Abstract
Objectives:
A changing sleep schedule that reduces sleep duration is thought to produce the increasing daytime sleepiness of adolescents. We tested the hypothesis that adolescent daytime sleepiness also results from adolescent brain maturational processes indexed by declining delta electroencephalographic (EEG) activity.
Design:
Data are from the first 3 years of a semilongitudinal study of EEG changes in adolescence. All-night EEG was recorded semiannually.
Setting:
EEG was recorded with ambulatory recorders in the subjects' homes.
Participants:
Thirty-one subjects were 9 years old (cohort C9), and 38 subjects were 12 years old (cohort C12) at the start of the study.
Measurements:
EEG power density (power/minute) was calculated for the first 5 hours of non-rapid eye movement sleep. Subjects rated sleepiness on a modified Epworth Sleepiness Scale. Habitual sleep schedules were assessed with self-reports and actigraphy.
Results:
In C9 subjects, sleepiness increased slightly and was related only to age. In C12 subjects, the increase in subjective sleepiness was related to changes in age, bedtime, time in bed, and a wide frequency range of EEG power density. Sleepiness was not related to rise time, non-rapid eye movement sleep duration, rapid eye movement sleep duration, or total sleep time. With sleep schedule measures statistically controlled, the increase in sleepiness in the C12 group was strongly related to declining delta power density and, unexpectedly, even more strongly related to declining theta power density.
Conclusions:
The data support our hypothesis that, independent of sleep schedule changes, increasing adolescent daytime sleepiness is related to brain maturational changes indexed by declining EEG power. Our working hypothesis is that the declines in delta and theta power are correlates of an adolescent synaptic pruning that reduces waking arousal levels.
Citation:
Campbell IG; Higgins LM; Trinidad JM; Richardson P; Feinberg I. The increase in longitudinally measured sleepiness across adolescence is related to the maturational decline in low-frequency EEG power. SLEEP 2007;30(12):1677-1687.
Keywords: Adolescent, sleepiness, homeostasis, slow wave
DECLINING SLEEP DURATION IS COMMONLY CITED AS THE REASON THAT DAYTIME SLEEPINESS EMERGES IN ADOLESCENTS (C.F. 1), I.E., ADOLESCENTS ARE BECOMING sleepy because they are becoming sleep deprived. It is certainly the case that sleep schedules change dramatically with age across adolescence. Compared to younger children, adolescents delay their bedtimes but wake up as early or earlier due to school schedules. This pattern is widespread in developed nations.2–11 It results in reduced time in bed and sleep duration. Actigraphy and polysomnography studies have documented reductions of about 1 hour in total sleep duration on school days across adolescence (reviewed in 12).
However, there is evidence that declining sleep duration is not the sole factor responsible for the development of daytime sleepiness in adolescence. In a study that fixed time in bed at 10 hours for both adolescents and preadolescents, adolescents had total sleep times as long as those of preadolescents but still had shorter sleep latencies in Multiple Sleep Latency Tests (MSLT), indicating increased sleepiness.13 Thus, adolescents had greater daytime sleepiness without decreased nocturnal sleep duration. This study indicates that some maturational factor must also play a role in the development of adolescent daytime sleepiness. This maturational factor could be a change in circadian or homeostatic regulation or a separate brain maturational change that reduces daytime arousal levels.
In addition to changes in sleep schedule, sleep duration, and daytime sleepiness, the sleep electroencephalogram (EEG) undergoes dramatic developmental changes during adolescence. The most well-described adolescent EEG change is a steep decline in non-rapid eye movement (NREM) slow-wave EEG (delta) activity.14, 15 We have proposed that this decline is one component of a widespread brain maturational process driven by synaptic pruning.15,16 According to our model, declining synaptic connectivity decreases the intensity of waking brain activity and, therefore, cerebral metabolic rate. It is now becoming recognized that the need for the sleep-dependent recuperation depends not only on waking duration, but also on the intensity of waking brain activity.17,18 A decrease in the intensity of waking neuronal activity should reduce the need for the recuperative processes of sleep and thus reduce the NREM delta intensity. We recently reported that delta EEG activity is constant between ages 9 and 11 years but declines steeply between ages 12 and 14 years.19 Another prominent change of adolescence is pubertal development and the associated hormonal changes driven by reactivation of gonadotropin-releasing hormone secretion.20 We found that the delta EEG decline between ages 12 and 14 is strongly related to age but is independent of pubertal development.19
Here we hypothesize that increasing daytime sleepiness is related to this decline in slow-wave EEG. Prior to adolescence, nocturnal sleep is very deep, waking brain activity (metabolic rate21) is very intense, and daytime sleepiness is normally absent. As the intensity of waking brain activity diminishes across adolescence, daytime sleepiness emerges. If our hypothesis is correct, daytime sleepiness in adolescents should be related to the decline in NREM delta power.
Thus, we propose that sleep-schedule changes and brain maturational processes independently contribute to the development of daytime sleepiness. Most previous studies on adolescent sleep have been cross-sectional. Longitudinal studies can more effectively determine how the development of sleepiness relates to changes in other variables. Thus the main goal of this study is to use longitudinal data to test the hypothesis that increasing sleepiness in adolescence is related to declining delta EEG independently of sleep-schedule changes. We also describe longitudinally measured changes, from ages 9 to 12 and 12 to 15 years, in the following variables: subjective daytime sleepiness, sleep schedule, sleep duration, and slow-wave EEG power.
METHODS
The data presented in this paper are from the first 6 semiannual recordings of our longitudinal study of sleep changes across adolescence.
Subjects
Subjects for this study were recruited with newspaper advertisements and word of mouth. Before enrollment, subjects were screened via a medical interview with the parents. Exclusion criteria included history of neurologic or psychiatric illness, sleep disorder, immediate family member with psychiatric illness, and illness requiring medication that affects the central nervous system. Urine tests for drug use were conducted at the time of each recording. Data here are from 69 subjects in 2 cohorts. We began recording the C9 (n = 31, 16 female, 15 male) cohort at approximately 9 years of age. We began recording the C12 (n = 38, 19 female, 19 male) cohort at approximately 12 years of age. Mean ages for the 2 cohorts at each of the 6 recordings are presented in Table 1. The UC Davis Institutional Review Board approved all procedures. Subjects were paid for their participation in the study.
Table 1.
