Abstract
Study Objectives:
To compare the time structure of leg movements (LM) during sleep of patients with rapid eye movement (REM) sleep behavior disorder (RBD) with that of patients with restless legs syndrome (RLS) or control subjects.
Design:
The polysomnographically recorded tibialis anterior activity during sleep was analyzed by means of a new approach able to consider duration, intermovement interval, sleep stage and time of night distribution, and periodicity.
Patients and Participants:
Twenty patients with idiopathic RBD, 37 with idiopathic RLS and 14 age-matched control subjects were consecutively recruited.
Measurements and Results:
Most patients with RBD (85%) presented periodic leg movements during sleep (PLMS). PLMS occurred more frequently during non-REM sleep in patients with RLS and during REM sleep in patients with RBD. PLMS were shorter in duration, less often bilateral, and with a higher intermovement interval in patients with RBD compared to those with RLS. The number of PLMS decreased across the night in patients with RBD and in those with RLS, but not in control subjects. In all subjects, LM periodicity clearly depended on sleep state, with higher values during non-REM than during REM sleep. Patients with RBD showed a lower LM periodicity, compared with patients with RLS, in each of the sleep states.
Conclusions:
Significant differences, together with some similarities in LM time structure, were observed between patients with RBD and those with RLS; for this reason, our approach seems to indicate that their phenotype might be dependent on 2 factors: disease and sleep stage.
Citation:
Manconi M; Ferri R; Zucconi M; Fantini ML; Plazzi G; Ferini-Strambi L. Time structure analysis of leg movements during sleep in REM sleep behavior disorder. SLEEP 2007;30(12):1779-1785.
Keywords: REM sleep behavior disorder, restless legs syndrome, periodic leg movements, sleep
INTRODUCTION
PERIODIC LEG MOVEMENTS DURING SLEEP (PLMS) ARE REPETITIVE LEG JERKS CHARACTERIZED BY A TRIPLE FLEXION OF LOWER LIMBS, WHICH ARE RECORDED during standard polysomnography by placing 2 electrodes over each anterior tibialis muscle.1 All anterior tibialis bursts with a duration from 0.5 to 51 or 102 seconds, separated by intervals ranging from 5 to 90 seconds and included in series of 4 or more consecutive leg movements (LM), are scored as PLMS. The pathologic meaning of PLMS is still debated; they are often associated with both autonomic and cortical electroencephalogram arousals, but the causal relationship between PLMS and sleep disruption is unclear.3–6 Some findings, in particular the rapid and dramatic suppression after a single low dose of a dopamine-agonist, suggest a possible role for a dopaminergic dysfunction in the pathogenesis of PLMS in patients with restless legs syndrome (RLS).7–9
Although they occur mainly in patients affected by RLS, PLMS can also be found in other sleep or neurologic disorders and in healthy subjects, especially in the elderly.10 Among sleep disorders, narcolepsy, sleep apnea syndrome, and rapid eye movement (REM) sleep behavior disorder (RBD) are well-established pathologic conditions associated with PLMS.11–13 In these disorders, specific PLMS features, such as duration, amplitude, periodicity, and distribution across the night, may be considerably different from those of RLS. For instance, it has been recently demonstrated that, in narcoleptic patients, LM are significantly less periodic and equally distributed across the night, compared with those of patients with RLS, who present highly periodic LM progressively decreasing in number throughout the night.14 These time-structure differences have been detected by applying a new computer-assisted analysis of nocturnal leg motor activity that is able to perform a new qualitative and quantitative differentiation between LM patterns in different disorders.15,16 The traditional method used to analyze PLMS is insufficient to capture the details of the differences in the electromyographic (EMG) signal, such as LM periodicity, area under the curve, distribution of intermovement interval, and in general LM time structure during sleep.16 These particular LM features may reflect a modulation effect of specific diseases on the same PLMS phenomenon; alternatively, they might suggest a different origin of LM in terms of mechanisms and pathogenesis. The presence of PLMS is not specific for a particular disorder; the time structure and qualitative pattern of PLMS, however, might be disease related. A detailed LM analysis has never been carried out in patients with RBD.
About 70% of patients with idiopathic RBD have a PLMS index (number of PLMS per hour of sleep) greater than 10, which, in the past, has been usually been considered to be pathologic.13 PLMS are not the sole feature shared by patients with RBD and RLS; in fact, in both diseases, a dopaminergic dysfunction is postulated to play an important role in the pathogenesis.9,17,18 Moreover, idiopathic forms of RBD may evolve into neurodegenerative α-synucleinopathies such as Parkinson disease, in which dopamine dysfunction is well demonstrated and the prevalence of PLMS is higher than in the general population.19,20
The aim of this study was to describe, in detail, LM activity during sleep in a group of patients with idiopathic RBD in comparison to age-matched healthy control subjects and patients with RLS.
