Abstract
Since clinical measures of bone mineral density do not necessarily predict whether a person will fracture a bone without an intervention, there is a need to find supplementary tools for assessing bone quality. Presently, we hypothesized that measures of mobile and bound water by a Nuclear Magnetic Resonance (NMR) technique are correlated with bone strength and toughness, respectively. To test this, bending specimens from the mid-diaphysis of 18 human femurs were collected from 18 male donors and divided into middle aged and elderly groups. After NMR measurements of each hydrated specimen, an inversion technique was used to convert the free induction decay data into a distribution of spin-spin (T2) relaxation rates. Then, the distribution resolved into three distinct components that likely represent solid hydrogen, water bound to bone tissue, and mobile water that occupy microscopic pores within the bone specimen. The integrated signal intensities of the bound and mobile components were normalized by the wet mass of the specimen. Following NMR measurements, three point bending tests were conducted to determine the modulus of elasticity, flexure strength, and work to fracture of each specimen. Next, the porosity, mineral-to-collagen ratio, and pentosidine concentration were measured. In this sample of human cortical bone, there was no age-related difference in the amount of mobile water, but the decrease in the amount of bound water with increasing age was statistically significant. Moreover, bound water was associated with both strength and work to fracture of bone, while mobile water was correlated with modulus of elasticity and appeared to quantify the level of microscopic pores within bone. On the other hand, bound water was correlated with the concentration of non-enzymatic collagen crosslinks. The results of this study indicate that quantifying mobile and bound water with magnetic resonance techniques could potentially serve as indicators of bone quality.
Keywords: Bone quality, NMR, Aging, Toughness, Water
INTRODUCTION
Traditionally, age-related loss of bone mineral density (BMD) has been viewed as the reason for the rising incidence of bone fractures in the elderly. However, quantifying low BMD by dual-energy X-ray absorptiometry alone is not sufficient to predict the risk of osteoporotic fractures [1]. Because of this, the concept of bone quality has emerged recognizing that fatigue microdamage, collagen integrity (crosslinking and content), and other inherent characteristics of bone tissue (besides BMD) are also important determinants of bone fracture susceptibility [2, 3]. Moreover, it provides insight into why the probability of fracture increases with age, but not necessarily related to the age-related decrease in BMD [4, 5]. In this study, use of Nuclear Magnetic Resonance (NMR) was explored to characterize the quality of human cortical bone tissue from two age groups.
The amount of water within bone tissue affects its material properties. A number of early studies demonstrated that dehydration decreases the strain at fracture and energy to fracture of cortical bone [6–11], and a recent study found that the contribution of water to bone toughness exists at multiple energy levels [12]. It has been shown that elevated drying of bone (70 °C) can cause significantly greater loss of bone toughness than moderate drying (room temperature), indicating that each of the hierarchical arrangements of water molecules bound to the collagen and mineral phases would likely influence the ability of bone to resist fracture. While we know that aging affects the energy to fracture of bone (or bone toughness) [13–16], whether aging affects these bound water interactions with bone tissue is unknown.
Recently, we developed a technique using NMR to non-destructively estimate the distribution of water in bone tissue [17]. When hydrogen nuclei absorb energy from a radio frequency (RF) pulse, their relaxation rate within a magnetic field depends on the surrounding diffusion characteristics and the energy of their chemical bonds with other elements. Thus, the spin direction of hydrogen atoms within bone precesses back to equilibrium at a rate that is dictated by the exponential exchange of energy to the surroundings. Covalently bonded hydrogen atoms, such as those comprising the amino acids of collagen or the hydroxyl group of mineral, relax much faster than those exhibiting van der Waals interactions between water molecules (mobile water). Exhibiting intermediate relaxation rates are those protons in the water molecules bonded to collagen and mineral (bound water).
Mobile water (also known as free water) exists in microscopic pores such as Haversian canals, canaliculi, and lacunae [18, 19]. Bound water exists in the extracellular matrix (ECM) of bone tissue since the residues of the collagen molecule form orderly ‘water bridges’ [20] and since surface charges (Ca2+ and PO4−) of bone mineral produces a hydration shell [21]. A quantification of mobile water by NMR would likely characterize the level of porosity within bone, while a quantification of bound water would characterize the degree of functionally hydrated bone tissue. Since porosity and strength of cortical bone increase and decrease, respectively, with age [14], we hypothesize that the NMR measure of mobile water increases with age and is inversely related to bone strength.
The age-related changes in the ECM that affect bound water are currently not clear. The early work of Robinson [18, 22, 23] indicates that water is displaced as osteoid mineralizes. This water is likely bound because the equatorial spacing of the collagen molecules is less in undemineralized bone than in demineralized bone [24, 25] as well as being less in dry bone than in wet bone [26]. Aging increases the fraction of hypermineralized bone [27, 28] and thus increases the fraction of partially dehydrated collagen within bone tissue. Skeletal maturation also appears to decrease water content as mineralization proceeds, as observed in growing dogs (8–44 weeks) [29]. Less clear is whether age-related increases in the concentrations of collagen crosslinks could alter the functional hydration of collagen. In intramuscular connective tissue, an inverse relationship was found between the level of crosslinking and the level of water binding collagen [30]. Since bone toughness decreases with age [14, 15, 31–33], we hypothesize that the NMR measure of bound water decreases with age and is directly related to the work-to-fracture of cortical bone.
To begin addressing the potential of NMR, and magnetic resonance in general, to quantify bone quality, we examined cortical bone specimens from middle aged and elderly male donors. These specimens were analyzed first by a NMR relaxation technique (Fig.1) [17] and then by three point bending so that correlations between NMR and mechanical properties could be tested for significance. Lastly, we measured selected compositional measures of bone as well as porosity to provide insight into the aging effects on mobile and bound water.
