Abstract
Attenuated recombinant Listeria monocytogenes (Lm) strains are a promising class of vaccine vectors that trigger protective antigen-specific CD8 T cells. Listeriolysin O (LLO) is an important Lm virulence determinant allowing the bacterium to escape from the endocytic vacuole into the cell cytoplasm in phagocytic cells. However in non-phagocytic cells, Lm phospholipase C can also mediate cytoplasmic entry. The ability of LLO-deficient Lm to confer long-term protection to infection is uncertain. Herein, we demonstrate that LLO-deficient Lm mutants can prime protective immunity to subsequent Lm infection and that Lm phospholipase C is required for protective immunity conferred by LLO-deficient Lm.
Keywords: bacteria, vaccine, T cell immunity
INTRODUCTION
Listeria monocytogenes (Lm) is a Gram-positive intracellular bacterium that causes serious infection in neonates and other immune compromised hosts. The pathogenesis and immune response to infection has been characterized using both in vitro and in vivo models of experimental infection [1,2]. Following infection with wildtype Lm, a protective T cell response is readily detected and characterized by the expansion of antigen-specific CD8 and CD4 T cells. For Lm infection, antigen-specific CD8 T cells confer the majority of the protective effects, whereas CD4 T cells have an important role in maintaining protective CD8 T cells into memory time points [3–6]. Additionally, many virulence determinants in Lm have been characterized, and the immune response to Lm mutants arrested in specific stages in the infection process have been used to characterize the immune response to foreign antigens in specific cellular compartments. Following passive uptake by phagocytic cells or active invasion of non-phagocytic cells, the bacterium initially resides within a membrane-bound vacuole. Depending on the type of infected cell, escape from this compartment into cell cytoplasm requires the hemolysin listeriolysin O (LLO) or either of two bacterial phospholipases C (PLCs) [7]. LLO is essential for cytoplasmic entry in phagocytic cells such as macrophages, while PLC can mediate cytoplasmic entry in non-phagocytic cells.
LLO mediated entry into the cytoplasm of professional phagocytic and antigen presenting cells such as macrophages and dendritic cells is required for optimal upregulation of costimulatory molecules, production of inflammatory cytokines important in priming naïve T cells, and dramatic changes in expression of immune regulated genes [8,9]. Accordingly, the long-standing paradigm has been that LLO mediated cytoplasmic entry by the bacterium was essential for priming Lm specific CD8 T cells and protective immunity to subsequent Lm infection [10,11]. This paradigm was revisited last year when two groups independently found that LLO-deficient Lm mutants can prime antigen-specific CD8 T cells and protective immunity after a single inoculation [12,13]. Compared to wildtype Lm, the high degree of attenuation of LLO-deficient Lm mutants make them attractive vaccine vectors to deliver T cell epitopes. However, the potential utility of LLO-deficient Lm as a vaccine vector is uncertain since the results of one study revealed only transient (lasting only up to seven days) protection [12], while another study reported no defects in long-term protective immunity conferred by infection with LLO-deficient Lm [13]. The reasons for these apparent discordant results are unclear. Furthermore, since neither study examined the role of PLC in LLO-deficient Lm mutants, the role of Lm cytoplasmic entry into any cell type in triggering protective immunity remains uncertain. Accordingly, in this study we examine the ability of LLO-deficient Lm mutants to prime protective immunity to subsequent infection with virulent Lm, as well as compare the immune response and protective effects of Lm mutants with targeted deficiency in LLO and mutants with combined defects in both LLO and PLC.
MATERIALS AND METHODS
The Lm mutant strains used were wildtype strain 10403s, or strains containing in-frame deletions in the actA gene (DPL1942, ΔactA), the hly gene (DPL2161, ΔLLO), or the hly, plcA, and plcB genes (DPL2319, ΔLLO/ΔPLC) that have been previously described [7,14]. For recombinant Lm, each Lm strain was transformed with a stable expression construct conferring chloramphenicol resistance containing the coding region of a hemagglutinin-tagged recombinant protein with the H-2Kb immune dominant peptide from Herpes simplex virus-1 glycoprotein B (gB) behind the hly promoter and signal sequence [15]. Western blotting was performed with supernatant protein preparations from bacteria in log-phase growth prepared by trichloroacetic acid precipitation (10%), separated by gel electophoresis, transferred to nitrocellulose, and probed with anti-hemagglutinin antibody as described [15].
