Abstract
Study Objectives:
Assessment of relationships between polysomnographic sleep, sex hormones, and core body temperature in postmenopausal women.
Design and Participants:
Ten women aged 57 to 71 years, at least 5 years past menopause.
Setting:
Laboratory of Human Chronobiology at Weill Cornell Medical College.
Interventions:
N/A.
Measurements and Results:
Lower estradiol (E2) and higher luteinizing hormone (LH) levels were significantly correlated with indices of poor sleep quality. Relationships between LH and polysomnographic variables were more robust than those for E2. Significant increases from basal LH levels (i.e., LH pulses) occurred more frequently after sleep onset than prior to sleep onset, and 30 of 32 of these LH pulses occurred prior to long awakenings from sleep. In addition, higher body core temperature prior to and during sleep was significantly correlated with poorer sleep efficiency and higher LH levels.
Conclusions:
Most investigations of relationships between sleep, sex hormones, and body temperature have focused on perimenopausal women, menopausal phenomena such as hot flashes, the role of declining estrogen, and treatment with exogenous estrogen. The current results suggest that altered levels of both sex steroids and gonadotropins may contribute to sleep disturbance in older women and confirm the results of previous studies indicating that higher body core temperature is associated with poorer sleep quality, even in women without vasomotor symptoms. The findings also raise the possibility of alternate treatment avenues for menopause- and age-related sleep disturbance that focus on altering LH levels.
Citation:
Murphy PJ; Campbell SS. Sex hormones, sleep, and core body temperature in older postmenopausal women. SLEEP 2007;30(12):1788-1794.
Keywords: Sex hormones, aging, postmenopausal women, body temperature
UP TO 50% OF MEDICALLY HEALTHY WOMEN OVER THE AGE OF 60 YEARS REPORT SLEEP DISTURBANCE.1–6 THESE SUBJECTIVE PERCEPTIONS OF POOR SLEEP QUALITY have been verified objectively in the laboratory. For example, with time in bed held constant, older women exhibit total sleep times approximately 2 hours shorter than those of young adults.7–9 Sleep also appears to become “shallower,” and more easily interrupted with age, as reflected in significant declines in electroencephalogram slow-wave activity, increases in wake time after sleep onset (WASO), and decreased arousal thresholds. 10–13 Older women who complain of sleep disturbance most often report difficulty maintaining, rather than initiating, sleep.14–16 They report particular difficulty maintaining sleep in the second half of the night and, as a result, often terminate their night's sleep prematurely. In the laboratory, not only do older women exhibit more spontaneous awakenings than do younger subjects, but they take almost 4 times longer to return to sleep than their younger counterparts.16
A constellation of risk factors for sleep problems in aging women has been identified,5, 17–19 and it is clear that several age-related physiologic changes interact to produce the disturbed regulation of sleep. One aspect of aging that has received sparse attention, however, with regard to effects on sleep, is the dramatic reregulation of reproductive hormones that occurs at menopause and continues throughout the postmenopausal period.
Few studies have measured gonadal hormones in women who are more than a couple of years postmenopause, and almost no studies have investigated, beyond menopause, whether endogenous levels of these hormones influence sleep. Yet, perimenopausal and postmenopausal women share many of the same features of sleep disturbance, such as awakening in the early morning hours and an inability to return to sleep. Furthermore, several studies have shown that hot flashes and other vasomotor symptoms that influence sleep continue years beyond menopause in up to 40% of postmenopausal women.20, 21 Thus, the sleep difficulties that emerge at menopause often do not abate and may become compounded by age-associated disruption of circadian and homeostatic processes that regulate sleep.22–24
Sleep disturbance during the climacteric has generally been associated with estrogen deficiency, and there is evidence that estrogen plays an important role. For example, exogenous estrogen therapy has been reported to improve subjective and, to a lesser extent, objective sleep, most often attributable to a decrease in hot flashes. Nevertheless, a substantial proportion of older women who take estrogen have residual sleep complaints. In addition, many older women experience sleep disturbance that may be associated with age-related changes in thermoregulation25 in the absence of hot flashes or night sweats. Whether the postmenopausal sex hormonal milieu is associated with sleep in these women has not been investigated to any extent. As emphasized by Moe (1999), “There is virtually no information available about how the postmenopausal hormone profile interacts with the sleep of older, asymptomatic postmenopausal women26, p344.”
The current study was undertaken to add to the literature relating to postmenopausal sex hormone levels and sleep. It was designed to exploit the well-documented variability in sleep among older women to examine potential relationships between objective sleep measures, nocturnal sex hormone levels, and the nocturnal course of body core temperature.
METHODS
Subjects
Ten postmenopausal women (mean age 65 ± 5 years; range: 57–71 years) were studied over 3 consecutive nights in the laboratory. They were fully informed of all study procedures and were compensated for their participation. The protocol was approved by the Institutional Review Board of Weill Cornell Medical College.