Figure 1.
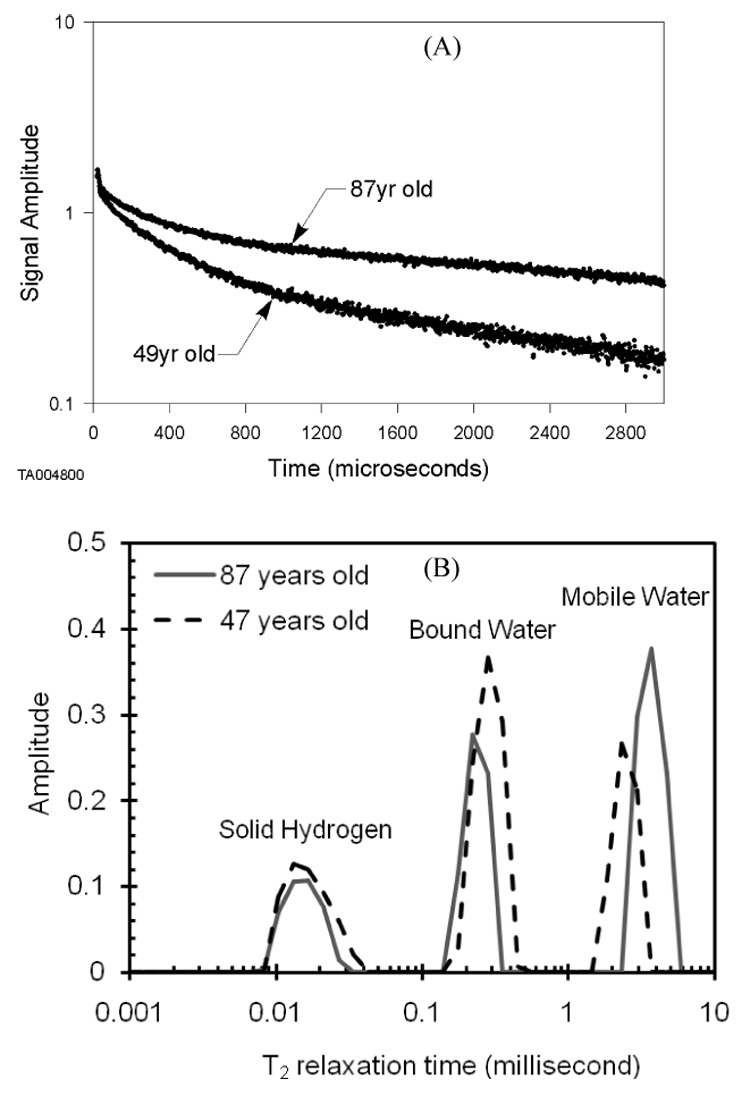
An example of Free Induction Decay (FID) signals are given for two specimens, one from each age group (A). Each signal generated a distribution of relaxation times in which the normalized signal intensity separated into three components (B).
MATERIALS AND METHODS
Specimen preparation
We collected 18 individual cadaveric femurs from two sources (National Disease Research Interchange, Philadelphia, PA and Willed Body Program, The University of Texas Southwestern Medical Center at Dallas, TX). The 18 male donors had no known bone disease. Based on donor age, the femurs were divided into two groups: middle aged included 8 ages of 47, 49, 49, 51, 55, 57, 59, and 59 years, while the elderly included 10 ages of 67, 69, 76, 76, 77, 77, 79, 79, 81, and 87 years. Cut from the medial quadrant of each mid-diaphysis, a longitudinal strip of bone was machined, while maintaining hydration, into a standard paralellelepiped specimen such that the osteons ran in the direction of the long axis. The nominal dimensions were 40.0 mm in length, 4.27 mm in width, and 2.07 mm in height (h). All specimens were wrapped in gauze, soaked in phosphate buffered saline, and stored at −20 °C until time of analysis.
NMR Analysis
In this study, we derived the NMR inversion T2 spectrum (Fig. 1B) from NMR free induction decay (FID) measurements (Fig. 1A) of each specimen. As previously described [17], this NMR technique quantifies the proton solid, bound and mobile components in bone when a broadline NMR system is set at a proton frequency of 27 MHz. Specifically, we used a 0.5 MHz to 40 MHz system built at Southwest Research Institute (San Antonio, TX) that has an electromagnet 19 inches in diameter with a 4 inch gap between the poles of the magnetic field. Each bone specimen was sealed in glass tube, and while within the magnetic field, 9.5 µs long RF-pulses caused 90° flips in the spin direction of the protons in bone. As the protons relaxed, the free induction decay (FID) data were recorded by the NMR receiver coil at 2 µs intervals, collecting 1500 data points over an approximate 3 millisecond window (Fig. 1A). Applying a mathematical, inversion relaxation technique to the FID data [34], we acquired for each specimen a T2 relaxation spectrum with 3 different components: fast decay indicating solid protons, intermediate decay indicating bound water, and slow decay indicating mobile water (Fig. 1B). To account for size variance among the specimens, the integrated intensity of each peak was normalized by wet bone mass and multiplied by 1000 (units of integrated signal intensity per 1000 per mg).
Mechanical Testing
After NMR analysis, each specimen was subjected to three point bending by a material testing system (EnduraTEC ELF 3300, Bose Corporation, Minnetonka, MN), which recorded the force-displacement data at 20 Hz. The span (L) was 20 mm; the load rate was 5mm/min, and hydration was maintained by dripping distilled water on the specimen during the test. All tests were performed at room temperature. Applying the flexure formula to the maximum force and applying the deflection equation to the slope of the linear section of the force-displacement curve provided the strength (σb) and modulus of elasticity (E), respectively (Fig. 2) [12]. Work-to-fracture (the area under the force-displacement curve) was normalized by the moment of inertia to account for slight differences in specimen dimensions. This normalization was an estimate of toughness.
