Abstract
In small birds, mass-dependent predation risk (MDPR) is known to make the trade-off between avoiding starvation and avoiding predation dependent on individual mass. This occurs because carrying increased fat reserves not only reduces starvation risk but also results in a higher predation risk due to reduced escape flight performance and/or the increased foraging exposure needed to maintain a higher body mass. In principle, the theory of MDPR could also apply to any animal capable of storing energy reserves to reduce starvation and whose escape performance decreases with increasing mass. We used a unique situation along certain parts of coastal Britain, where harbour porpoises (Phocoena phocoena) are pursued and killed but crucially not eaten by bottlenose dolphins (Tursiops truncatus), to investigate whether a MDPR effect can occur in non-avian species. We show that where high levels of dolphin ‘predation’ occur, porpoises carry significantly less energy reserves than would otherwise be expected and this equates to reducing by approximately 37% the length of time that a porpoise could survive without feeding. These results provide the first evidence that a mass-dependent starvation–predation risk trade-off may be a general ecological principle that can apply to widely different animal types rather than, as is currently thought, only to birds.
Keywords: energy reserves, starvation risk, starvation–predation risk trade-off, Phocoena phocoena, Tursiops truncatus
1. Introduction
The starvation–predation risk trade-off is a key challenge faced by living organisms and has the potential to influence the Darwinian fitness of virtually all animals (Houston et al. 1993; Lima 1998b). For birds, mass-dependent predation risk (MDPR) theory predicts that if the mass of an individual affects its likelihood of being caught and killed, individuals can reduce their predation mortality risk by keeping their mass, for any given body size, as low as possible (Lima 1986; Houston et al. 1993; Witter & Cuthill 1993). However, in order to avoid starvation in times of poor food availability, animals must carry energy reserves, usually in the form of fat, which can make up a considerable portion of their body mass (Lima 1986; Witter & Cuthill 1993). Theoretically, therefore, individuals could minimize their combined starvation and predation mortality risk by optimizing their fat reserves and body mass (Houston et al. 1993; Bednekoff & Houston 1994). Under such a strategy, an individual will carry increased energy reserves when starvation risk is high and decreased reserves when predation risk is high (Lima 1986; Bednekoff & Houston 1994). This concept of the starvation–predation risk trade-off being mass dependent was originally developed to explain the observation that in winter small birds do not carry as large energy reserves as would be expected if they were only trying to minimize their risk of starvation (Lima 1986).
For passerine birds, there is now a considerable body of evidence consistent with individuals in the wild managing their body mass over long (weeks and months) and short (hours and days) time scales in line with MDPR (Gosler et al. 1995; Adriaensen et al. 1998; Cresswell 1998; Gentle & Gosler 2001; Macleod et al. 2005c). The original classic study demonstrated that the average mass of wild great tits (Parus major) increased when their main predator, the sparrowhawk (Accipiter nisus), was absent and decreased when it was present (Gosler et al. 1995). Since then, other ecological studies have shown mass increasing and decreasing in line with predictors of starvation risk (such as season, weather and foraging uncertainty), and experimental studies have shown that when their perception of predation risk increases, birds decrease their mass and manage their daily acquisition of mass as predicted (Gentle & Gosler 2001; Gosler 2002; Macleod et al. 2005b,c). Two mechanisms are used to explain why birds respond in this way. First, escape flight performance is known to be reduced in heavier birds (Kullberg et al. 1996; Lind et al. 1999; MacLeod 2006), making them potentially more vulnerable to capture when attacked by a predator. Second, the extra foraging time assumed to be needed to maintain a higher body mass is thought to increase predation risk because it increases the time exposed to predators while feeding (Brodin 2001).
To date, the theory of MDPR has only been developed in birds and empirical evidence comes only from small passerines with masses between 10 and 150 g (Macleod et al. 2005b and references therein). However, according to Newtonian physics, when using maximum force, any object, including living animals, of any given body shape and size will accelerate and turn more slowly (or have a wider turning radius) if it is heavier (Witter et al. 1994). Therefore, there is no theoretical reason why mass-dependent predation should not apply to any animal that carries energy reserves to reduce starvation risk and uses rapid movement to escape from predators.
