Abstract
Objective
Several studies have demonstrated that Sirolimus-eluting stents reduce restenosis in patients with coronary artery disease. Here, we tested whether direct delivery of Sirolimus into the vessel wall during balloon angioplasty can modify vascular remodeling over several weeks.
Methods and Results
During angioplasty of the rabbit iliac artery we administered an intramural infusion of Sirolimus or its vehicle directly through a balloon catheter into the vessel wall. After 3 weeks neointimal formation was decreased (0.71±0.1 vs. 1.4±0.12 intima/media ratio), and this process was attributed to the inhibitory properties of Sirolimus on ECM deposition and smooth muscle cell proliferation. Sirolimus also significantly reduced the deposition of elastin, collagen III and fibronectin within the vascular wall. In parallel, proteomic profiles of arterial wall segments were obtained and 485 protein spots were consistently matched between non-dilated and dilated vessels. Differential expression of 12 proteins were observed between the groups and direct sequencing of digested peptides was performed. Local delivery of sirolimus during angioplasty attenuated the expression of structural proteins that included lamin A, vimentin, α-1-antitrypsin, and α-actin.
Conclusions
Local administration of Sirolimus during angioplasty prevents smooth muscle cell proliferation associated with vascular remodeling as well as the expression of extracellular matrix and structural proteins. Therefore, local injection of Sirolimus during balloon inflation may be an alternative therapeutic approach for preventing restenosis in small stenotic vessels (i.e., <2.5 mm).
Keywords: Angioplasty, Neointima formation, Sirolimus, 2D-proteome analysis, Vascular injury
1. Introduction
Restenosis is characterized as recurrent lumen narrowing upon angioplasty of a vascular stenosis. It can occur in up to 50% of patients that undergo percutaneous transluminal angioplasty (PTA) [1,2]. Lumen loss is initially caused by elastic recoil and in the long run to large extend by neointima formation. This vascular remodeling process is, in part, a result of dedifferentiation of vascular smooth muscle cells (VSMC) that migrate and proliferate into the intimal layer leading to the formation of a thickened neointima and consequent reduction in lumen diameter. VSMC proliferation and migration are controlled by dysregulated deposition of extracellular matrix (ECM) components [3–5] that also provide the architectural framework for the vessel wall [6].
Recent evidence has shed light on genes that govern restenosis. cDNA arrays and related technology have been used to define mRNAs that are differentially expressed in restenosed vessels following stent implantation [7] or balloon angioplasty [5]. Transcripts for ECM, ECM remodeling, and cell adhesion genes have been isolated in restenotic lesions [5]. The receptor for rapamycin (Sirolimus), FK506-binding protein 12 (FKBP12), has also been identified providing a rationale for the use of Sirolimus to prevent restenosis or neointima formation in patients undergoing balloon angioplasty and related procedures [7].
Most studies demonstrating the efficacy of Sirolimus in preventing restenosis have utilized continuous administration via eluting stents. Despite the undisputed advantage over balloon angioplasty, stents are associated with some shortcomings: they are more difficult to navigate to the lesion than plain balloons. Moreover, as data with various drugs (i.e., SIN-1 or Paclitaxel) eluting from a balloon catheter demonstrates significant reduction of neointima formation. With the chemical property advantages of Sirolimus, this might be the mode of application. Therefore this study was conducted to test whether a single injection of Siroliumus, administered intramurally during balloon angioplasty, prevents vascular remodeling associated with arterial injury. We found that intramural infusion of Sirolimus inhibited neointimal formation, smooth muscle cell proliferation, extracellular matrix synthesis, and the expression of several structural proteins.
2. Materials and methods
2.1. In vivo experiments
2.1.1. Balloon angioplasty of rabbit iliac artery
The investigations conform with the Guide for the Care and Use of Laboratory Animals, published by the State and University Review Animal Board. New Zealand White male rabbits (Charles River Germany, 2.8–3.8 kg) were housed individually in a controlled-temperature, standard light/dark environment and allowed to stabilize before any intervention. Rabbits were anesthetized with xylazinhydrochloride (4 mg/kg i.m., Bayer AG, Leverkusen, Germany) and pentobarbital (intramural: 10 mg/kg, supplementary: 4 mg/kg i.v., Sanofi, Nürtingen, Germany). Under sterile conditions the right femoral artery was isolated, a 2 cm incision was made, and a femoral arteriotomy was performed to advance the Transport Coronary Dilatation-Infusion Catheter (Transport®, Boston Scientific Corporation, Ratingen, Germany, 2 cm length, 1 cm for injection, diameter 2.5 and 3 mm balloon/vessel ratio was 1.25) retrogradely to an area within the iliac artery. The position of the balloon catheter was ascertained by anatomic landmarks. Thereafter the inner balloon was inflated with saline to a pressure of 6 atm. Through the outer balloon, rapamycin (Sirolimus; 2 μg/ml, 1 ml total; Calbiochem, Bad Soden, Germany) or its vehicle (saline) was infused into the vessel wall at a constant delivery rate (1 ml/min, 8 atm). The catheter was subsequently flushed with an additional ml of saline and after 2 min, the balloon catheter was deflated and removed. The femoral artery was sutured and the patency of the vessel was controlled by Doppler distal to the insertion site. The surgical incision was closed, the animals received fragmin (30 IU/kg s.c., Grünenthal, Aachen, Germany), aspirin (30 mg/kg i.v., Bayer AG), perioperative and postoperative gentamicin (6 mg/kg i.v. and i.m., Ratiopharm, Ulm, Germany). After the angioplasty procedure, the animals were allowed to recover and returned to their pens. Following 24 h, 1, 2, and 3 weeks following angioplasty, the vessels were harvested for further analysis. We studied 3 different groups, i.e., control animal, PTA plus vehicle and PTA plus Sirolimus. In each group we had at least 7 animals. A total of 30 animals (age 6–9 month) were analyzed for IM-ratio and 15 for proteome profiling.
2.1.2. Harvesting and fixation of injured vessels
Animals were anesthetized, fully heparinized (100 U/kg), euthanized, and perfusion-fixed at 100 mm Hg with 1 L cold (4 °C) 4% paraformaldehyde in PBS, pH 7.4. After perfusion fixation, the iliac arteries were excised, immersed in fresh fixative, and sectioned into 3-mm slices. Vessel sections were embedded in Immunobed (Polyscience Inc., Germany) at 4 °C for 12 h following acetone dehydration. Five μm-thick sections were cut using glass knives and transferred to coated slides. Slides were stained with hematoxylin–eosin.
2.1.3. Histomorphometric analysis of intima/media ratio
Two experienced observers, blinded to the treatment group, evaluated all vessel sections. Therefore, we were able to analyze the vessel over the entire dilated length. The sections were examined using a Zeiss microscope (Axioskop, Zeiss, Göttingen, Germany) with computer assisted morphometric analysis (Optimas-System). The cross-sectional area of the lumen, intima, and media were obtained to calculate the intima/media ratio.
2.1.4. Analysis of elastic fiber expression following vascular injury
Histochemical procedures for the staining of elastic fibers on plastic sections were performed using common protocols (Sigma Co., Deisenhofen, Germany). The sections were lightly counterstained with Gill's Hematoxylin 3 (Sigma Co., Deisenhofen, Germany), and examined using a Zeiss light microscope (Zeiss, Göttingen, Germany). The expression of elastic fibers was assessed in tissue sections as an intensity score ranging from 0 to 5, where 0 was equivalent to no staining and 5 indicated intense staining. The intensity of each section was analyzed, added and divided by the total number of vessels to get total protein expression intensity.
