Abstract
BACKGROUND AND PURPOSE: Escalation in monocyte trafficking from the bone marrow into the brain may play a critical role in central nervous system injury and cognitive deterioration in patients with HIV infection. This study tested the hypothesis that the mean diffusivity is sensitive to marrow changes in HIV patients and that these quantitative imaging measurements correlate with the severity of dementia.
METHODS: The mean diffusivity (MD), determined for clival and calvarial marrow regions, was compared in 11 HIV-infected patients and 9 control subjects. The imaging measurements were also evaluated for relationships with dementia severity and markers of disease progression (CD4 and viral load in plasma).
RESULTS: The MD was significantly reduced in both clival and calvarial marrow in HIV-infected patients (P =.006). Diffusion measurements for clival (P =.02) and for calvarial (P =.03) regions were significantly correlated with the severity of dementia.
CONCLUSION: The results of this investigation support the utility of diffusion strategies for monitoring the marrow and provide further evidence of a relationship between marrow status changes and neurologic progression in HIV patients.
Changes occurring in the marrow have been implicated in brain injury underlying cognitive deterioration and dementia in patients infected with HIV.1 For example, anemia before onset of AIDS is predictive of HIV dementia (HIV-D).2 Escalation in monocyte trafficking from the bone marrow into the brain in late-stage infection may represent a critical determinant of HIV-D neuropathogenesis.3 Significant numbers of monocytes entering the brain may initiate and maintain prolonged or self-sustaining states of deleterious immune activation. Quantitative imaging strategies can be used to acquire noninvasive measurements of the marrow in vivo. Measurements sensitive to marrow changes may represent potential imaging markers of neurologic progression in HIV patients. In this investigation, mean diffusivity (MD) measurements were determined in the clival and calvarial marrow regions of HIV patients. The study tested the hypothesis that the MD can be used to detect marrow changes and that these imaging measurements correlate with the severity of dementia in HIV patients.
Methods
Seropositive subjects (mean age, 49.45 ± 7.27 years; 9 men and 2 women) included 11 well-characterized, medically stable patients participating in a longitudinal investigation of the natural history of neurologic impairment in advanced HIV infection. Seropositivity was confirmed by enzyme-linked immunosorbent assay and Western blot. Study exclusion criteria included chronic neurologic disorders, current or past opportunistic central nervous system (CNS) infection, and psychosis at study entry. The control group included consecutive subjects presenting for participation in imaging studies (mean age, 39 ± 9.35 years; 7 men and 2 women). Subjects without history of neurologic illness were included; there were no additional inclusion criteria for the control group. All HIV subjects were receiving antiretroviral treatment (ARV), though one patient’s therapy was temporarily suspended at the time of the scan. Antiretroviral use was classified as dual therapy for one patient (1–2 ARVs) and as 3 or more ARVs (highly active antiretroviral therapy [HAART]) for 9 patients. Therapy for 9 (82%) of the 11 HIV subjects included protease inhibitors. Clinical assessments of the HIV subjects included the AIDS Clinical Trials Group Macro-Neurologic Examination and the motor portion of the Unified Parkinson’s Disease Rating Scale. Dementia severity was determined for all subjects by using standardized, operationalized definitions4 based on the Memorial Sloan Kettering (MSK) rating scale5 and the Karnofsky Performance Scale.6 CD4 counts for the HIV subjects ranged from 24/μL to 427/μL, plasma viral load ranged from undetectable to 5.49 log10 copies/mL, and hemoglobin levels ranged from 11 to 15.2. Cognitive and clinical information for the HIV patients is presented in the Table.