Marine mammals are several orders of magnitude larger than passerine birds (tens to thousands of kilograms versus hundredths to tenths of kilograms), live in a very different environment (aquatic versus terrestrial/aerial) and represent a very different evolutionary lineage (mammals versus birds; Brooke & Birkhead 1991; Rice 1998; Berta & Sumich 1999; Perrin et al. 2002). However, like birds and many other animals, marine mammals store energy reserves (in the form of blubber) on their bodies (Young 1976). Also like birds, marine mammals can use rapid movement to escape from predators. Marine mammals are predated by predators that they often cannot out-swim in a straight line (Domenici 2001). Therefore, their escape performance is expected to depend on manoeuvrability as well as speed, specifically acceleration, turning speed and/or turning radius. As predicted based on Newtonian physics, the turning speed and radius of marine mammals has been shown to be affected by their mass, with, all other things being equal, heavier individuals having larger turning circles and/or slower turning speeds than smaller ones of the same species (Fish et al. 2003) and lighter individuals being likely to have higher acceleration abilities (Domenici 2001). Marine mammals are, therefore, a good candidate for investigating whether the starvation–predation risk trade-off can be mass dependent in a taxonomic group other than passerine birds and in an environment very different from the terrestrial/aerial one where this influential phenomenon has previously been demonstrated.
However, exploring whether starvation–predation risk trade-off is mass dependent in marine mammals is difficult because most studies of predation on marine mammals are primarily based on opportunistic observations and wounds on living animals resulting from unsuccessful predation events (Heithaus 2001). As a result, the level of predation events experienced by marine mammal populations is often unknown and the overall importance of predation can be unclear because the carcass is normally consumed and disappears before it can be found by observers. To allow us to investigate whether mass-dependent predation might occur in a marine mammal species, we exploited an almost unique situation that exists on the east coast of Scotland. In this area, the main cause of death for harbour porpoises (Phocoena phocoena) is attacks by the much larger bottlenose dolphin, Tursiops truncatus (Ross & Wilson 1996; Patterson et al. 1998). These attacks are often, but not always, fatal and although this phenomenon is not precisely predation (since the porpoises are not consumed after being killed), the effect from the porpoise's (prey's) point of view is indistinguishable from actual predation events. The difference is, however, important for our study because the porpoise carcasses produced by lethal interactions with dolphins are frequently available for study once they are washed up along the coast as strandings, providing direct evidence of the frequency and location of such ‘predation’ events (Ross & Wilson 1996).
The harbour porpoise is a small marine mammal (adult weight usually between 45 and 60 kg) found throughout cooler waters of the Northern Hemisphere (Rice 1998). It is one of the most abundant cetaceans in the shelf waters of northwest Europe (Hammond et al. 2002) and is one of the best studied cetacean species. Harbour porpoises have relatively high daily energy demands. In captivity, they have been recorded consuming between 4 and 9.5% of their body weight in food per day and may need to consume more than this on a daily basis in the wild where they have to forage for live prey (mostly small fishes) and survive at lower temperatures (Kastelein et al. 1997). If a harbour porpoise does not capture enough prey to meet its daily energy demands, it can rely on stored energy to avoid starvation. This energy is primarily stored as blubber and it has been estimated that, on average, the blubber of a harbour porpoise contains enough energy to keep it alive for up to 3 to 5 days depending on age and initial physical condition (Kastelein et al. 1997). However, since blubber also has a role in thermoregulation, an animal may die of hypothermia before all the energy from the blubber has been used. Kastelein et al. (1997) estimate that harbour porpoise may have a life expectancy of as little as 3 days without food in waters of 20oC, and may notably lose condition after as little as 24 hours without eating. Therefore, the level of energy stores is a critical component of harbour porpoise survival, and in terms of surviving periods of low food availability, animals with higher energy stores can survive longer periods.
Dolphin-induced porpoise mortality has been commonly recorded at sites in Scottish North Sea coastal waters since 1991 and may have been occurring undetected before this (Ross & Wilson 1996; Patterson et al. 1998). Dolphin-induced porpoise mortality has also been recorded in other parts of Britain (along the coast of Wales and also in southwestern England; Jepson & Baker 1998), but these interactions have only become more frequent in this region since 1999 (Jepson 2006). Such interactions have not been recorded at other sites (Ross & Wilson 1996; Jepson 2006). Bottlenose dolphin attacks on harbour porpoises have been observed on a limited number of occasions, but accounts suggest that there is a pursuit phase when the porpoise attempts to escape the pursuing dolphins, presumably by attempting to outmanoeuvre them (Ross & Wilson 1996). Therefore, if MDPR applies, the theory predicts that when all else is equal, harbour porpoises that carry larger energy stores, and are therefore heavier for their size, will have a poorer turning ability and will not be able to escape bottlenose dolphins as easily as individuals with lower levels of energy stores.
To investigate whether MDPR could result in the starvation–predation risk trade-off being mass dependent in non-avian species, we studied the energy storage strategies of harbour porpoises in the presence and absence of lethal dolphin–porpoise interactions. Specifically, we tested the hypothesis that variations in harbour porpoise body mass can be predicted by the presence or absence of predation by bottlenose dolphins and that porpoises are lighter in the presence of dolphin predation than in its absence.
