Abstract
Synergies between global change and biological invasion have been identified as a major potential threat to global biodiversity and human welfare. The global change-type drought characteristic of many temperate terrestrial ecosystems is especially significant because it will apparently favour invasive over indigenous species, adding to the burden of conservation and compromising ecosystem service delivery. However, the nature of and mechanisms underlying this synergy remain poorly explored. Here we show that in a temperate terrestrial ecosystem, invasive and indigenous springtail species differ in the form of their phenotypic plasticity such that warmer conditions promote survival of desiccation in the invasive species and reduce it in the indigenous ones. These differences are consistent with significant declines in the densities of indigenous species and little change in those of invasive species in a manipulative field experiment that mimicked climate change trends. We suggest that it is not so much the extent of phenotypic plasticity that distinguishes climate change responses among these invasive and indigenous species, as the form that this plasticity takes. Nonetheless, this differential physiological response provides support for the idea that in temperate terrestrial systems experiencing global change-type drought, invasive species may well be at an advantage relative to their indigenous counterparts.
Keywords: acclimation, biological invasion, climate change, drought, soil fauna
1. Introduction
Invasive alien species constitute a significant environmental risk owing to their profound negative effects on species and ecosystems, and their economic costs to humankind (Mack et al. 2000; Pimental et al. 2005). In consequence, the conditions that promote invasion raise significant questions in conservation biology (Lockwood et al. 2005; Richardson & Pyšek 2006). An emerging concern is that modern environmental change will exacerbate the extent and impacts of biological invasions to the cost of indigenous biodiversity (Cannon 1998; Hobbs & Mooney 2005; Holzapfel & Vinebrooke 2005). Indeed, synergies between global change and biological invasion have recently been identified as a major threat to global biodiversity and human welfare (Millennium Ecosystem Assessment 2005). It is widely suggested that the warming and drying characteristic of climate change in many temperate ecosystems (global change-type drought; Easterling et al. 2000; Breshears et al. 2005) will favour invasive species over indigenous ones, adding to the burden of conservation and compromising ecosystem service delivery (Cannon 1998; Dukes & Mooney 1999; Walther et al. 2002). However, the nature of and mechanisms underlying such a synergy remain poorly explored (Agrawal 2001; Stachowicz et al. 2002).
Differences in phenotypic plasticity between introduced and indigenous species are likely to be particularly important in mediating responses to climate change. Short-term phenotypic plasticity is one of the most significant ways in which organisms react to environmental change (West-Eberhard 2003; Berrigan & Scheiner 2004; Chown & Terblanche 2007), and it is among the first in a graded set of responses that determine whether a population will persist following an alteration in its circumstances (Gabriel et al. 2005; Helmuth et al. 2005). The extent of phenotypic plasticity has also long been considered a major difference between introduced and indigenous plant species (Baker 1965; Daehler 2003), and while some studies have demonstrated the importance of plasticity for the invasion process in animals (Trussell & Smith 2000; Rosecchi et al. 2001; Duncan et al. 2003; Dzialowski et al. 2003), others have downplayed the role of plasticity and have emphasized strong responses to local selection (Davis & Shaw 2001; Lee 2002; Dybdahl & Kane 2005). Nonetheless, tests of whether systematic differences in performance and its plasticity exist between indigenous and exotic species are relatively uncommon, especially for terrestrial animals (Agrawal 2001; Lee et al. 2003). Moreover, they have seldom been undertaken within a climate change context, despite the obvious significance of doing so (Walther et al. 2002; Stillman 2003).
Here, we test two hypotheses concerning the climate change implications of differences in phenotypic plasticity among terrestrial indigenous and invasive species. First, we assess the idea that invasive species have greater plasticity per se than indigenous ones, which would enable the former to respond favourably to new conditions irrespective of what these are. This is the greater flexibility hypothesis first proposed by Baker (1965). Then, we determine whether the specific form of phenotypic plasticity differs among these groups in a way that would favour invasive species under climate change scenarios. Using terminology from the acclimation (a form of plasticity) literature, the question can be re-posed as whether, for invasive species, warmer environments will always be better (Huey et al. 1999).
The tests are effected by comparing the responses of desiccation resistance to thermal acclimation of springtails under laboratory conditions and by examining the responses of a springtail assemblage, of which the large majority of these species are members, to simulated climate warming and drying. Desiccation resistance is considered a fitness-enhancing trait in many arthropods (Hoffmann et al. 2003), including springtails (Kærsgaard et al. 2004) and it varies among species occupying moist and dry environments in a direction consistent with the assumptions that enhanced desiccation resistance increases fitness in dry environments (Leinaas & Sømme 1984; Addo-Bediako et al. 2001). Springtails are a globally significant group of soil organisms that play a major role in ecosystem functioning (Rusek 1998), and global change-type drought is forecast for many mid-latitude regions (Easterling et al. 2000; Breshears et al. 2005).
