Abstract
Medicinal plants constitute an important source of potential therapeutic agents for diabetes. In the present study, we investigated the effects of Moringa oleifera (MO) Lam, Moringacea, on glucose tolerance in Wistar rats and Goto-Kakizaki (GK) rats, modeled type 2 diabetes. Major polyphenols in MO powder were quercetin glucosides, rutin, kaempferol glycosides and chlorogenic acids by HPLC analysis. As the results of glucose tolerance test, MO significantly decreased the blood glucose at 20, 30, 45and 60 min for GK rats and at 10, 30 and 45 min for Wistar rats (p<0.05) compared to the both controls after glucose administration. The area under the curve of changes in the blood glucose was significantly higher in the GK control group than in the GK plus MO group (p<0.05) in the periods 30–60 min and 60–120 min. Furthermore, MO significantly decreased stomach emptying in GK rats (p<0.05). The results indicated that MO has an ameliorating effect for glucose intolerance, and the effect might be mediated by quercetin-3-glucoside and fiber contents in MO leaf powder. The action of MO was greater in GK rats than in Wistar rats.
Keywords: Moringa oleifera, Goto-Kakizaki rat, glucose tolerance, diabetes, quercetin glucoside
Introduction
Diabetes is a chronic metabolic disorder with impaired glucose tolerance and high risk of cardiovascular disease [1]. Many oral synthetic antidiabetic agents have been developed [2]. Hyperglycemia can be handled initially with oral agents and insulin therapy, which is sometimes required to achieve targeted glycemic levels. However, these synthetic agents produce some serious side effects and are relatively expensive for developing countries [3]. Therefore, searching for effective, low cost and less side effected hypoglycemic agents is important. Herbal remedies for diabetes are known since ancient times in different societies. Scientific data supported the antidiabetic effects of some medicinal plants [4]. Moringa oleifera (MO) Lam (drumstick tree and horseradish tree) belongs to Moringacea family witch accounts 14 species. MO is widely distributed in the tropics. MO has anti-cancer [5], anti-inflammatory [6] and thyroid status regulator [7] efficacies, and some researchers reported its hypoglycemic potential [8]. The aim of the present study was to examine the effects of MO on glucose tolerance in Goto-Kakizaki (GK) and normal Wistar rats. The GK rat is a relevant model to understand and study the human type 2 diabetes. The GK rat is obtained by selective breeding of normal Wistar rats, using glucose tolerance as the selection index [9]. In the early stages of the disease, GK rats do not present severe associated complications as compared to obese diabetic rats. Thus, they are very useful for understanding the onset of human type 2 diabetes.
Materials and Methods
Animals and diets
The MO leaves were harvested from different trees in the south region of Senegal. The leaves were dried in shade and pounded to yield a powder. Male spontaneously diabetic GK rats (body weight (BW): 150–170 g) and non-diabetic male Wistar rats as the control (BW: 190–210 g) were obtained from the Clea Japan, Inc. (Tokyo, Japan). The rats were housed in individual stainless cages in a temperature and humidity controlled room with a 12 h light/dark cycle. The animals were allowed free access to deionized water and the AIN 93G based diet during one week for acclimatization. Then, animals were randomly divided into two groups: control GK (G) and GK plus MO (GM). Control Wistar (C) and Wistar plus MO (CM) were also used for determining glucose tolerance in normal condition. This study was approved by the Tokyo University of Agriculture Animal Use Committee, and rats were maintained in accordance with the guidelines of the University for the Care and Use of Laboratory Animals.
Polyphenol analysis in MO
The types of polyphenols in MO leaf powder were determined by HPLC with photodiode array detector (Hitachi, Tokyo, Japan) [10].