2.1.5. Immunohistochemical analysis of ECM-expression following angioplasty
Immunohistochemical procedures for ECM on plastic sections were performed using the avidin–biotin immunoperoxidase technique (Vectastain ABC Reagent; Vector Laboratories, Burlingame, CA, USA). Immunohistochemical analysis was performed with antibodies directed against collagen III and fibronectin (Calbiochem, Bad Soden, Germany).
2.2. In vitro smooth muscle cell experiments
Smooth muscle cells were isolated from the thoracic aorta of white New Zealand rabbits by the explant technique and SMCs purity was characterized by positive staining with smooth muscle specific α-actin monoclonal antibodies (Sigma-Aldrich Co.). SMCs were maintained in RPMI-1640, (GIBCO BRL Life Technologies Inc., Eggenstein, Germany) and supplemented with 10% FCS (PAA Laboratories, Cölbe, Germany), Penicillin (100 IU/ml) and Streptomycin (50 μg/ml) (GIBCO BRL). Only cells of passages 3–5 were involved in the in vitro experiments.
Cell proliferation was determined using the colorimetric immunoassay (Roche, Mannheim, Germany) for the quantification of cell proliferation, based on the measurement of 5-bromo-2′-deoxyuridine (BrdU) incorporation during DNA synthesis. Briefly, rabbit SMCs were seeded in 96 well plates at a density of 1×104 cells per well then synchronized for 48 h with RPMI-1640 containing 0.1% FCS. Cell proliferation was then stimulated for 24 h with RPMI-1640 medium containing 10% FCS±Sirolimus (0.1 nM to 1 μM). BrdU incorporation was allowed for 4 h, before performing the ELISA following manufacturer’s instructions.
Programmed cell death was investigated with the photometric enzyme-immunoassay (Roche, Mannheim, Germany) for the qualitative and quantitative determination of cytoplasmatic histone-associated-DNA-fragments. Rabbit SMCs were seeded in 96 well plates, the next day incubated Sirolimus (10 nM to 100 μM), vehicle or treated with actinomycin D (100 ng/ml) as a positive control. DNA fragmentation was measured from lysates of cytoplasmic fractions after 24 h.
VSMC necrosis was investigated with the photometric enzyme-immunoassay (Roche, Mannheim, Germany) for the qualitative and quantitative determination of irreversible cellular necrosis. Cellular necrosis was determined from supernatant and lysates of cytoplasmic fractions 24 h after exposure to Sirolimus (10 nM to 100 μM).
2.3. Proteomic analysis of iliac arteries following vascular injury
2.3.1. Sample preparation
Proteome analysis was performed 3 weeks after PTA to detect the effect of Sirolimus. Iliac arteries (2 cm) were harvested 3 weeks after vascular injury where Sirolimus or its vehicle was delivered intramurally. Tissue samples were removed and flash-frozen in liquid nitrogen and kept at −80 °C until analysis. The samples were powderized and dissolved in 1000 μl lysis buffer containing 7 M urea, 2 M thiourea, 4% CHAPS, 40 mM TRIS (base) and 2 tablets of a protease inhibitor mix per 20 ml of buffer stock solution (complete mini®, Fa. Merck, Germany). Finally, 100 U/ml sample of DNAse/RNAse (Benzonase®) was added. The final protein concentration of each sample was determined by using the method of Popov et al. [8]. The protein concentration of most samples was approximately 2.5 μg/μl.
2.3.2. Two-dimensional Gel Electrophoresis (2D PAGE)
IEF (isoelectric focusing) was conducted using the IPGphor unit (Amersham Pharmacia) at a temperature of 20 °C. Immobilized gel strips pH 3–10 L were rehydrated for 12 h using a buffer containing 7 M urea, 2 M thiourea, 2% CHAPS, 0.5% IPG buffer pH 3–10 L, DTT 2.8 mg/ml and a few grains of bromophenol blue. The sample was applied as part of the rehydration solution. The protein concentration was 50 μg protein/13 cm strip for an analytical run and 250 μg for a preparative run. For both separations focusing was 2.0 h at 150 V, 1.0 h at 500 V, 1.0 h at 1000 Vand 2.0 h at 3000 V summing up to 7795 V h. For the second dimension (SDS PAGE) IPG-strips were equilibrated and SDS-gels 10% were used (160×160×1 mm). The gels were run for the first 15 min at 15 mA and thereafter at 30 mA until the dye front was approximately 1 mm from the bottom of the gels. During the second dimension the gels were continuously cooled to 17 °C. Gels were silver-stained according to Heukeshofen and Dernick [9] using silver staining kit (Amersham Pharmacia, Freiburg, Germany) and digitized with a scanner (AGFA, Germany). Gels were analyzed using the MELANIE III software package (genebio, Geneva, Switzerland).
A match set that contained digitized images from five gels per group was created. The number of valid spots was determined for each gel, as well as the number of spots matched to every gel, and qualitative and quantitative differences in the protein patterns between the control and treated vessels following balloon angioplasty were determined. Significant increases or decreases in protein expression between the groups were identified using the statistic part of the software package of MELANIE III.
2.3.3. Identification of 2D separated proteins
Protein spots were excised from the gels, washed with MeOH/H2O and ACN and digested with trypsin (Roche, Mannheim, Germany) overnight at 37 °C. For nanoHPLC/ESI/MS/MS analysis the peptides were separated using reversed phase chromatography (RP18-3, 100 Å, 15 cm, 75 μm i.d., Fa. LC-Packings). A 10 μl/min flow from an Eldex HPLC pump (Fa. SunChrom, Friedrichsdorf) was reduced using a custom-made split-system to achieve a flow rate across the column) of 150 nl/min. A gradient of 5–50% solvent A and B (A: 5% ACN, 95% H2O, 0.08% HCOOH; B: 5% H2O, 95% ACN, 0.08% HCOOH) was used for separation of trypsin-digested peptides. Peptide analysis was performed on a LCQ ion trap mass spectrometer (Fa. ThermoFinnigan, San Jose, USA) equipped with a gold-plated spray capillary (5 μm diameter, Fa. New Objective, USA). A mass spectrum in full-scan mode was followed by two MS/MS spectra of the most abundant peptide ions. This scan cycle was repeated to maximize data acquisition efficiency. Peptide tandem mass spectra were analyzed using the MASCOT software package (Matrix Science, Ltd, London, UK). For each peptide, a cut-off was used for positive protein identification using the MASCOT protein data base. In general, several peptides were analyzed. The significance of the protein match with the ion score was based on the Mowse scoring algorithm. A Score of >75 were judged as a significant match.
2.4. Statistical analysis
Data are presented as mean±SEM unless otherwise stated. The statistical significance of differences between the normal and treated groups was determined by a 1-way ANOVA. Differences were considered significant if P<0.05 by use of StatView 512+ statistical software (Brain Power, Inc.).
3. Results
3.1. Intramural administration successfully targets sirolimus to the vessel wall
For each study, 2 μg of Sirolimus was delivered directly to the injured vessel through the exterior portion of the balloon catheter (see Materials and methods). Fifteen min after administration, plasma levels of Sirolimus reached 2.1±0.4 ng/ml. When the same dose was injected intravenously, plasma levels of Sirolimus were significantly higher after 15 min (16.7±3.2 ng/ml). These pharmacokinetic observations, along with the data below, indicate that intramural administration of Sirolimus with the Transport Catheter successfully delivers a portion of the compound into the vessel wall.
