Abstract
Past studies have established that the cornea like the lens abundantly expresses a few water-soluble enzyme/proteins in a taxon specific fashion. Based on these similarities it has been proposed that the lens and the cornea form a structural unit the ‘refracton’ that has co-evolved through gene sharing to maximize light transmission and refraction to the retina. Thus far, the analogy between corneal crystallins and lens crystallins has been limited to similarities in the abundant expression, with few reports concerning their structural function. This review covers recent studies that establish a clear relationship between expression of corneal crystallins and light scattering from corneal stromal cells, i.e. keratocytes, that support a structural role for corneal crystallins in the development of transparency similar to that of lens crystallins that would be consistent with the ‘refracton’ hypothesis.
1. Introduction
Holt and Kinoshita [1] were the first to report that water-soluble extracts from the bovine cornea contained a single prominent protein fraction that was initially recognized as bovine corneal protein 54 (BCP54) [2, 3]. Later studies have identified BCP54 as aldehyde dehydrogenase 3A1 [4, 5] that comprises from 20-40% of the total water-soluble protein content of the bovine corneal epithelium. More recent studies have shown that unlike non-transparent tissues and organs other than lens, the corneas from a wide range of species abundantly express a few water-soluble enzyme/proteins (Table 1) [3, 6-14], many of which are identical to the abundantly expressed taxon-specific lens crystallins, including aldehyde dehydrogenase 1A1 (ALDH1A1)/η-crystallin, α-enolase/τ-crystallin, glutathione-S-transferase/Ω-crystallin, lactic dehydrogenase/ε-crystallin, glyceraldehyde-3-phosphate dehydrogenase (G3PDH)/π-crystallin, and arginino-succinate lyase/δ-crystallin [6, 8]. Interestingly, several of these corneal enzyme/proteins are abundantly expressed in the lens of the same species, notably arginino-succinate lyase/δ-crystallin in the chicken and glutathione-S-transferase/Ω-crystallin in the squid. Furthermore, the anuran (toad and frog) corneal epithelium, which can transdifferentiate to regenerate the lens, abundantly expresses ubiquitous, vertebrate lens α, β and γ crystallin in addition to the taxon-specific crystallin α-enolase/τ crystallin [10]. Overall, the similarity in expression of these proteins in the cornea and lens, both in abundance and taxon-specificity, has lead many investigators to refer to these proteins as corneal crystallins [15, 16].
Table 1.
Known Corneal Crystallins and Their Lens Counterparts
| Corneal Crystallin | Species | Ref. | Lens Crystallin | Species |
|---|---|---|---|---|
| ALDH1A1 | rabbit, human, pig | [8] | η-crystallin | elephant shrew |
| α-enolase | human, mouse, chicken, crocodile, toad/frog | [6, 9] | τ-crystallin | lamprey, crocodile |
| glutathione-S-transferase | squid, mouse | [6] | Ω-crystallin | cephalopods |
| lactic dehydrogenase | rabbit, human, chicken, pig | [7] | ε-crystallin | duck, crocodiles |
| G3PDH | rabbit, human, chicken | [7] | π-crystallin | geckos |
| arginino-succinate lyase | chicken | [6] | δ-crystallin | birds, reptiles |
| α, β, γ crystallin | Indian toad and frog | [10] | α, β, γ crystallin | mammals |
| triose phosphate isomerase | crocodile | [9] | ||
| TKT | mammals | [8, 11] | ||
| BCP54/ALDH3A1 | most mammals | [3, 6] | ||
| isocitrate dehydrogenase | bovine | [12] | ||
| gelsolin | fish | [13, 14] | ||
| actin | fish, mouse | [7, 13, 14] | ||
| peptidl-prolyl cistrans isomerase | chicken | [6] | ||
| pyruvate kinase | chicken | [7] | ||
| annexin II | chicken | [7] | ||
| protein disulfide isomerase | chicken | [7] |
While the critical function of corneal crystallins has yet to be established, the fact that the lens and cornea are unique in being transparent has prompted the hypothesis that they form a structural unit, the ‘refracton’, that has evolved through a process of gene sharing to meet the demands required for the development and maintenance of transparency and refraction of light necessary for vision [17, 18]. Indeed, many of the corneal and lens crystallins show important functional properties such as chaperones and metabolic enzymes that could play a role in protecting cells from light-induced stress. In mammals the most common corneal crystallin identified has been ALDH3A1, which has been shown to be protective against UV and oxidative stress-induced apoptosis [19-21]. ALDH3A1 and ALDH1A1 also metabolize hexanal and 4-hydroxynonenal and malondialdehyde, the major products of lipid peroxidation, while generating NADPH, a UV absorber [22-24]. ALDH3A1 may also directly absorb UV light and protects against UV-induced inactivation of other intracellular enzymes, either through UV-absorption or chaperone-like activity [22]. Overall, water-soluble proteins from the cornea while accounting for 20% of the total protein may account for over 50% of the total UVB-light absorption, and have been called “absorbins” by some investigators [25].
In addition to the metabolic and UV-absorptive roles, crystallins are thought to play a structural function by directly influencing light scattering within the cornea. In the lens, high concentration of crystallin proteins have been shown to is thought to provide short-range order within the cytoplasm of the lens fibers reducing light scattering from macromolecules as occur in more dilute solutions [26-29]. It has therefore been proposed that corneal crystallin proteins serve a similar function in corneal epithelial cells and stromal keratocytes (fibroblasts) by accumulating to a high proportion of the soluble protein and limiting light scattering [8, 30].
While there is strong theoretical basis supporting crystallin proteins, transparency and the ‘refracton’ theory, experimental evidence confirming these relationships are only now being reported. These studies suggest that there is an association between expression of corneal crystallins and cellular transparency, particular involving the stromal keratocyte. This paper reviews the evidence linking corneal crystallin protein expression and light scattering from the cornea and establishes that adult corneas that are transparent show high expression levels for corneal crystallins in stromal cells, i.e. keratocytes. More importantly, decreased corneal crystallin protein expression during early postnatal development or after injury is associated with loss of corneal transparency and marked light scattering from stromal keratocytes. Finally, decreased expression of corneal crystallins in cultured stromal keratocytes is associated with increased in vitro light scattering. Taken together these findings strongly support a role for corneal crystallins in the development and maintenance of transparency and the ‘refracton’ theory for the evolution of transparency and refraction within the eye.
2. Cellular Transparency in the Normal Cornea
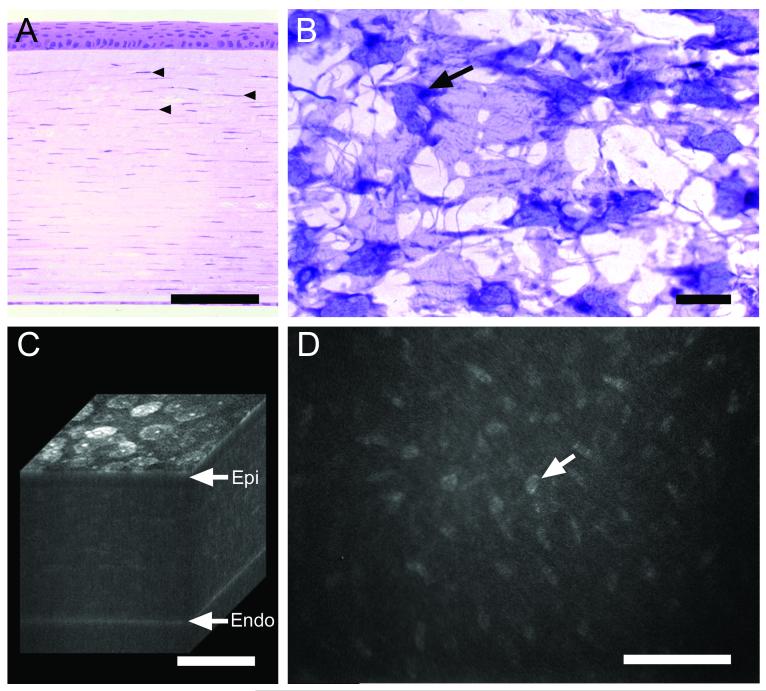
The normal cornea is composed of 3 distinct tissue layers (Fig. 1), the anterior corneal epithelium, the corneal stroma and the posterior corneal endothelium. While the thickness of the corneal epithelium is fairly constant, ranging from 45 to 50 μm in the human, mouse and rabbit [31-33], the corneal stromal thickness varies considerably depending on the species and can be quite thin in mice (70 to 90 μm depending on strain), thicker in rabbits and humans (325 μm to 500 μm, respectively) and very thick in pigs and cows (1 mm) [32-35]. The corneal stroma for the most part contains regularly arranged collagen fibers of uniform thickness and spacing that are organized into thicker collagen bundles or lamellae. Between lamellae are flattened corneal keratocytes that may appear sparse in cross-sections of cornea (Fig. 1A, arrowheads) but are quite dense as seen in coronal sections (Fig. 1B) having a broad cell body with multiple dendritic, interconnecting processes. Estimates of the volume of the corneal stroma occupied by keratocytes range from 9-17% [36], with densities ranging from 48,000 cells/mm3 in the mouse to 23,000 cells/mm3 in humans [36, 37].