Clinical, cognitive, and mean diffusivity measures for the HIV patients
| Patient No. | Clival | Calvarial | MSK | CD4 | Viral Load (log10) | Therapy |
|---|---|---|---|---|---|---|
| 1 | 0.000691 | 0.000854 | 2 | 174 | 3.914 | 3-TC, Kaletra,* Ziagen |
| 2 | 0.001017 | 0.000784 | 1 | 220 | 1.903 | 3-TC, Indinavir,* Nevirapine |
| 3 | 0.000907 | 0.000866 | 1 | 108 | 2.623 | ddl, Efavirenz, DMP266, Viread |
| 4 | 0.000771 | 0.001021 | 1 | 397 | 1.903 | Efavirenz, DMP266, Viread, Ziagen, Kaletra* |
| 5 | 0.000873 | 0.000851 | .5 | 81 | 3.204 | Kaletra,* Combivir |
| 6 | 0.000757 | 0.000770 | .5 | 373 | 1.903 | 3-TC, d4T, Viracept* |
| 7 | 0.000427 | 0.000729 | .5 | 427 | 1.903 | Ziagen, Kaletra* |
| 8 | 0.000822 | 0.000890 | .5 | 310 | 1.903 | Norvir,* Saquinavir,* Ziagen |
| 9 | 0.000941 | 0.000740 | .5 | 24 | 5.49 | None (drug holiday) |
| 10 | 0.000612 | 0.000844 | 1 | 122 | 4.699 | 3-TC, Norvir,* Amprenavir, Ziagen, Kaletra,* Tenofovir |
| 11 | 0.000632 | 0.001137 | 0.5 | 240 | 3.322 | 3-TC, AZT, ddl, Ziagen, Kaletra,* T20, Tenofovir |
Note:—MSK indicates Memorial Sloan Kettering rating scale.
Protease inhibitor.
MR Imaging
Imaging studies were performed on a 1.5T MR system with a gradient system achieving a maximum gradient strength of 40 mT/m and a maximum slew rate of 150 mT/m/m. A quadrature birdcage head coil was used for radio-frequency transmission and signal intensity reception. Diffusion tensor imaging was performed by using a diffusion sensitized echo-planar sequence with spectral selective pulses for fat suppression. Six diffusion-weighted images were acquired for each section, with a b value of 1000 seconds/mm2. Diffusion gradients applied along 6 directions: (1,0,1)/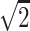 , (−1,0,1)/
, (−1,0,1)/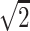 , (0,1,1)/
, (0,1,1)/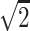 , (0,1,–1)/
, (0,1,–1)/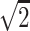 , (1,1,0)/
, (1,1,0)/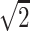 , (−1,1,0)/
, (−1,1,0)/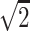 . A b = 0 reference image was also acquired. The entire brain was imaged inferior to superior from the base of the cerebellum to the top of the skull, by using 22 contiguous 7-mm axial sections with a field of view of 24 cm, matrix size of 128 × 128, and repetition time of 7000 milliseconds, with 4 averages.
. A b = 0 reference image was also acquired. The entire brain was imaged inferior to superior from the base of the cerebellum to the top of the skull, by using 22 contiguous 7-mm axial sections with a field of view of 24 cm, matrix size of 128 × 128, and repetition time of 7000 milliseconds, with 4 averages.
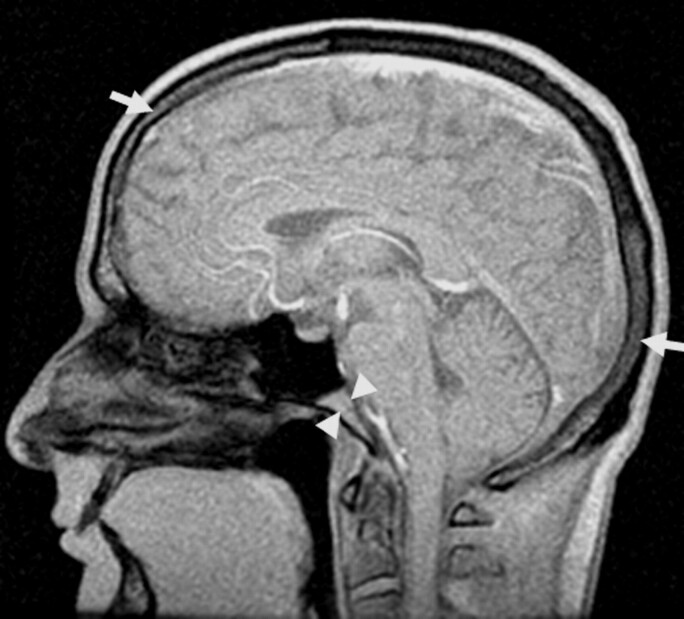
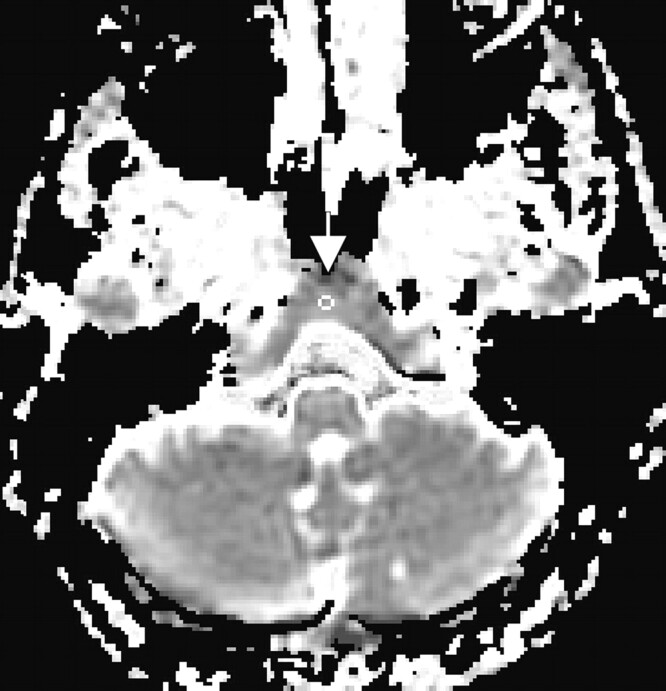