3.2. Intramural delivery of sirolimus inhibits neointima formation induced by arterial injury
All vessel segments analyzed for intimal proliferation after angioplasty were histologically characterized by disruption of the internal elastic lamina, with laceration of the tunica media and exposure of the external elastic lamina. Three weeks after angioplasty, both groups of animals showed a similar vessel size, assessed by the vascular area encircled by the external elastic lamina (data not shown). However, when the intima/media ratio (I/M-ratio) was evaluated, a statistically significant (P<0.05) reduction of I/M-ratio was observed in the sirolimus-treated animals compared with the vehicle treated animals (1.4±0.2 versus 0.75±0.1, respectively) (see Fig. 1). Representative photomicrographs of histological sections from the control (i.e., not dilated group), the PTA+vehicle, and the PTA+sirolimus-treated groups are depicted in the insets of Fig. 1. Only a few layers of vascular smooth muscle cells (VSMC) are found in the intima of normal vessels. Vehicle treated animals demonstrated significant VSMC proliferation in the intima following angioplasty. The newly formed proliferative tissue, characterized by spindle-shaped VSMC cells, filled gaps between medial tears and generally extended to adjacent medial areas that protruded into the lumen. Local delivery of Sirolimus significantly reduced VSMC proliferation and the formation of neointima that normally develops post-angioplasty (Fig. 1).
Fig. 1.
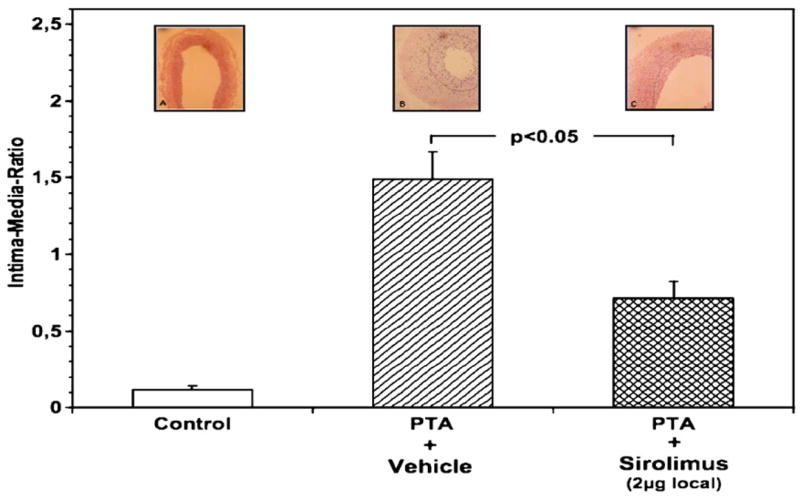
Sirolimus markedly attenuates formation of neointima induced by balloon angioplasty. Intima/media ratio was calculated after 3 weeks as described in Materials and methods. The ratio was obtained from uninjured vessels (control) or injured vessels that were treated with vehicle (PTA+vehicle) or sirolimus (PTA+sirolimus). Values are the mean±SEM of 7–9 independent experiments where statistical significance between the groups was set at P<0.05. Photomicrographs representing typical examples of each treatment are shown above the bars.
3.3. Sirolimus reduces ECM deposition within the vascular wall
We next determined if Sirolimus altered the expression of elastic fibers, collagen III, and fibronectin in the vessel wall of animals undergoing balloon angioplasty. Balloon angioplasty resulted in profound denovo synthesis of elastic fibers when compared to control vessels (Fig. 2A). Elastin expression increased with time, peaking at 3 weeks. Sirolimus markedly reduced the number of elastic fibers deposited in the vessel wall at each time point (Fig. 2A).
Fig. 2.
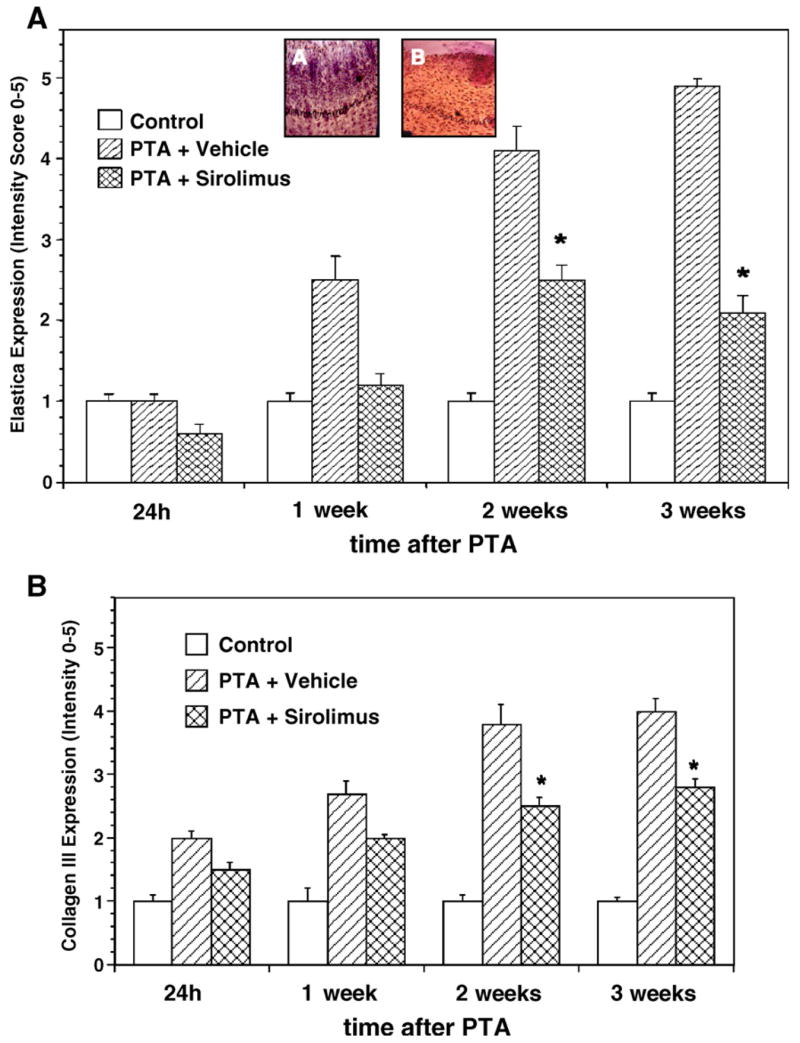
Sirolimus blocks the deposition of ECM in the walls of vessels injured by angioplasty. Vessels were left intact (control) or exposed to angioplasty where a intramural injection of sirolimus (PTA+sirolimus) or vehicle (PTA+vehicle) was delivered through the balloon catheter during the angioplasty procedure. The vessels were collected after 24 h, 1, 2, or 3 weeks for subsequent measurement of ECM components. (A) Bar graph showing the inhibitory effect of sirolimus on expression of elastin fibers following angioplasty at each time point. The insets are photomicrographs taken from representative experiments. Arrows point to elastic fibers that are stained in PTA+ vehicle or PTA+sirolimus treated vessels. (B) Bar graph showing the inhibitory effect of sirolimus on collagen III expression following angioplasty at each time point. The photomicrographs and bar graphs are representative examples of 5.
In addition to elastin, balloon angioplasty resulted in profound accumulation of collagen III when compared to uninjured vessels (Fig. 2B). Collagen III expression increased within 24 h post-angioplasty, and peaked after 2 weeks. Sirolimus attenuated, but did not completely block, collagen III expression at each time point (Fig. 2B). Similarly, the amount of fibronectin deposited in balloon injured vessels was significantly higher than uninjured vessels (data not shown). Sirolimus blocked fibronectin deposition throughout the post-angioplasty period (data not shown).
