Abstract
Complex interactions occur among embryonic, placental and maternal tissues during embryo implantation. Many of these interactions are controlled by growth factors, extracellular matrix and cell surface components that share the ability to bind heparan sulfate (HS) polysaccharides. HS is carried by several classes of cell surface and secreted proteins called HS proteoglycan that are expressed in restricted patterns during implantation and placentation. This review will discuss the expression of HS proteoglycans and various HS binding growth factors as well as extracellular matrix components and HS-modifying enzymes that can release HS-bound proteins in the context of implantation and placentation.
Keywords: Heparan sulfate proteoglycans, HIP/RPL29, heparanase, embryo implantation, placentation
I. Introduction
The processes of embryo implantation and placentation involve a complex coordination among multiple cell types of the embryo/fetus and mother [1]. Control of gene expression in these cell types involves the actions of transcription factors regulated, in many cases, by activation of various intracellular signal transduction pathways. Activation of many of these pathways involves interaction of cell surface receptors with extracellular matrix (ECM) components and growth factors which are often deposited in ECM. Binding to heparan sulfate proteoglycans (HSPGs) sequesters and concentrates growth factors in species ranging from Drosophila melanogaster and Caenorhabditis elegans to mammals [2]. HSPGs are proteins to which one or more heparan sulfate (HS) polysaccharides are covalently attached. In this review, we will focus on the role HSPGs play in modulating key events in embryo implantation and placentation as well as recent work on functions of novel HS binding proteins and extracellular HS modifying enzymes. The expression pattern and proposed functions of molecules involved in HS-dependent events in the fetal-maternal interface is summarized in Table I. Detailed descriptions of the cellular and morphological events that occur during embryo implantation and placentation are available [1,3,4].
Table I.
Expression of HS proteoglycans and HS-binding proteinsin the uteroplacental unit (partial list)1
| Molecule | Trophectoderm | Uterine Epithelia | Decidua | Trophoblast | Villus fibroblasts | Proposed function(s) |
|---|---|---|---|---|---|---|
| HSPGs | Tissue integrity, cell adhesion, growth factor binding | |||||
| • Syndecans | + | - | + (m,h) | + (h; Cyto, Syn) | + (h) | |
| • Glypicans | ? | ? | + (h) | + (h; Syn) | ? | |
| • Perlecan | + | + | + (m,h) | + (m, h; Cyto, Syn) | - (h) | |
| • Collagen XVIII | - | - | + (h) | - (h) | + (h) | |
| Heparanase | ? | + (m) | + (m) | + (m & h) | ? | Trophoblast invasion, angiogenesis, decidualization, remodeling of extracellular matrix, release of growth factors |
| Sulfs | ? | ? | + (m,h) | + (h, but location unknown) | ? | HS 6-O-desulfation, modulation of growth factor binding and signaling |
| HS-binding growth factors | ||||||
| • HBEGF | + | + | + (h) | + cyto(h) I-III | ? | Blastocyst attachment, trophoblast invasion, and maintenance of pregnancy [73,74] |
| • HGF | + | - | + (h) | + syn+cyto (h) | + (h) | Trophoblast invasion [75] |
| • FGF-2 | + (m) | + syn+cyto(h) I | + (h) | Placental growth and angiogenesis [76] | ||
| • FGF-10 | + (h)(I-III) | + syn+cyto (II-III) | Sprouting and branching of placental villi [77] | |||
| • VEGF | + (h) | + (h) | Placental angiogenesis and vasculogenesis [78] | |||
| • BMPs | + (m) | Decidualization, embryo spacing [79,80] | ||||
| • Wnts | + (m) | Labyrinth morphogenesis [79,81] | ||||
| • MK | - | Giant troph.(m) | Regulation of endometrial growth [82] | |||
| • PTN | +(m) | - | Decidualization [82] | |||
| HS-binding ECM components | ||||||
| • LAMA-111 | +BM | +BM | Post-implantation cyto invasion direction [83,84] | |||
| • FN | +(h) | Decidual cell differentiation and invasion [85-88] | ||||
| • Selectins | +(h) | - | +syn+cyto (h) | Attachment and establishment of pregnancy in human [89,90] | ||
| • CNN1 (Cyr61) | - | -syn(I-III)+cyto(III) | +(h)I-III | Vessel bifurcation in developing placenta[91] | ||
| Other HS-binding molecules | ||||||
| • HIP/RPL29 | + (m) | + (m) | + (m) | +cyto(h) | +(h) | Global protein synthesis and putative role in embryo attachment and invasion in human |
The symbols “+” or “- “ are used to indicate whether the corresponding molecule has been detected by any method with no attempt made to indicate relative levels of expression among these compartments. For trophoblast, the abbreviations “Cyto” and “Syn” are used to indicate whether expression has been observed in cytotrophoblasts or syncytiotrophoblast, respectively. The symbols “h” and “m” are used in parentheses to indicate whether the observations were made in human or mouse tissues, respectively. If differential expression is observed in human placental tissue during pregnancy, then Roman numerals I-III are used to discriminate among the first, second and third trimesters. For further details, see text. BM, basement membrane.
II. Heparan sulfate proteoglycans
Core proteins
There are three major classes of HSPG core proteins. The first is the syndecans, a family of four type I transmembrane proteins (reviewed in Beauvais and Rapraeger [5]). Only syndecans-1, -3 and -4 have been reported in the uteroplacental units of humans and rodents [6-10]. The characteristic short cytoplasmic tails of syndecans interact with cytoskeletal components and, in the case of syndecan-4, the signaling molecules PKCα and PIP2 (reviewed in Beauvais and Raprager, 2004 [5]). In the preimplantation mouse embryo, syndecan is detected at the surfaces of blastomeres at the morula stage, but becomes restricted to the inner surface of blastocoel cavity by the blastocyst stage [11]. Syndecans also are found in uterine stroma, syncytiotrophoblast and fetal vessels in the labyrinthine zone [6,8,12]. In the human fetal-maternal interface, syndecan-1 is restricted to the syncytiotrophoblast. Syndecan-4 is expressed in villous and extravillous cytotrophoblast early in pregnancy, but is lost from these regions later [10].