2. Material and methods
Since 1913, strandings of all cetaceans (including harbour porpoises) have been recorded by the Natural History Museum, London. Since 1990, post-mortem examinations have been conducted using standardized protocols to establish cause of death and to collect various biometric measurements (Jepson 2006). In this study we used data from 490 UK-stranded porpoises examined between 1990 and 2004. Neonate animals still completely dependent on their mother for survival were excluded from the analysis by selecting only animals of 1.0 m or greater in body length. We analysed all strandings for which the required biological variables (see below) were collected and that occurred in a contiguous coastal area extending north from the North Sea coasts of southern Britain (Kent), continuing around the eastern, northern and western coasts of Britain to the southwest coast of Scotland and including the coasts of the island groups of the Orkney, Shetland and Western Isles. Data from porpoises stranded along the coasts of Wales, western England and the English Channel were not used in the analysis because dolphin-induced mortality appears to be currently evolving in this area (Jepson 2006). As a result, the extent, timing and frequency of the bottlenose dolphin predation behaviour at sites in this latter area were not sufficiently clear for us to reliably identify when and where individual porpoises were likely to be exposed to predation pressure during the data collection period.
We used general linear modelling to test our hypothesis in a number of ways while controlling for other potentially confounding factors as follows. The dependent variable used in all cases was body mass. The independent variables included were body length, sex, age class, month, cause of death and the presence or absence of dolphin predation. Preliminary analysis using linear regression showed that (after exclusion of neonate animals) there was a strong linear relationship between body length and body mass in our porpoises and that 76% (F1,893=2829, p<0.001) of the variation in porpoise body mass was explained by overall body length. Using body length squared or body length cubed did not improve on this fit as both explained equal amounts of variation in body mass. We then used curve fitting to show that after using body length to control for size there were no significant linear, quadratic or cubic size-related patterns to the mass residuals (during curve fitting, minimum p-value was 0.947). We, therefore, used body length to control for the size of the animal. Sex, age class, month and cause of death were treated as fixed factors. Irrespective of their size, younger animals may carry different levels of energy reserves and therefore differ in mass simply because they are less able or experienced foragers. To control for this possibility, we classed animals as either juvenile (less than 5 years old) or adult (5 years and older), based on the age and/or age class determined during the post-mortem. Inclusion of month controlled for seasonal factors such as water temperature that will affect body mass because greater insulation is required at lower temperatures, as well as controlling for effects of the seasonal reproductive cycle on body weight. Based on the post-mortem results, we divided cause of death into two categories: those that died of sudden physical trauma (due to boat strikes, by-catch in fishing nets or dolphin predation) that causes a relatively sudden death and other mortality (including deaths due to starvation, disease and any other causes that could result in a more drawn-out death) that might be accompanied by a loss of body mass for reasons other than predation risk.
Finally, along with these potentially confounding variables, the presence of dolphin predation was included as a two-way fixed factor. The presence of dolphin predation was based on the recorded distribution of porpoise carcasses that exhibited evidence of bottlenose dolphin predation (Ross & Wilson 1996; Jepson & Baker 1998; Jepson 2006). This effectively consisted of sites along the east coast of Scotland from John O'Groats to the Scottish border being classed as experiencing dolphin predation and sites along the northern and western coasts of Scotland, around the Western, Shetland and Orkney Islands and along the eastern coast of England being classed as not experiencing dolphin predation.
Using these variables, the full dataset was analysed and then separate models were run focusing on subsets of the data or with subsets of the variables to test whether various alternative explanations for the results were possible. Alternatives considered included differences in environmental conditions between areas or in population substructuring of harbour porpoises in this region or other geographically related variables. Data analysis was performed using the SPSS statistical programs (SPSS 2001). Means are presented in the form mean±s.e.
3. Results
Preliminary analysis using linear regression confirmed that there is a highly significant positive relationship between porpoise mass variation and the size of the individual's energy reserves as measured by the mean of dorsal, ventral and lateral blubber thickness measurements (F1,1074=102.9, p<0.001). This variation in blubber explained 8.7% of the variation in porpoise body mass from the entire sample of UK-stranded porpoises.
The general linear model in table 1 shows that for the 490 harbour porpoises washed up around the coast of Scotland and eastern England, when all else is equal, porpoises were significantly lighter in the presence of dolphin predation than would otherwise have been expected. When the analysis was repeated focusing only on data for the 299 individuals found along the coasts of Scotland, the same pattern was obtained (table 2a). Similarly, when the analysis was repeated focusing only on data for the 408 individuals from the North Sea coast of Britain (from Kent to the Shetlands), the same result was again obtained (table 2b). Dolphin predation remained a significant effect with lower mass in the area experiencing predation (F1,225=5.3, p=0.023) when we considered only animals that had died suddenly of physical trauma and excluded all animals that might have reduced body mass because they had died slowly of starvation or disease. Focusing only on the animals in areas where dolphin predation occurred, the porpoises identified by post-mortem as killed by dolphins were, after controlling for the same factors as in the previous models in tables 1 and 2, significantly heavier than those not killed by dolphins (F1,183=19.0, p<0.001). Residual mass of porpoises killed by dolphins was 1.1±0.5 kg and those not killed by dolphins −1.2±0.5 kg.
