Abstract
The determinants of the different biological activities of progesterone receptors (PRs) vs. glucocorticoid receptors (GRs), which bind to the same DNA sequences, remain poorly understood. The mechanisms by which differential expression of a common target gene can be achieved by PR and GR include unequal agonist steroid concentrations for half maximal induction (EC50) and dissimilar amounts of residual partial agonist activity for antisteroids in addition to the more common changes in total gene induction, or Vmax. Several factors are known to alter some or all of these three parameters for GR-regulated gene induction and some (i.e., the corepressors NCoR and SMRT) modulate the EC50 and partial agonist activity for GR and PR induction of the same gene in opposite directions. The current study demonstrates that other factors (GME, GMEB-2, Ubc9, and STAMP) can also differentially interact with PRs and GRs or alter several of the above induction parameters under otherwise identical conditions. These results support the hypothesis that the modulation of EC50, partial agonist activity, and Vmax by a given factor is not limited to one receptor in a specific cell line. Furthermore, the number of factors that unequally modulate PR and GR induction parameters is now greatly expanded, thereby increasing the possible mechanisms for differential gene regulation by PRs vs. GRs.
Keywords: progesterone receptor, glucocorticoid receptor, transcription modulatory factor, GME, GMEB-2, Ubc9, STAMP
1. Introduction
The intracellular receptor proteins for glucocorticoid and progesterone steroid hormones are encoded by different genes but share a common domain organization, and regions of high homology, with the rest of the steroid/nuclear receptor superfamily. All steroid receptors are comprised of a hyper-variable N-terminal domain followed by highly conserved DNA binding domain (DBD) and finally a ligand binding domain (LBD) of varying homology. Glucocorticoid receptors (GRs) and progesterone receptors (PRs) are closely related. They are 90% identical in the DBD and 55% identical in the LBD (Evans, 1988). As might be expected from these degrees of similarily, GRs and PRs preferentially bind different steroids but can bind to, and activate transcription from, the same hormone response elements (HREs) (Cato et al., 1986; Lieberman et al., 1993). In this manner, much of the information contained in the structure of steroidal glucocorticoids vs. progestins appears to have been lost by activating the same HREs. Nevertheless, PRs and GRs display very different biological actions in cells and often regulate different endogenous genes (Wan and Nordeen, 2002). PR and GR can even have opposite effects, such as in bone where glucocorticoids inhibit (Ziegler and Kasperk, 1998) and progestins promote bone formation (Prior, 1990).
Several explanations have been proposed to account for the different biological activities of PRs and GRs, despite their binding to the same DNA sequences, including different levels of the receptor within the cell (Strahle et al., 1989). HRE mutations can variably affect GR vs. PR transactivation (Ricousse et al., 1996), although these differences appear to be less pronounced with similar receptor concentrations (Thackray et al., 1998). HRE flanking and spacer DNA can contribute to the affinity and specificity of receptor binding (Nelson et al., 1999). The DBD of androgen receptor (AR) has lower (76%) homology with the GR DBD (Chang et al., 1988), which seems to be sufficient for DNA binding specificity of AR vs. GR (Massaad et al., 2000; Schoenmakers et al., 2000). Thus subtle differences in HRE sequence may regulate the relative activities of GR and PR (Thackray et al., 1998). Another source of heterogeneity in PR vs. GR transactivation is their interactions with the burgeoning number of transcriptional coactivators, corepressor, and comodulators. Many factors equally affect the maximal levels of PR- and GR-mediated gene expression, or Vmax (McKenna et al., 1999; Giannoukos et al., 2001; Hosohata et al., 2003; Webster et al., 2003; Kino et al., 2004; Zhang et al., 2004; Dong et al., 2005). However, factors that preferentially alter the transactivation of PR vs. GR are being increasingly described (Tan et al., 2000; Fernandes et al., 2003; Metzger et al., 2003; Dhananjayan et al., 2006; Georgiakaki et al., 2006; Sanchez et al., 2007; Zhao et al., 2007). PR is reported to selectively recruit the coactivator SRC-1 and the comodulator CBP to acetylate K5 on histone H4 while GR preferentially recruits TIF2 and then PCAF to histone H3 to cause phosphorylation (S10) and acetylation (K14) and demethylation (K9) (Li et al., 2003). However, it now appears that a more complicated situation exists. The specific p160 coactivator (SRC-1, TIF2, or AIB1) that is utilized by GR is gene- and cell-specific (Trousson et al., 2007). Furthermore, the protein FLASH was found to bind the coactivators TIF2 and AIB1, but not SRC-1, and to repress transactivation by GR and PR (Kino et al., 2004). To the extent that cofactor binding occurs to the N-terminal domain, the low homology of the amino-terminal halves of GR and PR (<15%) offers an attractive mechanism for selective gene expression. Thus, the recent demonstration of coactivators and corepressors binding to different N-terminal regions of GR and PR (Wang et al., 2007) is especially relevant.
All of the above observations concern changes in the total activity, or Vmax, of GR vs. PR complexes. Two other transcriptional properties that have the potential to discriminate between GR and PR, even if the Vmax is the same, are the concentration of steroid required for half-maximal induction/repression (i.e., the EC50) and the amount of residual agonist activity of an antisteroid, or the partial agonist activity. Changes in EC50 of gene expression are particularly important for responses to the subsaturating concentrations of steroid that are present under physiological conditions. Depending on whether the EC50 for gene induction, or repression, is greater or less than the circulating steroid concentration, the regulated gene response will be significantly less or more respectively than the half-maximal activity. Thus, an EC50 for induction of a given gene that is 10-fold lower for GR, and 10-fold higher for PR, than the concentration of circulating steroid will afford >90% of full induction by GR but <10% of the maximal response by PR. Similarly, changes in the amount of partial agonist activity seen with the pharmacological concentrations of antisteroid encountered in endocrine therapies can theoretically increase the residual agonist activity of an antiglucocorticoid while decreasing the activity of an antiprogestin, or visa versa. Precisely these types of inverse effects have been documented for the corepressors NCoR and SMRT on GR and PR regulation of the same inducible gene in the same cells. These inverted responses were then shown to depend upon the joint actions of the N- and C-terminal domains of each receptor (Song et al., 2001). These results are consistent with the demonstration that corepressors interact with N-terminal regions of both GRs and PRs (Wang and Simons; Jr., 2005; Wang et al., 2007) in addition to the initially defined requirements of the C-terminal sequences of nuclear (Zamir et al., 1996) and steroid receptors (Cheng et al., 2002; Wang et al., 2004a; Wang and Simons; Jr., 2005; Frego and Davidson, 2006; Wu et al., 2006; Kroe et al., 2007; Wang et al., 2007).
It should be recognized that even relatively small differences in EC50 and partial agonist activity can have significant physiological consequences. The expression of numerous genes at different developmental stages of Drosophila larvae is exquisitely sensitive to concentration changes of the hormone ecdysone (Karim and Thummel, 1992). A three-fold change in the concentrations of Decapentaplegic or activin is sufficient for unequal developmental responses in Drosophila and Xenopus respectively (reviewed in Gurdon and Bourillot, 2001) while a 2- to 5-fold change in fibroblast growth factor is sufficient to trigger the differentiation of presomitic mesoderm into somites in mouse embryos (Dubrulle and Pourquie, 2004).
The objective of this study is to examine the consequences of several other factors previously shown to alter the EC50 and partial agonist activity of transiently transfected GR complexes and see if, under the same conditions, divergent responses are observed with the full-length PR, PR-B. The factors that we selected are: GME, GMEB2, Ubc9, and STAMP. GME, the first modulatory factor identified (Oshima and Simons; Jr., 1992), is a cis-acting DNA sequence that was isolated from the rat tyrosine aminotransferase (TAT) gene and acts in concert with two proteins that bind to the GME, i.e., GMEB-1 and -2 (Oshima et al., 1995; Zeng et al., 1998; Chen et al., 2002; Chen et al., 2004). GMEB-1 and -2 are also involved in parvovirus replication (Christensen et al., 1999; Christensen et al., 2001). Ubc9 is the human homolog of the E2 ubiquitin-conjugating enzymes of yeast that can transfer a ubiquitin-like molecule, called small ubiquitin-like modifier-1 (SUMO-1), to proteins in vertebrate cells (Desterro et al., 1997; Gong et al., 1997). However, Ubc9 also displays non-enzymatic effects on a variety of cellular actions (Hahn et al., 1997; Chakrabarti et al., 1999; Poukka et al., 1999; Kurtzman and Schechter, 2001) including GR-mediated transcription (Kaul et al., 2002; Cho et al., 2005). STAMP is a novel protein that was cloned by its ability to augment the modulatory activity of the coactivator TIF2 in both GR-mediated induction and GR-regulated repression of target genes (He and Simons; Jr., 2007). All of these factors are described in this study to display differences regarding PR-B vs. GR transactivation, thereby expanding the number of possible factors and mechanisms for eliciting divergent responses from PRs vs. GRs.
2. Materials and Methods
Unless otherwise indicated, all operations were performed at 0 °C or the temperature recommended by the supplier.
2.1. Chemicals
Dex was obtained from Sigma (St. Louis, MO), and promegestone (R5020) was from PerkinElmer Life Sciences (Boston, MA). RU486 was a gift from Etienne Baulieu (Paris, France). Dex-Ox (Pons and Simons; Jr., 1981) was prepared by Craig Thomas (NIDDK, NIH) and Dex-Mes (Simons; Jr. et al., 1980) was purchased from Steraloids (Newport, RI). Restriction enzymes and DNA polymerase were from New England Biolabs (Beverly, MA), Amersham Biosciences (Piscataway, NJ), or Promega (Madison, WI).
2.2. Plasmids
Renilla null luciferase reporter was purchased from Promega (Madison, WI). Renilla TS was a gift from Nasreldin M. Ibrahim, Otto Fröhlich, and S. Russ Price (Emory University School of Medicine). GREtkLUC (Sarlis et al., 1999), GMEGREtkLUC (Chen et al., 2000), GMEB-2 (Kaul et al., 2000), Ubc9 (Kaul et al., 2002), PR/GR chimera (Song et al., 2001), VP16/PR395C, /395-634, and /509C (Wang and Simons; Jr., 2005), and STAMP and GAL-DBD fusions of STAMP fragments (He et al., 2002) have been previously described. The cDNA plasmids of GR (pSVLGR from Keith Yamamoto, UCSF, San Francisco, CA), MMTVLuc (pLTRLuc; Gordon Hager, NIH, Bethesda, MD), TIF2 and the B form of human progesterone receptor (hPR-B; Hinrich Gronemeyer, IGBMC, Strasbourg, France), and VP16 activation domain fusions of PR fragments (PR-B, PR-A, PR-N535; Dean Edwards, Baylor College of Medicine, Houston, TX, and PR-N644; Kathryn Horwitz, Univ. of Colorado Health Sciences Center, Denver, CO) were received as gifts.