Mean Subject Ages and Tanner-stage Scores at Each Semiannual Recording
| Semiannual Recording |
||||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | |
| C9 Cohort | ||||||
| Age, y | ||||||
| Mean | 9.27 | 9.80 | 10.35 | 10.88 | 11.40 | 11.94 |
| SD | 0.27 | 0.22 | 0.23 | 0.23 | 0.25 | 0.30 |
| Tanner stage | ||||||
| Mean | 1.26 | 1.33 | 1.45 | 1.65 | 1.91 | 2.29 |
| SD | 0.39 | 0.46 | 0.69 | 0.67 | 0.79 | 0.91 |
| C12 Cohort | ||||||
| Age, y | ||||||
| Mean | 12.29 | 12.80 | 13.33 | 13.86 | 14.38 | 14.87 |
| SD | 0.19 | 0.19 | 0.20 | 0.21 | 0.23 | 0.22 |
| Tanner stage | ||||||
| Mean | 2.54 | 3.03 | 3.32 | 3.53 | 3.60 | 3.76 |
| SD | 1.0 | 0.86 | 0.80 | 0.62 | 0.57 | 0.53 |
Experimental Design
Sleep EEG was recorded semiannually in the subjects' homes using Grass H2O ambulatory EEG recorders (Astro-Med, Inc. West Warwick, RI). Sleep EEG was recorded for 4 consecutive nights. For the first 2 nights, the subjects maintained their habitual weekday bedtime and rise time. On the third and fourth nights, subjects kept their habitual weekday bedtime but were asked to sleep as late as possible on the following morning. For the 5 days prior to the recording, subjects maintained their habitual weekday sleep schedule and did not nap. Actigraphy watches (Minimitter A16 Actiwatch, Mini Mitter Co., Inc., Bend, Ore) confirmed subject compliance. Subjects who deviated from their habitual schedule were rescheduled for recording in a subsequent week.
Electrode Application and EEG Recording
Trained UC Davis undergraduates traveled to the subjects' homes to apply electrodes in the evenings before EEG recordings. Parents removed the electrodes in the morning. EEG electrodes were applied at Fz, Cz, C3, C4, O1, and O2 with A1 and A2 mastoid electrodes. Electrooculogram electrodes were applied at the left outer canthus and right outer canthus and referred to a forehead electrode. One ground electrode was applied to the face and another to the head. Electrode impedance was less than 5 KΩ at the start of the recording. The Grass H2O system uses the mean of all EEG electrodes as the reference. Electrode pairs, such as C3-A2, are obtained by subtraction (C3-reference minus A2-reference). The H2O recorder digitized all signals at 200 Hz and stored data to a removable hard disk for later analysis. We recently published a frequency response curve for the H2O recorder filters.22 The low-frequency filter is a single-pole, 0.3-Hz, one-half amplitude filter.
Sleep EEG Analysis
EEG data from the removable hard disk of the H2O were copied to the lab computer and analyzed with PASS PLUS (Delta Software, St. Louis, MO). Using an on-screen display of the digitized data, a trained rater scored each 20-second epoch as non-rapid eye movement (NREM), rapid eye movement (REM), stage 1 sleep, wake, or movement based on modified Rechtschaffen and Kales criteria.23 Independently of vigilance stage, epochs were scored for the presence of artifacts. A second rater checked the scoring and a third experienced rater reconciled any discrepancies.
Spectral analysis was performed on all artifact-free epochs in the first 5 hours of NREM sleep on baseline nights 1 and 2. Analysis was limited to the first 5 hours of NREM to control for changing sleep duration with age. Night 3 also provided baseline data because the first 5 hours of NREM preceded sleep extension. Night 4 data were not used for this report. The data point at each semiannual recording for each subject was the mean of the subject's baseline nights. Fast Fourier transform (FFT) parameters were as follows: 5.12-second Welch tapered windows with 2.62 seconds of overlap yielding 8 windows per 20-second epoch. These FFT parameters do not yield integer-value frequency-band limits. We define delta as 0.98 to 4.03 Hz and, for simplicity, will refer to it as 1 to 4 Hz. NREM delta power density is 1 to 4 Hz power divided by the number of minutes of artifact-free epochs.
All-night NREM duration, REM duration, and total sleep time were calculated from the visual scoring of the EEG. The sleep extension on night 3 affected these measures; therefore, only nights 1 and 2 were used for sleep-duration calculations. For nights 1 and 2, we also calculated all night 1 to 4 Hz power (total delta power, not power/min) in all artifact-free NREM epochs (i.e., not limited to the first 5 hours) using the above FFT parameters.
Pubertal Maturation Ratings and Age
Within 1 month of the EEG recording, subjects visited a physician who, during a physical exam, rated subjects' pubertal maturation using the Tanner-stage guidelines.24 The same physician performed all evaluations. Girls are rated on a 5-point scale for breast development and a 5-point scale for pubic hair growth. Boys are rated on a 5-point scale for genital development and a 5-point scale for pubic hair growth. For both girls and boys, the Tanner-stage score at each semiannual recording, shown in Table 1, is the average of the 2 ratings.
Age to the nearest day was also calculated at each recording session. For graphic display of the data in Figures 1 to 4, means are plotted for each recording session versus the average age at that recording session. For statistical analysis, age is a continuous, not a categorical, variable.
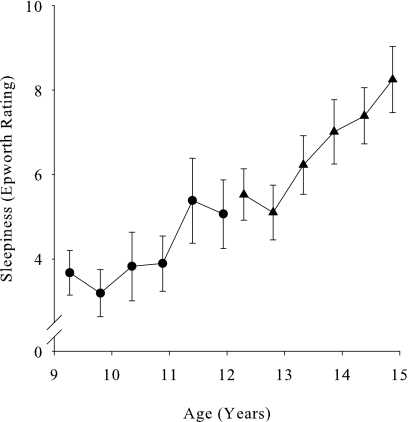
Figure 1.
Mean (± SEM) subjective daytime sleepiness rating plotted against subject age at each of 6 recordings for the C9 cohort (●) and the C12 cohort (▲). After an initial dip, sleepiness increased significantly with age in cohort C12. The increase with age in the C9 cohort was due to a large increase from the fourth to fifth recording.
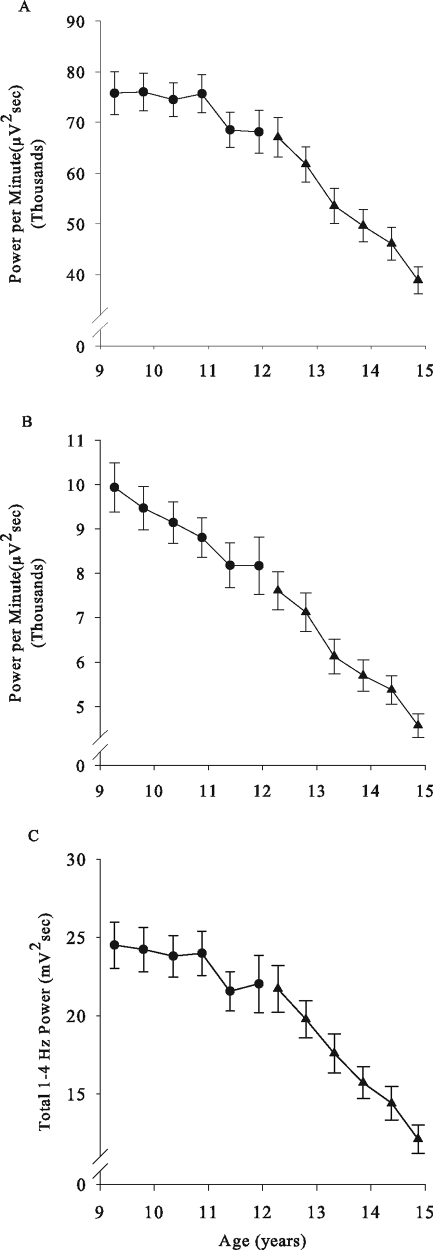
Figure 4.
