Abstract
To elucidate the roles of enteric bacteria and immunological interactions among liver, spleen and intestine in the pathogenesis of liver injury during obstructive jaundice, we studied the effects of antibiotics and splenectomy on bile-duct-ligated C57BL mice. When animals were subjected to bile-duct-ligation (BDL), plasma levels of bilirubin, alanine aminotransferase and aspartate aminotransferase increased markedly. However, the increases in plasma transaminases were significantly lower in splenectomized or antibiotics-treated groups than in the control BDL group. Histological examination revealed that liver injury was also low in the two groups. BDL markedly increased plasma level of interferon-γ (IFN-γ) and the expression of inducible nitric oxide synthase (iNOS) in liver and spleen. These changes were suppressed either by splenectomy or administration of antibiotics. Kinetic analysis revealed that BDL-induced liver injury and the increase of interleukin-10 (IL-10) and INF-γ were lower in iNOS−/− than in wild type animals. BDL markedly increased the expression of IgA in colonic mucosa. These observations suggest that enteric bacteria, nitric oxide and cytokines including IFN-γ and IL-10 derived from spleen and intestines form a critical network that determines the extent of liver injury during obstructive jaundice.
Keywords: obstructive jaundice, intestinal flora, endotoxemia, nitric oxide, interferon-γ
Introduction
The obstruction of the bile duct often occurs in various diseases including impaction of gallstones or hepato-cholangial and pancreatic tumors [1]. The biliary obstruction causes cholestatic liver injury, hepatocellular necrosis, proliferation of bile ductular epithelial cells, and activation of stellate cells followed by liver fibrosis [2, 3]. During cholestatic liver injury, both the hepatic expression of inducible nitric oxide synthase (iNOS) and the blood level of endotoxin markedly increase [4, 5]. Hepatocytes, macrophages and lymphocytes have been shown to express iNOS following the stimulation by viral and bacterial infections [6–8]. The expression of iNOS in rodents and humans is enhanced by various factors such as endotoxin and inflammatory cytokines [9–13]. Nitric oxide generated by iNOS plays two opposite roles. While it protects the liver and other tissues, it could also cause impairments of these tissues [14–19]. However, the roles of nitric oxide (NO) and extrahepatic tissues in liver injury during obstructive jaundice remain unclear. The impairment of bile secretion into the intestine enhances the translocation of bacteria across the intestinal mucosa and sometimes causes lethal endotoxemia during obstructive jaundice [20, 21]. Although the spleen produces various cytokines and plays important roles in host defense mechanism against pathogens, its roles in the etiology of liver injury during obstructive jaundice are not well understood. In order to elucidate the roles of enteric bacteria, iNOS-derived NO and the spleen in the pathogenesis of liver injury in obstructive jaundice, we undertook this project to study the effects of bile-duct-ligation (BDL), antibiotics, and splenectomy on liver functions of wild and iNOS−/− mice.
Materials and Methods
Reagents
Assay Kits for the determination of aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, and lipopolysaccharide (LPS) in the plasma were obtained from Wako Pure Chemical Co. (Osaka, Japan). Penicillin G potassium and streptomycin sulfate were obtained from Meiji Seika, LTD (Tokyo, Japan). Mouse monoclonal antibody against iNOS (610432), rabbit anti-iNOS polyclonal antibody (KAS-NO001), and goat anti-mouse immunoglobulin A (IgA) (62-6700) were obtained from BD Transduction Laboratories (San Jose, CA), Stressgen Biotechnologies (Victoria, Canada), and from Zymed Laboratories (San Francisco, CA), respectively. ELISA Kits for mouse interferon-γ (IFN-γ) and interleukin-10 (IL-10) were obtained from PIERCE ENDOGEN (Rockford, IL). Other reagents used were of the highest grade commercially available.
Animal experiments
Male C57BL/6J mice (20–25 g) and their iNOS−/− strain were purchased from SLC (Shizuoka, Japan) and Jackson Laboratories (Coldspring Habor, MA), respectively. Animals were allowed free access to laboratory chow (CE-2, Oriental Yeast Co., Tokyo, Japan) and water ad libitum during the experiments. All experiments were approved by the Animal Care and Use Committee of Osaka City University Medical School. Obstructive jaundice was elicited by ligating the common bile duct (BDL) as described previously [22]. Under light ether anesthesia, animals (80 animals/group) were subjected to BDL. In some animals, streptomycin (4 mg/ml) and penicillin G (2 mg/ml) were added in the drinking water during the experiments from one week before giving BDL. Another group of animals received both BDL and splenectomy. Sham-operation was performed as the control experiments. At the indicated times after giving BDL, animals were sacrificed to obtain blood and liver specimens for biochemical and histological analyses.