GAL/STAMPN518 was constructed by amplifying the fragment with primers 220F3 (5’ -TACGATATCGATGGCCCGGGACCTGGAGGAAAC-3’) and Xb1775R (5’-CGT CTA GAC ATC AGC AGT CAT TCT GTC CTG G-3’), cutting with EcoRV + XbaI, and inserting the fragment into the SmaI/XbaI digested site of PM plasmid. For GAL/STAMP956C, the STAMP 956C sequence was amplified by primers ER-3091F (5’-GGA ATT CCT GCC ACG CTG TCG ATC AGG AAG -3’ and 3’ primer 2 (5’-GCT CTA GAT GCA CCC AGG AGT GGT GAA CAG G-3’), digesting with EcoRI + XbaI, and inserting the fragment into PM plasmid cleaved with the same enzymes.
2.3. Cell culture and transfection
Monolayer cultures of Cos-7, CV-1, and 1470.2 cells were grown at 37 °C as described previously (Szapary et al., 1996; Giannoukos et al., 2001). Cells are transfected for 18 h using Lipofectamine (Life Technologies, Inc., Gaithersburg, MD) or FuGene6 (Roche Diagnostics, Indianapolis, IN) as recommended by the supplier. For each well of a 24-well plate, we use 100 ng of reporter (FRLUC, GMEGREtkLUC or GREtkLUC) and 5 or 10 ng Renilla-TS or Renilla null plus various combinations of other expression vectors. Equal molar amounts of expression vectors lacking GR, PR, or cofactors (e.g., pCMX-hSA, pSG5-hSA, VP16, or Gal) are included to keep the molar amount of each vector constant, with the total transfected DNA brought to 300 ng/well with pBSK+ unless otherwise indicated. The cells are then treated for 20-24 h with 0.1% ethanol ± steroids in media containing 10% FBS and harvested in 1x Passive Lysis Buffer (150 μl/well; Promega). Cell lysates (50 μl) are used to assay for luciferase activity using the Dual-Luciferase Assay System from Promega according to the supplier. The data are normalized for Renilla activity to correct for differences in transfection efficiency. The fold induction by a steroid is defined as (the activity with steroid)/(the basal level activity seen in the absence of hormone). The partial agonist activity of a steroid A (expressed as percent) is defined as follows: 100 × [(the activity with 1 μM steroid A) - (the basal level seen in the absence of hormone)]/[(the activity with 1 μM Dex) - (the basal level seen in the absence of hormone)]. The amount of each receptor plasmid used is determined from titration experiments to be less than that required for maximal gene induction, thus ensuring that the receptor protein is limiting for each condition. Dose-response curves were generated by KaleidaGraph (Synergy Software, Reading, PA) as the best fit for Michaelis-Menten kinetics (R2 usually ≥0.95). All errors are S.E.M. unless otherwise stated.
2.4. Statistical Analysis
Unless otherwise noted, all experiments were performed in triplicate several times. The values of n independent experiments are then analyzed for statistical significance by the two-tailed Student’s t test using the program InStat 3.0 for Macintosh (GraphPad Software, San Diego, CA). When the difference between the SDs of two populations is significantly different, then the Mann-Whitney test or the Alternate Welch t test is used.
3. Results
3.1. Effects of the GME on PR- vs. GR-mediated gene induction
A 21 bp element at -3.6 kb of the rat TAT gene, called the GME, modulates the position of the dose-response curve and the amount of partial agonist activity of GR complexes with homologous and heterologous promoters and reporters (Oshima and Simons; Jr., 1992; Oshima and Simons; Jr., 1993; Collier et al., 1996; Jackson et al., 1998; Chen et al., 2000; Kaul et al., 2000). In CV-1 cells, the GME is most active in modulating GR transcription properties when placed upstream of the GRE (Zeng et al., 2000). However, gene induction by the full-length PR (PR-B) is more robust in 1470.2 mouse mammary adenocarcinoma cells than in CV-1 green monkey kidney cells (Giannoukos et al., 2001). Also, GR and PR show different responses to corepressors in 1470.2 cells (Song et al., 2001). Therefore, we examined GR induction in 1470.2 cells of transiently transfected GREtkLUC reporter ± an upstream 5’ GME element. The GME yields an average of 1.74 ± 0.22 fold (n = 5, P = 0.029) left-shift in the GR dose-response curve to a lower EC50 (Fig. 1A) and 1.50 ± 0.13 (n = 5, P = 0.018) increase in total activity (Vmax). The partial agonist activity of the antiglucocorticoid Dex-Ox (Lamontagne et al., 1984) is weakly but significantly increased from an average of 7.6 ± 0.8 to 11.5 ± 0.8% (n = 5; P = 0.0072) (Fig. 1A). In contrast, the GME affords neither a decrease in the EC50 (0.83 ± 0.18 fold left-shift; n = 5, P = 0.40) nor an increase in Vmax (0.92 ± 0.13; n = 5, P = 0.58) for PR induction of GREtkLUC (Fig. 1B). Importantly, the GME-induced change in EC50 for GR vs. PR is significant at the level of P = 0.013 (n=5). Consistent with the different outcome of the GME on the PR EC50 and Vmax, the GME also has a negligible influence on the amount of partial agonist activity of the antiprogestin Dex-Mes (Giannoukos et al., 2001) with PR (Fig. 1B; 49.0 ± 4.0% with GME vs. 47.7 ± 6.4% without GME, n=5). We conclude that the GME alters several of the transactivation properties of GRs, but not PRs, with the same reporter in the same cells.
Fig. 1.
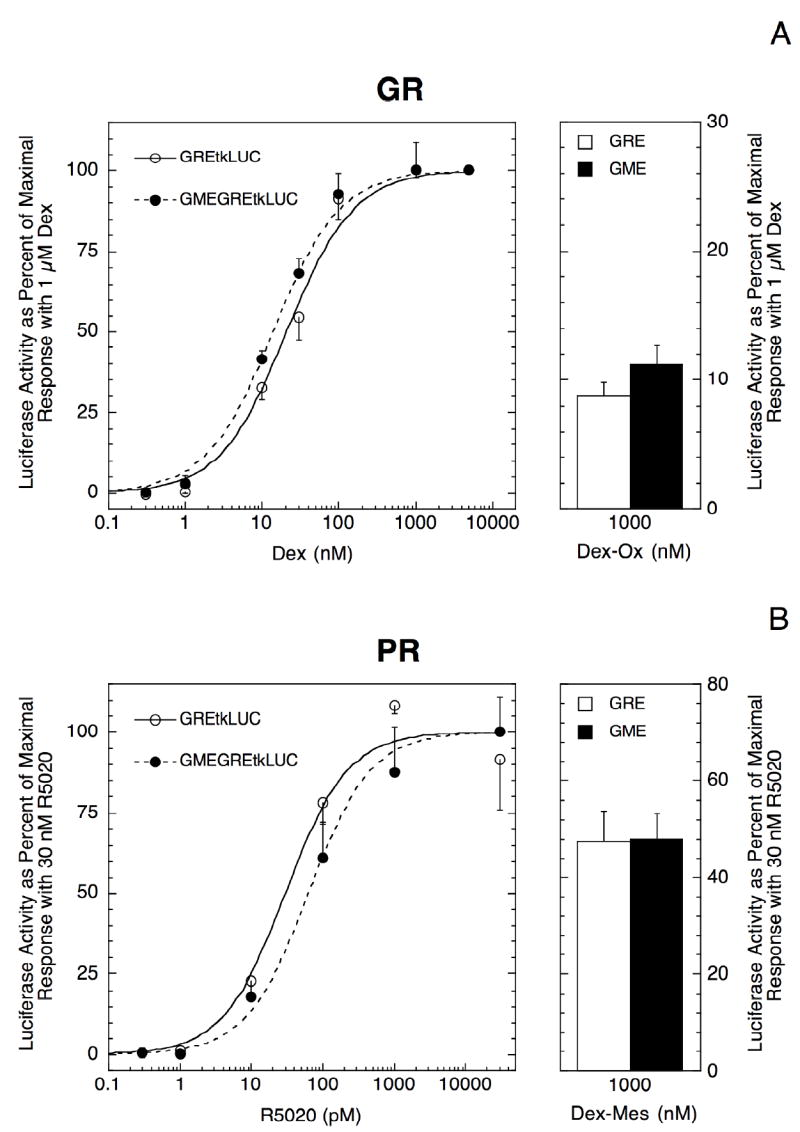
Influence of an upstream GME element in the GREtkLUC reporter on receptor-mediated transactivation. Triplicate wells of 1470.2 cells were transiently transfected with GREtkLUC ± an upstream GME element plus Renilla null luciferase controls along with (A) no receptor plasmid (for endogenous GR) or (B) 30 ng of co-transfected hPR-B plasmid. Cells were induced with varying concentrations of agonist and 1 μM antagonist (Dex and Dex-Ox respectively in A; R5020 and Dex-Mes in B), and assayed for luciferase and Renilla activities. The average values, normalized for cotransfected Renilla, were then plotted as percent of maximal induction above EtOH controls as described in Materials and Methods. The error bars represent the S.D. within a given experiment. Similar results were obtained in four additional experiments for both receptors.
3.2. Influence of GMEB-2 on GR vs. PR transactivation
Two proteins, GMEB-1 and -2, are involved in the expression of GME activity with GR (Oshima et al., 1995; Zeng et al., 1998; Kaul et al., 2000). Overexpression of each GMEB, either alone or in combination, causes a right-shift in the GR dose-response curve of a GMEGREtkLUC reporter in CV-1 cells, presumably due to squelching. Of the two proteins, GMEB-2 is the more potent (Kaul et al., 2000). In 1470.2 cells, 100 ng of GMEB-2 plasmid weakly increases the EC50 for GR induction to higher steroid concentrations for the same GMEGREtkLUC reporter (0.72 ± 0.09 fold left-shift, n = 5, P = 0.040; Fig. 2A) and reduces the Vmax to 26 ± 6% (n = 5, P = 0.0003) of the initial activity. The partial agonist activity of Dex-Ox is concomitantly repressed by 40 ± 12% (n = 5, P = 0.027; Fig. 2A). Lower concentrations of GMEB-2 (10 ng) produce a slightly weaker response for EC50 (0.78 ± 0.12 fold left-shift, n = 4, P = 0.14), Vmax (down to 50 ±14%, n = 5, P = 0.023), and partial agonist activity (31 ± 9% reduction, n = 5, P = 0.024). Thus, as was observed in CV-1 cells (Kaul et al., 2000), all concentrations of GMEB-2 are inhibitory of GR transactivation in 1470.2 cells, again apparently due to squelching.