The glypicans are a family of six HSPGs linked to the cell surface via glycosylphosphatidyl inositol anchors. Very little information is available on glypican expression in the utero-placental unit, but one report indicates that glypican-3, like syndecans, accumulates in syncytiotrophoblast [13] while another tentatively identified glypican-1 in human decidual tissues [9,10].
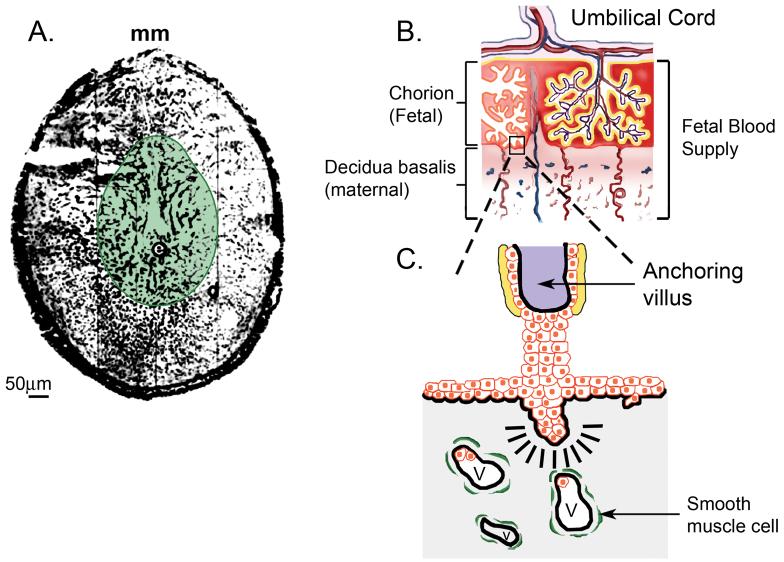
The third proteoglycan class is that of the ECM, namely agrin, collagen XVIII and perlecan. These proteoglycans all have relatively large protein cores and, in the cases of collagen type XVIII and perlecan, functions have been identified for proteolytic fragments derived from them which modulate angiogenesis [14]. There are no reports of agrin expression in the utero-placental unit. In contrast, collagen XVIII is expressed by decidua and villous fibroblasts, but not in trophoblastic tissue or cell lines [15,16]. Perlecan is more broadly expressed in basal lamina underlying uterine epithelia, trophoblast, maternal and placental vasculature and in decidua. In humans, perlecan also accumulates in the region of cytotrophoblast invasion into the decidua [17]. In the preimplantation mouse embryo, perlecan is transiently expressed before the morula stage [18]. During blastocyst development, perlecan expression is enhanced and is deposited on the external surface of the trophectoderm [19]. In delayed implantation blastocysts, perlecan mRNA accumulates, but the protein is not expressed until the blastocyst is activated indicating translational control [20]. The distribution of perlecan relative to syndecans and glypicans during the post-implantation and placentation periods in murine implantation site and the human fetal-maternal interface is illustrated in Figure 1.
Figure 1. Heparan sulfate proteoglycan distribution at implantation sites.
Panel A shows the pattern of perlecan (black areas) and syndecan-1 (green shaded area) in a cross section of a day 8 implantation site in the mouse. Perlecan is widely distributed in decidua as well as in both embryonic and maternal basal lamina. In contrast, syndecan-1 is restricted to the deciduas as well as embryonic tissue elements. Symbols: e, embryo; mm, mesometrial aspect of uterus. Panel B is a drawing representing main features of the human fetal-maternal interface showing the blood supply (red, arteries; blue, veins) leading to and from the umbilical cord and into the chorion as well as the maternal decidua. In panel C, an anchoring villus has been expanded from panel B to highlight the presence of invading cytotrophoblast (red cells), villar fibroblasts (purple shaded area), syncytiotrophoblast (yellow area), deciduas (gray area) and decidual vascular elements (V) surrounded by vascular smooth muscle cells (green). Perlecan is heavily deposited in areas indicated by the thick black lines. Syndecan-1 and -3 are found in the yellow region.
Gene knockouts have been generated for a variety of proteoglycan core proteins as well as HS biosynthetic enzymes (reviewed in Forsberg and Kjellen, 2001 [21]). None of these animals display an obvious implantation phenotype suggesting that HSPG-dependent functions are either dispensable or redundant at this early stage of development. A defect in placental development was noted in syndecan-4 nulls involving defects in fetal blood vessels [12]. Perlecan null mice display embryonic lethality with defects in formation of multiple fetal tissues [22]. These authors reported that the placental morphology through mid-gestation was similar to that of wild type and heterozygous animals. It should be noted that maternal RNA may support early murine embryonic development in certain cases [23] and it is possible that this occurs in the case of some proteoglycan nulls. If this is the case, then treatment of null embryos with the corresponding proteoglycan directed siRNA or ribozymes might reveal additional implantation/placentation phenotypes.
HS polysaccharides
The steps and genes involved in HS assembly have been reviewed in detail elsewhere [24]. An ordered series of reactions involving N-deacetylation/N-sulfation, uronic acid epimerization and O-sulfation occur that produce sequences within these large (20-50,00 MW) polysaccharides which provide binding sites for growth factors and ECM proteins. The particular balance in expression of these distinct activities gives rise to cell-type specific differences in expression of HS structures. Moreover, the size, charge and linearity of HS polysaccharides provide a capacity to bind more than one protein per polysaccharide chain [25,26]. Interestingly, several extracellular enzymes occur that can modify HS chains at the cell surface or ECM and modulate protein binding (see below). Thus, HS-dependent interactions with proteins can be dynamically modulated both at the synthetic and post-synthetic levels.