Quantitative image analysis was performed off-line by using a custom software package to compute the MD values.7 All image processing was performed by an operator who was blinded to diagnostic status. Clivus and calvaria were systematically identified on the axial MR images by using the sagittal localization as a reference (Fig 1). Uniform-sized (22 mm2) regions of interest were placed on the anatomic DTI b0 T2-weighted images. The region of interest for clival marrow was placed in the center of the narrow area anterior to the bright band of CSF and the dark band of compact bone tissue and posterior to the bilateral prevertebral muscles at the plane of medulla (Fig 2). The region of interest for calvarial marrow was placed on the left side of the frontal bone between the 2 layers of compact bone (Fig 3). Regions of interest were then projected onto maps to acquire the diffusion measurements.
Fig 1.
Clival (central arrowheads) and calvarial (outer arrows) marrow were systematically identified on the axial MR images by using the sagittal localization as a reference.
Fig 2.
Region of interest for the clival marrow shown on a diffusion map of an HIV patient.
Fig 3.
Region of interest for the calvarial marrow shown on a diffusion map of an HIV patient.
The primary dependent measures for analyses included the MD measures for clivus and calvaria. MD was calculated by using the average of the diagonal directions of the tensor matrix according to standard equations.8 From the eigenvalues, λ1, λ2, λ3, the trace of the diffusion tensor can be estimated, which is proportional to the MD.
Trace = λ1 + λ2 + λ3 and the MD = Trace/3
Cognitive (MSK and Karnofsky) and clinical status (CD4 counts, plasma viral load, body-mass index, hemoglobin, and hematocrit) measures were also examined. Relationships between the MD measurements and these variables were evaluated by using Pearson correlation coefficients or nonparametric measures of association where appropriate (eg, for MSK, Karnofsky, CD4, and viral load). Statistical analyses were executed with SPSS (SPSS, Inc., Chicago, Ill) by using 2-tailed tests and a significance level of .05. This investigation was conducted with approval from the institutional review board.
Results
The quantitative imaging measures were evaluated by using repeated measures analysis of variance with MD measures for clival and calvarial regions considered as a within-subject factor and HIV status as a between group factor, controlling for the effect of age, which was entered as a covariate. A significant main effect for group was obtained (F[1,17] = 9.925; P =.0058). MD values were significantly reduced in the HIV subjects in both marrow regions (clival: mean (SD) in units mm/s2 for HIV: 0.000768 [0.00017] vs controls: 0.001005 [0.00019]; t(18) = −2.909, P =.0094) and (calvarial: mean [SD] for HIV: 0.000862 [0.00012] vs controls: 0.001106 [0.00022)]; t[18] = −3.143, P =.0056).
Spearman correlation coefficients were used to examine the degree of relationship between the MD measures acquired in the marrow regions and the ordinal scale cognitive status ratings. Clival MD measurements were significantly associated with severity of dementia as indicated by MSK and Karnofsky measures (MSK: ρ = −.5165; P =.0197; Karnofsky: ρ = 0.4460, P =.0487). The relationship between calvarial MD values and dementia severity was also significant for the MSK measures (ρ = −.4741; P =.0347). Clinical status indices—including CD4 counts, viral load in plasma, body mass index, hemoglobin, and hematocrit—were not significantly associated with the bone marrow MD measures.
