Abstract
Soil organisms influence plant species coexistence and invasion potential. Plant–soil feedbacks occur when plants change soil community composition such that interactions with that soil community in turn may positively or negatively affect the performance of conspecifics. Theories predict and studies show that invasions may be promoted by stronger negative soil feedbacks for native compared with exotic species. We present a counter-example of a successful invader with strong negative soil feedbacks apparently caused by host-specific, pathogenic soil fungi. Using a feedback experiment in pots, we investigated whether the relative strength of plant–soil feedbacks experienced by a non-native woody invader, Sapium sebiferum, differed from several native tree species by examining their performance in soils collected near conspecifics (‘home soils’) or heterospecifics (‘away soils’) in the introduced range. Sapium seedlings, but no native seedlings, had lower survival and biomass in its home soils compared with soils of other species (‘negative feedback’). To investigate biotic agents potentially responsible for the observed negative feedbacks, we conducted two additional experiments designed to eliminate different soil taxa (‘rescue experiments’). We found that soil sterilization (pot experiment) or soil fungicide applications (pot and field experiments) restored Sapium performance in home soil thereby eliminating the negative feedbacks we observed in the original experiment. Such negative feedbacks apparently mediated by soil fungi could have important effects on persistence of this invader by limiting Sapium seedling success in Sapium dominated forests (home soils) though their weak effects in heterospecific (away) soils suggest a weak role in limiting initial establishment.
Keywords: biological invasions, Chinese tallow tree, pathogen accumulation, persistence, plant–soil feedbacks
1. Introduction
Interspecific interactions play an important role in mediating plant species coexistence and maintaining local biodiversity (Callaway & Maron 2006). Ecologists are increasingly aware that soil organisms may influence plant communities because they are involved in many processes crucial to plant survival and performance (e.g. Klironomos 2002; Bever 2003; van der Putten et al. 2005; Kardol et al. 2006; Levine et al. 2006; Reinhart & Callaway 2006). Moreover, the composition of the soil biota may in turn be affected by plant species. When plant species vary in their interactions with soil organisms, these interactions may cause species-specific feedbacks that in turn influence the future relative performances of multiple plant species (Bever et al. 1997). Positive or negative soil feedbacks occur when a plant promotes a soil community that in turn benefits or inhibits conspecific plant performance compared with heterospecifics, respectively. Differences in interactions between native versus exotic plants and resident soil organisms may have an important role in biological invasions and ultimately the persistence of exotic species (Callaway et al. 2004; Wolfe & Klironomos 2005; Levine et al. 2006).
Native and exotic plant species may differ in their interactions with the soil biota in the introduced range owing to differences in coevolutionary time scale (Richardson et al. 2000). When exotic species readily form associations with soil organisms in the introduced range (i.e. soil mutualists), it may facilitate invasion (Callaway et al. 2001, 2004). Further, novel interactions between invading plants and soil organisms in the introduced range could produce extreme benefits that generate strong positive feedbacks for the invader when these benefits are larger than those received by native plants (Beckstead & Parker 2003; Carey et al. 2004). However, interactions between invaders and soil organisms in the introduced range could also produce extreme costs (i.e. soil pathogens), which may help explain the failure of some plant invasions. This may occur when plant–soil feedbacks dominate early in the invasion process or may lead to a transient abundance of the invading species when feedbacks develop later in the invasion process. Accumulation or lack of host-specific soil-borne pathogens in the introduced range may play a role in the failure or success of exotic species' establishment and spread in the introduced range (Reinhart et al. 2003; Callaway et al. 2004; Knevel et al. 2004; Reinhart & Callaway 2004). To date, all studies comparing the relative strengths of negative feedbacks on co-occurring exotic and native plant species have shown stronger, more negative feedbacks for native species (Klironomos 2002; Callaway et al. 2004), suggesting that the soil biota increases the success of invading species.
This study had two goals. Using a feedback experiment with the woody invader Sapium sebiferum (Chinese tallow tree) and four co-occurring native tree species, we investigated whether soil feedbacks experienced by an invading species differed from multiple native plant species in the introduced range. In addition, we used field and pot rescue experiments to investigate biotic agents responsible for the negative feedbacks.
2. Material and methods
(a) Study site
This study used soils collected from the Big Thicket National Preserve (BTNP) in east Texas, USA. All the native trees used in this study, Quercus nigra (Water Oak, Fagaceae), Acer rubrum (Red Maple, Aceraceae), Liquidambar styraciflua (Sweetgum, Hamamelidaceae) and Pinus taeda (Loblolly Pine, Pinaceae), are common species in BTNP. Currently, mesic and flood plain forests of BTNP are heavily invaded by the exotic species S. sebiferum (Chinese tallow tree, Euphorbiaceae, synonyms include Croton sebiferum, Triadica sebifera and Stillingia sebifera; Harcombe et al. 1999). In BTNP, Sapium has steadily increased in abundance over the past 20 years. In some areas, it has become the most abundant understory tree, and is becoming increasingly common in the overstory.
