Abstract
The diverse and exceptionally well-preserved pycnogonids described herein from the Middle Jurassic La Voulte Lagerstätte fill a 400 Myr gap of knowledge in the evolutionary history of this enigmatic group of marine arthropods. They reveal very close morphological and functional (locomotion, feeding) similarities with present-day pycnogonids and, by contrast, marked differences with all Palaeozoic representatives of the group. This suggests a relatively recent, possibly Mesozoic origin for at least three major extant lineages of pycnogonids (Ammotheidae, Colossendeidae, Endeidae). Combined evidence from depositional environment, faunal associates and recent analogues indicate that the La Voulte pycnogonids probably lived in the upper bathyal zone (ca 200 m). Our results point to a remarkable morphological and ecological stability of this arthropod group over at least 160 Myr and suggest that the colonization of the deep sea by pycnogonids occurred before the Jurassic.
Keywords: Arthropoda, Pycnogonida, Jurassic, Callovian, Lagerstätte
1. Introduction
Present-day pycnogonids or sea spiders constitute a small group of more than 1300 described species (Arango & Wheeler 2007). They resemble arachnids and are particularly recognizable by their extremely long legs, prominent proboscis, ovigers and vestigial abdomen. They occupy a wide range of exclusively marine habitats from shallow water to abyssal depths. Details on their biology and body plan are summarized in Arnaud & Bamber (1987) and Vilpoux & Walossek (2003).
Pycnogonids have long been considered as an enigmatic group of arthropods. Recent attempts to clarify their affinities by using both morphological and molecular methods have provided a wealth of new information, but have still not resulted in a consensus. There are two different phylogenetic questions concerning the controversial affinities of pycnogonids: (i) the internal phylogeny of Pycnogonida mostly discussed from a cladistic perspective (Arango 2002, 2003; Arango & Wheeler 2007) and (ii) the position of pycnogonids within Arthropoda (Dunlop & Arango 2005). Pycnogonids are generally regarded as a sister group of the Euchelicerata or as the sister group of all other euarthropods (Siveter et al. 2004; Giribet et al. 2005; Maxmen et al. 2005; Hassanin 2006; Jager et al. 2006; Manuel et al. 2006). Obviously, a large part of the uncertainties and lack of consensus concerning the evolutionary history of pycnogonids is due to the patchiness of the fossil record. Two sets of fossil evidence both obtained recently from exceptional sites have been of crucial importance to this issue, but they concern only the early evolutionary stages of the group. (i) Three-dimensionally preserved undoubted pycnogonid larvae (Cambropycnogon klausmuelleri) from the ca 500 Myr old ‘Orsten’ deposits (Waloszek & Dunlop 2002) point to a remote origin of pycnogonids with a body plan established by at least the Cambrian times. (ii) The complete anatomy of an adult pycnogonid (Haliestes dasos) reconstructed digitally from a specimen embedded in Silurian (ca 425 Myr) carbonate concretion of the Herefordshire Lagerstätte (Siveter et al. 2004) demonstrates that the group was present in Lower Palaeozoic marine communities, with gross morphologies resembling those of recent pycnogonids, although lacking demonstrably close relations to any extant lineage. Similarly, the four described species (Palaeoisopus problematicus, Palaeopantopus maucheri, Palaeothea devonica and Flagellopantopus blocki) from the Devonian (ca 400 Myr) Hunsrück Slate Lagerstätte (Bergström et al. 1980; Bartels et al. 1998; Poschmann & Dunlop 2006) do not reveal the origin of recent forms (table 1). Fossil pycnogonids have been also reported from the Jurassic of Solnhofen, Germany, but these are now recognized as phyllosoma larvae of decapod crustaceans (Waloszek & Dunlop 2002). The group has no previous record in the Mesozoic or Tertiary.
Table 1.