For infection, female C57BL/6 (H-2Kb) mice were obtained from The Jackson Laboratory and used at 8 to 10 weeks. Lm was grown in brain heart infusion (BHI) media supplemented with chloramphenicol (20μg/ml), washed and diluted in saline, and injected through the lateral tail vein in 200 μl. At the indicated time points after infection, the gB498–505 -specific CD8 T cell response was examined directly ex-vivo in peripheral blood or splenocytes by staining with H-2Kb dimer X loaded with gB498–505 peptide, or after in vitro restimulation with gB498–505 peptide (10−6 M) followed by surface and intracellular cytokine staining using methods previously described [15]. The number of recoverable Lm in spleen and liver lysates from infected mice were quantified after serial dilution and plating onto BHI plates. Differences in numbers of recoverable CFUs were compared using the Student’s T test (GraphPad software) with p < 0.05 taken as statistically significant. All mice were maintained in specific pathogen-free facilities at the University of Washington, and experiments were performed under Institutional Animal Care and Use Committee-approved protocols.
RESULTS
Infection with Lm ΔactA that secretes a recombinant protein containing the H-2Kb peptide from HSV-1 glycoprotein B (gB498–505) primes a potent gB-specific CD8 T cell response that is readily tracked with gB-specific dimer [15]. We employed the same strategy to examine the immune response to LLO-deficient Lm and the additional role of PLC in immunity conferred by LLO-deficient Lm and transformed Lm ΔLLO (DPL2161) and Lm ΔLLO/ΔPLC (DPL2319) each with the gB498–505 expression construct. Compared with Lm ΔactA, both Lm ΔLLO and Lm ΔLLO/ΔPLC expressed and secreted the recombinant protein in culture supernatants to the same degree as determined by western blotting (Figure 1A). Consistent with results from our previous studies, 106 CFUs of Lm ΔactA primed a robust gB498–505 specific CD8 T cell response. At the peak of the T cell response (seven days after inoculation), ~3.5% of peripheral CD8 T cells were gB498–505 specific (Figure 1B). These cells contracted to ~10% of the peak response by day 14 and persisted at this level among peripheral T cells to at least day 28 after infection (Figure 1C). In striking contrast, at each of these time points after primary infection with either Lm ΔLLO or Lm ΔLLO/ΔPLC, only background levels of dimer staining were present. This apparent lack of gB498–505 specific CD8 T cell priming after infection with either Lm ΔLLO or Lm ΔLLO/ΔPLC compared with Lm ΔactA cannot be attributed to decreased antigen load related to the more severe attenuation of these mutants compared with Lm ΔactA; similar numbers of recoverable bacteria are found twenty-four hours after infection with 108 CFUs of Lm ΔLLO or Lm ΔLLO/ΔPLC compared with 106 CFUs Lm ΔactA (Figure 1D). This time point after infection was chosen for examination since antibiotic administration before 24 hours significantly reduces the magnitude of the CD8 T cell response, while antibiotics after 24 hours has no effect [16]. Therefore, the antigen load indicated by the number of live Lm CFUs directly correlates with the magnitude of the CD8 T cell response.
