Abstract
Understanding of the efficacy and mechanism for the reaction of the biologically important radicals with natural and/or synthetic antioxidants is the first step towards the development of future therapeutic agents. The kinetic parameters e.g., formation and decay rate constants predict the efficacy of an antioxidant and its fate after reaction. These parameters also dictate the ease with which competing reactions would occur in a bio-environment. The spectroscopic parameters provide the clue to the site of free radical attack to these antioxidants. Here, in this article an attempt has been made to show the use of physico-chemical methods in the evaluation of antioxidant activity of some important medicinal plants commonly used in India and the subcontinent. The systems chosen here for discussions are herbal extracts as such, curcumin from turmeric, methoxy phenols from Indian spices, dehydrogingerdione from ginger and bakuchiol from Psoralea corylifolia. All the examples shown in this article illustrate the potential of the pulse radiolysis coupled with kinetic spectroscopy and other physicochemical techniques for the study of antioxidants either in the form of mixture as in herbal extract or as an isolated compound.
Keywords: free radical, herbal extract, curcumin, dehydrogingerdione, bakuchiol
Introduction
Oxidation of biological molecules has been postulated to induce a variety of pathological events such as atherogenesis, carcinogenesis and ageing [1]. It has been proven beyond doubt that these damaging events are caused by free radicals [2]. The most important free radicals identified to induce such oxidative damage are commonly termed as reactive oxygen species (ROS) such as ·OH, HO2, O2−·, H2O2 etc and reactive nitrogen species (RNS) such as NO·, NO2·, ONOO− etc. Therefore the concept of pharmacological supplementation of a defense against ROS/RNS with antioxidants has become an intensive area of research and here lies the interest in the chemistry of antioxidants. The use of herbal products for improving one’s health has augmented in modern time. Indian medicinal plants provide a rich source of such products. Ayurveda and other systems of Indian medicine have identified a number of plants and their components that have curative values.
The measure of the antioxidant properties of the herbal products and their active components is important. Different biochemical as well as chemical methods can be employed for these studies. In vitro animal model systems such as rat brain and liver mitochondria, various cell culture systems are some of the subjects where these studies are carried out. The methodologies that have been used are immunocytochemistry, confocal microscopy and electron microscopy as morphological techniques to name a few. Several standard biochemical and molecular biological techniques are used to assess the damage caused by free radicals and its repair by the antioxidants. All these studies provide gross pictures about the events that take place involving free radicals and antioxidants in in vitro systems. Under this scenario, molecular level studies become indispensable. The physico-chemical parameters like redox potential, reactivity, half-life, chemical transformation and stable end products determine the positive and adverse effects of the test molecules in use. These studies might provide a clear view of the electron transfer processes that occur both in free radical induced damage as well as repair by antioxidants. Advanced techniques such as pulse radiolysis and cyclic voltammetry (CV) have been used extensively to determine redox properties of molecules in solution. The details of pulse radiolysis are given below. CV was extensively used by Chevion et al. [3] for the evaluation of antioxidant capacity of blood plasma, tissue homogenates and extracts of edible plants. Also some cyclic voltammograms were reported for beer samples as an example of a food product containing polyphenolic compounds [4]. It is also imperative to understand the mechanism involved in the antioxidant activity of such compounds. Kinetic spectroscopy in combination with pulse radiolysis is generally being used for such studies.
In this review we will focus on some recent results obtained in our laboratory. Mostly kinetic, spectroscopic and electrochemical methods were used to determine the antioxidant efficacy and mechanism of selected herbal extracts and their active ingredients. It must be noted that no attempt at a comprehensive survey of literature is made, nor is any claim made that the literature citations given are fully representative.
Pulse Radiolysis Setup and Measurement
In the pulse radiolysis setup used in this group at Bhabha Atomic Research Center [5], 7 MeV electrons come out from the waveguide through a titanium window with mean diameter of 2 mm. The machine is capable of producing electron pulses of widths between 50 ns to 2 µs with peak current ranging from 400 mA to 70 mA. The block diagram of the machine is shown in Fig. 1. In this technique free radicals are generated by employing electron pulse and the fate of these radicals are followed with time in absence and presence of scavengers. Pulse radiolysis is the most convenient method to generate detectable instantaneous concentrations of free radicals. Ever since its discovery, this technique has been used to study reactions of biological molecules. The simple reason is that one can mimic to some extent biological situations by studying the free radical induced reactions of bio-molecules in solutions. The intermediates, which are formed in vivo can be mimicked by studying redox reactions of biological molecule in vitro and can be assigned by the method like transient optical absorption technique in the present case. By knowing the intermediates, mechanism of the reaction can be delineated.
Fig. 1.