Palaeozoic fossil species of pycnogonids described in the literature.
| species | localities | formation and age | references |
|---|---|---|---|
| Flagellopantopus blocki | Germany | Hunsrück state. Lower Devonian (ca 400 Myr) | Poschmann & Dunlop (2006) |
| Palacoisopus problematicus | Germany | Hunsrück state. Lower Devonian (ca 400 Myr) | Bergström et al. (1980) |
| Palacoisopus devonica | Germany | Hunsrück state. Lower Devonian (ca 400 Myr) | Bergström et al. (1980) |
| Palaeothea devonica | Germany | Hunsrück state. Lower Devonian (ca 400 Myr) | Bergström et al. (1980) |
| Haliestes dasos | England | Herefordshire, Lower Silurian (ca 425 Myr) | Siveter et al. (2004) |
| Cambropycnogon klausmuelleri | Sweden | Orsten. Upper Cambrian (ca 500 Myr) | Waloszek & Dunlop (2002) |
We describe here three new species of pycnogonid from the ca 160 Myr Callovian Lagerstätte of La Voulte-sur-Rhône (France). Their excellent preservation allows detailed anatomical comparisons with both Palaeozoic and Recent forms. In addition to this, evidence from faunal associates and depositional environment indicate that pycnogonids were already members of the deep-water marine communities in the Jurassic.
2. Material and methods
Seventy complete or fragmentary specimens of pycnogonids come from the La Voulte-sur-Rhône Lagerstätte (Ardèche, SE France). They have been collected from a ca 4 m thick marly horizon in the Lower Callovian Gracilis Zone (Middle Jurassic, ca 160 Myr; see Fischer (2003) for lithological details). Pyritization mediated by bacteria combined with rapid burial in anoxic fine sediments probably led to the preservation of the soft-bodied and chitinous features (Wilby et al. 1996). The majority of specimens are preserved in dorsal view with their limbs spread out; only a few of them are curled up. In many cases, the mineralization obscures fine details such as of joints, ornament and setae. The absence of disarticulation in most specimens indicates that post-mortem transportation and decay were limited at the time of burial. Several rock slabs display numerous pycnogonids associated with abundant exceptionally preserved organisms on the surface of the same bedding plane (e.g. ophiuroids, coleoids, crustaceans, thylacocephalans), suggesting in situ assemblages. The material was mechanically prepared with fine needles and a sandblaster. Several rock slabs were X-rayed in order to study the three-dimensional aspect of pycnogonids embedded in sediment and to evaluate their numerical abundance. This method did not reveal supplementary external or internal anatomical details.
Recent pycnogonids from the zoological collections of the Muséum National d'Histoire Naturelle, Paris, were used for comparative studies. Fossil type specimens are registered in the public Collections of the Département de l'Ardèche, Privas (LAGER numbers).
3. Systematic palaeontology: description of new taxa
Phylum: Arthropoda, Subphylum: Chelicerata, Class: Pycnogonida (classification sensu Waloszek & Dunlop 2002)
(a) Uncertain family (possibly Ammotheidae)
(i) Palaeopycnogonides n. gen.
Type species. Palaeopycnogonides gracilis n. sp.
Derivation of name. Alluding to the antiquity of modern pycnogonids.
Diagnosis. Four pairs of long multi-articulated legs; short cylindrical proboscis with circular mouth; dome-shaped ocular tubercle equidistant to anterior and posterior margins of cephalosoma; single-segmented small chelifores at the base of the proboscis; three-segmented trunk with well-delineated lateral processes bearing distal rounded protuberances. Palps and ovigers absent or not preserved; abdomen lacking or vestigial.
(ii) Palaeopycnogonides gracilis n. sp.
figure 1a,b
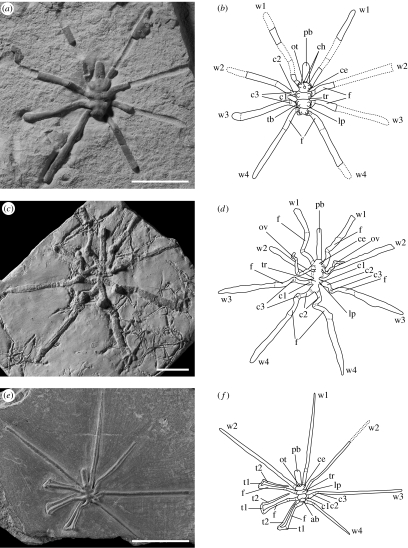
Figure 1.
