time to regain of righting reflex (RORR) was defined as the time post-injection when the rat could right itself onto all 4 paws two times within 60 seconds. Total alcohol-induced sleep time was calculated by subtracting the time to LORR from the time to RORR. Only rats that lost their righting reflex within 10 minutes of the injection were used in the analyses.
5.5. Alcohol administration
EEG studies: All subjects were alcohol-naive before acute alcohol administration (i.p) prior to EEG recordings. Based on the our previous studies and other studies (Ehlers et al., 1992,1998; Hetzler et al., 1981; Rodd et al., 2004; Slawecki, 2002; Slawecki et al., 2005; Varlinskaya and Spear, 2007), the 0.75 and 1.5 g/kg alcohol doses were used to test the low and moderate effects of alcohol on cortical EEG. 10 % and 20 % alcohol were used for 0.75 g/kg and 1.5 g/kg i.p. injection respectively. Injection volumes ranged from 1 to 4 mL based on the subjects’ free-moving body weight (range, 108–450 g). For vehicle injections, volumes were randomized within each group so that vehicle injections were equal in volume to one of the alcohol doses in each group of subjects. Alcohol and vehicle injections were administrated according to a pseudorandom design in their home cage.
5.6. Statistical Analysis
Two-way mixed analysis of variance was used to assess the effects of alcohol on the EEG (group X dose). The observed significant interactions between adolescent and adult in dose (0, 0.75 g/kg, and 1.5 g/kg of alcohol) were further analyzed by one-way ANOVA within each EEG band in adolescent and adult separately. Independent t-tests were used to assess baseline (with no alcohol administration) differences between adolescents and adults. To correct for multiple comparisons in frequency bands, p-value was set at p< 0.01 to determine the levels of statistical significance.
Acknowledgments
This work was supported by grants R01 AA014339, AA006059 to Cindy Ehlers from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Jerry P. Pian is supported by NIAAA training grant 5T32 AA007456-24. The authors thank Jennifer Roth and Derek Wills for their teaching and assistance in animal surgery, data collection and analyses. Dr. James Havstad developed the software used for EEG assessment.
Abbreviations
- EEG
electroencephalogram
- GABA
gamma-aminobutyric acid
- NMDA
N-methyl-D-aspartate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basar E, Yordanova J, Kolev V, Basar-Eroglu C. Is the alpha rhythm a control parameter for brain responses? Biol Cybern. 1997;76:471–480. doi: 10.1007/s004220050360. [DOI] [PubMed] [Google Scholar]
- Cha YM, Li Q, Wilson WA, Swartzwelder HS. Sedative and GABAergic effects of ethanol on male and female rats. Alcohol Clin Exp Res. 2006;30:113–117. doi: 10.1111/j.1530-0277.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- Cortese BM, Krahl SE, Berman RF, Hannigan JH. Effects of prenatal ethanol exposure on hippocampal theta activity in the rat. Alcohol. 1997;14:231–235. doi: 10.1016/s0741-8329(96)00147-4. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Dustman RE, LaMarche JA, Cohn NB, Shearer DE, Talone JM. Power spectral analysis and cortical coupling of EEG for young and old normal adults. Neurobiol Aging. 1985;6:193–198. doi: 10.1016/0197-4580(85)90049-1. [DOI] [PubMed] [Google Scholar]
- Dustman RE, Shearer DE, Emmerson RY. EEG and event-related potentials in normal aging. Prog Neurobiol. 1993;41:369–401. doi: 10.1016/0301-0082(93)90005-d. [DOI] [PubMed] [Google Scholar]
- Dustman RE, Shearer DE, Emmerson RY. Life-span changes in EEG spectral amplitude, amplitude variability and mean frequency. Clin Neurophysiol. 1999;110:1399–1409. doi: 10.1016/s1388-2457(99)00102-9. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Havstad JW. Characterization of drug effects on the EEG by power spectral band time series analysis. Psychopharmacology Bulletin. 1982;18:43–47. [Google Scholar]