Discussion
The brain is vulnerable to injury associated with HIV infection. Large natural history studies indicate that prognostic markers established before the introduction of HAART may no longer predict HIV-D in contemporary cohorts of HIV patients.9 The identification of predictive markers is a critical imperative to monitor HIV activity in the CNS, direct rational therapeutic intervention, and optimize treatment efficacy at the individual level. Formulations of HIV-D neuropathogenesis assign critical significance to changes occurring in the marrow in late-stage infection.3 MR measurements sensitive to changes in marrow status may represent potential imaging markers of neurologic progression in HIV patients.
In this investigation, measurements of the MD were significantly reduced in both clival and calvarial bone marrow regions and these measures were significantly correlated with the severity of dementia in HIV-infected patients. Membranes, membrane permeability, and the relative volume and morphology of the extracellular space are among determinants of the measured diffusivity.10 Interaction with cellular structures reduces the measured diffusion of protons in biologic tissues. Factors that restrict the mobility of protons, such as hypercellularity and extracellular hemosiderin, are among possible explanations for the diffusion abnormalities we observed. Extracellular hemosiderin, an iron storage protein commonly present in macrophages, has been identified in biopsies of HIV patients with marrow imaging abnormalities on conventional MR images,11,12 as has hypercellularity.11 Increased numbers of monocytes and macrophages13–15 have been seen in AIDS marrows and could contribute significantly to marrow hypercellularity. Whether a relationship exists between marrow changes detectable by histopathology and HIV-D is unclear at present, but HIV expression in marrow, which appears primarily restricted to macrophage lineage cells, has been found to be more common among individuals with HIV encephalitis,16 the pathologic correlate of HIV-D. Increased numbers of cellular structures, reduced extracellular space, and more tortuous diffusion pathways, therefore, could account for reduced diffusion in the marrow of the HIV-infected patients.
The marrow regions studied in this investigation represent significant sites of active hematopoiesis. The cranium and mandible together account for approximately 13% of active (red) marrow in adult humans.17 The changes observed in clival and calvarial marrow regions, therefore, likely reflect generalized alterations in hematopoiesis in these patients. Pathologic processes alter the composition of the bone marrow with relative increases in cellular, hematopoietic tissue and corresponding replacement of adipose tissue. Quantitative imaging studies using spectroscopic techniques have identified reduced fat fractions in the vertebral bone marrow of HIV patients.18,19 The prolonged T1 relaxation times of hematopoietic bone marrow may also alter the apparent signal intensity causing regions such as the clivus to appear mottled or hypointense on conventional (T1-weighted) images.20 Examinations of conventional MR images have identified signal intensity abnormalities in the clival11 and spinal marrow of HIV patients.12 Subtle changes in the marrow, however, may be difficult to detect on conventional MR images,21 and the marrow may appear normal in some HIV patients in advanced stages of immunosuppression.11 Such findings may reflect the insensitivity of conventional MR, the measurement error of subjective determinations of signal intensity abnormality, and/or meaningful variation associated with individual differences in vulnerability to HIV-D. MD measurements are based on physical properties of tissue (the apparent diffusion behavior of protons) that can be objectively quantified and do not require subjective grading relative to other imaged regions (eg, subcutaneous fat or white matter) necessitated by qualitative approaches.