2. Material and methods
(a) Site, species and acclimation conditions
This work was undertaken on sub-Antarctic Marion Island (46°54′ S, 37°45′ E), which has a cool, wet, windy climate that has shown substantial change over the last 50 years, including an increase in mean annual temperature of more than 1°C and a decline in precipitation of more than 500 mm per annum (Smith 2002; Le Roux & McGeoch 2007). In other words, it is showing global change-type drought. The island is home to 16 springtail species, of which five are invasive. The latter are typically Palearctic (though now virtually cosmopolitan) species thought to have been introduced following the establishment of the scientific station at the island in 1947 (Chown et al. 2002). We investigated the six most commonly found Arthropleona species: the invasive species Pogonognathellus flavescens Tullberg (Tomoceridae) (2127.53±104.49 μg; mean±s.e.) and Isotomurus cf. palustris Müller (Isotomidae) (564.65±36.58 μg); and the indigenous species Cryptopygus antarcticus Déharveng (Isotomidae) (71.48±4.76 μg), Cryptopygus dubius Déharveng (Isotomidae) (5.82±0.6 μg), Isotoma marionensis Déharveng (Isotomidae) (mass) (12.8±0.07 μg) and Tullbergia bisetosa Börner (Onychiuridae) (53.57±3.73 μg).
Adult specimens were collected from the field (below 25 m a.s.l.) with an aspirator and placed into 30 ml plastic vials with moist plaster-of-Paris substrates and small amounts of detritus as a food source and for shelter. Animals were transported to the laboratory within 5 h of collection. They were sorted into batches in vials, identical to those described above, for acclimation at either 5°C (9 L: 15 D photoperiod or 12 L : 12 D photoperiod) or 15°C (14 L : 10 D photoperiod or 12 L : 12 D) in climate chambers (LABCON, Johannesburg, South Africa, accurate to ±1°C) for no less than 7 and no more than 10 days. Over such a period, the acclimation response is typically asymptotic (for springtails, see Slabber et al. 2007; for other species, see Hoffmann & Watson 1993; Terblanche et al. 2006). Where differences among day length treatments were found (in a full factorial generalized linear model including day length treatment as a term), only data from the 12 L : 12 D experiments were used (invasive species). In all other cases, data were pooled, i.e. that of indigenous species, except C. dubius and I. marionensis which were only subjected to the 12 L : 12 D experiments. All the vials included detritus as a source of food and additional moisture. The vials occupied little space on a single incubator shelf and therefore shelf effects in the climate chambers were unlikely to have had any influence on the temperature at which the individuals were held. Moreover, the same small areas in the climate chambers were used for each species.
(b) Desiccation trials
The methods of Kærsgaard et al. (2004) were adapted slightly. A saturated NaCl solution was chosen as a desiccant because it provides consistent relative humidity (76%) at temperatures varying between 0 and 20°C. This humidity represents a value that is not atypical for the soil surface at Marion Island when springtails have been found to be active (approx. 70–90% RH, J.E. Lee, unpublished data). For each test temperature (5 or 15°C, maintained by the same glass-fronted cooled incubators for the larger species or by a custom-built water jacket connected to a Grant LTC 12 water bath for the two smallest species: C. dubius and I. marionensis), 10–40 springtails obtained at random from the vials held at each acclimation temperature (5 or 15°C) were used. The individuals were sorted into two groups and placed into two dry, transparent plastic 50 ml vials housed within a larger, transparent 300 ml vial that contained 70 ml saturated salt solution and had been pre-equilibrated for several days. The smaller vials were covered with 125 μm mesh to prevent individuals from escaping, and the larger vial was sealed with a plastic Petri dish lid. The survival of the individuals was assessed visually every 5 min (using a Leica dissection microscope for the two smallest species) without disturbing any of the containers. Individuals that showed no signs of movement were scored as dead.
For each species, the effects of treatment temperature, test temperature and their interactions on survival time (which is equivalent to count data) were examined using a generalized linear model assuming a Poisson error structure with a logarithmic link function and corrected for overdispersion. To assess the differences in absolute survival time among the indigenous and alien species groups, a generalized linear model assuming a Poisson error structure with a logarithmic link function and corrected for overdispersion was used, with species means of survival time for each treatment as the dependent variable, mean mass as covariate, and origin (indigenous versus invasive), acclimation temperature and test temperature as the independent variables. To determine whether plasticity (or flexibility) differed among the indigenous and alien species, the largest absolute difference in survival time between the two acclimation treatments was calculated per treatment temperature and was expressed in each case as a proportional increase over the shortest survival time at that temperature. A generalized linear model assuming a normal error structure with an identity link function was used to examine whether origin (indigenous versus invasive) and test temperature had an influence on the extent of plasticity expressed as a proportional increase in survival time.