3.4. The inhibitory properties of sirolimus are unlikely to be due to toxic apoptosis or cell necrosis
During angioplasty, Sirolimus delivered to the injured site may have approached 2 μM, although it is likely that individual cells are exposed to much lower concentrations of the drug in vivo. Nevertheless, we examined the possibility that the inhibitory properties of Sirolimus are due to cytotoxicity. Using a rabbit VSMC in vitro assay system, we first confirmed that Sirolimus blocks VSMC proliferation. The proliferative activity of the VSMCs, treated with increasing concentrations of sirolimus, was assessed following maximal stimulation with 10% FCS or PDGF (10 μg/ml). Treatment with Sirolimus led to a significant and concentration-dependent decrease in BrdU incorporation with an IC50 of 2.2 nM following FCS stimulation (Fig. 3A). Similarly, Sirolimus produced a significant and concentration-dependent decrease in PDGF-induced proliferative activity (IC50 of 5.8 nM; see Fig. 3B). Sirolimus had similar inhibitory effects when ADP (adenosine-di-phosphate) or thrombin was used to induce VSMC proliferation (data not shown).
Fig. 3.
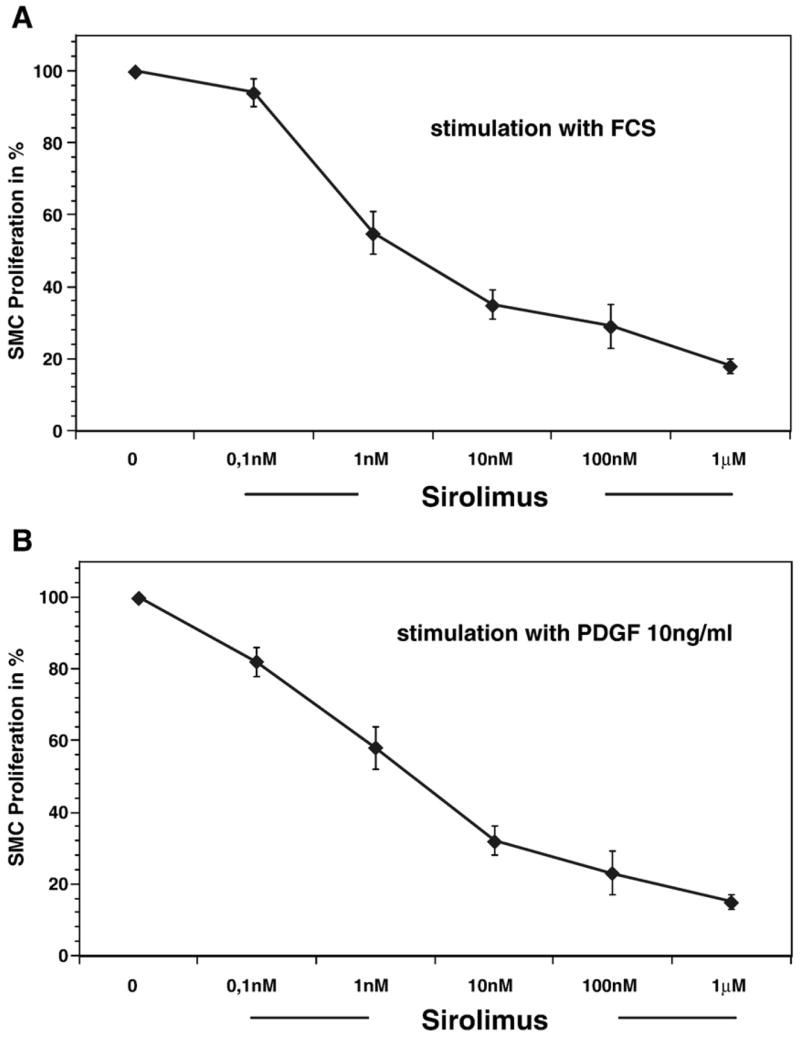
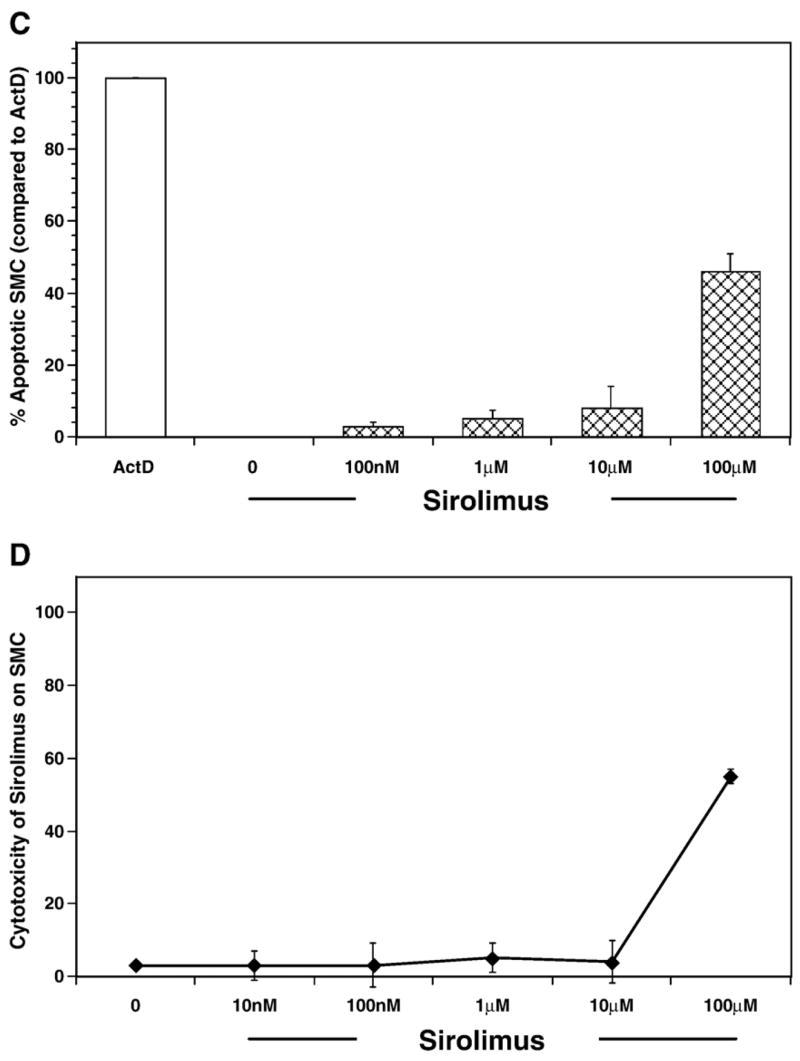
Sirolimus blocks smooth muscle proliferation independent of cell death. (A, B) Bar graph showing the concentration response for sirolimus-induced inhibition of cultured rabbit vascular smooth muscle cell (VSMC) proliferation. Cells were treated for 10 min with either vehicle or increasing concentrations of sirolimus and subsequently stimulated with either 10% FCS (A) or PDGF-α (10 ng/ml) (B). The results are the mean±SEM for 8 experiments. (C) Bar graph showing the percentage of cells that are apoptotic in actinomycin D or sirolimus treated smooth muscle cells. (D) Bar graph showing the cytotoxic effect of sirolimus on cultured rabbit aortic smooth muscle cells. The results are the mean±SEM of six independent experiments.
We next determined if the inhibitory properties of Sirolimus were due to cytotoxicity induced by the drug. Sirolimus did not induce DNA fragmentation, a measurement of programmed cell death, with concentrations approximately 50 times those used for intramural administration of the drug during angioplasty (see above and Fig. 3C). Moreover, only a very high concentration of Sirolimus induced VSMC necrosis (see Fig. 3D). These results indicate that the major effects of Sirolimus are not due to cellular cytotoxicity under the conditions of our study.