Pycnogonids from La Voulte (Jurassic): dorsal (a,b,e,f) and ventral (c,d) views and drawings from photographs. (a,b) Palaeopycnogonides gracilis n. gen. n. sp., holotype, LAGER.2007.1.1. (c,d) Colossopantopodus boissinensis n. gen. n. sp., holotype, LAGER.2007.1.4. (e,f) Palaeoendeis elmii n. gen. n. sp., holotype, LAGER.2007.1.5. ab, abdomen; ce, cephalosoma; ch, chelicera; c1–3, coxae 1–3; f, femur; lp, lateral process; ot, ocular tubercle; ov, oviger; pb, proboscis; tb, tubercle; tr, trunk; t1,2, tibiae 1,2; w1–w4, walking legs 1–4. Scale bars, 2 cm. (see electronic supplementary material 1 for additional figured specimens).
Type locality and stratigraphy. Ravin des Mines, near La Boissine, La Voulte-sur-Rhône, Ardèche, SE France; Middle Jurassic, Lower Callovian, Gracilis Biozone.
Type material. Holotype (LAGER.2007.1.1) and paratypes (LAGER.2007.1.2.1 and LAGER.2007.1.2.2 (both on the same slab), LAGER.2007.1.3), all from type locality (ca 50 additional specimens).
Derivation of name. Alluding to its slender walking legs and to the Gracilis ammonite Biozone (Callovian) in which this species occurs.
Diagnosis. As for genus.
Description. Body length (L; from distal trunk to proboscis tip) up to ca 1.8 cm with leg span ca 6.7 cm. Length of cephalosoma ca 10% L. Ocular tubercle as a small dome-like feature, equidistant to anterior and posterior margins of the cephalosoma. No discernable eyes. Short and cylindrical proboscis (ca 35% L), directed downwards in paratype LAGER.2007.1.2.1 (electronic supplementary material 1, figures a and b) with slightly thickened basis and distal circular oral opening. Cephalosoma appendages represented by 1-segmented minute chelifores and the first pair of walking legs. No palps and ovigers visible in any specimen (confirmed by X-ray). Trunk (ca 55% L) divided in three sub-cylindrical segments, each bearing a pair of walking legs attached to trunk via well-developed lateral processes. No abdomen visible. Each leg with nine segments (presumed joints indicated by folds) as in modern pycnogonids (three coxae, femur, first and second tibiae, tarsus, propodus and claw; respectively ca 0.2, 0.4, 0.2, 0.7, 0.6, 0.5, 0.2, 0.7 and 0.4 cm in paratype LAGER.2007.1.2.2 (electronic supplementary material 1, figures c and d)). No auxiliary claw(s) preserved. Walking legs of cephalosoma and trunk all identical. Lateral processes with distal rounded dorsal protuberances (electronic supplementary material 1, figures c–f). No cuticular external ornament (e.g. setae, spines) visible.
(b) Uncertain family (possibly Colossendeidae)
(i) Colossopantopodus n. gen.
Type species. Colossopantopodus boissinensis n.sp.
Derivation of name. From Greek kolossos (=giant) alluding to its large size.
Diagnosis. Four pairs of long, thick, multi-articulated walking legs; very long and sub-cylindrical proboscis; dorsal ocular tubercle not visible; long and 10-segmented ovigers; chelifores and palps absent or not preserved (confirmed by X-ray); thin trunk without external segmentation; abdomen lacking or vestigial.
(ii) Colossopantopodus boissinensis n. sp.
figure 1c,d
Type locality and stratigraphy. As for Palaeopycnogonides gracilis n. sp.
Type material. Holotype (LAGER.2007.1.4) from type locality. No other material available.
Derivation of name. From La Boissine, type locality.
Diagnosis. As for genus.