Mean (± SEM) electroencephalogram (EEG) power density in (A) 1- to 4-Hz and (B) 4- to 8-Hz frequency band is plotted against age with same format as in Figure 1. Delta (1–4 Hz) power density declined linearly across the 6 recordings in cohort C12, from age 12 to age 15 years. In cohort C9, the decline in delta power density with age was due to a decrease from the fourth to fifth recording. The 4- to 8-Hz power density declined linearly in both cohorts. (C) All-night total non-rapid eye movement sleep delta power showed the same relationship to age as did the delta power density.
Sleepiness ratings
In current sleep research, daytime sleepiness is measured objectively by determining how quickly a subject can fall asleep (MSLT25) or, less frequently, by how long a subject can remain awake (Maintenance of Wakefulness Test26) with EEG recorded in repeated sessions during the day. Sleepiness can also be measured with subjective ratings. The most widely used instrument, the Epworth Sleepiness Scale27 asks subjects how likely they are to fall asleep in various situations. Slightly adapted versions of this questionnaire have been used to measure sleepiness in adolescents, and the reliability has been demonstrated.3,28 The objective and subjective approaches measure different aspects of sleepiness. The objective MSLT and Maintenance of Wakefulness Test measures determine sleep propensity in a laboratory on the day of testing. In contrast, the Epworth Sleepiness Scale estimates sleep propensity in everyday life over a period of time.29
During the week of recording, subjects completed a sleepiness questionnaire based strongly on the Epworth Sleepiness Scale.27 The questionnaire asked subjects to consider their daytime sleepiness in the previous 2 weeks and to evaluate their likelihood of falling asleep in various everyday situations. The Epworth scale was slightly altered to make it appropriate for adolescents by replacing the question about falling asleep in a car stopped in traffic with 2 questions about falling asleep in school. The first asked how likely the subject was to fall asleep in class while the teacher was talking, and the second question asked how likely the subject was to fall asleep in class while working on his or her own. The additional question makes our questionnaire a 27-point scale rather than the original 24-point Epworth Scale.
Sleep Schedule Recordings
Subjects' sleep schedules were estimated in 2 ways. The subjects reported their habitual sleep schedules on a questionnaire that asked for bedtime and rise time. The subjects' bedtimes and rise times were also determined from the actigraphy recordings for the 3 nights prior to each recording.
Statistical Analyses
Mixed-effect analysis is particularly suited for longitudinal data.30,31 This statistical analysis is similar to regression but uses repeated measurements to model change within an individual. The output of linear mixed-effect analysis provides an estimate of the intercept and the slope of the relationship between the outcome variable and the predictor variable. The F and P values reported in this paper are for the slope and test for a significant relationship between outcome and predictor variables. The predictor variable is “centered” prior to analysis so that the intercept is meaningful.32 For example, when analyzing the relationship between sleepiness and age in the C12 cohort, we subtracted 12 from all ages so that the intercept is an estimate of sleepiness at age 12 rather than at age 0.
We applied mixed-effect analysis (SAS proc MIXED, SAS Institute, Inc., Cary, NC) to determine whether changes in sleepiness, EEG measures, and sleep-schedule measures were related to age and to describe these relationships. We also used mixed-effect analysis to test, within subjects, for relationships between changes in sleepiness and changes in EEG measures and between changes in sleepiness and changes in sleep schedule. The initial mixed-effect analyses showed a significant interaction between age and cohort. Therefore, we separately analyzed the C9 and C12 cohorts. Because of the large number of statistical tests, we adopted an α of 0.01.
RESULTS
Sleepiness Increased with Age
As shown in Figure 1, across the 3 years of data collection, sleepiness increased with age in both the C9 and C12 cohorts (F1,149 = 12.6, P = 0.0005 and F1,186 = 42.1, P < 0.0001 respectively). In the C9 cohort, sleepiness did not change significantly with age over the first 4 recordings (mixed-effect analysis using only recordings 1–4, F1,88 = 1.1, P = 0.31) but increased substantially between the fourth and fifth recordings. In the C12 cohort, following an initial nonsignificant (paired t-test of first and second recording, P = 0.37) dip, sleepiness increased linearly. The linear mixed-effect analysis of the C12 data estimated the following relationship between sleepiness and age over the entire 12-to-15-year age range: Epworth sleepiness rating at age 12 was 4.7 (± 0.66, SEM) points and increased 1.2 (± 0.2) points per year. Despite the robust increase in sleepiness between ages 12 and 15, the mean Epworth sleepiness rating at each of the 6 recordings remained in the range typical for normal subjects and was well below scores seen in patients with apnea or narcolepsy.27
Sleep Schedules Changed with Age
Bedtimes
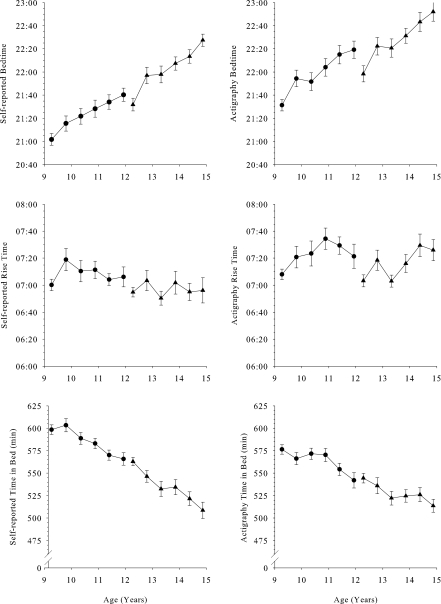
As shown in Figure 2, bedtimes became later with age. The trends were very similar for actigraphy and self-reported bedtimes, but the actigraphy-determined bedtime averaged about 30 minutes later than self-reported bedtime. Evaluated with mixed-effect analysis, the C9 self-reported bedtime (21:03 ±:06 at age 9) became 12.9 (± 1.8) minutes later each year (F1,149 = 50.6, P < 0.0001). The C9 actigraphy-determined bedtime (21:34 ±:07 at age 9) became 15.4 (± 2.2) minutes later each year (F1,144 = 48.9, P < 0.0001). In the C12 cohort, self-reported bedtime (21:33 ±:06 at age 12) became 18.5 (± 1.8) minutes later per year (F1,183 = 106, P < 0.0001), and the actigraphy-determined bedtime (21:58 ±:07 at age 12) became 19 (± 2.2) minutes later per year (F1,172 = 74.6, P < 0.0001).