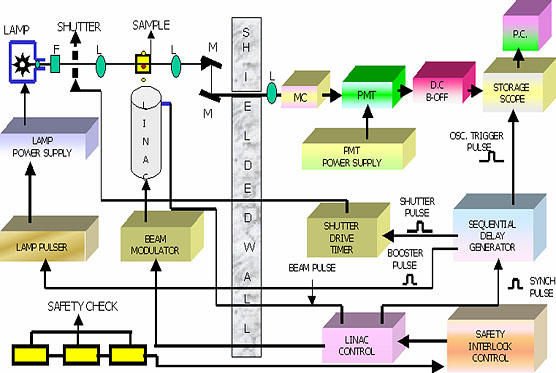
Block diagram of pulse radiolysis setup
Accurate measurements of kinetic parameters require knowledge of the concentrations of the reactants and the rate constants for the reactions so that importance of the potential competing reactions can be understood. The rate constants are determined by two methods, one is direct determination and the other is by competition kinetic method. In direct determination the principle of pseudo first order kinetics is generally followed. The substrate is used in large excess to scavenge all the free radicals (R·). The equations follow:
−d [R·]/dt = k1 [R·] (1)
Which can be interpreted in terms of
log [R·]0 − log [R·]t = k1t/2.303 (2)
Where, R0 is the initial free radical concentration and Rt is the same at time t. The pseudo first order rate constant is determined by ploting log [R·]0/[R·]t versus time (in practice absorbance of [R·]0 and [R·]t is used) to give final equation in terms of absorbance. The competition kinetic method will be discussed in detail with reference to herbal extracts in the subsequent section.
Studies with Herbal Extracts
Now a days plant extracts and herbal formulations, are gaining importance as less toxic antioxidants and radioprotectors [6, 7]. While there are several attempts to study the bio-chemical and pharmacological activities, there is a need to develop methods for evaluating the free radical scavenging ability of such extracts. Extracts contain mixture of compounds, with unknown compositions and also there is no direct method to estimate their concentration. In such case, a general practice is to express total concentration of the extract as µg/ml. Because of the multi-constituent mixture, it is not possible to determine the absolute rate constants for the reactions of extracts with free radicals. However, it is possible to estimate their relative reactivity in comparison with a standard, which can indirectly be used to indicate the total scavenging ability of the extract. Reactivity is a product of rate constant and the concentration and is a quantity, which can be directly compared when mixtures participate in a chemical or biological reaction. Some of the methods employed for studying free radical reactions are given below.
For the determination of hydroxyl radical (·OH) scavenging ability, potassium thiocyanate (KSCN or SCN−) is used as standard using pulse radiolysis. In this method ·OH is allowed to react with known concentration of KSCN at pH 7, in the absence and in the presence of various concentrations of extracts. In the absence of extract, the ·OH reacts completely with SCN− to produce (SCN)2·−. The formation of (SCN)2·− can be observed by monitoring its transient absorbance in microsecond time scales at 500 nm [8]. In the presence of extract, capable of reacting with ·OH, the absorbance due to (SCN)2·− at 500 nm decreases. From the extent of decrease in the absorbance at 500 nm, rate of scavenging of ·OH by extract (E) can be calculated. For these studies with ·OH radicals, it is necessary to use advanced techniques like pulse radiolysis, however it is possible to estimate the free radical scavenging ability using other radicals like 2,2'-diphenyl-1-picrylhydrazyl (DPPH) and 2,2'-azinobis(3-thylbenzthiazoline-6-sulfonic acid (ABTS·−). DPPH is purple in colour (λmax 517 nm) and in presence of a substance capable of donating an electron or a hydrogen atom, it loses its radical character and becomes colourless [9]. The total reactivity of the extract towards DPPH radical can be studied by following the kinetics of DPPH radical reaction in presence of the extracts. In general such reactions take place in time scales of fraction of a second to several hundred seconds and stopped-flow kinetic spectrometer is best suited for this.
ABTS·− radical is also a stable radical like DPPH, shows absorption in the wavelength region 400 nm to 750 nm, with absorption maxima at 417 nm (ε = 3.47 × 104 M−1cm−1), 645 nm (ε = 1.35 × 104 M−1cm−1) and at 728 nm (ε = 1.5 × 104 M−1cm−1) [10]. The reaction of ABTS·− radical with extracts can be followed by steady state or by time resolved methods. However, unlike DPPH, ABTS·− radical reactions involve electron transfer and take place at a much faster rate and hence depending on the time scale of reaction, it can be monitored by either pulse radiolysis or stopped-flow spectrometer.
Employing the above techniques, we were able to study a number of extracts such as Phyllanthus emblica (T1), Terminalia chebula (T2), Terminalia belerica (T3) and triphala (T4) an equi-proportional mixture of fruits of the three herbs [11–15]. Table 1 gives the ascorbate equivalents estimated by this method in different extracts studied in our laboratory. Further we were able to compare these results with their antioxidant activity like inhibition of lipid peroxidation in liposomes induced by γ-radiation. The IC50 value, i.e. the concentration of the extract required to inhibit lipid peroxidation by 50% was estimated and these are listed in Table 1. The values correlate well within experimental limits except in case of T2, which is being addressed in future. Cyclic votammetric studies were used to obtain the redox potential of the extracts. The reactions of the above extracts with different radicals are possible only when the reduction potential of constituents present in the extract is lower as compared to the reduction potential of the radicals. To determine the reduction potential, cyclic voltammetric traces of the extracts were recorded. None of them showed reversibility but broad peaks were observed at the peak potential values of 0.25 V, 0.3 V, and 0.24 V for T1, T2, and T3, respectively. The broad peaks have been attributed to the presence of large amount of oxidisable substrates present in these extracts. The low redox potential (0.2 to 0.3 V) of T1, T2, and T3 suggest that the extracts contain easily oxidisable substrates that help in neutralizing the oxidizing free radicals via electron transfer reaction. T4 being a mixture showed similar features, with major peak at 0.3 V.
