Abstract
Aurintricarboxylic acid (ATA), an inhibitor of endonuclease activity and other protein–nucleic acid interactions, blocks apoptosis in several cell types and prevents delayed death of hippocampal pyramidal CA1 neurons induced by transient global ischemia. Global ischemia in rats and gerbils induces down-regulation of GluR2 mRNA and increased α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-induced Ca2+ influx in CA1 before neurodegeneration. This result and neuroprotection by antagonists of AMPA receptors suggests that formation of AMPA receptors lacking GluR2, and therefore Ca2+ permeable, leads to excessive Ca2+ influx in response to endogenous glutamate; the resulting delayed neuronal death in CA1 exhibits many characteristics of apoptosis. In this study, we examined the effects of ATA on expression of mRNAs encoding glutamate receptor subunits in gerbil hippocampus after global ischemia. Administration of ATA by injection into the right cerebral ventricle 1 h before (but not 6 h after) bilateral carotid occlusion prevented the ischemia-induced decrease in GluR2 mRNA expression and the delayed neurodegeneration. These findings suggest that ATA is neuroprotective in ischemia by blocking the transcriptional changes leading to down-regulation of GluR2, rather than by simply blocking endonucleases, which presumably act later after Ca2+ influx initiates apoptosis. Maintaining formation of Ca2+ impermeable, GluR2 containing AMPA receptors could prevent delayed death of CA1 neurons after transient global ischemia, and block of GluR2 down-regulation may provide a further strategy for neuroprotection.
Keywords: transient ischemia, degeneration, AMPA receptors, GluR2, mRNA expression, excitotoxicity
Transient forebrain or global ischemia, induced in animals or occurring in humans as a result of cardiac arrest, is followed by delayed neurodegeneration in specific neuronal populations. Pyramidal neurons of the hippocampal CA1 are particularly vulnerable (for review, see ref. 1). During a brief episode of global ischemia (5–10 min) in rat or gerbil, extracellular concentrations of glutamate and K+ and intracellular levels of free Ca2+ rise throughout the forebrain, and neurons depolarize and become inexcitable. However, within minutes after reperfusion, the extracellular milieu is restored, cytoplasmic Ca2+ returns to resting levels, and neurons can again generate action potentials (2–4; see ref. 5). In the hippocampal CA1, this recovery is transient and after several days is followed by extensive degeneration of pyramidal neurons (6–8).
Global ischemia leads to specific down-regulation of GluR2 mRNA localized to the vulnerable CA1 before histologically detectable cell death (5, 9–12). Because α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors lacking the GluR2 subunit have markedly increased Ca2+ permeability (for review, see ref. 13), down-regulation of GluR2 mRNA while GluR1 mRNA levels are essentially unchanged would lead to increased formation of Ca2+ permeable AMPA receptors and increased toxicity of endogenous glutamate (the GluR2 hypothesis, refs. 14 and 15). In agreement with this prediction, postischemic CA1 neurons exhibit greater AMPA-induced Ca2+ influx than do control CA1 neurons, and this increase in Ca2+ influx occurs before obvious degeneration (5). The AMPA receptor-mediated excitatory post-synaptic currents in postischemic CA1 neurons show greater sensitivity to block by 1-naphthyl-acetyl-spermine (16), a channel blocker selective for Ca2+ permeable AMPA receptors (17, 18). The excitatory post-synaptic currents also are increased in duration, but this change has no obvious relation to the change in GluR2 expression.
An additional foundation of the GluR2 hypothesis is the observation that antagonists of AMPA and kainate receptors [but not of N-methyl-d-aspartate (NMDA) receptors] are effective in preventing or greatly delaying hippocampal degeneration after global ischemia, even when given 16 to 24 h after reperfusion (19–24; for review, see ref. 25). Neuroprotective doses of AMPA antagonists given at the time of reperfusion or 8 h later do not prevent down-regulation of GluR2 mRNA, suggesting that the protection results from blockade of Ca2+ permeable receptors rather than from prevention of their formation (10).
The mechanism of delayed neuronal death after ischemia remains somewhat controversial. There are alterations in gene and protein expression and fragmentation of DNA consistent with apoptosis (26–30), but others fail to find the characteristic morphological changes of this process (31).
Down-regulation of GluR2 also is thought to play a role in delayed neurodegeneration after kainic acid-induced status epilepticus; in this paradigm, the CA3 region exhibits delayed neurodegeneration and selective down-regulation of GluR2 (32). Furthermore, hippocampal neurons subject to oxygen and glucose deprivation in culture show increased Ca2+ permeability of AMPA receptors and greater sensitivity to kainate excitotoxicity (33).