(b) Feedback experiment
To test whether plant–soil feedbacks differed between the invader S. sebiferum and multiple native tree species, we grew each plant species in soil collected near conspecifics (‘home soil’) versus the other plant species (‘away soil’).
In June 2005, a 12-week (post-germination) pot study was conducted to test soil community feedbacks on seedling survival and performance using soil and roots collected at BTNP from under adult trees of the four natives (A. rubrum, L. styraciflua, P. taeda and Q. nigra) and the invader S. sebiferum. The entire experiment was a full factorial randomized block design with two rounds (block), two inoculum sources (whole soil versus roots only), five tree species as seedlings, six soil origins (five tree species and sterilized control (autoclaved)) and four replications (N=480).
Soil inoculum pots received fresh (active) soil and roots collected from beneath a particular tree species, whereas root inoculum pots received only fresh roots (root inoculum). The remainder of each pot was filled with homogenized, autoclaved soil and roots (inactive) from the remaining four species in the study to control for nutrient differences between the different soil types. Seedlings were planted in pots containing 1/5 active inoculum from under one tree species and 4/5 inactive inoculum from under the remaining four species (i.e. 1/5 from each). For the sterilized treatment, seedlings were planted in pots containing inactive inoculum from all five species.
All native seeds were purchased from the Louisiana Forest Seed Company (Lecompte, LA). Sapium seeds were collected from individual trees in Saratoga and Houston, TX. All seeds were treated with a 10% bleach rinse to remove surface contaminants and were germinated in batches on filter paper with perlite and watered daily with distilled water. At the beginning of each round (round 1—June and round 2—September 2005), soil and root inoculum were collected at BTNP from 6 to 10 parent trees of each species located in the Lance Rosier and Pine Island Bayou units. Soil was collected from below the canopy of individual parent trees of each species no more than 45 cm from the tree base. Two or three holes were dug around individual parent trees to a depth of 8–10 cm for soil collection. All soil and roots not used as fresh (active) inoculum were sterilized (twice autoclaved for 1.5 hours at 121°C). Autoclaved soil and roots for each species were then homogenized by passing through a 1 cm screen sieve to control for nutrient differences between soils of different species. Roots were cut into 1–2 cm fragments and homogenized in with the soil. Soil or roots used as fresh (active) inoculum were homogenized within a species (i.e. Sapium inoculum from Lance Rosier and Pine Island) to control for potential site differences. Root inoculum (fresh roots only) studies used a soil microbial backwash to control for the loss of the non-fungal soil community. The background microbial community was manipulated by mixing rinse water from all five root types and passing it through a 38 μm sieve (Johnson 1993).
At the beginning of each round, germinated seeds were planted directly into 410 ml Deepots (Stuewe & Sons, Corvallis, OR) and were randomized and periodically rotated. All species used in this experiment were germinating simultaneously at the beginning of each round and were the same ages during each round of the experiment. Plants were watered as needed and survival was checked weekly. At the end of each round, plants were harvested and aboveground and belowground dry masses were measured.
All species in this study are known to be endo- and/or ectomycorrhizal under field conditions (Nijjer et al. 2004). At each harvest, we randomly selected seedlings to analyse for endomycorrhizal (Acer, Liquidambar, Quercus and Sapium) or ectomycorrhizal colonization rates (Pinus and Quercus) using standard methodology (e.g. Bray et al. 2003; Callaway et al. 2003). However, when analysed by ANOVA, ecto- and endomycorrhizal colonization rates were independent of every factor (all p>0.11) and are not reported in detail here but can be obtained elsewhere (Nijjer 2006).
We used full factorial ANOVAs (type III sums of squares) to test the dependence of survival time, aboveground mass (log transformed) and belowground mass (log transformed) on experimental treatments. For this analysis, the soil origin factor was tested with three levels: as home (‘same species’—conspecific), away (‘different species’—heterospecific) and control (‘inactive’—sterilized). Fisher's LSD post hoc tests evaluated differences among treatment levels for significant main effects. Adjusted least-squares means post hoc tests were conducted to test for differences among treatment levels for significant interactions. We used SAS (SAS Institute, Cary, NC) for all analyses. Unless noted otherwise, analyses were balanced.
We conducted additional analyses to increase our confidence in the ANOVA results. Because survival time did not closely fit a normal distribution, it was ranked and the ANOVA was repeated (Conover & Iman 1981). The significance of every term in this analysis matched that in the first set of analyses. Because the analyses for mass were unbalanced (i.e. not all seedlings survived) and the significance of interaction terms can be sensitive to term inclusion in unbalanced designs, we repeated these analyses using sequential ANOVAs (type I sums of squares). The two three-way interactions that were significant for aboveground mass in the simultaneous ANOVA were not significant in the sequential ANOVA, but the significance of all other terms remained the same.