Table 1.
DPPH and ABTS−· radical reactions, redox potentials and antioxidant activities of plant extracts
| Extract | Cyclic Voltammetric Peaks | DPPH Assay (IC50 µg/ml) | ABTS·− assay Ascorbate equ. (%) | IC50 (µg/ml) to inhibit γ-radiation induced lipid peroxidation |
|---|---|---|---|---|
| Phyllanthus embilica, T1 | 0.25 V | 8 ± 2 | 33 ± 5 | 7 ± 3 |
| Terminalia chebula, T2 | 0.3 V | 6 ± 4 | 60 ± 5 | 11 ± 4 |
| Terminalia belerica, T3 | 0.24 V | 10 ± 2 | 35 ± 4 | 13 ± 3 |
| Triphala, T4 | 0.3 V | 7 ± 3 | 33 ± 6 | 10 ± 3 |
Studies with Curcumin
Turmeric is the root of the perennial herb Curcuma longa, belonging to the ginger family is cultivated in tropical regions of south and southeast Asia. It is commonly used as a spice and coloring agent in Indian cooking. Its dietary consumption is estimated to be ~1.5 g per person per day. Its utility in traditional Indian medicine is referred as old as second millennium BC. It is a household medicine widely used to cure biliary disorders, anorexia, cough, diabetic wounds, hepatic disorders, rheumatism and sinusitis [16–19]. The most active component of turmeric is curcumin, which makes upto 2–5% of the spice. Several studies have shown curcumin to be a potent antioxidant with its activity 10 times more than that of the well-known antioxidant vitamin E [20]. Recent phase I clinical trials indicate that human beings can tolerate a dose of curcumin as high as 8 g/day with no side effects [16, 21]. Curcumin has been shown to be an effective antioxidant under several conditions, where free radicals are generated and since the antioxidant activity is always associated with free radical scavenging activity, curcumin has been examined for free radical scavenging studies.
Using pulse radiolysis, reactions of superoxide, halocarbon peroxyl, lipidperoxyl, methyl and methyl peroxyl radicals, glutathione radicals and azide radicals have been reported [20, 22, 23]. The rate constants for the reactions of these radicals were determined in aqueous solutions by kinetic spectrophotometry, where the formation of transient at suitable wavelength is followed as a function of time. A transient having a lifetime of a few hundred microseconds absorbing from 250 to 600 nm with maximum absorption at 500 nm was characterized in all the reactions, with extinction coefficient of ~7300 M−1cm−1. In aqueous solutions the radicals of curcumin decay by second order kinetics with 2 k values of 7.5 × 108 M−1s−1. The reaction takes place by the radical-radical recombinations leading to the formation of products, one of them being a two electron oxidized species, which undergoes subsequent cleavage to produce species like, vanillin, ferulic acid etc. The second order radical decay suggests that the decay and lifetime of curcumin radical depend on the initial concentration of radicals (t1/2 = 0.693/2 k [radical]). Under cellular conditions, where very low concentration of radicals is formed (less than micromolar concentration), such reactions between two curcumin radicals do not take place, rather the radicals react with other cellular antioxidants and ultimately may be regenerated. In fact increase in lifetime by an order of magnitude was observed in TX-100 micellar systems.
Using stopped-flow spectrometry the reactions of DPPH radicals and superoxide radicals were studied and the rate constants reported [22, 24]. In all these cases decrease in the absorption of either the radical species or the parent curcumin were followed as a function of time. Using transient luminescence detection, the rate constant for the reaction of curcumin with singlet molecular oxygen was determined by following the decay of singlet oxygen emission at 1270 nm. All these rate constants are listed in Table 2. The radicals of curcumin absorbing at 500 nm have been attributed to phenoxyl radicals, as the decay of the radicals was not affected by the presence of oxygen and the radical absorption spectrum is not influenced by the change in pH from 3 to 10 and such radicals acquire resonance stability over the extended conjugation system.
Table 2.