Fig. 2.
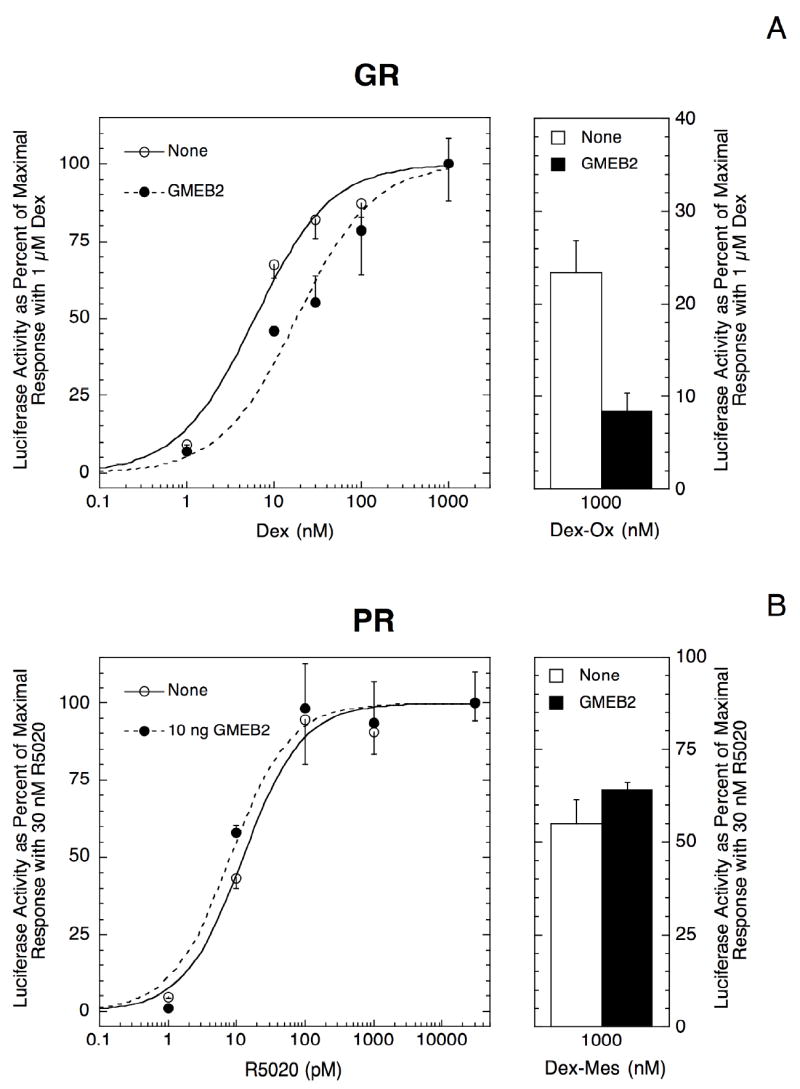
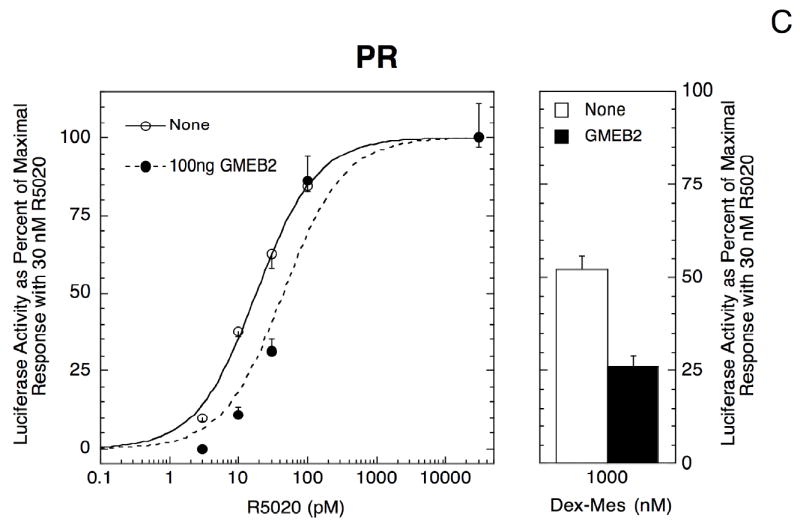
Response of receptor induction properties of GREtkLUC with different concentrations of GMEB-2. Triplicate wells of 1470.2 cells were transfected with 100 ng GREtkLUC and 20 ng Renilla null luciferase reporters to examine the induction properties of endogenous GR (A), and 30 ng of co-transfected hPR-B cDNA plasmid (B and C), in the presence of different concentrations of GMEB-2 plasmid: 10 ng in B or 100 ng in A and C. The responses after treatment with agonist (Dex (A) or R5020 (B, C)), or antagonist (Dex-Ox (A) or Dex-Mes (B, C)) were determined and plotted as in Fig. 1. Similar results were obtained in four (A) and three (B, C) additional experiments.
In contrast, the effects of GMEB-2 on PR induction properties depend on GMEB-2 concentration. Low levels of GMEB-2 (10 ng of transfected plasmid) yield a weak but significant left-shift (1.32 ± 0.07 fold, n=4, P = 0.018) and increased partial agonist activity of Dex-Mes (21 ± 7% increase, n=5, P=0.038) (Fig. 2B). GMEB-2 still decreases the total transactivation, though, by 60 ± 6% (n=5, P=0.0007). However, with higher levels of GMEB-2 (100 ng of plasmid), the dose-response curve shifts in the opposite direction (Fig. 2C, 0.44 ± 0.10 fold left-shift, n=4, P = 0.011), the partial agonist activity of Dex-Mes decreases by 46 ± 3% (n = 4, P=0.0008), while the Vmax of PR-mediated transactivation is further reduced by 77 ± 5% (n = 4, P = 0.0005). Thus, even though the presence of the GME in the regulated reporter construct does not alter PR induction properties, overexpression of GMEB-2, which binds to the GME sequence (Oshima et al., 1995; Zeng et al., 1998) and to GR (Kaul et al., 2000), exerts effects that are GMEB-2 dose-dependent. In summary, two transactivation properties of PR and GR (i.e., EC50 and partial agonist activity of antisteroids) respond equally to high GMEB-2 concentrations but in opposite manners to low GMEB-2 while the response of Vmax with each receptor is the same.
3.3. Modulatory activity of Ubc9 with PR vs. GR
Experiments in CV-1 cells have shown that Ubc9 reduces the EC50 for gene induction to lower concentrations of agonist steroid, and increases the partial agonist activity of antisteroids, only when GR concentrations are high (Kaul et al., 2002; Cho et al., 2005; Kim et al., 2006). The insensitivity to Ubc9 of low concentrations of GR has been ascribed to the presence of an inhibitory sequence in the N-terminal domain of GR. The absence of this inhibitory sequence in PRs led us to predict that Ubc9 actions with PRs would not be the same as with GRs (Cho et al., 2005). The data of Fig. 3 and Table 1 show that exogenous Ubc9 has approximately equal effects on PR and high concentrations of GR in CV-1 cells. The induction parameters of the PR/GR chimera, consisting of the PR N-terminal half fused to the GR C-terminal half, are similarly responsive to added Ubc9 (Fig. 3 and Table 1). In all three cases, the Vmax and partial agonist activity go up while the EC50 goes down. It should be noted that fold increase in partial agonist activity with added Ubc9 for GR and PR/GR is much less than the 7.9-fold seen with PR (Table 1). This is because the average agonist activity without Ubc9 is 24% for GR and 47% for PR/GR, so that the maximum possible increase is only 4- and 2-fold respectively.
Fig. 3.
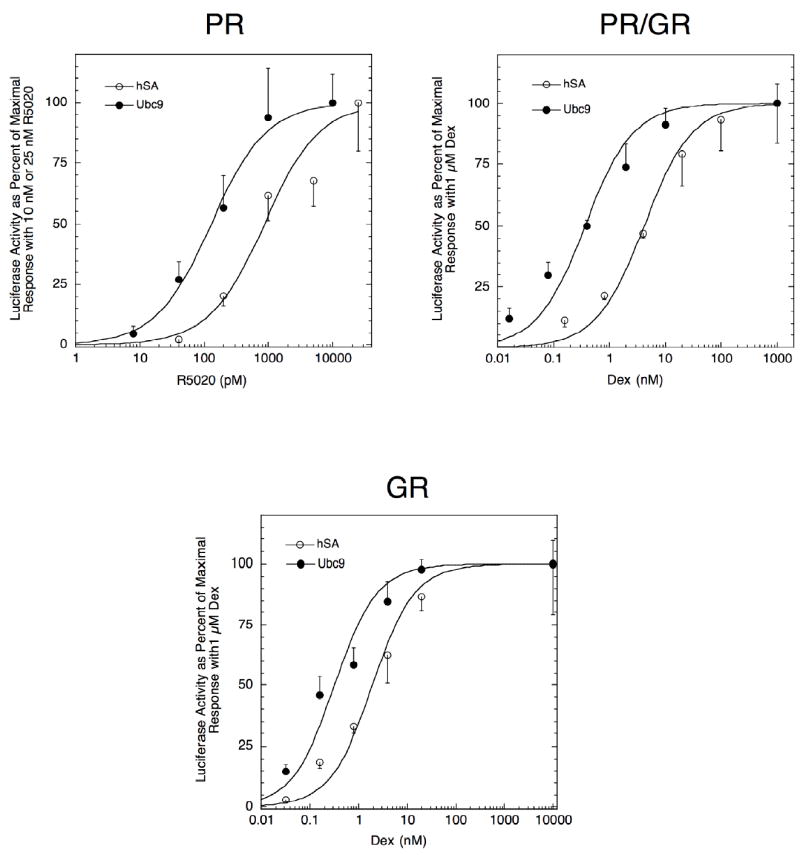
Effect of Ubc9 on PR and GR transactivation properties in CV-1 cells. Triplicate wells of CV-1 cells were transfected with 100 ng GREtkLUC and 20 ng Renilla null luciferase reporters plus 0.03 ng PR-B plasmid, 0.03 ng PR/GR plasmid, or 100 ng GR plasmid ± 150 ng Ubc9 plasmid (total plasmid for GR experiments = 403 ng). The cells were induced and analyzed, and the results plotted, as in Fig. 1. Similar results were obtained in 2-4 additional experiments.
Table 1.