Because species by treatment interactions were significant, individual species' three-way factorial ANOVAs were conducted to isolate effects by species. The soil origin factor was tested as described above (‘home’ versus ‘away’ versus ‘control’), as well as analysed as individual soil sources (five species and sterilized control) to ensure that the away soil response was not masking extremely high or low survival in soil originating from any one individual species.
(c) Pot rescue experiment
In May 2006, a 10-week pot study was conducted to test what biotic agents caused the observed negative feedback experienced by Sapium in home soils in feedback experiment (figure 2b). We term this study a ‘pot rescue experiment’ because the taxa whose elimination restores Sapium's survival and growth are likely to be responsible for Sapium's poor survival and growth in its own soils. We included seedlings from China to test whether Sapium's vulnerability to negative soil feedbacks differed between the native and non-native range because there is variation in herbivore resistance between the native and introduced range (Siemann & Rogers 2003b,c).
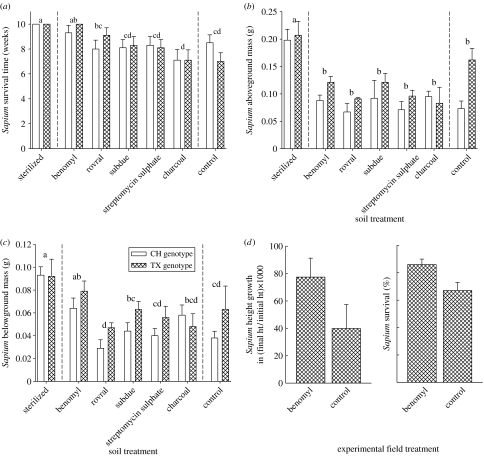
Figure 2.
The (a) survival, (b) aboveground and (c) belowground biomass of Sapium in conspecific treated soils (pot rescue experiment) and conspecific soils that received no treatment. White and hatched bars are China (CH) and Texas (TX) seedling origins, respectively. Letters denote treatments that did not differ in means contrast tests. +1 s.e. presented. (d) The growth and survival of Sapium in conspecific field soils (field rescue experiment).
The experiment consisted of 140 pots in a full factorial randomized design with seven treatment applications (control [target: untreated response], sterilized [target: active soil community], charcoal [target: allelochemicals], rovral fungicide [active ingredient: iprodione, target: pathogenic asco- and basidiomycete fungi], benomyl fungicide [active ingredient: benomyl, target: pathogenic asco- and basidiomycete fungi], subdue Maxx fungicide [active ingredient: mefenoxam, target: oomycete fungi] and Ferti-lome bactericide [active ingredient: streptomycin sulphate, target: soil bacterial pathogens]), two Sapium seedling origins (Texas or China) and 10 replications. Two fungicides were used to target pathogenic asco- and basidiomycete fungi because they included different fungal species on their product labels.
Soil was collected from beneath 10–15 parent trees in a 35-year-old Sapium monoculture at the University of Houston Coastal Center located 72 km south of Houston (Siemann & Rogers 2006). All soils were homogenized. Soils in the sterilized treatment were autoclaved at 121°C for 1.5 hours.
Texas Sapium seeds were collected from individual trees in Saratoga and Houston, TX (16 trees in total). Chinese Sapium seeds were collected from the Jiangsu (two trees), Zhejiang (three trees) and Anhui (three trees) provinces in east central China in the north of Sapium's native range. These provinces are probable sources of Sapium introductions in Texas (Dewalt et al. 2006). Chinese seedlings were distributed in a 3 : 4 : 3 ratio of the Jiangsu, Zhejiang and Anhui provinces for each treatment. Seeds were germinated in potting soil in the greenhouse on the Rice University campus in Houston, TX. Seedlings of the same size and age were treated with a 10% bleach rinse to remove surface contaminants prior to transplantation into 410 ml Deepots. Plants were watered, rotated and randomized. Survival was checked weekly. At the end of the experiment, dry aboveground and belowground masses were measured.
We used ANOVAs to examine the survival, aboveground mass and belowground mass using the same approach as in the feedback experiment (figure 2b). The same terms were significant for both ANOVAs for survival (i.e. time versus ranked) and aboveground and belowground masses (i.e. simultaneous versus sequential ANOVAs).
(d) Field rescue experiment
Owing to the success of eliminating Sapium's negative feedbacks in home soils with benomyl fungicide, we conducted a second ‘rescue’ experiment to verify that soil fungal suppression would increase Sapium seedling survival in field conditions.
Plots were established in the 35-year-old Sapium monoculture no more than 1–1.5 m from parent trees (12 0.25 m2 plots). Each week, benomyl fungicide was applied at a rate of 1.33 g m−2 as a soil drench in six plots. Controls received an equivalent amount of distilled water. Survival of all Sapium seedlings in each plot was recorded at the start of the experiment and each week thereafter. Within each plot, four randomly chosen first year Sapium seedlings were tagged for individual aboveground measurement. The height of these seedlings was measured at the start and end of the experiment. At the end of the experiment, we clipped these seedlings at ground level and measured dry aboveground biomass.