Biochemical analysis
The blood samples from BDL mice were diluted in 9 volumes of 3.8% sodium citrate and used for blood cell counting and chemiluminescence analysis. For cell counting, 50 µl of the blood samples were used for the analysis using a Celltac α (Nihon Koden MEK-6258, Tokyo, Japan). In chemiluminescence analysis, the blood samples (50 µl) were incubated in 0.5 ml of phosphate-buffered saline (PBS) containing 400 µM L-012, a highly sensitive chemiluminescence probe [23]. After incubation of the mixtures at 37°C for 3 min, the reaction was started by adding opsonized zymosan (5 mg/ml). During the incubation, chemiluminescence intensity was recorded continuously for 10 min using a Luminescence Reader BLR-201 (Aloka, Tokyo, Japan). Plasma levels of AST, ALT, total bilirubin, LPS, IFN-γ, and IL-10 were determined according to the manufacturer’s instructions.
Histological analysis
The liver specimens were fixed in phosphate-buffered formalin (10%), embedded in paraffin, and cut into 4-µm-thick sections. Thin sections were stained with hematoxylin-eosin and analyzed histologically to evaluate the degree of liver injury caused by BDL. The expression of iNOS was evaluated immunohistochemically under a fluorescent microscope as described previously [12]. Colon specimens were rapidly frozen in an OCT embedding medium (Tissue-Tek, Elkhart, IN) and stored at −80°C until use. Cryostat sections (6 µm thickness) were fixed in ice-cold acetone for 10 min. The expression of IgA was evaluated immunohistochemically under a fluorescent microscope as described previously [24].
Western blot analysis
The liver was homogenized in a lysis buffer containing 0.5% Nonidet P-40, 10% glycerol, 137 mM NaCl, 2 mM ethylendiamine-tetraacetic acid, and 50 mM Tris-HCl buffer (pH 8.0). After centrifugation at 3,000 × g for 10 min, the supernatant was separated and stored at −80°C. The stored specimens were subjected to 7.5% polyacrylamide gel electrophoresis (PAGE) in the presence of 0.1% SDS. The electrophoresed proteins in the gel were transferred to an Immobilon membrane (Millipore, Bedford, MA). The membrane was blocked with 5% skim milk at 4°C for overnight, subsequently incubated with primary antibodies at 25°C for 1 h and then with horseradish peroxidase-conjugated secondary antibodies. Immune complexes thus formed were detected with ECL reagents reagents (GE Healthcare Bio-Sciences, Piscataway, NJ).
Statistical analysis
All data were expressed as the mean ± SD. The results obtained from the four animal groups were analyzed by either Student’s t test or ANOVA using a computer software. Differences were considered significant when p<0.05.
Results
Roles of enteric bacteria and the spleen in BDL-induced liver injury
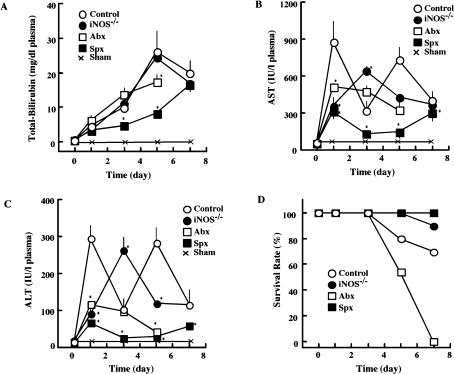
Since the obstruction of the bile duct impairs the secretion of the bile juice into the intestinal lumen, it decreases the bile-dependent intestinal functions, such as digestion of lipophilic nutrients and regulation of intestinal flora, and results in the regurgitation of hazardous bile acids and a variety of biliary metabolites. High concentrations of these metabolites often cause injury to the liver and other organs. To evaluate the effect of regurgitation of bile constituents on liver functions, changes in plasma levels of bilirubin and transaminases were determined after BDL. BDL increased the plasma levels of bilirubin in a time-dependent manner in control and iNOS−/− mice (Fig. 1). Although bilirubin levels also increased similarly in BDL-animals pretreated with antibiotics, splenectomy suppressed the rate of its increase. Despite the continuous increase of bilirubin levels in control animals, BDL increased the plasma levels of AST and ALT in a biphasic manner (the first peak followed by the second peak). Interestingly, BDL-induced increase in transaminases was significantly low in animals that had been received either antibiotics or splenectomy. The BDL-induced increases in transaminases occurred relatively slowly in iNOS−/− mice, peaked on day 3, and decreased thereafter. Thus, the area under the curve for the transaminases was slightly but significantly smaller in iNOS−/− than in control mice. BDL slightly decreased the survival rate of animals on day 5 and 6. Interestingly, the survival rate was significantly higher in iNOS−/− mice and splenectomized animals. In contrast, animals treated with antibiotics died within 7 days at the time when other BDL groups remain survived.
Fig. 1.