Fig. 1.
Normal rabbit cornea as shown by histology (A and B) and in vivo confocal microscopy (C and D). (A) Cross-section stained with hematoxylin & eosin showing anterior stratified corneal epithelium overlying corneal stroma with keratocyte nuclei (arrowheads) and posterior corneal endothelium. (B) Coronal section through the corneal stroma stained with gold-chloride showing keratocyte cell bodies and nuclei (arrow). (C) 3-D reconstruction of confocal images through a living rabbit cornea showing surface epithelial cells (Epi), underlying stroma and posterior corneal endothelium (Endo). (D) 2-D in vivo confocal image taken from the 3-D data set through the corneal stroma showing light scattering from the keratocyte nuclei. Bar = 100 μm.
In vivo confocal studies of the normal cornea and the theory of transparency
While transmission of light through the cornea is greater than 90% within the visible spectrum [38], a small amount of light scattering can be detected upon close examination using a slit-lamp biomicroscope [30]. Development of the reflectance confocal microscope, which provides high magnification, optical sectioning of tissue, has provided greater insight into the origins of the light scattering in living, intact corneas as seen by the slit-lamp (for review of confocal microscopy see [39]). Using the in vivo confocal microscope, the light scattering volume of the cornea can be 3-dimensionally reconstructed to show that the major sources of light scattering are the anterior superficial corneal epithelial cell layer and the posterior corneal endothelium (Fig. 1C). Scattering of light from these cellular structures is to be expected since they are at the interface between aqueous solutions which have a much lower refractive index (RI = 1.00) compared to cells (RI = 1.3). Within the cornea, little light scattering is detected from the stratified corneal epithelial layer. More interestingly however, is the finding that light scattering within the stroma is limited to the keratocyte nuclei; not from the broad cell bodies (Fig. 1D, arrow).
These light scattering observations provide important insights into the possible structural composition of both the corneal epithelial cells and keratocytes based on current theories of corneal transparency. These theories have primarily focused on studies describing light scattering from the extracellular matrix, indeed original theories ignored any cellular contribution to corneal transparency [40]. Since collagen and the surrounding ground substance that contains water, proteoglycans and glycosaminoglycans have different refractive indices of 1.47 and 1.35 respectively, the corneal stroma should be quite opaque, similar to the dermis and sclera of the eye. However, as noticed on transmission electron microscopy, the cornea as mentioned above has a uniform arrangement of collagen fibrils of very consistent size and spacing, unlike that of collagen in other tissues. This lattice arrangement of corneal collagen formed the original basis for the first explanation of corneal transparency as proposed by Maurice [40]. This ‘lattice theory’ proposed that the small size (32 nm) and uniform spacing (64 nm) led to lateral scattering of light by single collagen fibrils that were destructively interfered with by scattering from adjacent fibrils providing only forward transmission of light. Later, Goldman and Benedek modified the ‘lattice theory’ to include the fluctuation of the refractive index within the media as a function of distance [26, 41]. In general, this second theory proposed that light scattering in a media is dependent on the wavelength of light and the distance separating the fluctuations in refractive index and/or the magnitude of the refractive index fluctuations. In other words, when the distance between scattering structures such as a collagen fibrils is small or less the 1/2 the wavelength of visible light (400 - 700 nm) the media is transparent. However, when the refractive index fluctuates over distances greater that 1/2 the wavelength, or 200 nm, then significant scattering will be detected.
These theories can also be applied to light scattering from cells, particularly the corneal epithelium and keratocytes. As discussed by Moller-Pedersen [30], cells contain various structures including the plasma membrane, nuclei, mitochondrion, glycogen granules, large protein aggregates, and other inclusions bodies. While the refractive index of these structures are not known, it is likely that many of these structures are potential scatters, and would contribute significantly to light scattering within the cells. In addition to the intracellular scattering, keratocytes are also embedded in a collagen matrix with high refractive index. Physical separation of the collagen fibrils by the thickness of the keratocyte cell body, which may vary from 200 nm to 600 nm [42], should lead to maximum scattering along the lamellar interface. The lack of scattering detected in the epithelium and keratocytes, except for the nuclei, therefore indicate that the cells either have a uniform refractive index, which is not likely given the size and distance separating the larger subcellular structures, or that fluctuations in refractive index have been minimized. More importantly, in the keratocytes, the refractive index fluctuation between the collagen and the cell must also be minimized since the distances separating collagen on either side of the keratocyte are greater than those necessary to suppress light scattering.
2.2 Expression of corneal crystallins in corneal keratocytes
As mentioned above, the lens has evolved a mechanism to maximize transparency and minimize fluctuations in refractive index (even increase refractive index from 1.386 in the lens cortex to 1.4 in the lens nucleus) through the abundant expression of crystallin proteins that control the short range order within the lens cytoplasm [28, 29]. Since the corneal epithelium and the lens are both derived from ectoderm, it seems reasonable to propose that the evolution of transparency in these two structures may have co-evolved as an integrated ‘refracton’ unit. Indeed, data showing that the corneas from a diverse range of species including squid, fish, frogs, crocodiles, birds and mammals abundantly express a few water-soluble proteins is consistent with this theory [6, 7, 9-11, 13]. However, the vast majority of studies identifying corneal crystallin expression have used total corneal extracts or isolated corneal epithelium. It was therefore not initially clear that the ‘refracton’ unit would extend to the corneal keratocyte, which derives its embryological origins from migrating neural crest.
Abundant expression of a few water-soluble proteins in keratocytes was first identified in the rabbit [8]. In this initial study rabbit keratocytes, freshly isolated from live rabbit corneas were shown to abundantly express two water-soluble proteins, transketolase (TKT) and ALDH1A1/η-crystallin that comprised over 30% of the total water-soluble protein content (Fig. 2). Later studies showed that this pattern of expression in keratocytes was generally similar but not identical to that of the rabbit corneal epithelium [7]. While rabbit keratocytes expressed high levels of TKT and ALDH1A1/η-crystallin, comprising 14% and 12.7% of the total water-soluble protein respectively, rabbit corneal epithelial cells expressed predominantly lactic dehydrogenase/ε-crystallin (14.7%) and to a lesser extent TKT (7.5%), G3PDH/π-crystallin (6.9%), and α-enolase/τ-crystallin (4.5%). While these differences are likely related to the different embryologic origins, the marked similarity in abundant expression of water-soluble proteins in these two very different cells types (epithelial and mesenchymal) strongly suggest that they are under similar molecular control and have a similar functional significance.
Fig. 2.
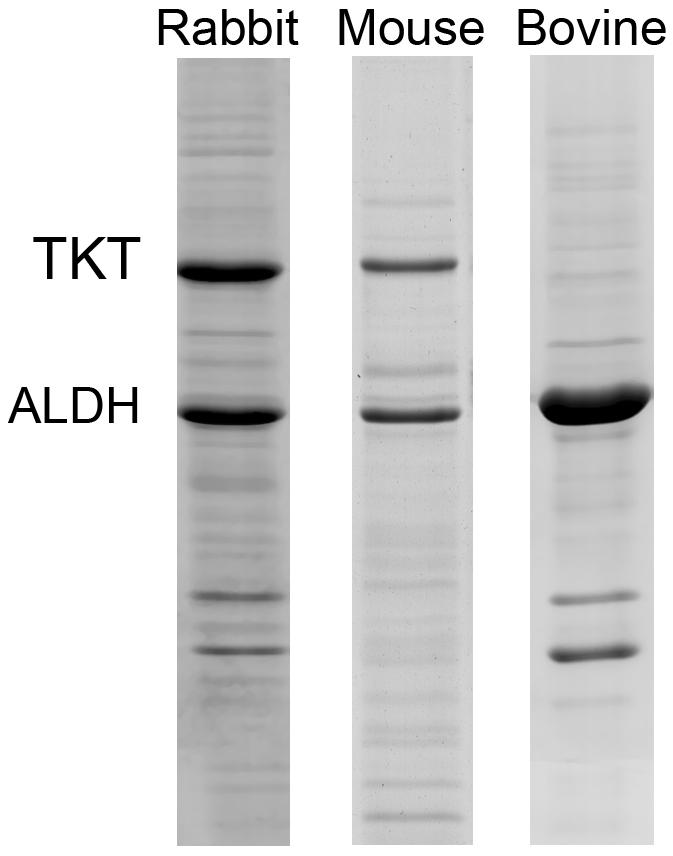
Coomassie-blue stained SDS-PAGE of water-soluble protein extracts keratocytes isolated fresh from rabbit, mouse and bovine. Note that TKT and ALDH is abundantly expressed in all three species, representing over 55% of the total water-soluble protein in bovine keratocytes. Bar = 100 μm.