We used ANOVA to test whether the survival percentage of naturally occurring seedlings (arcsine transformed) depended on treatment. We repeated this analysis with ranked data and obtained the same result. We used an ANOVA with a term for treatment and one for plot nested within treatment to examine the dependence of height growth (ln[end/start]) and aboveground biomass (log transformed) on benomyl application. We nested plot within the treatment term for biomass measurements to ensure that significance was not caused by the pseudoreplication of conspecific seedlings within plots.
3. Results
Soil communities that developed in the vicinity of different tree species had strong, species-specific effects on tree seedling survival and aboveground biomass production in this temperate forest community. Species differed in survival (figure 1a) and growth (figure 1b, aboveground mass; belowground mass), but there were no general, overall positive or negative soil feedbacks or inoculum type main effects (table 1). However, species varied in their feedbacks (species×soil origin term). Because species was a significant factor in multiple interaction terms (table 1), individual species analyses were conducted to identify the species driving significant species by experimental treatment interaction terms.
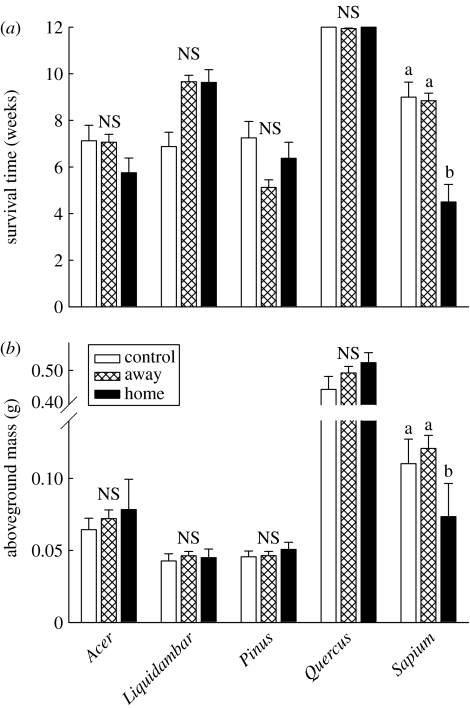
Figure 1.
The effect of soil origin on the (a) survival and (b) aboveground biomass of each species (feedback experiment). +1 s.e. are presented. White, hatched and black bars are control (sterilized), away (heterospecific) and home (conspecific) soil origins, respectively. Letters denote treatments that did not differ in within-species means contrast tests (α=0.05). NS indicates a lack of significant within-species differences.
Table 1.
ANOVA results of the feedback experiment: survival and aboveground and belowground biomass. (Significant results are shown in bold.)
| terms | df | survival | aboveground biomass | belowground biomass | |||
|---|---|---|---|---|---|---|---|
| Fx,360 | p | Fx,190 | p | Fx,190 | p | ||
| round | 1 | 0.6 | 0.46 | 1.4 | 0.24 | 1.2 | 0.28 |
| species | 4 | 17.0 | <0.0001 | 238.4 | <0.0001 | 304.4 | <0.0001 |
| inoculum | 1 | 0.2 | 0.69 | 0.2 | 0.66 | 1.8 | 0.18 |
| soil origin | 5 | 1.2 | 0.29 | 1.8 | 0.16 | 2.1 | 0.13 |
| round×species | 4 | 3.0 | <0.05 | 3.3 | <0.05 | 4.5 | <0.01 |
| round×inoculum | 1 | 2.4 | 0.12 | 0.1 | 0.79 | 2.1 | 0.15 |
| round×soil origin | 5 | 0.5 | 0.62 | 0.4 | 0.67 | 0.6 | 0.53 |
| species×inoculum | 4 | 1.1 | 0.36 | 2.2 | 0.08 | 2.3 | 0.06 |
| species×soil origin | 20 | 2.5 | <0.05 | 1.9 | 0.06 | 0.9 | 0.53 |
| inoculum×soil origin | 5 | 0.7 | 0.48 | 1.9 | 0.15 | 2.1 | 0.13 |
| round×species×inoculum | 4 | 1.0 | 0.40 | 2.7 | <0.05 | 0.4 | 0.84 |
| round×species×soil origin | 20 | 3.1 | <0.01 | 1.3 | 0.23 | 1.2 | 0.29 |
| round×inoculum×soil origin | 5 | 1.2 | 0.31 | 4.7 | <0.05 | 1.4 | 0.25 |
| species×inoculum×soil origin | 20 | 0.7 | 0.69 | 1.5 | 0.16 | 1.1 | 0.38 |
| round×species×inoculum×soil origin | 20 | 0.8 | 0.60 | 4.3 | <0.01 | 1.8 | 0.13 |
| model | 119 | 3.4 | <0.001 | 29.0 | <0.0001 | 38.1 | <0.0001 |
Only Sapium experienced negative feedbacks in its (‘home’) soil community (both root and soil inoculum) that significantly decreased growth and survival (figure 1). No other species' survival was significantly affected by soil feedbacks (i.e. soil origin terms were not significant). Sapium had a significant soil origin effect for survival and growth (survival: F2,84=4.5, p<0.05; aboveground mass: F2,51=3.3, p<0.05; figure 1). Means contrast tests showed that survival and aboveground mass in home soil was significantly lower than in away or control (sterilized) soils (figure 1a). Sapium survival in soils of individual species showed a significant individual source effect (F5,72=3.7, p<0.01) driven by lower survival rates of Sapium seedlings in Sapium soils compared with that in soils of other species. No other species had a significant parent source effect or a significant survival decrease in Sapium soils. These results implicate a biotic factor in the soil that is more abundant in soils beneath Sapium trees and that strongly affects only Sapium seedlings.