Figure 2.
Mean (± SEM) self-reported and actigraphy determined sleep-schedule measurements are plotted against age with format similar to Figure 1. With bedtime becoming later with age and rise time changing little, time in bed declined significantly with age in both the C9 and C12 cohorts.
Rise times
Age trends in rise times were much less pronounced than the age trends in bedtimes. In C9, neither self-reported (F1,149 = 0.2, P = 0.70) nor actigraphy-determined (F1,144 = 5.0, P = 0.026) rise time changed significantly with age. In C12, self-reported rise times did not change significantly with age (F1,182 = 0.03, P = 0.87), but the actigraphy-determined rise times (07:03 ±:06 at age 12) became 8.5 (± 2.7) minutes later per year (F1,172 = 9.7, P = 0.0021). Actigraphy rise time was about 15 minutes later than self-reported rise time. Mixed-effect analysis can distinguish the relationship of multiple time-linked variables. We evaluated the relationship of rise time to both age and bedtime. In C12, with bedtime controlled, actigraphy-determined rise time was unrelated to age (F1,171 = 0.00, P = 1). In contrast, with age controlled, rise time became 27 (± 4) minutes later for every hour delay in bedtime (F1,171 = 54.88, P < 0.0001). Applying the same analysis to self-reported rise time gave similar results. With age controlled, rise time became 30 (± 5) minutes later for every hour delay in self-reported bedtime (F1,181 = 36.24, P < 0.0001).
Time in Bed
Calculated as the time between bedtime and rise time, both actigraphy and self-reported time in bed decreased in both cohorts. In C9, self-reported time in bed decreased 13.7 (± 2.0) minutes per year (F1,149 = 47.5, P < 0.0001) from 607 (± 6) minutes at age 9, and actigraphy-determined time in bed decreased 10.5 (± 2.4) minutes per year (F1,144 = 19.6, P < 0.0001) from 580 (± 6) minutes at age 9. In C12, self-reported time in bed decreased 18.8 (± 6.7) minutes per year (F1,182 = 53.1, P < 0.0001) from 564 (± 7) minutes at age 12, and actigraphy determined time in bed decreased 10.2 (± 2.7) minutes per year (F1,172 = 14.22, P = 0.0002) from 545 (± 6) minutes at age 12.
Sleep Duration Declined with Age
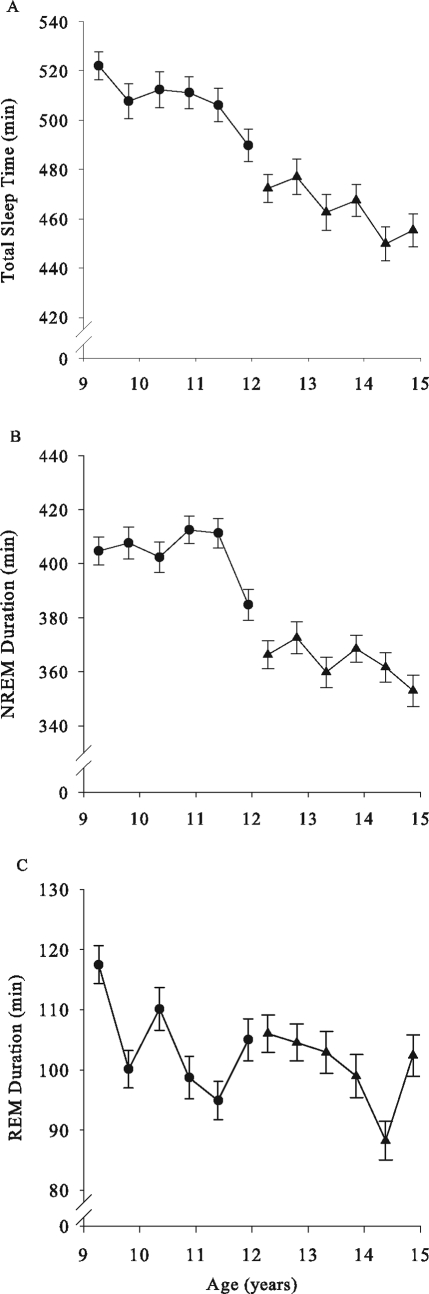
Total sleep time on school nights, measured polygraphically as NREM + REM duration, declined significantly across the 3 years of recording in both C9 and C12 (F1,149 = 14.5, P = 0.0002; F1,179 = 12.4, P = 0.0006). In C9, total sleep time declined 6% from a mean of 522 (± 6) minutes at the first recording at age 9 to 490 (± 8) minutes at the sixth recording at age 12 (Fig 3A). In C12, between the first and sixth recordings (ages 12 to 15), total sleep time declined 3.6% (472 ± 6 minutes to 455 ± 7 minutes) (Fig 3A). In C9, the NREM duration decline (Fig 3B) was entirely due to a 26-minute drop between the fifth and sixth recordings, and the decline over the 3 years did not reach significance (F1,149 = 4.6, P = 0.033). In C12, NREM duration (F1,179 = 7.3, P = 0.0076) declined significantly between ages 12 and 15 (Fig 3B). REM sleep duration declined across the 3 years of recording in C9 (F1,149 = 10.8, P = 0.0013) and in C12 (F1,179 = 11.1, P = 0.0011) (Figure 3C).
Figure 3.
Mean (± SEM) sleep durations are plotted against age with format as in Figure 1. Total sleep time declined significantly with age in both cohorts. Non-rapid eye movement sleep duration declined significantly in C12 but did not in C9. Despite a large increase in rapid eye movement duration at the sixth recording in C12, rapid eye movement sleep duration declined with age in both cohorts.
NREM EEG Power Density Declined with Age
Delta power density (DPD) showed a significant negative relationship to age in both C9 (F1,153 = 10.2, P = 0.0017) and C12 (F1,188 = 355, P < 0.0001). In C9, DPD was stable between ages 9 and 11 and then declined between ages 11 and 12 (Fig 4A). In the C12 cohort, DPD declined linearly from age 12 to 15 years (Figure 4A). The mixed-effect analysis estimated that DPD declined at a rate of 10,300 (± 500) μV2seconds per year from 69,400 (± 3,300) μV2seconds at age 12. The DPD decline was remarkably steep across this age range, dropping 42% over the 3- year period between the first and sixth recordings.
The decline in the EEG power density was not limited to the delta frequencies. In C12 between ages 12 and 15, power density declined significantly in all frequency bands analyzed between 0.3 and 30 Hz. Between ages 12 and 15, theta (4–8 Hz) power density declined significantly (F1,188 = 272, P < 0.0001) at a rate of 1,100 (±100) μV2seconds per year from 7,900 (± 400) μV2seconds at age 12. The 40% decline in theta power density between ages 12 and 15 (Figure 4B) was as large as that in DPD. It is important to note that, although DPD did not decline significantly in the 4 recordings between 9 and 11 years, theta power density declined significantly (F1,92 = 18.7, P < 0.0001) across the same age range.