Determination of blood glucose
After over night food privation, fasting blood glucose (BG) was determined and oral glucose tolerance test (OGTT) was performed with glucose D (+) (Kanto Chemical Co., INC, Tokyo, Japan). The control groups (C, G) received a single dose of glucose (2 g/kg BW), whereas a single dose of the solution glucose-MO (2 g/kg BW and 200 mg MO leaf powder/kg BW, respectively) was administered to MO groups (CM, GM). Blood glucose was reported at 10, 20, 30, 45, 60, 90 and 120 min via the tail vein with a blood glucose test meter (Medisafe-mini GR-102, TERUMO Co., Tokyo, Japan). The areas under the curve (AUC) of changes in the blood glucose were calculated by the following formula:
AUC = Σ{[(Cn − Co) + (Cn+1 − C0)] × (tn+1 − t0)]}
Weighing contents of stomach and intestine
At the end, the rats were anesthetized with diethyl ether, and blood was taken and centrifuged at 3,000 rpm for 15 min to obtain serum. The stomach, the small intestine and the caecum were taken, weighted and opened. Their contents were washed out with water, dried and weighted again. Their contents were estimated by weight differences.
Statistics
Data are expressed as means ± SEM. Two-way ANOVA was used to determine the main effects of MO and the time course, and their interaction in the changes of blood glucose in each strain. Significant differences between the groups were determined by Fischer’s PLSD test (SPSS version 14.0J, SPSS, Chicago, IL). Differences were considered significant at p<0.05.
Results and Discussion
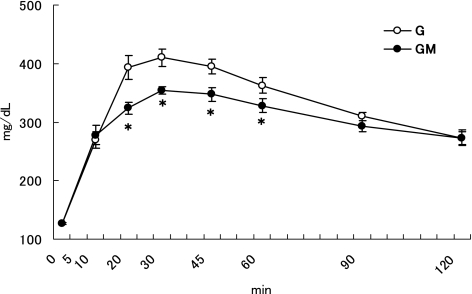
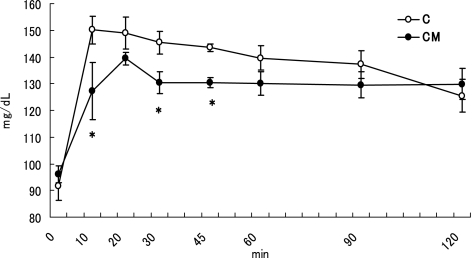
There were no significant differences on the body weight (GK: 190–192 g, Wistar: 207–208 g) between the two groups in both GK and Wistar rats. The Fig. 1 represents the results of the OGTT in GK rats. The initial BG (0 min) was 126.7 ± 1.5 mg/dl for the both two groups. The final BG (120 min) was 273.3 ± 14.0 and 272.8 ± 11.2 mg/dl for the G and GM, respectively. The two-way ANOVA showed that the two main effects of MO and time course, and also the interaction (MO and time course) were significant (p<0.05) for BG changes. MO significantly decreased BG at 20, 30, 45, and 60 min (p<0.05) compared to the control. The Fig. 2 represents the results of the OGTT in Wistar rats. The initial BG (0 min) was 91.6 ± 5.4 and 96.0 ± 3.1 mg/dl for the C and CM, respectively. The final BG (120 min) was 125.4 ± 6.1 and 129.8 ± 5.8 mg/dl for the C and CM, respectively. The two-way ANOVA showed that the two main effects (MO and time course) were significant (p<0.05) for OGTT. However, no significant interaction was found between these two factors for the BG changes. A significant difference in BG between the C and CM groups was noted at 10 30 and 45 min (p<0.05) after glucose administration. These results suggest that MO has a glucose intolerance ameliorating effect in both GK and Wistar rats. Furthermore, MO’s action was greater in GK rats than in Wistar rats.
Fig. 1.
Time course changes of blood glucose in GK rats after a single dose of a glucose or a glucose-MO solution. Values are means ± SEM, n = 6. Asterisk at a particular time point determines significant difference between the G and GM group, p<0.05.
Fig. 2.