III. HS Binding Proteins
HS chains interact with a variety of proteins such as growth and differentiation factors, morphogens, ECM proteins and others (reviewed in Bernfield et al., 1999 [27]). There are many examples of HS-binding protein expression in uteroplacental tissues and these are summarized in Table 1. These interactions have been shown to participate in both normal and pathological events and to require HS chains with specific oligosaccharide sequences and sulfation patterns that selectively bind to domains in interacting protein ligands. For example, recent studies using HS oligoaccharide libraries established the importance of either 2-O-sulfate and/or 6-O-sulfate, and N-sulfate groups in particular HS-binding protein-HS interactions [28,29]. Such specific HS-protein interactions serve to regulate the signal output of growth factor receptors as well as the storage and diffusion of extracellular protein effectors. Thus, HS chains of proteoglycans are believed to act as co-receptors which are capable of influencing cell fate by integrating cellular signals. Binding HS provides a means to both restrict the diffusion and concentrate of soluble proteins like growth factors. In addition, HS-binding by ECM components enhances the structural integrity of the tissue. In other cases, HS-binding may serve more complex functions as in the case of HIP/RPL29.
HIP/RPL29
Heparin/heparan sulfate interacting protein (HIP) is a highly conserved basic protein identical to ribosomal protein L29 (RPL29) found associated with perinuclear membranes and translationally active ribosomes after polysomal fractionation [30,31]. In human uterine epithelial all lines, HIP/RPL29 is found at the cell surface where it is believed to participate in HS-binding [32]. HIP RL29 also can modulate blood coagulation processes [33] and inhibits heparanase activity (see below). HIP/RPL29 is prominently expressed in uterine luminal and glandular epithelia in both humans and mice at all stages of the cycle including during the window of receptivity [32,34]. During the post-implantation period in mice, HIP/RPL29 accumulates in decidualizing stroma and actively dividing embryonic structures [34]. These expression patterns suggest an important role for HIP/RPL29 in the establishment and maintenance of pregnancy. In human placenta, HIP/RPL29 is expressed at high level by invasive cytotrophoblasts and to a lesser extent in syncytiotrophoblasts [17]. Hip/Rpl29 null mice display low birth weight followed by decreased fertility in adults [30]. Reduced rates of cellular proliferation and global protein synthesis appear to cause this runting phenotype. In utero, the growth defect of null animals becomes evident at 12.5 days of development and is systematically associated with decreased placental weight suggesting that the small phenotype might be the result of placental insufficiency.
IV. Heparanase
Growth factors and cytokines must be released from ECM sites of deposition, cell contacts with HS-bearing substrates must be broken and extracellular matrices must be remodeled during implantation and placentation. Heparanase (HPSE) is an endo-β-D-glucuronidase that cleaves HS at specific sites to generate oligosaccharides that are 10 - 20 sugar units in length [35]. Heparanase has been identified in a number of cell types and tissues, including the placenta [36-41]. The inactive 65 kD pro-enzyme subsequently is proteolytically cleaved to generate 50 kD and 8 kD polypeptides [35,42] that remain associated to provide catalytic activity [42]. HPSE displays optimal activity at pH 5 with an 80-90% activity reduction at neutral pH [35,43]. In this regard, HPSE binds HS and may function as a cell adhesion molecule at pH 7.0 [44]. Recently, an mRNA splice variant of human HPSE lacking exon 5 was identified that lacks HS hydrolytic activity, but may retain adhesion-promoting function [45].
Another member of the HPSE family, heparanase-2 (HPSE2), encodes for three different proteins due to alternative mRNA splicing [46]. Hpse2 mRNA is present in a number of human tissues, including uterus [46]. No information is available on HPSE2 protein expression, but we recently found that Hpse2 transcripts in human and baboon decidual tissue (S. D’Souza, A.T. Fazleabas and D.D. Carson, submitted).
HPSE can promote angiogenesis, a crucial process during placentation, by: 1) releasing HS:angiogenic growth factor complexes, allowing optimal binding between HS:growth factor and receptor; 2) promoting endothelial cells migration and; 3) degradation of the subendothelial basement membrane [47-49]. Little progress has been made in determining HPSE expression and function in implantation and placentation. HPSE is present in human and bovine placenta [50-53] as well as endometrium of cycling human uteri [54,55]. In transgenic mice, HPSE overexpression resulted in a significant increase in the number of implanted embryos [56]. Furthermore, exogenous HPSE supplementation increased the number of implantation sites and promoting embryo attachment and outgrowth in vitro [57]. Recently, we demonstrated large increases in HPSE expression and activity in decidua of mouse endometrium, in areas where the potential substrate, perlecan, also accumulates [58]. These studies also demonstrated that injection of the heparanase inhibitor, PI-88, severely inhibited embryo implantation and caused malformations of the implantation sites, supporting the notion that maintenance of HPSE activity is critical during this process. In baboon and human receptive phase uteri, HPSE is localized to the glandular epithelia and glandular secretions and at the maternal-fetal interface HPSE is localized in villi and decidua (manuscript submitted). Collectively, these studies indicate that HPSE plays a key role in early implantation as well as placentation in various species.
Two naturally occurring inhibitors of HPSE have been identified: eosinophil major basic protein [59] and HIP/RPL29 [34]. Human placenta express eosinophil basic protein mRNA [60] and a precursor form of eosinophil major basic protein is secreted constitutively by human trophoblast cells isolated from the first to third trimester as well as decidualizing human uterine stroma cells [61]. HIP/RPL29 accumulates in mouse decidua and is expressed in a complementary pattern to the HSPG, perlecan, at the human fetal-maternal interface [17]. Thus, these proteins are expressed in patterns consistent with a role in modulating HPSE activity during early pregnancy and placentation.