Keratocytes from other species have also been shown to abundantly express a few water-soluble proteins, including mouse, human, cow, pig and chicken (Fig. 2) [7, 43]. As in the rabbit, some differences in the expression pattern between keratocytes and corneal epithelial cells have been noted; however, on whole the patterns are remarkably similar. Specifically, mammalian keratocytes consistently show abundant expression of ALDH3A1/1A1 as does the corneal epithelium. Interestingly, keratocytes isolated from pig corneas showed the greatest expression of ALDH3A1/1A1 representing over 55% of the total water-soluble protein. Furthermore, as noted in other studies of the corneal epithelium, ALDH3A1/1A1 was not expressed in the chicken keratocyte, confirming taxon specificity in the expression patterns for keratocyte corneal crystallins, similar to that of the epithelial corneal crystallins.
Overall, these findings concerning the expression of corneal crystallins by keratocytes and corneal epithelial cells intuitively lead to several predictions based on the proposed ‘refracton’ theory and the role of corneal crystallins in the development and maintenance of transparency. First, if crystallin protein expression is associated with cellular transparency, then loss or decreased expression should lead to decreased transparency and increased light scattering by corneal cells. Second, if corneal crystallin expression is developmentally regulated as in the lens, then the development of corneal transparency should coincide with increasing expression of corneal crystallins. And finally, environmental modulation of the level of corneal crystallin protein expression in cells should modify the level of light scattering from cells.
3. Corneal haze, cellular light scattering and expression of corneal crystallins
Injury to the cornea, particularly the corneal stroma, frequently leads to corneal keratocyte activation, migration, and differentiation to fibroblasts and myofibroblast-like cells that participate in fibrotic wound healing responses producing corneal haze, scarring and loss of transparency [44]. This wound healing response has been extensively investigated and shown to be dependent on transforming growth factor beta (TGFβ) activation of normally quiescent stromal keratocytes. Addition of TGFβto cultures of corneal keratocytes initiates a SMAD dependent signal transduction cascade leading to expression of the integrin α5β1, fibronectin and collagen type I that downstream interact to induce focal adhesion formation, fibronectin fibril assembly and actin filament reorganization into prominent stress fibers containing the smooth muscle specific alpha isoform of actin (αSMA), a biomarker for myofibroblast differentiation [44]. While other growth factors such as fibroblast growth factor (FGF2) and platelet derived growth factor (PDGF) induce fibroblastic changes in keratocytes that lead to formation of focal adhesion and actin filament assembly, TGFβis the only growth factor thus far identified that induces αSMA expression and myofibroblast differentiation [45]. In the rabbit cornea the appearance of myofibroblasts as detected by immunostaining for αSMA occur as early as 3 days after incisional corneal injury and from 7 to 14 days after surface injury that damages the anterior corneal stroma and epithelial basement membrane. Importantly, the in vivo appearance of corneal myofibroblasts can be blocked by topically treating wounds with neutralizing antibodies to TGFβ, which dramatically reduces corneal scarring and haze [46].
For years, the development of corneal haze has been popularly explained by the abnormal deposition of collagen and proteoglycans by fibroblasts and myofibroblasts after injury. Normally, keratocytes synthesize type I and V collagen along with keratan sulfate proteoglycans (KSPG); KSPG, which is uniquely synthesized by keratocytes, appears to be particularly important in formation of collagen fibrils with uniform size and spacing [47]. On the other hand, keratocytes that have differentiated to fibroblasts and myofibroblasts lose the ability to synthesize KSPG and instead synthesize chondroitin sulfate proteoglycans (CSPG) along with type I and III collagen [48, 49]. This change in the extracellular matrix synthesis has historically been thought to be the underlying cause of corneal haze after injury due to the deposition of abnormal collagen fibril size and spacing.
3.1 The case for cellular light scattering as the basis for corneal haze
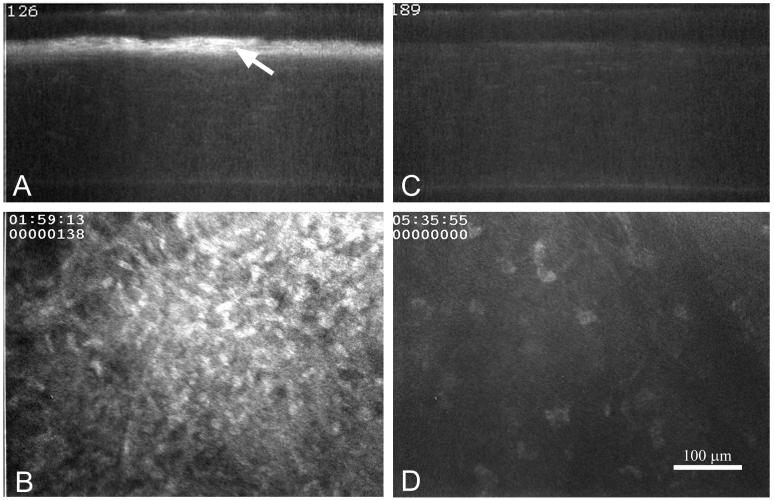
Recently there has been a resurgence of interest in the pathogenesis of corneal haze following the introduction of excimer lasers to photoablate the cornea and correct refractive errors. Since the initial procedures involved anterior surface ablation, many patients developed haze after surgery that was associated with keratocyte activation, migration and differentiation to corneal myofibroblasts. This haze in patients generally peaked within 1-2 months after surgery and then subsided over time [50]. A similar response to surface ablation was also detected in rabbits, albeit a more rapid response [32]. Interestingly, evaluation of haze by in vivo confocal microscopy in both patients and rabbits showed that light scattering originated from ‘cells’ rather than from the ‘extracellular matrix’ [32, 50]. Specifically, during the early wound healing response as cells migrated toward the wound there was an increase in light scattering from spindle- shaped migratory fibroblasts. Light scattering then dramatically increased and peaked as fibroblasts reached the wound surface and differentiated to myofibroblasts (Fig. 3A and B). Under some experimental conditions, light scattering from ‘cells’ stood in stark contrast to the transparency of the adjacent newly synthesized ‘extracellular matrix’ (see [51])
Fig. 3.
In vivo confocal images of light scattering from rabbit corneas 2 months after excimer laser surface ablation treated with vehicle (A and B) or 0.02% mitomycin C (C and D) to block myofibroblast differentiation. (A and C) X-Z slice through a 3-dimensional data set from the cornea. (B and D) X-Y optical plane from the 3-dimensional data set taken just below the corneal epithelium. Note the marked haze (A, arrow) below the epithelium and the cellular light scattering (B) in the vehicle treated eye. Bar = 100 μm.
Cellular light scattering leading to corneal haze can also be observed following more moderate injury where fibroblast migration but not myofibroblast differentiation is induced, such as after scrape or freeze injury that does not produce fibrosis [8, 51]. Interestingly, cellular light scattering and haze disappear as fibroblasts differentiate back to corneal keratocytes after stromal repopulation following these types of injury. Blocking myofibroblast differentiation by treating corneas with neutralizing antibodies to TGFβ [46] or blocking keratocyte activation using mitomycin C (Fig. 3C and D) also markedly reduces corneal haze and cellular light scattering.
Overall, with today’s increased use of in vivo confocal microscopy, light scattering from activated keratocytes is widely recognized as a major cause of some types of corneal haze. In addition to excimer laser surgery, cellular haze has been detected in patients with sterile keratitis and post penetrating keratoplasty [30], intrastromal lens implantation (Fig. 4) [52], and resolved herpetic keratitis [53] to name a few. This association of haze and cellular light scattering has prompted one investigator to suggest a new category be recognized for corneal haze that is associated solely with cellular light scattering along with the more traditionally accepted causes including edema, scarring and corneal dystrophy [30].
Fig. 4.