Rate constants for the reaction of different radicals with curcumin
| Radical | Medium | Rate constant M−1s−1 |
|---|---|---|
| N3· | 10% acetonitrile-water | 3.4 × 109 |
| N3· | Tx-100 micelles | 1.2 × 109 |
| CCl3O2 | 48% Isopropanol-water | 1.2 × 108 |
| Lipid peroxyl radical | Linoleic acid micelles | 7.3 × 105 |
| DPPH | Acetonitrile | 1.6 × 102 |
| Superoxide radical | Tx-100 Micelles | 4.6 × 104 |
| Superoxide radicals | Acetonitrile | 4.7 × 102 |
| Glutathione radical | 50% Methanol-water | 1.0 × 108 |
| Singlet oxygen | Acetonitrile | 1.3 × 106 |
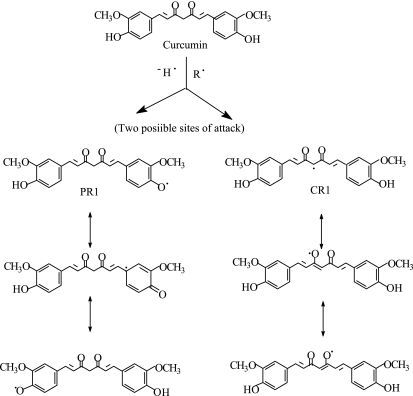
Phenoxyl radicals are formed by one of the two mechanisms such as direct hydrogen abstraction or by initial electron transfer to the free radical, where by a radical cation is formed, which subsequently undergoes a proton loss to produce a phenoxyl radical [25]. Jovanovic et al. also studied the free radical reaction mechanism of curcumin by pulse radiolysis and flashphotolysis and concluded that H atom transfer is the preferred antioxidant mechanism of curcumin [26]. They proposed that due to the availability of two methylenic hydrogens, the preferred site of free radical reaction is the CH2 group in the heptadienone link and not the phenolic OH group, which accounted for only 15% of the reaction. This was sharply contradicted later by many others. Berclay et al. [27], determined the inhibition of controlled initiation of styrene oxidation by curcumin and other synthetic non-phenolic curcuminoids having basic curcumin skeleton and do not possess phenolic groups and confirmed that curcumin is a classical phenolic chain-breaking antioxidant, donating H atoms from the phenolic OH groups and not the CH2 group. Later Jovanovic et al. [28] did some more supporting experiments and corrected their claims. Based on laser flash photolysis studies of curcumin and its mono methylated and trimethylated derivatives, they proposed that the radical formed is that of a phenoxyl radical, which is formed by direct one-electron oxidation of a methoxyphenol moiety or by that the initially formed β-oxo-alkyl radical undergoes an intramolecular H-atom transfer from the methoxyphenol. Later a conclusive evidence has been given by our group by the systematic comparison of the antioxidant activity and the free radical reactions of curcumin and dimethoxy curcumin, in which the two phenolic OH groups are blocked by the alkylation and the diketone moiety is intact, which makes an excellent comparison for this [22]. Three independent experiments were performed and compared for both the compounds. In these experiments, curcumin showed three times more efficacy in inhibiting lipid peroxidation than the dimethoxy derivative. Rate constants for hydrogen abstraction reaction with DPPH radical was 1800 times more than that with the dimethoxy derivative. Pulse radiolysis induced one-electron oxidation of dimethoxy derivative did not produce the characteristic 500 nm absorbing transient instead a week band at 310 nm was observed, that decayed in less than few microseconds. All these studies confirmed that phenolic OH makes major contribution in both antioxidant activity and hydrogen abstraction reactivity, while the methylenic group makes minor contribution for this and the 500 nm band observed in microseconds during one-electron oxidation corresponds to the phenoxyl radical. This was further confirmed by density functional theory (DFT), studies. The bond dissociation energies of the phenolic OH and the methylenic CH2 group in diketo form and that of CH group in the enolic form were estimated and found that the O-H bond dissociation energy of the phenolic group is 5.04 kcal/mol lower than the C-H bond dissociation energy (BDE) of the central CH2 of the diketo curcumin, suggesting that the phenolic OH is the most vulnerable target for free radicals in curcumin. The site for the free radical reaction of curcumin and the resonant structures of phenoxyl radicals are given in Fig. 2.
Fig. 2.
Reaction pathway of curcumin with oxidizing radicals
From the above studies, it was confirmed that the phenolic site is essential for the antioxidant activity. The diketone moiety of curcumin possesses chelating abilities towards transitions metals. This chelation of curcumin towards redox-active metals like iron and copper has been found to be useful in the treatment of Alzheimer’s disease and it is interesting to note that among the Indian populations, regular usage of turmeric is one of the reasons responsible for the reduced risk of Alzheimer’s disease. There is a similarity between drugs used for the treatment of Alzheimer’s disease and their usefulness as super oxide dismutase (SOD) mimics. This prompted us to develop new copper-curcumin complexes, which showed promising SOD activity and are found to be better antioxidants than curcumin at cellular level [29].
Studies with Other Methoxy Phenols: Correlating In-vitro Activity with Redox Potentials
In another study, four different o-methoxy phenols from Indian spices and plant products, viz., dehydrozingerone, brompentenone, eugenol and isoeugenol were examined for free radical scavenging reactions by pulse radiolysis [30]. In all these cases, one-electron oxidation produced phenoxyl radicals, having absorption spectrum in the range from 300 to 400 nm with absorption maximum at~350 nm. Further, the redox potentials of the compounds to form phenoxyl radicals from their parent phenols at pH 7 were estimated by pulse radiolysis. The reduction potentials are direct measure for their ability to undergo oxidation on reaction with oxidizing free radicals. This in turn is dependent on the O-H bond dissociation energy. This data was compared with the compound in vitro antioxidant ability like inhibition of free radicals induced lipid peroxidation in model systems. The results confirmed that a phenol, which can undergo easy oxidation, is more potent antioxidant. The comparative data is given in Table 3.
Table 3.