V. Sulf1 and Sulf2
Sulf1 and Sulf2 are secreted endosulfatases that remove 6-O-sulfate residues from HS [62]. They have been identified in a many species, including quail, zebrafish, C. elegans, Drosophila, mouse, rat and human [62-65]. Human SULFs have been shown to modulate binding of FGF-1 and VEGF to HS [66]. Sulf overexpression impacts signaling pathways including those activated by FGF-2, FGF-4, hepatocyte growth factor, Wnt, bone morphogenetic protein and HB-EGF [62,67-71]. The Sulf genes are expressed in various tissues during mouse embryogenesis including the nervous system, skeleton and heart [72]. In the pregnant mouse uterus, Sulf transcripts were detected in the decidual region [72]. In the same study, Sulf2 mRNA was detected in the spongiotrophoblast region of placenta while Sulf1 was not detected except in a subset of placental vasculature. Sulf transcripts have also been identified in human uterus and placenta [63]. Given their intriguing functions and expression patterns in the developing mouse embryo, SULF proteins may play a role in modulating HS-dependent processes.
Summary and Future Directions
HSPG expression changes dynamically during embryo implantation and placentation. Nonetheless, data is still incomplete on the expression of HSPG core proteins, particularly that of the glypicans and little is known about how the patterns of HS structures or the expression of key enzymes involved in HS assembly and extracellular modification change during these key biological events. HSPGs bind a wide array of growth factors and ECM components. In the former case, this binding serves to concentrate and restrict the diffusion of these growth factors to specific regions within the uteroplacental unit. Null mutations in genes encoding some proteoglycan core proteins have subtle effects suggesting they are not required or have redundant function in implantation and placentation; however, mutations in other HSPG-encoding genes have such massive developmental defects that it will be necessary to use more sophisticated methods, e.g., tissue-specific, inducible knockouts in the uterus or placenta, to dissect the role these gene products play in this process. Given the profound biological roles HS-binding growth factors and HSPGs are known to play in other biological events, expanding our knowledge of the expression and function of HSPGs, HS structures and HS modifying enzymes is likely to add significantly to our understanding of the biology of embryo implantation and placentation and pathologies associated with these processes, e.g., pre-eclampsia and intrauterine growth retardation.
Acknowledgements
The authors are grateful to Ms. Sharron Kingston and Mrs. Doreen Anderson for their excellent secretarial assistance. We also are indebted to Mrs. Margie Barrett for her graphics work. We appreciate the helpful discussions and critical reading of this manuscript of Dr. Mary C. Farach-Carson and Ms. JoAnne Julian. The authors were supported by NIH grant R35 HD25235 (to D.D.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, et al. Embryo implantation. Dev Biol. 2000;223(2):217–37. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- 2.Hacker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: the sweet side of development. Nature reviews. 2005;6(7):530–41. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- 3.Carter AM, Enders AC. Comparative aspects of trophoblast development and placentation. Reprod Biol Endocrinol. 2004;2:46. doi: 10.1186/1477-7827-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter AM. Animal models of human placentation - a review. Placenta. 2007;28(Suppl A):S41–7. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Beauvais DM, Rapraeger AC. Syndecans in tumor cell adhesion and signaling. Reprod Biol Endocrinol. 2004;2:3. doi: 10.1186/1477-7827-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jokimaa V, Inki P, Kujari H, Hirvonen O, Ekholm E, Anttila L. Expression of syndecan-1 in human placenta and decidua. Placenta. 1998;19(23):157–63. doi: 10.1016/s0143-4004(98)90004-2. [DOI] [PubMed] [Google Scholar]
- 7.Russo LA, Calabro SP, Filler TA, Carey DJ, Gardner RM. In vivo regulation of syndecan-3 expression in the rat uterus by 17 beta-estradiol. J Biol Chem. 2001;276(1):686–92. doi: 10.1074/jbc.M004106200. [DOI] [PubMed] [Google Scholar]
- 8.San Martin S, Soto-Suazo M, Zorn TM. Perlecan and syndecan-4 in uterine tissues during the early pregnancy in mice. Am J Reprod Immunol. 2004;52(1):53–9. doi: 10.1111/j.1600-0897.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 9.Hjelm Cluff A, Malmstrom A, Tingaker B, David G, Ekman-Ordeberg G. Normal labor associated with changes in uterine heparan sulfate proteoglycan expression and localization. Acta obstetricia et gynecologica Scandinavica. 2005;84(3):217–24. doi: 10.1111/j.0001-6349.2005.00484.x. [DOI] [PubMed] [Google Scholar]
- 10.Crescimanno C, Marzioni D, Paradinas FJ, Schrurs B, Muhlhauser J, Todros T, et al. Expression pattern alterations of syndecans and glypican-1 in normal and pathological trophoblast. The Journal of pathology. 1999;189(4):600–8. doi: 10.1002/(SICI)1096-9896(199912)189:4<600::AID-PATH440>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland AE, Sanderson RD, Mayes M, Seibert M, Calarco PG, Bernfield M, et al. Expression of syndecan, a putative low affinity fibroblast growth factor receptor, in the early mouse embryo. Development (Cambridge, England) 1991;113(1):339–51. doi: 10.1242/dev.113.1.339. [DOI] [PubMed] [Google Scholar]
