Abstract
Objective:
To evaluate the efficacy and tolerability of immediate release indiplon capsules in patients with chronic insomnia using an “as-needed” dosing strategy in response to difficulty falling back to sleep following a middle of the night, nocturnal awakening.
Methods:
Adult outpatients (N=264; 71% female; age, 46 years) who met DSM-IV criteria for primary insomnia, with average total sleep time (TST) <6.5 hours and >8 nights in the past month with nocturnal awakenings, were randomized to 4 weeks of double-blind treatment with 10mg or 20mg indiplon capsules, or placebo. The primary endpoint was latency to sleep onset post-dosing after a middle of the night awakening (LSOpd). Secondary endpoints included patients' subjective assessment of total sleep time (sTSTpd). Next day residual effects were evaluated by a 100mm Visual Analog Scale (VAS) rating of sleepiness.
Results:
Both doses of indiplon significantly reduced LSOpd at all time-points. Compared to placebo (45.2 min), the 4-week least squares (LS) mean LSOpd was 36.5 min in the indiplon 10mg group (P=0.0023) and 34.4 min in the indiplon 20mg group (P<0.0001). The 4-week LS mean sTSTpd was higher in the indiplon 10mg group (253 min) and 20mg group (278 min) compared to placebo (229 min; P<0.01 for both comparisons). There was no increase observed in VAS ratings of next-day sleepiness for either dose of indiplon when compared to placebo. Indiplon was well-tolerated at both doses.
Conclusions:
Patients with chronic insomnia with nocturnal awakenings achieved significant and sustained improvement in sleep parameters while utilizing an as-needed post bedtime dosing strategy with indiplon capsules. Indiplon was well-tolerated, with no self-rated, next-day residual effects.
Citation:
Roth T; Zammit GK; Scharf MB; Farber R. Efficacy and safety of as-needed, post bedtime dosing with indiplon in insomnia patients with chronic difficulty maintaining sleep. SLEEP 2007;30(12):1731-1738.
Keywords: Insomnia, indiplon, sleep maintenance disorders, awakenings
CHRONIC INSOMNIA, ASSOCIATED WITH IMPAIRMENT IN DAYTIME FUNCTIONING OR QUALITY OF LIFE, HAS A PREVALENCE OF 10–15%.1 RELATIVELY FEW epidemiologic studies are available which provide data on the proportion of patients with specific clinical subtypes of insomnia such as difficulty with sleep onset, difficulty maintaining sleep, or more than one insomnia complaint. Ohayon and Roth, in a large (N=26,400) cross-national community survey,2 reported that 55% of individuals with clinically significant levels of insomnia presented with difficulty maintaining sleep (nocturnal awakenings without accompanying sleep onset complaints).
Insomnia characterized by nocturnal awakenings occurs at a higher rate in women and in older age groups.2,3,3,4 In addition to being one of the most common clinical presentations of primary insomnia, difficulty maintaining sleep is frequently precipitated by disturbance in the sleep environment (e.g., excessive ambient noise), traumatic events or life stresses, pain or discomfort, medical conditions (e.g., arthritis, nocturia, hot flashes), and circadian disruptions (e.g., shift work, travel across time zones).5
Insomnia symptoms are not, typically, a nightly occurrence even among patients who meet DSM-IV criteria.6,7 Given the unpredictability in the occurrence of insomnia presenting as difficulty maintaining sleep, clinicians who prescribe bedtime dosing with sleep medications are confronted with a therapeutic dilemma: how to achieve effective treatment while avoiding use of hypnotics on nights when medication is not required. One possible treatment strategy is the use of “as-needed” (pro re nata, PRN) dosing limited to nights when patients have difficulty falling asleep or returning to sleep following frequent and/or prolonged awakenings. Preliminary studies suggest that patients with insomnia have a 25–45% lower weekly frequency of pill taking when permitted to use PRN regimens.8,9,10
To date, large, placebo-controlled clinical trials examining the efficacy of sedative hypnotics for patients reporting difficulty in maintaining sleep have exclusively used a bedtime dosing strategy.11,12,13 Furthermore, no placebo-controlled study has, to our knowledge, tested the efficacy of a true PRN, post bedtime dosing strategy in a clinical population meeting DSM-IV criteria for insomnia. A successful PRN, post bedtime dosing strategy requires a drug with rapid onset of hypnotic activity, and a sufficiently short elimination half-life that there is minimal-to-no risk of next-day impairment in cognitive and psychomotor function.
Indiplon is a pyrazolopyrimidine compound which is a benzodiazepine receptor agonist with high affinity and selectivity for the α1 subtype of the GABA-A receptor complex, which has been hypothesized to be the receptor subtype associated with sedative effects. The α1 subunit selectivity of indiplon is approximately 10-fold higher for α1 relative to the α2-subunit receptors, and 350-fold higher for α1 relative to α4 and α6-subunit receptors.14,15
The capsule formulation of indiplon has a Tmax of ∼1 hour, and an elimination half-life of 1.5 - 2 hours. It is metabolized by CYP3A4 and by carboxyesterase to inactive metabolites.16 Preliminary crossover studies in healthy volunteers have confirmed the absence of next-day residual effects when indiplon was administered 4 hours prior to awakening at doses of 5mg in the elderly and up to 20mg in adults.17,18 Thus, the pharmacokinetic profile of indiplon makes it a promising candidate for use in a PRN dosing strategy for sleep initiation at any time of the night, provided there are at least 4 hours of sleep time available.