In the pot rescue experiment, seven treatment applications targeted different biotic agents that could be responsible for observed negative feedbacks on Sapium. Survival time, aboveground mass and belowground mass depended on soil treatment (table 2). For survival time and belowground mass, benomyl fungicide and sterilized treatments were the only ones significantly greater than the control treatment in means contrasts tests (figure 2a,c). For aboveground biomass, only sterilization was significantly greater than the control treatment in means contrasts tests (figure 2b). We tested two Sapium genotypes (Texas and China) to test whether Sapium's vulnerability to negative soil feedbacks differed between the native and non-native ranges. Texas origin seedlings were larger than China ones, but survival did not vary (table 2). There was no interaction between genotype origin and soil treatment indicating that seedlings from populations in the native and introduced ranges did not differ in their susceptibility to fungal pathogens.
Table 2.
ANOVA results of the pot rescue experiment: survival and aboveground and belowground biomass. (Significant results are shown in bold.)
| df | survival | aboveground biomass | belowground biomass | ||||
|---|---|---|---|---|---|---|---|
| Fx,108 | p | Fx,74 | p | Fx,74 | p | ||
| model | 12 | 2.9 | <0.01 | 7.2 | <0.0001 | 5.3 | <0.0001 |
| treatment | 5 | 5.4 | <0.0001 | 12.3 | <0.0001 | 9.0 | <0.0001 |
| region | 2 | <0.1 | 0.90 | 15.1 | <0.001 | 5.9 | <0.01 |
| treatment×region | 5 | 0.9 | 0.52 | 1.3 | 0.26 | 1.6 | 0.17 |
In the field rescue experiment, Sapium seedling survival increased with suppression of fungal pathogens with benomyl fungicide (F1,10=5.0, p<0.05; figure 2d). The model for height growth was not significant (model F11,36=1.1, p=0.36) but the average growth rate of Sapium seedlings tended to be faster in benomyl fungicide soil compared with water controls (figure 2d). The model for aboveground mass was significant (model F11,36=3.5, p<0.01), but mass only varied with plot (F10,36=3.8, p<0.01) and not benomyl treatment (F1,36=0.1, p=0.72). Together the results of these two experiments implicate fungal pathogens as the probable cause of the strong negative soil feedback for Sapium.
4. Discussion
The effect of the soil community was not equal across plant species and has the potential to influence tree species composition. However, contrary to predictions, the negative soil feedbacks experienced by Sapium suggest that strongly negative soil communities hinder, rather than assist, Sapium's growth and persistence in sites where it has previously established (table 1 and figure 1). Although the range of soil feedbacks for exotics was expected to be larger than for natives owing to their shorter coevolutionary history with the soil biotic community, it was surprising that an invader as successful as Sapium was at the negative end of the range for the species tested here. However, it is consistent with low per capita success of Sapium seedlings compared with native seedlings in Sapium dominated forests (Siemann & Rogers 2006).
In primary invasions into prairie and forests, Sapium seedlings have comparable survival rates to native tree seedlings and much higher growth rates (Siemann & Rogers 2003a). In contrast, in the understory of first generation Sapium forests, such as the setting for the field study here, the pattern of seedling survival is very different despite similar light levels and soil types. Sapium seedlings have a 3-year survival rate that is 300-fold lower than that of native tree seedlings that leads to extremely poor recruitment of Sapium in such forests (Siemann & Rogers 2006). In fact, experimental additions of native tree seeds are sufficient to create a native tree dominated seedling community with these large differences in seedling survival rates (Siemann & Rogers 2006). So, these results together with the strong, significant increases in Sapium seedling survival with soil fungicide application that we report here in the field rescue experiment (figure 2d) and with soil sterilization or with soil fungicide in the pot rescue experiment (figure 2a) indicate that strongly negative soil feedbacks by inhibitory soil fungi may limit Sapium's ability to establish persistent monocultures. Indeed, such poor survival of exotic trees compared with native trees in the understory of forests dominated by conspecific primary invaders has been documented for other invasions (Lugo 2004). Moreover, in the case of Sapium, the greatest impact of aboveground herbivores and foliar fungal pathogens is also found under Sapium adults (Siemann & Rogers 2006), which suggests that aboveground and belowground enemies may have an additive effect on Sapium seedling survival in the introduced range.
