Abstract
Small intestinal resection rats are used widely as a malabsorption model, but the immunological changes are unclear. We examined the changes in systemic and mucosal immune status after a small intestinal resection in rats with a controlled nutritional status. Rats had 60% of their small intestine removed. At 5 days after the surgery, spleen cells and intraepithelial lymphocytes (IEL) were isolated. The phenotypes of spleen cells and IEL, the production patterns of Th1 and Th2 cytokines, and the proinflammatory cytokine levels in the plasma were measured. CD4+ T cells in the blood and spleen were significantly decreased in the Resection group (p<0.05). In contrast, IEL subpopulations were not different between the two groups. Interferon-γ production from the spleen cells was significantly decreased in the Resection group (p<0.05). Interleukin (IL)-4 production was not different between the two groups. Plasma IL-6 concentrations were significantly elevated in the Resection group 6 h after surgery (p<0.05). In conclusions, small intestinal resection in rats suppressed systemic immunity, and this model is useful as a surgical stress model.
Keywords: surgical stress, CD4, CD8, Th1/Th2, intraepithelial lymphocyte
Introduction
Both systemic and mucosal immunity play important roles in the host immune system. It has been reported that surgical stress induces alterations in systemic and mucosal immunity by using surgical stress models such as the gut ischemia-reperfusion model [1, 2] or the partial gastrectomy model [3, 4]. The small intestinal resection model is widely used as a postoperative malabsorption model [5, 6]. However, the immunological changes in this model remain unclear.
Surgical stress has been shown to affect the host immune system in human studies [7–9]. Surgical stress suppresses Th1 cytokine production, and the Th1/Th2 balance is shifted towards Th2 [10, 11]. These alterations are known to result in a susceptibility to infection.
It has been reported that the nutritional status also influences immune function [12, 13]. The immune status might be affected by both surgical stress and nutritional status. Therefore, we hypothesized that it would be necessary to examine the effects of surgical stress on the host immune system under the same nutritional status between operated and non-operated animals. In this study, we examined the influence of surgical stress on systemic and mucosal immunity in small intestinal resection rats under a controlled nutritional status. Lymphocyte phenotypes and the production patterns of cytokines were then determined.
Materials and Methods
Animals
Male, 6-week-old Sprague-Dawley rats (CLEA Japan, Inc) were housed in wire cages under controlled temperature (23 ± 2°C) and humidity conditions (50 ± 10%), with a 12-hour light-dark cycle. They were fed commercial laboratory chow, and were allowed drinking water ad libitum before the study began.
Experimental design
The experiments reported herein conform to the guideline for the care and use of laboratory animals established by the Animal Use and Care Committee of EN Otsuka Pharmaceutical Co., Ltd. Rats were randomized into two groups by body weight: the Control group (n = 6) consisting of non-operated controls, and the Resection group (n = 7) consisting of a 60% small intestine resection. Both groups of rats were fed liquid diets for 10 days, except for an overnight fast before the surgery. The animals of Resection group were anesthetized with pentobarbital, and a laparotomy was performed through a midline incision. Approximately 60% of the small intestine was removed from 15 cm distal to the ligament of Treitz to 10 cm above the cecum, and an end-to-end anastomosis was performed with eight interrupted 5-0 silk sutures. After surgery, all animals were fasted overnight, and on day 1 they were given the liquid diet described above. All animals were sacrificed on day 5, and the spleen and small intestine were obtained from each rat.
Cell isolation
Each spleen and small intestine was placed in tissue culture medium RPMI 1640 (Nissui Pharmaceutical, Tokyo, Japan) containing 10% fetal bovine serum (FBS), 2 mM glutamine, 100 g/L streptomycin, and 100,000 IU/L penicillin. The spleens were passed through a sterile wire mesh to remove any tissue debris. After centrifugation, the pellets were incubated with 0.83% NH4Cl Tris buffer to lyse the red blood cells. The cells were washed twice, and resuspended in tissue culture media.