- 12.Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Nakamura E, Ito M, et al. Syndecan-4 deficiency impairs the fetal vessels in the placental labyrinth. Dev Dyn. 2000;219(4):539–44. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1081>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 13.Khan S, Blackburn M, Mao DL, Huber R, Schlessinger D, Fant M. Glypican-3 (GPC3) expression in human placenta: localization to the differentiated syncytiotrophoblast. Histology and histopathology. 2001;16(1):71–8. doi: 10.14670/HH-16.71. [DOI] [PubMed] [Google Scholar]
- 14.Bix G, Iozzo RV. Matrix revolutions: “tails” of basement-membrane components with angiostatic functions. Trends in cell biology. 2005;15(1):52–60. doi: 10.1016/j.tcb.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Pollheimer J, Bauer S, Huber A, Husslein P, Aplin JD, Knofler M. Expression pattern of collagen XVIII and its cleavage product, the angiogenesis inhibitor endostatin, at the fetal-maternal interface. Placenta. 2004;25(10):770–9. doi: 10.1016/j.placenta.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Nasu K, Fujisawa K, Nishida Y, Kai S, Sugano T, Miyakawa I, et al. Expression of collagen XVIII mRNA and protein in human umbilical vein and placenta. Reproduction, fertility, and development. 2003;15(12):107–14. doi: 10.1071/rd02067. [DOI] [PubMed] [Google Scholar]
- 17.Rohde LH, Janatpore MJ, McMaster MT, Fisher S, Zhou Y, Lim KH, et al. Complementary expression of HIP, a cell-surface heparan sulfate binding protein, and perlecan at the human fetal-maternal interface. Biol Reprod. 1998;58(4):1075–83. doi: 10.1095/biolreprod58.4.1075. [DOI] [PubMed] [Google Scholar]
- 18.Dziadek M, Fujiwara S, Paulsson M, Timpl R. Immunological characterization of basement membrane types of heparan sulfate proteoglycan. The EMBO journal. 1985;4(4):905–12. doi: 10.1002/j.1460-2075.1985.tb03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carson DD, Tang JP, Julian J. Heparan sulfate proteoglycan (perlecan) expression by mouse embryos during acquisition of attachment competence. Dev Biol. 1993;155(1):97–106. doi: 10.1006/dbio.1993.1010. [DOI] [PubMed] [Google Scholar]
- 20.Smith SE, French MM, Julian J, Paria BC, Dey SK, Carson DD. Expression of heparan sulfate proteoglycan (perlecan) in the mouse blastocyst is regulated during normal and delayed implantation. Dev Biol. 1997;184(1):38–47. doi: 10.1006/dbio.1997.8521. [DOI] [PubMed] [Google Scholar]
- 21.Forsberg E, Kjellen L. Heparan sulfate: lessons from knockout mice. J Clin Invest. 2001;108(2):175–80. doi: 10.1172/JCI13561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costell M, Gustafsson E, Aszodi A, Morgelin M, Bloch W, Hunziker E, et al. Perlecan maintains the integrity of cartilage and some basement membranes. The Journal of cell biology. 1999;147(5):1109–22. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Payer B, Saitou M, Barton SC, Thresher R, Dixon JP, Zahn D, et al. Stella is a maternal effect gene required for normal early development in mice. Curr Biol. 2003;13(23):2110–7. doi: 10.1016/j.cub.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 24.Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annual review of biochemistry. 2002;71:435–71. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 25.Tumova S, Bame KJ. The interaction between basic fibroblast growth factor and heparan sulfate can prevent the in vitro degradation of the glycosaminoglycan by Chinese hamster ovary cell heparanases. J Biol Chem. 1997;272(14):9078–85. doi: 10.1074/jbc.272.14.9078. [DOI] [PubMed] [Google Scholar]
- 26.Harmer NJ, Robinson CJ, Adam LE, Ilag LL, Robinson CV, Gallagher JT, et al. Multimers of the fibroblast growth factor (FGF)-FGF receptor-saccharide complex are formed on long oligomers of heparin. Biochem J. 2006;393(Pt 3):741–8. doi: 10.1042/BJ20050985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, et al. Functions of cell surface heparan sulfate proteoglycans. Annual review of biochemistry. 1999;68:729–77. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 28.Ashikari-Hada S, Habuchi H, Kariya Y, Itoh N, Reddi AH, Kimata K. Characterization of growth factor-binding structures in heparin/heparan sulfate using an octasaccharide library. J Biol Chem. 2004;279(13):12346–54. doi: 10.1074/jbc.M313523200. [DOI] [PubMed] [Google Scholar]
- 29.Mahalingam Y, Gallagher JT, Couchman JR. Cellular adhesion responses to the heparin-binding (HepII) domain of fibronectin require heparan sulfate with specific properties. J Biol Chem. 2007;282(5):3221–30. doi: 10.1074/jbc.M604938200. [DOI] [PubMed] [Google Scholar]
- 30.Kirn-Safran CB, Oristian DS, Focht RJ, Parker SG, Vivian JL, Carson DD. Global growth deficiencies in mice lacking the ribosomal protein HIP/RPL29. Dev Dyn. 2007;236(2):447–60. doi: 10.1002/dvdy.21046. [DOI] [PubMed] [Google Scholar]
- 31.Hoke DE, Regisford EG, Julian J, Amin A, Begue-Kirn C, Carson DD. Murine HIP/L29 is a heparin-binding protein with a restricted pattern of expression in adult tissues. J Biol Chem. 1998;273(39):25148–57. doi: 10.1074/jbc.273.39.25148. [DOI] [PubMed] [Google Scholar]