METHODS
Subjects
A total of 71 adult subjects were recruited for this study, 20 (mean age 70.1, SD 4.6; 17 men, and 3 women) affected by RBD, 37 (mean age 67.8, SD 5.3; 17 men and 20 women) with RLS, and 14 (mean age 66.5, SD 5.9; 6 men and 8 women) control subjects. One subject, out of the 14 controls, reported that at least 1 of his first-degree relatives had RLS symptoms. A positive family history for RLS was reported by 51.3% (19 patients) of the subjects with RLS, whereas no patients with RBD reported RLS in their families. All subjects were medication free for at least 8 weeks prior to polysomnography. The diagnosis of RBD was based on the International Classification of Sleep Disorders21 criteria for RBD, including the presence of REM sleep without atonia, sleep-related injurious or disruptive behaviors by history or abnormal sleep behaviors documented during polysomnographic monitoring, absence of electroencephalogram epileptiform activity during REM sleep, and sleep disturbance not better explained by another sleep disorder, medical or neurologic disorder, mental disorder, medication use, or substance use disorder. Secondary forms of RBD were excluded on the basis on historical data, neurologic examination, hematologic tests, and encephalic magnetic resonance imaging findings. All patients with RBD with at least 1 subtentorial vascular lesion, at least 2 vascular supratentorial lesions greater than 0.5 cm, or at least 1 supratentorial lesion greater than 2 cm were excluded. Patients with RBD who reported symptoms that could indicate the presence of RLS were also excluded.
Inclusion criteria for RLS were those of the International RLS Study Group (IRLSSG); (1) leg restlessness, usually accompanied or caused by uncomfortable and unpleasant sensations in the legs; (2) beginning or worsening of this unpleasant sensation during rest or inactivity such as lying or sitting; (3) partial or total relief of the unpleasant sensations by movement, and (4) worsening or occurrence of the unpleasant sensations in the evening or night, compared to daytime.22 To be included in the study, the mean frequency of symptoms during the last 6 months had to be greater than 2 times per week. On the basis of historic data, neurologic examination, hematologic tests (including serum iron, transferrin and ferritin), and neurophysiologic investigation (EMG and electroneurography of the lower limbs), all patients with RLS with symptomatic forms were excluded. A mean score of 25.4 (SD 6.31) was obtained applying the IRLSSG rating scale23 to this group.
Patients with idiopathic RBD were consecutively enrolled from our sleep center, whereas patients with RLS were consecutively chosen among those admitted to our center in order to obtain a group with an age comparable to that of subjects with RBD.
Fourteen subjects served as a control group. None had any physical, neurologic or psychiatric disorder, or history of sleep problems, and none was taking medication at the time of recording. Exclusion criteria were (1) a sleep disorder diagnosis (including sleep apnea), (2) a major mental illness, (3) a significant history of cognitive difficulties, and (4) prior (within 1 year) or current use of a neuroleptic agent. All patients gave their written consent for the study, and the local ethics committee approved the investigation.
Nocturnal Polysomnography
All 71 subjects underwent a full-night video-polysomnographic study, which was carried out after a night of adaptation in a standard sound-attenuated (noise level to a maximum of 30 dB nHL) sleep laboratory room. Subjects were not allowed caffeinated beverages the afternoon preceding the recording and were allowed to sleep in until their spontaneous awakening in the morning. Lights-out time was based on individual habitual bed time and ranged between 2130 and 2330. The following signals were recorded: EEG (at least 3 channels, 1 frontal, 1 central and 1 occipital, referred to the contralateral earlobe); electrooculogram (electrodes placed 1 cm above the right outer cantus and 1 cm below the left outer cantus and referred to A1); electromyogram (EMG) of the submentalis muscle, EMG of the right and left tibialis anterior muscles (bipolar derivations with 2 electrodes placed 3 cm apart on the belly of the anterior tibialis muscle of each leg, impedance was kept less than 10 KΩ; and electrocardiogram (1 derivation). Sleep signals were sampled at 200 Hz and stored on hard disk in European data format (EDF, see Kemp et al.24 for details) for further analysis. Electromyogram signals, in particular, were digitally band-pass filtered at 10 to 100 Hz, with a notch filter at 50 Hz. The sleep respiratory pattern of each patient was monitored using oral and nasal airflow thermistors and/or nasal pressure cannula, thoracic and abdominal respiratory effort strain gauge, and pulse oximetry (oxygen saturation). This was performed in all subjects in a previous recording (within 1 week) or during the study recording; patients with an apnea-hypopnea index of 5 or greater were not included.
Sleep Scoring and Detection of Leg Movements
Prior to any recording, we verified that the EMG amplitude recorded from the 2 anterior tibialis muscles was below 2 μV at rest and exceeded 7 to 10 μV for small voluntary flexions of the foot. Sleep stages were visually scored following standard criteria25 at 30-second intervals using the sleep analysis software Hypnolab 1.2 (SWS Soft, Italy). LM during sleep were first detected by the same software that allows their computer-assisted detection. With this software, the detection is performed using a human-supervised automatic approach15 controlled by the scorer that uses World Association of Sleep Medicine-IRLSSG criteria.2 The performance of this system has been evaluated and validated, but, in this study, 1 scorer visually edited the detections proposed by the automatic analysis before computing a final result. In particular, the total LM Index was calculated to represent the total number of leg movements per hour of sleep, whereas the PLMS index was calculated as the number of LM included in a series of 4 of more, separated by more than 5 and less than 90 seconds, per hour of sleep.