Figure 2.
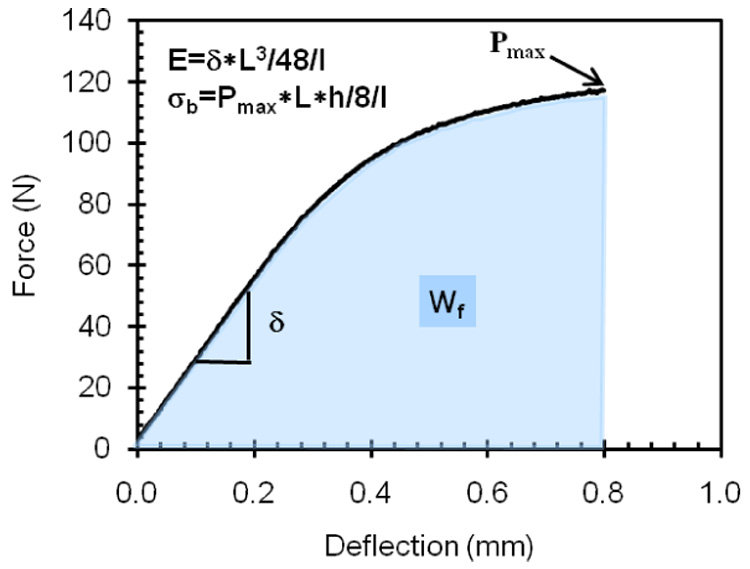
The mechanical properties of bone - modulus of elasticity (E), bending strength (σb), and work-to-fracture (Wf) or toughness - were determined from the resulting force-deflection curve and equations from beam theory. L is the span of the three point bending apparatus, h is the height of the bone specimen, and I is the moment of inertia, which for a parallelepiped is width*height3/12.
Compositional Analysis
After mechanical testing, a small piece of bone was cut from a non-loaded end of the specimen (e.g. outside the loading pins), and the concentration of pentosidine (PE, a marker of non-enzymatic crosslinks) was quantified for half of it following the protocol of Bank et al. [35] for high performance liquid chromatography. The concentration was measured in moles of PE per moles of collagen in which collagen was measured on the other half by a colorimetric assay of hydroxyproline (described in a previous study [36]). From sequential mass measurements of dry and ashed bone, we quantified the mineral-to-collagen ratio (Min/Org) using a small piece of specimen from the other non-loaded region of each specimen. Lastly, a portion of each broken specimen was embedded in cold mounting epoxy (EPOFIX, Struers, Denmark), and the fracture surface ground with successive grits of silicon carbide paper and then polished with 0.05 micron alumina slurry. As previously described in detail [12, 33], porosity (Po) was quantified from binary representations of digital images taken of the polished cross section.
Statistical analysis
A one factor ANOVA (StatView 5.0.1, Cary, NC) tested whether age affected the selected properties of bone. Correlation coefficients were generated for all property comparisons, but the values were only reported for those having statistical significance at a 90% or 95% confidence level. Lastly, a stepwise regression was performed to determine if the water content of bone explains the sum of mobile and bound water (i.e., NMR measure of Total water). Water content was the percent loss of mass due to drying at 100 °C for several days (equilibrium mass).
RESULTS
There was an age-related decrease in the NMR-derived bound water, but no change in the NMR-derived mobile water for the bone specimens tested in this study (Fig. 3). Of the mechanical properties, only toughness differed between middle aged and elderly bone with statistical significance, although there was a trend of decreasing flexure strength with increasing age (Table 1). Age also affected the concentration of pentosidine (PE) in the medial quadrant of the mid-diaphyseal femur (Table 1). In this sample of 18 femurs, intracortical porosity (Po) and the mineral-to-collagen ratio (Min/Org) did not increase with age (Table 1).
Figure 3.
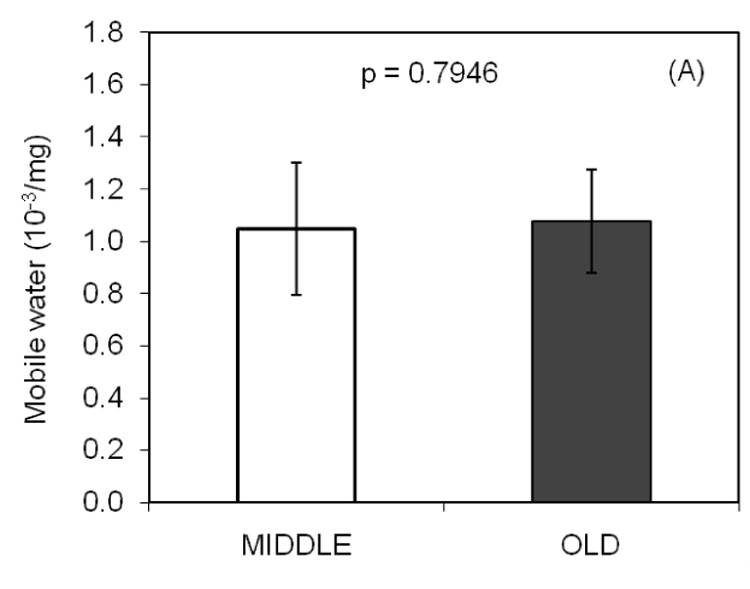
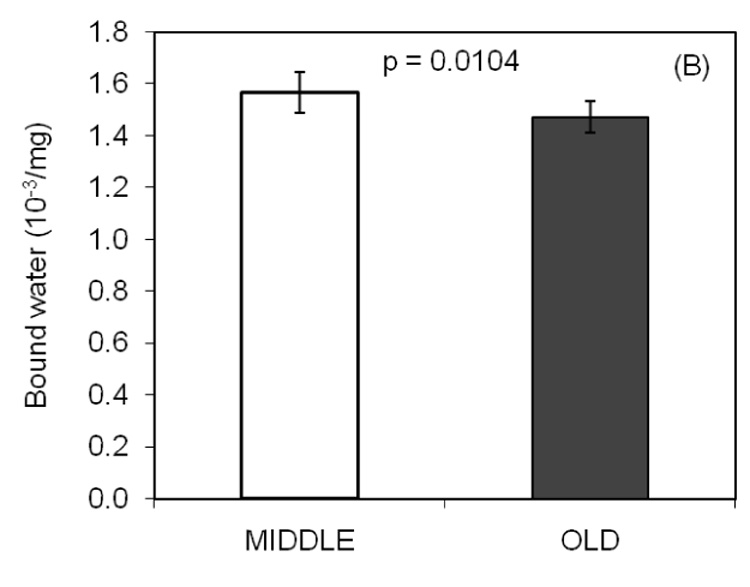
With a unit of integrated signal intensity per 1000 per mg, the NMR derived measure of mobile water (A) did not differ between the age groups. However, there was a statistically significant difference in bound water (B) between middle aged and elderly bone.