3.5. Sirolimus differentially blocks the expression of structural proteins in the post-angioplasty vessel wall
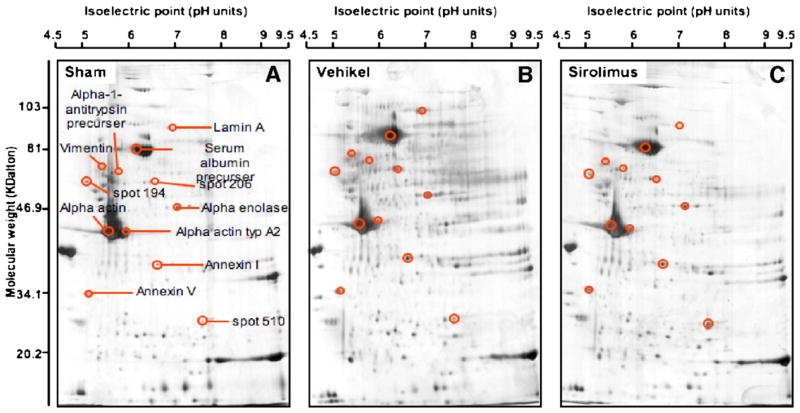
Vessel samples from each group (N= 5) were prepared as described in Materials and methods and the protein was normalized and separated by 2-D gel electrophoresis. A representative example of the silver stained gels obtained from segments of uninjured (sham) or injured vessels (i.e., dilated vessels) treated with vehicle or Sirolimus is shown in Fig. 4. On average, 485±15 protein spots were identified in the uninjured, sham-treated vessels (Fig. 4A). Following angioplasty, the expression of twelve proteins was consistently altered compared to uninjured vessels (see Fig. 4B and Table 1). Specifically, 9 proteins were increased in the group treated with vehicle alone when compared to uninjured vessels while 2 proteins were consistently decreased and 1 was not altered. These 12 proteins were subsequently identified by nanoHPLC/ESI-MS/MS. Nine of the proteins analyzed by using nanoHPLC/ESI-MS/MS could be matched to known protein sequences and unambiguously identified (Table 1). Using ESI-MS/MS and database search we were able to identify a total 7 to 19 peptides of these 9 proteins. Further we had a sequence coverage of 35 to 63% among these peptides.
Fig. 4.
Sirolimus differentially blocks the expression of structural proteins within the injured vessel wall. Vessels were left intact (sham) or exposed to angioplasty where an intramural injection of sirolimus (PTA+sirolimus) or vehicle (PTA+vehicle) was delivered through the balloon catheter during the angioplasty procedure. The vessels were collected after 3 weeks, proteins were harvested and normalized, and the proteins were separated by 2-D gel electrophoresis. Proteins on the gel were identified by silver stain as described in Materials and methods. (A) Sham (B) PTA+vehicle. (C) PTA+sirolimus. Size of original gel: 16×16×0.1 cm. Circled spots in panel B are proteins that were increased in angioplastied vessels compared to uninjured vessels (A). For comparison, the same areas are circled in panel C. Proteins that were eventually identified by nanospray tandem mass spectrometry (see Fig. 5 below) are labeled accordingly. This figure is representative of 5 independent experiments for each group.
Table 1.
Densitometric analysis and relative changes of all proteins analyzed
| Spot ID | Protein name | MW(Dalton) | IP(pH units) | Control
|
PTA+Vehicle
|
PTA+Sirolimus
|
|---|---|---|---|---|---|---|
| Optical density | Rel. changes vs. sham | Rel. changes vs. sham | ||||
| 104 | Lamin A | 65500 | 7.0 | 64.5±5.7 | 1.72 | 1.41 |
| 132 | Serum albumin precursor | 70900 | 6.3 | 178.5±11.9 | 0.77 | 1.08 |
| 156 | Vimentin | 53600 | 5.4 | 54.75±6.1 | 1.89 | 0.97 |
| 179 | Alpha-1-antitrypsin precursor | 46100 | 5.8 | 41.75±8.4 | 1.89 | 1.20 |
| 264 | Alpha enolase | 47400 | 7.1 | 141.00±10.4 | 1.03 | 1.11 |
| 294 | Alpha actin | 42600 | 5.5 | 187.5±9.3 | 1.30 | 0.74 |
| 307 | Alpha actin type A2 | 42500 | 6.0 | 161.75±7.8 | 1.25 | 0.92 |
| 379 | Annexin I | 39000 | 6.7 | 91.75±4.6 | 1.65 | 1.38 |
| 440 | Annexin V | 36000 | 5.2 | 71.25±3.2 | 1.69 | 1.37 |
| 194 | sequence tag | 57200 | 5.1 | 83.75±6.4 | 1.48 | 1.08 |
| 206 | sequence tag | 1754.8 | 6.4 | 56.5±4.2 | 1.47 | 1.75 |
| 510 | sequence tag | 29500 | 7.7 | 44.75±3.6 | 1.58 | 2.22 |
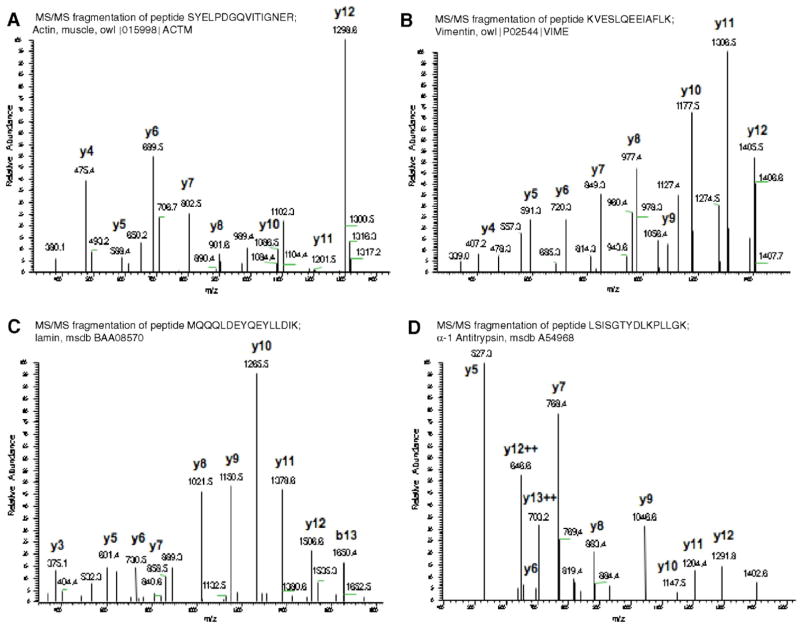
The peptide maps and sequences for α-actin, vimentin, lamin A, and α-1-antitrypsin are shown in Fig. 5A–D. The majority of proteins with specific matches regulate the structural integrity of vessel walls. Three additional proteins could not be found in the database (see Table 1). As shown in Fig. 5C, Sirolimus blocked the expression of a specific subset of proteins that were upregulated in injured vessels. Densitometric analyses of Sirolimus-sensitive proteins, which include lamin A, vimentin, α-1-antitrypsin, and α-actin, are found in Fig. 6A and B. Clearly Sirolimus treatment resulted in reduced expression of lamin A, vimentin, α-1-anti-trypsin precursor, and alpha actin. The expression of other proteins, including annexin I and annexin V, was not significantly altered by Sirolimus.
Fig. 5.
The expression of α-actin, vimentin, lamin A, and α-1-antitrypsin is increased in vessel walls following angioplasty. Tryptic maps from nanospray tandem mass spectrometry generated from protein spots in Fig. 1. The proteins were subsequently identified using MS-Tag database search as described in Materials and methods. The tryptic map for analysis for A, α-actin; B, vimentin; C, Lamin A; D, α-1-antitrypsin are shown. Proteins for each analysis were generated from 5 independent experiments, subsequently pooled, and analyzed as shown in the figure.