- 32.Rohde LH, Julian J, Babaknia A, Carson DD. Cell surface expression of HIP, a novel heparin/heparan sulfate binding protein, of human uterine epithelial cells and cell lines. J Biol Chem. 1996;271(20):11824–30. doi: 10.1074/jbc.271.20.11824. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, Zhou F, Hook M, Carson DD. A heparin-binding synthetic peptide of heparin/heparan sulfate-interacting protein modulates blood coagulation activities. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(5):1739–44. doi: 10.1073/pnas.94.5.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Julian J, Das SK, Dey SK, Baraniak D, Ta VT, Carson DD. Expression of heparin/heparan sulfate interacting protein/ribosomal protein l29 during the estrous cycle and early pregnancy in the mouse. Biol Reprod. 2001;64(4):1165–75. doi: 10.1095/biolreprod64.4.1165. [DOI] [PubMed] [Google Scholar]
- 35.Bame KJ. Heparanases: endoglycosidases that degrade heparan sulfate proteoglycans. Glycobiology. 2001;11(6):91R–8R. doi: 10.1093/glycob/11.6.91r. [DOI] [PubMed] [Google Scholar]
- 36.Nakajima M, Irimura T, Di Ferrante N, Nicolson GL. Metastatic melanoma cell heparanase. Characterization of heparan sulfate degradation fragments produced by B16 melanoma endoglucuronidase. J Biol Chem. 1984;259(4):2283–90. [PubMed] [Google Scholar]
- 37.Freeman C, Browne AM, Parish CR. Evidence that platelet and tumour heparanases are similar enzymes. Biochem J. 1999;342(Pt 2):361–8. [PMC free article] [PubMed] [Google Scholar]
- 38.Sewell RF, Brenchley PE, Mallick NP. Human mononuclear cells contain an endoglycosidase specific for heparan sulphate glycosaminoglycan demonstrable with the use of a specific solid-phase metabolically radiolabelled substrate. Biochem J. 1989;264(3):777–83. doi: 10.1042/bj2640777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laskov R, Michaeli RI, Sharir H, Yefenof E, Vlodavsky I. Production of heparanase by normal and neoplastic murine B-lymphocytes. Int J Cancer. 1991;47(1):92–8. doi: 10.1002/ijc.2910470117. [DOI] [PubMed] [Google Scholar]
- 40.Matzner Y, Bar-Ner M, Yahalom J, Ishai-Michaeli R, Fuks Z, Vlodavsky I. Degradation of heparan sulfate in the subendothelial extracellular matrix by a readily released heparanase from human neutrophils. Possible role in invasion through basement membranes. J Clin Invest. 1985;76(4):1306–13. doi: 10.1172/JCI112104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mollinedo F, Nakajima M, Llorens A, Barbosa E, Callejo S, Gajate C, et al. Major co-localization of the extracellular-matrix degradative enzymes heparanase and gelatinase in tertiary granules of human neutrophils. Biochem J. 1997;327(Pt 3):917–23. doi: 10.1042/bj3270917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levy-Adam F, Miao HQ, Heinrikson RL, Vlodavsky I, Ilan N. Heterodimer formation is essential for heparanase enzymatic activity. Biochem Biophys Res Commun. 2003;308(4):885–91. doi: 10.1016/s0006-291x(03)01478-5. [DOI] [PubMed] [Google Scholar]
- 43.Miao HQ, Navarro E, Patel S, Sargent D, Koo H, Wan H, et al. Cloning, expression, and purification of mouse heparanase. Protein Expr Purif. 2002;26(3):425–31. doi: 10.1016/s1046-5928(02)00558-2. [DOI] [PubMed] [Google Scholar]
- 44.Goldshmidt O, Zcharia E, Cohen M, Aingorn H, Cohen I, Nadav L, et al. Heparanase mediates cell adhesion independent of its enzymatic activity. Faseb J. 2003;17(9):1015–25. doi: 10.1096/fj.02-0773com. [DOI] [PubMed] [Google Scholar]
- 45.Nasser NJ, Avivi A, Shushy M, Vlodavsky I, Nevo E. Cloning, expression, and characterization of an alternatively spliced variant of human heparanase. Biochem Biophys Res Commun. 2007;354(1):33–8. doi: 10.1016/j.bbrc.2006.12.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKenzie E, Tyson K, Stamps A, Smith P, Turner P, Barry R, et al. Cloning and expression profiling of Hpa2, a novel mammalian heparanase family member. Biochem Biophys Res Commun. 2000;276(3):1170–7. doi: 10.1006/bbrc.2000.3586. [DOI] [PubMed] [Google Scholar]
- 47.Vlodavsky I, Goldshmidt O, Zcharia E, Metzger S, Chajek-Shaul T, Atzmon R, et al. Molecular properties and involvement of heparanase in cancer progression and normal development. Biochimie. 2001;83(8):831–9. doi: 10.1016/s0300-9084(01)01318-9. [DOI] [PubMed] [Google Scholar]
- 48.Elkin M, Ilan N, Ishai-Michaeli R, Friedmann Y, Papo O, Pecker I, et al. Heparanase as mediator of angiogenesis: mode of action. Faseb J. 2001;15(9):1661–3. doi: 10.1096/fj.00-0895fje. [DOI] [PubMed] [Google Scholar]
- 49.Gingis-Velitski S, Zetser A, Flugelman MY, Vlodavsky I, Ilan N. Heparanase induces endothelial cell migration via protein kinase B/Akt activation. J Biol Chem. 2004;279(22):23536–41. doi: 10.1074/jbc.M400554200. [DOI] [PubMed] [Google Scholar]
- 50.Goshen R, Hochberg AA, Korner G, Levy E, Ishai-Michaeli R, Elkin M, et al. Purification and characterization of placental heparanase and its expression by cultured cytotrophoblasts. Mol Hum Reprod. 1996;2(9):679–84. doi: 10.1093/molehr/2.9.679. [DOI] [PubMed] [Google Scholar]
- 51.Dempsey LA, Plummer TB, Coombes SL, Platt JL. Heparanase expression in invasive trophoblasts and acute vascular damage. Glycobiology. 2000;10(5):467–75. doi: 10.1093/glycob/10.5.467. [DOI] [PubMed] [Google Scholar]
- 52.Kizaki K, Nakano H, Takahashi T, Imai K, Hashizume K. Expression of heparanase mRNA in bovine placenta during gestation. Reproduction. 2001;121(4):573–80. [PubMed] [Google Scholar]