Table 1.
Mean ± standard deviation are given for selected properties of middle aged and elderly cortical bone with significance indicated at p < 0.05 (ANOVA). Otherwise, difference is not significant (NS)
| Property | Middle | Old | p-value |
|---|---|---|---|
| E (GPa) | 13.7 ± 0.8 | 13.8 ± 1.5 | NS |
| σb (MPa) | 201 ± 8 | 192 ± 16 | NS |
| Toughness (N/mm3) | 25.9 ± 5.8 | 20.2 ± 4.7 | 0.0341 |
| Po (%) | 6.0 ± 1.7 | 6.6 ± 1.4 | NS |
| Min/Org | 2.12 ± 0.09 | 2.13 ± 0.09 | NS |
| PE (mmol/mol) | 0.331 ± 0.111 | 0.794 ± 0.283 | 0.0005 |
At a confidence level of 95%, several statistically significant associations existed between the mechanical properties and the NMR measures. Bound water was directly related to bone strength and toughness, while mobile water was inversely related to modulus of elasticity (Table 2). The porosity and mineral-to-collagen ratio did not change with age in this study, and so they were found to have little association with any of the mechanical properties (Table 2). PE was, however, inversely related to bone toughness (Table 2).
Table 2.
Correlation coefficients are given for statistically significant associations (shade indicates significance at a 90% confidence level, otherwise 95%)
| E | σb | Toughness | Po | Mobile | Bound | Min/Org | PE | |
|---|---|---|---|---|---|---|---|---|
| E | 1 | 0.706 | NS | NS | −0.495 | NS | NS | NS |
| σb | 1 | 0.475 | NS | −0.456 | 0.599 | NS | −0.433 | |
| Toughness | 1 | NS | NS | 0.636 | NS | −0.700 | ||
| Po | 1 | 0.801 | −0.495 | NS | NS | |||
| Mobile | 1 | −0.460 | −0.511 | NS | ||||
| Bound | 1 | NS | −0.423 | |||||
| Min/Org | 1 | NS | ||||||
| PE 1 | 1 | |||||||
Mobile and bound water were directly and inversely related to porosity, respectively (Table 2). While Min/Org was not associated with porosity, it was inversely related to mobile water (Table 2). Bound water was inversely related to PE at the lower confidence level of 90%. In the stepwise regression analysis, water content as determined experimentally was found to best explain the variance in the NMR-derived total water when only the coefficient of the slope was included in the model (Fig. 3).
DISCUSSION
To the best of our knowledge, this is the first study to investigate the effects of aging on the water distribution within bone and to examine correlations between NMR derived measures of water and mechanical properties that characterize bone quality. Mobile water was not affected by age, but neither was intracortical porosity as determined by histomorphometry. Bound water did decrease with age, as hypothesized, but it was not associated with mineral-to-collagen ratio. Encouragingly, we observed direct relationships between bound water and two mechanical properties, namely flexure strength and work-to-fracture or toughness of cortical bone. Moreover, mobile water was associated with modulus of elasticity and, to a lesser extent, bending strength. Thus, NMR is a promising tool for characterizing bone quality, although more work is necessary to identify the exact nature of mobile and bound water as well to determine what age-related changes in bone tissue cause changes in bound water.
While not expected, our observation that porosity did not increase with age is consistent with studies of porosity throughout the entire cross-section of the femur at the level of the mid-diaphysis. In these studies, hundreds of cadaveric femurs were digitally examined for both female and male donors [37–39]. They revealed that 1) the rate increase in intracortical porosity with age is more gradual in males than in females [37], 2) age accounted for roughly 13.5% of the variance in porosity among males [38], and 3) the medial quadrant has the lowest porosity compared to the anterior and posterior quadrants [39]. Thus, to detect an age effect on Po, we would have needed to examine bone tissue from younger donors and perhaps examined several anatomical quadrants. Despite this limitation in the study, we do still find that Po and mobile water are directly correlated.