Further Analysis of LM Architecture
In this analysis, we used our newly described approach to study LM periodicity.16,15 These parameters are in line with those proposed by the World Association of Sleep Medicine -IRLSSG criteria2 and included the following. (a) Duration included all movements lasting 0.5 to 10 seconds. (b) Amplitude, as introduced above, was the amplitude of the EMG signal from the 2 anterior tibialis muscles below 2 μV at rest and exceeded 7 to 10 μV for small voluntary flexions of the foot. LM were included when the EMG increased to 8 μV or greater above the resting baseline (e.g.. 10 μV for a baseline of 2 μV, for our rectified signal). The ending point was when the EMG decreased to more than 2 μV above the resting level and remained below that value for 0.5 seconds. All these values were calculated on the rectified EMG signals. (c) Sleep stage was the leep stage in which each LM started. (d) Side was the right or left leg. (e) Start and ending time were the 2 values used for the calculation of the 2 intervals described next. (f) Interval 1 was the interval defined as the time between the onset of 2 subsequent LM and was used for the evaluation of their periodicity (see below). (g) Interval 2 was defined as the time between the end of 1 LM and the onset of the following LM; this interval was used for the separation of different LM intervening in the same leg on or the contralateral leg. (h) The minimum interval between 2 different LM was derived as follows: we applied a time resolution of 0.5 seconds for the detection of the presence of movement (see above), then we applied the same time resolution for the detection of the absence of movement. For this reason, the minimum interval between different LM (Interval 2) was set to 0.5 seconds. (i) Bilateral or unilateral movements were defined as 2 EMG bursts on the 2 legs separated (Interval 2) by at least 0.5 seconds for bilateral movements and as EMG bursts involving only 1 leg and separated by at least 0.5 seconds from any other LM for unilateral. (j) The Periodicity Index (PI) was defined as the number of sequences of 3 inter-LM intervals of at least 10 seconds but less than 91 seconds in duration divided by the total number of inter-LM intervals. This index can vary between 0 (absence of periodicity) to 1 (all intervals with lengths longer than 10 seconds but less than 91 seconds). PI is independent on the absolute number of LM recorded and was calculated for all the subjects included in this study for the total sleep period and, separately, for non-rapid eye movement (NREM) and REM sleep.
Statistical Analysis
The comparison between the different parameters derived from the classic PLMS analysis and the PI in the different patient groups was carried out by means of the nonparametric Kruskal-Wallis analysis of variance, followed by the nonparametric Mann-Whitney test for independent data sets (used as a posthoc test), in order to test the eventual differences between patients with RBD and control subjects or patients with RLS. Differences were considered statistically significant at P < 0.05. To compare the intermovement interval histograms of patients with RBD with those of patients with RLS and control subjects, we used the nonparametric Mann-Whitney test and applied a Bonferroni correction on 50 comparisons (statistical significance at P < 0.001; 0.05/50). For the comparison of the number of LM per hour of sleep of patients with RBD with that of patients with RLS and control subjects, we used a nonparametric Mann-Whitney test and applied a Bonferroni correction on 8 comparisons (statistical significance at P < 0.00625; 0.05/8). The commercially available software STATISTICA (data analysis software system), version 6, (StatSoft, Inc. Vigonza, Italy) was used for all statistical tests.
RESULTS
Regarding the sleep-staging results, except for a significant increase of slow-wave sleep percentage and of sleep instability (stage shift per hour) in the RBD group, no other differences among groups were detected. As expected, the PLMS index was greater than 15 in 34 (92%) of patients with RLS, whereas the same threshold was exceeded by 17 (85%) of the patients with RBD and by 5 (36%) of the control subjects.
As shown in Table 1, the total LM index was higher in the patients with RBD and RLS, as compared with the control subjects, but was not significantly different between the patients with RBD and RLS. These differences were due to the periodic LM component. In fact, the PLM index was significantly higher in patients with RLS and RBD, compared with normal subjects, but the isolated LM index did not differ among the 3 groups. The proportion of bilateral versus unilateral LM was higher in patients with RLS than in those RBD and, in both cases, was much higher than in control subjects. The highest value of unilateral LM was observed in the RBD group, but this was not significantly different from that of RLS and control subjects. Periodic LM were dependent on sleep states: total LM index and PLMS index were higher in patients with RLS, compared with those with RBD, during NREM sleep, whereas they were lower during REM sleep. The isolated LM index was not influenced by sleep states and was similar in the 3 groups of subjects. The number and duration of PLM sequences did not differ between patients with RLS and those with RBD. The mean duration of PLMS was shorter in the RBD than in RLS group, whereas the mean duration of isolated LM was similar in both groups. LM duration was significantly different between RBD and RLS only during NREM sleep.
Table 1.