As would be expected from the NREM DPD decline and NREM duration decline, total baseline NREM delta power (Figure 4C) declined significantly with age in both the C9 (F1,149 = 10.9, P = 0.0012) and C12 (F1,179 = 272, P < 0.0001) cohorts. Over the 3-year period, the average total NREM delta power declined by 10% in C9 and by 44% in C12.
Relationships Between Sleepiness and Other Time Dependent Variables
In C9, sleepiness was related only to age (F1,149 = 12.6, P = 0.0005). Sleepiness was not related to changes in pubertal stage or to any sleep-schedule variable, sleep-duration variable, or EEG variable. Table 2 shows that the story was different for C12. In this cohort, sleepiness was significantly related to pubertal stage, self-reported time in bed, self-reported bedtime, actigraphy-determined bedtime, DPD, all-night total delta power, and theta power density. Sleepiness was not related to self-reported or actigraphy-determined rise time or to actigraphy-determined time in bed. Curiously, daytime sleepiness was not significantly related to the changes in sleep-duration measures (NREM minutes, REM minutes, total sleep time) in either cohort.
Table 2.
For Cohort C12, Mixed-Effect Analysis of Relationship Between Subjective Sleepiness and Age, Pubertal Status, Sleep-Schedule, Sleep-Duration, and Electroencephalogram Predictor Variables
| Predictor Variable (fixed) | F | P |
|---|---|---|
| Age | 42.1 | < 0.0001 |
| Tanner stage | 14.9 | 0.0002 |
| Bedtime (self-reported) | 13.1 | 0.0004 |
| Rise time (self-reported) | 2.8 | 0.098 |
| Time in bed (self-reported) | 21.0 | < 0.0001 |
| Bedtime (actigraphy) | 7.1 | 0.0086 |
| Rise time (actigraphy) | 1.3 | 0.25 |
| Time in bed (actigraphy) | 1.8 | 0.18 |
| NREM duration | 1.3 | 0.26 |
| REM duration | 1.5 | 0.22 |
| TST (NREM + REM) | 2.0 | 0.16 |
| Delta (1–4 Hz) power density | 21.8 | < 0.0001 |
| Total delta (1–4 Hz) power | 27.9 | < 0.0001 |
| Theta (4–8 Hz) power density | 39.8 | < 0.0001 |
NREM refers to non-rapid eye movement sleep; REM, rapid eye movement sleep
As stated above, mixed-effect analysis can determine the relationship between an outcome variable (sleepiness) and multiple time-dependent predictor variables. For both C9 and C12, age was the dominant predictor variable for sleepiness. With age controlled, DPD, sleep-duration measures, sleep-schedule measures, and sexual maturation were unrelated to sleepiness. In contrast, the relationship between sleepiness and age remained strong with these other variables controlled.
We next removed age from the model and used mixed-effect analysis to test the main hypothesis of this article, whether the increase in sleepiness was related to the DPD decline independently of changes in sleep schedule. These results are presented in Table 3. In each analysis, sleepiness was strongly related to DPD with the sleep-schedule measure controlled. Thus, with sleep-schedule changes controlled, increasing sleepiness was still significantly related to declining DPD. With DPD controlled, only self-reported time in bed was significantly related to sleepiness. Other sleep-schedule measures, sexual maturation, and sleep-duration measures were unrelated to sleepiness with DPD controlled.
Table 3.
Results of Mixed-Effect Analyses Distinguishing the Effect of Delta (1–4 Hz) Power Density and Sleep Schedule Variables on Sleepiness in the C12 Cohort
| Delta power density |
Sleep-schedule variable |
|||
|---|---|---|---|---|
| Schedule Variable | F | P | F | P |
| Bed time (self-reported) | 19.1 | < 0.0001 | 2.3 | 0.13 |
| Rise time (self-reported) | 29.9 | < 0.0001 | 1.7 | 0.19 |
| Time in bed (self-reported) | 16.3 | < 0.0001 | 6.9 | 0.0092 |
| Bed time (actigraphy) | 24.2 | < 0.0001 | 0.4 | 0.54 |
| Rise time (actigraphy) | 31.2 | < 0.0001 | 0.8 | 0.37 |
| Time in bed (actigraphy) | 30.2 | < 0.0001 | 0.1 | 0.70 |
These analyses show that sleepiness is strongly related to delta power density (DPD) when each measure of the sleep schedule is controlled. Self-reported time in bed is the only sleep-schedule variable that retains a significant relationship to sleepiness when DPD is controlled.
As shown in Table 2, in C12, the F value for the relationship between sleepiness and theta power density, 39.8, was higher than that for sleepiness and DPD, 27.9. We, therefore, tested whether increasing sleepiness was more strongly related to declining delta or theta power density. With the decline in DPD controlled, increasing sleepiness was significantly (F1,185 = 6.87, P = 0.0095) related to declining theta power density. With the decline in theta power density controlled, sleepiness was not significantly related to declining DPD (F1,186 = 0.01, P = 0.94). In similar analyses with alpha, sigma, low beta, and high beta frequency bands, the relationship between delta and sleepiness persisted. Only theta was more strongly related to sleepiness than was delta.
DISCUSSION
The longitudinal data presented here support our hypothesis that the brain changes indexed by declining DPD contribute to increasing daytime sleepiness. Increasing sleepiness was strongly related to declining NREM delta power even with the changes in sleep schedule statistically controlled. Before interpreting this relationship, we briefly discuss the longitudinally measured changes in sleepiness, sleep schedule, sleep duration, and EEG power. In general, these longitudinal data confirm previous findings from studies that were primarily cross-sectional. Our data show that, across early adolescence, sleepiness increases, bedtimes become later, sleep durations decrease, and EEG power density declines.
Sleepiness Increases Across Adolescence
Our longitudinal data confirm previous findings that sleepiness increases with age across early adolescence. In pioneering studies, Carskadon et al13, 33 documented with the MSLT that adolescents were sleepier during the day than were preadolescents even when time in bed was set at 10 hours for both groups. They attributed the increasing sleepiness to pubertal development (Tanner Stage); however, they did not indicate how age and pubertal development effects were separated. Our longitudinal data show that subjective sleepiness is strongly related to age rather than pubertal development when the contributions of these variables are separated with mixed-effect analysis.
Change in Sleep Schedule with Age
Cross-sectional surveys of children in many industrialized societies have documented changes in sleep schedule in adolescence. Studies from Canada,3,5 Finland,7 Iceland,9 Japan,8 Korea,11 Taiwan2, Switzerland,4 and the United States10 have found later bedtimes with increasing age or grade level. Results were mixed as to whether rise times changed with age. Our longitudinal finding that bedtimes become progressively later on school nights in both the C9 and C12 cohorts fits well with these data. Our longitudinal data also confirm the cross-sectional finding that, even though rise time does not change significantly with age, it is significantly related to bedtime.6 Thus, even on school days, as adolescents go to bed later at night, they rise later the following morning. However, the change in rise time is smaller than the change in bedtime. As others have previously documented, a decline in sleep duration accompanies these changes in sleep schedule.