While mobile water is likely a measure of porosity in bone [40], bound water as presently quantified by NMR does not clearly relate to a specific property of bone because only a few compositional parameter were presently examined, with PE being weakly correlated (Table 2). The bulk measure of mineral-to-collagen was originally thought to influence bound water since mineral displaces water from collagen and collagen provides a number of sites for water to bind. The lack of correlation may reflect the likelihood that bulk measures do not adequately characterize the bone tissue at the proper hierarchical level. For example, increasing mineralization displaces water from collagen, but the mineral crystals are also sites for which water to bind. Moreover, bulk Min/Org does not necessarily affect the functional hydration of collagen in that it reflects both the amount of mineral within collagen fibrils (functional hydration) which displaces water from collagen and the compartment of the mineral existing between collagen fibrils which presumably does not affect collagen hydration.
Most likely, candidate properties affecting bound water content include those that affect hydration sites within the ECM of bone, and thus they could be characterized as properties of the bone ultrastructure. The three primary determinants of hydration sites (and therefore bound water) are likely to be 1) the quantity of collagen occupying a given volume (i.e., collagen density), 2) the quantity of crystals displacing water from the collagen, and 3) the available surface area of individual crystals to create water bridges (with other crystals or collagen). Conceivably, the packing of collagen molecules via intra- and inter-molecular crosslinks dictates collagen density and thus hydration sites. In other words, a highly crosslinked fiber could potentially hold less water than a poorly crosslinked fibers. This concept is currently unconfirmed. Nonetheless, with aging, there are increases in the concentration of non-enzymatic collagen crosslinks in bone [15] (Table 1), and perhaps this additional crosslinking decreases bound water with age and explains the correlation between bound water and PE (Table 2). With regards to the mineral phase, crystals occupy space within collagen fibrils as well as between fibrils [41] and thus mineral accumulation within the collagen fibrils would be expected to directly affect collagen hydration. In other words, with an increase in the number of crystals occupying the regular gaps that occur in the arrangement of collagen into a fibril, there could be a decrease in the level of collagen hydration, which is presumably important to bone toughness. In addition, the surfaces of crystals present sites for water molecules to interact, and so crystal size and crystallinity could conceivably affect the level of bound water as well. The question of how age affects bound water requires measurement techniques that quantify such ultrastructural properties as multiple collagen crosslink concentrations (both enzymatic and non-enzymatic), ratio of immature-to-mature crosslinks, spacing of collagen molecules or collagen density, crystal size, carbonate substitutions, and crystallinity.
Besides the lack of ultrastructural properties, this study has other limitations that are related to the NMR technique. Firstly, the signal intensity of each water component was normalized by the wet mass of the specimen. With precise measurements of bone tissue volume for each specimen, there would likely not be a correlation between Po and bound water. In other words, the tissue volume of the specimen would include mainly the collagen and mineral phases (not pores) and so the amount of bound water would be independent of specimen size. Another limitation is that the NMR-derived measure of bound water does not distinguish among the various energy levels at which water interacts with the ECM. Thus, the study does not reveal any mechanistic information on how aging affects bone quality. Nonetheless, an age-related change in the degree of hydrogen-bonding between water and collagen likely affects the mechanical properties of bone. As previously described, decreases in hydrogen-bonding (through moderate drying) causes bone to behave in a brittle fashion. Moreover, an age-related increase in the amide I band, as determined from deep-ultraviolet Raman spectra, was found to correlate with a decrease in fracture toughness of human cortical bone (i.e., increasing brittleness) [42]. This amide I chemical species is a product of the organic phase of bone, and its band is influenced by collagen crosslinking [43]. Interestingly, dehydration of dentin (elephant tusk) by polar solvents, which reduce hydrogen bonding between water and collagen, was also found to increase the amide I band [44].
Presently, the NMR technique does distinguish between three distinct T2 relaxation rates of protons. Nonetheless, each distribution of T2 does not necessarily produce a symmetric band (Fig 1A), and so multiple proton environments contribute to each relaxation profile. The so-called solid hydrogen band reflects protons of the collagen peptide and protons of the hydroxyapatite-related mineral, each with slightly different relaxation rates. Bound water band likely represents the so-called structural water layer bridging mineral and collagen that was reported to exist by Wilson et al. [45] who applied a cross-polarization NMR technique to cortical bone and synthetic apatite. Still, it also likely represents the number of water bridges (via hydrogen bonding) that exist between and within triple helical peptides of collagen [20, 46, 47]. Lastly, the mobile water band reflects differences in relaxation rates that are due to the variety of pores sizes in bone (canaliculi, lacunae, Haversian’s canals, Volkmann’s canals, resorption holes).
While both NMR and magnetic resonance imaging (MRI) have been used to characterize bone in a number of studies [40, 45, 48–56], the present work is the first to quantify water distribution in bone for two age groups. A previous study had actually characterized water distribution in dentin samples from a 20 year, 35 year, and 50 year old donor [50]. Applying a NMR spin-grouping technique to both hydrated and dehydrated specimens, Schreinder et al. [50] reported that 30% of the water was strongly bound to the apatite matrix (i.e., present in the dehydrated state), 52% of the water was hydrating the surfaces of the mineral crystals and the collagen protein, and the remaining percentage was mobile water occupying the dentinal tubules. In addition, the use of magnetic resonance to characterize mechanical properties is not entirely new. One previous study investigated connections between architectural indices of trabecular bone (as determined by MRI) and bone strength [54], while another related water content (as determined by NMR-derived diffusion characteristics of exchangeable water) to bone density and strength in an animal model of hypomineralization [49]. MRI studies of bone have been focused on assessing the structure or architecture of trabecular and cortical bone (see review by Wehrli for details [57]), but recently, water volume fraction in the mid-shaft of human tibiae was quantified by analyzing T2 protons with a 3 Tesla, clinical MRI scanner [58]. The effect of aging or water distribution however was not a focus in either of these studies, as it was here.