Subjects were amennorheic for a minimum of 5 years (mean = 14.4 ± 5 years). A physical and mental health examination prior to participation ensured the inclusion of healthy women with no contraindications for frequent blood sampling via indwelling catheter. The examination, conducted by a board-certified sleep medicine physician (n = 7) or geriatric psychiatrist (n = 3), included a sleep history interview in which potential participants self-reported current sleep quality. Of the 10 subjects studied, 3 reported excellent sleep quality with no complaints, 3 reported occasional (1–2 times per week) difficulty maintaining sleep, and 4 reported frequent difficulties maintaining sleep. One included subject reported significant sleep onset difficulties in addition to sleep maintenance problems. For approximately 2 weeks prior to the study, subjects maintained sleep diaries in which they reported bedtimes, estimated sleep-onset latencies, wake-up times, number of awakenings the previous night, and naps. In all 10 subjects, these diaries concurred with the self-reported sleep quality described during screening interviews.
Although complaints of sleep maintenance difficulties did not exclude a potential subject, the likely presence of sleep pathology, such as sleep apnea or periodic limb movements in sleep, as determined in the screening interview, was an exclusion criterion. Current mental health was assessed using the Mini-Mental State Exam27 to screen for dementia (score of > 29 required for study inclusion) and the 17-item Hamilton Depression Rating Scale28 to screen for depression (score of < 7 required for study inclusion). All subjects were free from use of psychotropic medications or medications that altered thyroid function. None had received any form of hormone therapy, including herbal preparations, for a minimum of 3 months prior to study participation.
Procedures
Participants arrived at the laboratory by 1900 on each of 3 consecutive nights. Body core temperature was recorded continuously throughout the subjects' time in the laboratory using an indwelling rectal thermistor. By 2100, an electrode montage for recording sleep-wake variables was attached at electroencephalogram sites F3, C3, O1, referenced to linked mastoids, and at bipolar electrooculograph and electromyograph sites. Subjects' bedtimes and wake times on each night were based on habitual times reported in the sleep diary completed by the subject for a minimum of 1 week prior to study participation. The first night provided adaptation to the laboratory and recording procedures and for additional screening for symptoms of sleep pathology. Specifically, pulse oximetry and tibialis electromyography variables were obtained throughout the sleep period. Oxygen desaturation below 90%, or a periodic limb movement index of more than 10 per hour of 5-second electromyogram contractions associated with an electroencephalogram-defined arousal, excluded subjects from further participation (resulting in dismissal of 2 subjects with significant symptoms of periodic limb movements of sleep). The second night provided further adaptation to the lab environment and to sleeping with the intravenous catheter (not inserted), arm board, and dressings required for remote blood sampling during sleep.
On the third night, an 18-gauge intravenous catheter, connected to a J-hook 12-foot extended polypropylene tube, was inserted into a forearm vein. From 2000 until the later of 0800 or habitual wake time, 5-mL blood samples were collected every 20 minutes. Samples were obtained remotely via the extended catheter tubing that passed through a portal in the wall to a sampling station outside the subject's bedroom. All samples were immediately centrifuged and separated, and plasma or serum aliquots were frozen at −20°C until assayed.
Wake and sleep electroencephalogram variables were recorded using LaMont Systems amplifiers connected to a LaMont HXAT/32 A-to-D board (Stellate Systems, Inc., Quebec, Ontario) in a locally served Ethernet network running the software program Harmonie (Stellate Systems, Inc., Quebec, Ontario). All sleep recordings were scored off line by trained scorers in 30-second epochs according to standard criteria.29
Body temperature was recorded every 2 minutes using disposable rectal thermistors (Yellow Springs International Series 4400, Yellow Springs, Ohio) connected to an ambulatory data-collection device (Minilogger 2000, Minimitter Corporation, Bend, Oregon). The rectal thermistor remained in place throughout the subjects' time in the laboratory, except for brief periods for personal hygiene.
Assays
Commercially available radioimmunoassay kits (Diagnostic Products Corporation, Los Angeles, CA) were used to measure estradiol (E2) and luteinizing hormone (LH) levels. We assayed E2 because it is the most biologically active estrogen in older women and because it provides negative feedback to LH releasing hormone neurons in the hypothalamus. LH was assayed based on literature suggesting that it (but not follicle stimulating hormone) may be associated with sleep disturbance.30–32 All of a subject's samples were assayed in the same run. Intraassay coefficients of variation averaged 7% ± 2% for E2 and 5% ± 1% for LH, whereas interassay coefficients of variation ranged from 5% to 14% for both E2 and LH. The E2 assays had a sensitivity of 1.6 pg/mL, and the LH assays had a lower sensitivity of 3 IU/L and upper sensitivity of 120 IU/L.
Data Analysis
Summary variables were calculated from the scored polysomnographic records. Sleep measures included sleep efficiency (the ratio of time spent asleep per time in bed), sleep period time (SPT: the interval from sleep onset to final morning awakening), total sleep time (TST: minutes of sleep stages 1, 2, 3, 4, and rapid eye movement [REM] during the SPT), sleep-onset latency (from bedtime until the first epoch of sleep stage 2, 3, 4, or REM), amount and percentage of WASO, percentages of each sleep stage as a proportion of SPT, REM sleep latency, and the number and duration of awakenings during the SPT.