Description. Body length (L; from distal trunk to proboscis tip) up to ca 4.6 cm with leg span up to 15 cm. Cephalosoma ca 15% L. No ocular tubercle preserved. Very long, cylindrical proboscis (ca 55% L), directed anteriorly with distal sub-circular oral opening. Ovigers inserted posteroventrally (length ca 3.2 cm), probably 10-segmented with segment 6 ca 0.5 cm. Other cephalic appendages (chelifores, palps) absent (confirmed by X-ray), except the first pair of walking legs. Trunk (ca 30% L), relatively narrow (width ca 0.6 cm), with no signs of external segmentation, bearing three pairs of identical, possibly nine-segmented walking legs (distal segmentation of the femur poorly preserved). Coxae 1, 2 and 3, respectively, ca 0.3, 1.1 and 0.6 cm long. Femur ca 1.4 cm long. Lateral processes poorly developed without ornament. Abdomen absent or not preserved. No cuticular external ornament visible.
(c) Uncertain family (possibly Endeidae)
(i) Palaeoendeis n. gen.
Type species. Palaeoendeis elmii n. sp.
Derivation of name. Alluding to the relationship to the Endeidae.
Diagnosis. Four pairs of long and slender multi-articulated walking legs; straight and cylindrical proboscis; dome-shaped ocular tubercle equidistant to anterior and posterior margins of cephalosoma; chelifores and palps absent; ovigers absent or not preserved; three-segmented narrow trunk with reduced lateral processes; abdomen present with rounded termination.
(ii) Palaeoendeis elmii n. sp.
Figure 1e,f
Type locality and stratigraphy. As for Palaeopycnogonides gracilis n. sp.
Type material. Holotype (LAGER.2007.1.5) and paratype (LAGER.2007.1.6) from type locality. No other material available.
Derivation of name. In honour of Prof. Serge Elmi (Université Lyon 1) who died in January 2007.
Diagnosis. As for genus.
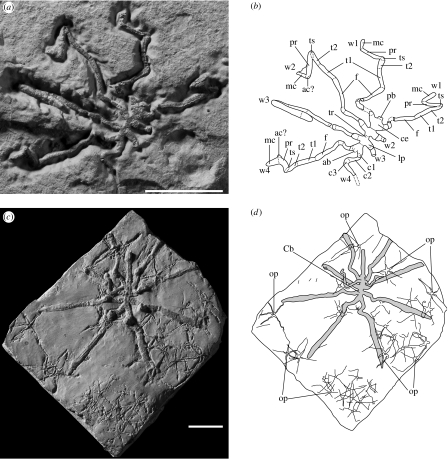
Description. Body length (L; from distal trunk to proboscis tip) up to 1.1 cm with leg span up to ca 6.7 cm. Cephalosoma compact (ca 15% L). Ocular tubercle as a rounded dome-like feature, equidistant to anterior and posterior margins of the cephalosoma. No discernable eyes. Long, straight and cylindrical proboscis (ca 45% L) directed frontally and possibly moveable; mouth not visible. No cephalic appendages (chelifores, palps and ovigers) visible, except the first pair of walking legs. Trunk (ca 40% L) divided into three segments, each bearing a pair of walking legs attached via reduced lateral processes. Last trunk segment bearing short abdomen (ca 0.8 mm in paratype LAGER.2007.1.6) with rounded termination and pointing upwards (figure 2a,b). Walking legs with nine segments (presumed joints indicated by folds) as in modern pycnogonids (three coxae, femur, first and second tibiae, tarsus, propodus and claw; respectively, ca 0.1, 0.4, 0.1, 0.7, 0.4, 0.3, 0.2, 0.5 and 0.3 cm long in paratype LAGER.2007.1.6). Small auxiliary claws may be present (figure 2a,b). Walking legs of cephalosoma and trunk all identical. No cuticular external ornament visible.
Figure 2.