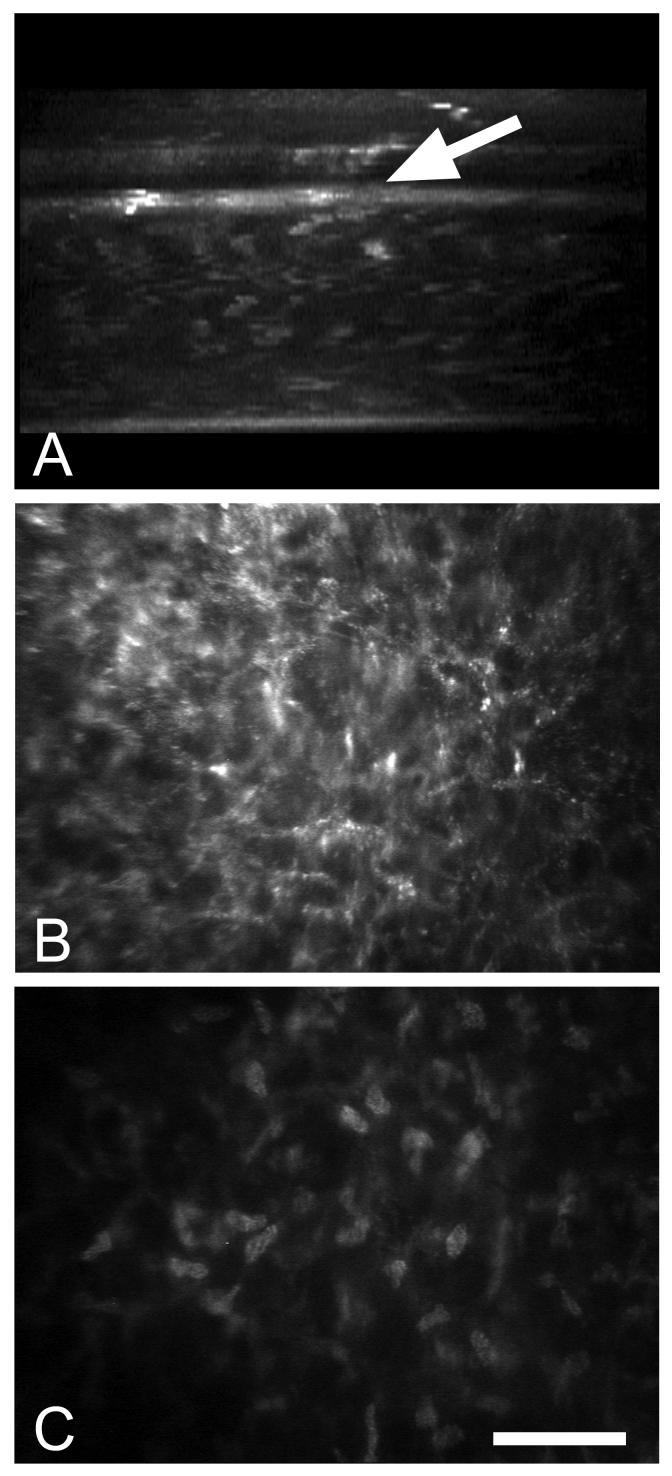
In vivo confocal microscopy of a patient following intrastromal implantation of a corneal lens. (A) X-Z projection through the 3-dimensional data set showing the anterior placement of the intracorneal lens (arrow) and the associated increased light scattering at the posterior margin of the lens. (B) X-Y plane taken from the 3-dimensional data set just posterior to the intracorneal lens showing marked light scattering from corneal keratocytes. (C) X-Y plane taken from the 3-dimensional data set further posterior within the normal stroma showing light scattering limited to keratocyte nuclei. Bar = 100 μm.
3.2 Corneal crystallin expression in hazy corneas
The first study to assess corneal crystallin protein expression in activated keratocytes and fibroblasts evaluated a freeze injury model in rabbits [8]. Transcorneal freeze injury is a simple, non-invasive, non-chemical injury that devitalizes the central cornea and is used to study non-fibrotic, non-inflammatory repair mechanisms in the corneal stroma and endothelium [54, 55]. In this type injury, surviving keratocytes adjacent to the frozen region become activated and migrate as fibroblasts into the devitalized corneal stroma. Importantly, migrating fibroblasts after this type of injury show marked light scattering by in vivo confocal microscopy. When cells from this area of injury were isolated and evaluated by SDS PAGE, migrating fibroblasts had a marked reduction in the level of expression of TKT and almost a complete loss in expression of ALDH1A1. This was in contrast to the high level of expression of TKT and ALDH1A1 in keratocytes isolated from the surrounding transparent region of the cornea in the same eyes [8].
Since this first report, keratocyte crystallin protein expression has been studied by many investigators using cell culture as a model to mimic keratocyte activation and fibroblast differentiation [56]. These studies have shown that keratocytes placed in serum culture or exposed to TGFβ to induce myofibroblast differentiation rapidly lose abundant expression of both TKT and ALDH1A1 protein and mRNA while keratocytes maintained in serum free conditions continue to abundantly express corneal crystallins as well as other keratocyte specific markers including keratan sulfate [7, 43, 57]. This loss is apparently due in part to ubiquination and proteosomal degradation of crystallin proteins upon keratocyte activation by serum containing growth factors [58]. There is also an apparent dilution of crystallins with cell division that occurs following stimulation with some growth factors, particularly FGF2 and PDGF [59]. Karring et al [60] has also performed a proteomic analysis of cultured human corneal fibroblasts to identify the most abundantly expressed proteins. In this study, no single protein band on 1 dimensional gel analysis was found to be abundantly expressed in human cultured corneal fibroblasts (abundant expression defined as >5% of the total water-soluble protein content). Furthermore, proteins with the highest expression were vimentin and actin, not TKT and ALDH3A1/1A1. Proteomic analysis also showed that fibroblasts expressed various proteins/enzymes that were protective against oxidative stress and protein misfolding including mitochondria and cytoplasmic glutathione S-transferases, thioredoxin, peroxiredoxin, superoxide dismutase, ubiquitin and prefoldin. Down regulation of crystallin RNA expression has also been identified using microarray analysis of cultured mouse keratocytes [61].
More recently immunocytochemistry has been used to evaluate expression of ALDH3A1 in human corneas that have been taken from patients having corneal haze following failed corneal transplantation or corneal scarring [57]. Using Thy-1 as a marker for the human corneal fibroblast phenotype [62], Pei et al showed that stromal cells in failed grafts that were Thy-1 positive were ALDH3A1 negative. Additionally in scarred corneas stained with antibodies against α-smooth muscle actin to identify corneal myofibroblasts, positive myofibroblasts in the region of scarring failed to stain for ALDH3A1. Overall, these findings clearly establish that keratocytes as they differentiate to fibroblasts and myofibroblasts in response to injury or serum culture lose the ability to highly express corneal crystallin proteins. Furthermore, in vivo differentiation of keratocytes to these alternate phenotypes is associated with a change in the light scattering properties of the cells from transparent to opaque. Taken together these data are consistent with the ‘refracton’ hypothesis and the prediction that decreased expression of corneal crystallins should be associated with increased light scattering from keratocytes.
4. Developmental regulation of transparency and expression of corneal crystallins
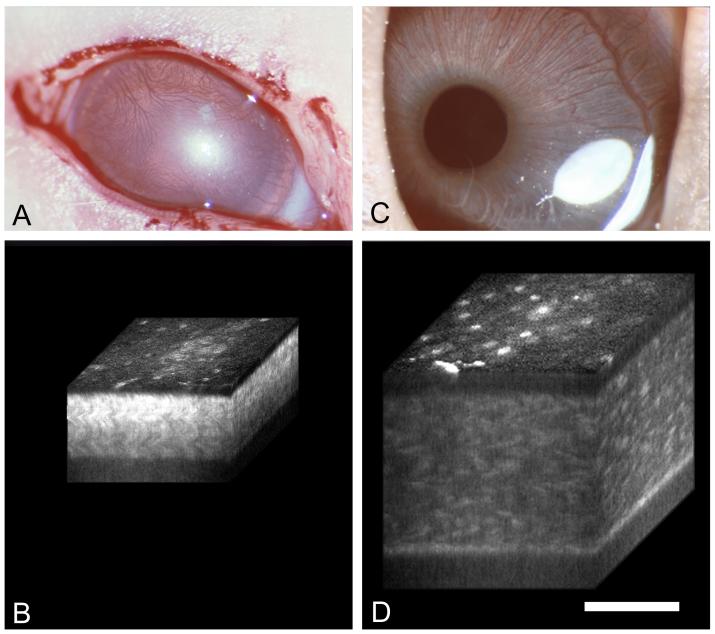
Previous studies have shown that expression of corneal crystallins in the mouse appear to increase around eyelid opening or postnatal day 12 [63, 64]. Since the cornea of most mammals is translucent at birth and only begins to develop transparency after eyelid opening [65, 66], a recent study investigated the relationship between the postnatal development of transparency and expression of corneal crystallin proteins in the rabbit [67]. In this study light scattering from the cornea was quantitatively measured using in vivo confocal microscopy and the changes in transparency correlated with the stromal and epithelial thickness, keratocyte cell density, cell cycle entry/exit and expression of corneal crystallin protein. The results showed that translucent corneas from birth to eyelid opening had high levels of light scattering from stromal cells in the cornea (Fig. 5A and B). After eyelid opening light scattering decreased from stromal cells and became increasing limited to the keratocyte nuclei (Fig. 5C and D). Furthermore, the decrease in light scattering showed a strong correlation with decreased cell density, however, the light scattering on a per cell basis remained constant until after eyelid opening after which the level dropped markedly. This drop in cellular light scattering was associate with two important events, first was the exit from the cell cycle by stromal cells based on Ki67 staining that has been used as a marker for cell cycle entry/exit. Second was the increasing expression of ALDH1A1 which occurred after eyelid opening at the time when cells showed lower light scattering.