To assess the effects of catheter insertion and frequent blood sampling on sleep, sleep efficiency values from the second and third night were compared. A paired t-test indicated slight, but nonsignificant, sleep disruption—mean sleep efficiency (± SD) was 81.2% ± 11.6% versus 79.6% ± 14.3% on the second versus third nights, respectively (P = 0.27). The only significant difference in sleep measures between nights was a lower percentage stage 1 on the third night (8.3% ± 4.1% vs 5.8% ± 1.9%, P < 0.05). Thus, sleep variables from the third night were considered representative of the subjects' laboratory sleep quality and were used in all subsequent analyses.
Mean E2 and LH levels were calculated for each subject as the average of all samples obtained during her sampling period (2000 to the later of 0800 or habitual wake time). Each hormone series was examined for significant pulses using a computerized program based on the cluster analysis method of Veldhuis and Johnson.33 As recommended by the authors, given our 20-minute blood sampling rate, a 2 × 1 cluster size (i.e., 2 consecutive samples for the upstroke and 1 sample for the down stroke) using assay replicate values was utilized, and t-values of 2.1 × 2.1 were applied as thresholds determining significance of the increase or decrease in the hormone level. This approach sets the false-positive error rate to 2.5%, although the false negative rate for LH, specifically, (i.e., not detecting pulses that are there) is likely to be substantially higher. However, logistic and subject safety considerations led to our limiting blood samples to every 20 minutes. Because the sampling rate was the same throughout the sampling interval both within and between subjects, comparisons both within the night for a given subject and between subjects is appropriate. Although the 20-minute sampling rate likely does not permit accurate characterization of LH pulse dynamics (i.e., absolute frequency or amplitude), examination of relative amplitudes and frequency is valid. Relative pulse amplitudes were calculated as the percentage increase from the prepeak valley and to the maximum value obtained, in picograms per milliliter (E2) or milli-international units per milliliter (LH).
Hormone levels and pulse frequencies were compared between the portion of the approximately 12-hour sampling period comprising the SPT (“during sleep”) and the portions comprising the intervals from 2000 to sleep onset and from final morning awakening-end of the sampling period (“during wakefulness”). To account for the fact that the SPT occupied the majority of the sampling period, the relative measure of number of pulses per hour of wakefulness and sleep were compared.
The raw body core temperature data were edited for missing (e.g., during removal of probe for personal hygiene) or artifact data (e.g., due to rectal probe slippage). The arithmetic mean of edited temperature data from 2000 to 0800 was calculated from the values of a 5-minute running average smoothed curve for each subject. The mean temperature during 3 intervals relative to sleep was also calculated: 2000 to polysomnographic sleep onset, sleep onset to morning awakening (i.e., during SPT), and during SPT minus during any interval of wakefulness lasting 5 minutes or longer.
Statistical analyses were computed using SAS JMP v. 5.0 (SAS Institute, Inc., Cary, NC). Results presented include those from all 10 subjects, unless otherwise stated. Regression analyses using hormone measures as the predictor variable were used to examine relationships among E2, LH, demographic, sleep, and temperature variables. All dependent variables were normally distributed as determined by Kolmogorov-Smirnov D test; thus, Pearson product-moment correlations are reported. Significant correlations are only reported as such if all data points fell within 90% bivariate confidence curves.
RESULTS
Hormone Levels
As expected, both E2 and LH levels were highly variable between individuals. Estradiol levels ranged from undetectable to an overnight average of 19.2 pg/mL, with a group mean of 10.2 ± 7.0 pg/mL. Overnight averages in LH levels ranged from 13.3 to 58.2 mIU/mL. The group average LH level was 33.8 ± 17.9 mIU/mL.
Sleep Measures
Table 1 lists group averages and range for polysomnographic measures. As evidenced in the table, there was substantial group variability, although sleep was disrupted relative to published values for healthy young women.1, 34, 35 As an example, 12 women aged 18 to 30 years studied in our lab under a similar protocol during the early follicular phase of the menstrual cycle exhibited sleep efficiency of 88.1% ± 6.0% (unpublished data).
Table 1.
Polysomnographic Variables for 10 Older Postmenopausal Women
| Variable | Mean (SD) | Range |
|---|---|---|
| Age, y | 64.7 (4.7) | 57 – 71 |
| Years since menopause | 14.4 (4.8) | 5 – 21 |
| Bedtime, clock time | 2333 (51m) | 2135 – 2419 |
| Wake-up time, clock time | 0759 (28m) | 0705 – 0831 |
| Time in bed, min | 507.1 (61.4) | 445.0 – 651.0 |
| Sleep period time, min | 485.0 (40.5) | 433.5 – 562.0 |
| Sleep-onset latency, min | 22.1 (25.7) | 8.0 – 89.0 |
| Total sleep time, min | 389.1 (47.6) | 284.0 – 430.0 |
| Sleep efficiency, % | 79.6 (9.0) | 61.8 – 89.5 |
| Sleep stage, min | ||
| WASO | 99.6 (44.9) | 49.0 – 175.5 |
| 1 | 27.7 (7.8) | 20.5 – 43.0 |
| 2 | 192.0 (43.0) | 119.5 – 268.0 |
| SWS | 73.3 (21.4) | 44.5 – 98.0 |
| REM | 92.7 (20.8) | 49.0 – 118.0 |
| Sleep stage, % | ||
| WASO | 20.4 (9.0) | 10.4 – 38.2 |
| 1 | 5.8 (1.9) | 3.8 – 9.9 |
| 2 | 39.6 (8.3) | 26.0 – 55.3 |
| SWS | 15.2 (4.7) | 9.3 – 20.7 |
| REM | 19.1 (4.3) | 10.7 – 25.3 |
| REM latency, min | 67.0 (15.3) | 45.0 – 91.5 |
| Awakenings | ||
| Number | ||
| Total | 11.4 (3.4) | 9 – 17 |
| > 5 min | 3.9 (2.0) | 1 – 6 |
| Duration, min | ||
| Total | 9.3 (19.8) | 2.3 – 13.3 |
| > 5 min | 25.7 (31.5) | 6.7 – 17.5 |
WASO refers to wake after sleep onset; SWS slow-wave sleep; REM rapid eye movement
Relationships Between Hormone Levels and Sleep Quality
Correlation analyses revealed no significant relationship between an individual's mean overnight E2 and LH levels (r = -.41, NS). In addition, neither chronologic age nor years since menopause were associated with either E2 or LH levels or with any sleep measures.