Pycnogonids from La Voulte (Jurassic), type specimens: ventral views and line drawings from photographs. (a,b) Palaeoendeis elmii n. sp. (paratype LAGER.2007.1.6), note the segmentation of the walking legs. (c,d) Colossopantopodus boissinensis n. sp (holotype, LAGER.2007.1.1) associated with numerous delicate ophiuroids (Ophiopinna elegans). Cb: C. boissinensis; ab, abdomen; ac?, possible auxiliary claw; ce, cephalosoma; c1–3, coxae 1–3; f, femur; lp, lateral process; mc, main claw; op, ophiuroid; pb, proboscis; pr, propodus; tr, trunk; ts, tarsus; t1,2, tibiae 1,2; w1–w4, walking legs 1–4. Scale bars, 2 cm.
4. General discussion
(a) Comparisons with Palaeozoic pycnogonids
Palaeopycnogonides gracilis, C. boissinensis and P. elmii resemble none of the six Palaeozoic species described in the literature (table 1). The exquisitely preserved H. dasos from the Silurian Herefordshire Lagerstätte is a microscopic animal (3.5 mm) that is 20–45 times smaller than the specimens from La Voulte. Haliestes dasos bears palps and has a segmented abdomen, which is not the case for P. gracilis and C. boissinensis. Its long ovigers are absent in P. gracilis and P. elmii and differ from those of C. boissinensis by their segmented pattern and segment proportion. Three species from the Devonian Hunsrück Lagerstätte have a size range more consistent with that of the pycnogonids from La Voulte but differ from them in important morphological traits. Palaeoisopus problematicus is a very large (40 cm) pycnogonid with a long multi-segmented abdomen and powerful raptorial chelifores with terminal chelae. Palaeopantopus maucheri has a three-segmented abdomen, whereas F. blocki bears a most peculiar flagellate telson 2.5 times longer than the body. None of these characters occur in the species from La Voulte. Palaeothea devonica (5 mm long) also from Hunsrück is represented by a unique, poorly preserved specimen with relatively short appendages and a body with a flattened shape. No detailed comparisons can be made with C. klausmuelleri from the Cambrian of Sweden that is known from a microscopic larval stage only.
(b) Comparisons with recent pycnogonids
Palaeopycnogonides gracilis shows remarkable similarities with typical representatives of the extant family Ammotheidae (compact trunk, often short proboscis, small reduced chelifores) such as Ammothea (for comparison, see Child (1994) and Munilla (2001)). Despite its reduced chelifores, its trunk shape, proboscis and dorsal protuberances are also reminiscent of Pycnogonum (Pycnogonidae; Arnaud & Bamber 1987). The absence of palps and ovigers, if confirmed by more specimens, might indicate that Palaeopycnogonides has closer affinities with Pycnogonidae than with Ammotheidae.
Colossopantopodus boissinensis is clearly different from P. gracilis chiefly in: (i) its unusually long and straight proboscis, (ii) its pair of ovigers, (iii) its unsegmented trunk, (iv) the absence of well-marked lateral processes, and (v) its larger size. Colossopantopodus boissinensis displays some of the diagnostic features of the extant family Colossendeidae, such as a pantograph-like shape, a very long proboscis, a narrow trunk without external segmentation, smooth lateral processes and 10-segmented ovigers. Indeed it closely resembles Colossendeis, a genus containing the largest representatives of living Pycnogonida. The relative proportions of the leg segments of C. boissinensis are very similar to those of Colossendeis (for comparison, see Bamber & Thurston (1995)). The wide leg span and the absence of chelifores that are typical features of adult colossendeids also occur in Colossopantopodus.
Palaeoendeis elmii differs from P. gracilis by: (i) its relatively long and straight proboscis; (ii) the absence of chelifores; and (iii) its relatively elongated trunk without well-marked lateral processes, and from C. boissinensis mainly by its shorter proboscis and distinctly segmented trunk. Close affinities with recent Endeidae (for comparison, see Fry & Hedgpeth 1969) are indicated by its spindly shape, relative proportion of trunk and proboscis, long walking legs, its distinctly segmented trunk, and the absence of palps and chelifores (lacking in all recent Endeidae).