- Ehlers CL, Foote SL. Ultradian periodicities in EEG and behavior in the Squirrel Monkey (Saimiri sciureus) American Journal of Primatology. 1984;7:381–389. doi: 10.1002/ajp.1350070407. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Schuckit MA. EEG spectral characteristics following ethanol administration in young men. Electroencephalogr Clin Neurophysiol. 1989;73:179–187. doi: 10.1016/0013-4694(89)90118-1. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI. EEG and ERP response to chronic ethanol exposure in rats. Psychopharmacology. 1991;104:67–74. doi: 10.1007/BF02244556. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Kaneko WM, Wall TL, Chaplin RI. Effects of dizocilpine (MK-801) and ethanol on the EEG and event-related potentials (ERPs) in rats. Neuropharmavology. 1992;31:369–378. doi: 10.1016/0028-3908(92)90069-2. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Somes C, Cloutier D. Are some of the effects of ethanol mediated through NPY? Psychopharmacology. 1998;139:136–144. doi: 10.1007/s002130050698. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Garcia-Andrade C, Phillips E. Effects of age and parental history of alcoholism on EEG findings in mission Indian children and adolescents. Alcoholism: Clin and Exp Res. 2001;25:672–679. [PubMed] [Google Scholar]
- Ehlers CL, Slutske WS, Gilder DA, Lau P, Wilhelmsen KC. Age at first intoxication and alcohol use disorders in Southwest California Indians. Alcoholism: Clin and Exp Res. 2006;30:1856–1865. doi: 10.1111/j.1530-0277.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Elul R. The genesis of the EEG. Int Rev Neurobiol. 1971;15:227–272. doi: 10.1016/s0074-7742(08)60333-5. [DOI] [PubMed] [Google Scholar]
- Engel AK, Konig P, Kreiter AK, Schillen TB, Singer W. Temporal coding in the visual cortex: new vista on integration in the nervous system. Trends Neurosci. 1992;15:218–226. doi: 10.1016/0166-2236(92)90039-b. [DOI] [PubMed] [Google Scholar]
- Ernst AJ, Dempster JP, Yee R, Dennis C, Nakano L. Alcohol toxicity, blood alcohol concentration and body water in young and adult rats. J Stud Alcohol. 1976;37:347–356. doi: 10.15288/jsa.1976.37.347. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Gasser T, Verleger R, Bacher P, Sroka L. Development of the EEG of school-age children and adolescents: I, analysis of band power. Electroenceph Clin Neurophysiolo. 1988;69:91–99. doi: 10.1016/0013-4694(88)90204-0. [DOI] [PubMed] [Google Scholar]
- Gitlow SE, Bentkover SH, Dziedzic SW, Khazan N. Persistence of abnormal REM sleep response to ethanol as a result of previous ethanol ingestion. Psychopharmacologia. 1973;33:135–170. doi: 10.1007/BF00429083. [DOI] [PubMed] [Google Scholar]
- Grieve S, Littleton J. Age and strain differences in the rate of development of functional tolerance to ethanol by mice. J Pharm Pharmacol. 1979;31:696–700. doi: 10.1111/j.2042-7158.1979.tb13631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol. Psychopharmacologia. 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol. 2005;116:2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Hetzler BE, Heilbronner RL, Griffin J, Griffin G. Acute effects of alcohol on evoked potentials in visual cortex and superior colliculus of the rat. Electroencephalogr Clin Neurophysiol. 1981;51:69–79. doi: 10.1016/0013-4694(81)91510-8. [DOI] [PubMed] [Google Scholar]
- Hollstedt C, Olsson O, Rydberg U. Effects of ethanol on the developing rat. II. Coordination as measured by the tilting-plane test. Med Biol. 1980;58:164–168. [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex-developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kaneko WM, Phillip EL, Riley EP, Ehlers CL. EEG findings in fetal alcohol syndrome and Down syndrome children. Electroencephalogr Clin Neurophysiol. 1996;98:20–28. doi: 10.1016/0013-4694(95)00189-1. [DOI] [PubMed] [Google Scholar]
- Katada A, Ozaki H, Suzuki H, Suhara K. Developmental characteristics of normal and mentally retarded children’s EEGs. Electroenceph Clin Neurophsiol. 1981;52:192–201. doi: 10.1016/0013-4694(81)90166-8. [DOI] [PubMed] [Google Scholar]
- Killam KF, Jr, Slikker W, Jr, Brocco MJ, Gehrmann JE. Correlation of behavioral and EEG effects during interaction studies with secobarbital and psychomotor stimulants. Proc west Pharmacol Soc. 1976;19:432–434. [PubMed] [Google Scholar]
- Li Q, Wilson WA, Swartzwelder HS. Developmental differences in the sensitivity of hippocampal GABAA receptor-mediated IPSCS to ethanol. Alcohol Clin Exp Res. 2003;27:2017–2022. doi: 10.1097/01.ALC.0000108390.62394.71. [DOI] [PubMed] [Google Scholar]