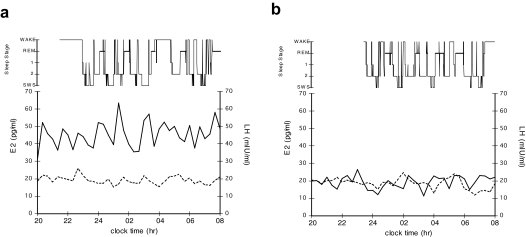
Correlations between polysomnographic measures and overnight average E2 or LH level are shown in Table 2. Although there was a significant positive relationship between E2 levels and sleep efficiency, no other sleep measures were significantly related to subjects' nocturnal E2 levels. In contrast, higher LH levels were significantly associated with lower sleep efficiency (and positively associated, r = 0.64 with its inverse, percentage of WASO, not shown in table), more minutes of WASO, and the number of awakenings longer than 5 minutes. Figure 1 shows sleep and hormone levels for 2 representative subjects. Note that although both subjects had similar E2 levels throughout the night, the first (Figure 1a) had LH levels more than twice as high as the second (Figure 1b). Sleep efficiency was 76% for the woman with higher LH levels, compared with 84% for the woman with lower LH levels.
Table 2.
Pearson Correlations Between Sleep and Overnight Hormone Levels in 10 Postmenopausal Women
| Polysomnographic variable | Mean E2, pg/mL | Mean LH, mIU/mL |
|---|---|---|
| Total sleep time | 0.34 | −0.33 |
| Sleep period time | −0.01 | 0.27 |
| Sleep-onset latency | 0.40 | 0.29 |
| Sleep efficiency | 0.53 | −0.64a |
| Sleep stage, min | ||
| WASO | −0.47 | 0.66a |
| 1 | 0.24 | −0.61 |
| 2 | 0.22 | −0.23 |
| SWS | 0.10 | 0.27 |
| REM | 0.37 | −0.46 |
| REM latency | −0.17 | 0.60 |
| Awakenings, no. > 5 min | −0.21 | 0.85b |
E2 refers to estradiol; LH, luteinizing hormone; WASO, wake after sleep onset; SWS, slow-wave sleep; REM, rapid eye movement.
p < 0.01
p < 0.05
Figure 1.
Hypnogram and plasma levels of estradiol (E2; dotted line) or luteinizing hormone (LH; solid line) for (a) a 67-year-old woman, 14 years after menopause with a sleep efficiency (total sleep time/sleep period time)=76%, mean E2=19.2 pg/mL, and mean LH=45.5 mIU/mL and (b) a 61-year-old woman, 13 years after menopause with a sleep efficiency=84%, mean E2=18.6 pg/mL, and mean LH=19.3 mIU/mL.
Hormone Pulses
Table 3 lists E2 and LH pulse characteristics for the entire sampling period and for the sampling intervals during wakefulness (from 2000–sleep onset plus final awakening–0800) and during the sleep period time. For E2, a group mean of 5.1 (median: 5) peaks were detected. These pulses were generally of low amplitude, with an average increase from prepulse E2 level of 12% ± 12%.
Table 3.
Estradiol and LH Pulse Characteristics During Wakefulness Versus Sleep in 10 Postmenopausal Women
| E2 | LH | |
|---|---|---|
| Pulses, no. | 51 | 49 |
| Pulses, mean | 5.1 | 4.9 |
| Pulses, median | 5 | 5 |
| Pulses during wakefulness, no. | 19 | 9 |
| Pulses during SPT, no. | 32 | 40 |
| Pulses during wakefulness, % | 37 | 18 |
| Pulses during SPT, % | 63 | 82 |
| Hours of sampling interval comprised by wakefulness (proportion) | 3.82 ± 1.16 (32%) | |
| Hours of sampling interval comprised by SPT (proportion) | 8.19 ± 1.16 (68%) | |
| Pulses per h wakefulness, no. | 0.48 | 0.17 |
| Pulses per h SPT, no. | 0.44 | 0.51 |
E2 refers to estradiol; LH, luteinizing hormone; SPT, sleep period time.