Fig. 5.
Clinical photographs (A and C) and 3-D reconstructions (B and D) of postnatal rabbit eyes at 4 and 20 days after birth. Note prominent light scattering through out the cornea from the eyes at 4 days compared to the keratocyte nuclear scattering seen at 20 days. Bar = 100 μm.
Overall, the findings of this study are consistent with previous work in the mouse showing up-regulation in TKT and ALDH3A1 expression after eyelid opening [63, 64, 68]. While these investigators didn’t measure light scattering, such measurements have been made in postnatal mice and shown to follow a similar course as that identified in the rabbit [69]. Interestingly, the level of TKT expression in light/dark raised mice is 2.1 fold higher than that of dark raised pups 2 weeks after eyelid opening suggesting that expression of TKT can in part be manipulated environmentally [64]. Furthermore, early eyelid opening induced by injection of EGF results in earlier expression of TKT, further suggesting that light may in part modify expression of corneal crystallins. Similarly, cultured rat corneal epithelial cells maintain elevated expression of ALDH3A1 longer when cultured under light/dark conditions compared to constant dark [70] indicating that expression of both TKT and ALDH3A1 maybe sensitive to light conditions. However, it should be noted that light deprivation in adult animals does not apparently affect the levels of corneal crystallin protein expression, at least for periods up to 2-8 weeks, and that light deprivation only partially blocks postnatal up-regulation of TKT.
This environmental regulation of corneal crystallins is in apparent contrast to that of lens crystallins which show early embryologic up-regulation. However, the finding that expression of corneal crystallins may be dependent on cell cycle exit, interestingly parallels the finding in the lens that expression of ubiquitous crystallins become up-regulated after cell cycle withdrawal of lens epithelial cells and the onset of cell elongation to form lens fibers [71, 72]. More interesting is the finding that transgenic expression of immortalizing oncogenes such as the large T antigen effects lens differentiation leading to reduced lens crystallin expression [73]. This effect on lens fiber development is similar to the effect of cell activation on keratocyte corneal crystallin expression either by corneal injury in vivo or serum culture. Finally a recent study by Pappa et al [21] showed that induced expression and nuclear localization of ALHD3A1 in a transfected human corneal epithelial cell line inhibited cell proliferation and slowed cycle progression. Similar affects have been noted in corneal keratocyte cell lines transfected with expression vectors for ALDH3A1 and ALDH1A1 (data not shown). While the loss of differentiation related protein expression is a common finding as cells enter the cell cycle [70, 74], growth down-regulation following induced expression of crystallin proteins has not been previously reported. Pappa et al [21] therefore speculate that corneal crystallins may have important affects on growth control necessary to maintain transparency.
Overall, the data supports the second prediction of the ‘refracton’ hypothesis that development of corneal transparency should by related to increased expression of corneal crystallin proteins during development. Furthermore, the relationship between the development of cellular transparency and cell cycle exit clearly suggest that keratocyte differentiation to an adult transparent phenotype is heralded by the onset of increased corneal crystallin protein expression, remarkably similar to that of the crystallin lens; albeit during postnatal rather than embryologic development. This suggests similar regulatory mechanisms and signals used in lens development may also be used by the cornea. Importantly, the postnatal differentiation is most likely the result of proliferative demands on the corneal keratocytes to increase corneal thickness and control corneal shape for the proper refraction of light at the time of eyelid opening. The role of light in modifying this last stage of differentiation remains to be clarified, and whether eyelid opening results in feedback control from the retina to the cornea as occurs in the sclera during the development of form-deprivation myopia [75] is unknown and needs further study.
In counterpoint to these findings is the report that ALDH3a1 null mice appear to have structurally normal corneas [76]. However, more recently a double knock out ALDH3a1/1a1 mouse has been generated that shows early development of cataracts and increased susceptibility to UV light damage in both the lens and cornea suggesting that corneal crystallins may help in maintaining transparency through light filtering (UV absorption) and enzymatic detoxification. While corneal transparency has not been rigorously evaluated in these mice, it is difficult to completely abolish crystallin protein expression from corneal cells since knockout of TKT is lethal [77], and other water-soluble proteins may compensate for the absence of ALDH3a1/1a1. Regarding the latter possibility, analysis of mouse water-soluble proteins show other enzymes to be abundantly expressed in addition to TKT and ALDH3a1 including glutathione-S-transferase/Ω-crystallin and pyruvate kinase (Table 1). A final caveat in studying corneal transparency using the mouse as a model is the difficulty in assessing light scattering with such a thin tissue. Since the effect of scattering on transparency is not linear [78, 79], the demand for suppressing cellular light scattering in tissue ranging in thickness from 70 to 90 μm is probably orders of magnitude less than that required for human corneas (∼500 μm) or pigs (∼1 mm). This may explain why keratocytes in the pig cornea show such high levels of ALDH3a1/1a1 expression compared to human or mouse.
5. In vitro light scattering and crystallin protein expression
As mentioned above, culture of corneal keratocytes under serum-free conditions maintains keratocyte differentiation and elevated expression levels for differentiation markers ALDH1A1 and KSPG [43, 80]. Growth under such conditions has then been used to study phenotypic modulation of keratocytes to fibroblasts and myofibroblasts under the influence of different growth factors including FGF2, PDGF, IGFII and TGFβ1 [45]. Using this approach, a recent study evaluated quantitatively the in vitro light scattering properties of keratocytes, fibroblasts and myofibroblasts using reflectance confocal microscopy by plating cells on collagen coated polyacrylamide gels to reduce light scattering from the cell substrate [7]. For keratocytes grown in the absence of growth factors, cells maintained their normal dendritic appearance and showed very low levels of light scattering while maintaining high levels of expression of TKT and ALDH1A1 (Fig. 6A). On the other hand, keratocytes that were phenotypically modulated to differentiate into myofibroblasts by the addition of TGFβ1 (1 ng/ml) showed increased cell spreading and significantly greater light scattering (> 50%, p < 0.05) while expressing 45% less corneal crystallin, TKT and ALDH1A1. Phenotypic modulation of keratocytes to fibroblasts by FGF2 and PDGF also caused reduced expression of crystallin proteins and increased light scattering, but the levels were not as great as with TGFβ1 and were not significantly different.
Fig. 6.