Table 1.
Harbour porpoise mass around Britain is predicted by the presence and absence of bottlenose dolphin ‘predation’ (see values in italics). (GLM of factors predicting mass variation, dependent variable is body mass, N=490 individual harbour porpoises, adjusted R2=0.81. Parameter estimates given are for mortality other than physical trauma (fixed factor: cause of death; presence of dolphin ‘predation’ (dolphin predation); juveniles (age class); females (sex).)
| source of variation | sum of squares | d.f. | F | p | parameter estimate±s.e. |
|---|---|---|---|---|---|
| body length | 40 617 | 1 | 1388.9 | <0.001 | 0.60±0.2 |
| cause of death | 3057 | 1 | 104.5 | <0.001 | −5.47±0.54 |
| dolphin ‘predation’ | 173 | 1 | 5.9 | 0.015 | −1.29±0.53 |
| age class | 85 | 1 | 2.9 | 0.089 | −1.13±0.66 |
| sex | 1 | 1 | 0.1 | 0.841 | −0.10±0.51 |
| month | 341 | 11 | 1.1 | 0.392 | |
| model | |||||
| explained | 60 518 | 16 | 129.3 | <0.001 | |
| residual | 13 832 | 473 | |||
| total | 74 350 | 489 |
Table 2.
Focusing within areas, harbour porpoise mass is still predicted by the presence and absence of bottlenose dolphin ‘predation’ (see values in italics) around (a) Scotland (N=299, adjusted R2=0.82) and (b) the North Sea (N=408, adjusted R2=0.80). (GLM of factors predicting mass variation, dependent variable is body mass. Parameter estimates given are for mortality other than physical trauma (fixed factor: cause of death; presence of dolphin ‘predation’ (dolphin predation); juveniles (age class); females (sex).)
| source of variation | sum of squares | d.f. | F | p | parameter estimate±s.e. |
|---|---|---|---|---|---|
| (a) | |||||
| body length | 23 425 | 1 | 804.2 | <0.001 | 0.59±0.02 |
| cause of death | 1198 | 1 | 41.1 | <0.001 | −4.69±0.73 |
| dolphin ‘predation’ | 123 | 1 | 4.2 | 0.041 | −1.50±0.73 |
| age class | 68 | 1 | 2.3 | 0.127 | −1.27±0.83 |
| sex | 6 | 1 | 0.2 | 0.661 | −0.29±0.66 |
| month | 311 | 11 | 1.0 | 0.472 | |
| model | |||||
| explained | 39 246 | 16 | 84.2 | <0.001 | |
| residual | 8214 | 282 | |||
| total | 47 460 | 298 | |||
| (b) | |||||
| body length | 33 347 | 1 | 1114 | <0.001 | 0.60±0.02 |
| cause of death | 2560 | 1 | 85.5 | <0.001 | −5.44±0.69 |
| dolphin ‘predation’ | 138.7 | 1 | 4.6 | 0.032 | −1.22±0.57 |
| age class | 31 | 1 | 1.0 | 0.311 | −0.78±0.77 |
| sex | 3 | 1 | 0.1 | 0.743 | 0.19±0.57 |
| month | 229 | 11 | 0.7 | 0.743 | |
| model | |||||
| explained | 47 736 | 16 | 99.7 | <0.001 | |
| residual | 11 704 | 391 | |||
| total | 59 440 | 407 | |||
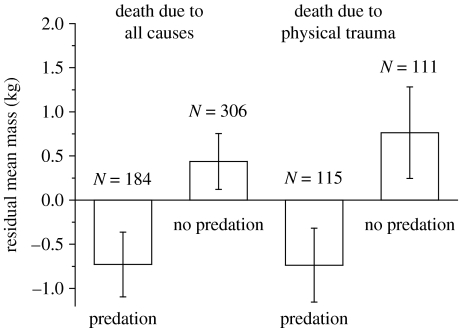
Using the full dataset and after controlling for size, sex, age class, time of year and whether cause of death was rapid or slower, harbour porpoises living in areas of dolphin predation were significantly lighter than would otherwise have been expected (figure 1). Those harbour porpoises living at sites where dolphin-induced mortality was occurring had a mean residual mass of 1.2±0.5 kg lighter than elsewhere (independent t-test: t1,488=2.3, p=0.019). Similarly, when considering only animals that had died suddenly of physical trauma, those experiencing dolphin predation had a mean residual mass of 1.5±0.7 kg lighter than those elsewhere (independent t-test: t1,224=2.3, p=0.025).