As shown in Table 3, 37% of pulses occurred during wakefulness (which comprised 32% of the sampling interval) and 63% during sleep (which comprised 68% of the sampling interval). To account for the different durations of wakefulness versus sleep, the number of E2 pulses per hour of wakefulness, or sleep, was calculated. Estradiol pulses were distributed evenly between the wakefulness and sleep portions of the sampling period—0.48 pulses per hour of wakefulness versus 0.44 pulses per hour of sleep (paired t-test P = 0.13).
For LH pulse activity, a mean of 4.9 (median: 5) peaks per subject were detected. These peaks were generally of higher amplitude than E2 and averaged an increase of 47% ± 19% above the prepulse LH level. Also in contrast to E2, only 18% of LH pulses occurred outside of the SPT, whereas 82% occurred during the sleep period. There were 0.17 LH pulses per hour of wakefulness versus 0.51 LH pulses per hour of sleep (t10 = 2.42, P = 0.04), indicating that LH pulses occurred disproportionately during sleep.
Concurrence of LH Pulses and Awakenings
LH pulses that occurred during the sleep period almost invariably heralded a long awakening from sleep. Ninety-three percent of LH pulses that were detected during sleep were followed, within 20 minutes (the window of time circumscribed by the blood sampling rate), by an awakening of at least 5 minutes in duration. In other words, although not every long awakening was preceded by an LH pulse, 30 of 32 LH pulses were followed closely in time—an average of 8.0 ± 6.2 minutes—by a significant disruption of sleep. (The other 2 LH pulses were from the same subject during ongoing sleep; neither a brief nor a long awakening occurred within an hour of those 2 pulses.) The mean duration of awakenings following an LH pulse was 18 ± 19 minutes.
Body Temperature, Sleep Measures, and Hormone Levels
Body core temperature data from Night 3 were available for 8 of the 10 subjects. Mean body temperature prior to sleep onset was significantly correlated with subsequent sleep efficiency (−.73, P < 0.05). Mean temperature during the SPT was significantly correlated with sleep efficiency (r = −.74, P < 0.05 and its inverse, percentage of WASO, r = 0.74, P < 0.05). When temperature values corresponding to periods of wakefulness longer than 5 minutes were removed from each subject's dataset, and mean temperature was recalculated, the correlation between temperature during the SPT and sleep efficiency remained significant (r = −.72, P < 0.05).
Body temperature was also correlated with hormone measures. There was a strong positive relationship between temperature during sleep and overnight LH levels (r = 0.89, P < 0.01). This correlation was reduced to r = 0.60 (NS) when temperature during intervals of wakefulness longer than 5 minutes was removed from the datasets. Estradiol levels were not significantly associated with any temperature measures.
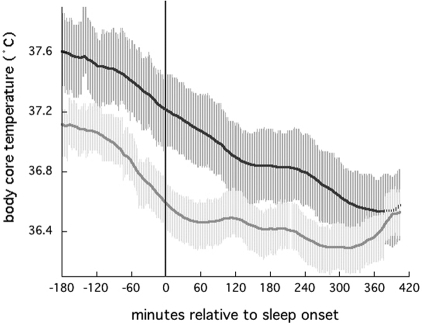
The subset of 8 women with body temperature data included 4 with a sleep efficiency higher than 85% and 4 with a sleep efficiency less than 77%. As illustrated in Figure 2, there were obvious differences between these groups in body core temperature levels. Despite the small size of these subgroups, there was sufficient power to detect significant differences between them (i.e., with an α = 0.05, β was approximately 0.75 for all t-tests described below). Table 4 lists comparisons of average core body temperature levels for these subgroups prior to sleep onset, during sleep, and during sleep minus during awakenings that lasted 5 minutes or longer. Average body temperature prior to sleep onset was significantly higher for women with lower sleep efficiency for all 3 intervals compared. In addition, mean LH levels were significantly higher in the women with lower sleep efficiency and higher body temperature. Although mean E2 levels were lower in those with poorer sleep efficiency, this difference did not reach statistical significance.
Figure 2.
Average core body temperature aligned to individual subjects' sleep-onset times (vertical line), for 8 postmenopausal women, divided into subgroups of 4 women with sleep efficiency < 77%, mean = 72.3% ± 7% (upper, dark gray line) and 4 women with sleep efficiency > 85%, mean = 88.0% ± 2% (lower, light gray line).
Table 4.