Correlation between reduction potential and the IC50 value to inhibit lipid peroxidation in microsomes
| Phenol | Reduction potential at pH 7 vs NHE | IC50 (µM) to inhibit Fe+2 induced lipid peroxidation |
|---|---|---|
| Dehydrozingerone | ~1 V | 30 ± 1 |
| Bromopentenone | 0.9 V | — |
| Eugenol | 0.8 V | 11.0 ± 0.3 |
| Isoeugenol | 0.7 V | 3.0 ± 0.4 |
Studies with Dehydrogingerdione
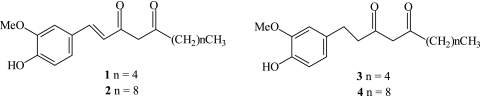
Ginger (Zingiber officinale Roscoe) is one of the world’s best-known spices, and it is universally used throughout history for its health benefits. The dried extract of ginger contains monoterpenes and sesquiterpenes. The main antioxidant principles in ginger are the gingerols, shogaols and some related phenolic ketone derivatives. Ginger extract possesses antioxidative characteristics, since it can scavenge superoxide anion and hydroxyl radicals. Ginger alone contains up to 12 compounds providing as much as 40-times higher antioxidant activity than vitamin E. The National Cancer Institute has identified ginger as one of the top-10 anti-cancer foods. As with other botanicals and herbs, ginger also offers numerous other benefits and could easily be placed in the cardiovascular category. Dehydrogingerdione (1), one of the constituents of ginger, possesses a partially methylated catechol moiety, a 1,3-diketo functionality and an extended conjugation (Fig. 3).
Fig. 3.
Chemical structure of ginger compounds (1–4)
In principle, compounds possessing multiple double bonds and especially with active hydrogen can act as radical scavengers via addition to double bonds and/or abstraction of hydrogen atom from the allylic position as is the case with lipids. The positive role of the β-diketone moiety in the antioxidant action of four ginger-derived phenolic diketones (1–4) (Fig. 3) against lipid peroxidation were reported [31] using biologically relevant model systems and pulse radiolysis. In iron-independent peroxidation, however, 1 showed activity comparable to that of curcumin. This was ascribed due to its higher affinity for the lipid peroxyl radical due to higher hydrophobicity. The antioxidant/prooxidant activity of a test compound in iron-mediated lipid peroxidation in a biological system is critically governed by factors such as the concentration of endogenous reducing systems and/or redox couples. Thus, the true antioxidant activity of 1 was assessed by use of a biologically relevant liposomal system, devoid of reductants such as ascorbate, reduced form of nicotinamide adenine dinucleotide phosphate (NADPH), etc., and by carrying out the Fe2+-mediated peroxidation at pH 5.0, at which value Fe2+ remains unoxidized, for at least 3 h, during which considerable peroxidation took place. This study not only ensured minimization of the prooxidant effect of ascorbate, but also assisted in the quantitative comparison of the antioxidant activities of 1 and ascorbate, individually and in combination. The protection offered by vitamin C (15 µM) alone was considerably less than that offered by 1 even at lower concentrations (5 and 10 µM). More interestingly, the antioxidant activity of 1 increased drastically in the presence of vitamin C at increasing concentrations. These results strongly suggest that 1 scavenges the LOO· radical more quickly than vitamin C does, but the radical generated from 1 then reacts with vitamin C to regenerate 1. This established a synergistic effect of vitamin C on the antioxidant activity of 1. The above results were also confirmed by pulse radiolytic studies of the reaction between 1 and the trichloromethylperoxyl radical (CCl3O2·). In spite of its higher reduction potential value (1.5 V), compared to the physiologically relevant peroxides, the CCl3O2· radical is extensively used as a representative peroxyl radical because of the inherent simplicity in performing the experiments, and it was indeed used earlier to study a very important biophysical phenomenon, namely free radical interaction between vitamin E and vitamin C [32]. The radical can be generated in aerated water/isopropanol/acetone mixtures containing carbon tetrachloride. A very high rate constant (2 × 109 M−1s−1) for the reaction between 1 and CCl3O2· established the superior antioxidant efficacy of 1 in comparison to vitamins C and E. This study also established a synergistic effect of vitamin C on the antioxidant activity of 1. On the basis of pulse radiolysis study, liposomal peroxidation study and HPLC analysis of the products, a mechanism for the antioxidant action of 1 has been proposed, suggesting the contribution of the phenolic group as well as the active methylene group of the 1,3-diketones [31].
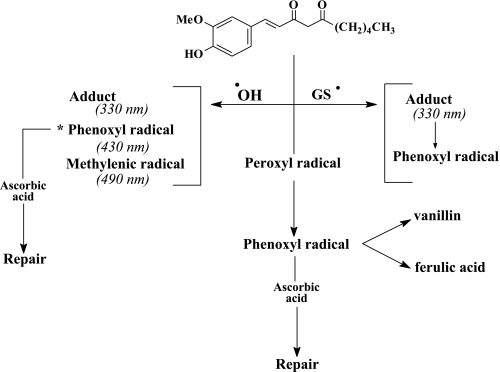
The pulse radiolysis studies revealed its high reactivity with the hydroxyl and glutathiyl radicals. With the hydroxyl radicals, it initially formed three species, an adduct, a phenoxyl and a methylenic radical. The bimolecular rate constants for the formation of these species were the same (6.8 × 109 M−1s−1). At a later stage a phenoxyl radical is produced through an intramolecular transformation from the initial radicals [33]. In the case of glutathiyl-radical-induced oxidation, a carbon-centered radical was inferred from the result of the oxygen effect on the decay of the radical. A suitable mechanism for all the reactions mentioned for the oxidation of 1 has been proposed (Fig. 4).