Change in induction parameters with added Ubc9 in CV-1 cells
| Fold Increase in
|
Fold Decrease in
|
|||
|---|---|---|---|---|
| Receptor
|
Fold Induction
|
Vmax |
Partial Agonist Activity
|
EC50 |
| PR (0.03 ng) | 0.78 ± 0.21
(0.35) |
5.12 ± 0.29
(0.0001) |
7.94 ± 2.57
(0.054) |
4.62 ± 0.87
(0.025) |
| PR/GR (0.03 ng) | 0.14 ± 0.04
(0.0022) |
1.91 ± 0.32
(0.11) |
1.76 ± 0.17
(0.048) |
10.60 ± 0.83
(0.0074) |
| GR (100 ng) | 0.15 ± 0.02
(<0.0001) |
1.47 ± 0.14
(0.028) |
3.62 ± 0.42
(0.0034) |
6.48 ± 0.54
(0.0005) |
Average values of fold induction, increase in Vmax, and decrease in EC50 from N = 4-5 (PR and GR) and 3 (PR/GR) independent experiments such as in Fig. 3 are listed. At the same time, the average fold increase in partial agonist activity of 1 μM antiprogestins (Dex-Mes) with PR, and antiglucocorticoid (Dex-Ox) with PR/GR and GR was determined as described in Materials and Methods. P values for statistical significance are in parentheses.
To further test our hypothesis that Ubc9 can unequally affect GR- vs. PR-transactivation under the appropriate conditions, we looked in another cell line, 1470.2 cells, which contain GR but not PR. More importantly, 1470.2 cells permit gene induction by a wider range of PR concentrations, thereby permitting a rigorous test of whether Ubc9 can affect both low and high concentrations of PRs. Transfected GR in 1470.2 cells causes squelching of the induction of transfected GREtkLUC reporter (data not shown), indicating that the endogenous levels of GR are already maximal. These are conditions where Ubc9 is expected to significantly decrease the EC50 and increase both the Vmax and partial agonist activity (Kaul et al., 2002; Cho et al., 2005; Kim et al., 2006). As listed in Table 2, only the Vmax of GR induction increases significantly. In contrast, added Ubc9 convincingly augments the Vmax and partial agonist activity and lowers the EC50 with low concentrations of PR (0.3 ng of plasmid, see ref. Giannoukos et al., 2001). Thus the inhibitory effects with low amounts of GR in CV-1 cells are not seen with low PR concentrations in 1470.2 cells, as previously predicted (Cho et al., 2005). At high PR concentrations, Ubc9 still causes a left-shift in the dose-response curve, although there is no longer any rise in the partial agonist activity. The amount of partial agonist activity of Dex-Mes without Ubc9 (59 ± 4%) it is not so high that we could not see a further increase. Why we do not see such an increase is presently unclear. Nonetheless, the responses of PR are different from those of GR in that Ubc9 is able to alter the EC50 and partial agonist activity of low concentrations of PR, but not GR, and Ubc9 shifts the dose-response curve to lower EC50s under all conditions, including those where there is a negligible effect on the EC50 of GR-mediated induction.
Table 2.
Modulation of induction parameters with added Ubc9 in 1470.2 cells
| Fold Increase in
|
Fold Decrease in
|
|||
|---|---|---|---|---|
| Receptor
|
Fold Induction
|
Vmax |
Partial Agonist Activity
|
EC50 |
| PR (0.3ng) | 0.84 ± 0.08
(0.11) |
1.85 ± 0.15
(0.0012) |
1.28 ± 0.05
(0.0016) |
2.58 ± 0.37
(0.0055) |
| PR (10 ng) | 0.54 ± 0.06
(0.0014) |
1.47 ± 0.22
(0.10) |
0.95 ± 0.07
(0.51) |
3.07 ± 0.64
(0.032) |
| GR (endog.) | 1.14 ± 0.08
(0.19) |
2.74 ± 0.74
(0.10) |
0.56 ± 0.18
(0.087) |
1.15 ± 0.05
(0.051) |
The average values listed are from N independent experiments similar to those of Fig. 3 but now in 1470.2 cells. The experiments were performed and analyzed as in Table 1 with the indicated amounts of transfected PR plasmid or endogenous GR (n = 7 for 0.3 ng PR, = 5 for 10 ng PR, and = 4 for GR). P values for statistical significance are in parentheses.
3.4. Modulation of PR and GR induction properties by STAMP
STAMP was recently discovered as a cofactor that assists TIF2 in modulating GR transcriptional properties (He and Simons; Jr., 2007). STAMP was also found to interact with PR in a mammalian two-hybrid assay. We therefore tested our prediction that STAMP would alter PR transcriptional properties. In CV-1 cells, GR induction of exogenous GREtkLUC reporter displays the usual additive effects of STAMP and TIF2 on EC50, partial agonist activity, and Vmax (Figs. 4A&C). Under the same conditions, PR shows a qualitatively identical but slightly reduced response (Fig. 4B&C).
Fig. 4.
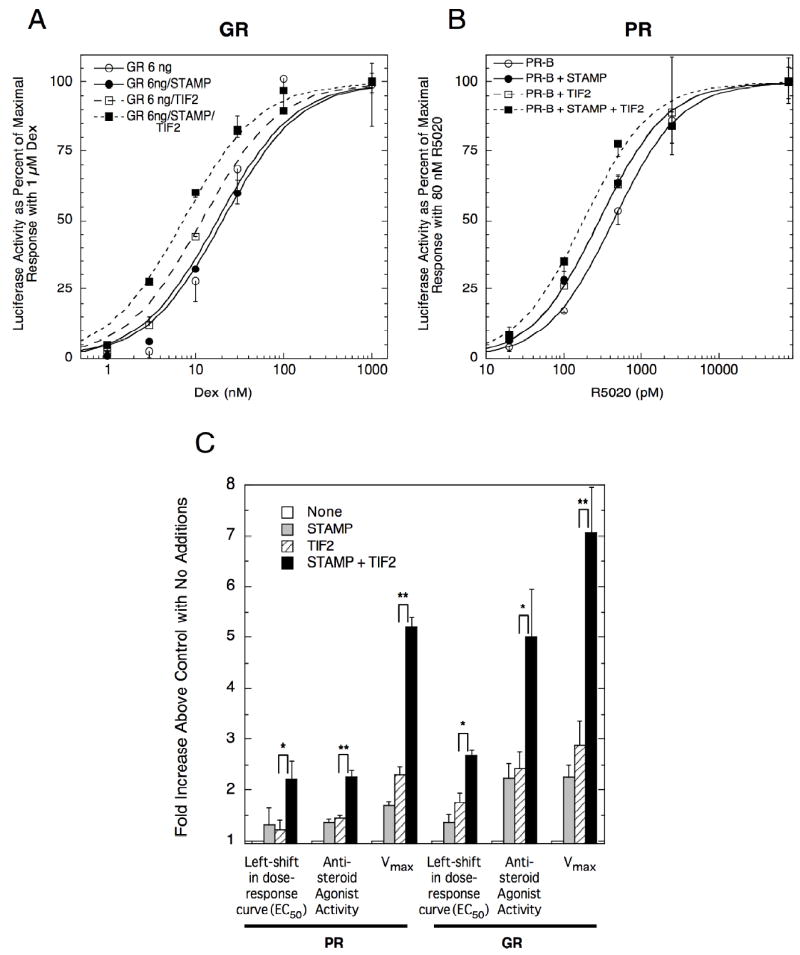
Ability of STAMP ± TIF2 to alter PR and GR induction properties in CV-1 cells. Triplicate wells of CV-1 cells were transfected with 100 ng GREtkLUC and 20 ng Renilla null luciferase reporters plus 0.3 ng PR (A), or 6 ng GR (B), ± 20 ng TIF2 and ± 160 ng Flag/STAMP. The cells were induced and analyzed, and the results plotted, as in Fig. 1. (C) Fold changes in PR and GR induction parameters by STAMP and TIF2. The average fold-increase in each parameter from 4-5 experiments like Figs. 4A & B were determined as follows: for EC50s, fold increase = [EC50]receptor/[EC50] receptor +factor; for partial agonist activity and Vmax (or total agonist activity), fold increase = activityreceptor+factor/activityreceptor).
We next used a mammalian two-hybrid assay of chimeras of the VP16 activation domain with full-length PR or GR and GAL4 DBD fused to various STAMP fragments to determine what region(s) of STAMP interacts with each receptor. As previously seen for GR in CV-1 cells (He and Simons; Jr., 2007), the C-terminal fragment 834C (amino acids 834-1277) displays the greatest steroid-inducible response with each receptor in Cos-7 cells (Fig. 5). Thus, the interactions of STAMP with GR are not dependent upon the cell line used. However, the importance of regions within STAMP 834C for this interaction varies with the receptor. The amino acids 956-1127 and 1127C afford 2-19% of the steroid-inducible response of 834C with PR vs. 39-68% respectively with GR (Fig. 5). This indicates that these regions of STAMP have unequal effects in mediating the association with PR vs. GR.
Fig. 5.