Core Body Temperature, Estradiol Levels, and Luteinizing Hormone Levels in 8 Postmenopausal Women Stratified by Sleep Efficiency
| Sleep efficiency, % | t-test | ||
|---|---|---|---|
| > 85 (n = 4) |
< 77 (n = 4) |
||
| Time of Tr measurement | Core temperature average, °C | ||
| Prior to sleep onset | 36.97 ± 0.15 | 37.43 ± 0.34 | t6 = 2.5, P < 0.05 |
| During SPT | 36.57 ± 0.19 | 37.05 ± 0.34 | t6 = 2.5, P < 0.05 |
| During SPT minus during awakenings > 5 min | 36.43 ± 0.09 | 36.92 ± 0.40 | t6 = 2.8, P < 0.05 |
| Hormone measured | Mean hormone levels obtained from 2000 - 0800, pg/mL | ||
| Luteinizing hormone | 21.7 ± 11.6 | 50.6 ± 5.7 | t6 = 4.5, P < 0.01 |
| Estradiol | 14.25 ± 4.70 | 7.62 ± 8.01 | t6 = 1.4, NS |
DISCUSSION
In these postmenopausal women, nocturnal levels of the sex steroid estradiol and the gonadotropin LH were significantly associated with objective sleep measures, and LH levels were significantly correlated with sleep-related body temperature variables. These results are consistent with those of previous studies reporting that high LH levels are associated with disturbed sleep in women. For example, an investigation of the relationship between gonadotropins and sleep in perimenopausal women reported that daytime levels of LH, but not FSH, were positively correlated with the number of awakenings from nocturnal sleep, percentages of sleep stages 1 and 2, REM sleep latency, and the number of shifts between sleep stages.32 In addition, the daily peak in LH pulse amplitudes occurred during the afternoon hours in premenopausal women in the early follicular phase, but during the early morning hours in postmenopausal women.36 This peak in LH pulsatile activity corresponds to the time during which many older women experience difficulty maintaining sleep.e.g.24
In our study, significant increases in LH above basal levels occurred more frequently during the nocturnal sleep period than during the evening hours prior to bedtime or after morning awakening. This result is in apparent opposition to the “sleep-related inhibition” of LH that has been observed in young women during the early follicular phase.36–38 Interestingly, Hall and colleagues recently found that, in premenopausal women, the probability of wakefulness within a sleep episode increased significantly prior to an LH pulse. The authors interpreted the result as suggesting that wakefulness was the primary event and that an awakening from sleep permitted release from the inhibitory effect of sleep on hypothalamic control of LH pulsatility. Because many of the older women in our study had frequent and long awakenings, it is quite possible that this same temporal relationship—wakefulness permitting an LH pulse—occurred in our sample. However, our relatively infrequent sampling rate does not allow us to adequately confirm this possibility. Additional studies of sex hormones, body temperature, and sleep, in which the variables are measured across the circadian day, both in the presence and absence of sleep (i.e., during sleep deprivation, sleep reversal, or daytime napping), might also clarify the direction of relationships between the variables.
One limitation of this study is the possibility that women with sleep-disordered breathing may have been included. Although we excluded any woman with a diagnosis of sleep apnea or other medical sleep disorder and, further, included only those whose pulse oximetry values did not drop below 90% at any time during the night, we did not measure additional respiratory variables that may have revealed the presence of sleep-disordered breathing. Another limitation of the study is that no systematic measures of subjective sleep quality were obtained. Although subjects' self-perceptions of sleep quality obtained during screening examinations were generally confirmed by polysomnographic results, the important question of whether there are relationships among subjective sleep quality, hormone levels, and body temperature could not be examined.
Mechanisms by which steroid hormones influence sleep have been studied to some extent, in contrast with the possible contribution of gonadotropins to regulation of central nervous system activities. It has long been known that estrogen receptors are present in sleep- and temperature-regulating areas of the hypothalamus. Recently, LH receptors have also been identified in the human brain, including the hypothalamus and pineal,39, 40 and they have been shown to influence synthesis of melatonin in rats.41 One means by which pituitary LH release could affect sleep comes from studies reporting that LH levels are positively related to the frequency and severity of hot flashes, even in the presence of relatively high estrogen levels.42–44 Although LH pulses are not the proximate cause of hot flashes, pituitary LH release occurs concomitantly with the dramatic central and body core temperature increase at their onset45 and LH receptors located in distal vasculature may interfere with peripheral heat loss mechanisms.40 Combined with evidence that altered temperature regulation contributes to some forms of sleep disturbance,46 it is conceivable that high LH levels influence sleep via thermoregulatory mechanisms. Congruent with this notion, we found that poor sleep quality was associated with both higher LH levels and higher body core temperature. One intriguing question is whether LH pulsatile activity is associated with acute changes in body temperature that lead to sleep disruption or, perhaps, whether gonadotropin release has more long-term effects on, for example, the circadian course of body temperature.