Fig. 4.
Oxidation mechanism of dehydrogingerdione [33]
Studies with Bacuchiol
Besides phenolics, antioxidant activities of other classes of compounds including terpenoids are also reported [34, 35] in literature. In this section the mechanism for the antioxidant activity of the meroterpene, bakuchiol, an important constituent of the plant Psoralea corylifolia (P. corylifolia) is discussed. P. corylifolia is an ancient medicinal plant found in India and China and is still being cultivated for medicinal purposes in China today. It is abundant in various parts of India, and its seeds have enjoyed an honoured place in Ayurvedic medicine, particularly for the treatment of inflammatory and skin diseases. Bakuchiol, one of the important constituents of P. corylifolia seeds, is also reported to possess anti-inflammatory and lipid protective activities. The protective activity of bakuchiol against oxidative damages to lipids and proteins has been investigated [36] and rationalized based on the scavenging activity against various oxidizing radicals (CCl3OO·, LOO·, hydroxyl and glutathiyl radicals). The rate constants of the scavenging reactions (Table 4), transients formed in these reactions and their mechanistic pathways had been probed using optical pulse radiolysis (PR) technique.
Table 4.
Rate constants for the reactions of bakuchiol (5), methoxybakuchiol (6) and the analog (3) with different radicals
| Radical | pH | λ (nm) | kf (M−1s−1) | kd (s−1) | |
|---|---|---|---|---|---|
| 5 | ·OH | 10 | 360 | 3.6 × 109 | 2.3 × 103 |
| N3· | 11 | 360 | 1.3 × 109 | 1.0 × 103 | |
| GS· | 5.4 | 300, 360 | 9.0 × 107 | 1.8 × 103 | |
| CCl3O2· | 10.9 | 350 | 2.8 × 108 | 3.0 × 103 | |
| 6 | GS· | 5.4 | 300 | 8 × 107 | 2.55 × 103 |
| 7 | GS· | 5.4 | 300 | 1.2 × 108 | 2.6 × 103 |
kf and kd are the bimolecular rate constant for the formation of the scavengers radical and the decay constant of this radical, respectively.
Bakuchiol inhibits microsomal lipid peroxidation in a concentration-dependent manner showing 74.7% protection at a concentration of 10 µM. In view of its solubility in both lipid and water (at higher pH), it is expected to be distributed in both these phases. This may account for its low IC50 = 6.1 ± 0.2 µM value against lipid peroxidation. Thus, the protective effect of 5 against lipid peroxidation was explained on the basis of radical scavenging only. Surprisingly, the methyl ether of bakuchiol (6) also prevented Fenton-mediated peroxidation of rat brain homogenate (RBH), with an IC50 value of 50.2 ± 5.3 µM. The activity of 6 was explained by abstraction of its allylic hydrogen by the lipid peroxyl radical (LOO·). This was more evident from the subsequent studies on the reactions of different radicals with 5.
Bakuchiol reacted with CCl3O2· producing radical with absorption in the visible region. Based on the absorption, formation and decay constants it was proposed that only the phenoxyl radical of bakuchiol was generated. Similarly the azidyl and hydroxyl radicals also produce the phenoxyl radicals.
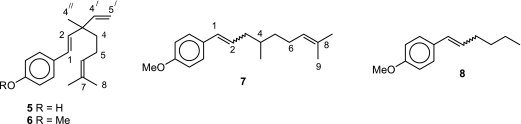
Studying the dynamics of the reaction between 5 and glutathiyl radical (GS·) revealed that two species are formed initially, one being the phenoxyl radical other is an allylic radical. Further confirmation of the generation of the allylic radical in 5 was confirmed by studying the reaction of GS· with the protected phenol derivative 6. As expected, 6 produced only one transient, the C-centered radical at the C-5 position of its terpenoid chain. This was further corroborated based on the reaction between glutathiyl radicals and two other congeners, 7 and 8 (Fig. 5). On the basis of strong optical absorption signals with 5–7 as compared to that in the case of 8, formation of the allylic radical adjacent to the trisubstituted olefin function in 5–7 was envisaged. In the case of 5, the allylic radical was found to be transformed into the phenoxyl radical at a later stage. It revealed that 5 not only scavenged GS· radicals but might also repair it by abstracting a hydrogen atom from its terpenoid chain. This repair phenomenon is very attractive especially with respect to its biological activity. These results showed the importance of the terpenoid moiety of bakuchiol in controlling its antioxidant action via radical scavenging [36].
Fig. 5.
Chemical structure of bakuchiol (5), methoxy bakuchiol (6), congeners (7, 8)
Conclusions
Measurements of kinetic parameters and physico-chemical properties can be useful in understanding the radical scavenging efficiency of herbal extracts. This in turn provides an idea about the antioxidant activity of the extracts as such. Even though the extracts are generally mixture of many compounds, rate constant for the reaction of hydroxyl radical with the extract can be determined by competition kinetic method. Such studies help in corroborating the results obtained from same sample in biochemical and chemical assays for antioxidant activities.