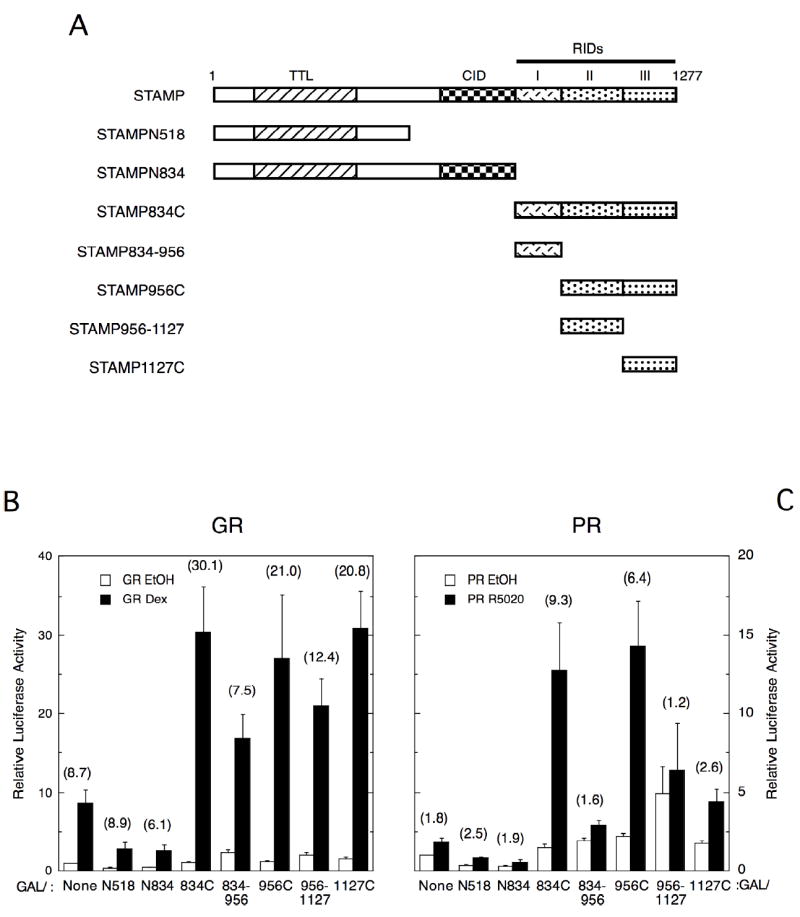
Regions of STAMP that associate with PR and GR in two-hybrid assays. (A) Cartoons show regions of STAMP that are retained in each of the constructs used below. Plasmid names are that of STAMP followed by the sequence retained, with the letters N and C designating the N- and C-terminal residues of the receptor respectively. Abbreviations of STAMP domains: TTL = tubulin tyrosine ligase, CID = coactivator interaction domain, RIDs = receptor interaction domains, which is comprised of three subdomains I-III (He and Simons; Jr., 2007). (B&C) Cos-7 cells were transiently transfected with 86 ng VP16/GR ± 1 μM Dex (B) or 1 ng VP16/PR-B ± 20 nM R5020 (C) plus GAL fusions of STAMP segments (molar equivalent to 80 ng of GAL/STAMP834C) and 100 ng of FRLuc reporter. The average relative luciferase activities (n = 2-6 for B; = 3-7 for C) were determined as in Fig. 1 and plotted. The values in parentheses above the bars indicate the fold increased interaction ± 1 μM Dex (B) or 20 nM R5020 (C).
Because different RID segments of STAMP are involved in binding to PRs and GRs, we used the maximally binding segment of STAMP, 834C, in the same mammalian two-hybrid assay to ask whether the interactions proceeds via similar or non-homologous domains of the two receptors. No specific binding of the GR N-terminal construct (GRN523; Fig. 6A) to STAMP was obtained (Fig. 6B). With the C-terminal fragment, GR407C, the amount of steroid-inducible binding to GAL/STAMP834C was only slightly above the non-specific association with GAL. Thus no one region of GR retains the ability of full length GR to bind to STAMP. PR-A, which is missing the first 164 amino acids of PR-B, affords only 50% of the response with wt PR-B while all response is eliminated with deletion of the first 394 residues (Fig. 6C). Nevertheless, the isolated N-terminal fragments PR-BN535 and PR-BN645 displayed no interaction and neither did the C-terminal fragment PR509C (Figs. 6C&D). Therefore, for both GR (Fig. 6B and ref. He and Simons; Jr., 2007) and PR (Figs. 6C&D), it appears that the entire receptor sequence is needed for maximal, steroid-inducible interaction with STAMP in this system.
Fig. 6.
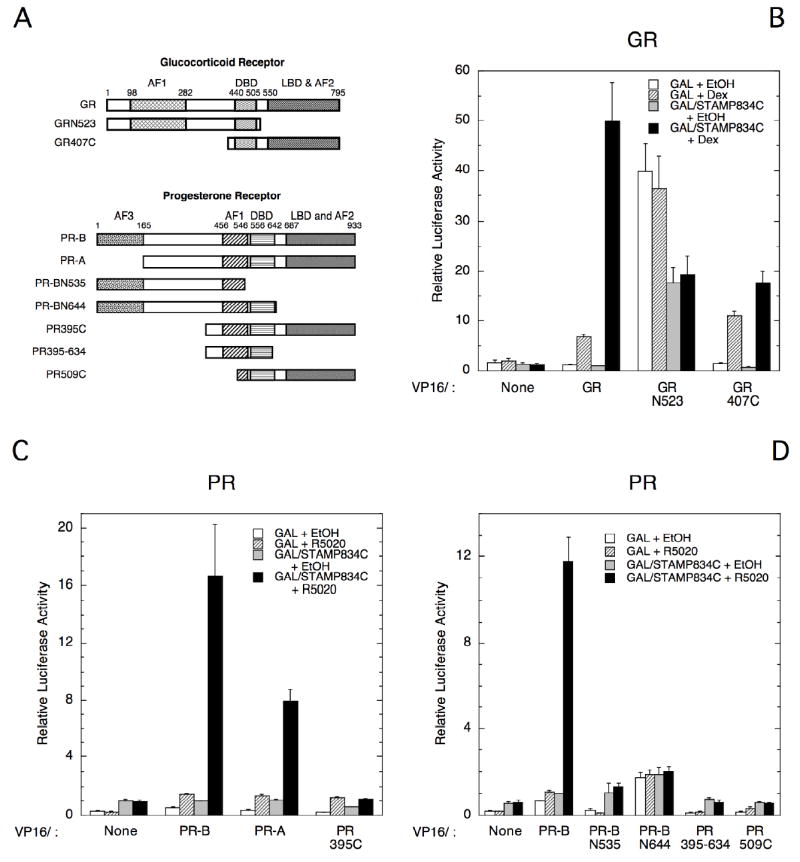
Regions of PR and GR interacting with STAMP in two-hybrid assays. (A) Cartoons show regions of GR and PR that are retained in each of the constructs used below, with the numbers above each structure indicate the amino acid position of each domain boundary. Plasmid names are that of the receptor followed by the sequence retained, with the letters N and C designating the N- and C-terminal residues of the receptor respectively. (B-D) Cos-7 cells were transiently transfected with GAL (57 ng) or GAL/STAMP834C (80 ng) plus VP16 fusions of GR segments (molar equivalent to 86 ng VP16/GR; B) of PR segments (molar equivalent to 1 ng VP16/PR-B; C&D) and 100 ng of FRLuc reporter ± 1 μM Dex (B) or 20 nM R5020 (C&D). The average relative luciferase activities from three independent experiments were determined and plotted as in Fig. 5.
4. Discussion
The ability of various factors to alter the EC50 for gene expression by agonists, and the amount of residual or partial agonist activity by antagonists, plays a major role in the amount of response elicited by subsaturating, physiological concentrations of agonist steroid and by the saturating concentrations of antisteroids used in endocrine therapies respectively. In this study, each factor (GME, GMEB-2, Ubc9, and STAMP) that we have previously reported to modulate the EC50, partial agonist activity, and Vmax of GR transactivation (Oshima and Simons; Jr., 1992; Kaul et al., 2000; Zeng et al., 2000; He et al., 2002; Kaul et al., 2002; Cho et al., 2005; Kim et al., 2006; He and Simons; Jr., 2007) is found to have some different effect on PR-mediate induction or receptor binding under identical transient transfection conditions (Table 3A). Assay conditions under which the GME alters all three properties of GRs have negligible, if any effect, on PRs. High concentrations of GMEB-2 shift the dose-response curve to higher EC50s for GR and PR, and reduce the partial agonist activity. Low amounts of GMEB-2 cause opposite responses of the EC50 of the two receptors: the EC50 is decreased for PRs but remains the same, or slightly increases, for GRs (see also below). The disparate consequences of elevated Ubc9 (changes in PR partial agonist activity and/or EC50 under conditions where no change is observed for GRs) confirms our earlier predictions that were based on the lack of an appropriate inhibitory sequence in the N-terminus of PRs (Cho et al., 2005). The effects of STAMP on PRs and GRs are similar. However, differences in the regions of STAMP that interact with each receptor suggest that the STAMP sequences mediating, and thus the conditions producing, these changes can be different. Thus, the GME, GMEB-2, and Ubc9 join the corepressors NCoR and SMRT (Song et al., 2001) as factors that can produce unequal effects on PR- vs. GR-regulated gene induction, while it is likely that STAMP has similarly selective actions under the appropriate conditions. The additional factors of the present study greatly increases the number of demonstrated receptor-selective factors and further supports the hypothesis that many of the cellular differences between PR- and GR-mediated responses may derive from unequal interpretations of PR and GR interactions with the same cofactors.
Table 3.
Summary of Cofactor Interactions with PR vs. GR
| EC50 |
% Agonist Activity
|
Vmax |
|||||
|---|---|---|---|---|---|---|---|
| Factor
|
Response of PR vs. GR
|
PR
|
GR
|
PR
|
GR
|
PR
|
GR
|
| A. GME | Different | = | - | = | + | = | + |
| GMEB-2 | |||||||
| Low | Different | - | ≥ | + | - | - | - |
| High | Same | + | + | - | - | - | - |
| Ubc 9 | More sensitive | - - | - | + | ≈ | + | + |
| STAMP | Same† | - | - | + | + | + | + |
| † different domains | |||||||
| B. Receptor | Same | - | - | + | + | + | + |
| TIF2 | Same | - | - | + | + | + | + |
| NCoR | Different | - | + | + | - | - | - |
| SMRT | Different | + | - | - | + | ≈ | - |
Summary is a composite of results from (A) this study and (B) previous studies for PR and GR (Szapary et al., 1996; Szapary et al., 1999; Giannoukos et al., 2001), TIF2 (Szapary et al., 1999; Giannoukos et al., 2001), and NCoR and SMRT (Song et al., 2001). Definition of symbols describing effect on parameters following addition of various factors: --, much less than; -, less than; =, equal to; ≈, approximately equal to; ≥, greater than or equal to; +, more than.
The unequal effects of increasing GMEB-2 on the EC50 and partial agonist activity of GRs and PRs in Fig. 2 is most likely due to differential sensitivities of the receptors to squelching. Because GMEB-2 binds to the GME (Oshima et al., 1995), which causes a decrease in the EC50, and in increase in the partial agonist activity, of GR-regulated transactivation (Oshima and Simons; Jr., 1992), it was expected that added GMEB-2 would produce the same effect. The fact that increased GMEB-2 had the opposite effects with GRs (increased EC50 and decreased partial agonist activity) was ascribed GMEB-2 not being a limiting factor in CV-1 cells and thus squelching when present in higher concentrations (Kaul et al., 2000). The same squelching effects of GR-mediated gene induction by GMEB-2 are again seen in the present study in 1470.2 cells. A similar squelching with PR transactivation is also seen here with high GMEB-2 concentrations. However, the opposite effects on PR gene induction with low amounts of exogenous GMEB-2 suggest that, in this system, GMEB-2 is not in excess for PR-regulated transactivation. Thus, the opposite responses of GR and PR to low amounts of exogenous GMEB-2 indicate a different concentration requirement of GMEB-2 for GR- vs. PR-controlled expression of the same gene in the same cell.