5. Evolutionary and palaeoecological implications
(a) Fossil and extant pycnogonid lineages
The fossil species from La Voulte provide the first set of key information on the composition and diversity of the Mesozoic pycnogonid fauna. Comparative morphology would advocate placing them within extant families (Ammotheidae, Colossendeidae, Endeidae, Pycnogonidae?). By contrast, they display resemblances with none of the best documented Palaeozoic taxa (e.g. H. dasos and P. problematicus). Their possible assignment to modern families (e.g. Endeidae, Colossendeidae, Ammotheidae, Pycnogonidae) might be tested by cladistic analyses if more morphological characters were made available. Although excellently preserved, the pycnogonids from La Voulte have far fewer usable features than their modern analogues. Numerous tiny features of importance in the systematics of recent Pycnogonida are simply absent due to preservation. Clearly, combining uneven fossil and recent data in a cladistic analysis may lead to misleading interpretations concerning the relationships of fossil taxa to extant clades. In the most recent cladistic analysis of Pycnogonida (Arango & Wheeler 2007) based on 78 morphological characters, unfortunately inapplicable in their vast majority to the fossil taxa (e.g. cement glands, genital pores, larval type), four Palaeozoic species (versus 59 living taxa) are grouped with extant species, instead of emerging as an early derived group near the base of the tree as in previous phylogenies (see Poschmann & Dunlop 2006).
Our opinion, supported by the new line of fossil evidence from La Voulte, is that the evolutionary history of Pycnogonida followed two major steps that gave rise successively to (i) a Palaeozoic fauna and (ii) a post-Palaeozoic fauna including extant forms. The most probable hypothesis, also expressed by Giribet et al. (2001) and Arango (2003), is that extant pycnogonid lineages evolved from a common ancestor via a possible Mesozoic radiation of the group.
(b) Mode of life and habitat of Mesozoic sea spiders
The three species from La Voulte have long legs that may be slender (P. elmii), thicker (P. gracilis) or have a very large leg span (C. boissinensis). This appendage design is characteristic of epibenthic walkers. Most recent long-legged pycnogonids live on the muddy bottom of bathyal to abyssal environments (Arnaud & Bamber 1987). Direct evidence for the diet of the La Voulte pycnogonids is not available. However, potential prey such as ophiuroids, small bivalves and polychaetes are abundant at La Voulte and are found to be associated with pycnogonids (Fischer 2003). The sucking up of small organisms from mud via a well-developed proboscis may have been one of the feeding strategies of the pycnogonids from La Voulte as observed in Colossendeis colossea (Arnaud & Bamber 1987). Colossopantopodus boissinensis, which has a long proboscis, is preserved in association with numerous ophiuroids (figure 2c,d) and, alternatively, might have fed on proteinaceous mucus often produced on the surface of these small echinoderms. Colossopantopodus boissinensis and P. elmii were possibly solitary forms, whereas P. gracilis, with a palaeodensity of ca 55 individuals m−2 (estimated from two large slabs), was apparently gregarious.
Convergent palaeoenvironmental data obtained from both crinoids and siliceous sponges (Charbonnier et al. 2007) support the notion that the La Voulte area was situated in the upper bathyal zone near the slope–basin transition with a water depth most probably exceeding 200 m. The fauna from the Lagerstätte itself includes ophiuroids in high concentrations, vampyromorph squids and deep-sea cirrate octopods (Fischer 2003), thylacocephalan arthropods (Vannier et al. 2006) with hypertrophied eyes resembling those of recent deep-sea crustaceans, and eryonid crustaceans. All are indicative of a bathyal environment and dysphotic or aphotic conditions. The pycnogonids, which show close similarities with the recent deep-sea representatives of the group, provide further evidence for a relatively deep marine palaeoenvironment.