Effect of BDL on plasma bilirubin, transaminases, and survival rate. Under light ether anesthesia, animals were subjected to bile-duct-ligation (BDL). At the indicated times after BDL, plasma levels of total-bilirubin (A), AST (B), and ALT (C), were measured as described in the text. During the experiment, survival rate of animals was analyzed (D). Open circles: control mice (BDL alone); Open squares, animals pretreated with antibiotics from 1 week before BDL (Abx); Closed squares, animals splenectomized just before BDL (Spx); Closed circles, iNOS−/− mice which received BDL (iNOS−/−). Crosses, sham-operated mice. Statistical difference between control (BDL alone) and experimental groups is analyzed by Student’s t test. *p<0.05
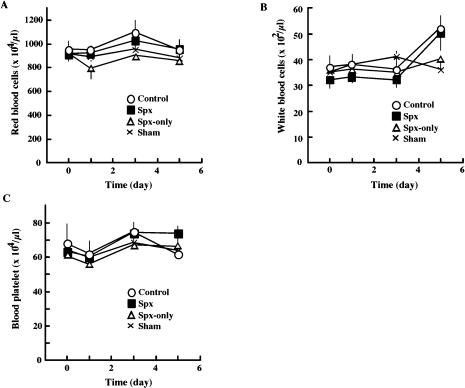
Since the spleen is responsible for the elimination of injured blood cells including erythrocytes, splenectomy may affect the profiles of blood cells in the circulation. To test this possibility, we analyzed the profiles of the circulating blood cells after splenectomy and/or BDL. However, populations of blood cells including platelets remained unchanged during the experiments (Fig. 2).
Fig. 2.
Effect of splenectomy on the population of the circulating blood cells. At the indicated times after BDL, blood samples were obtained. The population of blood cells was analyzed as described in the text. Open circles, control mice (BDL alone); Closed squares, animals splenectomized just before BDL (Spx); Open triangle, splenectomized animals without BDL (Spx-only); Crosses, sham-operated mice.
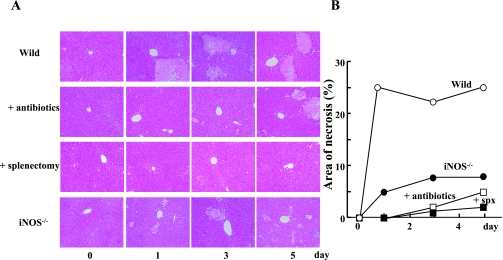
Histological examination revealed that BDL rapidly induced liver injury in the control group (Fig. 3). Consistent with the difference in the increase of plasma transaminases, liver injury was less apparent with animals that had been received either antibiotics or splenectomy. Liver injury was also mild in iNOS−/− mice as compared to control BDL animals. These observations suggest that the intestinal flora, iNOS-derived NO and the immune system in the spleen play critical roles in the determination of obstructive liver injury.
Fig. 3.
Histological examination of liver specimens after BDL. At the indicated times after BDL, the liver specimens were obtained, fixed with 10% formalin, and embedded in paraffin. Thin sections of the liver specimens were stained with hematoxylin-eosin (A). Area of necrosis was determined by using a Image J Software and expressed as % of total area (B). Statistical difference between control (BDL alone) and experimental groups is analyzed by Student’s t test. *p<0.05. All symbols and other conditions were as in Fig. 1. Scale bar = 100 µm.
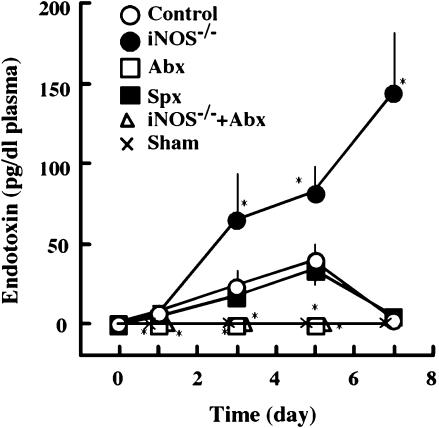
BDL increased the plasma level of endotoxin
Since bile acids have a detergent-like activity and function as bacteriostatic compounds, lack of bile juice may affect enteric flora and increase the incidence of bacterial translocation across the intestinal mucosa [20, 21]. To test this possibility, we measured the plasma levels of endotoxin before and after BDL (Fig. 4). As expected, the plasma level of endotoxin in control mice increased after BDL, peaked on day 5 and decreased thereafter. The level of endotoxin in animals administered with antibiotics was below detectable levels during the experiments. Although splenectomy did not affect the BDL-induced increase in plasma endotoxin, the extent of the increase was significantly high with iNOS−/− mice. The marked increase in plasma endotoxin in iNOS−/− mice was strongly inhibited by antibiotics. These observations suggest that BDL changed the enteric flora and enhanced bacterial translocation across the intestinal mucosa by a mechanism that was suppressed by antibiotics and iNOS-derived NO.
Fig. 4.