Only a few prior studies have documented decreased growth and survival of an invading species due to negative soil feedbacks in the introduced range and none have shown more strongly negative feedbacks compared with co-occurring native species. The beach grass, Ammophila arenaria, had negative feedbacks in introduced California (Beckstead & Parker 2003) and RSA (Knevel et al. 2004) soils, but in RSA only a few sites exhibited pathogenic activity. The diversity of specialist nematodes feeding on native plants is greater than that of those feeding on A. arenaria in its introduced ranges (van der Putten et al. 2005), which suggests that soil nematode feedbacks may also be stronger on native plants. Acer spp. have negative feedbacks with soil communities of congeners in the introduced range (Reinhart & Callaway 2004). However, the positive feedback effects of the soil community Acer experienced under different plant species in the introduced range (negative effect of soil sterilization) enhanced its biomass and height growth (Reinhart & Callaway 2004), perhaps promoting invasion. Sapium experienced decreased survival and biomass in home soils (positive effect of sterilization relative to home soil) but did not experience an increased growth or survival response in soils of native plant species compared with sterilized soils (a negative effect of sterilization relative to away soil) in the introduced range (figure 1). Unlike Acer spp., this suggests that Sapium does not experience a beneficial effect from the soil biotic community under the native tree species used in this study, perhaps in part due to the lack of congeners or confamilial trees in the introduced range and taxonomically restricted associations.
Decreased survival of Sapium seedlings in home soil compared with soils of native tree species suggests accumulation of high densities of host-specific soil pathogens beneath Sapium adults. This is consistent with the Janzen–Connell hypothesis that predicts reduced offspring success beneath parent trees caused by the accumulation of distance- and/or density-dependent mortality factors like host-specific soil pathogens (Janzen 1970; Connell 1978; Augspurger 1983; Packer & Clay 2000, 2003). A number of fungal pathogens are known to associate with Sapium in the introduced range (Alfieri et al. 1994). Sapium's success with benomyl fungicide compared with rovral fungicide suggests (figure 2a,c) that they are selectively targeting different soil fungal pathogens, which may help determine taxa responsible for negative feedbacks we observed. Experiments in the native range of Sapium would bring additional insights into the role of soil pathogens in its success.
The similar responses of Sapium in both soil and root inoculum in the feedback experiment (figure 2b) suggest that the effects of beneficial mutualists were not strong enough to influence negative feedback responses. Sapium can be endomycorrhizal in field conditions and can have high levels of mycorrhizal colonization corresponding to increased aboveground biomass when planted in homogenized, sieved field soil in greenhouse studies (Nijjer et al. 2004). However, no significant correlations between Sapium aboveground and belowground biomass and beneficial mycorrhizal mutualists were found here. There is no evidence of allelopathy decreasing Sapium survival under parent trees (Conway et al. 2002; figure 2a,c).
The feedbacks exerted by soil communities on exotic species can have important consequences for establishment and persistence of invading species. Sapium sebiferum, a successful invader in southeastern US temperate forests and coastal prairies, displays highly negative feedback responses to conspecific soil communities that may decrease its longer-term persistence in invaded communities by limiting self-replacement. Our results, consistent across three experiments, provide evidence that Sapium does not escape from belowground natural enemies in the introduced range for multiple generations in a single location. The next step is to understand what causes the soil biota to facilitate some invasions in some conditions or stages of invasions, but to reduce the success of exotic plants in other conditions. Because invasive species are such an important problem and the soil biota can have such strong effects, this has the potential to increase our ability to predict future invaders and manage current problem species.
Acknowledgments
The research was supported by a Wray Todd Fellowship and Autrey Fellowship to S.N. and support from NSF to E.S. and W.E.R. We thank: Big Thicket National Preserve and University of Houston Coastal Center for access; R. Callaway for charcoal; C. LaMaur, D. Mee, J. Nijjer, J. Orr, M. Rua and I. White for assistance; P. Avis, A. Tuininga, N. Kleczewski, A. Antoninka and S. Bray for training in mycorrhizal identification and procedures; and K. Clay, J. Rudgers, N. Holland and T. Jones for comments.