The objective of this study was to evaluate the efficacy, tolerability, and safety of 4 weeks of treatment with indiplon capsules using an as-needed dosing strategy in adult patients with chronic insomnia characterized by nocturnal awakenings with difficulty falling back to sleep.
METHODS
Study Design
The study design was a randomized, double-blind, placebo-controlled, parallel-group trial intended to assess the efficacy, tolerability, and safety of 4 weeks of as-needed, post-bedtime dosing with indiplon (immediate release) 10mg or 20mg capsules in outpatients meeting DSM-IV criteria for primary insomnia characterized by nocturnal awakenings with difficulty falling back to sleep. The study was conducted at 15 sites in the United States. The protocol was approved by an Institutional Review Board (Ethics Committee) for each site, and study conduct was consistent with the Declaration of Helsinki. The study was explained to prospective participants, who were recruited principally by advertisement. Written informed consent was obtained prior to study entry and all subjects received nominal compensation for their participation.
After completing a drug-free screening period, up to 14 days in duration, eligible patients entered a 2-week, single-blind placebo lead-in period. Patients who continued to meet study entry criteria at the end of 2 weeks were randomized (1:1:1) to receive 4 weeks of double-blind treatment with indiplon 10mg, indiplon 20mg, or placebo.
Patients were instructed to take their assigned study medication if a ≥10 minute nighttime awakening occurred, as long as they could remain in bed for ≥4 hours after dosing and the time was before 4:00 am. After taking study drug, patients were instructed to remain awake for 20 minutes. During this 20 minute interval, patients were instructed to complete their sleep diary, and perform a standardized paper and pencil activity which consisted of a simple word search task. At the end of 20 minutes, patients were instructed to turn off the lights, lie down in bed, and attempt to go back to sleep.
At the end of 4 weeks of study treatment (or at the time of early discontinuation), unused study medication was collected and final safety assessments were performed.
Subjects
Men and non-pregnant women (using medically acceptable contraception unless surgically sterilized or ≥2 years post-menopausal) ages 18–64, inclusive, were enrolled if they met DSM-IV criteria for primary insomnia characterized by the following: (1) ≥3 months duration; (2) ≥8 nights per month (∼2 nights per week) with nighttime awakenings, with ≥4 awakenings per month lasting 60 minutes or longer; (3) average subjective total sleep time (sTST) <6.5 hours; and (4) usual time spent in bed was 7–9 hours. Usual bedtime was required to be between 2100 and 2400 hours, and could not vary by more than 2 hours on 5 nights per week. Key exclusion criteria included the following: (1) presence of any clinically significant or unstable medical disorder in the past 30 days; (2) any history of epilepsy or serious head injury; (3) presence of any clinically significant abnormal finding on physical examination, laboratory testing, or electrocardiogram (ECG); (4) any past history of sensitivity to benzodiazepines or other drugs acting at the GABA receptor; (5) history in past 2 years of alcohol or substance dependence or abuse as defined by DSM-IV criteria, or have a positive urine drug screen; (6) consumption of ≥5 alcoholic beverages per day, or ≥14 alcoholic beverages per week; (7) use of (in the 2 weeks prior to single-blind placebo period) any anxiolytics, antidepressants, anticonvulsants, histamine-1 receptor antagonists (except loratidine and fexofenadine), narcotic analgesics, or potent P450 3A4 inhibitors and inducers; (8) night or rotating shift work; and (9) comorbid history (in past 5 years) of chronic pain, uncontrolled benign prostatic hypertrophy, major depressive disorder, schizophrenia, panic disorder, generalized anxiety disorder, or dementia.
To be eligible for randomization, patients were required to meet the following 2 prospective criteria during the 2-week single-blind placebo lead-in period: (1) at least 2 nighttime awakenings of ≥60 minutes in duration after placebo administration; and (2) at least 2 additional nighttime awakenings of ≥30 minutes in duration after placebo administration as determined by responses on a questionnaire filled out upon arising.
Efficacy Evaluations
The primary outcome measure was the patient-rated estimate of the amount of time to fall asleep after taking study drug (latency to sleep onset post-dosing; LSOpd). Secondary patient-rated outcome measures consisted of: (1) the subjective estimate of total sleep time post-dose (sTSTpd); (2) the subjective estimate of the total amount of time awake after returning to sleep post-dose (sWASOpd); (3) the subjective estimate of the number of awakenings after returning to sleep post-dose (sNAASOpd); and (4) sleep quality, rated in the morning on a 7-point scale ranging from 1=excellent to 7=extremely poor. On nights when study medication was consumed, all patient rated measures were completed and recorded on paper in a sleep diary in the morning upon arising.
Next Day Residual Effects
To evaluate next-day residual effects, patients completed a visual analog scale (VAS) for sleepiness the morning after dosing which consisted of a 100mm horizontal line anchored on the left side by the label “very alert” and on the right side by the label “very sleepy.”
Safety Evaluations
During the screening period, safety evaluations to assess patient eligibility included physical and neurological examinations, vital signs, routine laboratory tests (hematology, serum chemistry and urinalysis), Hepatitis B surface antigen and Hepatitis C antibody test, pregnancy test for fertile females, 12-lead ECG, and urine drug screen. Vital signs (blood pressure, heart rate, respiratory rate and oral temperature) were repeated at each visit; weight was measured at screening and end of study. Laboratory tests deemed clinically significant by the investigator were repeated until normal. The physical and neurological examinations, routine laboratory tests and ECG were repeated at end of study. All observed or reported adverse events, regardless of suspected causality, were recorded and rated as to severity.