The intraepithelial lymphocytes (IEL) were isolated using the method described by Ishikawa et al. [14] with some modification. After removing the small intestine, it was placed in Hank’s balanced salt solution (HBSS, Gibco, GrandIsland, NY) containing 5% FBS. The intestine was turned inside out, cut into 2 cm pieces and incubated with HBSS (Ca, Mg free, 5% FBS, 5 mM EDTA) for 45 min at 37°C in a water shaker (150 rpm). The supernatants were then filtered through a glass wool column. After centrifugation, the pellets were suspended in 44% Percoll (Amersham Bioscience AB, Uppsala, Sweden), and the cell suspensions were overlaid on 70% Percoll. After centrifugation for 20 min at 600 g at 20°C, the lymphocytes were recovered from the 44/70% interface and washed in tissue culture media. The lymphocytes were finally resuspended in tissue culture media.
Flow cytometry
Fluorescein isothiocyanate (FITC)-conjugated anti-rat CD4 and CD8 antibodies were obtained from Immunoteck (Marseille, France), and FITC-conjugated anti-rat T-cell receptor (TCR) αβ and TCR γδ were obtained from Oxford Biotechnology (Oxford, UK). CD4 and CD8 were used as general markers of T cell subsets. TCRαβ and TCRγδ were used to identify the αβ chain of T-cell receptor and the γδ chain of T-cell receptor of IEL. The blood samples, spleen cells and IEL were incubated with monoclonal antibodies for 20 min in the dark at 4°C. The antigen expressions were then analyzed on a flow cytometer FC500 (Coulter, Miami, FL).
Measurement of cytokines
For plasma interleukin (IL)-6 measurements, blood samples were collected 6 h after surgery, and were centrifuged for 15 min at 3,000 rpm. The supernatants were collected and stored at −80°C until measurement of IL-6 concentration. The spleen cells were cultured for 48 h at 37°C in a 24 well flat bottom plate at 1 × 106 cells/ml/well in the presence of 2.5 µg/ml concanavalin A (ConA). The culture supernatants were then collected and stored at −80°C until measurement of interferon (IFN)-γ and IL-4. The cytokine concentrations were determined by an enzyme-linked immunosorbent assay (ELISA) kit (BioSource International, CA), according to the manufacturer’s instructions.
Statistical analysis
The results were expressed as means ± SD. The statistical analysis was performed by Student’s t test and p values less than 0.05 were considered to be statistically significant.
Results
Food Intake and body weight change
Both groups of rats were fed the same volume of iquid diets, so the energy intakes during the experimental period were almost precisely the same between the two groups.
Body weight was decreased in both groups after surgery (Table 1). There were no significant differences in body weight during the experimental period between the two groups.
Table 1.
Body weight changes during the experimental period
| Before surgery |
After surgery |
||
|---|---|---|---|
| Day 0 | Day 1 | Day 5 | |
| Control (n = 6) | 181.73 ± 10.08 | 167.73 ± 9.87 | 176.30 ± 9.45 |
| Resection (n = 7) | 185.72 ± 9.11 | 163.10 ± 8.36 | 175.36 ± 11.82 |
Values are expressed in grams (means ± SD).
Lymphocyte phenotypes
In the Resection group, the percentage of CD4+ T cells in the blood and spleens were significantly decreased, but there were no significant differences in CD8+ T cell percentages between the two groups (Table 2). CD4/CD8 ratio in blood and spleens were lower in the Resection group, but these differences were not statistically significant. CD8+, TCRαβ+, and TCRγδ+ T cell percentages in IEL were lower in the Resection group than in the Control group, but no significant differences were found between these two groups.
Table 2.
Changes in the percentages of lymphocyte phenotypes and CD4/CD8 ratio in the blood, spleen and intraepithelial lymphocytes
| Control (n = 6) | Resection (n = 7) | ||
|---|---|---|---|
| blood | CD4+ | 50.3 ± 3.9 | 39.2 ± 7.7* |
| CD8+ | 13.5 ± 6.8 | 13.5 ± 7.8 | |
| CD4/CD8 | 4.62 ± 2.18 | 3.77 ± 2.01 | |
| spleen | CD4+ | 33.6 ± 3.9 | 26.5 ± 5.0* |
| CD8+ | 20.7 ± 3.1 | 17.3 ± 3.2 | |
| CD4/CD8 | 1.67 ± 0.36 | 1.55 ± 0.31 | |
| IEL | CD4+ | 12.8 ± 5.8 | 14.9 ± 5.1 |
| CD8+ | 57.5 ± 12.1 | 47.9 ± 7.0 | |
| TCRαβ+ | 60.0 ± 8.4 | 51.8 ± 6.2 | |
| TCRγδ+ | 9.4 ± 1.5 | 8.9 ± 3.2 | |
| CD4/CD8 | 0.24 ± 0.14 | 0.31 ± 0.11 |
Values are means ± SD.