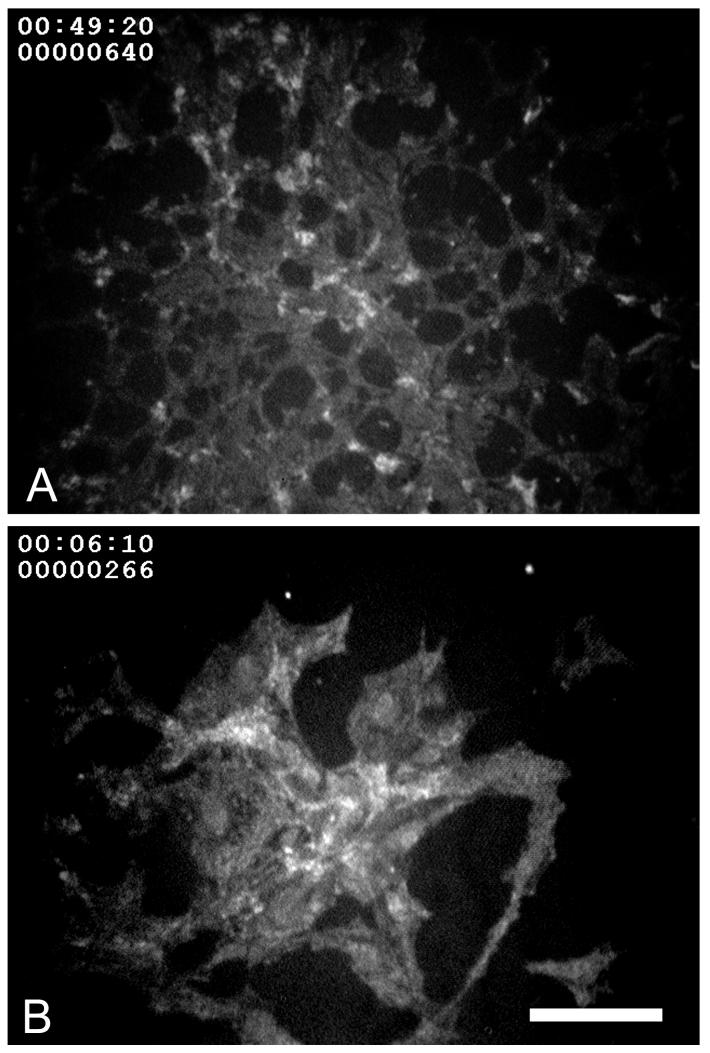
Reflectance confocal micrographic of corneal keratocytes treated with control serum-free media (A) and TGFβ1 (1 ng/ml) to induce myofibroblast differentiation (B). Note that myofibroblasts appear much larger and spread out then keratocytes and appear to scatter more light. Bar = 100 μm.
Overall, these data suggest that there is a relationship between the level of expression of corneal crystallins and light scattering as predicted by the ‘refracton’ hypothesis. While it is tempting to propose that the differences are directly related to corneal crystallin protein expression it should be remembered that levels of TKT and ALDH1A1 are only one of the many characteristics that distinguish keratocytes from fibroblasts and myofibroblasts. Most notably, there are also differences in proliferation potential, [43, 80], and biosynthetic capability [81, 82]. Importantly, there are also major differences in actin filament assembly [45], particularly for stress fibers, which are present in fibroblasts and even more prominent in myofibroblasts but absent in corneal keratocytes. It is therefore not possible at this time to rule out stress fibers as playing a role in the increased light scattering from myofibroblasts compared to keratocytes.
6. Future Directions
Thus far, studies of corneal crystallin expression and cellular light scattering from normal transparent corneas or hazy and opaque corneas after injury or during early postnatal development indicate that keratocytes with elevated expression appear transparent, while developing keratocytes or fibroblasts and myofibroblasts with reduced corneal crystallin expression appear to markedly scatter light and are opaque. These findings strongly support a structural role for corneal crystallins that is similar to that of crystallins in the lens which are thought to provide short range order within the cytoplasm to facilitate transparency. These findings are also consistent with the ‘refracton’ theory for the evolution of transparency in the lens and cornea, and many of the developmental events such as cell cycle entry/exit regulating corneal crystallin expression appear to have parallels in the crystallin lens that may underlie the co-evolution of transparency in these two structures. However, the exact structural role of crystallins and their requirement for transparency remains to be fully determined.
Reports indicating that double knockout of ALDH3a1 and ALDH1a1 leads to early cataract and increased sensitivity to UV damage in mice [83] suggest that corneal crystallins also provide important enzymatic functions. However, it seems paradoxical that the function of corneal crystallins’ is solely enzymatic and involved in protecting against oxidative and UV-stress, since injury leads to loss of these protective crystallin enzymes while at the same time there is up-regulation of other protective anti- oxidative stress and protein misfolding enzyme/proteins as identified by proteomic analysis [60] that do not maintain transparency.
Clearly, there is much to learn about both the function of these proteins in the cornea as well as the regulatory signals that control their expression. Studies are needed to identify how light interacts with corneal cells to modify the expression of corneal crystallin during development. Does light deprivation lead to continued keratocyte cell proliferation, delaying cell cycle exit and final keratocyte differentiation? Does the corneal stromal thickness change in response to dark conditions and are these responses modified by some retinal feedback mechanism that influences transparency and the shape of the cornea? How does cell light scattering change as a function of corneal crystallin concentration? Why is it that up-regulation of crystallin expression alters the cell cycle and slows growth? These are only some of the questions that need to be addressed if we are going to understand more clearly the role of corneal crystallin in the development and maintenance of corneal transparency. Fortunately, with development of knockout mice and in vitro light scattering models available, many of these questions can begin to be answered.
Acknowledgement
The author would like to thank past collaborators that have helped in the pursuit of understanding the keratocyte role in corneal transparency, in particular Dwight Cavanagh, Matt Petroll and Torben Moller-Pedersen; also Joram Piatigorsky for providing a key insight into corneal crystallins and Vasilis Vasiliou for his energetic and enthusiastic support.
Supported in part by NIH grant EY13215, EY 17963 and EY16663, Unrestricted Grant from Research to Prevent Blindness, Inc. and The Skirball Program in Molecular Ophthalmology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Holt WS, Kinoshita JH. The soluble proteins of the bovine cornea. Invest Ophthalmol Vis Sci. 1973;12:114. [PubMed] [Google Scholar]
- [2].Alexander RJ, Silverman B, Henley WL. Isolation and characterization of BCP 54, the major water soluble protein of bovine cornea. Exp Eye Res. 1981;32:205. doi: 10.1016/0014-4835(81)90009-9. [DOI] [PubMed] [Google Scholar]
- [3].Silverman B, Alexander RJ, Henley WL. Tissue and species specificity of BCP 54, the major soluble protein of bovine cornea. Exp Eye Res. 1981;33:19. doi: 10.1016/s0014-4835(81)80078-4. [DOI] [PubMed] [Google Scholar]
- [4].Abedinia M, Pain T, Algar EM, Holmes RS. Bovine corneal aldehyde dehydrogenase: the major soluble corneal protein with a possible dual protective role for the eye. Exp Eye Res. 1990;51:419. doi: 10.1016/0014-4835(90)90154-m. [DOI] [PubMed] [Google Scholar]
- [5].Cooper DL, Baptist EW, Enghild JJ, Isola NR, Klintworth GK. Bovine corneal protein 54 K (BCP540 is a homologue of the tumor-associated (class 3) rat aldehyde dehydrogenase (TAT_ALD) Gene. 1991;98:201. doi: 10.1016/0378-1119(91)90174-a. [DOI] [PubMed] [Google Scholar]
- [6].Cuthbertson RA, Tomarev SI, Piatigorsky J. Taxon-specific recruitment of enzymes as major soluble proteins in the corneal epithelium of three mammals, chicken, and squid. Proc Natl Acad Sci U S A. 1992;89:4004. doi: 10.1073/pnas.89.9.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jester JV, Budge A, Fisher S, Huang J. Corneal keratocytes: phenotypic and species differences in abundant protein expression and in vitro light-scattering. Invest Ophthalmol Vis Sci. 2005;46:2369. doi: 10.1167/iovs.04-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jester JV, et al. The cellular basis of corneal transparency: evidence for ‘corneal crystallins’. J Cell Sci. 1999;112(Pt 5):613. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- [9].Kathiresan T, et al. Triose phosphate isomerase, a novel enzyme-crystallin, and tau-crystallin in crocodile cornea. High accumulation of both proteins during late embryonic development. Febs J. 2006;273:3370. doi: 10.1111/j.1742-4658.2006.05344.x. [DOI] [PubMed] [Google Scholar]
- [10].Krishnan K, et al. Ubiquitous Lens {alpha}-, beta-, and {gamma}-Crystallins Accumulate in Anuran Cornea as Corneal Crystallins. J Biol Chem. 2007;282:18953. doi: 10.1074/jbc.M609275200. [DOI] [PubMed] [Google Scholar]
- [11].Sax CM, et al. Transketolase is a major protein in the mouse cornea. Journal of Biological Chemistry. 1996;271:33568. doi: 10.1074/jbc.271.52.33568. [DOI] [PubMed] [Google Scholar]