Figure 1.
Mass difference between harbour porpoises in the presence and absence of bottlenose dolphin ‘predation’. The significantly higher mass of individuals living in areas without the risk of predation by dolphins suggests that porpoises are reducing mass in response to a mass-dependent-type predation risk. Error bars indicate ±s.e.
4. Discussion
As predicted by our MDPR hypothesis, harbour porpoises are significantly lighter in the presence of lethal porpoise–dolphin interactions and this difference is not confounded by size, sex or age class of the individuals involved, the time of year or cause of their death. In addition, and again as predicted by MDPR, the porpoises actually killed by dolphins were heavier than the other animals in the same area. Our results also confirm along with previous work (Kastelein et al. 1997) that as in other animals, the mass of individual porpoises changes with the amount of stored energy reserves. Therefore, after controlling for size differences, changes in energy reserves will be reflected by changes in mass (Lima 1986; Houston et al. 1993; Gosler et al. 1995). Our results are, therefore, consistent with our hypothesis that MDPR can lead to a starvation–predation risk trade-off that is mass dependent in non-avian species and outside the aerial environment favoured by birds.
By focusing on subsets of the data, our analysis also allowed us to consider, and rule out, a number of alternative explanations for harbour porpoises being lighter and carrying less energy reserves where dolphin predation occurred. First, finding the same result when focusing only on Scotland (table 2a) showed that differences in mass are unlikely to be related to latitude, since latitude is similar between the areas with and without dolphin predation. Similarly, finding the same result when we focus only on the environmentally similar North Sea coast (table 2b) showed that differences in longitude are unlikely to be driving the results. We were also able to use the analysis in table 2a,b to rule out several alternative explanations that depend on differences in energy requirements or food availability between dolphin predation sites and other areas.
When energy requirements are lower or food availability is higher, animals need to carry lower energy reserves to avoid starvation (Lima 1986; Houston et al. 1993; Witter & Cuthill 1993). We therefore considered whether lower mass could be explained by less need for insulation and lower energy requirements due to differences in water temperature between sites where there was and was not bottlenose dolphin predation. We were able to discount this potential explanation because table 2a shows that despite the west coast of Scotland sites being the warmer water environment due to the Gulf Stream, the porpoises in those sites were heavier compared with our dolphin predation risk sites on the east coast rather than lighter as might otherwise be expected. The results in table 2b allowed us to rule out greater primary productivity providing more abundant food, and therefore requiring less stored energy reserves, in the dolphin predation sites. In the North Sea, primary productivity is higher in the southern areas (including along the east coast of England) than in the northern areas (including along the east coast of Scotland; Moll 1998). However, despite the resulting probable lower risk of starvation, and therefore needing to carry less energy reserves to avoid this risk, the porpoises are relatively heavier in the more productive southern region. The fact that our results remained significant even when we excluded all animals that had shown signs of starvation also allowed us to rule out a third alternative explanation; that is, lower prey availability in the predation area might have meant that porpoises were lighter simply because more starving animals were being measured. Ruling out another related alternative explanation, the results in table 2b suggest that competition with human fisheries for prey does not explain the findings of this study since there are no substantial differences in the level of fishing effort between the southern and northern North Sea (Jennings et al. 1999).
We were also able to discount the possibility of direct competition from bottlenose dolphins depriving the porpoises of sufficient feeding opportunities to build up energy reserves, because the bottlenose dolphin population in the area where dolphin predation occurs is estimated to be 110 and 174 individuals (Wilson et al. 1999) compared with many thousands of porpoises (Hammond et al. 2002). It would be impossible for so few bottlenose dolphins to have a large direct competitive effect on so many porpoises over such a wide area. Similarly, we can discount increased energetic costs from porpoises being directly chased by bottlenose dolphins preventing extra reserves being carried, because again there are too few bottlenose dolphins to be able to chase individual porpoises on a regular basis. It also seems unlikely that indirect competition between bottlenose dolphins and porpoises might somehow drive the result because both occur together in other parts of the study area (e.g. on the west coast of Scotland; S. Bannon 2006, personal communication), so it is only the predation behaviour that is unique to the predation factor in our models and not the presence of dolphins. It therefore seems reasonable to assume that the effect of the dolphins is a result of their predation on the porpoises.