Our study reveals the remarkable morphological and ecological stability of Pycnogonida over at least 160 Myr. The occupation of the deep sea by these organisms is not a recent event and may have taken place before the Mesozoic. Palaeozoic pycnogonids such as those from the Silurian Herefordshire (Siveter et al. 2004) and Devonian Hunsrück Slate (Poschmann & Dunlop 2006) Lagerstätten are associated with relatively deep marine settings. The colonization of shallow water niches by pycnogonids may find its origin in a post-Jurassic ecological shift of the group from an originally bathyal stock.
Acknowledgments
The authors thank F. Arnaud for her expertise on pycnogonids, M. Sutton and three anonymous reviewers for comments made on their manuscript, R. Cleva for access to recent material, and N. Podevigne for photography. Contribution UMR5125-07.034.
Supplementary Material
Type specimens of Palaeopycnogonides gracilis n. sp.
References
- Arango C.P. Morphological phylogenetics of the sea spiders (Arthropoda: Pycnogonida) Org. Divers. Evol. 2002;2:107–125. doi:10.1078/1439-6092-00035 [Google Scholar]
- Arango C.P. Molecular approach to the phylogenetics of sea spiders (Arthropoda: Pycnogonida) using partial sequences of nuclear ribosomal DNA. Mol. Phylogenet. Evol. 2003;28:588–600. doi: 10.1016/s1055-7903(03)00069-1. doi:10.1016/S1055-7903(03)00069-1 [DOI] [PubMed] [Google Scholar]
- Arango C.P, Wheeler W.C. Phylogeny of the sea spiders (Arthropoda, Pycnogonida) based on direct optimization of six loci and morphology. Cladistics. 2007;23:255–293. doi: 10.1111/j.1096-0031.2007.00143.x. doi:10.1111/j.1096-0031.2007.00143.x [DOI] [PubMed] [Google Scholar]
- Arnaud F, Bamber R.N. The biology of Pycnogonida. Adv. Mar. Biol. 1987;24:1–96. [Google Scholar]
- Bamber R.N, Thurston M.H. The deep-water pycnogonids (Arthropoda: Pycnogonida) of the northeastern Atlantic Ocean. Zool. J. Linn. Soc. 1995;115:117–162. doi:10.1006/zjls.1995.0035 [Google Scholar]
- Bartels C, Briggs D.E.G, Brassel G. Marine life in the Devonian. 1st edn. Cambridge University Press; Cambridge, UK; New York, NY: 1998. The fossils of the Hunsrück Slate. [Google Scholar]
- Bergström J, Stürmer W, Winter G. Palaeoisopus, Palaeopantopus, Palaeothea, pycnogonid arthropods from the Lower Devonian Hunsrück Slate, West Germany. Paläont. Zeit. 1980;54:7–54. [Google Scholar]
- Charbonnier S, Vannier J, Gaillard C, Bourseau J.-P, Hantzpergue P. The La Voulte Lagerstätte (Callovian): evidence for a deep water setting from sponge and crinoid communities. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007;250:216–236. doi:10.1016/j.palaeo.2007.03.013 [Google Scholar]
- Child C.A. Antarctic and subantarctic Pycnogonida 1, the family Ammotheidae. Antarct. Res. Ser. 1994;63:1–48. [Google Scholar]
- Dunlop J.A, Arango C.P. Pycnogonid affinities: a review. J. Zool. Syst. Evol. Res. 2005;43:8–21. doi:10.1111/j.1439-0469.2004.00284.x [Google Scholar]
- Fischer J.C. Invertébrés remarquables du Callovien inférieur de La Voulte-sur-Rhône (Ardèche France) Ann. Paléontol. 2003;89:223–252. doi:10.1016/j.annpal.2003.09.001 [Google Scholar]
- Fry W.G, Hedgpeth J.W. Pycnogonida, 1. Colossendeidae, Pycnogonidae, Endeidae, Ammotheidae. Fauna of the Ross Sea, 7. Mem. N. Z. Oceanogr. Inst. 1969;79:1–139. [Google Scholar]