The modulatory activity of all factors in this study has now been observed in multiple cell lines with multiple receptors. The GME is active with GRs in Fu5-5 rat hepatoma (Oshima and Simons; Jr., 1992) and CV-1 (Chen et al., 2000) in addition to influencing GRs and PRs in the 1470.2 cells of the current study. GMEB-2 alters the properties of GRs in CV-1 (Kaul et al., 2000) and both GRs and PRs in 1470.2 cells (Fig. 2). The initial activity of Ubc9 with GRs in CV-1 cells (Kaul et al., 2002; Cho et al., 2005) is restricted in 1470.2 cells but is very similar for PRs in both cell lines (Tables 1 and 2). STAMP modulatory activity with GRs has been reported in CV-1 and U2OS human osteosarcoma cells (He and Simons; Jr., 2007) and with PRs in CV-1 cells. Thus, the effects of these factors are not limited to a single steroid receptor or a single cell line. Further studies are required to determine the breadth of cells and receptors with which these factors display modulatory activity.
The current studies were conducted with transiently transfected reporter genes and thus cannot assess possible contributions of chromatin structure and/or reorganization to differences in PR vs. GR transcription properties (Chen et al., 2006). However, the GME appears to be equally active with both endogenous and synthetic reporter genes (Oshima and Simons; Jr., 1992; Szapary et al., 1992; Collier et al., 1996). Furthermore, it is clear from microarray studies that endogenous genes yield a spectrum of responses that resist generalization (Mittelstadt and Ashwell, 2003; Rogatsky et al., 2003; Donn et al., 2007; Kininis et al., 2007). Therefore, the present results establish that various factors can unequally alter the transcription properties of PR vs. GR in a model system that most likely is recapitulated by some endogenous regulated genes. In several cases of the present study, the magnitude of the changes is small. However, it should be remembered that similar differences in the concentration of morphogens have been shown to by physiologically relevant and to have dramatic effects on the development of flies, frogs, and mice (Karim and Thummel, 1992; Gurdon and Bourillot, 2001; Dubrulle and Pourquie, 2004).
The only cis-acting element other than the GME of which we are aware with differential modulatory activity is a 37 bp element in the distal promoter of the gene encoding the PR, which affects estrogen receptor induction of the PR gene (Montano et al., 1997). However, we have not seen any report that this 37 bp element has differential activity with another receptor. The GME is part of the upstream enhancer region of the endogenous TAT gene (Oshima and Simons; Jr., 1992) and its effects with GR appear to be mediated by two proteins, GMEB-1 and -2, that bind to the GME as a heterooligomer (Oshima et al., 1995). The presence of the GME in other genes is, unfortunately, difficult to predict due to the frequency with which the four base binding sequence occurs (Oshima et al., 1995; Zeng et al., 1998), usually as a tandem repeat with a flexible spacing of from 1 to as much as 15 bp (Christensen et al., 1999). Therefore, the frequency with which the GME occurs in other regulated genes to afford possible differences between PR- and GR-mediated induction is currently unknown.
STAMP was discovered as a cofactor that increased the modulatory activity of the coactivator TIF2 in GR-mediated induction and repression (He and Simons; Jr., 2007). The more than additive effects on the EC50, partial agonist activity, and Vmax for GR transactivation are confirmed here and extended to PR gene induction, although the magnitude of change with PR is somewhat less for each parameter. The major difference for STAMP actions with PRs vs. GRs is that two C-terminal regions of STAMP (956-1127, and 1127C) interact strongly with GR either separately or in combination while both domains have to be present for a strong association with PR (Fig. 5). While interactions in mammalian two hybrid assays do not establish a direct interaction between the proteins and can instead result from the mutual binding to an adapter protein, we suspect a direct interaction on the basis of GR binding to STAMP also in pulldown and co-immunoprecipitation assays (He and Simons; Jr., 2007). Establishing the molecular cause for the different binding affinity of PR vs. GR to the C-terminal domains of STAMP will not be easy, though, because multiple regions of each receptor are also required (Fig. 6). This, in turn, suggests that STAMP association and activity with PRs and GRs are sensitive to the overall confirmation of each receptor, which can be unequally altered by the variety of known binding proteins and DNAs.
A recurrent theme with these modulatory proteins is that the Vmax can be altered independently of EC50 and partial agonist activity (Oshima and Simons; Jr., 1993; Szapary et al., 1996; Szapary et al., 1999; Chen et al., 2000; Zeng et al., 2000; Giannoukos et al., 2001; Song et al., 2001; He et al., 2002; Kaul et al., 2002; Wang et al., 2004a; Wang et al., 2004b; Cho et al., 2005; Kim et al., 2006). This phenomenon is again observed here with GMEB-2 (Table 3A). With both receptors, low amounts of GMEB-2 cause a decrease in Vmax but the effects on EC50 and partial agonist activity are diametrically opposed for PRs and GRs. Even with the same receptor (PRs), the modulatory activity of GMEB-2 on EC50 and partial agonist activity is reversed upon increasing the GMEB-2 concentration from 10 to 100 ng of plasmid while there is little difference on the Vmax of PRs. These results continue to support our working hypothesis that different pathways may determine the Vmax vs. EC50 and partial agonist activity of steroid receptor-mediated gene induction.
In summary, we have documented several new factors (Table 3A) that, like the previously identified factors (Table 3B), differentially modulate the EC50, partial agonist activity, and Vmax of PR- vs. GR-mediated induction. These are critical parameters for determining the level of gene transcription in response to a single concentration of both endogenous agonist steroid and added antisteroid. The unequal changes in these parameters produced by the factors of this study provide additional mechanistic avenues for PRs and GRs responding differently in the common environment of intact cells and tissues, thereby differentially altering gene expression during development, differentiation, homeostasis, and endocrine therapies. It will be interesting to see if other factors differentially modify gene transcription not only for PR but also for the closely related mineralocorticoid receptors, which responds similarly to GRs with added TIF2, SRC-1, and SMRT (Wang et al., 2004b). However, the factor ELL reduces the EC50 for gene induction by mineralocorticoid receptor by a factor of ≈10 and increases both the Vmax for aldosterone and the partial agonist activity of the antagonist spironolactone while exhibiting no effect on androgen receptors or PRs and decreasing the Vmax for GRs (Pascual-Le Tallec et al., 2005). GT198 produces a left-shift in GR dose-response curve to lower EC50 values (~2 fold) (Ko et al., 2002). The ability of CBP to modulate GR properties (Szapary et al., 1999) has been confirmed and shown to reduce the EC50 by ~6-fold, while TRBP produced a 16-fold left-shift (Ko et al., 2002). Thus the possible receptor-specific modulatory effects of additional factors remain to be examined.
Acknowledgments
We thank Trevor Archer (NIEHS, NIH, NC) for critical review of the manuscript and Etienne Baulieu, Dean Edwards, Otto Fröhlich, Hinrich Gronemeyer, Gordon Hager, Kathryn Horwitz, Nasreldin M. Ibrahim, S. Russ Price, and Keith Yamamoto for the generous donation of reagents. This research was supported by the Intramural Research Program of the NIH, NIDDK.
Footnotes
Competing Interests Statement The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cato ACB, Miksicek R, Schutz G, Arnemann J, Beato M. The hormone regulatory element of mouse mammary tumour virus mediates progesterone induction. EMBO J. 1986;5:2237–2240. doi: 10.1002/j.1460-2075.1986.tb04490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti SR, Sood R, Ganguly S, Bohlander S, Shen Z, Nucifora G. Modulation of TEL transcription activity by interaction with the ubiquitin-conjugating enzyme UBC9. Proc Natl Acad Sci U S A. 1999;96:7467–7472. doi: 10.1073/pnas.96.13.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kokontis J, Liao S. Structural analysis of complementary DNA and amino acid sequences of human and rat androgen receptors. Proc Natl Acad Sci USA. 1988;85:7211–7215. doi: 10.1073/pnas.85.19.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, He Y, Simons SS., Jr Structure/activity relationships for GMEB-2: the second member of the glucocorticoid modulatory element binding complex. Biochemistry. 2004;43:245–255. doi: 10.1021/bi035311b. [DOI] [PubMed] [Google Scholar]
- Chen J, Kaul S, Simons SS., Jr Structure/activity elements of the multifunctional protein, GMEB-1. Characterization of domains relevant for the modulation of glucocorticoid receptor transactivation properties. J Biol Chem. 2002;277:22053–22062. doi: 10.1074/jbc.M202311200. [DOI] [PubMed] [Google Scholar]
- Chen J, Kinyamu HK, Archer TK. Changes in attitude, changes in latitude: nuclear receptors remodeling chromatin to regulate transcription. Mol Endocrinol. 2006;20:1–13. doi: 10.1210/me.2005-0192. [DOI] [PubMed] [Google Scholar]
- Chen S, Sarlis NJ, Simons SS., Jr Evidence for a common step in three different processes for modulating the kinetic properties of glucocorticoid receptor-induced gene transcription. J Biol Chem. 2000;275:30106–30117. doi: 10.1074/jbc.M005418200. [DOI] [PubMed] [Google Scholar]
- Cheng S, Brzostek S, Lee SR, Hollenberg AN, Balk SP. Inhibition of the dihydrotestosterone-activated androgen receptor by nuclear receptor corepressor. Mol Endocrinol. 2002;16:1492–1501. doi: 10.1210/mend.16.7.0870. [DOI] [PubMed] [Google Scholar]