(c) Manipulative field experiments
The field experiment was conducted over an area of approximately 100×150 m on Skua Ridge on the eastern side of Marion Island, with the aim of investigating the effects of drying, warming and shading on a keystone plant species, Azorella selago, and the arthropods typical of this environment. The experimental approach and outcomes for the plant and arthropod community as a whole are reported in detail by Le Roux et al. (2005) and McGeoch et al. (2006). Here we provide a brief description of the experimental design and the data we used to assess springtail responses to global change-type drought.
Azorella selago plants for the experiment were selected from the median size category of plants on Skua Ridge, were at a minimum distance of 5 m from each other, were not sheltered by rocks or other plants and showed no signs of damage or senescence. There were no a priori differences in plant size, nearest neighbour characteristics, soil depth or epiphyte loads between treatment plants (Le Roux et al. 2005).
Since we were interested only in dry and warm conditions (and not shading which was also a treatment in the larger experiment), we compared the two treatment categories from the larger experiment in which plants were assigned randomly to treatment groups, i.e. dry-warm (n=16) and control (n=16) groups (Le Roux et al. 2005). Dry–warm plants were covered by clear polycarbonate sheets. These cloches (rainout shelters, dry–warm treatment) were secured and supported on each corner, open-sided, positioned at a minimum distance of 0.1 m above the experimental plant and slightly angled so that surface run-off was displaced down slope and away from other experimental plants. The cloches were designed to shelter plants from direct precipitation and not to eliminate all water sources. The effect of each treatment on temperature was quantified using iButton dataloggers (Thermocron DS1921G, Dallas Semi-Conductors, 0.5°C resolution). These were placed 15 mm below the dorsal plant surface of eight plants in each treatment for a period of several days in each season (February, April, August and December) with readings taken at hourly intervals (full details provided in Le Roux et al. 2005).
On removal of the treatments in April 2003, microarthropods were sampled from the centre of each of the 57 experimental plants using an O'Connor split corer (70 mm internal diameter). All arthropods were extracted from these cores (including surface leaves and underlying decomposing plant material) in a MacFadyen high-gradient extractor and were identified to species level. No specimens of P. flavescens were recorded at this site (McGeoch et al. 2006). For this study, we examined the treatment effects on the densities of indigenous and invasive Arthropleona species using a generalized linear model assuming Poisson errors and using a log link function. We report differences in relative density between control and treatment plots.
3. Results
(a) Desiccation trials
In all cases, test temperature had a significant effect on survival time as might be expected given the increasing saturation deficit at the higher temperatures. No difference was found among indigenous and invasive species in the absolute duration of survival once mass (which was significant: Χ1,152=22.3, p=0.00002) had been taken into consideration (Χ1,152=3.01, p=0.08). Likewise, the two groups did not differ in plasticity, expressed as a proportional change in survival time (Χ1,82=1.0, p=0.316), although plasticity was smaller at the higher test temperature (Χ1,82=6.07, p=0.014).
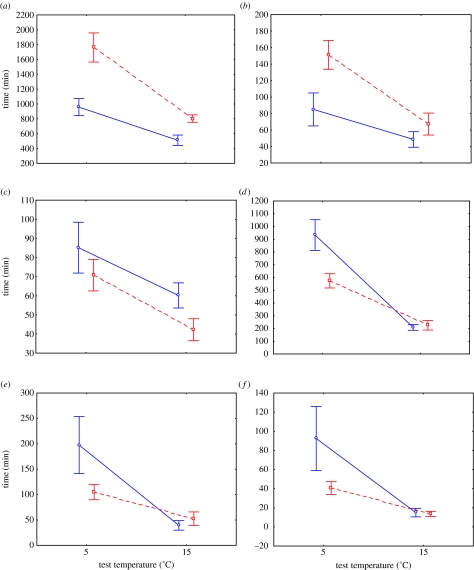
The six springtail species can be separated into two (or if C. dubius is distinguished from the remaining species, three) major groups based on their responses to acclimation and acclimation×test temperature interaction (table 1). In the first group, the interactions were not significant (P. flavescens, I. cf. palustris and T. bisetosa), whereas in the second group they were significant (C. antarcticus, C. dubius and I. marionensis). These response types do not completely distinguish the indigenous from the invasive species because T. bisetosa showed a response similar to those demonstrated by the invasive species (figure 1). In the first response type, individuals of the invasive species acclimated to 15°C always survived longer than those acclimated to 5°C, whereas the opposite was true for T. bisetosa (figure 1). In the second response type, individuals of the indigenous species acclimated to 5°C had significantly and substantially (double) longer survival times at 5°C than those acclimated to 15°C, whereas little difference in survival time occurred among acclimation treatments at a test temperature of 15°C (figure 1). The exception is C. dubius, in which individuals acclimated at the two temperatures differed at both test temperatures in such a way that the interaction term in the model remained significant (table 1) but the acclimation terms did not. In other words, the ‘reaction norms’ crossed (figure 1).