In the case of known antioxidant like curcumin, methoxy phenols, gingeroids, and bakuchiol a proper mechanism can be delineated based on the kinetic spectroscopic results. Certainly parallel studies on the redox potential, products formed during the reaction and theoretical modeling should substantiate the proposed mechanism. Towards the development of future therapeutic agents as antioxidants and radioprotectors, the methodology described in the article may be very important but preliminary steps to achieve the goal.
Acknowledgments
The authors gratefully acknowledge Drs. Hari Mohan and S. Chattopadhyay for fruitful discussion and suggestions during the course of the experiments and also in the process of preparation of the manuscript.
References
- 1.Finkel T., Holbrook N.J. Oxidants, oxidative stress and biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B., Gutteridge J.M.C. Free radicals in biology and medicine. Clarendon Press; Oxford: 1993. p. 416. [Google Scholar]
- 3.Chevion S., Roberts M. A., Chevion M. The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free Rad. Biol. Med. 2000;28:860–870. doi: 10.1016/s0891-5849(00)00178-7. [DOI] [PubMed] [Google Scholar]
- 4.Marian F. Electrochemical analysis of polyphenolic compounds. Anal. SCI. 2001;17:S1667–S1670. [Google Scholar]
- 5.Mukherjee T. In: Some recent studies of molecular dynamics at BARC. in Atomic, Molecular and Cluster Physics. Ahmad S.A., editor. Narosa; New Delhi: 1997. pp. 299–316. [Google Scholar]
- 6.Weiss J.F., Landauer M.R. Protection against ionising radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. doi: 10.1016/s0300-483x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 7.Arora R., Gupta D., Chawla R., Sagar R., Sharma A., Kumar R., Prasad J., Singh S., Samanta N., Sharma R.K. Radioprotection by plant products: present status and future prospects (review) Phytother. Res. 2005;19:1–22. doi: 10.1002/ptr.1605. [DOI] [PubMed] [Google Scholar]
- 8.Spinks J.W.T., Woods R.J. In: Water and inorganic aqueous systems. in Introduction to Radiation Chemistry. Wiley J., editor. New York: 1991. pp. 243–313. [Google Scholar]
- 9.Kumar S.S., Priyadarsini K.I., Sainis K.B. Free radical scavenging activity of vanillin and o-vanillin using 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical. Redox Rep. 2002;7:35–40. doi: 10.1179/135100002125000163. [DOI] [PubMed] [Google Scholar]
- 10.Scott S.L., Chen W.L., Espenson J.H. Spectroscopic parameters, electrode potentials, acid ionization constants, and electron exchange rates of the 2,2'-azino-bis (3-thylbenz-thiazoline-6-sulfonic acid) radicals and ions. J. Phys. Chem. 1993;97:6710–6714. [Google Scholar]
- 11.Naik G.H., Priyadarsini K.I., Bhagirathi R.G., Mishra B., Mishra K.P., Banavalikar M.M., Mohan H. In-vitro antioxidant studies and free radical reactions of triphala, an ayurvedic formulation and its constituents. Phytother. Res. 2005;19:582–586. doi: 10.1002/ptr.1515. [DOI] [PubMed] [Google Scholar]
- 12.Naik G.H., Priyadarsini K.I., Satav J.G., Banavalikar M.M., Sohani D.P., Biyani M.K., Mohan H.A. Comparative antioxidant activity of individual herbal components used in ayurvedic medicine. Phytochemistry. 2003;63:97–104. doi: 10.1016/s0031-9422(02)00754-9. [DOI] [PubMed] [Google Scholar]
- 13.Khopde S.M., Priyadarsini K.I., Mohan H., Gawandi V.B., Satav J.G., Yakhmi J.V., Banavaliker M.M., Biyani M.K., Mittal J.P. Characterising the antioxidant activity of amla (phyllanthus emblica) extract. Current Science. 2001;81:185–190. [Google Scholar]
- 14.Naik G.H., Priyadarsini K.I., Gangabhagirathi R., Mohan H. Studies on the aqueous extract of Terminalia chebula as a potent antioxidant and a probable radioprotector. Phytomedicine. 2004;11:530–538. doi: 10.1016/j.phymed.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Naik G.H., Priyadarsini K.I., Mohan H. Free radical scavenging reactions and phytochemical analysis of triphala, an ayurvedic formulation. Current Science. 2006;90:1100–11051. [Google Scholar]
- 16.Shishodia S., Sethi G., Aggarwal B.B. Curcumin: Getting back to the roots. Ann. N. Y Acad. Sci. 2005;1056:206–217. doi: 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal B.B., Kumar A., Bharti A.C. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 18.Sharma R.A., Gescher A.J., Steward W.P. Curcumin: The story so far. Eur. J. Cancer. 2005;41:1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Ammon H.P.T., Wahl M.A. Pharmacology of Curcuma longa. Planta. Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 20.Khopde S.M., Priyadarsini K.I., Venkatesan P., Rao M.N.A. Free radical scavenging ability and antioxidant efficiency of curcumin and its substituted analogue. Biophys. Chem. 1999;80:85–91. doi: 10.1016/s0301-4622(99)00070-8. [DOI] [PubMed] [Google Scholar]