- Cho S, Kagan BL, Blackford JA, Jr, Szapary D, Simons SS., Jr Glucocorticoid receptor ligand binding domain is sufficient for the modulation of both the dose-response curve of receptor-agonist complexes and the partial agonist activity of receptor-antisteroid complexes by glucocorticoid receptors, coactivator TIF2, and Ubc9. Mol Endo. 2005;19:290–311. doi: 10.1210/me.2004-0134. [DOI] [PubMed] [Google Scholar]
- Christensen J, Cotmore SF, Tattersall P. Two new members of the emerging KDWK family of combinatorial transcription modulators bind as a heterodimer to flexibly spaced PuCGPy half-sites [In Process Citation] Mol Cell Biol. 1999;19:7741–7750. doi: 10.1128/mcb.19.11.7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Cotmore SF, Tattersall P. Minute virus of mice initiator protein NS1 and a host KDWK family transcription factor must form a precise ternary complex with origin DNA for nicking to occur. J Virol. 2001;75:7009–7017. doi: 10.1128/JVI.75.15.7009-7017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier CD, Oshima H, Simons SS., Jr A negative tyrosine aminotransferase gene element that blocks glucocorticoid modulatory element-regulated modulation of glucocorticoid-induced gene expression. Mol Endocrinol. 1996;10:463–476. doi: 10.1210/mend.10.5.8732678. [DOI] [PubMed] [Google Scholar]
- Desterro JM, Thomson J, Hay RT. Ubch9 conjugates SUMO but not ubiquitin. FEBS Lett. 1997;417:297–300. doi: 10.1016/s0014-5793(97)01305-7. [DOI] [PubMed] [Google Scholar]
- Dhananjayan SC, Ramamoorthy S, Khan OY, Ismail A, Sun J, Slingerland J, O’Malley BW, Nawaz Z. WW domain binding protein-2, an E6-associated protein interacting protein, acts as a coactivator of estrogen and progesterone receptors. Mol Endocrinol. 2006;20:2343–2354. doi: 10.1210/me.2005-0533. [DOI] [PubMed] [Google Scholar]
- Dong X, Shylnova O, Challis JR, Lye SJ. Identification and characterization of the protein-associated splicing factor as a negative co-regulator of the progesterone receptor. J Biol Chem. 2005;280:13329–13340. doi: 10.1074/jbc.M409187200. [DOI] [PubMed] [Google Scholar]
- Donn R, Berry A, Stevens A, Farrow S, Betts J, Stevens R, Clayton C, Wang J, Warnock L, Worthington J, Scott L, Graham S, Ray D. Use of gene expression profiling to identify a novel glucocorticoid sensitivity determining gene, BMPRII. FASEB J. 2007;21:402–414. doi: 10.1096/fj.06-7236com. [DOI] [PubMed] [Google Scholar]
- Dubrulle J, Pourquie O. fgf8 mRNA decay establishes a gradient that couples axial elongation to patterning in the vertebrate embryo. Nature. 2004;427:419–422. doi: 10.1038/nature02216. [DOI] [PubMed] [Google Scholar]
- Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes I, Bastien Y, Wai T, Nygard K, Lin R, Cormier O, Lee HS, Eng F, Bertos NR, Pelletier N, Mader S, Han VK, Yang XJ, White JH. Ligand-dependent nuclear receptor corepressor LCoR functions by histone deacetylase-dependent and -independent mechanisms. Mol Cell. 2003;11:139–150. doi: 10.1016/s1097-2765(03)00014-5. [DOI] [PubMed] [Google Scholar]
- Frego L, Davidson W. Conformational changes of the glucocorticoid receptor ligand binding domain induced by ligand and cofactor binding, and the location of cofactor binding sites determined by hydrogen/deuterium exchange mass spectrometry. Protein Sci. 2006;15:722–730. doi: 10.1110/ps.051781406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiakaki M, Chabbert-Buffet N, Dasen B, Meduri G, Wenk S, Rajhi L, Amazit L, Chauchereau A, Burger CW, Blok LJ, Milgrom E, Lombes M, Guiochon-Mantel A, Loosfelt H. Ligand-controlled interaction of histone acetyltransferase binding to ORC-1 (HBO1) with the N-terminal transactivating domain of progesterone receptor induces steroid receptor coactivator 1-dependent coactivation of transcription. Mol Endocrinol. 2006;20:2122–2140. doi: 10.1210/me.2005-0149. [DOI] [PubMed] [Google Scholar]
- Giannoukos G, Szapary D, Smith CL, Meeker JEW, Simons SS., Jr New antiprogestins with partial agonist activity: potential selective progesterone receptor modulators (SPRMs) and probes for receptor- and coregulator-induced changes in progesterone receptor induction properties. Mol Endocrinol. 2001;15:255–270. doi: 10.1210/mend.15.2.0596. [DOI] [PubMed] [Google Scholar]
- Gong L, Kamitani T, Fujise K, Caskey LS, Yeh ET. Preferential interaction of sentrin with a ubiquitin-conjugating enzyme, Ubc9. J Biol Chem. 1997;272:28198–28201. doi: 10.1074/jbc.272.45.28198. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Bourillot PY. Morphogen gradient interpretation. Nature. 2001;413:797–803. doi: 10.1038/35101500. [DOI] [PubMed] [Google Scholar]
- Hahn SL, Wasylyk B, Criqui-Filipe P, Criqui P. Modulation of ETS-1 transcriptional activity by huUBC9, a ubiquitin-conjugating enzyme. Oncogene. 1997;15:1489–1495. doi: 10.1038/sj.onc.1201301. [DOI] [PubMed] [Google Scholar]
- He Y, Simons SS., Jr STAMP: a novel predicted factor assisting TIF2 actions in glucocorticoid receptor-mediated induction and repression. Mol Cell Biol. 2007;27:1467–1485. doi: 10.1128/MCB.01360-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Szapary D, Simons SS., Jr Modulation of induction properties of glucocorticoid receptor-agonist and -antagonist complexes by coactivators involves binding to receptors but is independent of ability of coactivators to augment transactivation. J Biol Chem. 2002;277:49256–49266. doi: 10.1074/jbc.M205536200. [DOI] [PubMed] [Google Scholar]
- Hosohata K, Li P, Hosohata Y, Qin J, Roeder RG, Wang Z. Purification and identification of a novel complex which is involved in androgen receptor-dependent transcription. Mol Cell Biol. 2003;23:7019–7029. doi: 10.1128/MCB.23.19.7019-7029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DA, Collier CD, Oshima H, Simons SS., Jr Modulation of tat gene induction by glucocorticoids involves a neutralizing sequence. J Steroid Biochem Molec Biol. 1998;66:79–91. doi: 10.1016/s0960-0760(98)00048-x. [DOI] [PubMed] [Google Scholar]
- Karim FD, Thummel CS. Temporal coordination of regulatory gene expression by the steroid hormone ecdysone. EMBO J. 1992;11:4083–4093. doi: 10.1002/j.1460-2075.1992.tb05501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul S, Blackford JA, Jr, Chen J, Ogryzko VV, Simons SS., Jr Properties of the glucocorticoid modulatory element binding proteins GMEB-1 and -2: potential new modifiers of glucocorticoid receptor transactivation and members of the family of KDWK proteins. Mol Endocrinol. 2000;14:1010–1027. doi: 10.1210/mend.14.7.0494. [DOI] [PubMed] [Google Scholar]
- Kaul S, Blackford JA, Jr, Cho S, Simons SS., Jr Ubc9 is a novel modulator of the induction properties of glucocorticoid receptors. J Biol Chem. 2002;277:12541–12549. doi: 10.1074/jbc.M112330200. [DOI] [PubMed] [Google Scholar]
- Kim Y, Sun Y, Chow C, Pommier YG, Simons SS., Jr Effects of acetylation, polymerase phosphorylation, and DNA unwinding in glucocorticoid receptor transactivation. J Steroid Biochem Molec Biol. 2006;100:3–17. doi: 10.1016/j.jsbmb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Kininis M, Chen BS, Diehl AG, Isaacs GD, Zhang T, Siepel AC, Clark AG, Kraus WL. Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol Cell Biol. 2007;27:5090–5104. doi: 10.1128/MCB.00083-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T, Ichijo T, Chrousos GP. FLASH interacts with p160 coactivator subtypes and differentially suppresses transcriptional activity of steroid hormone receptors. J Steroid Biochem Mol Biol. 2004;92:357–363. doi: 10.1016/j.jsbmb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Ko L, Cardona GR, Henrion-Caude A, Chin WW. Identification and characterization of a tissue-specific coactivator, GT198, that interacts with the DNA-binding domains of nuclear receptors. Mol Cell Biol. 2002;22:357–369. doi: 10.1128/MCB.22.1.357-369.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroe RR, Baker MA, Brown MP, Farrow NA, Gautschi E, Hopkins JL, Lafrance RR, Kronkaitis A, Freeman D, Thomson D, Nabozny G, Grygon CA, Labadia ME. Agonist versus antagonist induce distinct thermodynamic modes of co-factor binding to the glucocorticoid receptor. Biophys Chem. 2007;128:156–164. doi: 10.1016/j.bpc.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Kurtzman AL, Schechter N. Ubc9 interacts with a nuclear localization signal and mediates nuclear localization of the paired-like homeobox protein Vsx-1 independent of SUMO-1 modification. Proc Natl Acad Sci U S A. 2001;98:5602–5607. doi: 10.1073/pnas.101129698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne N, Mercier L, Pons M, Thompson EB, Simons SS., Jr Glucocorticoid vs antiglucocorticoid activity: can a single functional group modification of glucocorticoid steroids always convey antiglucocorticoid activity? Endocrinology. 1984;114:2252–2263. doi: 10.1210/endo-114-6-2252. [DOI] [PubMed] [Google Scholar]
- Li X, Wong J, Tsai SY, Tsai MJ, O’Malley BW. Progesterone and glucocorticoid receptors recruit distinct coactivator complexes and promote distinct patterns of local chromatin modification. Mol Cell Biol. 2003;23:3763–3773. doi: 10.1128/MCB.23.11.3763-3773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman BA, Bona BJ, Edwards DP, Nordeen SK. The constitution of a progesterone response element. Mol Endocrinol. 1993;7:515–527. doi: 10.1210/mend.7.4.8388996. [DOI] [PubMed] [Google Scholar]
- Massaad C, Garlatti M, Wilson EM, Cadepond F, Barouki R. A natural sequence consisting of overlapping glucocorticoid-responsive elements mediates glucocorticoid, but not androgen, regulation of gene expression. Biochem J. 2000;350:123–129. MEDLINE record in process. [PMC free article] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O’Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocrine Reviews. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- Metzger E, Muller JM, Ferrari S, Buettner R, Schule R. A novel inducible transactivation domain in the androgen receptor: implications for PRK in prostate cancer. EMBO J. 2003;22:270–280. doi: 10.1093/emboj/cdg023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelstadt PR, Ashwell JD. Disruption of glucocorticoid receptor exon 2 yields a ligand-responsive C-terminal fragment that regulates gene expression. Mol Endo. 2003;17:1534–1542. doi: 10.1210/me.2002-0429. [DOI] [PubMed] [Google Scholar]