Fig. 6.
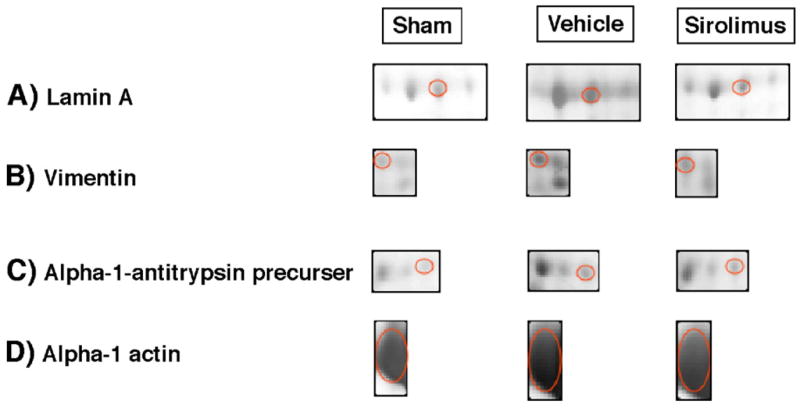
Sirolimus differentially blocks the expression of structural proteins in angioplastied vessels. Protein expression for lamin A, vimentin, α-1-antitrypsin, and α-actin in control vessels, PTA+vehicle, and PTA+sirolimus using 2D-electrophoresis with computer assisted densitometry. (A) Magnification of gel spots for all three groups, (B) Densitometric analysis, the bars in the graph depict the mean±SEM for 5 independent experiments. *P<0.05.
4. Discussion
High restenosis rates continue to be the major setback for balloon angioplasty and related procedures. The primary goal of this study was to determine if intramural delivery of Sirolimus, a reagent known to prevent restenosis when administered in drug-eluting stents, prevents neointimal formation [10–12]. Sirolimus is a macrolide antibiotic with antifungal and immunosuppressive activities that is being used in liver, kidney, and heart transplantation [13]. Sirolimus also inhibits the development of arteriopathy after allograft transplantation [14,15] and has anti-angiogenic effects [16]. It prevents cellular proliferation by binding to FK506-binding protein 12 (FKBP12) [13], a protein that is markedly upregulated in restenotic lesions [7]. The FKBP12–rapamycin complex inhibits cellular growth and proliferation by inhibiting mTOR (mammalian Target of Rapamycin) activity and interfering with the progression of G1-phase cells into S phase [17,18]. mTOR is a phosphatidylinositol-related kinase that limits the translation of a specific subset mRNAs and thereby controls the rate of passage of mitogen-stimulated cells through the mid/late G1-phase checkpoint [19]. In addition, Sirolimus selectively inhibits synthesis of proteins that alter cellular phenotype in the absence of cell cycle progression or cell growth [20]. In our studies, Sirolimus blocked the expression of lamin A, vimentin, α-actin, α-actin type 2A, and α-1-antitrypsin. It also inhibited ECM deposition and neoinitimal proliferation following angioplasty. Presumably these inhibitory effects are due to reduction of SMC proliferation within the injured vessel. However, decreased translation of Sirolimus-sensitive transcripts that encode proteins involved in arterial remodeling might be another explanation.
Previous studies have demonstrated that Sirolimus attenuates arterial late lumen loss following angioplasty, but administration of the drug was initiated 3 days prior to the procedure to ascertain that VSMCs would be maintained in a quiescent state from the moment of injury. Sirolimus administration was then maintained for an additional 14 days to prevent any growth stimulus that might occur after the procedure [21,22]. Similarly, delivery of Sirolimus in coronary stents with gradual elution also blocks restenosis, further demonstrating that continuous treatment affords protection against neoinitima proliferation [11,12]. Our results indicate that chronic Sirolimus treatment may not be a necessary requisite for its anti-restenotic properties. Indeed, intramural delivery of Sirolimus during balloon dilatation successfully reduced intimal hyperplasia, although it is not clear how long the drug remains at the injured site. Nevertheless, Sirolimus has a half-life exceeding 95 h.
Consistent with its inhibitory effect on neointimal formation, Sirolimus decreased deposition of elastin, collagen III, and fibronectin. Synthesis of these ECM components critically regulates neointima formation [23–26] and leukocyte and VSMC migration within the vascular wall [6]. Of interest in our study, Sirolimus reduced neointimal formation in the face of decreased elastin deposition, an event that enhances vascular smooth muscle deposition in developmental models [4]. This indicates that Sirolimus has effects on cellular and matrix components of the restenostic lesion that favorably influence the vascular remodeling process. Our observations provide a rationale for additional investigation of intramural delivery of Sirolimus during angioplasty to prevent restenosis in patients undergoing this procedure.
The molecular mechanisms that govern arterial remodeling are just being discovered. cDNA array technology has been used successfully to identify differentially expressed genes within restenotic lesions [7]. Identification of these transcripts has been used to predict the expression of corresponding proteins. In many cases, mRNA and protein levels parallel one another. However, in other instances translation of mRNA into protein does not occur in the absence of critical signaling events, and numerous examples from our laboratory, as well as others, demonstrate that primary human cells control expression of specific gene products at the translational level, as do cell lines and neoplastic cells [27–31]. Proteomic profiling of restenotic arteries allowed us to generate expression data for over 500 gene products. Twelve of these proteins were differentially expressed in restenotic vessels when compared to uninjured vessels and subsequently identified by nano HPLC/ESI-MS/MS. Sequencing of the fragmented peptides demonstrated that the majority of proteins serve structural functions by regulating vascular wall integrity. Sirolimus inhibited some, but not all of the differentially expressed proteins.
Given the amount of arterial remodeling that occurs following angioplasty, it is not surprising that the majority of peptide sequences matched proteins that regulate vessel wall structure. Lamin A and vimentin were two of these proteins. Previous studies have demonstrated that both are upregulated in proliferating fibroblasts and vascular smooth muscle cells [32,33]. Vimentin provides support for the contractile apparatus [34] and is overexpressed in VSMC’s that comprise human restenotic lesions [35]. Proliferating VSMCs simultaneously undergo contractile behavior changes [6], a response that is consistent with increased expression of α-actin, α-actin type A2, and annexins in our restenosis model. Actin family members are compartmentalized to distinct intracellular regions of proliferating rabbit VSMCs, a process that modulates cell signaling events [34]. Annexins bind calcium and control intracellular Ca2+ signaling, a process that leads to structural rearrangements within the sarcolemma of contracting VSMC’s [36,37]. We also found that α1-antitrypsin was increased by nearly 2-fold in the vehicle group when compared to control vessels. This anti-protease regulates ECM deposition, and recently it has been reported that α-1-anti-trypsin induces fibroblast proliferation and collagen production via classical mitogen-activated (MAP) signaling pathways [38]. Increased levels of α-1-antitrypsin and lamin A, a nuclear intermediate filament protein that structurally supports the lamina network [39], are thought to play pivotal roles in vivo tissue repair and ECM production [38,39]. A recent report also demonstrates that a carboxyl-terminal fragment of α-1-antitrypsin is present in atherosclerotic plaques where it regulates transcription factor activity in monocytes [40].