Time course changes of blood glucose in Wistar rats after a single dose of a glucose or a glucose-MO solution. Values are means ± SEM, n = 5. Asterisk at a particular time point determines significant difference between the C and CM group, p<0.05.
The Table 1 shows the AUC of BG levels in GK and Wistar rats. The two-way ANOVA showed that the main two effects (MO and time course) were significant (p<0.05), but the interaction was not significant for AUC. For GK rats, the AUC was significantly lower in the GM group than in the G group (p<0.05) in the periods 30–60 min and 60–120 min. The total AUC was also significantly lower in the GM group than in the G group (p<0.05). For Wistar rats, the two-way ANOVA showed a significant effect in the factor of time course (p<0.05). On the basis of BG level of the CM group compared to the control (C), no significant differences were noted during the periods 0–30 min, 30–60 min and 60–120 min after the administration. After an oral ingestion of glucose, the maintenance of glucose tolerance depends on the insulin secretion, the hepatic glucose production, the glucose absorption rate in the digestive tract and the glucose uptake by peripheral tissues. Impaired insulin secretion or insulin resistant in GK rats explains the glucose intolerance of these animals. The restitution of insulin secretion in GK rats normalizes the defective glucose responsiveness [11]. Although serum insulin was not determined in this study, but MO may influence the secretion and action of insulin like other plant products [12, 13].
Table 1.
Blood glucose calculated from areas under the courves of blood concentrations at specific time periods
| GK |
Wistar |
||||
|---|---|---|---|---|---|
| G | GM | C | CM | ||
| 0–30 min | 5506.6 ± 309.9 | 4621.6 ± 203.9 | 1429.0 ± 126.7 | 1152.0 ± 33.0 | |
| 30–60 min | 7916.2 ± 298.0 | 6522.5 ± 221.5* | 1543.5 ± 152.6 | 1339.5 ± 181.0 | |
| 60–120 min |
11227.5 ± 500.9 |
10207.5 ± 364.4* |
2595.0 ± 408.4 |
2694.0 ± 565.2 |
|
| Total | 24650.4 ± 838.5 | 21351.6 ± 499.8* | 5567.5 ± 646.7 | 5185.5 ± 750.1 | |
Values are means SEM, n = 6 (GK) or 5 (Wistar). Asterisk at a particular time point determines significant difference between the G and GM group, p<0.05.
The dark chocolate polyphenols [14] and some other polyphenols [15, 16] were described as hypoglycemic agents. We analyzed polyphenols in the MO leaf powder. The analysis showed a high concentration of quercetin-3-glycoside (Q-3-G: 1494.2 µmol/100 g dry weight(dw)), rutin (1446.6 µmol/100 g dw), kaempferol glycosides (394.4 µmol/100 g dw), and other polyphenols were almost chlorogenic acid (134.5 µmol/100 g dw). In plants and most plant-derivated foods, flavonoids are largely present as conjugates with the flavonoid linked to a variable sugar moiety by a beta-glycosidic bond [17]. Q-3-G was reported to be more bioavailable than the quercetin aglycone [18]. From these findings, the involvement of the intestinal sodium glucose transporter-1 (SGLT-1) in the absorption of quercetin glucosides was suggested [19, 20]. Ader et al. [21] suggested that quercetin glucosides named Q3G (isoquercitrin) and quercetin-4'-glucoside (spiraeosid) competitively inhibit sodium (Na+) dependent mucosal uptake of the non-metabolisable glucose analogue methyl-α-d-glucopyranoside via SGLT-1 using rat mid-jejunum, whereas quercetin (aglycone) and rutin had no effect. Furthermore, Cermak et al. [22], in a similar experiment with SGLT-1-containing brush-border-membrane vesicles from porcine jejunum, have shown that Q3G inhibits not only Na+-dependent glucose uptake but also Na+-independent, non-saturable uptake of glucose. They suggest that this uptake component could be due to passive glucose diffusion or to glucose transport via a Na+-independent carrier, which does not saturate at the glucose concentrations used in their study. The hypoglycemic effect of kaempferol-3,7-O-(α)-dirhamnoside (kaempferitrin) has been also reported by using alloxan-induced diabetic rats [23]. Thus, MO flavonoids, especially Q3G, might affect glucose uptake by intestinal mucosa and therefore affect the time course of appearance of glucose in the blood and their availability to other parts of the body.