- Li Q, Wilson WA, Swartzwelder HS. Developmental differences in the sensitivity of spontaneous and miniature IPSCs to ethanol. Alcoholism: Clin and Exp Res. 2006;30:119–126. doi: 10.1111/j.1530-0277.2006.00006.x. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Rakic P. Synchronized overproduction of neurotransmitter receptors in diverse regions of the primate cerebral cortex. Proc Natl Acad Sci USA. 1991;88:10218–10221. doi: 10.1073/pnas.88.22.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Lovinger DR. 5-HT3 receptors and the neural actions of alcohols: an increasingly exciting topic. Neurochem Int. 1999;35:125–130. doi: 10.1016/s0197-0186(99)00054-6. [DOI] [PubMed] [Google Scholar]
- Matsuura M, Yamamoto K, Fukuzawa H, Okubo Y, Uesugi H, Moriiwa M, Kojima T, Shimazono Y. Age development and sex differences of various EEG elements in healthy children and adult-quantification by a computerized wave form recognition method. Electroenceph Clin Neurophysiol. 1985;60:394–406. doi: 10.1016/0013-4694(85)91013-2. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Silverstein FS, Johnston MV. Neuroprotective effects of MK-801, TCP, PCP and CPP against N-methyl-D-aspartate induced neurotoxicity in an in vivo perinatal rat model. Brain Res. 1989;490:33–40. doi: 10.1016/0006-8993(89)90427-7. [DOI] [PubMed] [Google Scholar]
- Mercia H, Gaillard JM. The EEG of the sleep onset period in insomnia: a discriminant analysis. Physiol Behav. 1992;52:199–204. doi: 10.1016/0031-9384(92)90258-4. [DOI] [PubMed] [Google Scholar]
- Mercia H, Fortune RD. State transitions between wake and sleep, and within the ultradian cycle, with focus on the link to neuronal activity. Sleep med. 2004;8:473–485. doi: 10.1016/j.smrv.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res Rev. 1999;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- Mihic SJ. Acute effects of ethanol on GABAA and glycine receptor function. Neurochem Int. 1999;35:115–123. doi: 10.1016/s0197-0186(99)00053-4. [DOI] [PubMed] [Google Scholar]
- Moy SS, Duncan GE, Knapp DJ. Sensitivity to ethanol across development in rats: comparison to [3H]zolpidem binding. Alcohol Clin Exp Res. 1998;22:1485–1492. [PubMed] [Google Scholar]
- Padmanabhapillai A, Tang Y, Ranganathan M, Rangaswamy M, Jones KA, Chorlian DB, Kamaraian C, Stimus A, Kuperman S, Rohrbaugh J, O’connor SJ, Bauer LO, Schuckit MA, Begleiter H, Porjesz B. Evoked gamma band response in male adolescent subjects at high risk for alcoholism during a visual oddball task. Int Psychophysiol. 2006;62:262–271. doi: 10.1016/j.ijpsycho.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2. Sydney: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Proctor WR, Soldo BL, Allan AM, Dunwiddie TV. Ethanol enhances synaptically evoked GABAA receptor-mediated responses in cerebral cortical neurons in rat brain slices. Brain Res. 1992;595:220–227. doi: 10.1016/0006-8993(92)91053-h. [DOI] [PubMed] [Google Scholar]
- Purves D, Lichtman JW. Elimination of synapses in the developing nervous system. Science. 1980;210:153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Robledo P, Lumeng L, Li T-K, Ehlers CL. Effects of oral ethanol self-administration on the EEG of alcohol preferring and –nonpreferring rats. Psychopharmacology. 1993;113:60–66. doi: 10.1007/BF02244335. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McKinzie DL, Webster AA, Murphy JM, Lumeng L, Li TK, McBride WJ. Low-dose stimulatory effects of ethanol during adolescence in rat lines selectively bred for high alcohol intake. Alcohol Clin Exp Res. 2004;28:535–543. doi: 10.1097/01.alc.0000122107.08417.d0. [DOI] [PubMed] [Google Scholar]
- Roubicek J. The electroencephalogram in the middle-aged and elderly. J American Geri Society. 1977;XXV:145–152. doi: 10.1111/j.1532-5415.1977.tb00282.x. [DOI] [PubMed] [Google Scholar]