Table 1.
The effects of acclimation temperature, test temperature and their interactions on survival time in each of the invasive and indigenous springtail species.
| variable | Χ2 | p | Χ2 | p |
|---|---|---|---|---|
| invasive species | ||||
| Pogonognathellus flavescens | Isotomurus cf. palustris | |||
| acclimation | 88.4 | 0.00001 | 24.9 | 0.00001 |
| test | 158.1 | 0.00001 | 60.3 | 0.00001 |
| interaction | 1.83 | 0.18 | 1.86 | 0.17 |
| d.f. | 1, 72 | 1, 71 | ||
| indigenous species | ||||
| Cryptopygus antarcticus | Tullbergia bisetosa | |||
| acclimation | 10.3 | 0.0013 | 15.7 | 0.00008 |
| test | 410.2 | 0.00001 | 41.1 | 0.00001 |
| interaction | 19.8 | 0.00001 | 1.52 | 0.22 |
| d.f. | 1, 126 | 1, 119 | ||
| Cryptopygus dubius | Isotoma marionensis | |||
| acclimation | 1.09 | 0.29 | 11.4 | 0.0008 |
| test | 65.2 | 0.00001 | 160.8 | 0.00001 |
| interaction | 9.0 | 0.003 | 7.8 | 0.005 |
| d.f. | 1, 47 | 1, 52 | ||
Figure 1.
Mean±s.e. of survival time (at 76% relative humidity) following acclimation at 5°C (blue solid line) or at 15°C (red stippled line) at test temperatures of 5 and 15°C in the invasive (a) P. flavescens and (b) Isotomurus cf. palustris, and the indigenous (c) T. bisetosa, (d) C. antarcticus, (e) C. dubius and (f) I. marionensis. Note that the lines for the acclimations have been staggered slightly on the x-axis for ease of interpretation.
(b) Manipulative field experiments
The dry–warm treatment plants received 2034 mm less direct precipitation than other treatment plants over the period May 2002–April 2003, but did receive water from lateral soil water movement, surface flow and condensation (see also Le Roux et al. 2005). The effect of the dry–warm treatment on temperature was an average warming of 0.25°C (s.e. 0.01) relative to control plants over the year.
The response of the arthropod community to the experimental treatment was considerable, but was more pronounced for springtails than for mites (McGeoch et al. 2006). At this site, several Arthropleona species were found, including five of those examined in the desiccation trials, but excluding P. flavescens. Nonetheless, the response to the year of drying and warming was typically consistent: the density of the indigenous species declined dramatically, while invasive species density was unaffected (figure 2).
Figure 2.
Proportional (relative to controls) decline (less than 1) or increase (more than 1) in the density of the Arthropleona springtail species recorded in the experimental field site. The first four species are indigenous and members of the genera Cryptopygus, Tullbergia and Isotoma. The fifth species is the invasive Isotomurus cf. palustris. *Significant differences (p<0.05) in absolute densities between experimental and control sites, assessed using a generalized model assuming Poisson errors and using a log link function.
4. Discussion
Since the extent of phenotypic plasticity and absolute survival time did not differ among the indigenous and invasive species, Baker's (1965) greater flexibility hypothesis was not supported for these species. Although some support exists for this idea in plants (Daehler 2003) and aquatic animals (Trussell & Smith 2000), its significance is now being widely questioned. In many cases, strong selection (though not for flexibility) appears to account for the success of organisms encountering new environments (Davis & Shaw 2001; Lee et al. 2003, 2007). However, the greater flexibility hypothesis has not been widely assessed in an explicit way in terrestrial animals (Duncan et al. 2003; Gilchrist & Lee 2007). Our findings suggest that it might not be of relevance to understanding differential climate change responses among invasive and indigenous springtail species. However, greater numbers of invasive species need to be examined before the idea can be firmly discounted.