Statistical Methods
Demographic and other baseline clinical information obtained during the screening period were summarized using descriptive statistics. Efficacy analyses were performed on all patients who were randomized, received study drug, and provided at least one LSOpd value for the double-blind treatment period. The safety analysis set included all randomized patients who received at least one dose of double-blind study drug. The pre-specified primary efficacy variable was LSOpd (4-week average), which was log10-transformed for the purpose of analysis and reported for each treatment group as a least squares (LS) mean value. The primary and secondary outcome variables were analyzed using an analysis of covariance (ANCOVA) model that included fixed effects for treatment and pooled site and the baseline value of the outcome variable as a covariate. For the primary outcome measure, LSOpd, the maximum experiment-wise Type I error rate was maintained at α = 0.05 using Hochberg's method. Categorical variables were analyzed using the Cochran-Mantel-Haenszel (CMH) test.
RESULTS
Patient Characteristics
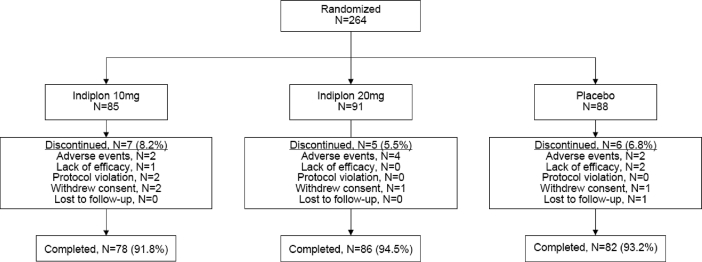
Two-hundred sixty-four patients completed 2 weeks of single-blind, placebo lead-in and were randomized to study treatment; 4 patients never received study medication and were excluded from the analysis (Figure 1). Although a numerical difference was observed for some data points at baseline, the 3 treatment groups were not significantly different on any of the key demographic and baseline clinical variables (Table 1). During the placebo lead-in screening period, the typical patient had 1–2 nocturnal awakenings per night, with only ∼20% of patients reporting 2 or more awakenings on ≥50% of nights per week. In addition, the average LSOpd after placebo dosing was approximately 75 minutes during the 2-week lead-in period (Table 1).
Figure 1.
Patient Disposition
Table 1.
Baseline Demographic and Sleep Characteristics (Safety Sample)
| Placebo (N=86) | Indiplon 10 mg (N=84) | Indiplon 20 mg (N=90) | P-value* | |
|---|---|---|---|---|
| Female | 74% | 70% | 70% | 0.8434 |
| Age, yr; mean ± sd | 46.7 ± 9.5 | 45.2 ± 12.3 | 45.4 ± 11.0 | 0.7198 |
| Race, % | ||||
| White | 73% | 67% | 80% | |
| Black | 19% | 20% | 16% | |
| Other | 8% | 13% | 4% | 0.1331 |
| Body mass index, mean ± sd | 28.6 ± 6.9 | 27.7 ± 6.2 | 27.4 ± 6.7 | 0.9550 |
| Sleep Characteristics After Post-Bedtime Dosing During Placebo Lead-in | ||||
| LSOpd, min | ||||
| Mean ± sd | 72.4 ± 36.8 | 72.2 ± 29.2 | 80.2 ± 48.2 | |
| LSOPD ≥60 min in ≥50% of nights, %† | 58.1% | 54.8% | 61.1% | 0.2497 |
| sWASOpd, min | ||||
| Mean ± sd | 27.7 ± 37.4 | 31.8 ± 28.8 | 37.4 ± 55.6 | |
| sWASOPD ≥30 min in ≥50% of nights, %† | 20.9% | 28.6% | 17.8% | 0.2809 |
| sNAASOpd | ||||
| Mean ± sd | 1.2 ± 1.3 | 1.4 ± 1.2 | 1.5 ± 1.5 | |
| sNAASOPD ≥2-times in ≥50% of nights, %† | 16.3% | 20.2% | 20.0% | 0.3890 |
| sTSTpd, min | ||||
| Mean ± sd | 203.9 ± 8.5 | 202.2 ± 8.5 | 198.4 ± 8.4 | 0.8542 |
| Sleep Quality, | ||||
| Mean ± sd | 4.2 ± 1.1 | 4.3 ± 1.2 | 4.3 ± 1.1 | |
| Fair-to-extremely-poor, % | 73.3.% | 72.6% | 80.0% | 0.6221 |
P-values for gender and race were obtained from a generalized CMH procedure with study site as a stratification variable, comparing across treatment groups. P-values for the sleep parameters were obtained from an ANOVA model using log-transformed (base 10) data with main effects for treatment and study site, comparing across treatment groups. Sleep parameters were measured from when study drug was taken at the time of nighttime awakening
Proportion of patients reporting awakenings in ≥50% of the 2-week single-blind, placebo-controlled run-in period
Discontinuation rates during the 4 weeks of study treatment were similar among the treatment groups (Figure 1).