Effect of BDL on plasma level of endotoxin. The plasma level of endotoxin was measured as described in the text. Open circles: control mice (BDL alone); Open squares, animals pretreated with antibiotics from 1 week before BDL; Closed squares, animals splenectomized just before BDL; Closed circles, iNOS−/− mice which received BDL. Open triangles, iNOS−/− mice treated with antibiotics 1 week before BDL Cross: sham-operated mice. Statistical analysis carried out as in Fig. 1. *p<0.05
BDL increased plasma levels of cytokines
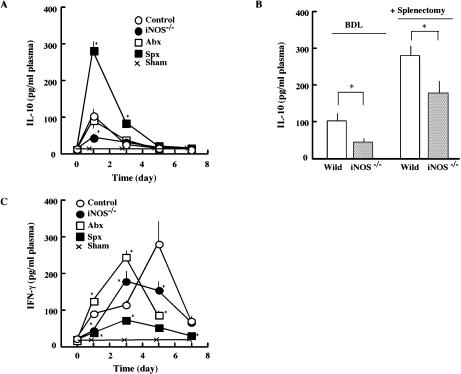
Since endotoxin stimulates a variety of leucocytes via the receptor-mediated mechanism, we determined the changes in plasma levels of cytokines. The plasma level of IL-10 rapidly increased after BDL, peaked on day 1 and decreased thereafter. Although the BDL-induced increase in plasma IL-10 remained unaffected by antibiotics, it increased markedly in splenectomized animals (Fig. 5). As shown in the two columns under the sign of “BDL”, the BDL-induced increase in plasma IL-10 was lower in iNOS−/− mice than in control mice. Splenectomy performed right before BDL (+Splenectomy) enhanced the BDL-induced increases of IL-10 remarkably on day 1 in both animal groups. The plasma level of IFN-γ increased after BDL, peaked on day 5 and decreased thereafter. In the antibiotics-treated group and the iNOS−/− group, the BDL-induced increase in IFN-γ occurred slightly faster. It peaked on day 3 and decreased thereafter. The BDL-induced increase in IFN-γ was slightly smaller with iNOS−/− mice than with the control group. Splenectomy markedly inhibited the BDL-induced increase in plasma IFN-γ.
Fig. 5.
Effect of BDL on plasma level of cytokines. Plasma levels of IL-10 (A and B) and INF-γ (C) were measured after BDL as described in the text. In panel B, the plasma levels of IL-10 in BDL mice (left two columns) and splenectomized-BDL wild mice and iNOS−/− mice (right two columns) are also shown. All symbols are the same as those in Fig. 1. All values are shown as the mean ± SD. Statistical analysis carried out as in Fig. 1. *p<0.05
BDL induced the expression of IgA in the intestine
Changes in intestinal flora have been known to modulate mucosal lymphocytes that secrete Th2-type cytokines and antibodies, such as IL-10 and IgA, respectively [25]. Thus, we analyzed the expression of IgA in the colon from BDL-treated animals. Colonic expression of IgA markedly increased after BDL. As shown in Fig. 6, the expression of IgA in the control group increased after BDL, peaked on day 3, and decreased thereafter. However, the expression of IgA in animals pretreated with antibiotics was extremely low. The expression in splenectomized-BDL mice peaked on day 1, but it decreased thereafter. A possible relationship between the IgA peak at day 1 and an extremely high level of IL-10 in splenectomized-BDL wild mice (Fig. 5B) will be discussed in the Discussion section. Although the expression of IgA was low in iNOS−/− mice during the first 3 days, it increased significantly 5 days after BDL.
Fig. 6.
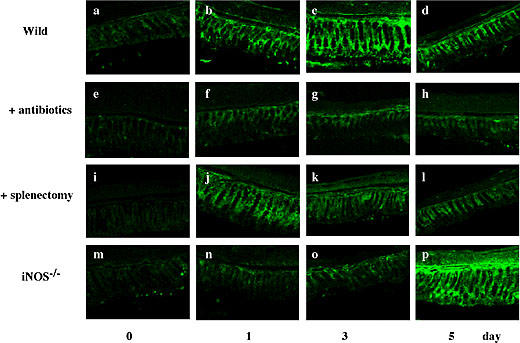
Histological examination of the expression of IgA in the colon. At the indicated times after BDL, colon specimens were frozen, cut into thin sections, treated with anti-IgA antibody and then stained with FITC-conjugated second antibody. Other conditions were the same as in Fig. 1. Scale bar = 50 µm.
BDL induced ROS generation from circulating neutrophils
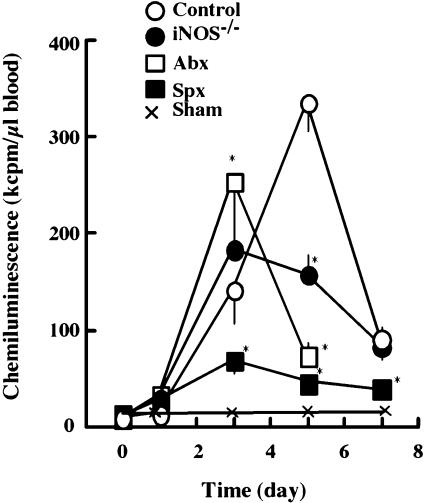
Since the BDL-induced cytokines have potent activities to stimulate leucocytes and modulate their metabolism, we analyzed the activity of circulating neutrophils in generating reactive oxygen species (ROS) using L-012-dependent chemiluminescence. The ROS generation by neutrophils in the blood increased after BDL, peaked on day 5 and decreased thereafter (Fig. 7). It decreased slightly in mice treated with antibiotics and greatly in splenectomized animals. In iNOS−/− mice, it peaked on day 3, and the peak value was lower than that in control animals.
Fig. 7.