The present study was undertaken to investigate further the molecular mechanisms underlying ischemia-induced neuronal cell death. By using the two-vessel occlusion model in gerbils, we examined the effect of aurintricarboxylic acid (ATA) on changes in AMPA- and NMDA-receptor gene expression induced by global ischemia. ATA administered 1 h before induction of global ischemia prevents the delayed neuronal cell death in the hippocampal CA1 (measured by spectrin breakdown, ref. 34; and by histologic examination, ref. 30). ATA also reduces delayed neurodegeneration after retinal ischemia (35). ATA blocks apoptosis in PC12 cells and neuronal cultures (36, 37) and can act as an endonuclease inhibitor (38). ATA binds nonspecifically to nucleic acids as well as proteins and can inhibit other protein–nucleic acid interactions, such as transcription and protein synthesis (e.g., refs. 39–41). ATA apparently can act as a competitive antagonist at NMDA receptors (42), but, as noted above, NMDA antagonists appear not to be neuroprotective in global ischemia (10, 21).
We show here that ATA sufficient to protect CA1 neurons against delayed neurodegeneration after global ischemia prevents the down-regulation of GluR2 mRNA. Moreover, ATA administered 6 h after reperfusion neither prevents down-regulation of GluR2 nor provides neuroprotection. These results suggest that ATA given before the insult protects by blocking the transcriptional changes that lead to GluR2 down-regulation and only indirectly prevents the later changes in gene expression that immediately precede cell death (30, 43, 44). These results provide additional support for the hypothesis that reduction in GluR2 expression and formation of Ca2+ permeable AMPA receptors play a causal role in the selective cell death of CA1 pyramidal cells after global ischemia.
MATERIALS AND METHODS
Induction of Ischemia and Drug Administration.
Adult male (60–80 g) Mongolian gerbils (Tumblebrook Farms, Westbrookfield, MA) were maintained in a temperature and light-controlled environment with a 14-h light/10-h dark cycle. Animals were treated in accordance with the principles and procedures of the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Before the induction of global ischemia, animals were fasted overnight and then anesthetized with i.p. injection of ketamine (80 mg/kg) and xylazine (6 mg/kg). ATA (4 μg in 1 μl saline, n = 7) or saline (1 μl, n = 8) was injected into the right lateral ventricle of the brain (intracerebroventricular, i.c.v., injection; ref. 34). Examination of the brains showed that ATA injected into one ventricle spread to the contralateral ventricle. The concentration of ATA used is neuroprotective in the gerbil, whereas 10 times lower or higher concentrations are ineffective; the higher concentration may be toxic (30).
One hour after i.c.v. injection of ATA or saline or with no injection, gerbils were subjected to forebrain ischemia by temporary bilateral occlusion of the carotid arteries (n = 7 for ATA, n = 4 for saline, and n = 4 for no injection). The carotids were occluded with nontraumatic aneurism clips, which were removed after 5 min to allow cerebral reperfusion. Body temperature was maintained at 37.5°C with a rectal thermistor and heating lamp until thermal homeostasis was restored. Sham-operated control gerbils (n = 4) underwent the same surgical procedure except for occlusion of the carotids and received no i.c.v. injection. In another series of seven animals, ATA was injected 6 h after induction of ischemia; there was one sham-operated control. After the procedures, the gerbils were housed individually. For in situ hybridization, animals were killed at 48 h, a time at which there is no histologically detectable degeneration (5). After decapitation under halothane anesthesia, the brains were rapidly removed and frozen in isopentane over dry ice. For assay of neurodegeneration animals were killed at 7 d when cell loss is extensive. After induction of halothane anesthesia, the animals were perfused intracardially with phosphate buffered 10% formalin, and the brains were removed immediately, sectioned at 3 mm in coronal planes, and postfixed in the same fixative.
Histological Analysis.
Neuronal cell loss in the hippocampus was assessed by histological examination of coronal sections. Thionin staining was performed before in situ hybridization on air dried sections from all of the animals killed at 48 h. Coronal blocks from brains of animals killed 7 d after ischemia were embedded in paraffin, sectioned at 7 μm, and stained with hematoxylin/eosin.
In Situ Hybridization.
[35S]UTP-labeled RNA probes were transcribed from the cDNAs for the GluR1 and GluR2 AMPA-receptor subunits and for the NR1011 NMDA receptor subunit. cDNA templates were incubated with the appropriate polymerase (T7 for GluR1–2; T3 for NR1) and labeled and unlabeled nucleotides (1 h, 37°C), using a Stratagene transcription kit. RNA probes were purified by phenol/chloroform extraction.