Effect of As-Needed Post Bedtime Dosing with Indiplon on Sleep Onset
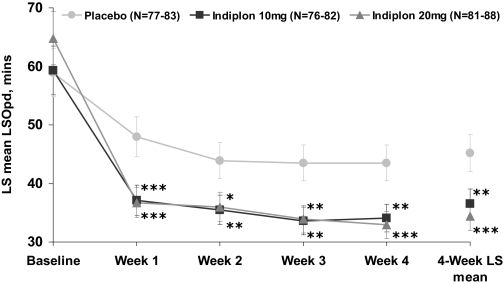
Treatment with indiplon 10mg and 20mg was associated with significant improvement relative to placebo in the primary outcome, the 4-week average LSOpd (Figure 2, Table 2). LSOpd was significantly improved on both doses of indiplon at the 1-week time point and significance was maintained during each week of the 4-week study. The magnitude of improvement was comparable in the 10mg and 20mg treatment groups.
Figure 2.
LSOpd: weekly LS mean ± SE for MOTN Dosing Nights * P<0.05; ** P<0.01; ***P<0.001
pd = post-dose; MOTN = middle of the night; SE = standard error; LS means are from an ANCOVA model with baseline used as a covariate
Table 2.
Results of an ANCOVA Analysis Evaluating the Efficacy of Indiplon vs. Placebo on Primary and Secondary Sleep Parameters (intent to treat): LS mean ± SE
| Placebo | Indiplon 10 mg | Indiplon 20 mg | Significance testing, Indiplon vs. placebo |
||||
|---|---|---|---|---|---|---|---|
| N=86 | N=84 | N=90 | 10 mg |
20 mg |
|||
| t | P | t | P | ||||
| LSOpd, min | |||||||
| Baseline | 58.9 ± 2.7 | 59.3 ± 2.8 | 64.8 ± 3.0 | ||||
| 4-week average | 45.2 ± 2.2 | 36.5 ± 1.8 | 34.4 ± 1.7 | −3.08 | 0.0023 | −3.96 | <0.0001 |
| sWASOpd, min | |||||||
| Baseline | 27.5 ± 4.6 | 31.7 ± 4.7 | 37.7 ± 4.6 | ||||
| 4-week average | 24.4 ± 3.5 | 16.2 ± 3.6 | 12.0 ± 3.5 | −1.65 | 0.1000 | −2.52 | 0.0122 |
| sNAASOpd | |||||||
| Baseline | 1.22 ± 0.14 | 1.44 ± 0.15 | 1.48 ± 0.14 | ||||
| 4-week average | 0.85 ± 0.09 | 0.74 ± 0.09 | 0.54 ± 0.09 | −0.90 | 0.3712 | −2.52 | 0.0125 |
| sTSTpd, min | |||||||
| Baseline | 204 ± 8.4 | 203 ± 8.5 | 198 ± 8.2 | ||||
| 4-week average | 229 ± 6.6 | 253 ± 6.8 | 278 ± 6.5 | 2.60 | 0.0099 | 5.28 | <0.0001 |
| Sleep Quality | |||||||
| Baseline | 4.2 ± 0.1 | 4.3 ± 0.1 | 4.3 ± 0.1 | ||||
| 4-week average | 3.8 ± 0.1 | 3.3 ± 0.1 | 3.0 ± 0.1 | −3.44 | 0.0007 | −5.83 | <0.0001 |
All values are reported as LS means ± standard error of the mean (SE) 4-week average LS means are from an ANCOVA model with baseline values used as a covariate. For LSOpd the ANCOVA was performed on log-transformed data, and then antilog transformed.
Effect of Indiplon on Secondary Sleep Parameters
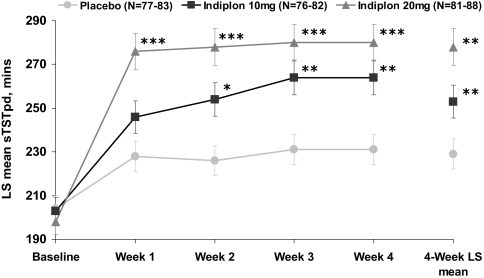
Treatment with the 20mg dose of indiplon produced significant improvement in the 4-week LS mean data for sTSTpd and all other secondary post-dose sleep measures (Table 2, Figure 3). Treatment with the 10mg dose also produced significant 4-week LS mean improvement in sTSTpd, but was not significant for sWASOpd or sNAASOpd measurements (Table 2). Furthermore, sleep quality was rated as significantly improved on both PRN doses of indiplon over the 4 weeks of study treatment (Table 2).
Figure 3.
sTSTpd: weekly LS mean ± SE for MOTN Dosing Nights
*P<0.05; **P<0.01; ***P<0.001
pd = post-dose; MOTN = middle of the night; SE = standard error; LS means are from an ANCOVA model with baseline used as a covariate
Pattern Analysis of As-Needed Post Bedtime Dosing
For most patients, the typical time of dose administration was between 12:00 and 2:00 am (Table 3). The mean (± sd) number of doses taken per week was 3.9 ± 1.9 for the indiplon 10mg group, 3.7 ± 1.7 for the 20mg group, and 3.7 ± 1.9 for placebo. Approximately 15% of patients, regardless of treatment, averaged 6 or more doses per week across the 4 weeks of study treatment.
Table 3.