- [12].Sun L, Sun TT, Lavker RM. Identification of a cytosolic NADP+-dependent isocitrate dehydrogenase that is preferentially expressed in bovine corneal epithelium. A corneal epithelial crystallin. J Biol Chem. 1999;274:17334. doi: 10.1074/jbc.274.24.17334. [DOI] [PubMed] [Google Scholar]
- [13].Swamynathan SK, Crawford MA, Robison WG, Jr., Kanungo J, Piatigorsky J. Adaptive differences in the structure and macromolecular compositions of the air and water corneas of the “four-eyed” fish (Anableps anableps) Faseb J. 2003;17:1996. doi: 10.1096/fj.03-0122com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xu YS, Kantorow M, Davis J, Piatigorsky J. Evidence for gelsolin as a corneal crystallin in zebrafish. J Biol Chem. 2000;275:24645. doi: 10.1074/jbc.M001159200. [DOI] [PubMed] [Google Scholar]
- [15].Piatigorsky J. Multifunctional lens crystallins and corneal enzymes. More than meets the eye. Ann N Y Acad Sci. 1998;842:7. doi: 10.1111/j.1749-6632.1998.tb09626.x. [DOI] [PubMed] [Google Scholar]
- [16].Piatigorsky J. Gene sharing in lens and cornea: facts and implications. Prog Retin Eye Res. 1998;17:145. doi: 10.1016/s1350-9462(97)00004-9. [DOI] [PubMed] [Google Scholar]
- [17].Piatigorsky J. Enigma of the abundant water-soluble cytoplasmic proteins of the cornea: the “refracton” hypothesis. Cornea. 2001;20:853. doi: 10.1097/00003226-200111000-00015. [DOI] [PubMed] [Google Scholar]
- [18].Piatigorsky J. The diversity of protein functions. Harvard University Press; Cambridge, Massachusetts: 2007. Gene Sharing and Evolution. [Google Scholar]
- [19].Lassen N, et al. Antioxidant function of corneal ALDH3A1 in cultured stromal fibroblasts. Free Radic Biol Med. 2006;41:1459. doi: 10.1016/j.freeradbiomed.2006.08.009. [DOI] [PubMed] [Google Scholar]
- [20].Manzer R, et al. Ultraviolet radiation decreases expression and induces aggregation of corneal ALDH3A1. Chem Biol Interact. 2003;143-144:45. doi: 10.1016/s0009-2797(02)00171-0. [DOI] [PubMed] [Google Scholar]
- [21].Pappa A, et al. Human aldehyde dehydrogenase 3A1 inhibits proliferation and promotes survival of human corneal epithelial cells. J Biol Chem. 2005;280:27998. doi: 10.1074/jbc.M503698200. [DOI] [PubMed] [Google Scholar]
- [22].Estey T, et al. Mechanisms involved in the protection of UV-induced protein inactivation by the corneal crystallin ALDH3A1. J Biol Chem. 2007;282:4382. doi: 10.1074/jbc.M607546200. [DOI] [PubMed] [Google Scholar]
- [23].Estey T, Piatigorsky J, Lassen N, Vasiliou V. ALDH3A1: a corneal crystallin with diverse functions. Exp Eye Res. 2007;84:3. doi: 10.1016/j.exer.2006.04.010. [DOI] [PubMed] [Google Scholar]
- [24].Manzer R, et al. Molecular cloning and baculovirus expression of the rabbit corneal aldehyde dehydrogenase (ALDH1A1) cDNA. DNA Cell Biol. 2003;22:329. doi: 10.1089/104454903322216671. [DOI] [PubMed] [Google Scholar]
- [25].Mitchell J, Cenedella RJ. Quantitation of ultraviolet light-absorbing fractions of the cornea. Cornea. 1995;14:266. doi: 10.1097/00003226-199505000-00007. [DOI] [PubMed] [Google Scholar]
- [26].Benedek G. Theory of the transparency of the eye. Appl Optics. 1971;10:459. doi: 10.1364/AO.10.000459. [DOI] [PubMed] [Google Scholar]
- [27].Bettelheim FA. Physical basis of lens transparency. Marcel Dekker, Inc.; New York and Basel: 1985. [Google Scholar]
- [28].Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- [29].Tardieu A. alpha-Crystallin quaternary structure and interactive properties control eye lens transparency. Int J Biol Macromol. 1998;22:211. doi: 10.1016/s0141-8130(98)00018-x. [DOI] [PubMed] [Google Scholar]
- [30].Moller-Pedersen T. Keratocyte reflectivity and corneal haze. Exp Eye Res. 2004;78:553. doi: 10.1016/s0014-4835(03)00208-2. [DOI] [PubMed] [Google Scholar]
- [31].Cavanagh HD, et al. Effects of daily and overnight wear of a novel hyper oxygen-transmissible soft contact lens on bacterial binding and corneal epithelium: a 13-month clinical trial. Ophthalmology. 2002;109:1957. doi: 10.1016/s0161-6420(02)01278-2. [DOI] [PubMed] [Google Scholar]
- [32].Moller-Pedersen T, Li HF, Petroll WM, Cavanagh HD, Jester JV. Confocal microscopic characterization of wound repair after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 1998;39:487. [PubMed] [Google Scholar]
- [33].Robertson DM, et al. Bcl-2 and Bax regulation of corneal homeostasis in genetically altered mice. Eye Contact Lens. 2006;32:3. doi: 10.1097/01.icl.0000156216.37737.b3. [DOI] [PubMed] [Google Scholar]
- [34].Moller-Pedersen T, et al. Quantification of stromal thinning, epithelial thickness, and corneal haze after photorefractive keratectomy using in vivo confocal microscopy. Ophthalmology. 1997;104:360. doi: 10.1016/s0161-6420(97)30307-8. [DOI] [PubMed] [Google Scholar]
- [35].Bartholomew LR, Pang DX, Sam DA, Cavender JC. Ultrasound biomicroscopy of globes from young adult pigs. Am J Vet Res. 1997;58:942. [PubMed] [Google Scholar]
- [36].Hahnel C, Somodi S, Weiss DG, Guthoff RF. The keratocyte network of human cornea: a three-dimensional study using confocal laser scanning fluorescence microscopy. Cornea. 2000;19:185. doi: 10.1097/00003226-200003000-00012. [DOI] [PubMed] [Google Scholar]
- [37].McLaren JW, Nau CB, Kitzmann AS, Bourne WM. Keratocyte density: comparison of two confocal microscopes. Eye Contact Lens. 2005;31:28. doi: 10.1097/01.icl.0000151948.92593.c3. [DOI] [PubMed] [Google Scholar]
- [38].Boettner EA, Wolter JR. Transmission of the Ocular Media. Invest Ophthalmol Vis Sci. 1962;1:776. [Google Scholar]
- [39].Cavanagh HD, Petroll WM, Jester JV. Cornea. 2nd Edition Elsevier-Mosby; Philadephia: 2005. Confocal Microscopy; p. 283. [Google Scholar]
- [40].Maurice DM. The structure and transparency of the cornea. J Physiol. 1957;136:263. doi: 10.1113/jphysiol.1957.sp005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Goldman JN, Benedek GB. The relationship between morphology and transparency in the nonswelling corneal stroma of the shark. Invest Ophthalmol Vis Sci. 1967;6:574. [PubMed] [Google Scholar]
- [42].Muller LJ, Pels L, Vrensen GFJM. Novel aspects of the ultrasturctural organization of human corneal keratocytes. Invest Ophthalmol Vis Sci. 1995;36:2557. [PubMed] [Google Scholar]
- [43].Beales MP, Funderburgh JL, Jester JV, Hassell JR. Proteoglycan synthesis by bovine keratocytes and corneal fibroblasts: maintenance of the keratocyte phenotype in culture. Invest Ophthalmol Vis Sci. 1999;40:1658. [PubMed] [Google Scholar]
- [44].Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999;18:311. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- [45].Jester JV, Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- [46].Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Neutralizing antibody to TGFbeta modulates stromal fibrosis but not regression of photoablative effect following PRK. Curr Eye Res. 1998;17:736. [PubMed] [Google Scholar]
- [47].Funderburgh JL. Keratan sulfate: structure, biosynthesis, and function. Glycobiology. 2000;10:951. doi: 10.1093/glycob/10.10.951. [DOI] [PubMed] [Google Scholar]
- [48].Cintron C, Hong BS, Covington HI, Macarak EJ. Heterogeneity of collagens in rabbit cornea: type III collagen. Invest Ophthalmol Vis Sci. 1988;29:767. [PubMed] [Google Scholar]
- [49].Hassell JR, Cintron C, Kublin C, Newsome DA. Proteoglycan changes during restoration of transparency in corneal scars. Arch Biochem Biophys. 1983;222:362. doi: 10.1016/0003-9861(83)90532-5. [DOI] [PubMed] [Google Scholar]
- [50].Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: a 1-year confocal microscopic study. Ophthalmology. 2000;107:1235. doi: 10.1016/s0161-6420(00)00142-1. [DOI] [PubMed] [Google Scholar]