Glutamate receptor mRNA expression was measured by in situ hybridization on sections of control and postischemic gerbil brains by a modification of Pellegrini-Giampietro et al. (45). In brief, coronal sections (20 μm) of frozen brains from sham-operated control (n = 4 with saline injection and n = 4 without) and 48-h postischemic gerbils treated with saline (n = 8) or ATA (n = 7) were hybridized with [35S]UTP-labeled RNA probes directed against the GluR1, GluR2, and NR1 subunit mRNAs. Before application of RNA probe, sections were subjected to acetylation and incubated for 2 h at 50°C with 100 μl of prehybridization solution. For hybridization, slides were incubated overnight at 50°C with the 35S-labeled RNA probe (106 cpm/section, 1 ng/μl). Sections were treated with RNase A (20 μg/ml) and dehydrated in ethanol. Slides were apposed to Kodak XAR-5 film for 24–72 h. To enable comparisons between groups for any given RNA probe, brain sections from control and postischemic gerbils with different treatments were cut in the same experimental session, incubated with the same solution of probe on the same day, and apposed to the same sheet of film.
Signal specificity was assessed in a parallel study by competition experiments in which radiolabeled probes were hybridized to sections in the presence of excess (100-fold) of the same unlabeled probe (5). This procedure resulted in virtually blank autoradiograms. Similarly, sections pretreated with RNase A (100 μg/ml) showed no detectable labeling by the probes (5). Conditions were of sufficiently high stringency as to rule out cross-hybridization among GluR1, GluR2, and GluR3 (45) and more distantly related glutamate receptor subunits (GluR5–GluR7, KA1, and KA2). The GluR1 and GluR2 probes are “pan” probes (46) in that they label both “flip” and “flop” splice variants. The NR1 probe is a “pan” probe in that it labels all of the splice variants.
For quantification of mRNA expression levels, autoradiograms were analyzed with a Molecular Dynamics 300A Computing Densitometer and the National Institutes of Health image software. Films were scanned at 2,000 dots/inch resolution, and images of each section (≈106 pixels) were created. Optical densities of the CA1 and CA3 pyramidal cell layers and of the dentate granule cell layer from two or three sections from each animal for each probe were background subtracted and averaged. Optical density values were expressed as grand means (±SEM) of individual means from each group of gerbils.
Mean optical density readings were statistically analyzed by the Student’s unpaired t test. Percentage change in optical densities for postischemic gerbils was expressed relative to optical densities for the corresponding regions of the control gerbil in the same film. The rationale of the quantitative analysis was based on the following factors. (i) Optical density readings taken from each hippocampal region of interest varied little in different sections from the same animals. (ii) The concentration of RNA probe used (106 cpm/section) produced saturating levels of hybridization and the maximal signal-to-noise ratio for the probes used. (iii) Use of [35S]UTP-labeled brain paste standards by Pellegrini-Giampietro et al. (45) indicated that exposure times were in the linear response range of the film.
RESULTS
ATA Protects Hippocampal Neurons from Damage Induced by Global Ischemia.
Seven days after 5-min global ischemia in gerbils (with saline injection 1 h before), there was extensive neurodegeneration of CA1 pyramidal cells in the hippocampus with little or no loss in CA3 or the dentate gyrus (Fig. 1 C and D, n = 8, sham-operated controls without injection in Fig. 1 A and B, n = 4). Only a few surviving neurons remained in CA1 that also may have been deteriorating. Histological signs of degeneration in these neurons were not apparent at 72 h after the ischemic insult (in toluidine blue-stained eponate-embedded sections, ref. 5). These results confirm the findings of others (1).
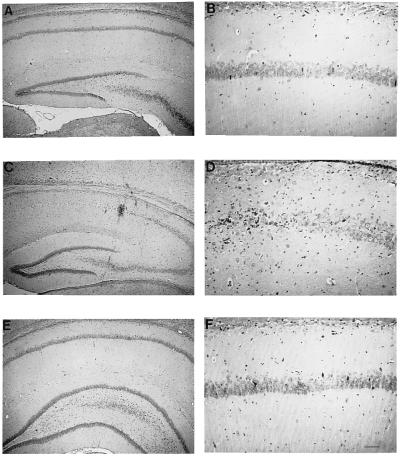
Figure 1.