Comparison between the traditional PLM parameters found in controls, in the group of patients with REM sleep behavior disorder and in those with restless legs syndrome
| PLM parameter | Group |
Kruskal-Wallis ANOVA P value < | Mann-Whitney test comparing P < |
|||
|---|---|---|---|---|---|---|
| 1. Controls n=14 | 2. RBD n=20 | 3. RLS n=37 | 1 vs 2 | 2 vs 3 | ||
| Index during total sleep time | ||||||
| Total LM | 26.9 (21.22) | 49.9 (31.05) | 67.5 (50.27) | 0.0015 | 0.01 | NS |
| PLMS | 18.0 (19.51) | 40.7 (30.38) | 59.6 (49.53) | 0.0007 | 0.01 | NS |
| Isolated LM | 8.9 (4.20) | 9.2 (3.88) | 7.9 (3.85) | NS | - | - |
| Number of LM during total sleep time | ||||||
| Unilateral | 124.8 (150.86) | 190.3 (108.02) | 184.4 (147.74) | NS | - | - |
| Bilateral | 26.2 (32.35) | 73.9 (48.19) | 159.3 (173.18) | 0.0001 | 0.002 | 0.04 |
| Index During NREM sleep | ||||||
| Total LM | 26.1 (18.48) | 44.7 (30.52 | 72.8 (50.42) | 0.0004 | 0.05 | 0.03 |
| PLMS | 17.5 (17.54) | 36.4 (29.67 | 65.2 (49.61) | 0.0001 | 0.05 | 0.02 |
| Isolated LM | 8.5 (4.41) | 8.3 (3.82) | 7.5 (4.20) | NS | - | - |
| Index During REM sleep | ||||||
| Total LM | 31.0 (47.21) | 66.1 (53.79) | 30.8 (36.44) | 0.0055 | 0.01 | 0.003 |
| PLMS | 19.8 (43.56) | 54.0 (55.28) | 21.8 (36.62) | 0.0077 | 0.01 | 0.007 |
| Isolated LM | 11.2 (6.41) | 12.2 (7.31) | 9.0 (7.11) | NS | - | - |
| PLMS sequences | ||||||
| Number | 7.4 (6.27) | 13.0 (8.58) | 13.4 (7.19) | 0.03 | 0.05 | NS |
| Duration, s | 104.8 (220.59) | 152.9 (239.39) | 105.2 (172.41) | NS | - | - |
| PLMS duration, s | ||||||
| During total sleep time | ||||||
| All PLMS | 2.2 (0.82) | 2.1 (0.51) | 2.6 (0.74) | 0.05 | NS | 0.026 |
| Isolated LM | 2.2 (0.90) | 2.2 (0.61) | 2.7 (0.90) | NS | - | - |
| During NREM sleep | ||||||
| All PLMS | 2.1 (0.51) | 2.2 (0.69) | 2.6 (0.74) | 0.04 | NS | 0.03 |
| Isolated LM | 2.3 (0.80) | 2.3 (0.62) | 2.7 (0.88) | NS | - | - |
| During REM sleep | ||||||
| All PLMS | 1.6 (1.48) | 2.1 (0.81) | 2.0 (1.45) | NS | - | - |
| Isolated LM | 2.0 (1.11) | 1.9 (0.96) | 2.0 (1.49) | NS | - | - |
Data are expressed as mean and SD. RBD refers to rapid eye movement sleep behavior disorder; RLS, restless legs syndrome; ANOVA, analysis of variance; PLMS, periodic limb movements of sleep; LM, leg movements.
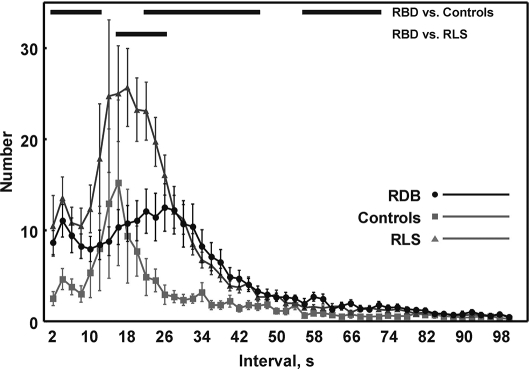
The distribution of inter-LM intervals in control subjects, patients with RBD, and patients with RLS is shown in Figure 1. Interesting differences in profiles were noted between the 3 groups. A bimodal distribution is clear for the all groups, with the first smallest peak in the range of the shortest intervals (1 < I ≤ 3 seconds). The second peak occurs at intervals around longer than 17 but shorter than 21 seconds in control subjects and in RLS subjects (higher values in RLS), whereas, in patients with RBD, it appears to be more flattened and occurs at intervals of approximately 26 seconds. The profiles of the 3 groups are indistinguishable for interval classes longer than 66 seconds. Although still clearly recognizable, the bimodal distribution was less evident in subjects with RBD. Patients with RLS had values significantly higher than patients with RBD, and these, in turn, had values significantly higher than control subjects, mostly for the first peak.
Figure 1.
Comparison between the distribution of inter-leg-movement intervals in controls, patients with rapid eye movement (REM) sleep behavior disorder (RBD) and patients with restless legs syndrome (RLS); values are shown as mean and standard error (whiskers).