4.1. Unknown features and variables
The goal of this study was to determine if intramural delivery of Sirolimus during angioplasty inhibits neointimal formation and associated expression of structural factors. We found that proteomic profiling of excised vessels was highly reproducible. Expression patterns between animals were consistent, and only a small number of differentially expressed proteins were observed between control and injured vessels. In addition, the number of altered proteins is almost certainly underestimated in our study because of the limits of detection of silver staining. Due to the complexity of the procedure and proteomic profiling, we conducted our analysis at a single time point — 3 weeks post-angioplasty. It is likely that there are significant temporal changes in protein expression patterns post-injury. In this regard, measurement of temporal gene expression by real-time PCR revealed that more ECM-related transcripts are upregulated 1 day following angioplasty than any other time point [5], consistent with marked leukocyte infiltration. A number of these mRNAs were still induced or repressed over 4 weeks, indicating that a return to pre-injury expression patterns and steady state transcription are not achieved within the first month [5]. Our findings suggest that continuous translation of mRNAs into proteins involved in restenosis also occurs post-angioplasty.
Precise identification of the cells synthesizing each protein, complicated by the lack of antibodies that recognize rabbit antigens, and the specific cell types influenced by Sirolimus are additional unknown features. Since VSMC are the predominant cell within the neointima, it is likely that the majority of protein expression signals are derived from these vascular cells although this, too, will vary with time. In addition, Sirolimus is reported to exert a major inhibiting effect on smooth muscle cell proliferation and migration in vascular stent models [41]. Nevertheless, Sirolimus may also influence other cells that are active in the complex restenotic process. We found that Sirolimus markedly decreased the initial accumulation of leukocytes in vessels at 24 and 48 h after angioplasty (M. Buerke et al., unpublished data). Sirolimus inhibits synthesis of urokinase plasminogen activator receptor, an adhesion molecule that recognizes vitronectin in human monocytes in vitro. It may have similar inhibitory effects in vivo. In addition, Sirolimus differentially blocks synthesis of specific proteins in human platelets and neutrophils [20,42].
In summary, we found that intramural delivery of Sirolimus during balloon angioplasty inhibited neointimal formation and the expression of associated structural proteins. Taken together, these results suggest that local administration of Sirolimus may be a useful approach to prevent restenosis in small vessels (<2.5 mm) exposed to balloon angioplasty.
Abbreviations
- ECM
extracellular matrix
- PDGF
platelet derived growth factor
- PTA
percutaneous transluminal angioplasty
- VSMC
vascular smooth muscle cells
- FKBP12
FK506-binding protein 12
- I/M-ratio
intima/media ratio
- 2D PAGE
Two-dimensional Gel Electrophoresis
- FCS
fetal calf serum
References
- 1.Fuster V, Falk E, Fallon JT, Badimon L, Chesebro JH, Badimon JJ. The three processes leading to post PTCA restenosis: dependence on the lesion substrate. Thromb Haemost. 1995;74:552–559. [PubMed] [Google Scholar]
- 2.Landau C, Lange RA, Hillis LD. Percutaneous transluminal coronary angioplasty. N Engl J Med. 1994;330:981–993. doi: 10.1056/NEJM199404073301407. [DOI] [PubMed] [Google Scholar]
- 3.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 4.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 5.Tai JT, Brooks EE, Liang S, Somogyi R, Rosete JD, Lawn RM, Shiffman D. Determination of temporal expression patterns for multiple genes in the rat carotid artery injury model. Arterioscler Thromb Vasc Biol. 2000;20:2184–2191. doi: 10.1161/01.atv.20.10.2184. [DOI] [PubMed] [Google Scholar]
- 6.Raines EW. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int J Exp Pathol. 2000;81:173–182. doi: 10.1046/j.1365-2613.2000.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zohlnhofer D, Klein CA, Richter T, Brandl R, Murr A, Nuhrenberg T, Schomig A, Baeuerle PA, Neumann FJ. Gene expression profiling of human stent-induced neointima by cDNA array analysis of microscopic specimens retrieved by helix cutter atherectomy: detection of FK506-binding protein 12 upregulation. Circulation. 2001;103:1396–1402. doi: 10.1161/01.cir.103.10.1396. [DOI] [PubMed] [Google Scholar]
- 8.Popov N, Schmitt M, Schulzeck S, Matthies H. Reliable micromethod for determination of the protein content in tissue homogenates. Acta Biol Med Ger. 1975;34:1441–1446. [PubMed] [Google Scholar]
- 9.Heukeshoven J, Dernick R. Improved silver staining procedure for fast staining in PhastSystem Development Unit. I. Staining of sodium dodecyl sulfate gels. Electrophoresis. 1988;9:28–32. doi: 10.1002/elps.1150090106. [DOI] [PubMed] [Google Scholar]
- 10.Sousa JE, Costa MA, Abizaid A, Abizaid AS, Feres F, Pinto IM, Seixas AC, Staico R, Mattos LA, Sousa AG, Falotico R, Jaeger J, Popma JJ, Serruys PW. Lack of neointimal proliferation after implantation of sirolimus-coated stents in human coronary arteries: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation. 2001;103:192–195. doi: 10.1161/01.cir.103.2.192. [DOI] [PubMed] [Google Scholar]
- 11.Sousa JE, Costa MA, Abizaid AC, Rensing BJ, Abizaid AS, Tanajura LF, Kozuma K, Van Langenhove G, Sousa AG, Falotico R, Jaeger J, Popma JJ, Serruys PW. Sustained suppression of neointimal proliferation by sirolimus-eluting stents: one-year angiographic and intravascular ultrasound follow-up. Circulation. 2001;104:2007–2011. doi: 10.1161/hc4201.098056. [DOI] [PubMed] [Google Scholar]
- 12.Rensing BJ, Vos J, Smits PC, Foley DP, van den Brand MJ, van der Giessen WJ, de Feijter PJ, Serruys PW. Coronary restenosis elimination with a sirolimus eluting stent: first European human experience with 6-month angiographic and intravascular ultrasonic follow-up. Eur Heart J. 2001;22:2125–2130. doi: 10.1053/euhj.2001.2892. [DOI] [PubMed] [Google Scholar]
- 13.Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annu Rev Immunol. 1996;14:483–510. doi: 10.1146/annurev.immunol.14.1.483. [DOI] [PubMed] [Google Scholar]
- 14.Gregory CR, Huie P, Billingham ME, Morris RE. Rapamycin inhibits arterial intimal thickening caused by both alloimmune and mechanical injury. Its effect on cellular, growth factor, and cytokine response in injured vessels. Transplantation. 1993;55:1409–1418. doi: 10.1097/00007890-199306000-00037. [DOI] [PubMed] [Google Scholar]
- 15.Meiser BM, Billingham ME, Morris RE. Effects of cyclosporin, FK506, and rapamycin on graft-vessel disease. Lancet. 1991;338:1297–1298. doi: 10.1016/0140-6736(91)92594-r. [DOI] [PubMed] [Google Scholar]