By contrast, the indigenous and invasive species typically responded in very different ways to acclimation. The invasive species showed no interaction effects among acclimation temperature and test temperature, and individuals of both species always survived longer following acclimation to a higher temperature. While the lack of interaction effect also characterized T. bisetosa, individuals of this indigenous species always survived longer following acclimation to the lower temperature. The remaining indigenous species were characterized by a significant interaction between acclimation and test temperature. Individuals survived longer at the low test temperature following acclimation at that temperature, with little performance difference between acclimation treatments at the higher test temperature. The sole exception was C. dubius, where differences in survival time were significant at both test temperatures. In the language of the beneficial acclimation hypothesis and its alternatives (Huey et al. 1999; Deere & Chown 2006), it appears that ‘hotter is better’ for the invasive species, while ‘colder is better’ for the indigenous ones, with the possible exception of C. dubius that seems to show evidence for beneficial acclimation. However, this evidence is weak owing to the minor differences in the performance at the high test temperature by comparison with those at the low test temperature. Overall, these results suggest that it is not the extent of phenotypic plasticity that differs between these indigenous and invasive species, but rather the form that this plasticity takes. If these differences in the form of plasticity mediate differential responses to climate change, then warming and drying, which is typical of the system inhabited by these species (Le Roux & McGeoch 2007), should have negative consequences for the indigenous species, but little effect on the invasive ones.
The manipulative field experiment demonstrates that this may be the case. The density of indigenous species declined following a year of drying and warming, while the invasive species density was unaffected. Species-specific springtail predators or parasites are not found in this ecosystem (Chown et al. 2002), so it is not an experimentally induced change at a higher trophic level that is driving this response. Rather, differences in the form of phenotypic plasticity among the indigenous and invasive species appear to mediate their ecological responses to simulated climate change.
Disparity in plasticity among the indigenous and invasive species might be attributable to variation in the vertical distribution of the species or to some other consistently varying trait, such as geographical range size or variable establishment success of introduced species (Simons 2003). The former seems unlikely because vertical distribution preferences have little effect on desiccation resistance in some springtails (Kærsgaard et al. 2004). The latter is more difficult to assess. No information exists for this system on the number of successful introductions relative to the total number of introductions. Likewise, the ranges of these springtail species have not been fully documented. Nonetheless, both groups of species are wide ranging. With the exception of I. marionensis, the indigenous species are typically widely distributed in the higher latitudes of the Southern Hemisphere (Deharveng 1981; Stevens et al. 2006), and in their natural ranges the invasive species are common across the Holarctic (Gisin 1960; Potapow 2001). Even so, evidence that consistent differences in physiological traits exist between broadly and narrowly distributed species is equivocal (Spicer & Gaston 1999; Pohlman et al. 2005). Therefore, variation in the form of plasticity appears to constitute a first-order difference among these indigenous and invasive species.
In recent years, the idea that climate change will differentially benefit invasive species over indigenous ones has been widely discussed (Walther et al. 2002; Hobbs & Mooney 2005). While firm evidence exists that this is sometimes the case in marine species (Stachowicz et al. 2002), few studies have presented evidence for such an effect, especially for terrestrial animals. We do so here, providing initial support for previous assumptions that climate change and biological invasions are likely to act synergistically to compromise terrestrial ecosystems. More importantly, we show that the major differences between these indigenous and invasive terrestrial species appear to be vested not be so much in their absolute tolerances or extent of phenotypic plasticity, but rather in the form that the plasticity takes. Our work therefore bears out assertions that the complexity of biological responses to climate change requires further investigation (Davis & Shaw 2001; Walther et al. 2002; Hoffmann et al. 2003), particularly the role played by phenotypic plasticity in mediating these responses (Stillman 2003). How the differences in response are likely to play out in the context of population differentiation (Kristensen et al. 2007) is especially significant, given that in many invasive invertebrates genetic diversity is low in comparison with similar indigenous species (Myburgh et al. 2007).
Acknowledgments
We thank Erika Nortje for assistance in the laboratory, and Peter le Roux, Elizabeth Hugo and Mawethu Nyakatya for assistance in the field. Janne Bengtsson and several anonymous referees provided helpful comments on an earlier version of this manuscript. This work was supported by the DST-NRF Centre of Excellence for Invasion Biology and a National Research Foundation–Norwegian Research Council Science Liaison grant.