NREM EEG Power Density Declines with Age
We recently reported that NREM DPD does not change between ages 9 and 11 but declines steeply between ages 12 and 14.19 The current study expands the time frame of these longitudinal recordings by 1 year. In the C9 cohort, DPD declines significantly between ages 11 and 12, confirming our supposition that this age range would mark the start of the DPD decline. In the C12 cohort, the linear DPD decline we previously described for ages 12 to 14 continues through age 15. Total NREM delta power also declines steeply between ages 12 and 15 years. Total delta power is the product of all night DPD and NREM duration. If the homeostatic model of delta is correct, total delta power reflects the total nightly brain recuperation. The data show that this index of recuperation changes with age in nearly the exact same manner as DPD in the first 5 hours of NREM. The similarity of the age effects on these 2 measures is not surprising. For the C12 cohort, the first 5 hours of NREM contain 90% to 95% of the total delta in the night.
The steep linear delta decline (∼10,000 μV2second per year) observed in C12 between ages 12 and 15 cannot be maintained across the remainder of adolescence. At this rate, DPD would reach zero before age 20. We hypothesize that the decline will slow markedly shortly after age 15. If so, the ages 12 to 15 will mark the peak period of the maturational brain reorganization reflected by the delta EEG decline.
The power density decline is not limited to the delta frequencies. Power density in all frequency bands between 0.3 and 30 Hz decline significantly between ages 12 and 15. This decline is greatest in frequencies less than 12 Hz, the same frequencies that respond homeostatically to sleep deprivation.34 Sleep EEG changes across childhood and adolescence have also been noted with cyclic alternating pattern analysis.35,36 Cyclic alternating pattern subtype A1 is lower in teenagers than in children and lower in adults than in teenagers.
As noted in the beginning of this paper, we propose that the DPD decline reflects adolescent brain maturation driven by synaptic pruning.15,16 A decrease in synaptic connectivity could decrease slow-wave EEG activity by 2 mechanisms. First, decreases in connectivity would decrease the size of the pool of neurons capable of synchronous oscillation. The amplitude of the EEG recorded at the scalp partly depends on the number of cortical neurons oscillating in unison. This mechanism should affect the amplitude of the entire EEG spectrum. Second, synaptic pruning should reduce the homeostatic need for sleep. Decreased connectivity would reduce homeostatic need because it would reduce the intensity of waking brain activity; waking brain activity along with waking duration determine the need for recuperation.17 The fact that age-related changes in synaptic density parallel the decline in waking cerebral metabolic rate16 is consistent with our view. Thus, as synaptic connectivity and the intensity of waking brain activity decline across adolescence, the need for the sleep-dependent recuperation (and delta EEG) diminishes. This mechanism should disproportionately reduce power in homeostatic EEG frequencies. These include, notably, theta as well as delta EEG.34
In C12, theta power density declined across ages 12 to 15 years in parallel with DPD. However, the patterns of decline differed in the C9 cohort. C9 showed a robust decrease in theta across ages 9 to 11 years, an age range across which delta did not change. This dissociation has not previously been observed.
One would expect theta to be affected by both mechanisms proposed above to explain the delta decline. It seems possible that different processes or sites govern the generation of theta waves and delta waves. This proposal is consistent with findings from multilead recordings of EEG topography that show a frontal predominance for NREM delta and an occipital predominance for NREM theta.37 The cyclic alternating pattern A1 subtype also shows frontal predominance.38 Although the EEG amplitude recorded at an electrode is affected by volume conduction of activity from other areas of the brain as well as from the areas under the electrode, the different EEG topography of delta and theta supports the idea that these frequencies are generated in different brain areas. Therefore, the different patterns of theta and delta decline across adolescence may represent differences in the timing of synaptic pruning in different brain regions. A longitudinal structural magnetic resonance imaging study found different patterns of change in grey-matter thickness across adolescence that were attributed to regional differences in synaptic pruning.39 The decline in frontal grey-matter thickness closely matches the timing and the sex difference in the delta EEG decline. Parietal grey-matter thickness begins to decline slightly earlier than does frontal, and occipital grey-matter thickness increases across adolescence.
Although our working hypothesis is that synaptic pruning causes the adolescent EEG power density decline, we acknowledge that other factors may be involved. Other cerebral factors could decrease the average membrane change per neuron, an effect that would also reduce EEG amplitudes. However, it is unlikely that extracerebral factors such a skull thickening can explain the adolescent EEG power-density decline. Such factors could not explain the different behavior of theta and delta in C9 and could not explain our recent finding that delta-wave incidence, as well as amplitude, declines significantly across adolescence.
Increasing Sleepiness is Related to Changing Sleep Schedule
In the C12 cohort, increasing sleepiness was related to both actigraphy and self-reported bedtimes. Sleepiness was also related to self-reported time in bed. Surprisingly, we found no significant relationship between sleepiness and any measure of sleep duration (total sleep time, NREM duration, or REM duration). Therefore, the relationships between increasing sleepiness and sleep-schedule changes are not due to the decline in sleep duration associated with the change in schedule. This result is less surprising when one considers that, in C12, average total sleep time declined by only 17 minutes between ages 12 and 15, whereas bedtime became 56 minutes later. In addition to the difference in the magnitudes of the sleep-duration and bedtime changes, we propose 2 possible explanations for the paradox that sleepiness is related to bedtime but not sleep duration.
Sleepiness is related to bedtime but not sleep duration because a circadian phase shift produces both later bedtimes and daytime sleepiness. Relative to preadolescents, adolescents have a phase shift to later times.1, 40 This circadian phase shift either allows or causes later bedtimes. The phase shift, accompanied by a fixed wake time, would also result in waking near the peak in circadian propensity for sleepiness. This would produce intense sleepiness in the morning hours. Unfortunately we cannot yet evaluate this possibility because our sleepiness questionnaire did not address the timing of daytime sleepiness. The questionnaire has been modified so that we can examine this possibility in the C9 cohort as the subjects enter the age range of strongly increasing sleepiness.
Sleepiness is related to bedtime but not sleep duration because later bedtimes more accurately reflect the slower accumulation of sleep need. A sleep-deprivation study confirmed that, at clock times between 22:30 and 02:30, sleep latency was shorter in prepubertal children than in mature adolescents.41 Relative to preadolescents, adolescents have a slower rate of delta accumulation (“Process S” in the 2-process model42) during waking.43 The slower rate of Process S accumulation should delay the time at which Process S accumulates to the point of inducing sleep, allowing delayed bedtimes. This would be consistent with our hypothesis that decreased intensity of waking brain activity underlies the reduced rate at which the need for recuperation accumulates, and also reduces daytime arousal level. Thus, decreased waking brain activity could contribute to the relationship between sleepiness and bedtime. Decreased waking brain activity could permit increased daytime sleepiness by reducing daytime arousal level. It could also lead to later bedtimes because sleep need accumulates more slowly.