References
- Alfieri S.A, Langdon K.R, Kimbrough J.W, El-Gholl N.E, Whelberg C, editors. Diseases and disorders of plants in Florida. Division of Plant Industry, Florida Department of Agriculture and Consumer Services; Gainesville, FL: 1994. p. 1114. [Google Scholar]
- Augspurger C.K. Seed dispersal of the tropical tree Platypodium elegans, and the escape of its seedlings from fungal pathogens. J. Ecol. 1983;71:759–771. doi:10.2307/2259591 [Google Scholar]
- Beckstead J, Parker I.M. Invasiveness of Ammophila arenaria: release from soil-borne pathogens? Ecology. 2003;84:2824–2831. doi:10.1890/02-0517 [Google Scholar]
- Bever J.D. Soil community feedback and the coexistence of competitors: conceptual frameworks and empirical tests. New Phytol. 2003;157:465–473. doi: 10.1046/j.1469-8137.2003.00714.x. doi:10.1046/j.1469-8137.2003.00714.x [DOI] [PubMed] [Google Scholar]
- Bever J.D, Westover K.M, Antonovics J. Incorporating the soil community into plant population dynamics: the utility of the feedback approach. J. Ecol. 1997;85:561–573. doi:10.2307/2960528 [Google Scholar]
- Bray S.R, Kitajima K, Sylvia D.M. Mycorrhizae differentially alter growth, physiology, and competitive ability of an invasive shrub. Ecol. Appl. 2003;13:565–574. doi:10.1890/1051-0761(2003)013[0565:MDAGPA]2.0.CO;2 [Google Scholar]
- Callaway R.M, Maron J.L. What have exotic plant invasions taught us over the past 20 years? Trends Ecol. Evol. 2006;21:369–374. doi: 10.1016/j.tree.2006.04.008. doi:10.1016/j.tree.2006.04.008 [DOI] [PubMed] [Google Scholar]
- Callaway R.M, Newingham B, Zabinski C.A, Mahall B.E. Compensatory growth and competitive ability of an invasive weed are enhanced by soil fungi and native neighbours. Ecol. Lett. 2001;4:429–433. doi:10.1046/j.1461-0248.2001.00251.x [Google Scholar]
- Callaway R.M, Mahall B.E, Wicks C, Pankey J, Zabinski C. Soil fungi and the effects of an invasive forb on grasses: neighbor identity matters. Ecology. 2003;84:129–135. doi:10.1890/0012-9658(2003)084[0129:SFATEO]2.0.CO;2 [Google Scholar]
- Callaway R.M, Thelen G.C, Rodriguez A, Holben W.E. Soil biota and exotic plant invasion. Nature. 2004;427:731–733. doi: 10.1038/nature02322. doi:10.1038/nature02322 [DOI] [PubMed] [Google Scholar]
- Carey E.V, Marler M.J, Callaway R.M. Mycorrhizae transfer carbon from a native grass to an invasive weed: evidence from stable isotopes and physiology. Plant Ecol. 2004;172:133–141. doi:10.1023/B:VEGE.0000026031.14086.f1 [Google Scholar]
- Connell J.H. Diversity in tropical rain forests and coral reefs—high diversity of trees and corals is maintained only in a non-equilibrium state. Science. 1978;199:1302–1310. doi: 10.1126/science.199.4335.1302. doi:10.1126/science.199.4335.1302 [DOI] [PubMed] [Google Scholar]
- Conover W.J, Iman R.L. Rank transformations as a bridge between parametric and nonparametric statistics. Am. Stat. 1981;35:124–129. doi:10.2307/2683975 [Google Scholar]
- Conway W.C, Smith L.M, Bergan J.F. Potential allelopathic interference by the exotic Chinese tallow tree (Sapium sebiferum) Am. Midl. Nat. 2002;148:43–53. doi:10.1674/0003-0031(2002)148[0043:PAIBTE]2.0.CO;2 [Google Scholar]
- Dewalt S.J, Siemann E, Rogers W.E. Microsatellite markers for an invasive tetraploid tree Chinese tallow (Triadica sebifera) Mol. Ecol. Notes. 2006;6:505–507. doi:10.1111/j.1471-8286.2006.01296.x [Google Scholar]
- Harcombe P.A, Hall R.B.W, Glitzenstein J.S, Cook E.S, Krusic P, Fulton M. Sensitivity of Gulf Coast forests to climate change. In: Gunterspergen G, Varain B.A, editors. Vulnerability of coastal wetlands in the southeastern United States: climate change research results. Biological science report USGS/BRD/BSR-1998-0002. National Wetlands Research Center; Lafayette, LA: 1999. pp. 47–67. [Google Scholar]
- Janzen D.H. Herbivores and number of tree species in tropical forests. Am. Nat. 1970;104:501–528. doi:10.1086/282687 [Google Scholar]
- Johnson N.C. Can fertilization of soil select less mutualistic mycorrhizae. Ecol. Appl. 1993;3:749–757. doi: 10.2307/1942106. doi:10.2307/1942106 [DOI] [PubMed] [Google Scholar]
- Kardol P, Bezemer T.M, van der Putten W.H. Temporal variation in plant–soil feedback controls succession. Ecol. Lett. 2006;9:1080–1088. doi: 10.1111/j.1461-0248.2006.00953.x. doi:10.1111/j.1461-0248.2006.00953.x [DOI] [PubMed] [Google Scholar]
- Klironomos J.N. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature. 2002;417:67–70. doi: 10.1038/417067a. doi:10.1038/417067a [DOI] [PubMed] [Google Scholar]
- Knevel I.C, Lans T, Menting F.B.J, Hertling U.M, van der Putten W.H. Release from native root herbivores and biotic resistance by soil pathogens in a new habitat both affect the alien Ammophila arenaria in South Africa. Oecologia. 2004;141:502–510. doi: 10.1007/s00442-004-1662-8. doi:10.1007/s00442-004-1662-8 [DOI] [PubMed] [Google Scholar]
- Levine J.M, Pachepsky E, Kendall B.E, Yelenik S.G, Lambers J.H.R. Plant–soil feedbacks and invasive spread. Ecol. Lett. 2006;9:1005–1014. doi: 10.1111/j.1461-0248.2006.00949.x. doi:10.1111/j.1461-0248.2006.00949.x [DOI] [PubMed] [Google Scholar]
- Lugo A.E. The outcome of alien tree invasions in Puerto Rico. Front. Ecol. Environ. 2004;2:265–273. doi:10.1890/1540-9295(2004)002[0265:TOOATI]2.0.CO;2 [Google Scholar]
- Nijjer, S. 2006 Understanding belowground community regulation in an invaded forest. PhD thesis, Rice University, Houston.