ATA prevents ischemia-induced degeneration of CA1 pyramidal neurons of gerbil. Photomicrographs of hematoxylin/eosin-stained coronal sections of rat brain at the level of the hippocampus, higher magnification in the right column. (A and B) Representative sham-operated control. (C and D) One week after saline injection and 5-min occlusion of the carotids, most neurons in the CA1 have degenerated, whereas CA3 and dentate gyrus appear unaffected. (E and F) One week after ATA injection followed after 1 h by 5-min occlusion of the carotids, neurons in CA1, as well as CA3 and dentate gyrus, appear unaffected. (Scale bar in F: 240 μm for A, C, and E; 60 μm for B, D, and F.)
I.c.v. injection of ATA 1 h before the 5-min ischemic period almost completely prevented the delayed cell death in CA1 (Fig. 1 E and F, n = 16, see ref. 30).
GluR2 mRNA Is Specifically Down-Regulated in the PostIschemic CA1 Region before Neurodegeneration.
Expression of glutamate receptor mRNAs in gerbil brain was examined by in situ hybridization with riboprobes specific for GluR1, GluR2, and NR1 mRNAs. Autoradiograms of coronal sections of brains of sham-operated gerbils showed hippocampal distributions of GluR1, GluR2, and NR1 mRNAs (Fig. 2 A, D and G; see also ref. 5) similar to those observed in the rat (45, 47, 48). GluR1 and GluR2 were expressed at high levels in the pyramidal cell layer of CA1 and CA3 and in the granule cell layer of the dentate gyrus (Fig. 2 A and D). NMDAR1 mRNA had a similar distribution (Fig. 2G).
Figure 2.
Pretreatment with ATA prevents ischemia-induced down-regulation of GluR2 mRNA in CA1 pyramidal neurons of gerbil. Determinations at 48 h after 5 min of ischemia, 1 h after i.c.v. injection of ATA. Photomicrographs of autoradiograms of GluR1, GluR2, and NMDAR1 mRNAs detected by in situ hybridization in coronal sections at the level of the hippocampus. Levels are essentially uniform through the hippocampal cell layers in sham-operated (control) animals (Left). (A–C) After 48 h, GluR1 mRNA is at similar levels in sham-operated gerbils (A) and in gerbils injected i.c.v. with either saline (B) or ATA (C) 1 h before 5 min of ischemia. (D–F) GluR2 mRNA was dramatically reduced in CA1 (arrowhead), but not in the other hippocampal regions after ischemia with saline pretreatment (E). Pretreatment with ATA prevented the ischemia-induced decrease in GluR2 mRNA in CA1 and had no effect on GluR2 expression in CA3 and dentate gyrus (F). (G–I) NR1 mRNA was unaffected by ischemia with saline (H) or with ATA pretreatment (I).
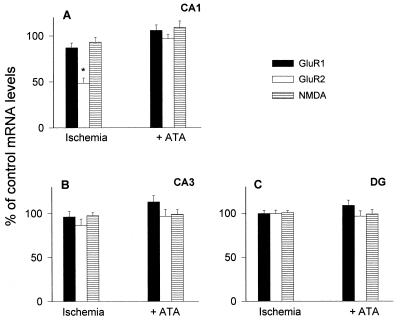
At 48 h after 5-min global ischemia, GluR2 mRNA was specifically decreased in the pyramidal cell layer of the vulnerable CA1 (Fig. 2E) (to 42 ± 7% of control levels, n = 8), whereas GluR1 and NR1 mRNAs were little changed in this region (Fig. 2 B and H) (to 87 ± 5% and 93 ± 5% of control, respectively, n = 6, Fig. 3A). All three mRNAs were essentially unchanged in the pyramidal cell layer of CA3 and the granule cell layer of dentate gyrus (Fig. 2 B, E, and H; Fig. 3 B and C). Thionin staining of the sections revealed no neuronal degeneration at this time. These data confirm the findings of Gorter et al. (5). The selective decrease in GluR2 expression after ischemia is localized to CA1 pyramidal neurons as demonstrated by examination of emulsion-dipped sections (5).
Figure 3.
Expression of GluR1, GluR2, and NR1 mRNAs in gerbil hippocampus 48 h after global ischemia with and without ATA pretreatment. Ischemia was followed by down-regulation of GluR2 specific to CA1; this effect was blocked by i.c.v. injection of ATA 1 h before the ischemic episode. (A) CA1. (B) CA3. (C) dentate gyrus. Optical density values are plotted as percentage (± SEM) of densities in sham-operated animals. Gerbils were injected i.c.v. with either saline (n = 4, bars labeled ischemia) or ATA (n = 8; bars labeled +ATA) 10 min before induction of 5 min of ischemia. Four additional animals subjected to ischemia but with no injection are included with the saline-injected animals from which they did not differ. DG, dentate gyrus. ∗, P < 0.01 GluR2 in CA1 ischemia vs. sham-operated and vs. ATA- injected.