The findings of the present work suggest that the techniques in magnetic resonance related to relaxation rates are a potential tool for assessing bone quality beyond architectural characteristics in a non-destructive fashion. Currently, NMR has distinguished mobile water from bound water in such a way that each phase was associated with at least one mechanical property of cortical bone. Mobile water likely represents porosity, while bound water likely represents a combination of ultrastructural properties of bone tissue.
Figure 4.
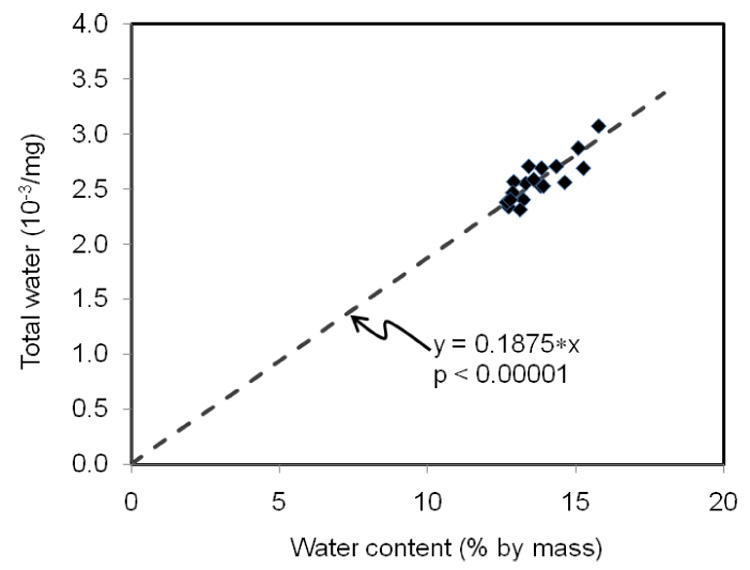
Water content, as measured by mass loss due to drying, best explained the variance in total water, as measured by NMR, when there was no y-intercept in the linear regression model.
ACKNOWLEDGEMENTS
Grants from NIH/NIA (AG), NIH/NIAMS (AR049627), and the San Antonio Area Foundation supported the present research. We thank Ms. Rae Acuna, Mr. Joseph Beccerril, and Mr. Jerrod Tyler for generating and processing specimens.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Miller PD, McClung M. Prediction of fracture risk. I: Bone density. Am J Med Sci. 1996;312:257–259. doi: 10.1097/00000441-199612000-00003. [DOI] [PubMed] [Google Scholar]
- 2.van der Meulen MC, Jepsen KJ, Mikic B. Understanding bone strength: Size isn't everything. Bone. 2001;29:101–104. doi: 10.1016/s8756-3282(01)00491-4. [DOI] [PubMed] [Google Scholar]
- 3.Burr DB, Forwood MR, Fyhrie DP, Martin RB, Schaffler MB, Turner CH. Bone microdamage and skeletal fragility in osteoporotic and stress fractures. J Bone Miner Res. 1997;12:6–15. doi: 10.1359/jbmr.1997.12.1.6. [DOI] [PubMed] [Google Scholar]
- 4.De Laet CE, Van Hout BA, Burger H, Weel AE, Hofman A, Pols HA. Hip fracture prediction in elderly men and women: Validation in the rotterdam study. J Bone Miner Res. 1998;13:1587–1593. doi: 10.1359/jbmr.1998.13.10.1587. [DOI] [PubMed] [Google Scholar]
- 5.Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to bmd and diagnostic thresholds. Osteoporos Int. 2001;12:989–995. doi: 10.1007/s001980170006. [DOI] [PubMed] [Google Scholar]
- 6.Evans FG, Lebow M. Regional differences in some of the physical properties of the human femur. J Appl Physiol. 1951;3:563–572. doi: 10.1152/jappl.1951.3.9.563. [DOI] [PubMed] [Google Scholar]
- 7.Dempster WT, Liddicoat RT. Compact bone as a non-isotropic material. Am J Anat. 1952;91:331–362. doi: 10.1002/aja.1000910302. [DOI] [PubMed] [Google Scholar]
- 8.Smith JW, Walmsley R. Factors affecting the elasticity of bone. J Anat. 1959;93:503–523. [PMC free article] [PubMed] [Google Scholar]
- 9.Sedlin ED, Hirsch C. Factors affecting the determination of the physical properties of femoral cortical bone. Acta Orthop Scand. 1966;37:29–48. doi: 10.3109/17453676608989401. [DOI] [PubMed] [Google Scholar]
- 10.Yamada H, Evans FG. Strength of biological materials. Baltimore: Williams & Wilkins; 1970. [Google Scholar]
- 11.Evans FG. Mechanical properties of bone. Springfield: Thomas; 1973. [Google Scholar]
- 12.Nyman JS, Roy A, Shen X, Acuna RL, Tyler JH, Wang X. The influence of water removal on the strength and toughness of cortical bone. J Biomech. 2006;39:931–938. doi: 10.1016/j.jbiomech.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burstein AH, Reilly DT, Martens M. Aging of bone tissue: Mechanical properties. J Bone Joint Surg Am. 1976;58:82–86. [PubMed] [Google Scholar]
- 14.McCalden RW, McGeough JA, Barker MB, Court-Brown CM. Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. J Bone Joint Surg Am. 1993;75:1193–1205. doi: 10.2106/00004623-199308000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- 16.Zioupos P, Currey JD. Changes in the stiffness, strength, and toughness of human cortical bone with age. Bone. 1998;22:57–66. doi: 10.1016/s8756-3282(97)00228-7. [DOI] [PubMed] [Google Scholar]
- 17.Ni Q, Nyman JS, Wang X, De Los Santos A, Nicolella DP. Assessment of water distribution changes in human cortical bone by nuclear magnetic resonance. Meas Sci Technol. 2007;18:715–723. [Google Scholar]
- 18.Robinson RA. Bone tissue: Composition and function. Johns Hopkins Med J. 1979;145:10–24. [PubMed] [Google Scholar]
- 19.Tami AE, Schaffler MB, Knothe Tate ML. Probing the tissue to subcellular level structure underlying bone's molecular sieving function. Biorheology. 2003;40:577–590. [PubMed] [Google Scholar]