Effect of BDL on ROS generation by circulating neutrophils. At the indicated times after BDL, 0.5 ml of blood samples were obtained from mice in 50 µl of 3.8% sodium citrate. The blood samples (50 µl) were incubated in 0.5 ml of phosphate-buffered saline in the presence of 400 µM L-012. After incubation of the mixture for 3 min at 37°C, the reaction was started by adding 5 µg/ml of opsonized zymosan. All symbols are the same as those in Fig. 1. Statistical analysis carried out as in Fig. 1. *p<0.05
BDL induced expression of iNOS in the liver
Since BDL caused significant differences between wild mice and iNOS−/− mice in terms of the plasma levels of endotoxin and cytokines as well as the degree of liver injury, we analyzed the expression of iNOS in liver specimens from BDL-treated wild mice and iNOS−/− mice (Fig. 8). Immunohistochemical and Western blotting analysis revealed that BDL markedly induced iNOS in the liver of wild mice. The expression increased with time, peaked on day 3 and decreased thereafter. The BDL-induced expression of iNOS was inhibited significantly in animals pretreated with antibiotics and completely in splenectomized animals. Naturally, no iNOS was induced by BDL in the knockout mice.
Fig. 8.
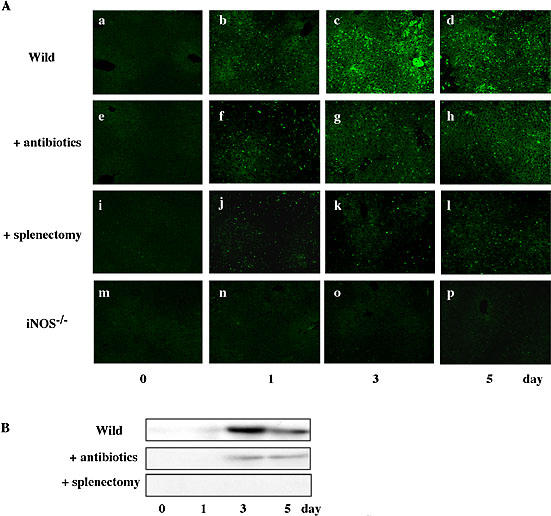
Effect of BDL on hepatic expression of iNOS. At the indicated times after BDL, liver specimens were incubated with anti-iNOS antibody and then stained with FITC-conjugated second antibody (A). Expression of iNOS was determined by Western blot analysis (B). Equal amounts of protein (30 µg/lane) were subjected to a 7.5% SDS-PAGE, and the electrophoresed proteins were transferred onto Immobilon-P membranes, and analyzed using specific anti-iNOS antibody. The bands with 130 kDa responsible for iNOS were seen in liver specimens from BDL mice. Data show one typical result out of 3 representative experiments. Scale bar = 100 µm.
Discussion
The present work shows that obstruction of the bile duct impaired the liver in a biphasic manner by a mechanism that was inhibited strongly by splenectomy and mildly by the administration of antibiotics. It has been well documented that a high concentration of bile acids has a detergent-like activity and exerts toxic effects on biological membranes [26–28]. Thus, the initial increase of plasma transaminases (Fig. 1) seems to reflect the toxic effects of the regurgitated bile acids to the liver. Although hyperbilirubinemia has been used for a long time as a marker for heme degradation and/or disturbance of biliary elimination pathway, conjugated bilirubin is no-toxic antioxidant to suppress oxidative stress [29]. It should be noted that conjugated bilirubin is predominantly responsible for the hyperbilirubinemia induced by BDL [30]. Thus, it is not surprising that plasma bilirubin level increased fairly slowly while plasma transaminases, a marker for necrotic injury of hepatocytes, increased rapidly in a biphasic manner.
The increase in AST on day 1 was about three-fold higher than that of ALT, suggesting an occurrence of mild hemolysis in BDL-mice. Since the spleen eliminates injured erythrocytes from the circulation, this organ might be responsible for the hemolysis during the early period of obstructive jaundice. Consistent with this notion is the findings that splenectomy markedly inhibited the BDL-induced increase in plasma levels of transaminases and bilirubin. Since the population of blood cells including erythrocytes remained unchanged after splenectmy in all animal groups, the spleen might induce minimum hemolysis without affecting cell kinetics of leukocytes and platelets in the circulation (Fig. 2).
High concentrations of bile acids have been known to exhibit bacteriostatic activity [31, 32]. Administration of antibiotics has been shown to decrease the number of enteric bacteria [33, 34]. Light microscopic examination revealed that, under the present experimental conditions, the administration of antibiotics for 1 week decreased the number of enteric bacteria to less than 0.0001% of the initial level (data not shown). A lack of bile secretion caused by obstructive jaundice enhanced bacterial translocation across the intestinal mucosa [20, 21]. In fact, the pretreatment of animals with antibiotics completely inhibited the BDL-induced elevation of endotoxin in the plasma (Fig. 4). Since the administration of antibiotics significantly suppressed the BDL-induced increase of plasma transaminases, changes in the intestinal flora would also participate in the modulation of liver injury during obstructive jaundice. It should be noted that the intestinal mucosa is one of the major sites for the immunological defense against pathogens. Hence, mucosal tissues are highly enriched with dendritic cells for the recognition of antigens and with lymphocytes for the elimination of pathogens. The administration of antibiotics seems to affect the status of intestinal flora and, hence, they may modulate the mucosal immune reactions. All of these factors seem to underlie the mechanism by which antibiotics significantly inhibited the BDL-induced liver injury (Figs. 1 and 3).