At a minimum, these data provide evidence to warrant continued investigation of how age-related changes in the output of the hypothalamic-pituitary-gonadal axis, including both gonadal steroids and gonadotropins, impact sleep quality in aging women, with the aim of developing novel approaches to the treatment of menopause- and age-related sleep disturbance.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Bixler E, Kales A, Soldato C, Kales D, Healey S. Prevalence of sleep disorders in the Los Angeles metropolitan area. Am J Psychiatry. 1979;136:1262–75. doi: 10.1176/ajp.136.10.1257. [DOI] [PubMed] [Google Scholar]
- 2.Buysse DJ, Reynolds CD, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–8. [PubMed] [Google Scholar]
- 3.Carskadon M, van den Hoed J, Dement W. Sleep and daytime sleepiness in the elderly. J Geriatr Psychiat. 1980;13:135–51. [PubMed] [Google Scholar]
- 4.Dement WC, Miles LE, Carskadon MA. “white paper” on sleep and aging. J Am Geriatr Soc. 1982;30:25–50. doi: 10.1111/j.1532-5415.1982.tb03700.x. [DOI] [PubMed] [Google Scholar]
- 5.Foley D, Monjan A, Broen S, Simonsick E, Wallace R, Blazer D. Sleep complaints among elderly persons: An epidemiologic study of three communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 6.Schubert CR, Cruickshanks KJ, Dalton DS, Klein BE, Klein R, Nondahl DM. Prevalence of sleep problems and quality of life in an older population. Sleep. 2002;25:889–93. [PubMed] [Google Scholar]
- 7.Campbell SS, Dawson D. Aging young sleep: A test of the phase advance hypothesis of sleep disturbance in the elderly. Journal of Sleep Research. 1992;1:205–210. doi: 10.1111/j.1365-2869.1992.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 8.Buysse DJ, Browman KE, Monk TH, Reynolds CD, Fasiczka AL, Kupfer DJ. Napping and 24-hour sleep/wake patterns in healthy elderly and young adults. J Am Geriatr Soc. 1992;40:779–86. doi: 10.1111/j.1532-5415.1992.tb01849.x. [DOI] [PubMed] [Google Scholar]
- 9.Webb WB. Sleep in older persons: Sleep structures of 50- to 60-year-old men and women. J Gerontol. 1982;37:581–6. doi: 10.1093/geronj/37.5.581. [DOI] [PubMed] [Google Scholar]
- 10.Pivik RT, Joncas S, Busby KA. Sleep spindles and arousal: The effects of age and sensory stimulation. Sleep Res Online. 1999;2:89–100. [PubMed] [Google Scholar]
- 11.Reynolds CD, Kupfer DJ, Taska LS, et al. EEG sleep in elderly depressed, demented, and healthy subjects. Biol Psychiatry. 1985;20:431–42. doi: 10.1016/0006-3223(85)90045-9. [DOI] [PubMed] [Google Scholar]
- 12.Busby K, Mercier L, Pivik R. Ontogenetic variations in auditory arousal threshold during sleep. Psychophysiology. 1994;31:182–188. doi: 10.1111/j.1469-8986.1994.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 13.Webb WB, Campbell SS, Hendlin R. The termination of extended sleep. Biological Psychology. 1980;11:45–48. doi: 10.1016/0301-0511(80)90025-3. [DOI] [PubMed] [Google Scholar]
- 14.Campbell S, Dawson D, Anderson M. Alleviation of sleep maintenance insomnia with timed exposure to bright light. J American Geriatric Society. 1993;41:829–836. doi: 10.1111/j.1532-5415.1993.tb06179.x. [DOI] [PubMed] [Google Scholar]
- 15.Campbell SS, Gillin JC, Kripke DF, Erikson P, Clopton P. Gender differences in the circadian temperature rhythms of healthy elderly subjects: Relationships to sleep quality. Sleep. 1989;12:529–36. [PubMed] [Google Scholar]
- 16.Webb W, Campbell S. Awakenings and return to sleep in an older population. Sleep. 1980;3:41–6. [PubMed] [Google Scholar]
- 17.McCrae CS, Wilson NM, Lichstein KL, et al. ‘young old’ and ‘old old’ poor sleepers with and without insomnia complaints. J Psychosom Res. 2003;54:11–19. doi: 10.1016/s0022-3999(02)00543-3. [DOI] [PubMed] [Google Scholar]
- 18.Myers BL, Badia P. Changes in circadian rhythms and sleep quality with aging: Mechanisms and interventions. Neurosci Biobehav Rev. 1995;19:553–71. doi: 10.1016/0149-7634(95)00018-6. [DOI] [PubMed] [Google Scholar]
- 19.Weitzman ED, Moline ML, Czeisler CA, Zimmerman JC. Chronobiology of aging: Temperature, sleep-wake rhythms and entrainment. Neurobiol Aging. 1982;3:299–309. doi: 10.1016/0197-4580(82)90018-5. [DOI] [PubMed] [Google Scholar]
- 20.Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006;166:1262–8. doi: 10.1001/archinte.166.12.1262. [DOI] [PubMed] [Google Scholar]
- 21.Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351–8. doi: 10.1016/s0029-7844(00)00930-3. [DOI] [PubMed] [Google Scholar]
- 22.Czeisler CA, Dumont M, Duffy JF, et al. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–6. doi: 10.1016/0140-6736(92)92817-y. [DOI] [PubMed] [Google Scholar]
- 23.Dijk DJ, Duffy JF, Czeisler CA. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol Int. 2000;17:285–311. doi: 10.1081/cbi-100101049. [DOI] [PubMed] [Google Scholar]