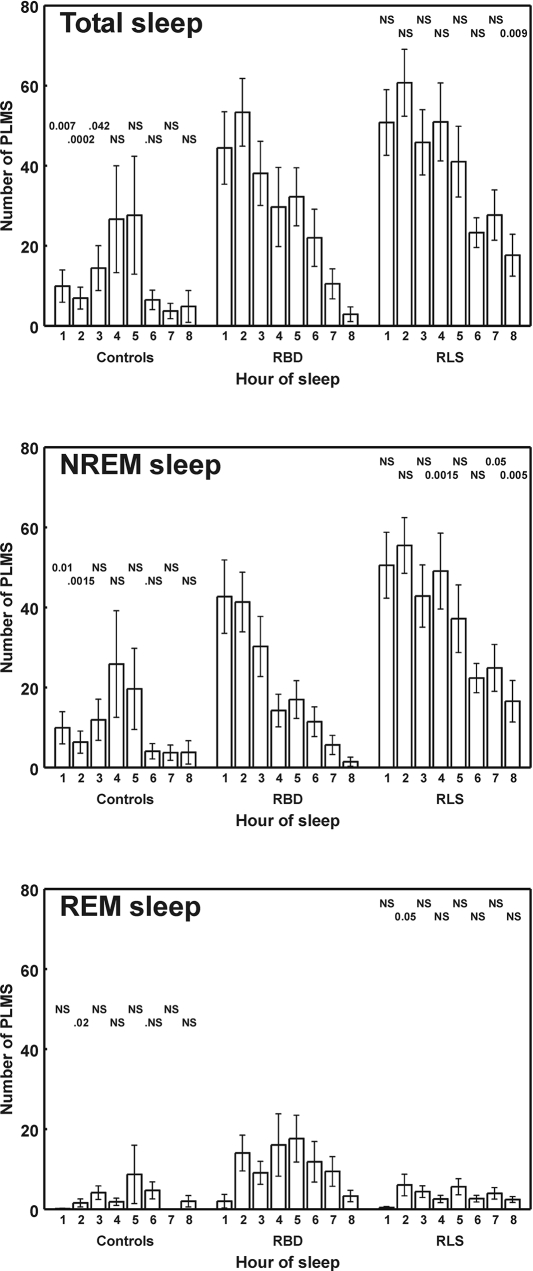
Figure 2 shows the comparison of the distribution of number of PLM per hour of sleep for the first 8 hours among control subjects and patients with RBD or RLS. A bell-shaped distribution can be seen for control subjects; in contrast, patients with RLS and RBD show a progressive decrease in the number of PLM per hour of sleep throughout the night. In both groups, the same decreasing trend is confirmed during NREM sleep, whereas it disappears when considering separately only REM sleep of patients with RBD.
Figure 2.
Comparison between the distribution of number of periodic limb movements per hour of sleep (PLMS; first 8 hours shown) in normal control subjects and patients with rapid eye movement (REM) sleep behavior disorder (RBD) or restless legs syndrome (RLS). The results of the statistical analysis of the differences between RBD and RLS or controls is shown at the top of each histogram bar (P values; NS = not significant). All values are shown as mean and standard error (whiskers). NREM refers to non-rapid eye movement sleep.
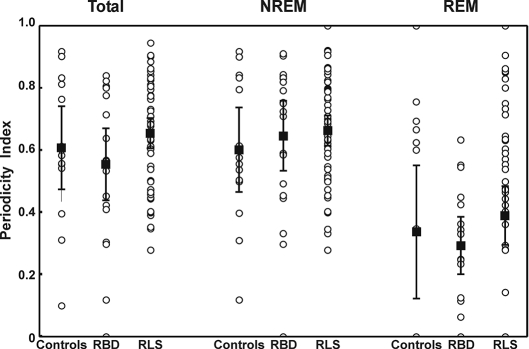
PI, which is inversely correlated to the entropy of the EMG burst time structure,16 was calculated in each of the 3 groups and is shown in figure 3. The total PI was significantly highest for patients with RLS (mean PI 0.671, SD 0.164) and lowest for patients with RBD (mean PI 0.553, SD 0.248), whereas it fell in the middle for control subjects (mean PI 0.606, SD 0.232). Different values were obtained calculating PI separately for each sleep states: during REM sleep, the distance between low PI values in RBD versus high values in patients with RLS was confirmed; NREM sleep almost abolished this difference. However, it should be noted that none of these group comparisons reached statistical significance; on the contrary, the comparison between REM and NREM PI within the same group was significant (P < 0.035 in control subjects, P < 0.00015 in patients with RBD, and P < 0.00005 in patients with RLS) by means of the nonparametric Wilcoxon test for paired data sets.
Figure 3.
Periodicity index in controls, patients with rapid eye movement (REM) sleep behavior disorder (RBD), and patients with restless legs syndrome (RLS); values are shown as mean (black-filled squares) and 95% confidence intervals (whiskers). Individual values are also shown (circles). NREM refers to non-rapid eye movement sleep.