References
- Addo-Bediako A, Chown S.L, Gaston K.J. Revisiting water loss in insects: a large scale view. J. Insect Physiol. 2001;47:1377–1388. doi: 10.1016/s0022-1910(01)00128-7. doi:10.1016/S0022-1910(01)00128-7 [DOI] [PubMed] [Google Scholar]
- Agrawal A.A. Phenotypic plasticity in the interactions and evolution of species. Science. 2001;294:321–326. doi: 10.1126/science.1060701. doi:10.1126/science.1060701 [DOI] [PubMed] [Google Scholar]
- Baker H.G. Characteristics and modes of origin of weeds. In: Baker H.G, Stebbins G.L, editors. The genetics of colonizing species. Academic Press; New York, NY: 1965. pp. 147–168. [Google Scholar]
- Berrigan D.M, Scheiner S.M. Modelling the evolution of phenotypic plasticity. In: DeWitt T.J, Scheiner S.M, editors. Phenotypic plasticity. Functional and conceptual approaches. Oxford University Press; Oxford, UK: 2004. pp. 82–97. [Google Scholar]
- Breshears D.D, et al. Regional vegetation die-off in response to global-change-type drought. Proc. Natl Acad. Sci. USA. 2005;102:15 144–15 148. doi: 10.1073/pnas.0505734102. doi:10.1073/pnas.0505734102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon R.J.C. The implications of predicted climate change for insect pests in the UK, with emphasis on non-indigenous species. Glob. Change Biol. 1998;4:785–796. doi:10.1046/j.1365-2486.1998.00190.x [Google Scholar]
- Chown S.L, McGeoch M.A, Marshall D.J. Diversity and conservation of invertebrates on the sub-Antarctic Prince Edward Islands. Afr. Entomol. 2002;10:67–82. [Google Scholar]
- Chown S.L, Terblanche J.S. Physiological diversity in insects: ecological and evolutionary contexts. Adv. Insect Physiol. 2007;33:50–152. doi: 10.1016/S0065-2806(06)33002-0. doi:10.1016/S0065-2806(06)33002-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daehler C.C. Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annu. Rev. Ecol. Evol. Syst. 2003;34:183–211. doi:10.1146/annurev.ecolsys.34.011802.132403 [Google Scholar]
- Davis M.B, Shaw R.G. Range shifts and adaptive responses to quaternary climate change. Science. 2001;292:673–679. doi: 10.1126/science.292.5517.673. doi:10.1126/science.292.5517.673 [DOI] [PubMed] [Google Scholar]
- Deere J.A, Chown S.L. Testing the beneficial acclimation hypothesis and its alternatives for locomotor performance. Am. Nat. 2006;168:630–644. doi: 10.1086/508026. doi:10.1086/508026 [DOI] [PubMed] [Google Scholar]
- Deharveng L. Collemboles des îles subantarctiques de l'Océan Indien Mission J. Travé 1972–1973. Comité. Natl Fran. Recherches Antarct. 1981;48:33–108. [Google Scholar]
- Dukes J.S, Mooney H.A. Does global change increase the success of biological invaders? Trends Ecol. Evol. 1999;14:135–139. doi: 10.1016/s0169-5347(98)01554-7. doi:10.1016/S0169-5347(98)01554-7 [DOI] [PubMed] [Google Scholar]
- Duncan R.P, Blackburn T.M, Sol D. The ecology of bird introductions. Annu. Rev. Ecol. Evol. Syst. 2003;34:71–98. doi:10.1146/annurev.ecolsys.34.011802.132353 [Google Scholar]
- Dybdahl M.F, Kane S.L. Adaptation vs. phenotypic plasticity in the success of a clonal invader. Ecology. 2005;86:1592–1601. doi:10.1890/04-0898 [Google Scholar]
- Dzialowski A.R, Lennon J.T, O'Brien W.J, Smith V.H. Predator-induced phenotypic plasticity in the exotic cladoceran Daphnia lumholtzi. Freshw. Biol. 2003;48:1593–1602. doi:10.1046/j.1365-2427.2003.01111.x [Google Scholar]
- Easterling D.R, Meehl G.A, Parmesan C, Changnon S.A, Karl T.R, Mearns L.O. Climate extremes: observations, modeling, and impacts. Science. 2000;289:2068–2074. doi: 10.1126/science.289.5487.2068. doi:10.1126/science.289.5487.2068 [DOI] [PubMed] [Google Scholar]
- Gabriel W, Luttbeg B, Sih A, Tollroan R. Environmental tolerance, heterogeneity, and the evolution of reversible plastic responses. Am. Nat. 2005;166:339–353. doi: 10.1086/432558. doi:10.1086/432558 [DOI] [PubMed] [Google Scholar]
- Gilchrist G.W, Lee C.E. All stressed out and nowhere to go: does evolvability limit adaptation in invasive species? Genetica. 2007;129:127–132. doi: 10.1007/s10709-006-9009-5. doi:10.1007/s10709-006-9009-5 [DOI] [PubMed] [Google Scholar]