In this investigation, diffusion measures in clival and calavarial bone marrow correlated with the severity of dementia. Monocytes develop within marrow and circulate in blood for only a short time before migrating into the tissues. Perturbations in monocyte production and egress from marrow could influence the levels and activation state of these cells in blood. Increased numbers of activated monocytes correlate with HIV-D22 and HIV encephalitis, characterized by increased numbers of infiltrating blood-derived monocytes, and are strongly associated with the presence of clinical dementia.23,24 Gradual failure of the immune system in HIV infection may set the stage for macrophage dysregulation, involving pathologic cycles of monocyte recruitment, cellular activation, and the CNS production of potentially toxic secretions.2 Monocytes infiltrating the brain can accumulate within the perivascular space (thus becoming perivascular macrophages), and activate microglia and other macrophages. These activated cells can subsequently produce proinflammatory cytokines (eg, tumor necrosis factor-α) and other mediators of inflammation, including chemokines (eg, monocyte chemoattractant protein-1 [MCP-1]) that attract more monocytes into the brain. Levels of MCP-1 and specific metalloproteinases relevant to blood-brain barrier integrity and monocyte ingress are elevated in HIV patients with cognitive impairment.25,26 Prolonged or unrelenting stimulation of macrophages may result in extensive injury to brain tissue.27,28 In addition, the activation of brain macrophages and microglia infected with HIV can initiate virus replication with the consequent production of neurotoxic HIV proteins. The high levels of HIV in brain parenchyma present in some individuals in final stages of infection may also be accounted for by this model. The relative proportions of HIV infected to uninfected monocytes entering the brain under conditions of normal versus enhanced trafficking is unknown, but it is highly unlikely that the proportion of infected cells entering is reduced under conditions of accelerated trafficking. It is important to note that this model can account for the neuroprotective benefits of HAART therapy despite poor CNS penetrance.29 This benefit could result from the control of peripheral factors (ie, limiting the activation and subsequent trafficking of bone marrow-derived monocytes into the brain).30
These findings are based on a small sample of HIV patients. Further studies are necessary to replicate and extend the results. The heterogeneity of the skull, user-dependent region of interest placement, and the possibility of marrow abnormalities in the control participants are possible sources of measurement error. Prospective studies in larger samples of HIV and well-characterized control subjects will be necessary to establish the predictive significance of marrow diffusion measurements for neurologic progression.
Conclusion
This investigation indicates that the MD can be used to detect abnormalities in the clival and calvarial bone marrow and these imaging measurements are significantly correlated with dementia severity in HIV patients. Diffusion strategies may have considerable promise for monitoring changes in marrow during the course of HIV infection. The noninvasive imaging measurements can be acquired in vivo during a neuroradiologic examination in marrow regions not easily accessible to biopsy or aspiration. Quantitative imaging studies of the marrow may yield insights concerning factors underlying neurologic progression in HIV infection and other CNS disorders involving shared neuroimmune mechanisms.
Acknowledgments
The National Institute of Mental Health (grants MH66705 and MH63039) and the National Institute of Neurologic Disorders and Stroke (grants NS36519 and NS049465) provided funding or support for this study. We would like to express appreciation to Pottumarthi Prasad, Linda Reisberg, and Linda Pierchala for assistance.
References
- 1.McArthur J, Hoover D, Bacellar H, et al. Dementia in AIDS patients: incidence and risk factors: Multicenter AIDS Cohort Study. Neurology 1993;43:2245–52 [DOI] [PubMed] [Google Scholar]
- 2.Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annu Rev Neurosci 2002;25:537–62 [DOI] [PubMed] [Google Scholar]
- 3.Gartner S. HIV infection and dementia. Science 2000;287:602–604 [DOI] [PubMed] [Google Scholar]
- 4.Marder K, Albert SM, McDermott MP, et al. Inter-rater reliability of a clinical staging of HIV-associated cognitive impairment. Neurology 2003;60:1467–73 [DOI] [PubMed] [Google Scholar]