Trends in Post-Bedtime Dosing Frequency and Timing Over 4 Weeks of Study Treatment
| Placebo | Indiplon 10 mg | Indiplon 20 mg | |
|---|---|---|---|
| N=86 | N=84 | N=90 | |
| Timing of post-bedtime dosing, % † | |||
| Before midnight | 6.9% | 8.7% | 6.5% |
| 12:00–02:00 | 54.6% | 53.6% | 60.5% |
| After 02:00 | 39.6% | 37.7% | 33.0% |
| Percent of total post-bedtime doses | |||
| Total sample: | |||
| Week 1 | 25.7% | 26.3% | 24.9% |
| Week 2 | 26.7% | 24.9% | 23.8% |
| Week 3 | 23.2% | 24.4% | 24.3% |
| Week 4 | 24.5% | 24.4% | 26.9% |
| Total post-bedtime doses taken | 100% | 100% | 100% |
| Percent of total post-bedtime doses | |||
| Responder subgroup:†† | |||
| Week 1 | 24.1% | 26.5% | 24.2% |
| Week 2 | 26.7% | 24.8% | 23.8% |
| Week 3 | 23.0% | 24.2% | 24.0% |
| Week 4 | 26.2% | 24.5% | 28.0% |
| Total post-bedtime doses taken | 100% | 100% | 100% |
| Percent of total post-bedtime doses | |||
| Non-responder subgroup: | |||
| Week 1 | 26.5% | 25.8% | 27.0% |
| Week 2 | 26.7% | 25.2% | 23.7% |
| Week 3 | 23.2% | 24.7% | 25.1% |
| Week 4 | 23.6% | 24.3% | 24.2% |
| Total post-bedtime doses taken | 100% | 100% | 100% |
Data from all four weeks of double-blind treatment
≥ 1/3 improvement compared to baseline in LSOpd
To evaluate whether dosing frequency changed during the 4 weeks of study treatment, a frequency distribution of dosing nights across all weeks was analyzed for the total intent-to-treat sample, and for the subgroups of responders and non-responders. As can be seen in Table 3, a comparable percentage of total dosing nights, for each week, were observed for each of the three study treatment groups. This pattern was seen in the total sample and the responder and non-responder subgroups (where responder was defined, post-hoc, as those whose LSOpd was reduced by at least one third when compared to baseline).
Next Day Residual Effects
To evaluate the next-morning drowsiness, a 100mm VAS was used to rate drowsiness/alertness (higher number indicates greater drowsiness). The LS mean (± se) VAS rating, averaged over the 4 weeks of treatment, was lower for 10mg (40.5 ± 1.9; P=0.15) and significantly lower for the 20mg indiplon group (38.7 ± 1.8; P=0.03) relative to placebo (44.4 ± 1.9). The proportion of patients achieving a 10mm or greater improvement in next-day alertness on their VAS scale (“responders”) was higher in the 10mg (45.2%) and 20mg (43.3%) indiplon groups relative to placebo (26.7%; P < 0.05 for both comparisons). Among the subgroup of patients reporting elevated levels of daytime sleepiness at pre-treatment baseline (VAS-sleepiness ≥60mm), the proportion achieving ≥10mm improvement was even higher for the 10mg (65.5%) and 20mg (69.2%) indiplon groups relative to placebo (26.9%; P < 0.01 for both comparisons).
Safety Evaluations
Both doses of indiplon were well-tolerated (Table 4). Only one adverse event (somnolence) occurred with an incidence of ≥5% on the 10mg dose of indiplon. One serious adverse event (not treatment-related) occurred in a patient receiving indiplon who was hospitalized during week 2 of study treatment with a diagnosis of stage III-C ovarian cancer. Two laboratory abnormalities considered clinically significant also occurred during double-blind therapy. One patient on indiplon 10mg had an isolated low blood glucose level (49 mg/dL), and one patient on placebo had several mildly elevated gamma glutamyl transferase levels. There were no other clinically significant treatment-emergent abnormalities in ECG, vital signs, or physical or neurological examinations.
Table 4.
Treatment-emergent adverse events (all-causality, ≥5%)
| Placebo | Indiplon | Indiplon | |
|---|---|---|---|
| 10 mg | 20 mg | ||
| (N=86) | (N=84) | (N=90) | |
| Somnolence | 3.5% | 6.0% | 7.8% |
| Headache | 2.3% | 2.4% | 6.7% |
| Any adverse event | 26% | 39% | 34% |
| Any severe adverse event | 3.5% | 4.8% | 5.6% |
DISCUSSION
This study is the first placebo-controlled clinical trial to evaluate the efficacy and tolerability of as-needed post bedtime dosing with a sedative-hypnotic administered only in response to middle of the night, nocturnal awakenings in outpatients with a DSM-IV diagnosis of primary insomnia. When compared to placebo, treatment with both the 10mg and 20mg doses of indiplon significantly reduced the time to return to sleep and significantly increased the amount of sleep obtained in the remaining time left in bed, following a nocturnal awakening. Significant improvement in sleep quality was also reported on both doses of indiplon. Improvement in wake time after returning to sleep, and number of awakenings was significant for the 20mg dose, but not for the 10mg dose.