- 21.Cheng A.L., Hsu C.H., Lin J.K., Hsu M.M., Ho Y.F., Shen T.S., Ko J.Y., Lin J.T., Lin B.R., Wu M.S., Yu H.S., Jee S.H., Chen G.S., Chen T.M., Chen C.A., Lai M.K., Pu Y.S., Pan M.H., Wang Y.J., Tsai C.C., Hsieh C.Y. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anti-cancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 22.Priyadarsini K.I., Maity D.K., Naik G.H., Sudheer Kumar M., Unnikrishan M.K., Satav J.G., Mohan H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003;35:475–484. doi: 10.1016/s0891-5849(03)00325-3. [DOI] [PubMed] [Google Scholar]
- 23.Priyadarsini K.I. Free radical reactions of curcumin in membrane models. Free Rad. Biol. Med. 1997;23:838–843. doi: 10.1016/s0891-5849(97)00026-9. [DOI] [PubMed] [Google Scholar]
- 24.Mishra B., Priyadarsini K.I., Bhide M.K., Kadam R.M., Mohan H. Reaction of superoxide radicals with curcumin: Probable mechanisms by optical spectroscopy and EPR. Free Radic. Res. 2004;38:355–362. doi: 10.1080/10715760310001660259. [DOI] [PubMed] [Google Scholar]
- 25.Gorman A.A., Hamblett V.S., Srinivasan V.S., Wood P.D. Curcumin derived transients: A pulsed laser and pulse radiolysis study. Photochem. Photobiol. 1994;59:389–398. doi: 10.1111/j.1751-1097.1994.tb05053.x. [DOI] [PubMed] [Google Scholar]
- 26.Jovanovic S.V., Steenken S., Borne C.W., Simic M.G. H-atom transfer is a preferred antioxidant mechanism of curcumin. J. Am. Chem. Soc. 1999;121:9677–9681. [Google Scholar]
- 27.Barclay L.R.C., Vinqvist M.R., Mukai K., Goto H., Tokunaga A., Uno H. On the antioxidant mechanism of curcumin: classical methods are needed to determine antioxidant mechanism and activity. Org. Lett. 2000;2:2841–2843. doi: 10.1021/ol000173t. [DOI] [PubMed] [Google Scholar]
- 28.Jovanovic S.V., Boone C.W., Steenken S., Trinoga M., Kaskey R.B. How curcumin works preferentially with water soluble antioxidants. J. Am. Chem. Soc. 2001;123:3064–3068. doi: 10.1021/ja003823x. [DOI] [PubMed] [Google Scholar]
- 29.Barik A., Mishra B., Shen L., Mohan H., Kadam R.M., Dutta S., Zhang H-Y., Priyadarsini K.I. Evaluation of a new copper(II)-curcumin complexes as superoxide dismutase mimic and its free radical reactions. Free Radic. Biol. Med. 2005;39:811–822. doi: 10.1016/j.freeradbiomed.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Priyadarsini K.I., Guha S.N., Rao M.N.A. Physico-chemical properties and antioxidant activities of methoxy phenols. Free. Rad. Biol. Med. 1998;24:933–941. doi: 10.1016/s0891-5849(97)00382-1. [DOI] [PubMed] [Google Scholar]
- 31.Patro B.S., Rele S., Chintalwar G.J., Chattopadhyay S., Adhikari S., Mukherjee T. Lipid protective activities of dehydrogingerdiones and probable mode of action. Chem. Bio. Chem. 2002;3:364–370. doi: 10.1002/1439-7633(20020402)3:4<364::AID-CBIC364>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 32.Packer J.E., Slater T.F., Willson R.L. Direct observation of a free radical interaction between vitamin E and C. Nature. 1979;278:737–738. doi: 10.1038/278737a0. [DOI] [PubMed] [Google Scholar]
- 33.Patro B.S., Adhikari S., Chintalwar G.J., Chattopadhyay S., Mukherjee T. The radioprotection and antioxidant properties of dehydrogingerdione. Res. Chem. Interm. 2005;31:667–678. [Google Scholar]
- 34.Ng T.B., Liu F., Wang Z.T. Antioxidant activity of natural products from plants. Life Sci. 2000;66:709–723. doi: 10.1016/s0024-3205(99)00642-6. [DOI] [PubMed] [Google Scholar]
- 35.Liebgott T., Miollan M., Berchadsky Y., Drieu K., Culcasi M., Pietri S. Complementary cardioprotective effects of flavonoid metabolites and terpenoid constituents of ginkgo biloba extract (Egb761) during ischemia and reperfusion. Basic. Res. Cardiol. 2000;95:368–377. doi: 10.1007/s003950070035. [DOI] [PubMed] [Google Scholar]
- 36.Adhikari S., Joshi R., Patro B.S., Ghanty T.K., Chintalwar G.J., Sharma A., Chattopadhyay S., Mukherjee T. Antioxidant activity of bakuchiol: Experimental evidences and theoretical treatments on the possible involvement of the terpenoid chain. Chem. Res. Toxicol. 2003;16:1062–1069. doi: 10.1021/tx034082r. [DOI] [PubMed] [Google Scholar]