The mere presence of a predator can change the prey's behaviour (e.g. Lima & Dill 1990), so we have considered whether there are any behavioural changes other than those predicted to be induced by a MDPR, that might also result in a reduction in mass reserves. Predators can influence growth of their prey, but this does not explain our results since the mass difference we find is a change in residual mass after controlling for the size of the animals. Reviews of the behavioural responses to predators suggest that MDPR is the only known way in which predators produce a reduction in the mass reserves of their prey (Witter & Cuthill 1993; Lima 1998a,b). The fact that our results remained significant even when we excluded all animals that had shown signs of starvation suggests it is unlikely that the predator's presence is producing a reduction in mass by forcing the porpoises to feed in suboptimal habitats where lower prey availability means there are more starving animals. Greater competition with other marine mammal species also seems unlikely to be able to drive the results since there is a higher biodiversity of marine mammal species on the west and north coasts of Scotland than along the east coast (Reid et al. 2003; MacLeod et al. 2005a).
Finally, on an unrelated possibility, we considered whether a genetically distinct porpoise population might exist in our predation area that was lighter than the rest of the population due to phenotypic reasons. However, harbour porpoises from around the coast of Scotland are relatively genetically similar (Walton 1997), suggesting that genetic substructuring of porpoise populations is unlikely to account for the observed effect.
Based on stranding data, 11% of porpoise mortality around Britain was due to starvation and the lethal porpoise–dolphin interactions that we use as our proxy for predation risk account for 64% of known harbour porpoise mortality where this behaviour occurs (Ross & Wilson 1996). With such a high mortality due to predation, porpoises will be experiencing a very strong pressure to adopt any adaptive behaviour that could reduce this mortality risk. Carrying less energy reserves should provide just such a benefit and figure 1 shows that the sample of harbour porpoises that died of sudden physical trauma, which are therefore probably the most representative of the normal healthy individuals in our study, was 1.5 kg lighter in the presence of dolphin predation risk than elsewhere. This equates to 4.4% of the mean mass of the porpoises in our study. As porpoises are known to need to consume a minimum of approximately 4% of their body mass daily to balance their energy budget (Kastelein et al. 1997), this represents a reduction of at least 1.1 days worth of energy reserves. This is a minimum estimate because it ignores the fact that food intake will not be converted to energy with 100% efficiency. Kastelein et al. (1997) estimated a maximum harbour porpoise life expectancy of 3 days without eating, due to death by hypothermia as blubber reserves are used up. Based on this, we can estimate that the porpoises are reducing their energy reserves by more than a third (37%) in response to dolphin predation and will consequently face a considerably higher risk of starvation when foraging conditions are poor. A 4.4% reduction in body mass is very similar to that found for birds in the original study of great tits and their main predator the sparrowhawk, which provided the first empirical evidence of MDPR (Gosler et al. 1995). That study showed the Wytham Woods population of great tits weighed on average 19.65 g at the start of the 1970s when their main predator was absent (due to organochlorine pesticide poisoning) and then showed a 0.9 g decrease in residual mass over the following decade as sparrowhawks re-established a breeding population in the area. This gives an effect size of a 4.6% decrease in mass compared with the 4.4% decrease in mass we find in the marine environment between areas where dolphin predation occurs and where it is absent. Although these are only two results and it is too early to draw a firm conclusion, it is interesting that the apparent effect size of MDPR is so similar in two very different environments and involving species very different in size and evolutionary history.
In conclusion, we suggest that the results of this study provide the first evidence that the starvation–predation risk trade-off is mass dependent in the marine environment and in animals other than birds. Given the fundamental nature of the starvation–predation risk trade-off in the lives of virtually all animals, the occurrence of MDPR in animals as different as small birds and porpoises suggests that mass-dependent behaviour is likely to play an important and previously unrecognized role in many parts of the animal kingdom. Although the study is correlative and needs to be supported by experimental evidence, it appears that the effect of lethal bottlenose dolphin attacks on harbour porpoises not only results in high direct mortality but is also likely to increase starvation mortality because it forces porpoises to stay thin.
Acknowledgments
Many thanks to all the observers throughout Britain who reported strandings over many years and allowed the data that form the basis of this study to be collected. Thanks also to the two anonymous referees whose suggestions allowed us to make significant improvements to this paper. Post-mortem examinations of UK-stranded cetaceans were conducted under contract to the UK Department for Environment, Food and Rural Affairs (Defra) as part of the UK Government's commitment to a number of international conservations agreements (e.g. ASCOBANS). Work by J.A.L. was funded by the BIOCET project. R.M. is supported by Glasgow University.