Our results showed that MO tended to retain longer the food consumption in the stomach (Table 2). The weight of stomach contents of the GM group was significantly higher than that of the G group, and the weight tended to be higher in the CM group than the C group, whereas no significant differences were found for the weights of small intestine and caecum contents between the two groups in both GK and Wistar rats. Rapid gastric emptying rates would result in increased absorption of food factors, and therefore a higher postprandial glycemia would be observed. The delay of gastric emptying may play an important role in glycemic control in diabetes. Slowing the stomach emptying can improve glycemia control by prolonging the postprandial absorption. Thus, MO might decrease the velocity of gastric emptying, a major determinant of postprandial glycemia [24]. Some herbal medicines were reported to inhibit gastric emptying and also glucose uptake in the small intestine [25]. High fiber diets decrease postprandial blood glucose by slowing the rate of food passage from the stomach to the small intestine [26]. Other fibers from rice and soybean decrease plasma glucose after glucose loading in diabetic rats [27]. MO leaf powder contains around 4% of fiber.
Table 2.
Weights of Stomach, Small intestine and Caecum contents
| GK |
Wistar |
||||
|---|---|---|---|---|---|
| G | GM | C | CM | ||
| Stomach content (g) | 1.8 ± 0.2 | 2.5 ± 0.2* | 0.4 ± 0.1 | 0.9 ± 0.2 | |
| Intestine content (g) | 1.8 ± 0.2 | 1.6 ± 0.1 | 2.4 ± 0.1 | 2.3 ± 0.1 | |
| Caecum content (g) | 0.7 ± 0.1 | 0.7 ± 0.1 | 1.4 ± 0.1 | 1.32 ± 0.1 | |
Values are means ± SEM, n = 6 (GK) or 5 (Wistar). Asterisk determines significant difference between the G and GM group, p<0.05.
We speculated hypoglycemic effect of MO might be due to an inhibition of glucose uptake with Q3G and slowing gastric emptying with fiber in MO leaf powder, although other active components [27, 28] are existed in MO.
In conclusion, we investigated the effects of oral administration of MO leaf powder on glucose tolerance in GK and Wistar rats. Our results showed that MO has a glucose intolerance ameliorating effect in both GK and Wistar rats, although the action of MO was greater in GK rats than in Wistar rats. The MO’s action might be mediated by Q3G and fiber contents. However, further studies are needed to identify the certain active components of MO for glucose intolerance ameliorating action.