Early but Not Late Application of ATA Prevents the PostIschemic Decrease in GluR2 mRNA.
I.c.v. injection of ATA 1 h before induction of ischemia virtually abolished the subsequent decrease in expression of GluR2 mRNA in CA1. In CA1, as well as in CA3 and dentate gyrus, GluR1, GluR2, and NR1 mRNAs were at or near control levels (Fig. 2 C, F, and I, Fig. 3A–C).
ATA injected intraventricularly 6 h after ischemia had no protective effect; CA1 showed virtually complete cell loss at 7 d (30). Moreover, at 48 h, GluR2 was down-regulated in CA1 to a similar extent compared with CA3 and dentate gyrus as in postischemic animals that were not injected with ATA (n = 7, Fig. 4). These observations suggest that ATA maybe neuroprotective because it prevents the down-regulation of GluR2.
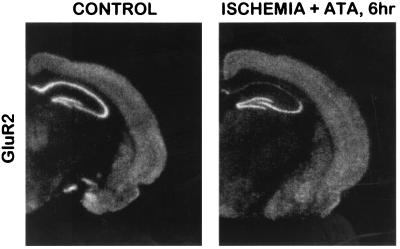
Figure 4.
ATA injected 6 h after an ischemic episode does not prevent ischemia-induced down-regulation of GluR2 in CA1. (A) Autoradiogram of sham-operated control, as in Fig. 2. (B) Autoradiogram from gerbil injected i.c.v. with ATA 6 h after 5 min of ischemia and killed at 48 h. GluR2 mRNA is markedly reduced in CA1 but is at control levels in CA3 and dentate gyrus.
Injection of ATA at the dose used for neuroprotection by itself had no obvious effect on hippocampal neurons examined histologically 7 d later (not shown, n = 2, see ref. 30).
DISCUSSION
The present study confirms that global ischemia induces a marked and selective reduction of GluR2 mRNA in pyramidal neurons of the hippocampal CA1 (5, 9–12). Moreover, ATA given i.c.v. before ischemia prevents the down-regulation of GluR2 and is neuroprotective (see also ref. 30). As noted above, the ischemia-induced down-regulation of GluR2 mRNA, although delayed by 24–48 h, precedes histologically detectable cell death in the CA1. Because AMPA receptors lacking the GluR2 subunit are relatively Ca2+ permeable (see ref. 13), the decrease in GluR2 mRNA could lead to increased formation of Ca2+ permeable AMPA receptors and increased Ca2+ influx in response to endogenous glutamate. This abnormal Ca2+ influx could cause or contribute to the observed delayed neuronal death. Consistent with this hypothesis, antagonists selective for AMPA and kainate receptors protect against postischemic cell death in CA1 of gerbils and rats (19–24; for review, see ref. 25). However, in contrast to our result with ATA, neuroprotective doses of AMPA-receptor antagonists given at the time of reperfusion or 8 h later do not affect the ischemia-induced down-regulation of GluR2 mRNA (10). A plausible explanation is that the AMPA antagonists remain in the body for some days and neuroprotect by blocking Ca2+ influx through AMPA receptors formed after the down-regulation of GluR2.
Localized injection of 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline (NBQX) into the area tempestas of the prepiriform cortex is neuroprotective of CA1 when given just before an ischemic insult (49). Because injection at this site blocks the ischemia-induced expression of heat shock protein in the hippocampus (50), it also may block down-regulation of GluR2. It remains to be determined whether injection of NBQX into the area tempestas is neuroprotective at the later times when systemic NBQX is effective. It is possible that systemic NBQX is neuroprotective through its action on the area tempestas, activation of which leads to activation of the hippocampus (see ref. 50).