References
- Adriaensen F, Dhondt A.A, Van Dongen S, Lens L, Matthysen E. Stabilizing selection on blue tit fledgling mass in the presence of sparrowhawks. Proc. R. Soc. B. 1998;265:1011–1016. doi:10.1098/rspb.1998.0392 [Google Scholar]
- Bednekoff P.A, Houston A.I. Optimizing fat reserves over the entire winter—a dynamic-model. Oikos. 1994;71:408–415. doi:10.2307/3545828 [Google Scholar]
- Berta A, Sumich J.L. Academic Press; San Diego, CA: 1999. Marine mammals: evolutionary biology. [Google Scholar]
- Brodin A. Mass-dependent predation and metabolic expenditure in wintering birds: is there a trade-off between different forms of predation? Anim. Behav. 2001;62:993–999. doi:10.1006/anbe.2001.1844 [Google Scholar]
- Brooke M, Birkhead T. Cambridge University Press; Cambridge, UK: 1991. The Cambridge encycolpedia of ornithology. [Google Scholar]
- Cresswell W. Diurnal and seasonal mass variation in blackbirds Turdus merula: consequences for mass-dependent predation risk. J. Anim. Ecol. 1998;67:78–90. doi:10.1046/j.1365-2656.1998.00174.x [Google Scholar]
- Domenici P. The scaling of locomotory preformance in predator–prey encounters: from fish to killer whales. Comp. Biochem. Physiol. A. 2001;131:222–227. doi: 10.1016/s1095-6433(01)00465-2. [DOI] [PubMed] [Google Scholar]
- Fish F.E, Hurley J, Costa D.P. Maneuverability by the sea lion Zalophus californianus: turning performance of an unstable body design. J. Exp. Biol. 2003;206:667–674. doi: 10.1242/jeb.00144. doi:10.1242/jeb.00144 [DOI] [PubMed] [Google Scholar]
- Gentle L.K, Gosler A.G. Fat reserves and perceived predation risk in the great tit, Parus major. Proc. R. Soc. B. 2001;268:487–491. doi: 10.1098/rspb.2000.1405. doi:10.1098/rspb.2000.1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosler A.G. Strategy and constraint in the winter fattening response to temperature in the great tit Parus major. J. Anim. Ecol. 2002;71:771–779. doi:10.1046/j.1365-2656.2002.00642.x [Google Scholar]
- Gosler A.G, Greenwood J.J.D, Perrins C. Predation risk and the cost of being fat. Nature. 1995;377:621–623. doi:10.1038/377621a0 [Google Scholar]
- Hammond P.S, et al. Abundance of harbour porpoise and other cetaceans in the North Sea and adjacent waters. J. Appl. Ecol. 2002;39:361–376. doi:10.1046/j.1365-2664.2002.00713.x [Google Scholar]
- Heithaus M.R. Predator–prey and competitive interactions between sharks (order Selachii) and dolphins (suborder Odontoceti): a review. J. Zool. 2001;253:53–68. doi:10.1017/S0952836901000061 [Google Scholar]
- Houston A.I, McNamara J.M, Hutchinson J.M.C. General results concerning the trade-off between gaining energy and avoiding predation. Phil. Trans. R. Soc. B. 1993;341:375–397. doi:10.1098/rstb.1993.0123 [Google Scholar]
- Jennings S, Alvsvaga J, Cotter A.J.R, Ehrich S, Greenstreet S.P.R, Jarre-Teichmann A, Mergardt N, Rijnsdorp A.D, Smedstad O. Fishing effects in northeast Atlantic shelf seas: patterns in fishing effort, diversity and community structure. III. International trawling effort, in the North Sea: an analysis of spatial and temporal trends. Fish. Res. 1999;40:125–134. doi:10.1016/S0165-7836(98)00208-2 [Google Scholar]
- Jepson, P. D. 2006 Cetacean strandings investigation and co-ordination in the UK 2000–2004. Final report to Department for Food and Rural Affairs, pp. 1–79. See http://www.defra.gov.uk/wildlife-countryside/resprog/findings/index.htm
- Jepson P.D, Baker J.R. Bottlenosed dolphins (Tursiops truncatus) as a possible cause of acute traumatic injuries in porpoises (Phocoena phocoena) Vet. Rec. 1998;143:614–615. doi: 10.1136/vr.143.22.614. [DOI] [PubMed] [Google Scholar]
- Kastelein R.A, Hardemann J, Boer H. Food consumption and body weight of harbour porpoises (Phocoena phocoena) In: Read A.J, Wiepkema P.R, Nachtigall P.E, editors. The biology of the harbour porpoise. De Spil Publishers; Woerden, The Netherlands: 1997. pp. 217–234. [Google Scholar]
- Kullberg C, Fransson T, Jakobsson S. Impaired predator evasion in fat blackcaps (Sylvia atricapilla) Proc. R. Soc. B. 1996;263:1671–1675. doi:10.1098/rspb.1996.0244 [Google Scholar]