*p<0.05 compared with the Control group.
Cytokine production
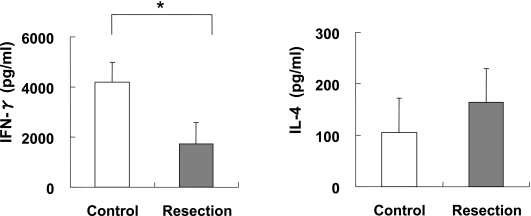
IFN-γ concentrations in the ConA stimulated spleen cell cultures were significantly decreased in the Resection group (Fig. 1). In contrast, IL-4 concentrations were not decreased in the Resection group.
Fig. 1.
Cytokine production by rat spleen cells. Rats spleen cells were isolated and cultured (1 × 106 cells/well) in the presence of 2.5 µg/ml concanavalin A (ConA) for 48 h at 37°C. The supernatants were then collected and stored at −80°C. The cytokine concentrations were determined by ELISA. Values are means ± SD. *p<0.05 compared with the Control group. (A) Interferon (IFN)-γ secretion for the Control (n = 6) and Resection groups (n = 7). (B) Interleukin (IL)-4 secretion for the Control (n = 6) and Resection groups (n = 7).
Plasma IL-6 concentrations
Plasma IL-6 concentrations were significantly elevated in the Resection group 6 h after surgery (Table 3). Before surgery and 3 days after surgery, plasma IL-6 levels were not detectable in both groups.
Table 3.
Changes in plasma Interleukin (IL)-6 concentrations
| Before surgery |
After surgery |
||
|---|---|---|---|
| Day 1 | 6 h | Day 3 | |
| Control (n = 6) | N.D. | N.D. | N.D. |
| Resection (n = 7) | N.D. | 81.5 ± 52.0*(pg/ml) | N.D. |
Values are means ± SD.
N.D.: not detected.
*p<0.05 compared with the Control group.
Discussion
Small intestinal resection in rats significantly suppressed systemic CD4+ T cells. In addition, Th1 cytokine IFN-γ production from the spleen cells decreased. In contrast, no significant changes were observed in mucosal lymphocytes IEL.
A decrease in CD4+ T cells after surgery has been previously reported in studies of operated patients. Ogawa et al. [7] reported that the number of peripheral blood lymphocytes decreased after surgery in patients with gastrointestinal cancer, and that this change was mainly attributed to the decrease in CD3+ and CD4+ T cells. In our study, CD4+ T cells in the blood and spleens were significantly decreased after surgery. This change in the T cells may be associated with increasing post operative infectious complications. In contrast, no significant changes were observed in IEL. The gut-associated lymphoid tissue (GALT) is a barrier in the intestine. IEL are present in the innermost layer of the GALT, and represent a rich source of cytokines [15]. A depletion of CD4+ and CD8+ T cells within the GALT has been reported to increase bacterial translocation [16, 17]. Gryglewski et al. [4] reported that lymphocytes in the peripheral blood and GALT (Peyer’s patches and mesenteric lymph nodes) changed significantly three days after a gastrectomy. We examined the changes in the lymphocyte phenotypes 5 days after surgery. Whether the changes in mucosal immunity after a small intestinal resection occur earlier than the systemic immunity changes remains unknown. Further studies are needed to clarify the changes in IEL.
CD4+ T cells are divided into Th1 and Th2 subsets based on their cytokine production. The Th1/Th2 balance is regulated by various hormones and cytokines in the homeostasis of the host. Th1 subsets are characterized by cell-mediated immunity, and by the production of IFN-γ, IL-2, and IL-12. Th2 subsets are characterized by the cytokines IL-4 and IL-10, and by antibody production [18, 19]. In our study, IFN-γ production by spleen cells decreased significantly after surgery. In contrast, IL-4 production did not change after surgery, and the Th1/Th2 balance was shifted towards Th2. It has been reported that a down-regulation of the Th1 cytokine response or cell-mediated immunity makes patients more susceptible to infections with viruses, protozoa and intracellular bacteria [10]. Tatsumi et al. [11] reported that IFN-γ-producing CD4+ T (Th1) cells were suppressed on postoperative day 7 in patients who developed postoperative infections. This shift of the Th1/Th2 balance to Th2 after small intestinal resection will also affect the incidence of postoperative infections.