- 53.Kizaki K, Yamada O, Nakano H, Takahashi T, Yamauchi N, Imai K, et al. Cloning and localization of heparanase in bovine placenta. Placenta. 2003;24(4):424–30. doi: 10.1053/plac.2002.0909. [DOI] [PubMed] [Google Scholar]
- 54.Hasengaowa, Kodama J, Kusumoto T, Seki N, Matsuo T, Ojima Y, et al. Heparanase expression in both normal endometrium and endometrial cancer. Int J Gynecol Cancer. 2006;16(3):1401–6. doi: 10.1111/j.1525-1438.2006.00614.x. [DOI] [PubMed] [Google Scholar]
- 55.Xu X, Ding J, Rao G, Shen J, Prinz RA, Rana N, et al. Estradiol induces heparanase-1 expression and heparan sulphate proteoglycan degradation in human endometrium. Hum Reprod. 2007;22(4):927–37. doi: 10.1093/humrep/del483. [DOI] [PubMed] [Google Scholar]
- 56.Zcharia E, Metzger S, Chajek-Shaul T, Aingorn H, Elkin M, Friedmann Y, et al. Transgenic expression of mammalian heparanase uncovers physiological functions of heparan sulfate in tissue morphogenesis, vascularization, and feeding behavior. Faseb J. 2004;18(2):252–63. doi: 10.1096/fj.03-0572com. [DOI] [PubMed] [Google Scholar]
- 57.Revel A, Helman A, Koler M, Shushan A, Goldshmidt O, Zcharia E, et al. Heparanase improves mouse embryo implantation. Fertil Steril. 2005;83(3):580–6. doi: 10.1016/j.fertnstert.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 58.D’Souza SS, Daikoku T, Farach-Carson MC, Carson DD. Heparanase Expression and Function During Early Pregnancy in Mice. Biol Reprod. 2007 doi: 10.1095/biolreprod.107.061317. [DOI] [PubMed] [Google Scholar]
- 59.Temkin V, Aingorn H, Puxeddu I, Goldshmidt O, Zcharia E, Gleich GJ, et al. Eosinophil major basic protein: first identified natural heparanase-inhibiting protein. J Allergy Clin Immunol. 2004;113(4):703–9. doi: 10.1016/j.jaci.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 60.Plager DA, Adolphson CR, Gleich GJ. A novel human homolog of eosinophil major basic protein. Immunological reviews. 2001;179:192–202. doi: 10.1034/j.1600-065x.2001.790119.x. [DOI] [PubMed] [Google Scholar]
- 61.Giudice LC, Conover CA, Bale L, Faessen GH, Ilg K, Sun I, et al. Identification and regulation of the IGFBP-4 protease and its physiological inhibitor in human trophoblasts and endometrial stroma: evidence for paracrine regulation of IGF-II bioavailability in the placental bed during human implantation. The Journal of clinical endocrinology and metabolism. 2002;87(5):2359–66. doi: 10.1210/jcem.87.5.8448. [DOI] [PubMed] [Google Scholar]
- 62.Dhoot GK, Gustafsson MK, Ai X, Sun W, Standiford DM, Emerson CP., Jr. Science. 5535. Vol. 293. New York, NY: 2001. Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase; pp. 1663–6. [DOI] [PubMed] [Google Scholar]
- 63.Morimoto-Tomita M, Uchimura K, Werb Z, Hemmerich S, Rosen SD. Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. J Biol Chem. 2002;277(51):49175–85. doi: 10.1074/jbc.M205131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nagamine S, Koike S, Keino-Masu K, Masu M. Expression of a heparan sulfate remodeling enzyme, heparan sulfate 6-O-endosulfatase sulfatase FP2, in the rat nervous system. Brain Res Dev Brain Res. 2005;159(2):135–43. doi: 10.1016/j.devbrainres.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 65.Ohto T, Uchida H, Yamazaki H, Keino-Masu K, Matsui A, Masu M. Identification of a novel nonlysosomal sulphatase expressed in the floor plate, choroid plexus and cartilage. Genes Cells. 2002;7(2):173–85. doi: 10.1046/j.1356-9597.2001.00502.x. [DOI] [PubMed] [Google Scholar]
- 66.Uchimura K, Morimoto-Tomita M, Bistrup A, Li J, Lyon M, Gallagher J, et al. HSulf-2, an extracellular endoglucosamine-6-sulfatase, selectively mobilizes heparin-bound growth factors and chemokines: effects on VEGF, FGF-1, and SDF-1. BMC Biochem. 2006;7:2. doi: 10.1186/1471-2091-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ai X, Do AT, Lozynska O, Kusche-Gullberg M, Lindahl U, Emerson CP., Jr. QSulf1 remodels the 6-O sulfation states of cell surface heparan sulfate proteoglycans to promote Wnt signaling. The Journal of cell biology. 2003;162(2):341–51. doi: 10.1083/jcb.200212083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lai J, Chien J, Staub J, Avula R, Greene EL, Matthews TA, et al. Loss of HSulf-1 up-regulates heparin-binding growth factor signaling in cancer. J Biol Chem. 2003;278(25):23107–17. doi: 10.1074/jbc.M302203200. [DOI] [PubMed] [Google Scholar]
- 69.Lai JP, Chien J, Strome SE, Staub J, Montoya DP, Greene EL, et al. HSulf-1 modulates HGF-mediated tumor cell invasion and signaling in head and neck squamous carcinoma. Oncogene. 2004;23(7):1439–47. doi: 10.1038/sj.onc.1207258. [DOI] [PubMed] [Google Scholar]
- 70.Viviano BL, Paine-Saunders S, Gasiunas N, Gallagher J, Saunders S. Domain-specific modification of heparan sulfate by Qsulf1 modulates the binding of the bone morphogenetic protein antagonist Noggin. J Biol Chem. 2004;279(7):5604–11. doi: 10.1074/jbc.M310691200. [DOI] [PubMed] [Google Scholar]