Timing of As-Needed Dosing in Insomnia: Bedtime Vs. Nighttime Awakening
Between 40–80% of individuals with insomnia report that their episodes of insomnia do not occur every night.7,8,19 Indeed, only 15% of patients in the current study reported utilizing indiplon or placebo ≥6 nights of dosing per week during the 28-day period. For the past two decades, as-needed treatment with hypnotics has been recommended as a preferred therapeutic option,20,21,22,23 but we are unaware of any large, randomized, placebo-controlled trials which have tested the efficacy of this recommendation, using middle of the night dosing as needed for nocturnal awakenings, in patients with insomnia. Several placebo-controlled trials have evaluated the efficacy of bedtime treatment with a fixed intermittent dosing schedule (5 consecutive nights per week with 2 nights of placebo vs nightly),24 or bedtime dosing with as-needed treatment, but restricted to 3–5 pills per week. 25,26,27 The current study thus provides the first data which evaluate the efficacy of unrestricted PRN hypnotic treatment combined with a middle of the night dosing occurring at the time of a nocturnal awakening. The sleep quality results in the current study suggest that an as-needed middle of the night dosing strategy might achieve clinical benefit comparable to nightly bedtime dosing in patients with chronic nocturnal awakening difficulties,28,29 but with notably reduced overall medication utilization, in the range of 3.7–3.9 pills per week. The fixed or restricted regimens of previous intermittent dosing trials, as well as their use of bedtime dosing,24–27 does not allow for direct comparison to the current, post-bedtime dosing regimen. The latter regimen would appear to generalize more readily to “real world” clinical practice in which insomniacs have been reported to self-administered a (bedtime) hypnotic medication on 56–73% of nights over a 7 day free choice period.8–10 The results of the current study suggest that PRN dosing may be an alternative to the more standard nightly, bedtime dosing regimen.
Use of a middle of the night dosing strategy is optimal in drugs with a rapid Tmax (<1 hour) and a relatively short elimination half-life (≤2 hours). Drugs with intermediate-or-longer elimination half-lives (eg, zopiclone, eszopiclone, diphenhydramine, trazodone, and many of the benzodiazepine hypnotics) appear to be less suitable for use in a nighttime awakening treatment strategy, and in fact are not indicated for such use since their longer elimination half-lives have been associated with significant next-day residual effects when dosed within 4–6 hours of rise time.30,31,32,33,34 Indiplon has a pharmacokinetic profile appropriate for a middle of the night dosing strategy. The compound is rapidly absorbed, with 70% of Cmax reached within 30 minutes, and is associated with no next-day impairment when administered 4 hours before awakening.17,18 The results of the current study confirm the potentially favorable pharmacokinetic and pharmacodynamic profile for PRN middle of the night use. Among available hypnotics, the elimination half-life of zolpidem is most similar to that of indiplon. Zolpidem has been reported to reduce sleep latency after experimentally induced nocturnal awakenings in patients with sleep-maintenance insomnia.32 However, studies have also reported that zolpidem 10 mg was associated with residual sedation17,32 and/or performance impairment29,32,35 up to 7 hours after middle of the night dosing.
In reporting the current post bedtime dosing results, it is important to note the standardized treatment convention that was used. Patients took study medication after 10 minutes of post bedtime awakening, and then stayed awake at the bedside completing their sleep diary (and other quiet activities) for 20 minutes before lights out. While the patients were encouraged to complete the quiet activities, it is acknowledged that getting out of bed, turning on the lights, completing activities is a deviation from real-world scenarios in which the patient would simply attempt to return to sleep with as little disruption to the night as possible.
Effects of 4 Weeks of Treatment
The efficacy of indiplon was sustained over the 4 weeks of study treatment, with no evidence of tolerance in either sleep onset or sleep maintenance parameters. The current study permitted as-needed dosing, with patients asked to take study medication whenever the duration of a nighttime awakening exceeded 10 minutes. Given the elective nature of dosing, it is notable that patients did not escalate their frequency of dosing from week 1 to week 4, but instead maintained a consistent dosing frequency. This suggests that as-needed, middle of the night dosing may be an efficient and novel alternative to typical nightly bedtime treatment.
CONCLUSIONS
Over a 4-week period, as-needed dosing with indiplon 10mg and 20mg resulted in significant and sustained improvement in both the ability to return to sleep and the amount of sleep obtained in the remaining time left in bed, following a nocturnal awakening. Indiplon was well-tolerated, with no evidence of self-rated, next-day residual effects. Further research is needed to determine the efficacy and safety of an as-needed post-bedtime dosing strategy during longer-term treatment, and in key clinical populations such as the elderly, in whom nocturnal awakenings are common. Additionally, studies in both adults and elderly could evaluate sleep parameters on non-dosing nights to better understand the effect of a PRN dosing schedule on the natural progression of insomnia. In conclusion, as-needed post-bedtime dosing with indiplon appears to provide a safe and effective method for treating patients suffering from insomnia characterized by middle of the night, nocturnal awakenings.
ACKNOWLEDGMENTS
Funding was provided by Neurocrine Biosciences, Inc
The authors wish to acknowledge Phil Jochelson, MD for contributions to the original study design; and Edward Schweizer, MD for assistance in the preparation of the manuscript.