Plasma IL-6 concentrations were significantly elevated 6 h after small intestinal resection. Surgical stress induces the release of cytokines such as TNF-α, IL-1, IL-6 and IL-8 from the operated site or the damaged tissues. IL-6 is the main mediator of the host acute phase responses, and stimulates hepatocytes to produce acute phase reactants such as immunosuppressive acid protein (IAP), C-reactive protein (CRP), and others [20, 21]. IAP is reported to modulate the CD4 antigen expression by lymphocytes [21]. Therefore, the increase in IL-6 levels after small intestinal resection might be one of the factors that suppress lymphocyte function, such as CD4+ T cell depletion or a shift to Th2.
In conclusion, the present study demonstrated that surgical stress in a rat small intestinal resection model decreases systemic CD4+ T cells and suppresses Th1 cytokine production without increasing Th2 cytokine. These changes may affect the host immune defense, and increase the incidence of postoperative infections. Our study indicated that small intestinal resection rats are useful not only as a malabsorption model, but also as a surgical stress model. Further studies are needed on the immunological changes induced by surgical stress, and effective nutrients for the recovery of immune function.
Abbreviations
- IFN
Interferon
- IL
interleukin
- IEL
intraepithelial lymphocytes
- FBS
fetal bovine serum
- HBSS
Hank’s balanced salt solution
- FITC
Fluorescein isothiocyanate
- TCR
T-cell receptor
- ConA
concanavalin A
- IAP
immunosuppressive acid protein
- CRP
C-reactive protein
References
- 1.Fukatsu K., Ueno C., Maeshima Y., Hara E., Nagayoshi H., Omata J., Mochizuki H., Hiraide H. Effects of L-arginine infusion during ischemia on gut blood perfusion, oxygen tension, and circulating myeloid cell activation in a murine gut ischemia/reperfusion model. J. Parenter. Enteral Nutr. 2004;28:224–230. doi: 10.1177/0148607104028004224. [DOI] [PubMed] [Google Scholar]
- 2.Fukatsu K., Sakamoto S., Hara E., Ueno C., Maeshima Y., Matsumoto I., Mochizuki H., Hiraide H. Gut ischemia-reperfusion affects gut mucosal immunity: a possible mechanism for infectious complications after sever surgical insults. Crit. Care Med. 2006;34:182–187. doi: 10.1097/01.ccm.0000196207.86570.16. [DOI] [PubMed] [Google Scholar]
- 3.Gryglewski A., Marcinkiewicz J., Popiela T., Ptak W. Effect of surgical trauma (gastrectomy) on cell-mediated and humoral response in mice. Clin. Exp. Immunol. 1985;59:50–54. [PMC free article] [PubMed] [Google Scholar]
- 4.Gryglewski A., Szczepanik M., Majcher P., Popiela T., Ptak W. Different patterns of γδ and αβ T cell redistribution in the mouse after partial gastrectomy. J. Surg. Res. 1997;73:137–142. doi: 10.1006/jsre.1997.5220. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura T., Yoshihara D., Ohmori T., Yanai M., Takeshita Y. Effects of diet high in medium-chain triglyceride on plasma ketone, glucose, and insulin concentrations in enterectomized and normal rats. J. Nutr. Sci. Vitaminol. 1994;40:147–159. doi: 10.3177/jnsv.40.147. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura T., Yoshihara D., Yanai M., Kawanishi G., Katayama S. Effects of medium-chain triglyceride on lipid metabolism in gastrectomized rats. J. Jpn. Soc. Nutr. Food Sci. 1987;40:485–495. [Google Scholar]
- 7.Ogawa K., Hirai M., Katsube T., Murayama M., Hamaguchi K., Shimakawa T., Naritake Y., Hosokawa T., Kajiwara T. Suppression of cellular immunity by surgical stress. Surgery. 2000;127:329–336. doi: 10.1067/msy.2000.103498. [DOI] [PubMed] [Google Scholar]
- 8.Gupta A., Watson D.I. Effect of laparoscopy on immune function. Br. J. Surg. 2001;88:1296–1306. doi: 10.1046/j.0007-1323.2001.01860.x. [DOI] [PubMed] [Google Scholar]