- 24.Campbell SS, Murphy PJ. Relationships between sleep and body temperature in middle-aged and older subjects. J Am Geriatr Soc. 1998;46:458–62. doi: 10.1111/j.1532-5415.1998.tb02466.x. [DOI] [PubMed] [Google Scholar]
- 25.Van Someren EJ, Raymann RJ, Scherder EJ, Daanen HA, Swaab DF. Circadian and age-related modulation of thermoreception and temperature regulation: Mechanisms and functional implications. Ageing Res Rev. 2002;1:721–78. doi: 10.1016/s1568-1637(02)00030-2. [DOI] [PubMed] [Google Scholar]
- 26.Moe KE. Reproductive hormones, aging, and sleep. Semin Reprod Endocrinol. 1999;17:339–48. doi: 10.1055/s-2007-1016243. [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. «mini-mental state». A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton M. A rating scale for depression. J Neurol Neurosur Psychiat. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rechtschaffen A, Kales A. Washington D.C: National Institute of Health; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Vol. publ. 204. [DOI] [PubMed] [Google Scholar]
- 30.Ushiroyama T, Okamoto Y, Okazaki T, Sugimoto O. Correlation between climacteric disorder and endocrine features-longitudinal study for climacteric women with symptoms. Advances in Obstetrics and Gynecology. 1991;43:202–206. [Google Scholar]
- 31.Bellipanni G, Bianchi P, Pierpaoli W, Bulian D, Ilyia E. Effects of melatonin in perimenopausal and menopausal women: A randomized and placebo controlled study. Exp Gerontol. 2001;36:297–310. doi: 10.1016/s0531-5565(00)00217-5. [DOI] [PubMed] [Google Scholar]
- 32.Hoffmann G, Wauter H, Pauwels E, Buytaert G, Uyttenbroeck F, Petre-Quadens O. Sleep and pituitary hormones: A comparison study between premenopause and menopause. Waking – Sleeping. 1978;2:157–167. [Google Scholar]
- 33.Veldhuis J, Johnson M. Cluster analysis: A simple, versatile, and robust algorithm for endocrine pulse detection. American Journal of Physiology. 1986;250:E486–E493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- 34.Ehlers CL, Kupfer DJ. Effects of age on delta and REM sleep parameters. Electroencephalogr Clin Neurophysiol. 1989;72:118–25. doi: 10.1016/0013-4694(89)90172-7. [DOI] [PubMed] [Google Scholar]
- 35.Hume KI, Van F, Watson A. A field study of age and gender differences in habitual adult sleep. J Sleep Res. 1998;7:85–94. doi: 10.1046/j.1365-2869.1998.00103.x. [DOI] [PubMed] [Google Scholar]
- 36.Rossmanith WG. The impact of sleep on gonadotropin secretion. Gynecol Endocrinol. 1998;12:381–9. doi: 10.3109/09513599809012840. [DOI] [PubMed] [Google Scholar]
- 37.Hall JE, Sullivan JP, Richardson GS. Brief wake episodes modulate sleep-inhibited luteinizing hormone secretion in the early follicular phase. J Clin Endocrinol Metab. 2005;90:2050–5. doi: 10.1210/jc.2004-2033. [DOI] [PubMed] [Google Scholar]
- 38.Kapen S, Boyar R, Hellman L, Weitzman ED. The relationship of luteinizing hormone secretion to sleep in women during the early follicular phase: Effects of sleep reversal and a prolonged three-hour sleep-wake schedule. J Clin Endocrinol Metab. 1976;42:1031–40. doi: 10.1210/jcem-42-6-1031. [DOI] [PubMed] [Google Scholar]
- 39.Bhatnagar KP, Li X, Lei ZM, Rao CV. Human pineal luteinizing hormone receptors. Biotech Histochem. 2002;77:223–8. [PubMed] [Google Scholar]
- 40.Toth P, Lukacs H, Gimes G, et al. Clinical importance of vascular LH/Hcg receptors—a review. Reprod Biol. 2001;1:5–11. [PubMed] [Google Scholar]
- 41.Itoh MT, Hosaka T, Takahashi N, Ishizuka B. Expression of luteinizing hormone/chorionic gonadotropin receptor in the rat pineal gland. J Pineal Res. 2006;41:35–41. doi: 10.1111/j.1600-079X.2006.00329.x. [DOI] [PubMed] [Google Scholar]
- 42.Fossum G, Greep N, Kletzky O. Patterns of luteinizing and follicle-stimulating hormone pulsatility in menopausal women: Correlation with plasma testosterone level and vasomotor instability episodes. Am J Obstet Gynecol. 1995;41:795–800. doi: 10.1016/0002-9378(95)90343-7. [DOI] [PubMed] [Google Scholar]
- 43.Ushiroyama T, Yoshikawa M, Saeki M, Sugimoto O. Hypergonadotropinemia with estradiol secretion in peri- and postmenopausal period. Acta Obstetrica Gynecolica Scandinavica. 1989;68:139–143. doi: 10.3109/00016348909009901. [DOI] [PubMed] [Google Scholar]
- 44.Ushiroyama T, Ikeda A, Ueki M. Evidence for attenuation of gonadotropin pulse frequency in hypergonadotropic women with estradiol secretion in the menopausal transition. Psychoneuroendocrinology. 1999;24:85–97. doi: 10.1016/s0306-4530(98)00038-9. [DOI] [PubMed] [Google Scholar]
- 45.Casper RF, Yen SS, Wilkes MM. Menopausal flushes: A neuroendocrine link with pulsatile luteninizing hormone secretion. Science. 1979;205:823–5. doi: 10.1126/science.462193. [DOI] [PubMed] [Google Scholar]
- 46.Lushington K, Dawson D, Lack L. Core body temperature is elevated during constant wakefulness in elderly poor sleepers. Sleep. 2000;23:504–10. [PubMed] [Google Scholar]