- Montano MM, Kraus WL, Katzenellenbogen BS. Identification of a novel transferable cis element in the promoter of an estrogen-responsive gene that modulates sensitivity to hormone and antihormone. Mol Endocrinol. 1997;11:330–341. doi: 10.1210/mend.11.3.9899. [DOI] [PubMed] [Google Scholar]
- Nelson CC, Hendy SC, Shukin RJ, Cheng H, Bruchovsky N, Koop BF, Rennie PS. Determinants of DNA sequence specificity of the androgen, progesterone, and glucocorticoid receptors: evidence of differential steroid receptor response elements. Mol Endocrinol. 1999;13:2090–2107. doi: 10.1210/mend.13.12.0396. [DOI] [PubMed] [Google Scholar]
- Oshima H, Simons SS., Jr Modulation of transcription factor activity by a distant steroid modulatory element. Mol Endocrinol. 1992;6:416–428. doi: 10.1210/mend.6.3.1584217. [DOI] [PubMed] [Google Scholar]
- Oshima H, Simons SS., Jr Sequence-selective interactions of transcription factor elements with tandem glucocorticoid-responsive elements at physiological steroid concentrations. J Biol Chem. 1993;268:26858–26865. [PubMed] [Google Scholar]
- Oshima H, Szapary D, Simons SS., Jr The factor binding to the glucocorticoid modulatory element of the tyrosine aminotransferase gene is a novel and ubiquitous heteromeric complex. J Biol Chem. 1995;270:21893–21910. doi: 10.1074/jbc.270.37.21893. [DOI] [PubMed] [Google Scholar]
- Pascual-Le Tallec L, Simone F, Viengchareun S, Meduri G, Thirman MJ, Lombes M. The elongation factor ELL (eleven-nineteen lysine-rich leukemia) is a selective coregulator for steroid receptor functions. Mol Endocrinol. 2005;19:1158–1169. doi: 10.1210/me.2004-0331. [DOI] [PubMed] [Google Scholar]
- Pons M, Simons SS., Jr Facile, high yield synthesis of spiro C-17-steroidal oxetan-3’-ones. J Org Chem. 1981;46:3262–3264. [Google Scholar]
- Poukka H, Aarnisalo P, Karvonen U, Palvimo JJ, Janne OA. Ubc9 interacts with the androgen receptor and activates receptor-dependent transcription. J Biol Chem. 1999;274:19441–19446. doi: 10.1074/jbc.274.27.19441. [DOI] [PubMed] [Google Scholar]
- Prior JC. Progesterone as a bone-trophic hormone. Endocr Rev. 1990;11:386–398. doi: 10.1210/edrv-11-2-386. [DOI] [PubMed] [Google Scholar]
- Ricousse SL, Gouilleux F, Fortin D, Joulin V, Richard-Foy H. Glucocorticoid and progestin receptors are differently involved in the cooperation with a structural element of the mouse mammary tumor virus promoter. Proc Natl Acad Sci USA. 1996;93:5072–5077. doi: 10.1073/pnas.93.10.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogatsky I, Wang JC, Derynck MK, Nonaka DF, Khodabakhsh DB, Haqq CM, Darimont BD, Garabedian MJ, Yamamoto KR. Target-specific utilization of transcriptional regulatory surfaces by the glucocorticoid receptor. Proc Natl Acad Sci U S A. 2003;100:13845–13850. doi: 10.1073/pnas.2336092100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M, Sauve K, Picard N, Tremblay A. The hormonal response of estrogen receptor beta is decreased by the phosphatidylinositol 3-kinase/Akt pathway via a phosphorylation-dependent release of CREB-binding protein. J Biol Chem. 2007;282:4830–4840. doi: 10.1074/jbc.M607908200. [DOI] [PubMed] [Google Scholar]
- Sarlis NJ, Bayly SF, Szapary D, Simons SS., Jr Quantity of partial agonist activity for antiglucocorticoids complexed with mutant glucocorticoid receptors is constant in two different transactivation assays but not predictable from steroid structure. J Steroid Biochem Molec Biol. 1999;68:89–102. doi: 10.1016/s0960-0760(99)00021-7. [DOI] [PubMed] [Google Scholar]
- Schoenmakers E, Verrijdt G, Peeters B, Verhoeven G, Rombauts W, Claessens F. Differences in DNA binding characteristics of the androgen and glucocorticoid receptors can determine hormone-specific responses. J Biol Chem. 2000;275:12290–12297. doi: 10.1074/jbc.275.16.12290. [DOI] [PubMed] [Google Scholar]
- Simons SS, Jr, Pons M, Johnson DF. α-Keto mesylate: a reactive thiol-specific functional group. J Org Chem. 1980;45:3084–3088. [Google Scholar]
- Song L-N, Huse B, Rusconi S, Simons SS., Jr Transactivation specificity of glucocorticoid vs. progesterone receptors: role of functionally different interactions of transcription factors with amino- and carboxyl-terminal receptor domains. J Biol Chem. 2001;276:24806–24816. doi: 10.1074/jbc.M102610200. [DOI] [PubMed] [Google Scholar]
- Strahle U, Boshart M, Klock G, Stewart F, Schutz G. Glucocorticoid- and progesterone-specific effects are determined by differential expression of the respective hormone receptors. Nature. 1989;339:629–632. doi: 10.1038/339629a0. [DOI] [PubMed] [Google Scholar]
- Szapary D, Huang Y, Simons SS., Jr Opposing effects of corepressor and coactivators in determining the dose-response curve of agonists, and residual agonist activity of antagonists, for glucocorticoid receptor regulated gene expression. Mol Endocrinol. 1999;13:2108–2121. doi: 10.1210/mend.13.12.0384. [DOI] [PubMed] [Google Scholar]
- Szapary D, Oshima H, Simons SS., Jr Modulation of glucocorticoid induction of stably transfected tyrosine aminotransferase gene constructs involves elements up-stream of the glucocorticoid responsive element. Endocrinology. 1992;130:3492–3502. doi: 10.1210/endo.130.6.1350762. [DOI] [PubMed] [Google Scholar]
- Szapary D, Xu M, Simons SS., Jr Induction properties of a transiently transfected glucocorticoid-responsive gene vary with glucocorticoid receptor concentration. J Biol Chem. 1996;271:30576–30582. doi: 10.1074/jbc.271.48.30576. [DOI] [PubMed] [Google Scholar]
- Tan J-a, Hall SH, Hamil KG, Grossman G, Petrusz P, Liao J, Shuai K, French FS. Protein inhibitor of activated STAT-1 (signal transducer and activator of transcription-1) is a nuclear receptor coregulator expressed in human testis. Mol Endo. 2000;14:14–26. doi: 10.1210/mend.14.1.0408. [DOI] [PubMed] [Google Scholar]
- Thackray VG, Lieberman BA, Nordeen SK. Differential gene induction by glucocorticoid and progesterone receptors. J Steroid Biochem Molec Biol. 1998;66:171–178. doi: 10.1016/s0960-0760(98)00044-2. [DOI] [PubMed] [Google Scholar]
- Trousson A, Grenier J, Fonte C, Massaad-Massade L, Schumacher M, Massaad C. Recruitment of the p160 coactivators by the glucocorticoid receptor: Dependence on the promoter context and cell type but not hypoxic conditions. J Steroid Biochem Mol Biol. 2007;104:305–131. doi: 10.1016/j.jsbmb.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Wan Y, Nordeen SK. Overlapping but distinct gene regulation profiles by glucocorticoids and progestins in human breast cancer cells. Mol Endocrinol. 2002;16:1204–1214. doi: 10.1210/mend.16.6.0848. [DOI] [PubMed] [Google Scholar]
- Wang D, Simons SS., Jr Corepressor binding to progesterone and glucocorticoid receptors involves the AF-1 domain and is inhibited by molybdate. Mol Endo. 2005;19:1483–1500. doi: 10.1210/me.2005-0012. [DOI] [PubMed] [Google Scholar]
- Wang D, Wang Q, Awasthi S, Simons SS., Jr Amino-terminal domain of TIF2 is involved in competing for corepressor binding to glucocorticoid and progesterone receptors. Biochemistry. 2007;48:8036–8049. doi: 10.1021/bi7004575. [DOI] [PubMed] [Google Scholar]
- Wang Q, Blackford JA, Jr, Song L-N, Huang Y, Simons SS., Jr Equilibrium interactions of corepressors and coactivators modulate the properties of agonist and antagonist complexes of glucocorticoid receptors. Mol Endocrinol. 2004a;18:1376–1395. doi: 10.1210/me.2003-0421. [DOI] [PubMed] [Google Scholar]
- Wang Q, Richter WF, Anzick SL, Meltzer PS, Simons SS., Jr Modulation of transcriptional sensitivity of mineralocorticoid and estrogen receptors. J Steroid Biochem Molec Biol. 2004b;91:197–210. doi: 10.1016/j.jsbmb.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Webster JI, Tonelli LH, Moayeri M, Simons SS, Jr, Leppla SH, Sternberg EM. Anthrax lethal factor represses glucocorticoid and progesterone receptor activity. Proc Natl Acad Sci U S A. 2003;100:5706–5711. doi: 10.1073/pnas.1036973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Kawate H, Ohnaka K, Nawata H, Takayanagi R. Nuclear compartmentalization of N-CoR and its interactions with steroid receptors. Mol Cell Biol. 2006;26:6633–6655. doi: 10.1128/MCB.01534-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir I, Harding HP, Atkins GB, Horlein A, Glass CK, Rosenfeld MG, Lazar MA. A nuclear hormone receptor corepressor mediates transcriptional silencing by receptors with distinct repression domains. Mol Cell Biol. 1996;16:5458–5465. doi: 10.1128/mcb.16.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Jackson DA, Oshima H, Simons SS., Jr Cloning and characterization of a novel binding factor (GMEB-2) of the glucocorticoid modulatory element. J Biol Chem. 1998;273:17756–17762. doi: 10.1074/jbc.273.28.17756. [DOI] [PubMed] [Google Scholar]
- Zeng H, Plisov SY, Simons SS., Jr Ability of the glucocorticoid modulatory element (GME) to modify glucocorticoid receptor transactivation indicates parallel pathways for the expression of GME and glucocorticoid response element activities. Mol Cell Endo. 2000;162:221–234. doi: 10.1016/s0303-7207(99)00208-7. [DOI] [PubMed] [Google Scholar]
- Zhang A, Yeung PL, Li CW, Tsai SC, Dinh GK, Wu X, Li H, Chen JD. Identification of a novel family of ankyrin repeats containing cofactors for p160 nuclear receptor coactivators. J Biol Chem. 2004;279:33799–33805. doi: 10.1074/jbc.M403997200. [DOI] [PubMed] [Google Scholar]
- Zhao X, Patton JR, Ghosh SK, Fischel-Ghodsian N, Shen L, Spanjaard RA. Pus3p- and Pus1p-dependent pseudouridylation of steroid receptor RNA activator controls a functional switch that regulates nuclear receptor signaling. Mol Endocrinol. 2007;21:686–699. doi: 10.1210/me.2006-0414. [DOI] [PubMed] [Google Scholar]
- Ziegler R, Kasperk C. Glucocorticoid-induced osteoporosis: prevention and treatment. Steroids. 1998;63:344–348. doi: 10.1016/s0039-128x(98)00022-1. [DOI] [PubMed] [Google Scholar]