DISCUSSION
This study confirms the high prevalence of PLMS in RBD13 and the significant differences in PLMS time structure that emerged between patients with RBD and those with RLS. First of all, PLMS were clearly associated with NREM sleep in RLS and with REM sleep in RBD, at least in terms of number per hour. Moreover, PLMS were shorter, were less often bilateral, and had with a higher intermovement interval in patients with RBD compared to those with RLS. The LM periodicity was lower in patients with RBD than in those with RLS, especially during REM sleep, even if the results were not statistically significant. The reason of the lack of statistical significance is that age is an important factor influencing periodicity of LM not only in RLS,16 but also in control subjects,26 and, in agreement with the particular age of our subjects, we found relatively high values of PI in all of our groups. Finally, the trend of the number of PLM per hour decreased similarly throughout the night in both patients with RBD and those with RLS, but, when it was calculated separately during REM and NREM sleep, it became remarkably different during REM sleep, with patients with RBD showing clearly higher values organized in a bell-shaped distribution. The above-mentioned differences were not detected when considering isolated LM, which were equally represented in all groups (RBD, RLS, and control subjects) and did not segregate with sleep states.
The high PLMS occurrence in RBD, especially during REM, might be triggered by the absence of brainstem inhibition on spinal motoneurons, which is the main feature of RBD.27
Several pieces of evidence suggest that dopaminergic dysfunction plays a crucial role in RLS and PLMS pathogenesis: both RLS and PLMS are markedly improved by low doses of dopamine agonists since the first night of treatment,7,8 they worsen after administration of antidopaminergic drugs,28 are highly represented in patients affected by Parkinson disease,29 can be induced by iron deficiency (iron is the coenzyme of the tyrosine hydroxylase, the key enzyme in the dopamine anabolism pathway),30 and finally show a circadian pattern opposite to that of serum dopamine levels.31 Also, in patients with RBD, the frequent occurrence of PLMS may be a consequence of a disease-related dysfunction in dopamine regulation. Neuroimaging investigations have demonstrated reduced striatal dopamine transporters32 and innervation18 in idiopathic RBD. Furthermore, pramipexole (a D3 preferential agonist) decreases the frequency and the intensity of REM motor episodes in patients with RBD.33
The absence of any pattern of expression across the night, together with the recent demonstrated low response after treatment by dopamine-agonists,33 indicates a separate neurotransmitter and perhaps separate pathway in the regulation of nonperiodic LM, both in RLS and RBD.
To the best of our knowledge, only 1 paper has focused on PLMS in RBD.13 The authors found that, compared with patients with RLS, PLMS are more frequent in REM sleep in patients with RBD, are not clearly responsive to pramipexole, and seem to be less frequently associated with cortical and autonomic arousals. The hypothesis of a possible impairment in cortical and autonomic reactivity to internal stimuli in RBD patients was strengthened by the tendency in idiopathic RBD to evolve in neurodegenerative disorders which may impair cognitive and vegetative functions.34 An alternative hypothesis might be that, although classified as PLMS by the standard criteria, LM in RBD may be different, at least in part, in terms of pathogenesis, neurotransmitter implication, and the central nervous system pacemaker involved, compared with those observed in RLS. The reduced response to dopamine agonists in RBD versus RLS,33 as well as the discrepancies in EMG phenotype between these 2 sleep disorders, agrees with the theory that we are in front of 2 at least partially different motor phenomena, which consequently may have different correlates in the cortical and autonomic system mechanisms and structures. This may also explain why most patients affected by Parkinson disease, although usually treated with high doses of dopamine agonists, continue to have high PLMS indexes.35,36 In these patients, the persistence of PLMS may cooperate with other causes of sleep fragmentation (sleep apnea, difficulty in changing body position, pharmacologic side effects) in disrupting hypnic structures and provoking insomnia and excessive daytime sleepiness.37
The differences between PLMS phenotype in RBD and RLS, observed by the above-mentioned study,13 and those demonstrated in the present investigation may also be explained by 2 other hypotheses: (1) PLMS in RBD and RLS may share the same pathogenic origin, but their EMG phenotypes appear diverse because of the influences operated by each disease—in other words, these differences may be disease-related; and (2) PLMS in RBD and RLS are homogeneous under the pathogenic profile, but their differences may be sleep–state-related. Although the present study does clearly support any of the previous theories, our results seem to agree better with the last hypothesis. Some of the qualitative features of PLMS, such as periodicity, but mostly their distribution across the night and mean duration, which appear to be quite different between RLS and RBD, become very similar if they are considered only during NREM sleep. From this point of view, the influence of sleep state seems to be stronger than the influence of disease. It seems that REM sleep per se, in both RLS and RBD, increases the entropy of anterior tibialis activity, maybe by deactivating a possible central pacemaker. Other periodic phenomena, such as the cortical activity known as cyclic alternating pattern,38 the respiratory pattern,39 or few autonomic functions (heart-rate variability, b-waves in systemic blood pressure, arteriolar sphinteric activity)40 become extremely irregular passing from NREM to REM sleep. Therefore, the final phenotype of the total PLMS is more influenced by REM sleep in RBD and by NREM sleep in RLS, simply because PLMS occur more frequently during REM sleep in RBD and during NREM sleep in RLS. Under this point of view, the disease per se may be responsible for moving PLMS from NREM to REM sleep and REM sleep per se in changing PLMS phenotype. In any case, our study provides neurophysiologic information on the complex interaction between the 2 main factors influencing the differential expression of PLMS in these 2 conditions: disease and sleep stage.