Ischemia-induced cell death is thought to involve activation of immediate early genes (51–54). Calcium-dependent mechanisms have been implicated (2, 44, 55, 56), but chelation of Zn2+ during an ischemic episode is neuroprotective, and the toxic ion that enters the cells at this time may be Zn2+ (57); Zn2+ chelation is not neuroprotective at 1h after ischemia, but later times have not been tested. A transient rise of intracellular Ca2+ (or Zn2+) during global ischemia may trigger a series of transcriptional and translational events that ultimately lead to reduced expression of GluR2 mRNA in CA1 pyramidal neurons. In the present study, we show that intracerebral treatment of gerbils with ATA just before global ischemia virtually abolishes ischemia-induced changes in GluR2 expression in CA1. This effect may result from inhibition by ATA of transcription of immediate early genes, because ATA administered 6 h after ischemia is not neuroprotective and does not prevent the down-regulation of GluR2. ATA protects PC12 cells and sympathetic neurons in culture from apoptotic cell death induced by serum deprivation (36, 37). The lack of protection by ATA given 6 h after ischemia (and failure to block down-regulation of GluR2) suggests that ATA just before the ischemic episode does not act by directly blocking apoptosis (or other cell death program) in CA1 neurons, which is likely to be initiated after the formation of GluR2 lacking Ca2+ permeable AMPA receptors. Possibly, ATA may be protective whether given at a still later time when frank degeneration is beginning. Retinal ischemia is followed by delayed neurodegeneration, and ATA is protective when given just before or 6 h after the ischemic insult (35). The mechanisms of the degeneration have not been explored, and changes in GluR2 (58, 59) and subsequent stages in degeneration may be earlier or later than in the hippocampus; in addition, ATA may be retained better in the affected tissue when injected into the globe rather than into the cerebral ventricles.
Block of GluR2 down-regulation induced by ischemia is observed with neuroprotection by administration of activators of adenosine A1 receptors and of KATP channels and by conditioning with a sublethal period of ischemia (12). Our results using ATA, particularly in combination with those of Heurteaux et al. (12), afford additional support for the GluR2 hypothesis in respect to global ischemia and indicate that prevention of down-regulation of GluR2 may be an important therapy in the prevention of delayed neurodegeneration after ischemia and other neurological insults.
Acknowledgments
We are indebted to Mr. James Anthony for expert technical assistance. This work was supported by the American Heart Association of New York City (Grant-in-Aid to D.M.R.), by National Institutes of Health Grants NS 20778, NS 20013, and NS 34758 (to J.A.K.), NS 07412 (to M.V.L.B.), NS 20752 and NS 31282 (to R.S.Z.), and EY 11253 (to D.M.R.), by an Aaron Diamond Postdoctoral Fellowship Award (to E.M.A.), and by a Human Frontier Science Program Award (to J.A.G). M.V.L.B. is the Sylvia and Robert S. Olnick Professor of Neuroscience.
ABBREVIATIONS
- ATA
aurintricarboxylic acid
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- i.c.v.
intracerebroventricular
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoylbenzo[f]quinoxaline
- NMDA
N-methyl-d-aspartate
References
- 1.Schmidt-Kastner R, Freund T F. Neuroscience. 1991;40:599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- 2.Silver I A, Erecinska M. J Cereb Blood Flow Metab. 1992;12:759–772. doi: 10.1038/jcbfm.1992.107. [DOI] [PubMed] [Google Scholar]
- 3.Torp R, Arvin B, Le Peillet E, Chapman A G, Ottersen O P, Meldrum B S. Exp Brain Res. 1993;96:365–376. doi: 10.1007/BF00234106. [DOI] [PubMed] [Google Scholar]
- 4.Xu Z C, Pulsinelli W A. J Neurophysiol. 1996;76:1689–1697. doi: 10.1152/jn.1996.76.3.1689. [DOI] [PubMed] [Google Scholar]