Increasing Sleepiness is Related to Declining NREM EEG Power Density
The main hypothesis of this study was that increasing daytime sleepiness would be related to declining DPD independent of changes in sleep schedule. The data here strongly support this hypothesis. In the C12 cohort, sleepiness was significantly related to DPD with each measure of sleep-schedule change controlled. However, delta was not the only EEG frequency to which sleepiness was related. Increasing sleepiness was significantly related to the decline in power in all EEG frequencies between 0.3 and 30 Hz. To our surprise, sleepiness was more strongly related to theta than to delta power. The basis for the stronger relationship to theta is not obvious. It is tempting to propose that the well-established relationships between waking theta and episodic memory and attention (cf 44,45) bear on the relationship between increasing sleepiness and declining NREM theta-power density. However, we know of no studies suggesting that NREM theta and waking theta are related. In fact, intracranial EEG recordings from epileptic patients have found that hippocampal and neocortical theta waves recorded during REM sleep and quiet waking were absent during NREM sleep.46
We speculate that adolescent brain maturation produces the relationship between increasing sleepiness and declining EEG power density. According to this speculation, prior to adolescence, more-intense waking brain activity (associated with higher cerebral metabolic rate) produces a level of arousal that precludes daytime sleepiness. We propose that this same intense waking brain activity produces a greater need for the recuperative processes of sleep, accounting for the higher levels of delta and theta EEG power in preadolescents. As synaptic pruning occurs during adolescence, EEG power density declines and waking brain activity declines in intensity, allowing daytime sleepiness to emerge. This indirect link between EEG power and sleepiness may explain why sleepiness was more strongly related to age than to any other predictor variable, i.e., waking brain activity is a missing covariate. Alternatively, the strong relationship of increasing sleepiness to declining theta may indicate that the brain regions giving rise to NREM theta are particularly important for arousal and that pruning in these regions diminish arousal levels. These 2 interpretations cannot be directly tested because current methods of measuring waking brain metabolism require radioactive tracers that preclude measurement in normal children and synaptic density measurements require histologic examination of brain sections. Until synaptic density or waking brain metabolism can be measured indirectly, it remains possible that the sleepiness increase, the delta power decrease, the synaptic connectivity decrease, and the waking brain metabolic rate decrease are independent maturational brain changes controlled by biologic age through mechanisms yet to be elucidated.
A less plausible interpretation of the relationship between increasing sleepiness and decreasing delta and theta power density is that adolescents become sleepier during the day because their nighttime sleep provides less recuperation. This explanation seems unlikely, in part, because sleepiness and delta are not tightly linked. Partial sleep deprivation strongly increases sleepiness even though delta power remains at baseline levels.47 Furthermore, delta behaves homeostatically. If the need for recuperation were to increase, DPD should increase to meet this need. We think it more likely that the declining delta reflects a declining need for brain recuperation, associated with decreasing synaptic density and waking brain metabolic rate.
Limitations
As noted above, the topography of EEG changes across adolescence could have been evaluated with a greater number of EEG electrodes. Although we present only central derivations in this paper, we did record from Fz and from O1 and O2. Future analysis of signals from these leads will provide some gross assessment of topographic changes. We also noted above that the Epworth sleepiness ratings are subjective and do not provide an indication of the timing of daytime sleepiness. It would be interesting if an objective measure, such as the MSLT, shows the same relationship to the EEG changes of adolescence. Finally, we note that although ambulatory EEG recordings provide a measure of a subject's sleep EEG in his or her natural environment, these recordings occur without the careful control provided by a laboratory setting. All of these limitations arise because of compromises we thought necessary for a longitudinal study. If we had increased the burden on the subjects, falling retention rates might have precluded the analyses necessary to address the main hypothesis of this article.
CONCLUSIONS
This study shows that increasing daytime sleepiness in adolescents is related to declining EEG power density independent of concomitant changes in sleep schedule. We found no relationship between increasing daytime sleepiness and declining sleep duration. These longitudinal data do not contradict the cross-sectional evidence that subjects who sleep less are sleepier during the day (cf 3) or that this sleepiness can impair their daytime functioning.3, 10 Our data show that the increase in sleepiness within subjects is unrelated to their sleep-duration decline (which averaged only 17 minutes between ages 12 and 15 years). These results support the hypothesis that intrinsic brain maturation processes in adolescence contribute to increasing daytime sleepiness. These findings add to the public health concerns about adolescent sleepiness that have previously focused on changes in sleep schedule. Adolescents should be aware that they have a biologic tendency toward daytime sleepiness and that insufficient nighttime sleep would compound this condition.
ACKNOWLEDGMENTS
This research was supported by PHS grant R01 MH62521. We thank UC Davis faculty member Rahman Azari for his consultation on statistical methods. We also thank Lisa Khaw who helped with scheduling and recording for the first 2 years. We also thank the subjects who volunteered for this study and their parents for their generous cooperation.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21:871–881. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- 2.Gau SF, Soong WT. Sleep problems of junior high school students in Taipei. Sleep. 1995;18:667–73. doi: 10.1093/sleep/18.8.667. [DOI] [PubMed] [Google Scholar]
- 3.Gibson ES, Powles AC, Thabane L, et al. “Sleepiness” is serious in adolescence: two surveys of 3235 Canadian students. BMC Public Health. 2006;6:116. doi: 10.1186/1471-2458-6-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: Reference Values and generational Trends. Pediatrics. 2003;111(2):302–307. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- 5.Laberge L, Petit D, Simard C, Vitaro F, Tremblay RE, Montplaisir J. Development of sleep patterns in early adolescence. J Sleep Res. 2001;10:59–67. doi: 10.1046/j.1365-2869.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- 6.Reid A, Maldonado CC, Baker FC. Sleep behavior of South African adolescents. Sleep. 2002;25:423–7. [PubMed] [Google Scholar]