- Scher MS, Richardson GA, Robles N, Geva D, Goldschmidt L, Dahl RE, Sclabassi RJ, Day NL. Effects of prenatal substance exposure: altered maturation of visual evoked potentials. Ped Neurol. 1998;18:236–243. doi: 10.1016/s0887-8994(97)00217-8. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control patients. Arch Gen Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. New findings in the genetics of alcoholism. JAMA. 1999;281:1875–1876. doi: 10.1001/jama.281.20.1875. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of rapid tolerance to the hypnotic effects of ethanol. Alcohol Clin Exp Res. 1999;23:1180–1184. [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. The effects of NMDA and GABAA pharmacological manipulations on ethanol sensitivity in immature and mature animals. Alcohol Clin Exp Res. 2002;26:449–456. [PubMed] [Google Scholar]
- Slawecki CJ, Somes C, Ehlers CL. Effects of chronic ethanol exposure on neurophysiological responses to corticotropin-releasing factor and neuropeptide Y. Alcohol alcohol. 1999;34:289–299. doi: 10.1093/alcalc/34.3.289. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Walpole T, Purdy RH, Ehlers CL. Comparison of the neurophysiological effects of allopregnanolone and ethanol in rats. Psychopharmacology. 2000;149:351–359. doi: 10.1007/s002139900365. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Maury C, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Developmental Brain Research. 2001;128:63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ. Altered EEG responses to Ethanol in Adult Rats Exposed to Ethanol During Adolescence. Alcohol clin Exp Res. 2002;26:246–254. [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell A, Ehlers CL. Antagonism of neuropeptide YY1 receptors does not inhibit ethanol’s effects on cortical EEG and ERPs in Wistar rats. J Stud Alcohol. 2005;66:559–566. doi: 10.15288/jsa.2005.66.559. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Roth J, Gilder A. Neurobehavioral profiles during the acute phase of ethanol withdrawal in adolescent and adult Sprague-Dawley rats. Behav Brain Res. 2006;170:41–51. doi: 10.1016/j.bbr.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestation. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steriade M, Gloor P, Llinas RR, Lopes de Silva FH, Mesulam MM. Report of IFCN Committee on Basic Mechanisms. Basic mechanisms of cerebral rhythmic activities. Electroencephalogr Clin Neurophysiol. 1990;76:481–508. doi: 10.1016/0013-4694(90)90001-z. [DOI] [PubMed] [Google Scholar]
- Swartzwelder HS, Richardson RC, Markwiese B, Wilson WA, Little PJ. Developmental differences in the acquisition of tolerance to ethanol. Alcohol. 1998;15:311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Chronic tolerance to the social consequences of ethanol in adolescent and adult Sprague-Dawley rats. Neurotoxicol Teratol. 2007;29:23–30. doi: 10.1016/j.ntt.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Zhang L, Carlen PL. Potentiation of GABAA –mediated synaptic current by ethanol in hippocampal CA1 neurons: possible role of protein kinase C. J Pharmacol Exp Ther. 1994;268:1388–1395. [PubMed] [Google Scholar]
- Weiner JL, Gu C, Dunwiddie TV. Differential ethanol sensitivity of subpopulations of GABAA synapses onto rat hippocampal CA1 pyramidal neurons. J Neurophysiol. 1997;77:1306–1312. doi: 10.1152/jn.1997.77.3.1306. [DOI] [PubMed] [Google Scholar]
- White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern of ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000;24:1251–1256. [PubMed] [Google Scholar]
- Whitford TJ, Rennie CJ, Grieve SM, Clark CR, Gordon E, William LM. Brain Maturation in Adolescence: Concurrent Changes in Neuroanatomy and Neurophysiology. Human Brain Mapping. 2007;28:228–237. doi: 10.1002/hbm.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ED. Mechanisms of alcohol abuse and alcoholism in adolescents: a case for developing animal models. Behav Neural Biol. 1994;62:168–177. doi: 10.1016/s0163-1047(05)80015-9. [DOI] [PubMed] [Google Scholar]
- Wright JM, Peoples RW, Wright FF. Single-channel and whole-cell analysis of ethanol inhibition of NMDA-activated currents in cultured mouse cortical and hippocampal neurons. Brain Res. 1996;738:249–256. doi: 10.1016/s0006-8993(96)00780-9. [DOI] [PubMed] [Google Scholar]
- York JL, Chan AWK. Age-related differences in sensitivity to alcohol in the rat. Alcohol Clin Exp Res. 1993;17:864–869. doi: 10.1111/j.1530-0277.1993.tb00855.x. [DOI] [PubMed] [Google Scholar]