- Gisin H. Museum Histoire Naturelle; Genève, Switzerland: 1960. Collembolanfauna Europas. [Google Scholar]
- Helmuth B, Kingsolver J.G, Carrington E. Biophysics, physiological ecology, and climate change: does mechanism matter? Annu. Rev. Physiol. 2005;67:177–201. doi: 10.1146/annurev.physiol.67.040403.105027. doi:10.1146/annurev.physiol.67.040403.105027 [DOI] [PubMed] [Google Scholar]
- Hobbs R.J, Mooney H.A. Invasive species in a changing world: the interactions between global change and invasives. In: Mooney H.J, Mack R.N, McNeely J.A, Neville L.E, Schei P.J, Waage J.K, editors. Invasive alien species. A new synthesis. Island Press; Washington, DC: 2005. pp. 310–331. [Google Scholar]
- Hoffmann A.A, Hallas R.J, Dean J.A, Schiffer M. Low potential for climatic stress adaptation in a rainforest Drosophila species. Science. 2003;301:100–102. doi: 10.1126/science.1084296. doi:10.1126/science.1084296 [DOI] [PubMed] [Google Scholar]
- Hoffmann A.A, Watson M. Geographical variation in the acclimation responses of Drosophila to temperature extremes. Am. Nat. 1993;142:S93–S113. doi: 10.1086/285525. doi:10.1086/285525 [DOI] [PubMed] [Google Scholar]
- Holzapfel A.M, Vinebrooke R.D. Environmental warming increases invasion potential of alpine lake communities by imported species. Glob. Change Biol. 2005;11:2009–2015. [Google Scholar]
- Huey R.B, Berrigan D, Gilchrist G.W, Herron J.C. Testing the adaptive significance of acclimation: a strong inference approach. Am. Zool. 1999;39:323–336. [Google Scholar]
- Kærsgaard C.W, Holmstrup M, Malte H, Bayley M. The importance of cuticular permeability, osmolyte production and body size for the desiccation resistance of nine species of Collembola. J. Insect Physiol. 2004;50:5–15. doi: 10.1016/j.jinsphys.2003.09.003. doi:10.1016/j.jinsphys.2003.09.003 [DOI] [PubMed] [Google Scholar]
- Kristensen T.N, Loeschcke V, Hoffmann A.A. Can artificially selected phenotypes influence a component of field fitness? Thermal selection and fly performance under thermal extremes. Proc. R. Soc. B. 2007;274:771–778. doi: 10.1098/rspb.2006.0247. doi:10.1098/rspb.2006.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux P.C, McGeoch M.A, Nyakatya M.J, Chown S.L. Effects of a short-term climate change experiment on a keystone plant species in the sub-Antarctic. Glob. Change Biol. 2005;11:1628–1639. doi:10.1111/j.1365-2486.2005.001022.x [Google Scholar]
- Lee C.E. Evolutionary genetics of invasive species. Trends Ecol. Evol. 2002;17:386–391. doi:10.1016/S0169-5347(02)02554-5 [Google Scholar]
- Lee C.E, Remfert J.L, Chang Y.-M. Response to selection and evolvability of invasive populations. Genetica. 2007;129:179–192. doi: 10.1007/s10709-006-9013-9. doi:10.1007/s10709-006-9013-9 [DOI] [PubMed] [Google Scholar]
- Lee C.E, Remfert J.L, Gelembiuk G.W. Evolution of physiological tolerance and performance during freshwater invasions. Integr. Comp. Biol. 2003;43:439–449. doi: 10.1093/icb/43.3.439. doi:10.1093/icb/43.3.439 [DOI] [PubMed] [Google Scholar]
- Leinaas H.P, Sømme L. Adaptations in Xenylla maritima and Anurophorus laricis (Collembola) to lichen habitats on alpine rocks. Oikos. 1984;43:197–206. doi:10.2307/3544769 [Google Scholar]
- Le Roux P.C, McGeoch M.A. Changes in climatic extremes, variability and signature on sub-Antarctic Marion Island. Clim. Change. 2007 doi:10.1007/s10584-007-9259-y [Google Scholar]
- Lockwood J.L, Cassey P, Blackburn T.M. The role of propagule pressure in explaining species invasions. Trends Ecol. Evol. 2005;20:223–228. doi: 10.1016/j.tree.2005.02.004. doi:10.1016/j.tree.2005.02.004 [DOI] [PubMed] [Google Scholar]
- Mack R.N, Simberloff D, Lonsdale W.M, Evans H, Clout M, Bazzaz F.A. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl. 2000;10:689–710. [Google Scholar]
- McGeoch M.A, le Roux P.C, Hugo E.A, Chown S.L. Species and community responses to short-term climate manipulation: microarthropods in the sub-Antarctic. Aust. Ecol. 2006;31:719–731. doi:10.1111/j.1442-9993.2006.01614.x [Google Scholar]