- 20.Bella J, Brodsky B, Berman HM. Hydration structure of a collagen peptide. Structure. 1995;3:893–906. doi: 10.1016/S0969-2126(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 21.Timmins PA, Wall JC. Bone water. Calcif Tissue Res. 1977;23:1–5. doi: 10.1007/BF02012759. [DOI] [PubMed] [Google Scholar]
- 22.Robinson RA. Crystal collagen water relationships in bone matrix. Clin Orthop. 1960;17:69–76. [Google Scholar]
- 23.Robinson RA. Physiocochemical structure of bone. Clin Orthop. 1975:263–315. [PubMed] [Google Scholar]
- 24.Bonar LC, Lees S, Mook HA. Neutron diffraction studies of collagen in fully mineralized bone. J Mol Biol. 1985;181:265–270. doi: 10.1016/0022-2836(85)90090-7. [DOI] [PubMed] [Google Scholar]
- 25.Lees S, Hukins DW. X-ray diffraction by collagen in the fully mineralized cortical bone of cow tibia. Bone Miner. 1992;17:59–63. doi: 10.1016/0169-6009(92)90710-u. [DOI] [PubMed] [Google Scholar]
- 26.Lees S, Mook HA. Equatorial diffraction spacing as a function of water content in fully mineralized cow bone determined by neutron diffraction. Calcif Tissue Int. 1986;39:291–292. doi: 10.1007/BF02555221. [DOI] [PubMed] [Google Scholar]
- 27.Boyce TM, Bloebaum RD. Cortical aging differences and fracture implications for the human femoral neck. Bone. 1993;14:769–778. doi: 10.1016/8756-3282(93)90209-s. [DOI] [PubMed] [Google Scholar]
- 28.Simmons ED, Jr, Pritzker KP, Grynpas MD. Age-related changes in the human femoral cortex. J Orthop Res. 1991;9:155–167. doi: 10.1002/jor.1100090202. [DOI] [PubMed] [Google Scholar]
- 29.Jonsson U, Ranta H, Stromberg L. Growth changes of collagen cross-linking, calcium, and water content in bone. Arch Orthop Trauma Surg. 1985;104:89–93. doi: 10.1007/BF00454244. [DOI] [PubMed] [Google Scholar]
- 30.Kopp J, Bonnet M, Renou JP. Effect of collagen crosslinking on collagen-water interactions (a DSC investigation) Matrix. 1989;9:443–450. doi: 10.1016/s0934-8832(11)80013-2. [DOI] [PubMed] [Google Scholar]
- 31.Nalla RK, Kruzic JJ, Kinney JH, Ritchie RO. Effect of aging on the toughness of human cortical bone: Evaluation by r-curves. Bone. 2004;35:1240–1246. doi: 10.1016/j.bone.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Zioupos P, Currey JD, Hamer AJ. The role of collagen in the declining mechanical properties of aging human cortical bone. J Biomed Mater Res. 1999;45:108–116. doi: 10.1002/(sici)1097-4636(199905)45:2<108::aid-jbm5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 33.Nyman JS, Roy A, Tyler JH, Acuna RL, Gayle HJ, Wang X. Age-related factors affecting the postyield energy dissipation of human cortical bone. J Orthop Res. 2007;25:646–655. doi: 10.1002/jor.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Labadie C, Lee JH, Vetek G, Springer CS., Jr Relaxographic imaging. J Magn Reson B. 1994;105:99–112. doi: 10.1006/jmrb.1994.1109. [DOI] [PubMed] [Google Scholar]
- 35.Bank RA, Beekman B, Verzijl N, de Roos JA, Sakkee AN, TeKoppele JM. Sensitive fluorimetric quantitation of pyridinium and pentosidine crosslinks in biological samples in a single high-performance liquid chromatographic run. J Chromatogr B Biomed Sci Appl. 1997;703:37–44. doi: 10.1016/s0378-4347(97)00391-5. [DOI] [PubMed] [Google Scholar]
- 36.Nyman JS, Roy A, Acuna RL, Gayle HJ, Reyes MJ, Tyler JH, et al. Age-related effect on the concentration of collagen crosslinks in human osteonal and interstitial bone tissue. Bone. 2006;39:1210–1217. doi: 10.1016/j.bone.2006.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feik SA, Thomas CD, Clement JG. Age-related changes in cortical porosity of the midshaft of the human femur. J Anat. 1997;191(Pt 3):407–416. doi: 10.1046/j.1469-7580.1997.19130407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bousson V, Meunier A, Bergot C, Vicaut E, Rocha MA, Morais MH, et al. Distribution of intracortical porosity in human midfemoral cortex by age and gender. J Bone Miner Res. 2001;16:1308–1317. doi: 10.1359/jbmr.2001.16.7.1308. [DOI] [PubMed] [Google Scholar]
- 39.Thomas CD, Feik SA, Clement JG. Regional variation of intracortical porosity in the midshaft of the human femur: Age and sex differences. J Anat. 2005;206:115–125. doi: 10.1111/j.1469-7580.2005.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Ni Q. Determination of cortical bone porosity and pore size distribution using a low field pulsed nmr approach. J Orthop Res. 2003;21:312–319. doi: 10.1016/S0736-0266(02)00157-2. [DOI] [PubMed] [Google Scholar]