- Giribet G, Edgecombe G.D, Wheeler W.C. Arthropod phylogeny based on eight molecular loci and morphology. Nature. 2001;413:157–161. doi: 10.1038/35093097. doi:10.1038/35093097 [DOI] [PubMed] [Google Scholar]
- Giribet G, Richter S, Edgecombe G.D, Wheeler W.C. The position of crustaceans within Arthropoda—evidence from nine molecular loci and morphology. In: Koeneman S, Jenner R.A, editors. Crustacea and arthropod relationships. Taylor & Francis; Boca Raton, FL: 2005. pp. 307–352. [Google Scholar]
- Hassanin A. Phylogeny of Arthropoda inferred from mitochondrial sequences: strategies for limiting the misleading effects of multiple changes in pattern and rates of substitution. Mol. Phylogenet. Evol. 2006;38:100–116. doi: 10.1016/j.ympev.2005.09.012. doi:10.1016/j.ympev.2005.09.012 [DOI] [PubMed] [Google Scholar]
- Jager M, Murienne J, Clabaut C, Deutsch J, Le Guyader H, Manuel M. Homology of arthropod anterior appendages revealed by Hox gene expression in a sea spider. Nature. 2006;441:506–508. doi: 10.1038/nature04591. doi:10.1038/nature04591 [DOI] [PubMed] [Google Scholar]
- Manuel M, Jager M, Murienne J, Clabaut C, Le Guyader H. Hox genes in sea spiders (Pycnogonida) and the homology of arthropod head segments. Dev. Genes Evol. 2006;216:481–491. doi: 10.1007/s00427-006-0095-2. doi:10.1007/s00427-006-0095-2 [DOI] [PubMed] [Google Scholar]
- Maxmen A, Browne W.E, Martindale M.Q, Giribet G. Neuroanatomy of sea spiders implies an appendicular origin of the protocerebral segment. Nature. 2005;437:1144–1148. doi: 10.1038/nature03984. doi:10.1038/nature03984 [DOI] [PubMed] [Google Scholar]
- Munilla T. A new species of Ammothea (Pycnogonida) and others pycnogonids from Livingston Island and surrounding waters (South Shetland Islands Antarctica) Antarct. Sci. 2001;13:144–149. doi:10.1017/S0954102001000220 [Google Scholar]
- Poschmann M, Dunlop J.A. A new sea spider (Arthropoda: Pycnogonida) with a flagelliform telson from the Lower Devonian Hunsrück Slate, Germany. Palaeontology. 2006;49:983–989. doi:10.1111/j.1475-4983.2006.00583.x [Google Scholar]
- Siveter D.J, Sutton M.D, Briggs D.E.G, Siveter D.J. A Silurian sea spider. Nature. 2004;431:978–980. doi: 10.1038/nature02928. doi:10.1038/nature02928 [DOI] [PubMed] [Google Scholar]
- Vannier J, Chen J.-Y, Huang D.-Y, Charbonnier S, Wang X.-Q. Thylacocephalan arthropods: their Early Cambrian origin and evolutionary significance. Acta Paleontol. Pol. 2006;51:1–14. [Google Scholar]
- Vilpoux K, Waloszek D. Larval development and morphogenesis of the sea spider Pycnogonum litorale (Ström, 1762) and tagmosis of the body of Pantopoda. Arthrop. Struct. Dev. 2003;32:349–383. doi: 10.1016/j.asd.2003.09.004. doi:10.1016/j.asd.2003.09.004 [DOI] [PubMed] [Google Scholar]
- Waloszek D, Dunlop J.A. A larval sea spider (Arthropoda: Pycnogonida) from the Upper Cambrian ‘Orsten’ of Sweden, and the phylogenetic position of pycnogonids. Palaeontology. 2002;45:421–446. doi:10.1111/1475-4983.00244 [Google Scholar]
- Wilby P.R, Briggs D.E.G, Riou B. Mineralization of soft-bodied invertebrates in a Jurassic metalliferous deposit. Geology. 1996;24:847–850. doi:10.1130/0091-7613(1996)024<0847:MOSBII>2.3.CO;2 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Type specimens of Palaeopycnogonides gracilis n. sp.