Footnotes
Disclosure Statement
This study was Funded by Neurocrine Biosciences, Inc. Dr. Roth has received research support from Aventis, Cephalon, GlaxoSmithKline, Neurocrine, Pfizer, Sanofi-Aventis, Schering-Plough, Sepracor, Somaxon, Syrex, Takeda, TransOral, Wyeth, and XenoPort; is a consultant for Abbott, Accadia, Acoglix, Actelion, Alchemers, Alza, Ancil, Arena, AstraZeneca, Aventis, BMS, Cephalon, Cypress, Dove, Elan, Eli Lilly, Evotec, Forest, GlaxoSmithKline, Hypnion, Jazz, Johnson – Johnson, King, Lundbeck, Mc-Neil, MedicNova, Merck, Neurim, Neurocrine, Neurogen, Novartis, Orexo, Organon, Orginer, Prestwick, Proctor and Gamble, Pfizer, Purdue, Restiva, Roche, Sanofi, ShoeringPlough, Sepracor, Servier, Shire, Somaxon, Syrex, Takeda, TransOral, Vanda, Vivometrics, Wyeth, Yamanuchi, and Xenoport; and has participated in speaking engagements supported by Sanofi and Takeda. Dr. Zammit has received research support from Ancile Pharmaceuticals, Arena, Aventis, Cephalon, Elan, Epix, Forest, GlaxoSmithKline, H. Lundbeck A/S, King, Merck, Neurim, Neurocrine Biosciences, Neurogen, Organon, Orphan Medical, Pfizer, Respironics, Sanofi-Aventis, Sanofi-Synthelabo, Schering-Plough, Sepracor, Somaxon, Takeda, UCB Pharma, Predix, Vanda, and Wyeth-Ayerst; has consulted for Aventis, Cephalon, Elan, GlaxoSmithKline, Jazz, King, Merck, Neurocrine Biosciences, Organon, Pfizer, Sanofi-Aventis, Select Comfort, Sepracor, Shire, and Takeda; has participated in speaking engagements for Neurocrine Biosciences, Kink, McNeil, Sanofi0Aventis, Sanofi-Synthelabo, Sepracor, Takeda, Vela, and Wyeth-Ayerst; and has ownership/directorship in Clinilabs, Inc., Clinilabs IPA, Inc., and Clinilabs Physician Services, PC. Dr. Scharf has received research support from GlaxoSmithKline, Sanofi-Aventis, Merck, Resmed, Respironics, Transoral, Neurogen, Evotek, and Takeda and has consulted and participated in speaking engagements for Jazz, Sepracor, Neurocrine Biosciences, and Transoral. Dr. Farber is a full-time employee of Neurocrine Biosciences.
REFERENCES
- 1.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 2.hayon MM, Roth T. What are the contributing factors for insomnia in the general population? J Psychosom Res. 2001;51:745–755. doi: 10.1016/s0022-3999(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 3.Novak M, Mucsi I, Shapiro CM, Rethelyi J, Kopp MS. Increased utilization of health services by insomniacs--an epidemiological perspective. J Psychosom Res. 2004;56:527–536. doi: 10.1016/j.jpsychores.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Ohayon MM, Hong SC. Prevalence of insomnia and associated factors in South Korea. J Psychosom Res. 2002;53:593–600. doi: 10.1016/s0022-3999(02)00449-x. [DOI] [PubMed] [Google Scholar]
- 5.Roehrs T, Zorick F, Roth T. Transient and short-term insomniacs. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Third edition. Philadelphia: W. B. Saunders Co.; 2000. pp. 624–632. [Google Scholar]
- 6.Washington, DC: National Sleep Foundation; 2002. Sleep in America Poll. [Google Scholar]
- 7.Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. II. Sleep. 1999;22(Suppl 2):S354–358. [PubMed] [Google Scholar]
- 8.Roehrs T, Bonahoom A, Pedrosi B, Rosenthal L, Roth T. Treatment regimen and hypnotic self-administration. Psychopharmacology. 2001;155:11–17. doi: 10.1007/s002130000661. [DOI] [PubMed] [Google Scholar]
- 9.Roehrs T, Pedrosi B, Rosenthal L, Roth T. Hypnotic self-administration and dose escalation. Psychopharmacology. 1996;127:150–154. doi: 10.1007/BF02805988. [DOI] [PubMed] [Google Scholar]
- 10.Roehrs T, Merlotti L, Zorick FJ, Roth T. Rebound insomnia and hypnotic self-administration. Psychopharmacology. 1992;107:480–484. doi: 10.1007/BF02245259. [DOI] [PubMed] [Google Scholar]
- 11.Holm KJ, Goa KL. Zolpidem: an update of its pharmacology, therapeutic efficacy and tolerability in the treatment of insomnia. Drugs. 2000;59:865–889. doi: 10.2165/00003495-200059040-00014. [DOI] [PubMed] [Google Scholar]
- 12.Dooley M, Plosker GL. Zaleplon: a review of its use in the treatment of insomnia. Drugs. 2000;60:413–445. doi: 10.2165/00003495-200060020-00014. [DOI] [PubMed] [Google Scholar]
- 13.Krystal AD, Walsh JK, Laska E, Caron J, Amato DA, Wessel TC, Roth T. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26:793–799. doi: 10.1093/sleep/26.7.793. [DOI] [PubMed] [Google Scholar]
- 14.Foster AC, Pelleymounter MA, Cullen MJ, Lewis D, Joppa M, Chen TK, Bozigian HP, Gross RS, Gogas KR. In vivo pharmacological characterization of indiplon, a novel pyrazolopyrimidine sedative-hypnotic. J Pharmacol Exp Ther. 2004;311:547–559. doi: 10.1124/jpet.103.063487. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan SK, Petroski RE, Verge G, Gross RS, Foster AC, Grigoriadis DE. Characterization of the interaction of indiplon, a novel pyrazolopyrimidine sedative-hypnotic, with the GABAA receptor. J Pharmacol Exp Ther. 2004;311:537–546. doi: 10.1124/jpet.104.071282. [DOI] [PubMed] [Google Scholar]