- Millennium Ecosystem Assessment. World Resources Institute; Wahsington, DC: 2005. Ecosystems and human well-being: biodiversity synthesis. [Google Scholar]
- Myburgh M, Chown S.L, Daniels S.R, Jansen van Vuuren B. Population structure, propagule pressure and conservation biogeography: lessons from indigenous and invasive springtails. Divers. Distrib. 2007;13:143–154. [Google Scholar]
- Pimental D, Zuniga R, Morrison D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005;52:273–288. [Google Scholar]
- Pohlman C.L, Nicotra A.B, Murray B.R. Geographic range size, seedling ecophysiology and phenotypic plasticity in Australian Acacia species. J. Biogeogr. 2005;32:341–351. doi:10.1111/j.1365-2699.2004.01181.x [Google Scholar]
- Potapow M. Staatliches Museum für Naturkunde; Görlitz, Germany: 2001. Synopses on palaearctic collembola Isotomidae. [Google Scholar]
- Richardson D.M, Pyšek P. Plant invasions: merging the concepts of species invasiveness and community invisibility. Prog. Phys. Geogr. 2006;30:409–431. doi:10.1191/0309133306pp490pr [Google Scholar]
- Rosecchi E, Thomas F, Crivelli A.J. Can life-history traits predict the fate of introduced species? A case study of two cyprinid fish in southern France. Freshw. Biol. 2001;46:845–853. doi:10.1046/j.1365-2427.2001.00715.x [Google Scholar]
- Rusek J. Biodiversity of Collembola and their functional role in the ecosystem. Biodiv. Conserv. 1998;7:1207–1219. doi:10.1023/A:1008887817883 [Google Scholar]
- Simons A.M. Invasive aliens and sampling bias. Ecol. Lett. 2003;6:278–280. doi:10.1046/j.1461-0248.2003.00430.x [Google Scholar]
- Slabber S, Worland M.R, Leinaas H.P, Chown S.L. Acclimation effects on thermal tolerances of springtails from sub-Antarctic Marion Island: indigenous and invasive species. J. Insect Physiol. 2007;53:113–125. doi: 10.1016/j.jinsphys.2006.10.010. doi:10.1016/j.jinsphys.2006.10.010 [DOI] [PubMed] [Google Scholar]
- Smith V.R. Climate change in the sub-Antarctic: an illustration from Marion Island. Clim. Change. 2002;52:345–357. doi:10.1023/A:1013718617277 [Google Scholar]
- Spicer J.I, Gaston K.J. Blackwell Science; Oxford, UK: 1999. Physiological diversity and its ecological implications. [Google Scholar]
- Stachowicz J.J, Terwin J.R, Whitlatch R.B, Osman R.W. Linking climate change and biological invasions: ocean warming facilitates nonindigenous species invasions. Proc. Natl Acad. Sci. USA. 2002;99:15 497–15 500. doi: 10.1073/pnas.242437499. doi:10.1073/pnas.242437499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M.I, Greenslade P, Hogg I.D, Sunnucks P. Southern Hemisphere springtails: could any have survived glaciation of Antarctica? Mol. Biol. Evol. 2006;23:874–882. doi: 10.1093/molbev/msj073. doi:10.1093/molbev/msj073 [DOI] [PubMed] [Google Scholar]
- Stillman J.H. Acclimation capacity underlies susceptibility to climate change. Science. 2003;301:65. doi: 10.1126/science.1083073. doi:10.1126/science.1083073 [DOI] [PubMed] [Google Scholar]
- Terblanche J.S, Klok C.J, Krafsur E.S, Chown S.L. Phenotypic plasticity and geographic variation in thermal tolerance and water loss of the tsetse fly Glossina pallidipes (Diptera: Glossinidae): implications for distribution modelling. Am. J. Trop. Med. Hyg. 2006;74:786–794. [PMC free article] [PubMed] [Google Scholar]
- Trussell G.C, Smith L.D. Induced defenses in response to an invading crab predator: an explanation of historical and geographic phenotypic change. Proc. Natl Acad. Sci. USA. 2000;97:2123–2127. doi: 10.1073/pnas.040423397. doi:10.1073/pnas.040423397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther G.-R, Post E, Convey P, Menzel A, Parmesan C, Beebee T.J.C, Fromentin J.-M, Hoegh-Guldberg O, Bairlein F. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. doi:10.1038/416389a [DOI] [PubMed] [Google Scholar]
- West-Eberhard M.J. Oxford University Press; New York, NY: 2003. Developmental plasticity and evolution. [Google Scholar]