In the control (BDL alone) mice, the bilirubin level peaked on day 5. The transaminase levels had two peaks, namely, on day 1 and day 5. On day 5, we observed that the ROS generation by circulating neutrophils also increased markedly by a mechanism that was inhibitable by splenectomy. The histological examination demonstrated that necrosis was markedly enhanced in the liver by a mechanism that could be significantly inhibited by splenectomy and by antibiotics. Thus, the initial phase of BDL-induced liver injury on day 1 seems to elicit long-lasting inflammatory reactions through day 2 to day 5 and activated immunocytes in the spleen and other tissues to regulate the generations of ROS, NO and various cytokines including IFN-γ. This hypothesis is consistent with our findings that splenectomy strongly inhibited (i) the BDL-induced increase in ROS generation by the circulating neutrophils (Fig. 7), (ii) the elevation of plasma IFN-γ (Fig. 5) and (iii) the enhancement of the expression of hepatic iNOS (Fig. 8). All of these effects seem to be the causes for the observed suppression of liver injury.
The present work demonstrates that splenectomy enhanced the BDL-induced increase of IL-10, an anti-inflammatory cytokine (Fig. 5). Th1- and Th2-type lymphocytes cross-talk with each other to regulate a sequence of inflammatory reactions to favor the survival of animals [35, 36]. Since the plasma level of IL-10 markedly increased on day 1 by a mechanism that was inhibited by splenectomy, Th2-type lymphocytes in extrasplenic tissue(s) might be activated during the initial period of obstructive jaundice. It has been known that Th2-type lymphocytes and plasma cells in the intestinal mucosa secrete IL-10 and IgA, respectively [25]. We also found that BDL markedly increased the mucosal expression of IgA in the colon by a mechanism that was suppressed by antibiotics (Fig. 6). These observations suggest that a lack of bile juice changed the intestinal flora, thereby stimulating Th2-type lymphocytes in the intestinal mucosa to produce IL-10 and subsequently increase IgA secretion. IFN-γ derived from the activated splenocytes seems to suppress the intestinal secretion of IL-10 from Th2-type lymphocytes and maintain the inflammatory reactions in the liver, particularly during an early period of obstructive jaundice.
Endotoxin activates Kupffer cells and a variety of immunocytes to stimulate cytokine production and iNOS expression in the liver and other tissues [13]. Although BDL increased endotoxin levels in plasma more markedly with iNOS−/− mice than with wild-type animals, the liver injury was relatively mild with the former as compared with the latter (Fig. 4). The BDL-induced elevation of IFN-γ and IL-10 was also lower with iNOS−/− mice than with wild-type mice. Although iNOS-derived NO play a role in the regulation of ROS and cytokines, the elevation of plasma endotoxin per se may not be essential for the deterioration of BDL-induced liver injury.
The present work also revealed that the pretreatment of animals with antibiotics suppressed both the BDL-enhanced expression of iNOS in the liver and ROS generation by the circulating neutrophils. However, all animals treated with antibiotics died within 7 days at the time when other BDL groups survived. In rodents, the antibiotics used for the present experiments are excreted predominantly by the liver through organic anion transport systems [37]. Although the kidney also excretes these antibiotics particularly when hepatic excretion is decreased, a hepato-renal syndrome induced by liver injury and hyperbilirubinemia might inhibit renal functions to excrete xenobiotics [30]. In fact, biochemical analysis revealed that plasma levels of BUN (10–14 mg/dl in intact animals) increased rapidly after BDL and peaked on day 3 (40–50 mg/dl) both in control and antibiotics-treated groups. Thus, BDL might also inhibit the excretory systems for antibiotics in the kidney, thereby enhancing the retention of antibiotics to exhibit the toxic effects of the drugs to increase the mortality of animals. The mechanism by which antibiotics increased the mortality of BDL animals should be studied further.
In summary, our observations suggest that obstructive liver injury is enhanced by INFγ-dependent splenic response to bacteria and iNOS-derived NO, but suppressed by IL-10 generated by intestinal mucosal lymphocytes. Thus, immunological cooperation and cross-talk among the intestine, liver, spleen and kidney, and the use of antibiotics play critical roles in the determination of clinical course of patients with obstructive jaundice.
Acknowledgments
This work was supported by a Special Coordination Fund for Promoting Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology (16590252 and 14370062), and 21st Century COE Program “Base to Overcome Fatigue” supported by MEXT, Japan. The authors thank Prof. S. Tsuyoshi Ohnishi for his critical reading of the manuscript.