Further studies evaluating the LM activity in symptomatic RBD cases (i.e., Parkinson disease, Lewy-body body dementia, and multiple system atrophy) and changes in LM induced by pharmacologic treatments may contribute to explaining the differences between RBD and RLS and, eventually, to distinguishing different RBD subtypes and might have important prognostic implications.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Recording and scoring leg movements. The Atlas Task Force. Sleep. 1993;16:748–59. [PubMed] [Google Scholar]
- 2.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Sforza E, Juony C, Ibanez V. Time-dependent variation in cerebral and autonomic activity during periodic leg movements in sleep: implications for arousal mechanisms. Clin Neurophysiol. 2002;113:883–91. doi: 10.1016/s1388-2457(02)00066-4. [DOI] [PubMed] [Google Scholar]
- 4.Sforza E, Jouny C, Ibanez V. Time course of arousal response during periodic leg movements in patients with periodic leg movements and restless legs syndrome. Clin Neurophysiol. 2003;114:1116–24. doi: 10.1016/s1388-2457(03)00077-4. [DOI] [PubMed] [Google Scholar]
- 5.Winkelman JW. The evoked heart rate response to periodic leg movements of sleep. Sleep. 1999;22:575–80. doi: 10.1093/sleep/22.5.575. [DOI] [PubMed] [Google Scholar]
- 6.Ferri R, Zucconi M, Rundo F, Spruyt K, Manconi M, Ferini-Strambi L. Heart rate and spectral EEG changes accompanying periodic and non-periodic leg movements during sleep. Clin Neurophysiol. 2007;118:438–48. doi: 10.1016/j.clinph.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Manconi M, Ferri R, Zucconi M, et al. First night efficacy of pramipexole in restless legs syndrome and periodic leg movements. Sleep Med. 2007 doi: 10.1016/j.sleep.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Saletu M, Anderer P, Saletu B, et al. Sleep laboratory studies in restless legs syndrome patients as compared with normals and acute effects of ropinirole. 2. Findings on periodic leg movements, arousals and respiratory variables. Neuropsychobiology. 2000;41:190–9. doi: 10.1159/000026659. [DOI] [PubMed] [Google Scholar]
- 9.Rye DB. Parkinson's disease and RLS: the dopaminergic bridge. Sleep Med. 2004;5:317–28. doi: 10.1016/j.sleep.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Hornyak M, Feige B, Riemann D, Voderholzer U. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Med Rev. 2006;10:169–77. doi: 10.1016/j.smrv.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Nicpon KW. [Re: Restless legs syndrome and periodic limb movements during sleep in a patient with obstructive sleep apnea] Neurol Neurochir Pol. 2005;39:429–30. [PubMed] [Google Scholar]
- 12.Wetter TC, Pollmacher T. Restless legs and periodic leg movements in sleep syndromes. J Neurol. 1997;244:S37–S45. doi: 10.1007/BF03160570. [DOI] [PubMed] [Google Scholar]
- 13.Fantini ML, Michaud M, Gosselin N, Lavigne G, Montplaisir J. Periodic leg movements in REM sleep behavior disorder and related autonomic and EEG activation. Neurology. 2002;59:1889–94. doi: 10.1212/01.wnl.0000038348.94399.f6. [DOI] [PubMed] [Google Scholar]
- 14.Ferri R, Zucconi M, Manconi M, et al. Different periodicity and time structure of leg movements during sleep in narcolepsy/cataplexy and restless legs syndrome. Sleep. 2006;29:1587–94. doi: 10.1093/sleep/29.12.1587. [DOI] [PubMed] [Google Scholar]
- 15.Ferri R, Zucconi M, Manconi M, et al. Computer-assisted detection of nocturnal leg motor activity in patients with restless legs syndrome and periodic leg movements during sleep. Sleep. 2005;28:998–1004. doi: 10.1093/sleep/28.8.998. [DOI] [PubMed] [Google Scholar]
- 16.Ferri R, Zucconi M, Manconi M, Plazzi G, Bruni O, Ferini-Strambi L. New approaches to the study of periodic leg movements during sleep in restless legs syndrome. Sleep. 2006;29:759–69. [PubMed] [Google Scholar]
- 17.Matheson JK, Saper CB. REM sleep behavior disorder: a dopaminergic deficiency disorder? Neurology. 2003;61:1328–9. doi: 10.1212/wnl.61.10.1328. [DOI] [PubMed] [Google Scholar]
- 18.Albin RL, Koeppe RA, Chervin RD, et al. Decreased striatal dopaminergic innervation in REM sleep behavior disorder. Neurology. 2000;55:1410–2. doi: 10.1212/wnl.55.9.1410. [DOI] [PubMed] [Google Scholar]