Abbreviations
- AUC
areas under the curve
- BG
blood glucose
- BW
body weight
- GK
Goto-Kakizaki
- MO
Molinga oleifera
- OGTT
oral glucose tolerance test
- Q3G
quercetin-3-glucoside
- SGLT-1
Sodium/glucose co-transporter
References
- 1.Schnell O., Standl E. Impaired glucose tolerance, diabetes, and cardiovascular disease. Endocr. Pract. 2006;12:16–19. doi: 10.4158/EP.12.S1.16. [DOI] [PubMed] [Google Scholar]
- 2.Defronzo R.A. Pharmacologic therapy for type 2 diabetes mellitus. Ann. Intern. Med. 1999;131:281–303. doi: 10.7326/0003-4819-131-4-199908170-00008. [DOI] [PubMed] [Google Scholar]
- 3.Rubin R.J., Altman W.M., Mendelson D.N. Health care expenditures for people with diabetes mellitus, 1992. J. Clin. Endocrinol. Metab. 1994;78:809A–809F. doi: 10.1210/jcem.78.4.8157701. [DOI] [PubMed] [Google Scholar]
- 4.Grover J.K., Yadav S., Vats V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002; 81:81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 5.Guevara A.P., Vargas C., Sakurai H., Fujiwara Y., Hashimoto K., Maoka T., Kozuka M., Ito Y., Tokuda H., Hishino H. An antitumor promoter from moringa oleifera Lam. Mutat. Res. 1999;440:181–188. doi: 10.1016/s1383-5718(99)00025-x. [DOI] [PubMed] [Google Scholar]
- 6.Kurma S.R., Mishra S.H. Anti inflammatory and hepatoprotective activities of fruits of Moringa pterygosperma Gaerth. Ind. J. Nat. Prod. 1998;14:3–10. [Google Scholar]
- 7.Tahiliani P., Kar A. Role of Moringa oleifera leaf extract in the regulation of thyroid hormone status in adult male and female rats. Pharmacol. Res. 2000;41:319–323. doi: 10.1006/phrs.1999.0587. [DOI] [PubMed] [Google Scholar]
- 8.Kar A., Choudhary B.K., Bandyopadhyay N.G. Comparative evaluation of hypoglycaemic activity of some Indian medicinal plants in alloxan diabetic rats. J. Ethnopharmacol. 2003; 84:105–108. doi: 10.1016/s0378-8741(02)00144-7. [DOI] [PubMed] [Google Scholar]
- 9.Goto Y., Kakizaki M., Masaki N. Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J. Exp. Med. 1976;119:85–90. doi: 10.1620/tjem.119.85. [DOI] [PubMed] [Google Scholar]
- 10.Sakakibara H., Honda Y., Nakagawa S., Ashida H., Kanazawa K. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J. Agric. Food Chem. 2003;51:571–581. doi: 10.1021/jf020926l. [DOI] [PubMed] [Google Scholar]
- 11.Dolz M., Bailbe D., Giroix M.H., Calderari S., Gangnerau M.N., Serradas P., Rickenbach K., Irminger J.C., Portha B. Restitution of defective glucose-stimulated insulin secretion in diabetic GK rat by acetylcholine uncovers paradoxical stimulatory effect of beta-cell muscarinic receptor activation on cAMP production. Diabetes. 2005;54:3229–3237. doi: 10.2337/diabetes.54.11.3229. [DOI] [PubMed] [Google Scholar]
- 12.Achrekar S., Kaklij G.S., Pote M.S., Kelkar S.M. Hypoglycemic activity of Eugenia jambolana and ficus bengalensis: mechanism of action. In Vivo. 1991;5:143–147. [PubMed] [Google Scholar]
- 13.Attele A.S., Zhou Y.P., Xie J.T., Wu J.A., Zhang L., Dey L., Pugh W., Rue P.A., Polonsky K.S., Yuan C.S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–1858. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- 14.Grassi D., Lippi C., Necozione S., Desideri G., Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensibility and a decrease in blood pressure in healthy persons. Am. J. Clin. Nutr. 2005;81:611–614. doi: 10.1093/ajcn/81.3.611. [DOI] [PubMed] [Google Scholar]