- 5.Price RW, Brew BJ. The AIDS dementia complex. J Infect Dis 1988;158:1079–83 [DOI] [PubMed] [Google Scholar]
- 6.Karnofsky DA, Abelman WH, Craver LF, et al. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer 1948;1:634–56 [Google Scholar]
- 7.DPTools. Version 1.4. Paris: INFORMAG;2001
- 8.Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med 1998;39:928–34 [DOI] [PubMed] [Google Scholar]
- 9.McArthur JC, McDermott MP, McClernon D, et al. Attenuated central nervous system infection in advanced HIV/AIDS with combination antiretroviral therapy. Arch Neurol 2004;61:1687–96 [DOI] [PubMed] [Google Scholar]
- 10.Beaulieu C. The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed 2002;15:435–55 [DOI] [PubMed] [Google Scholar]
- 11.Eustace S, McGrath D, Albrecht M, et al. Clivical marrow changes in AIDS: findings at MR imaging. Radiology 1994;193:623–27 [DOI] [PubMed] [Google Scholar]
- 12.Geremia GK, McCluney KW, Adler SS, et al. The magnetic resonance hypointense spine of AIDS. J Comput Assist Tomogr 1990;14:785–89 [DOI] [PubMed] [Google Scholar]
- 13.Schneider DR, Picker LJ. Myelodysplasia in the acquired immune deficiency syndrome. Am J Clin Pathol 1985;84:144–52 [DOI] [PubMed] [Google Scholar]
- 14.Kaloutsi V, Kohlmeyer U, Maschek H, et al. Comparison of bone marrow and hematological findings in patients with human immunodeficiency virus infection and those with myelodysplastic syndromes and infectious diseases. Am J Clin Pathol 1994;101:123–29 [DOI] [PubMed] [Google Scholar]
- 15.Titius BR, Thiele J, Schaefer H, et al. Ki-S1 and proliferating cell nuclear antigen expression of bone marrow macrophages: immunohistochemical and morphometric study including reactive (inflammatory) myelitis, secondary aplastic anemia, AIDS, myelodysplastic syndromes and primary (idiopathic) osteomyelofibrosis. Acta Haematol 1994;91:144–49 [DOI] [PubMed] [Google Scholar]
- 16.Weiser B, Burger H, Campbell P, et al. HIV type 1 RNA expression in bone marrows of patients with a spectrum of disease. AIDS Res Hum Retroviruses 1996;12:1551–58 [DOI] [PubMed] [Google Scholar]
- 17.Wintrobe MM, Lee GR, Boggs DR, et al. Origin and development of the blood and bloodforming tissues. In: Clinical hematology. 8th ed. Philadelphia: Lea & Febiger;1981. :52
- 18.Huang JS, Mulkern RV, Grinspoon S. Reduced intravertebral bone marrow fat in HIV-infected men. AIDS 2002;16:1265–69 [DOI] [PubMed] [Google Scholar]
- 19.Mulkern RV, Huang JS, Vajapeyam S, et al. Fat fractions and spectral T2 values in vertebral bone marrow in HIV- and non-HIV-infected men: a 1H spectroscopic imaging study. Magn Reson Med 2004;52:552–58 [DOI] [PubMed] [Google Scholar]
- 20.Loevner LA, Tobey JD, Yousem DM, et al. MR imaging characteristics of cranial bone marrow in adult patients with underlying systemic disorders compared with healthy control subjects. AJNR Am J Neuroradiol 2002;23:267–72 [PMC free article] [PubMed] [Google Scholar]
- 21.Takagi S, Tsunoda S, Tanaka O. Bone marrow involvement in lymphoma: the importance of marrow magnetic resonance imaging. Leuk Lymphoma 1998;29:515–22 [DOI] [PubMed] [Google Scholar]
- 22.Pulliam L, Gascon R, Stubblebine M, et al. Unique monocyte subset in patients with AIDS dementia. Lancet 1997;349:692–95 [DOI] [PubMed] [Google Scholar]
- 23.Wiley C, Achim C. Human immunodeficiency virus encephalitis is the pathological correlate of dementia in acquired immunodeficiency syndrome. Ann Neurol 1994;36:673–76 [DOI] [PubMed] [Google Scholar]
- 24.Glass JD, Fedor H, Wesselingh SL, et al. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol 1995;38:755–62 [DOI] [PubMed] [Google Scholar]
- 25.Conant K, Garzino-Demo A, Nath A, et al. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci U S A 1998;95:3117–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conant K, McArthur JC, Griffin DE, et al. Cerebrospinal fluid levels of MMP-2, 7 and 9 are elevated in association with HIV-dementia. Ann Neurol 1999;46:391–98 [DOI] [PubMed] [Google Scholar]
- 27.Nath A, Conant K, Chen P, et al. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes: a hit and run phenomenon. J Biol Chem 2001;274:17098–102 [DOI] [PubMed] [Google Scholar]
- 28.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 2001;410:988–94 [DOI] [PubMed] [Google Scholar]
- 29.Gartner S, Liu Y. Insights into the role of immune activation in HIV neuropathogenesis. J of Neurovirol 2002;8:69–75 [DOI] [PubMed] [Google Scholar]
- 30.Clifford DB. AIDS dementia. Med Clin North Am 2002;86:537–50 [DOI] [PubMed] [Google Scholar]