- 19.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol. 2006;5:572–7. doi: 10.1016/S1474-4422(06)70476-8. [DOI] [PubMed] [Google Scholar]
- 20.Plazzi G. REM sleep behavior disorders in Parkinson's disease and other Parkinsonian disorders. Sleep Med. 2004;5:195–9. doi: 10.1016/j.sleep.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Diagnostic and Coding Manual. Westchester IL: American Academy of Sleep Medicine; 2005. International Classification of Sleep Disorders; p. 2. [Google Scholar]
- 22.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 23.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 24.Kemp B, Varri A, Rosa A, Nielsen K, Gade J. A simple format for exchange of digitized polygraphic recordings. Electroencephalogr Clin Neurophysiol. 1992:391–3. doi: 10.1016/0013-4694(92)90009-7. [DOI] [PubMed] [Google Scholar]
- 25.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system of sleep stages of human subjects. 1968 doi: 10.1046/j.1440-1819.2001.00810.x. [DOI] [PubMed] [Google Scholar]
- 26.Pennestri M, Whittom S, Benoit A, Petit D, Carrier J, Montplaisir J. PLMS and PLMW in healthy subjects as a function of age: Prevalence and interval distribution. Sleep. 2006:1183–7. doi: 10.1093/sleep/29.9.1183. [DOI] [PubMed] [Google Scholar]
- 27.Mahowald MW, Schenck CH. Rem sleep without atonia—from cats to humans. Arch Ital Biol. 2004;142:469–78. [PubMed] [Google Scholar]
- 28.Walters AS, Hening W, Rubinstein M, Chokroverty S. A clinical and polysomnographic comparison of neuroleptic-induced akathisia and the idiopathic restless legs syndrome. Sleep. 1991;14:339–45. [PubMed] [Google Scholar]
- 29.Garcia-Borreguero D, Odin P, Serrano C. Restless legs syndrome and PD: a review of the evidence for a possible association. Neurology. 2003;61:S49–S55. doi: 10.1212/wnl.61.6_suppl_3.s49. [DOI] [PubMed] [Google Scholar]
- 30.Allen R. Dopamine and iron in the pathophysiology of restless legs syndrome (RLS) Sleep Med. 2004;5:385–91. doi: 10.1016/j.sleep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Michaud M, Dumont M, Selmaoui B, Paquet J, Fantini ML, Montplaisir J. Circadian rhythm of restless legs syndrome: relationship with biological markers. Ann Neurol. 2004;55:372–80. doi: 10.1002/ana.10843. [DOI] [PubMed] [Google Scholar]
- 32.Eisensehr I, Linke R, Noachtar S, Schwarz J, Gildehaus FJ, Tatsch K. Reduced striatal dopamine transporters in idiopathic rapid eye movement sleep behaviour disorder. Comparison with Parkinson's disease and controls. Brain. 2000;123(Pt 6):1155–60. doi: 10.1093/brain/123.6.1155. [DOI] [PubMed] [Google Scholar]
- 33.Fantini ML, Gagnon JF, Filipini D, Montplaisir J. The effects of pramipexole in REM sleep behavior disorder. Neurology. 2003;61:1418–20. doi: 10.1212/wnl.61.10.1418. [DOI] [PubMed] [Google Scholar]
- 34.Sudarsky L, Friedman J. REM sleep behavior disorder: a possible early marker for synucleinopathies. Neurology. 2006;67:2090–1. doi: 10.1212/01.wnl.0000250652.65675.7f. [DOI] [PubMed] [Google Scholar]
- 35.Poewe W, Hogl B. Akathisia, restless legs and periodic limb movements in sleep in Parkinson's disease. Neurology. 2004;63:S12–S16. doi: 10.1212/wnl.63.8_suppl_3.s12. [DOI] [PubMed] [Google Scholar]
- 36.Askenasy JJ, Weitzman ED, Yahr MD. Are periodic movements in sleep a basal ganglia dysfunction? J Neural Transm. 1987;70:337–47. doi: 10.1007/BF01253608. [DOI] [PubMed] [Google Scholar]
- 37.Thorpy MJ. Sleep disorders in Parkinson's disease. Clin Cornerstone. 2004;6(Suppl 1A):S7–15. doi: 10.1016/s1098-3597(04)90013-0. [DOI] [PubMed] [Google Scholar]
- 38.Terzano MG, Parrino L, Smerieri A, et al. CAP and arousals are involved in the homeostatic and ultradian sleep processes. J Sleep Res. 2005;14:359–68. doi: 10.1111/j.1365-2869.2005.00479.x. [DOI] [PubMed] [Google Scholar]
- 39.Remmers JE, Issa FG, Suratt PM. Sleep and respiration. J Appl Physiol. 1990;68:1286–9. doi: 10.1152/jappl.1990.68.3.1286. [DOI] [PubMed] [Google Scholar]
- 40.Penzel T, Kantelhardt JW, Grote L, Peter JH, Bunde A. Comparison of detrended fluctuation analysis and spectral analysis for heart rate variability in sleep and sleep apnea. IEEE Trans Biomed Eng. 2003;50:1143–51. doi: 10.1109/TBME.2003.817636. [DOI] [PubMed] [Google Scholar]