- 15.Al-Awwadi N., Azay J., Poucheret P., Cassanas G., Krosniak M., Auger C., Gasc F., Rouanet J.M., Cros G., Teissedre P.L. Antidiabetic activity of red wine polyphenolic extract, ethanol, or both in streptozotocin-treated rats. J. Agric. Food Chem. 2004;52:1008–1016. doi: 10.1021/jf030417z. [DOI] [PubMed] [Google Scholar]
- 16.Moharram F.A., Marzouk M.S., Al-Toumy S.A., Ahmed A.A., Aboutabl E.A. Polyphenols of Melaleuca quinquenerva leaves pharmacological studies of grandinin. Phytother. Res. 2003;17:767–773. doi: 10.1002/ptr.1214. [DOI] [PubMed] [Google Scholar]
- 17.Kuhnau J. The flavonoids: A class of semi-essential food components: Their role in human nutrition. World Rev. Nutr. Diet. 1976;24:117–120. [PubMed] [Google Scholar]
- 18.Morand C., Manach C., Crespy V., Remesy C. Quercetin 3-O-beta-glucoside is better absorbed than other quercetin forms and is not present in rat plasma. Free Radic Res. 2000;33:667–676. doi: 10.1080/10715760000301181. [DOI] [PubMed] [Google Scholar]
- 19.Noteborn H.P.J.M., Jansen E., Benito S., Mengelers M.J.B. Oral absorption of quercetin and sugar-conjugated derivatives in specific transport systems. Cancer Lett. 1997;114:175–177. doi: 10.1016/s0304-3835(97)04655-7. [DOI] [PubMed] [Google Scholar]
- 20.Gee J.M., Du Pont M.S., Rhodes M.J.C., Johnson I.T. Quercetin glucosides interact with the intestinal glucose transport pathway. Free Radic. Biol. Med. 1998;25:19–25. doi: 10.1016/s0891-5849(98)00020-3. [DOI] [PubMed] [Google Scholar]
- 21.Ader P., Block M., Pietzsch S., Wolffram S. Interaction of quercetin glucosides with the intestinal sodium/glucose co-transporter (SGLT-1) Cancer Lett. 2001;162:175–180. doi: 10.1016/s0304-3835(00)00645-5. [DOI] [PubMed] [Google Scholar]
- 22.Cermak R., Landgraf S., Wolffram S. Quercetin glucosides inhibit glucose uptake into brush-border-membrane vesicles of porcine jejunum. Br. J. Nutr. 2004;91:849–855. doi: 10.1079/BJN20041128. [DOI] [PubMed] [Google Scholar]
- 23.De Sousa E., Zanatta L., Seifriz I., Creczynski-Pasa T.B., Pizzolatti M.G., Szpoganicz B., Silva F.R. Hypoglycemic effect and antioxidant potential of kaempferol-3,7-O-(α)-dirhamnoside from Bauhinia forficata leaves. J. Nat. Prod. 2004;67:829–832. doi: 10.1021/np030513u. [DOI] [PubMed] [Google Scholar]
- 24.Horowitz M., O’Donovan D., Jones K.L., Feinle C., Rayner C.K., Samsom M. Gastric emptying in diabetes: clinical significance and treatment. Diabetes Med. 2002;19:177–194. doi: 10.1046/j.1464-5491.2002.00658.x. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda H., Murakami T., Li Y., Yamahara J., Yoshikawa M. Mode of action of escins Ia and IIa and E,Z-Senegin II on glucose absorption in gastrointestinal tract. Bioorg. Med. Chem. 1998;6:1019–1023. doi: 10.1016/s0968-0896(98)00054-6. [DOI] [PubMed] [Google Scholar]
- 26.Tsai A.C., Peng B. Effects of locust bean gum on glucose tolerance, sugar digestion, and gastric motility in rats. J. Nutr. 1981;111:2152–2156. doi: 10.1093/jn/111.12.2152. [DOI] [PubMed] [Google Scholar]
- 27.Madar Z. Effect of brown rice and soybean dietary fiber on the control of glucose and lipid metabolism in diabetic rats. Am. J. Clin. Nutr. 1983;38:388–393. doi: 10.1093/ajcn/38.3.388. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu K., Ozeki M., Tanaka K., Itoh K., Nakajyo S., Urakawa N., Atsuchi M. Suppression of glucose absorption by extracts from the leaves of Gymnema inodorum. J. Vet. Med. Sci. 1997;59:753–757. doi: 10.1292/jvms.59.753. [DOI] [PubMed] [Google Scholar]