- Lima S.L. Predation risk and unpredictable feeding conditions—determinants of body-mass in birds. Ecology. 1986;67:377–385. doi:10.2307/1938580 [Google Scholar]
- Lima S.L. Nonlethal effects in the ecology of predator–prey interactions—what are the ecological effects of anti-predator decision-making? Bioscience. 1998a;48:25–34. doi:10.2307/1313225 [Google Scholar]
- Lima S.L. Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Adv. Study Behav. 1998b;27:215–290. [Google Scholar]
- Lima S.L, Dill L.M. Behavioral decisions made under the risk of predation—a review and prospectus. Can. J. Zool. -Revue Canadienne De Zoologie. 1990;68:619–640. [Google Scholar]
- Lind J, Fransson T, Jakobsson S, Kullberg C. Reduced take-off ability in robins (Erithacus rubecula) due to migratory fuel load. Behav. Ecol. Sociobiol. 1999;46:65–70. doi:10.1007/s002650050593 [Google Scholar]
- MacLeod R. Why does diurnal mass change not appear to affect alarmed flight performance? Anim. Behav. 2006;71:523–530. doi:10.1016/j.anbehav.2005.04.020 [Google Scholar]
- MacLeod C.D, Bannon S.M, Pierce G.J, Schweder C, Learmonth J.A, Reid R.J, Herman J.S. Climate change and the cetacean community of northwest Scotland. Biol. Conserv. 2005a;124:477–483. doi:10.1016/j.biocon.2005.02.004 [Google Scholar]
- Macleod R, Barnett P, Clark J.A, Cresswell W. Body mass change strategies in blackbirds Turdus merula: the starvation–predation risk trade-off. J. Anim. Ecol. 2005b;74:292–302. doi:10.1111/j.1365-2656.2005.00923.x [Google Scholar]
- Macleod R, Gosler A.G, Cresswell W. Diurnal mass gain strategies and perceived predation risk in the great tit Parus major. J. Anim. Ecol. 2005c;74:956–964. doi:10.1111/j.1365-2656.2005.00993.x [Google Scholar]
- Moll A.I. Regional distribution of primary production in the North Sea simulated by a three-dimensional model. J. Mar. Syst. 1998;16:151–170. doi:10.1016/S0924-7963(97)00104-8 [Google Scholar]
- Patterson I.A.P, Reid R.J, Wilson B, Grellier K, Ross H.M, Thompson P.M. Evidence for infanticide in bottlenose dolphins: an explanation for violent interactions with harbour porpoises? Proc. R. Soc. B. 1998;265:1167–1170. doi: 10.1098/rspb.1998.0414. doi:10.1098/rspb.1998.0414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin W.F, Wursig B, Thewissen J.G.M. Academic Press; San Diego, CA: 2002. Encycolpedia of marine mammals. [Google Scholar]
- Reid J.B, Evans P.G.H, Northridge S.P. Joint Nature Conservation Committee; Peterborough, UK: 2003. Atlas of cetacean distribution in north-west European waters. [Google Scholar]
- Rice D.W. The Society for Marine Mammalogy; San Francisco, CA: 1998. Marine mammals of the world: systematics and distribution. Special publication. [Google Scholar]
- Ross H.M, Wilson B. Violent interactions between bottlenose dolphins and harbour porpoises. Proc. R. Soc. B. 1996;263:283–286. doi: 10.1098/rspb.1998.0414. doi:10.1098/rspb.1996.0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS. SPSS, Inc; Chicago, IL: 2001. SPSS for windows. [Google Scholar]
- Walton M. Population structure of harbour porpoises Phocoena phocoena in the seas around the UK and adjacent waters. Proc. R. Soc. B. 1997;264:89–94. doi: 10.1098/rspb.1997.0013. doi:10.1098/rspb.1997.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B, Hammond P.S, Thompson P.M. Estimating size and assessing status of a coastal bottlenose dolphin population. Ecol. Appl. 1999;9:288–300. doi:10.1890/1051-0761(1999)009[0288:ESAATI]2.0.CO;2 [Google Scholar]
- Witter M.S, Cuthill I.C. The ecological costs of avian fat storage. Phil. Trans. R. Soc. B. 1993;340:73–92. doi: 10.1098/rstb.1993.0050. doi:10.1098/rstb.1993.0050 [DOI] [PubMed] [Google Scholar]
- Witter M.S, Cuthill I.C, Bonser R.H.C. Experimental investigations of mass-dependent predation risk in the European starling, Sturnus vulgaris. Anim. Behav. 1994;48:201–222. doi:10.1006/anbe.1994.1227 [Google Scholar]
- Young R.A. Fat, energy and mammalian survival. Am. Zool. 1976;16:699–710. [Google Scholar]