- 7.Saarenpaa-Heikkila OA, Rintahaka PJ, Laippala PJ, Koivikko MJ. Sleep habits and disorders in Finnish schoolchildren. J Sleep Res. 1995;4:173–182. doi: 10.1111/j.1365-2869.1995.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 8.Shinkoda H, Matsumoto K, Park YM, Nagashima H. Sleep-wake habits of schoolchildren according to grade. Psychiatry Clin Neurosci. 2000;54:287–9. doi: 10.1046/j.1440-1819.2000.00681.x. [DOI] [PubMed] [Google Scholar]
- 9.Thorleifsdottir B, Bjornsson JK, Benediktsdottir B, Gislason T, Kristbjarnarson H. Sleep and sleep habits from childhood to young adulthood over a 10-year period. J Psychosom Res. 2002;53:529–37. doi: 10.1016/s0022-3999(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 10.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Development. 1998;69:875–887. [PubMed] [Google Scholar]
- 11.Yang CK, Kim JK, Patel SR, Lee JH. Age-related changes in sleep/wake patterns among Korean teenagers. Pediatrics. 2005;115:250–6. doi: 10.1542/peds.2004-0815G. [DOI] [PubMed] [Google Scholar]
- 12.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 13.Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, Dement WC. Pubertal changes in daytime sleepiness. Sleep. 1980;2:453–460. doi: 10.1093/sleep/2.4.453. [DOI] [PubMed] [Google Scholar]
- 14.Coble PA, Reynolds CF, III, Kupfer DJ, Houck P. Electroencephalographic sleep of healthy children. Part II: Findings using automated delta and REM sleep measurement methods. Sleep. 1987;10:551–562. [PubMed] [Google Scholar]
- 15.Feinberg I, March JD, Flach K, Maloney T, Chern WJ, Travis F. Maturational changes in amplitude, incidence and cyclic pattern of the 0 to 3 Hz (Delta) electroencephalogram of human sleep. Brain Dysfunction. 1990;3:183–192. [Google Scholar]
- 16.Feinberg I, Thode HC, Chugani HT, March JD. Gamma distribution model describes maturational curves for delta wave amplitude, cortical metabolic rate and synaptic density. Journal of Theoretical Biology. 1990;142:149–161. doi: 10.1016/s0022-5193(05)80218-8. [DOI] [PubMed] [Google Scholar]
- 17.Feinberg I. Changes in sleep cycle patterns with age. Journal of Psychiatric Research. 1974;10:283–306. doi: 10.1016/0022-3956(74)90011-9. [DOI] [PubMed] [Google Scholar]
- 18.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Feinberg I, Higgins LM, Khaw WY, Campbell IG. The adolescent decline of NREM delta, an indicator of brain maturation, is linked to age and sex but not to pubertal stage. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1724–9. doi: 10.1152/ajpregu.00293.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebling FJ. The neuroendocrine timing of puberty. Reproduction. 2005;129:675–83. doi: 10.1530/rep.1.00367. [DOI] [PubMed] [Google Scholar]
- 21.Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Annals of Neurology. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- 22.Darchia N, Campbell IG, Tan X, Feinberg I. Kinetics of NREM delta EEG power density across NREM periods depend on age and on delta band designation. Sleep. 2007;30:71–79. doi: 10.1093/sleep/30.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring Systems for Sleep Stages of Human Subjects. Washington, D.C.: Public Health Services, U.S.Government Printing Office; 1968. [Google Scholar]
- 24.Grumbach MM, Styne DM. Puberty: Ontogeny, neuroendocrinology, physiology, and disorders. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR, editors. Williams Textbook of Endocrinology. 9th ed. Philadelphia: W.B. Saunders Company; 1998. pp. 1509–1551. [Google Scholar]
- 25.Carskadon MA, Dement WC. The multiple sleep latency test: What does it measure? Sleep. 1982;5:s67–s72. doi: 10.1093/sleep/5.s2.s67. [DOI] [PubMed] [Google Scholar]
- 26.Mitler MM, Gujavarty KS, Sampson MG, Browman CP. Multiple daytime nap approaches to evaluating the sleepy patient. Sleep. 1982;5:s119–s127. doi: 10.1093/sleep/5.s2.s119. [DOI] [PubMed] [Google Scholar]
- 27.Johns MW. A new method for measuring sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 28.Melendres MC, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004;114:768–75. doi: 10.1542/peds.2004-0730. [DOI] [PubMed] [Google Scholar]
- 29.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: failure of the MSLT as a gold standard. Journal of Sleep Research. 2000;9:5–11. doi: 10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 30.Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics. 1998;24:323–355. [Google Scholar]
- 31.Twisk J. Applied longitudinal data analysis for epidemiology. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- 32.Willett JB, Singer JD, Martin NC. The design and analysis of longitudinal studies of development and psychopathology in context: statistical models and methodological recommendations. Dev Psychopathol. 1998;10:395–426. doi: 10.1017/s0954579498001667. [DOI] [PubMed] [Google Scholar]
- 33.Carskadon MA, Orav EJ, Dement WC. Evolution of sleep and daytime sleepiness in adolescents. In: Guilleminault C, Lugaresi E, editors. Sleep/Wake disorders: natural history, epidemiology, and long-term evolution. New York: Raven Press; 1983. pp. 201–216. [Google Scholar]
- 34.Borbely A, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep-deprivation: effect on sleep stages and EEG power density in man. Electroencephalography and clinical Neurophysiology. 1981;51:483–493. doi: 10.1016/0013-4694(81)90225-x. [DOI] [PubMed] [Google Scholar]
- 35.Bruni O, Ferri R, Miano S, et al. Sleep cyclic alternating pattern in normal school-age children. Clin Neurophysiol. 2002;113:1806–14. doi: 10.1016/s1388-2457(02)00265-1. [DOI] [PubMed] [Google Scholar]
- 36.Parrino L, Boselli M, Spaggiari MC, Smerieri A, Terzano MG. Cyclic alternating pattern (CAP) in normal sleep: polysomnographic parameters in different age groups. Electroencephalogr Clin Neurophysiol. 1998;107:439–50. doi: 10.1016/s0013-4694(98)00108-4. [DOI] [PubMed] [Google Scholar]
- 37.Finelli L, Borbely AA, Achermann P. Functional topography of the human nonREM sleep electroencephalogram. European Journal of Neuroscience. 2001;13:2282–2290. doi: 10.1046/j.0953-816x.2001.01597.x. [DOI] [PubMed] [Google Scholar]
- 38.Ferri R, Bruni O, Miano S, Terzano MG. Topographic mapping of the spectral components of the cyclic alternating pattern (CAP) Sleep Med. 2005;6:29–36. doi: 10.1016/j.sleep.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 40.Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms. 1997;12:278–89. doi: 10.1177/074873049701200309. [DOI] [PubMed] [Google Scholar]
- 41.Taylor DJ, Jenni OG, Acebo C, Carskadon MA. Sleep tendency during extended wakefulness: insights into adolescent sleep regulation and behavior. J Sleep Res. 2005;14:239–44. doi: 10.1111/j.1365-2869.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- 42.Borbely A. A two-process model of sleep regulation. Human Neurobiology. 1982;1:195–204. [PubMed] [Google Scholar]
- 43.Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–54. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- 44.Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci Lett. 1999;259:165–8. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- 45.Klimesch W, Schimke H, Schwaiger J. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalogr Clin Neurophysiol. 1994;91:428–41. doi: 10.1016/0013-4694(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 46.Cantero JL, Atienza M, Stickgold R, Kahana MJ, Madsen JR, Kocsis B. Sleep-dependent theta oscillations in the human hippocampus and neocortex. J Neurosci. 2003;23:10897–903. doi: 10.1523/JNEUROSCI.23-34-10897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]