- Nijjer S, Rogers W.E, Siemann E. The effect of mycorrhizal inoculum on the growth of five native tree species and the invasive Chinese tallow tree (Sapium sebiferum) Tex. J. Sci. 2004;56:357–368. [Google Scholar]
- Packer A, Clay K. Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature. 2000;404:278–281. doi: 10.1038/35005072. doi:10.1038/35005072 [DOI] [PubMed] [Google Scholar]
- Packer A, Clay K. Soil pathogens and Prunus serotina seedling and sapling growth near conspecific trees. Ecology. 2003;84:108–119. doi:10.1890/0012-9658(2003)084[0108:SPAPSS]2.0.CO;2 [Google Scholar]
- Reinhart K.O, Callaway R.M. Soil biota facilitate exotic Acer invasions in Europe and North America. Ecol. Appl. 2004;14:1737–1745. doi:10.1890/03-5204 [Google Scholar]
- Reinhart K.O, Callaway R.M. Soil biota and invasive plants. New Phytol. 2006;170:445–457. doi: 10.1111/j.1469-8137.2006.01715.x. doi:10.1111/j.1469-8137.2006.01715.x [DOI] [PubMed] [Google Scholar]
- Reinhart K.O, Packer A, Van der Putten W.H, Clay K. Plant–soil biota interactions and spatial distribution of black cherry in its native and invasive ranges. Ecol. Lett. 2003;6:1046–1050. doi:10.1046/j.1461-0248.2003.00539.x [Google Scholar]
- Richardson D.M, Allsopp N, D'Antonio C.M, Milton S.J, Rejmanek M. Plant invasions—the role of mutualisms. Biol. Rev. 2000;75:65–93. doi: 10.1017/s0006323199005435. doi:10.1017/S0006323199005435 [DOI] [PubMed] [Google Scholar]
- Siemann E, Rogers W.E. Herbivory, disease, recruitment limitation, and success of alien and native tree species. Ecology. 2003a;84:1489–1505. doi:10.1890/0012-9658(2003)084[1489:HDRLAS]2.0.CO;2 [Google Scholar]
- Siemann E, Rogers W.E. Increased competitive ability of an invasive tree may be limited by an invasive beetle. Ecol. Appl. 2003b;13:1503–1507. doi:10.1890/03-5022 [Google Scholar]
- Siemann E, Rogers W.E. Reduced resistance of invasive varieties of the alien tree Sapium sebiferum to a generalist herbivore. Oecologia. 2003c;135:451–457. doi: 10.1007/s00442-003-1217-4. [DOI] [PubMed] [Google Scholar]
- Siemann E, Rogers W.E. Recruitment limitation, seedling performance and persistence of exotic tree monocultures. Biol. Invas. 2006;8:979–991. doi:10.1007/s10530-005-0825-9 [Google Scholar]
- van der Putten W.H, Yeates G.W, Duyts H, Reis C.S, Karssen G. Invasive plants and their escape from root herbivory: a worldwide comparison of the root-feeding nematode communities of the dune grass Ammophila arenaria in natural and introduced ranges. Biol. Invas. 2005;7:733–746. doi:10.1007/s10530-004-1196-3 [Google Scholar]
- Wolfe B.E, Klironomos J.N. Breaking new ground: soil communities and exotic plant invasion. Bioscience. 2005;55:477–487. doi:10.1641/0006-3568(2005)055[0477:BNGSCA]2.0.CO;2 [Google Scholar]