- 16.Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M, Jauch KW, Geissler EK. Rapamycin inhibits primary and metastatic tumor growth by anti-angiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–135. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- 17.Morice WG, Brunn GJ, Wiederrecht G, Siekierka JJ, Abraham RT. Rapamycin-induced inhibition of p34cdc2 kinase activation is associated with G1/S-phase growth arrest in T lymphocytes. J Biol Chem. 1993;268:3734–3738. [PubMed] [Google Scholar]
- 18.Albers MW, Brown EJ, Tanaka A, Williams RT, Hall FL, Schreiber SL. An FKBP-rapamycin-sensitive, cyclin-dependent kinase activity that correlates with the FKBP-rapamycin-induced G1 arrest point in MG-63 cells. Ann N Y Acad Sci. 1993;696:54–62. doi: 10.1111/j.1749-6632.1993.tb17142.x. [DOI] [PubMed] [Google Scholar]
- 19.Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- 20.Weyrich AS, Dixon DA, Pabla R, Elstad MR, McIntyre TM, Prescott SM, Zimmerman GA. Signal-dependent translation of a regulatory protein, Bcl-3, in activated human platelets. Proc Natl Acad Sci U S A. 1998;95:5556–5561. doi: 10.1073/pnas.95.10.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallo R, Padurean A, Jayaraman T, Marx S, Roque M, Adelman S, Chesebro J, Fallon J, Fuster V, Marks A, Badimon JJ. Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation. 1999;99:2164–2170. doi: 10.1161/01.cir.99.16.2164. [DOI] [PubMed] [Google Scholar]
- 22.Burke SE, Lubbers NL, Chen YW, Hsieh GC, Mollison KW, Luly JR, Wegner CD. Neointimal formation after balloon-induced vascular injury in Yucatan minipigs is reduced by oral rapamycin. J Cardiovasc Pharmacol. 1999;33:829–835. doi: 10.1097/00005344-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Strauss BH, Chisholm RJ, Keeley FW, Gotlieb AI, Logan RA, Armstrong PW. Extracellular matrix remodeling after balloon angioplasty injury in a rabbit model of restenosis. Circ Res. 1994;75:650–658. doi: 10.1161/01.res.75.4.650. [DOI] [PubMed] [Google Scholar]
- 24.Ju H, Dixon IM. Extracellular matrix and cardiovascular diseases. Can J Cardiol. 1996;12:1259–1267. [PubMed] [Google Scholar]
- 25.Lafont A, Durand E, Samuel JL, Besse B, Addad F, Levy BI, Desnos M, Guerot C, Boulanger CM. Endothelial dysfunction and collagen accumulation: two independent factors for restenosis and constrictive remodeling after experimental angioplasty. Circulation. 1999;100:1109–1115. doi: 10.1161/01.cir.100.10.1109. [DOI] [PubMed] [Google Scholar]
- 26.Imanaka-Yoshida K, Matsuura R, Isaka N, Nakano T, Sakakura T, Yoshida T. Serial extracellular matrix changes in neointimal lesions of human coronary artery after percutaneous transluminal coronary angio-plasty: clinical significance of early tenascin-C expression. Virchows Arch. 2001;439:185–190. doi: 10.1007/s004280000390. [DOI] [PubMed] [Google Scholar]
- 27.Pradet-Balade B, Boulme F, Beug H, Mullner EW, Garcia-Sanz JA. Translation control: bridging the gap between genomics and proteomics? Trends Biochem Sci. 2001;26:225–229. doi: 10.1016/s0968-0004(00)01776-x. [DOI] [PubMed] [Google Scholar]
- 28.Lindemann S, Tolley ND, Dixon DA, McIntyre TM, Prescott SM, Zimmerman GA, Weyrich AS. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J Cell Biol. 2001;154:485–490. doi: 10.1083/jcb.200105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindemann S, Tolley ND, Eyre JR, Kraiss LW, Mahoney TM, Weyrich AS. Integrins regulate the intracellular distribution of eukaryotic initiation factor 4E in platelets. A checkpoint for translational control. J Biol Chem. 2001;276:33947–33951. doi: 10.1074/jbc.M104281200. [DOI] [PubMed] [Google Scholar]
- 30.Kraiss LW, Weyrich AS, Alto NM, Dixon DA, Ennis TM, Modur V, McIntyre TM, Prescott SM, Zimmerman GA. Fluid flow activates a regulator of translation, p70/p85 S6 kinase, in human endothelial cells. Am J Physiol, Heart Circ Physiol. 2000;278:H1537–H1544. doi: 10.1152/ajpheart.2000.278.5.H1537. [DOI] [PubMed] [Google Scholar]
- 31.Dixon DA, Tolley ND, King PH, Nabors LB, McIntyre TM, Zimmerman GA, Prescott SM. Altered expression of the mRNA stability factor HuR promotes cyclooxygenase-2 expression in colon cancer cells. J Clin Invest. 2001;108:1657–1665. doi: 10.1172/JCI12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dyer JA, Kill IR, Pugh G, Quinlan RA, Lane EB, Hutchison CJ. Cell cycle changes in A-type lamin associations detected in human dermal fibro-blasts using monoclonal antibodies. Chromosome Res. 1997;5:383–394. doi: 10.1023/a:1018496309156. [DOI] [PubMed] [Google Scholar]
- 33.Kocher O, Gabbiani F, Gabbiani G, Reidy MA, Cokay MS, Peters H, Huttner I. Phenotypic features of smooth muscle cells during the evolution of experimental carotid artery intimal thickening. Biochemical and morphologic studies. Lab Invest. 1991;65:459–470. [PubMed] [Google Scholar]
- 34.Worth NF, Rolfe BE, Song J, Campbell GR. Vascular smooth muscle cell phenotypic modulation in culture is associated with reorganisation of contractile and cytoskeletal proteins. Cell Motil Cytoskelet. 2001;49:130–145. doi: 10.1002/cm.1027. [DOI] [PubMed] [Google Scholar]
- 35.Inoue K, Nakamura N, Kakio T, Suyama H, Tanaka S, Goto Y, Nakazawa Y, Yamamoto Y, Nagamatsu T. Serial changes of coronary arteries after percutaneous transluminal coronary angioplasty: histopathological and immunohistochemical study. J Cardiol. 1994;24:279–291. [PubMed] [Google Scholar]
- 36.Hawkins TE, Merrifield CJ, Moss SE. Calcium signaling and annexins. Cell Biochem Biophys. 2000;33:275–296. doi: 10.1385/cbb:33:3:275. [DOI] [PubMed] [Google Scholar]
- 37.Babiychuk EB, Draeger A. Annexins in cell membrane dynamics. Ca(2+)-regulated association of lipid microdomains. J Cell Biol. 2000;150:1113–1124. doi: 10.1083/jcb.150.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dabbagh K, Laurent GJ, Shock A, Leoni P, Papakrivopoulou J, Chambers RC. Alpha-1-antitrypsin stimulates fibroblast proliferation and procollagen production and activates classical MAP kinase signalling pathways. J Cell Physiol. 2001;186:73–81. doi: 10.1002/1097-4652(200101)186:1<73::AID-JCP1002>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 39.Mounkes LC, Burke B, Stewart CL. The A-type lamins: nuclear structural proteins as a focus for muscular dystrophy and cardiovascular diseases. Trends Cardiovasc Med. 2001;11:280–285. doi: 10.1016/s1050-1738(01)00126-8. [DOI] [PubMed] [Google Scholar]
- 40.Dichtl W, Moraga F, Ares MP, Crisby M, Nilsson J, Lindgren S, Janciauskiene S. The carboxyl-terminal fragment of alpha1-antitrypsin is present in atherosclerotic plaques and regulates inflammatory transcription factors in primary human monocytes. Mol Cell Biol Res Commun. 2000;4:50–61. doi: 10.1006/mcbr.2000.0256. [DOI] [PubMed] [Google Scholar]
- 41.Marx SO, Jayaraman T, Go LO, Marks AR. Rapamycin-FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ Res. 1995;76:412–417. doi: 10.1161/01.res.76.3.412. [DOI] [PubMed] [Google Scholar]
- 42.Yost CC, Denis MM, Lindemann S, Buerke RFJMGKM, McIntyre TM, Weyrich AS, Zimmerman GA. Activated polymorphonuclear leucocytes rapidly synthesize retinoic acid receptor-alpha: a mechanism for translational control of transcriptional events. J Exp Med. 2004;200:671–680. doi: 10.1084/jem.20040224. [DOI] [PMC free article] [PubMed] [Google Scholar]