- 5.Gorter J A, Petrozzino J J, Aronica E M, Rosenbaum D M, Opitz T, Bennett M V L, Connor J A, Zukin R S. J Neurosci. 1997;17:6179–6188. doi: 10.1523/JNEUROSCI.17-16-06179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pulsinelli W A, Brierley J B, Plum F. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 7.Kirino T. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- 8.Hsu M, Sik A, Gallyas F, Horvath Z, Buzsaki G. Ann NY Acad Sci. 1994;743:121–139. doi: 10.1111/j.1749-6632.1994.tb55790.x. [DOI] [PubMed] [Google Scholar]
- 9.Pellegrini-Giampietro D E, Zukin R S, Bennett M V L, Cho S, Pulsinelli W A. Proc Natl Acad Sci USA. 1992;89:10499–10503. doi: 10.1073/pnas.89.21.10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pellegrini-Giampietro D E, Pulsinelli W A, Zukin R S. J Neurochem. 1994;62:1067–1073. doi: 10.1046/j.1471-4159.1994.62031067.x. [DOI] [PubMed] [Google Scholar]
- 11.Pollard H, Heron A, Moreau J, Ben-Ari Y, Khrestchatisky M. Neuroscience. 1993;57:545–554. doi: 10.1016/0306-4522(93)90004-y. [DOI] [PubMed] [Google Scholar]
- 12.Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. Proc Natl Acad Sci USA. 1995;92:4666–4670. doi: 10.1073/pnas.92.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bettler B, Mulle C. Neuropharmacology. 1995;34:123–139. doi: 10.1016/0028-3908(94)00141-e. [DOI] [PubMed] [Google Scholar]
- 14.Bennett M V L, Pellegrini-Giampietro D E, Gorter J A, Aronica E, Connor J A, Zukin R S. Cold Spring Harbor Symp Quant Biol. 1996;61:373–384. [PubMed] [Google Scholar]
- 15.Pellegrini-Giampietro D E, Gorter J A, Bennett M V L, Zukin R S. Trends Neurosci. 1997;20:464–470. doi: 10.1016/s0166-2236(97)01100-4. [DOI] [PubMed] [Google Scholar]
- 16.Tsubokawa H, Oguro K, Masuzawa T, Nakaima T, Kawai N. J Neurophysiol. 1995;74:218–225. doi: 10.1152/jn.1995.74.1.218. [DOI] [PubMed] [Google Scholar]
- 17.Blaschke M, Keller B U, Rivosecchi R, Hollmann M, Heinemann S, Konnerth A. Proc Natl Acad Sci USA. 1993;90:6528–6532. doi: 10.1073/pnas.90.14.6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herlitze S, Raditsch M, Ruppersberg J P, Jahn W, Monyer H, Schoepfer R, Witzemann V. Neuron. 1993;10:1131–1140. doi: 10.1016/0896-6273(93)90061-u. [DOI] [PubMed] [Google Scholar]
- 19.Sheardown M J, Nielsen E O, Hansen A J, Jacobsen P, Honore T. Science. 1990;247:571–574. doi: 10.1126/science.2154034. [DOI] [PubMed] [Google Scholar]
- 20.Buchan A M, Li H, Cho S, Pulsinelli W A. Neurosci Lett. 1991;132:255–258. doi: 10.1016/0304-3940(91)90314-j. [DOI] [PubMed] [Google Scholar]
- 21.Buchan A, Li H, Pulsinelli W A. J Neurosci. 1991;11:1049–1056. doi: 10.1523/JNEUROSCI.11-04-01049.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diemer N H, Jorgensen M B, Johansen F F, Sheardown M, Honore T. Acta Neurol Scand. 1992;86:45–49. doi: 10.1111/j.1600-0404.1992.tb08052.x. [DOI] [PubMed] [Google Scholar]
- 23.Le Peillet E, Arvin B, Moncada C, Meldrum B S. Brain Res. 1992;571:115–120. doi: 10.1016/0006-8993(92)90516-c. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Buchan A M. Can J Neurol Sci. 1995;22:S47. (abstr.). [Google Scholar]
- 25.Ginsberg M D. Neuroscientist. 1995;1:95–103. [Google Scholar]
- 26.MacManus J P, Hill I E, Preston E, Rasquinha I, Walker T, Buchan A M. J Cereb Blood Flow Metab. 1995;15:728–737. doi: 10.1038/jcbfm.1995.93. [DOI] [PubMed] [Google Scholar]
- 27.Nitatori T, Sato N, Waguri S, Karasawa Y, Araki H, Shibanai K, Kominami E, Uchiyama Y. J Neurosci. 1995;15:1001–1011. doi: 10.1523/JNEUROSCI.15-02-01001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honkanieme J, Massa S M, Breckinridge M, Sharp F R. Mol Brain Res. 1996;42:79–88. doi: 10.1016/s0169-328x(96)00121-0. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Jin K, Chen M, Pei W, Kawaguchi K, Greenberg D A, Simon R P. J Neurochem. 1997;69:232–245. doi: 10.1046/j.1471-4159.1997.69010232.x. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbaum, D. H., D’Amore, J., Llena, J., Rybak, S., Balkany, A. & Kessler, J. A. (1998) Ann. Neurol., in press. [DOI] [PubMed]