- 41.Hellmich C, Ulm FJ. Average hydroxyapatite concentration is uniform in the extracollagenous ultrastructure of mineralized tissues: Evidence at the 1–10-microm scale. Biomech Model Mechanobiol. 2003;2:21–36. doi: 10.1007/s10237-002-0025-9. [DOI] [PubMed] [Google Scholar]
- 42.Ager JW, Nalla RK, Breeden KL, Ritchie RO. Deep-ultraviolet raman spectroscopy study of the effect of aging on human cortical bone. J Biomed Opt. 2005;10:034012. doi: 10.1117/1.1924668. [DOI] [PubMed] [Google Scholar]
- 43.Paschalis EP, Verdelis K, Doty SB, Boskey AL, Mendelsohn R, Yamauchi M. Spectroscopic characterization of collagen cross-links in bone. J Bone Miner Res. 2001;16:1821–1828. doi: 10.1359/jbmr.2001.16.10.1821. [DOI] [PubMed] [Google Scholar]
- 44.Nalla RK, Balooch M, Ager JW, 3rd, Kruzic JJ, Kinney JH, Ritchie RO. Effects of polar solvents on the fracture resistance of dentin: Role of water hydration. Acta Biomater. 2005;1:31–43. doi: 10.1016/j.actbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Wilson EE, Awonusi A, Morris MD, Kohn DH, Tecklenburg MM, Beck LW. Highly ordered interstitial water observed in bone by nuclear magnetic resonance. J Bone Miner Res. 2005;20:625–634. doi: 10.1359/JBMR.041217. [DOI] [PubMed] [Google Scholar]
- 46.Nomura S, Hiltner A, Lando JB, Baer E. Interaction of water with native collagen. Biopolymers. 1977;16:231–246. doi: 10.1002/bip.1977.360160202. [DOI] [PubMed] [Google Scholar]
- 47.Pineri MH, Escoubes M, Roche G. Water--collagen interactions: Calorimetric and mechanical experiments. Biopolymers. 1978;17:2799–2815. doi: 10.1002/bip.1978.360171205. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez-Seara MA, Wehrli SL, Wehrli FW. Diffusion of exchangeable water in cortical bone studied by nuclear magnetic resonance. Biophys J. 2002;82:522–529. doi: 10.1016/S0006-3495(02)75417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandez-Seara MA, Wehrli SL, Takahashi M, Wehrli FW. Water content measured by proton-deuteron exchange nmr predicts bone mineral density and mechanical properties. J Bone Miner Res. 2004;19:289–296. doi: 10.1359/JBMR.0301227. [DOI] [PubMed] [Google Scholar]
- 50.Schreiner LJ, Cameron IG, Funduk N, Miljkovic L, Pintar MM, Kydon DN. Proton nmr spin grouping and exchange in dentin. Biophys J. 1991;59:629–639. doi: 10.1016/S0006-3495(91)82278-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu Y, Ackerman JL, Kim HM, Rey C, Barroug A, Glimcher MJ. Nuclear magnetic resonance spin-spin relaxation of the crystals of bone, dental enamel, and synthetic hydroxyapatites. J Bone Miner Res. 2002;17:472–480. doi: 10.1359/jbmr.2002.17.3.472. [DOI] [PubMed] [Google Scholar]
- 52.Fantazzini P, Brown RJ, Borgia GC. Bone tissue and porous media: Common features and differences studied by nmr relaxation. Magn Reson Imaging. 2003;21:227–234. doi: 10.1016/s0730-725x(03)00129-2. [DOI] [PubMed] [Google Scholar]
- 53.Wilson EE, Awonusi A, Morris MD, Kohn DH, Tecklenburg MM, Beck LW. Three structural roles for water in bone observed by solid-state nmr. Biophys J. 2006;90:3722–3731. doi: 10.1529/biophysj.105.070243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chung H, Wehrli FW, Williams JL, Kugelmass SD. Relationship between nmr transverse relaxation, trabecular bone architecture, and strength. Proc Natl Acad Sci U S A. 1993;90:10250–10254. doi: 10.1073/pnas.90.21.10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Majumdar S, Newitt D, Mathur A, Osman D, Gies A, Chiu E, et al. Magnetic resonance imaging of trabecular bone structure in the distal radius: Relationship with x-ray tomographic microscopy and biomechanics. Osteoporos Int. 1996;6:376–385. doi: 10.1007/BF01623011. [DOI] [PubMed] [Google Scholar]
- 56.Wehrli FW, Gomberg BR, Saha PK, Song HK, Hwang SN, Snyder PJ. Digital topological analysis of in vivo magnetic resonance microimages of trabecular bone reveals structural implications of osteoporosis. J Bone Miner Res. 2001;16:1520–1531. doi: 10.1359/jbmr.2001.16.8.1520. [DOI] [PubMed] [Google Scholar]
- 57.Wehrli FW. Structural and functional assessment of trabecular and cortical bone by micro magnetic resonance imaging. J Magn Reson Imaging. 2007;25:390–409. doi: 10.1002/jmri.20807. [DOI] [PubMed] [Google Scholar]
- 58.Techawiboonwong A, Song HK, Wehrli FW. In vivo mri of submillisecond t2 species with two-dimensional and three-dimensional radial sequences and applications to the measurement of cortical bone water. NMR in Biomedicine. doi: 10.1002/nbm.1179. In press. [DOI] [PubMed] [Google Scholar]