Abbreviations
- BDL
bile duct ligation
- iNOS
inducible nitric oxide synthase
- NO
nitric oxide
- IL-10
interleukin 10
- IFN-γ
interferon-γ
- LPS
lipopolysaccharide
- ALT
alanine aminotransferase
- AST
aspartate amionotransferase
- ROS
reactive oxygen species
- BUN
blood urea nitrogen
References
- 1.Hofmann A.F. Cholestatic liver disease: pathophysiology and therapeutic options. Liver. 2002;22:14s–19s. doi: 10.1034/j.1600-0676.2002.00002.x. [DOI] [PubMed] [Google Scholar]
- 2.Scobie B.A., Summerskill W.H. Hepatic Cirrhosis secondary to obstruction of the biliary system. Am. J. Dig. Dis. 1965;10:135–146. doi: 10.1007/BF02236664. [DOI] [PubMed] [Google Scholar]
- 3.Kountouras J., Billing B.H., Scheuer P.J. Prolonged bile duct obstruction: a new experimental model for cirrhosis in the rat. Br. J. Exp. Pathol. 1984;65:305–311. [PMC free article] [PubMed] [Google Scholar]
- 4.Wei C.L., Khoo H.E., Lee K.H., Hon W.M. Differential expression and localization of nitric oxide synthases in cirrhotic livers of bile duct-ligated rats. Nitric Oxide. 2002;7:91–102. doi: 10.1016/s1089-8603(02)00103-9. [DOI] [PubMed] [Google Scholar]
- 5.Wei C.L., Hon W.M., Lee K.H., Khoo H.E. Temporal expression of hepatic inducible nitric oxide synthase in liver cirrhosis. World J. Gastroenterol. 2005;11:362–367. doi: 10.3748/wjg.v11.i3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood E.R., Berger H., Jr ., Sherman P.A., Lapetina E.G. Hepatocytes and macrophages express an identical cytokine inducible nitric oxide synthase gene. Biochem. Biophys. Res. Commun. 1993;191:767–774. doi: 10.1006/bbrc.1993.1283. [DOI] [PubMed] [Google Scholar]
- 7.Denis M. Human monocytes/macrophages: NO or no NO? J. Leukoc. Biol. 1994;55:682–684. doi: 10.1002/jlb.55.5.682. [DOI] [PubMed] [Google Scholar]
- 8.Mannick J.B., Asano K., Izumi K., Kieff E., Stamler J.S. Nitric oxide produced by human B lymphocytes inhibits apoptosis and Epstein-Barr virus reactivation. Cell. 1994;79:1137–1146. doi: 10.1016/0092-8674(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 9.Bredt D.S., Hwang P.M., Glatt C.E., Lowenstein C., Reed R.R., Snyder S.H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991;351:714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- 10.Lamas S., Marsden P.A., Li G.K., Tempst P., Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc. Natl. Acad. Sci. U.S.A. 1992;89:6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowenstein C.J., Glatt C.S., Bredt D.S., Snyder S.H. Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc. Natl. Acad. Sci. U.S.A. 1992;89:6711–6715. doi: 10.1073/pnas.89.15.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yagnik G.P., Takahashi Y., Tsoulfas G., Reid K., Murase N., Geller D.A. Blockade of the L-arginine/NO synthase pathway worsens hepatic apoptosis and liver transplant preservation injury. Hepatology. 2002;36:573–581. doi: 10.1053/jhep.2002.35058. [DOI] [PubMed] [Google Scholar]
- 13.Schweyer S., Mihm S., Radzun H.J., Hartmann H., Fayyazi A. Liver infiltrating T lymphocytes express interferon gamma and inducible nitric oxide synthase in chronic hepatitis C virus infection. Gut. 2000;46:255–259. doi: 10.1136/gut.46.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rai R.M., Lee F.Y., Rosen A., Yang S.Q., Lin H.Z., Koteish A., Liew F.Y., Zaragoza C., Lowenstein C., Diehl A.M. Impaired liver regeneration in inducible nitric oxide synthase deficient mice. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13829–13834. doi: 10.1073/pnas.95.23.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nanji A.A., Greenberg S.S., Tahan S.R., Fogt F., Loscalzo J., Sadrzadeh S.M., Xie J., Stamler J.S. Nitric oxide production in experimental alcoholic liver disease in the rat: role in protection from injury. Gastroenterology. 1995;109:899–907. doi: 10.1016/0016-5085(95)90400-x. [DOI] [PubMed] [Google Scholar]
- 16.Bohlinger I., Leist M., Barsig J., Uhlig S., Tiegs G., Wendel A. Interleukin-1 and nitric oxide protect against tumor necrosis factor alpha-induced liver injury through distinct pathways. Hepatology. 1995;22:1829–1837. [PubMed] [Google Scholar]
- 17.Muriel P. Nitric oxide protection of rat liver from lipid peroxidation, collagen accumulation, and liver damage induced by carbon tetrachloride. Biochem. Pharmacol. 1998;56:773–779. doi: 10.1016/s0006-2952(98)00220-2. [DOI] [PubMed] [Google Scholar]
- 18.Menezes J., Hierholzer C., Watkins S.C., Lyons V., Peitzman A.B., Billiar T.R., Tweardy D.J., Harbrecht B.G. A novel nitric oxide scavenger decreases liver injury and improves survival after hemorrhagic shock. Am. J. Physiol. 1999;277:G144–151. doi: 10.1152/ajpgi.1999.277.1.G144. [DOI] [PubMed] [Google Scholar]