- [51].Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Corneal haze development after PRK is regulated by volume of stromal tissue removal. Cornea. 1998;17:627. doi: 10.1097/00003226-199811000-00011. [DOI] [PubMed] [Google Scholar]
- [52].Petroll WM, et al. Confocal assessment of the corneal response to intracorneal lens insertion and laser in situ keratomileusis with flap creation using IntraLase. J Cataract Refract Surg. 2006;32:1119. doi: 10.1016/j.jcrs.2006.01.093. [DOI] [PubMed] [Google Scholar]
- [53].Rosenberg ME, Tervo TM, Muller LJ, Moilanen JA, Vesaluoma MH. In vivo confocal microscopy after herpes keratitis. Cornea. 2002;21:265. doi: 10.1097/00003226-200204000-00006. [DOI] [PubMed] [Google Scholar]
- [54].Ichijima H, et al. Actin filament organization during endothelial wound healing in the rabbit cornea: comparison between transcorneal freeze and mechanical scrape injuries. Invest Ophthalmol Vis Sci. 1993;34:2803. [PubMed] [Google Scholar]
- [55].Ichijima H, et al. In vivo confocal microscopic studies of endothelial wound healing in rabbit cornea. Cornea. 1993;12:369. doi: 10.1097/00003226-199309000-00001. [DOI] [PubMed] [Google Scholar]
- [56].Jester JV, et al. Corneal keratocytes: in situ and in vitro organization of cytoskeletal contractile proteins. Invest Ophthalmol Vis Sci. 1994;35:730. [PubMed] [Google Scholar]
- [57].Pei Y, Reins RY, McDermott AM. Aldehyde dehydrogenase (ALDH) 3A1 expression by the human keratocyte and its repair phenotypes. Exp Eye Res. 2006;83:1063. doi: 10.1016/j.exer.2006.05.011. [DOI] [PubMed] [Google Scholar]
- [58].Stramer BM, Cook JR, Fini ME, Taylor A, Obin M. Induction of the ubiquitin-proteasome pathway during the keratocyte transition to the repair fibroblast phenotype. Invest Ophthalmol Vis Sci. 2001;42:1698. [PubMed] [Google Scholar]
- [59].Stramer BM, Fini ME. Uncoupling keratocyte loss of corneal crystallin from markers of fibrotic repair. Invest Ophthalmol Vis Sci. 2004;45:4010. doi: 10.1167/iovs.03-1057. [DOI] [PubMed] [Google Scholar]
- [60].Karring H, Thogersen IB, Klintworth GK, Enghild JJ, Moller-Pedersen T. Proteomic analysis of the soluble fraction from human corneal fibroblasts with reference to ocular transparency. Mol Cell Proteomics. 2004;3:660. doi: 10.1074/mcp.M400016-MCP200. [DOI] [PubMed] [Google Scholar]
- [61].Chakravarti S, Wu F, Vij N, Roberts L, Joyce S. Microarray studies reveal macrophage-like function of stromal keratocytes in the cornea. Invest Ophthalmol Vis Sci. 2004;45:3475. doi: 10.1167/iovs.04-0343. [DOI] [PubMed] [Google Scholar]
- [62].Pei Y, Sherry DM, McDermott AM. Thy-1 distinguishes human corneal fibroblasts and myofibroblasts from keratocytes. Exp Eye Res. 2004;79:705. doi: 10.1016/j.exer.2004.08.002. [DOI] [PubMed] [Google Scholar]
- [63].Pappas P, Stephanou P, Vasiliou V, Karamanakos P, Marselos M. Ontogenesis and expression of ALDH activity in the skin and the eye of the rat. Adv Exp Med Biol. 1997;414:73. doi: 10.1007/978-1-4615-5871-2_10. [DOI] [PubMed] [Google Scholar]
- [64].Sax CM, et al. Transketolase gene expression in the cornea is influenced by environmental factors and developmentally controlled events. Cornea. 2000;19:833. doi: 10.1097/00003226-200011000-00014. [DOI] [PubMed] [Google Scholar]
- [65].Smelser K, Ozanics V. Studies on the differentiation of the cornea and sclera of the rabbit. Anatomical Records. 1956;124:362. [Google Scholar]
- [66].Zinn KM, Mockel-Pohl S. Fine structure of the developing cornea. Int Ophthalmol Clin. 1975;15:19. doi: 10.1097/00004397-197501510-00004. [DOI] [PubMed] [Google Scholar]
- [67].Jester JV, et al. Development of postnatal corneal transparency is associated with keratocyte cell cycle exit and marked expression of ALDH1A1. Invest Ophthalmol Vis Sci. doi: 10.1167/iovs.07-0431. in Press. [DOI] [PubMed] [Google Scholar]
- [68].Downes J, Holmes R. Development of aldehyde dehydrogenase and alcohol dehydrogenase in mouse eye: evidence for light-induced changes. Biol Neonate. 1992;61:118. doi: 10.1159/000243539. [DOI] [PubMed] [Google Scholar]
- [69].Song J, et al. Neonatal corneal stromal development in the normal and lumican-deficient mouse. Invest Ophthalmol Vis Sci. 2003;44:548. doi: 10.1167/iovs.02-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Boesch JS, Lee C, Lindahl RG. Constitutive expression of class 3 aldehyde dehydrogenase in cultured rat corneal epithelium. J Biol Chem. 1996;271:5150. doi: 10.1074/jbc.271.9.5150. [DOI] [PubMed] [Google Scholar]
- [71].McAvoy JW. Cell division, cell elongation and the co-ordination of crystallin gene expression during lens morphogenesis in the rat. J Embryol Exp Morphol. 1978;45:271. [PubMed] [Google Scholar]
- [72].McAvoy JW. Cell division, cell elongation and distribution of alpha-, beta- and gamma-crystallins in the rat lens. J Embryol Exp Morphol. 1978;44:149. [PubMed] [Google Scholar]
- [73].Griep AE, Kuwabara T, Lee EJ, Westphal H. Perturbed development of the mouse lens by polyomavirus large T antigen does not lead to tumor formation. Genes Dev. 1989;3:1075. doi: 10.1101/gad.3.7.1075. [DOI] [PubMed] [Google Scholar]
- [74].Rana B, Mischoulon D, Xie Y, Bucher NL, Farmer SR. Cell-extracellular matrix interactions can regulate the switch between growth and differentiation in rat hepatocytes: reciprocal expression of C/EBP alpha and immediate-early growth response transcription factors. Mol Cell Biol. 1994;14:5858. doi: 10.1128/mcb.14.9.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Tejedor J, de la Villa P. Refractive changes induced by form deprivation in the mouse eye. Invest Ophthalmol Vis Sci. 2003;44:32. doi: 10.1167/iovs.01-1171. [DOI] [PubMed] [Google Scholar]
- [76].Nees DW, Wawrousek EF, Robison WG, Jr., Piatigorsky J. Structurally normal corneas in aldehyde dehydrogenase 3a1-deficient mice. Mol Cell Biol. 2002;22:849. doi: 10.1128/MCB.22.3.849-855.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Xu ZP, Wawrousek EF, Piatigorsky J. Transketolase haploinsufficiency reduces adipose tissue and female fertility in mice. Mol Cell Biol. 2002;22:6142. doi: 10.1128/MCB.22.17.6142-6147.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Farrell RA, McCally RL, Tatham PER. Wavelength Dependencies of Light Scattering in Normal and Cold-Swollen Rabbit Corneas and their Structural Implications. J Physiol (Lond) 1973;233:589. doi: 10.1113/jphysiol.1973.sp010325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].McCally RL, et al. Light Scattering and Ultrastructure of Healed Penetrating Corneal Wounds. Invest Ophthalmol Vis Sci. 2007;48:157. doi: 10.1167/iovs.06-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM. Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea. 1996;15:505. [PubMed] [Google Scholar]
- [81].Hassell JR, et al. Biosynthesis of stromal matrix proteoglycans and basement membrane components by human corneal fibroblasts. Invest Ophthalmol Vis Sci. 1992;33:547. [PubMed] [Google Scholar]
- [82].Ohji M, SundarRaj N, Thoft RA. Transforming growth factor-beta stimulates collagen and fibronectin synthesis by human corneal stromal fibroblasts in vitro. Curr Eye Res. 1993;12:703. doi: 10.3109/02713689308995765. [DOI] [PubMed] [Google Scholar]
- [83].Lassen N, et al. Multiple and additive functions of ALDH3A1 and ALDH1A1: cataract phenotype and ocular oxidative damage in aldh3a1(-/-)/aldh1a1(-/-) knockout mice. J Biol Chem. 2007 doi: 10.1074/jbc.M702076200. [DOI] [PMC free article] [PubMed] [Google Scholar]