- 9.Shimaoka M., Hosotsubo K., Sugimoto M., Sakaue G., Taenaka N., Yoshiya I., Kiyono H. The influence of surgical stress on T cells: enhancement of early phase lymphocyte activation. Anesth. Analg. 1998;87:1431–1435. doi: 10.1097/00000539-199812000-00043. [DOI] [PubMed] [Google Scholar]
- 10.Decker D., Schöndorf M., Bidlingmaier F., Hirner A., von Ruecker A.A. Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up-regulation of antibody-mediated immunity commensurate to the trauma. Surgery. 1996;119:316–325. doi: 10.1016/s0039-6060(96)80118-8. [DOI] [PubMed] [Google Scholar]
- 11.Tatsumi H., Ura H., Ikeda S., Yamaguchi K., Katsuramaki T., Asai Y., Hirata K. Surgical influence on Th1/Th2 balance and monocyte surface antigen expression and its relation to infectious complications. World J. Surg. 2003;27:522–528. doi: 10.1007/s00268-003-6813-2. [DOI] [PubMed] [Google Scholar]
- 12.Manhart N., Vierlinger K., Bergmeister H., Boltz-Nitulescu G., Spittler A., Roth E. Influence of short-term protein malnutrition of mice on the phenotype and costimulatory signals of lymphocytes from spleen and Peyer’s patches. Nutrition. 2000;16:197–201. doi: 10.1016/s0899-9007(99)00279-8. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda S., Saito H., Fukatsu K., Inoue T., Han I., Furukawa S., Matsuda T., Hidemura A. Dietary restriction impairs neutrophil exudation by reducing CD11b/CD18 expression and chemokine production. Arch. Surg. 2001;136:297–304. doi: 10.1001/archsurg.136.3.297. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa H., Li Y., Abeliovich A., Yamamoto S., Kaufmann S.H., Tonegawa S. Cytotoxic and interferon gamma-producing activities of gamma delta T cells in the mouse intestinal epithelium are strain dependent. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8204–8208. doi: 10.1073/pnas.90.17.8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiyono H., Fujihashi K., Taguchi T., Aicher W.K., McGhee J.R. Regulatory functions for murine intraepithelial lymphocytes in mucosa responses. Immunol. Res. 1991;10:324–330. doi: 10.1007/BF02919716. [DOI] [PubMed] [Google Scholar]
- 16.Gautreaux M., Deitch E.A., Berg R.D. T lymphocytes in host defense against bacterial translocation from the gastrointestinal tract. Infect. Immun. 1994;62:2874–2884. doi: 10.1128/iai.62.7.2874-2884.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gautreaux M., Deitch E., Berg R. Adoptive transfer of T lymphocytes to T-cell-depleted mice inhibits Escherichia coli translocation from the gastrointestinal tract. Infect. Immun. 1995;63:3827–3834. doi: 10.1128/iai.63.10.3827-3834.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Prete G., Maggi E., Romagnani S. Human Th1 and Th2 cells: functional properties, mechanisms of regulation, and role in disease. Lab. Invest. 1994;70:299–306. [PubMed] [Google Scholar]
- 19.Manetti R., Gerosa F., Giudizi M.G. Interleukin 12 induces stable priming for interferon γ (IFN-γ) production during differentiation of human T helper (Th) cells and transient IFN-γ production in established Th2 cell clones. J. Exp. Med. 1994;179:1273–1283. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murata A., Ogawa M., Yasuda T., Nishijima J., Oka Y., Ohmachi Y., Hiraoka N., Niinobu T., Uda K., Mori T. Serum interleukin 6, C-reactive protein and pancreatic secretory trypsin inhibitor (PSTI) as acute phase reactants after major thoraco-abdominal surgery. Immunol. Invest. 1990;19:271–278. doi: 10.3109/08820139009041842. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi Y., Miyahara E., Funakoshi M., Takeshima I., Kawami H., Sato Y., Toge T. Modulation of CD4 antigen expression on the lymphocyte surface in advanced cancer patients. Oncology. 1995;52:1–6. doi: 10.1159/000227418. [DOI] [PubMed] [Google Scholar]