- 71.Wang S, Ai X, Freeman SD, Pownall ME, Lu Q, Kessler DS, et al. QSulf1, a heparan sulfate 6-O-endosulfatase, inhibits fibroblast growth factor signaling in mesoderm induction and angiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(14):4833–8. doi: 10.1073/pnas.0401028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lum DH, Tan J, Rosen SD, Werb Z. Gene trap disruption of the mouse heparan sulfate 6-O-endosulfatase gene, Sulf2. Molecular and cellular biology. 2007;27(2):678–88. doi: 10.1128/MCB.01279-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raab G, Kover K, Paria BC, Dey SK, Ezzell RM, Klagsbrun M. Development. 2. Vol. 122. Cambridge, England: 1996. Mouse preimplantation blastocysts adhere to cells expressing the transmembrane form of heparin-binding EGF-like growth factor; pp. 637–45. [DOI] [PubMed] [Google Scholar]
- 74.Leach RE, Kilburn B, Wang J, Liu Z, Romero R, Armant DR. Heparin-binding EGF-like growth factor regulates human extravillous cytotrophoblast development during conversion to the invasive phenotype. Dev Biol. 2004;266(2):223–37. doi: 10.1016/j.ydbio.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 75.Patel Y, Kim H, Rappolee DA. A role for hepatocyte growth factor during early postimplantation growth of the placental lineage in mice. Biol Reprod. 2000;62(4):904–12. doi: 10.1095/biolreprod62.4.904. [DOI] [PubMed] [Google Scholar]
- 76.Muhlhauser J, Marzioni D, Morroni M, Vuckovic M, Crescimanno C, Castellucci M. Codistribution of basic fibroblast growth factor and heparan sulfate proteoglycan in the growth zones of the human placenta. Cell and tissue research. 1996;285(1):101–7. doi: 10.1007/s004410050625. [DOI] [PubMed] [Google Scholar]
- 77.Anteby EY, Natanson-Yaron S, Hamani Y, Sciaki Y, Goldman-Wohl D, Greenfield C, et al. Fibroblast growth factor-10 and fibroblast growth factor receptors 1-4: expression and peptide localization in human decidua and placenta. European journal of obstetrics, gynecology, and reproductive biology. 2005;119(1):27–35. doi: 10.1016/j.ejogrb.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 78.Demir R, Kayisli UA, Cayli S, Huppertz B. Sequential steps during vasculogenesis and angiogenesis in the very early human placenta. Placenta. 2006;27(67):535–9. doi: 10.1016/j.placenta.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 79.Paria BC, Ma W, Tan J, Raja S, Das SK, Dey SK, et al. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(3):1047–52. doi: 10.1073/pnas.98.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pfendler KC, Yoon J, Taborn GU, Kuehn MR, Iannaccone PM. Nodal and bone morphogenetic protein 5 interact in murine mesoderm formation and implantation. Genesis. 2000;28(1):1–14. doi: 10.1002/1526-968x(200009)28:1<1::aid-gene10>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 81.Cross JC, Nakano H, Natale DR, Simmons DG, Watson ED. Branching morphogenesis during development of placental villi. Differentiation; research in biological diversity. 2006;74(7):393–401. doi: 10.1111/j.1432-0436.2006.00103.x. [DOI] [PubMed] [Google Scholar]
- 82.Fan QW, Muramatsu T, Kadomatsu K. Distinct expression of midkine and pleiotrophin in the spinal cord and placental tissues during early mouse development. Development, growth & differentiation. 2000;42(2):113–9. doi: 10.1046/j.1440-169x.2000.00497.x. [DOI] [PubMed] [Google Scholar]
- 83.Miner JH, Li C, Mudd JL, Go G, Sutherland AE. Development. 10. Vol. 131. Cambridge, England: 2004. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation; pp. 2247–56. [DOI] [PubMed] [Google Scholar]
- 84.Scheele S, Falk M, Franzen A, Ellin F, Ferletta M, Lonai P, et al. Laminin alpha1 globular domains 4-5 induce fetal development but are not vital for embryonic basement membrane assembly. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(5):1502–6. doi: 10.1073/pnas.0405095102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kayisli UA, Korgun ET, Akkoyunlu G, Arici A, Demir R. Expression of integrin alpha5 and integrin beta4 and their extracellular ligands fibronectin and laminin in human decidua during early pregnancy and its sex steroid-mediated regulation. Acta histochemica. 2005;107(3):173–85. doi: 10.1016/j.acthis.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 86.Rider V, Carlone DL, Witrock D, Cai C, Oliver N. Uterine fibronectin mRNA content and localization are modulated during implantation. Dev Dyn. 1992;195(1):1–14. doi: 10.1002/aja.1001950102. [DOI] [PubMed] [Google Scholar]
- 87.Schultz JF, Mayernik L, Rout UK, Armant DR. Integrin trafficking regulates adhesion to fibronectin during differentiation of mouse peri-implantation blastocysts. Developmental genetics. 1997;21(1):31–43. doi: 10.1002/(SICI)1520-6408(1997)21:1<31::AID-DVG4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 88.Lessey BA, Castelbaum AJ. Integrins and implantation in the human. Reviews in endocrine & metabolic disorders. 2002;3(2):107–17. doi: 10.1023/a:1015450727580. [DOI] [PubMed] [Google Scholar]
- 89.Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, et al. Science. 5605. Vol. 299. New York, NY: 2003. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface; pp. 405–8. [DOI] [PubMed] [Google Scholar]
- 90.Prakobphol A, Genbacev O, Gormley M, Kapidzic M, Fisher SJ. A role for the L-selectin adhesion system in mediating cytotrophoblast emigration from the placenta. Dev Biol. 2006;298(1):107–17. doi: 10.1016/j.ydbio.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 91.Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF. CYR61 (CCN1) is essential for placental development and vascular integrity. Molecular and cellular biology. 2002;22(24):8709–20. doi: 10.1128/MCB.22.24.8709-8720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]