- 16.Jochelson P, Chen TK, Farber R, Campbell B. Lack of Pharmacological and Pharmacokinetic Tolerance Following Repeat Dosing of Indiplon (NBI-34060). Poster presentation at the 17th annual meeting of the Associated Professional Sleep Societies; June 3–8, 2003; Chicago, Il. [Google Scholar]
- 17.Garber M, Burke J, Farber R, Jochelson P. Residual effects of middle of the night dosing: a placebo-controlled crossover study of indiplon-IR, zolpidem, and zopiclone. Poster presentation, 157th annual meeting of the American Psychiatric Association; May 1–6, 2004; New York. [Google Scholar]
- 18.Jochelson P, Gately N, Garber M, Farber R. Residual effects of middle of the night dosing: a placebo-controlled cross-over study of indiplon-IR and zopiclone in elderly volunteers. Poster presentation, 156th annual meeting of the American Psychiatric Association; May 17–22, 2003; San Francisco. [Google Scholar]
- 19.Estivill E. Behaviour of insomniacs and implication for their management. Sleep Med Rev. 2002;6(Suppl 1):S3–6. doi: 10.1016/s1087-0792(02)80001-6. [DOI] [PubMed] [Google Scholar]
- 20.World Psychiatric Association: Task force on sedative hypnotics: A Report. Eur Psychiatry. 1993;8:45–49. [Google Scholar]
- 21.National Institutes of Health. Consensus Development Statement: The treatment of sleep disorders of older people. Sleep. 1991;14:169–177. [PubMed] [Google Scholar]
- 22.National Institute of Mental Health. Consensus Development Conference: Drugs and insomnia: The use of medications to promote sleep. JAMA. 1984;251:2410–2414. [PubMed] [Google Scholar]
- 23.Dundar Y, Boland A, Strobl J, Dodd S, Bagust A, Haycox A, Bogg J, Dickson R, Walley T. Newer hypnotic drugs for the short term management of insomnia: a rapid and systematic review. Health Technol Assess. 2004;8:1–125. doi: 10.3310/hta8240. [DOI] [PubMed] [Google Scholar]
- 24.Cluydts R, Peeters K, De Bouyalsky I, Lavoisy J. Comparison of continuous versus intermittent administration of zolpidem in chronic insomniacs: a double-blind, randomized pilot study. J Int Med Res. 1998;26:3–24. doi: 10.1177/030006059802600102. [DOI] [PubMed] [Google Scholar]
- 25.Hajak G, Cluydts R, Declerck A, Estivill SE, Middleton A, Sonka K, Unden M. Continuous versus non- nightly use of zolpidem in chronic insomnia: results of a large- scale, double-blind, randomized, outpatient study. Int Clin Psychopharmacol. 2002;17:9–17. doi: 10.1097/00004850-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Walsh JK, Roth T, Randazzo A, Erman M, Jamieson A, Scharf M, Schweitzer PK, Ware JC. Eight weeks of non-nightly use of zolpidem for primary insomnia. Sleep. 2000;23:1087–1096. [PubMed] [Google Scholar]
- 27.Perlis ML, McCall WV, Krystal AD, Walsh JK. Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry. 2004;65:1128–1137. doi: 10.4088/jcp.v65n0816. [DOI] [PubMed] [Google Scholar]
- 28.Scharf MB, Roth T, Walsh, Walsh JK, Jochelson P, Garber M. Efficacy of indiplon-MR in inducing and maintaining sleep in patients with chronic sleep maintenance insomnia. Poster presentation at the 157th annual meeting of the American Psychiatric Association; May 1–6, 2004; New York, NY. for the 0221 Study Group. [Google Scholar]
- 29.Zoladult Study Group. Roth T, Soubrane C, Titeux L, Walsh JK. Efficacy and safety of zolpidem-MR: A double-blind, placebo-controlled study in adults with primary insomnia. Sleep Medicine. 2006;7:397–406. doi: 10.1016/j.sleep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Vermeeren A. Residual effects of hypnotics: epidemiology and clinical implications. CNS Drugs. 2004;18:297–328. doi: 10.2165/00023210-200418050-00003. [DOI] [PubMed] [Google Scholar]
- 31.Verster JC, Veldhuijzen DS, Volkerts ER. Residual effects of sleep medication on driving ability. Sleep Med Rev. 2004;8:309–325. doi: 10.1016/j.smrv.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Basu R, Dodge H, Stoehr GP, Ganguli M. Sedative-hypnotic use of diphenhydramine in a rural, older adult, community-based cohort: effects on cognition. Am J Geriatr Psychiatry. 2003;11:205–213. [PubMed] [Google Scholar]
- 33.Agostini JV, Leo-Summers LS. Inouye S: Cognitive and other adverse effects of diphenhydramine use in hospitalized older patients. Arch Intern Med. 2001;161:2091–2097. doi: 10.1001/archinte.161.17.2091. [DOI] [PubMed] [Google Scholar]
- 34.Zammit GK, Corser B, Doghramji K, Fry JM, James S, Krystal A, Mangano RM. Sleep and residual sedation after administration of zaleplon, zolpidem, and placebo during experimental middle-of-the-night awakening. J Clin Sleep Med. 2006;2:417–423. [PubMed] [Google Scholar]
- 35.Danjou P, Paty I, Fruncillo R, Worthington P, Unruh M, Cevallos W, Martin P. A comparison of the residual effects of zaleplon and zolpidem following administration 5 to 2 h before awakening. Br J Clin Pharmacol. 1999;48:367–374. doi: 10.1046/j.1365-2125.1999.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]