- 19.Thiemermann C., Ruetten H., Wu C.C., Vane J.R. The multiple organ dysfunction syndrome caused by endotoxin in the rat: attenuation of liver dysfunction by inhibitors of nitric oxide synthase. Br. J. Pharmacol. 1995;116:2845–2851. doi: 10.1111/j.1476-5381.1995.tb15935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouma D.J., Coelho J.C., Schlegel J.F., Li Y.F., Moody F.G. The effect of preoperative internal and external biliary drainage on mortality of jaundiced rats. Arch. Surg. 1987;122:731–734. doi: 10.1001/archsurg.1987.01400180113022. [DOI] [PubMed] [Google Scholar]
- 21.Ding J.W., Andersson R., Soltesz V., Willen R., Bengmark S. The role of bile and bile acids in bacterial translocation in obstructive jaundice in rats. Eur. Surg. Res. 1993;25:11–19. doi: 10.1159/000129252. [DOI] [PubMed] [Google Scholar]
- 22.Miyoshi H., Rust C., Roberts P.J., Burgart L.J., Gores G.J. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology. 1999;117:669–677. doi: 10.1016/s0016-5085(99)70461-0. [DOI] [PubMed] [Google Scholar]
- 23.Imada I., Sato E.F., Miyamoto M., Ichimori Y., Minamiyama Y., Konaka R., Inoue M. Analysis of reactive oxygen species generated by neutrophils using a chemiluminescence probe L-012. Anal. Biochem. 1999;271:53–58. doi: 10.1006/abio.1999.4107. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto M., Kweon M.N., Rennert P.D., Hiroi T., Fujihashi K., McGhee J.R., Kiyono H. Role of gut-associated lymphoreticular tissues in antigen-specific intestinal IgA immunity. J. Immunol. 2004;173:762–769. doi: 10.4049/jimmunol.173.2.762. [DOI] [PubMed] [Google Scholar]
- 25.Fagarasan S., Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat. Rev. Immunol. 2003;3:63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- 26.Popper H. Cholestasis. Annu. Rev. Med. 1968;19:39–56. doi: 10.1146/annurev.me.19.020168.000351. [DOI] [PubMed] [Google Scholar]
- 27.Greim H., Trulzsch D., Roboz J., Dressler K., Czygan P., Hutterer F., Schaffner F., Popper H. Mechanism of cholestasis. 5. Bile acids in normal rat livers and in those after bile duct ligation. Gastroenterology. 1972;63:837–845. [PubMed] [Google Scholar]
- 28.Scholmerich J., Becher M.S., Schmidt K., Schubert R., Kremer B., Feldhaus S., Gerok W. Influence of hydroxylation and conjugation of bile salts on their membrane-damaging properties—studies on isolated hepatocytes and lipid membrane vesicles. Hepatology. 1984;4:661–666. doi: 10.1002/hep.1840040416. [DOI] [PubMed] [Google Scholar]
- 29.Stocker R., Yamamoto Y., McDonagh A.F., Glazer A.N., Ames B.N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 30.Sugi K., Inoue M., Morino Y. Degradation of plasma bilirubin by a bilirubin oxidase derivative which has a relatively long half-life in the circulation. Biochim. Biophys. Acta. 1989;991:405–409. doi: 10.1016/0304-4165(89)90065-2. [DOI] [PubMed] [Google Scholar]
- 31.Gouma D.J., Coelho J.C., Fisher J.D., Schlegel J.F., Li Y.F., Moody F.G. Endotoxemia after relief of biliary obstruction by internal and external drainage in rats. Am. J. Surg. 1986;151:476–479. doi: 10.1016/0002-9610(86)90107-8. [DOI] [PubMed] [Google Scholar]
- 32.Greve J.W., Maessen J.G., Tiebosch T., Buurman W.A., Gouma D.J. Prevention of postoperative complications in jaundiced rats. Internal biliary drainage versus oral lactulose. Ann. Surg. 1990;212:221–227. doi: 10.1097/00000658-199008000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo W., Ding J., Huang Q., Jerrells T., Deitch E.A. Alterations in intestinal bacterial flora modulate the systemic cytokine response to hemorrhagic shock. Am. J. Physiol. 1995;269:G827–832. doi: 10.1152/ajpgi.1995.269.6.G827. [DOI] [PubMed] [Google Scholar]
- 34.Deitch E.A., Maejima K., Berg R. Effect of oral antibiotics and bacterial overgrowth on the translocation of the GI tract microflora in burned rats. J. Trauma. 1985;25:385–392. doi: 10.1097/00005373-198505000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Moore K.W., O’Garra A., de Waal Malefyt R., Vieira P., Mosmann T.R. Interleukin-10. Annu. Rev. Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 36.Benbernou N., Esnault S., Shin H.C., Fekkar H., Guenounou M. Differential regulation of IFN-gamma, IL-10 and inducible nitric oxide synthase in human T cells by cyclic AMP-dependent signal transduction pathway. Immunology. 1997;91:361–368. doi: 10.1046/j.1365-2567.1997.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yabuuchi H., Tamai I., Morita K., Kouda T., Miyamoto K., Takeda E., Tsuji A. Hepatic sinusoidal membrane transport of anionic drugs mediated by anion transporter Npt1. J. Pharmacol. Exp. Ther. 1998;286:1391–1396. [PubMed] [Google Scholar]