- 31.Petito C K, Torres-Munoz J, Roberts B, Olarte J P, Nowak T S J, Pulsinelli W A. J Cereb Blood Flow Metab. 1997;17:967–976. doi: 10.1097/00004647-199709000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Friedman L K, Pellegrini-Giampietro D E, Sperber E F, Bennett M V L, Moshe S L, Zukin R S. J Neurosci. 1994;14:2697–2707. doi: 10.1523/JNEUROSCI.14-05-02697.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ying H S, Weishaupt J H, Grabb M, Canzoniero L M T, Sensi S L, Sheline C T, Monyer H, Choi D W. J Neurosci. 1997;15:9536–9544. doi: 10.1523/JNEUROSCI.17-24-09536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts-Lewis J M, Marcy V R, Zhao Y, Vaught J L, Siman R, Lewis M E. J Neurochem. 1993;61:378–381. doi: 10.1111/j.1471-4159.1993.tb03583.x. [DOI] [PubMed] [Google Scholar]
- 35.Rosenbaum D M, Rosenbaum P S, Gupta A, Michaelson M D, Hall D H, Kessler J A. Vision Res. 1997;37:3445–3451. doi: 10.1016/S0042-6989(96)00328-8. [DOI] [PubMed] [Google Scholar]
- 36.Batistatou A, Greene L A. J Cell Biol. 1991;115:461–471. doi: 10.1083/jcb.115.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mesner P W, Winters T R, Green S H. J Cell Biol. 1992;119:1669–1680. doi: 10.1083/jcb.119.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hallick R B, Chelm B K, Gray P W, Orozco E M. Nucleic Acids Res. 1977;4:3055–3064. doi: 10.1093/nar/4.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegelman F, Apirion D. J Bacteriol. 1971;105:902–907. doi: 10.1128/jb.105.3.902-907.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Givens J F, Manly K F. Nucleic Acids Res. 1976;172:1298–1303. doi: 10.1093/nar/3.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzales R G, Haxo R S, Schleich T. Biochemistry. 1980;19:4299–4303. doi: 10.1021/bi00559a023. [DOI] [PubMed] [Google Scholar]
- 42.Zeevalk G D, Schoepp D, Nicklas W J. J Neurochem. 1993;61:386–389. doi: 10.1111/j.1471-4159.1993.tb03585.x. [DOI] [PubMed] [Google Scholar]
- 43.Bredesen D E. Ann Neurol. 1995;38:839–851. doi: 10.1002/ana.410380604. [DOI] [PubMed] [Google Scholar]
- 44.Choi D W. Curr Opin Neurobiol. 1996;6:667–672. doi: 10.1016/s0959-4388(96)80101-2. [DOI] [PubMed] [Google Scholar]
- 45.Pellegrini-Giampietro D E, Bennett M V L, Zukin R S. Proc Natl Acad Sci USA. 1991;88:4157–4161. doi: 10.1073/pnas.88.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sommer B, Keinanen K, Verdoorn T A, Wisden W, Burnashev N, Herb A, Kohler M, Takagi T, Sakmann B, Seeburg P H. Science. 1990;249:1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- 47.Keinanen K, Wisden W, Sommer B, Werner P, Herb A, Verdoorn T A, Sakmann B, Seeburg P H. Science. 1990;249:556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- 48.Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Nature (London) 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- 49.Kawaguchi K, Simon R P. J Cereb Blood Flow Metab. 1997;17:356–360. doi: 10.1097/00004647-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Kawaguchi K, Simon R P. Brain Res. 1997;746:59–62. doi: 10.1016/s0006-8993(96)01096-7. [DOI] [PubMed] [Google Scholar]
- 51.Jorgensen M B, Deckert J, Wright D C, Gehlert D R. Brain Res. 1898;484:393–398. doi: 10.1016/0006-8993(89)90388-0. [DOI] [PubMed] [Google Scholar]
- 52.Heurteaux C, Bertaina V, Widmann C, Lazdunski M. Proc Natl Acad Sci USA. 1993;90:9431–9435. doi: 10.1073/pnas.90.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamme F, Campbell K, Wieloch T. Eur J Neurosci. 1995;7:2007–2016. doi: 10.1111/j.1460-9568.1995.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 54.Choi D W. Trends Neurosci. 1995;18:58–60. [PubMed] [Google Scholar]
- 55.Deshpande J K, Siesjo B K, Wieloch T. J Cereb Blood Flow Metab. 1987;7:89–95. doi: 10.1038/jcbfm.1987.13. [DOI] [PubMed] [Google Scholar]
- 56.Siesjo B K, Siesjo P. Eur J Anaesthesiol. 1996;13:247–268. [PubMed] [Google Scholar]
- 57.Koh J Y, Suh S W, Gwag B J, He Y Y, Hsu C Y, Choi D W. Science. 1996;17:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- 58.Hamassaki-Britto D E, Hermans-Borgmeyer I, Heinemann S, Hughes T E. J Neurosci. 1993;13:1888–1898. doi: 10.1523/JNEUROSCI.13-05-01888.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng Y W, Blackstone C D, Huganir R L, Yau K W. Neuroscience. 1995;66:483–497. doi: 10.1016/0306-4522(94)00569-q. [DOI] [PubMed] [Google Scholar]