Figure 1.
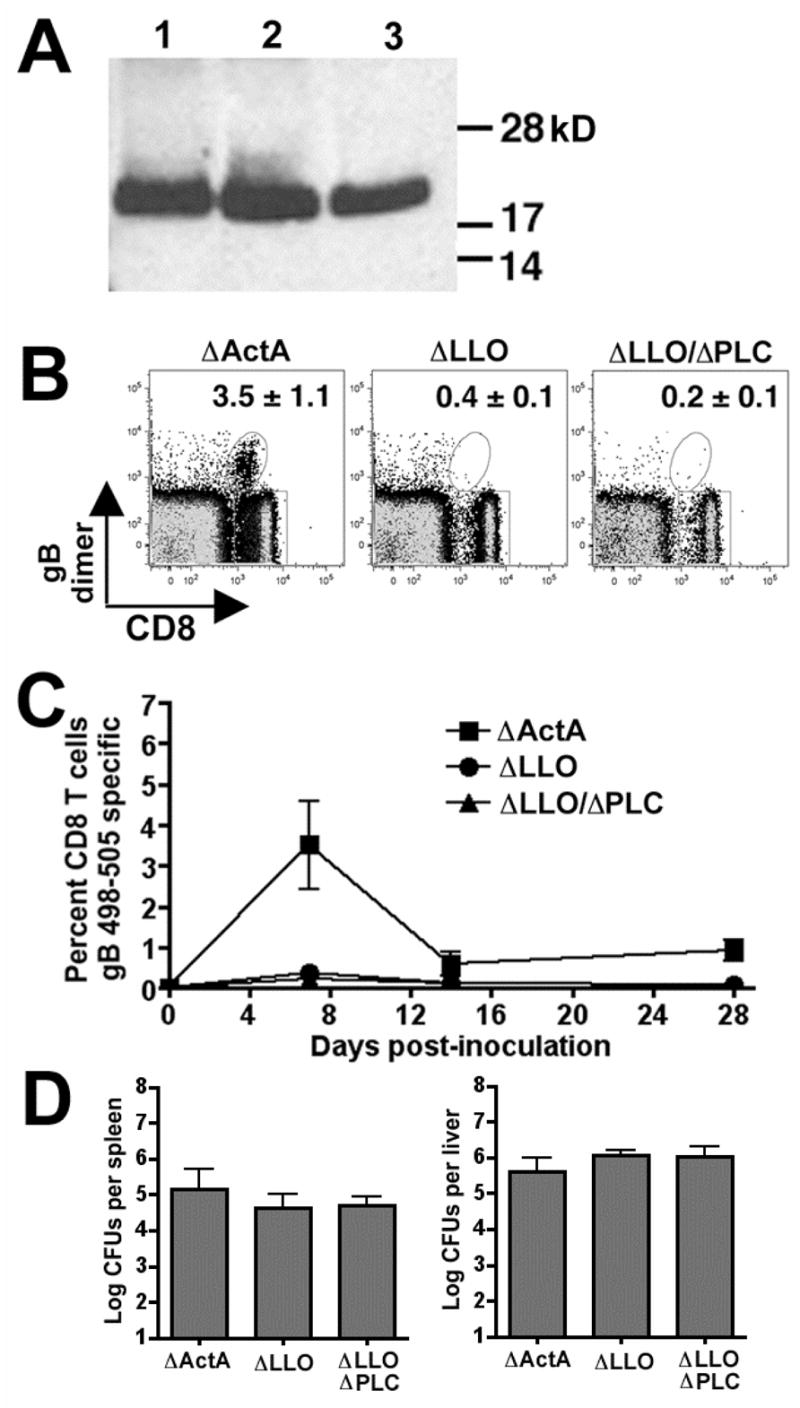
A. Western blot of supernatant protein probed with anti-HA antibody from Lm strains DPL1942 (ΔactA, lane 1), DPL2161 (ΔLLO, lane 2), and DPL2319 (ΔLLO/ΔPLC, lane 3) transformed with the expression construct encoding an HA-tagged recombinant protein containing the HSV gB498–505 peptide antigen. B. FACS plot of peripheral blood leukocytes after staining with H-2Kb dimer loaded with gB498–505 peptide day 7 after infection with 106 Lm ΔactA, 108 Lm ΔLLO, or 108 Lm ΔLLO/ΔPLC. Numbers in the upper right hand quadrant indicate the mean percentage (± standard error) of dimer+ cells among total CD8 T cells for seven to nine mice per group from two separate experiments. C. The percentage of gB498–505 dimer+ CD8 T cells in the peripheral blood at the indicated time points after infection for these same groups of mice. D. Number of recoverable bacterial CFUs in the spleen and liver 24 hours after infection with 106 Lm ΔactA, 108 Lm ΔLLO, or 108 Lm ΔLLO/ΔPLC. Bar, ± one standard error.
There is no apparent correlation between the ability to prime antigen-specific CD8 T cell responses after primary Lm infection with development and maintenance of protective immunity at memory time points. For example, a relatively low inocula of Lm ΔLLO does not prime a detectable CD8 T cell response yet confers protective immunity when challenged 28 days after infection [13], while another study revealed that there was no protection from challenge 25 days after initial infection even though Lm ΔLLO could prime a robust T cell response [12]. Accordingly, we examined the level of protection conferred by Lm ΔLLO and Lm ΔLLO/ΔPLC compared with Lm ΔactA 28 days after initial priming by challenge with wildtype Lm. To allow examination of the gB498–505 specific secondary response after challenge in these same mice, the virulent Lm strain used for challenge was transformed with the gB498–505 expression construct. As predicted, mice primed with Lm ΔactA compared with naïve mice had marked reduction in recoverable Lm CFUs in the livers and spleens day 4 after challenge with wildtype Lm, indicating protection (p < 0.05) (Figure 2A,B). Consistent with the results reported by Hamilton et al., mice primed with Lm ΔLLO compared with naïve mice also had marked reductions in Lm CFUs after challenge, and the level of protection in mice primed with Lm ΔLLO and Lm ΔactA was indistinguishable. In contrast, protection was significantly reduced for mice primed with Lm ΔLLO/ΔPLC compared with Lm ΔactA as significantly increased numbers of bacteria were recovered from both the livers and spleens after challenge (P< 0.05) (Figure 2A). These results reveal an important role of PLC in the ability of LLO-deficient Lm to trigger protective immunity to infection.
Figure 2.
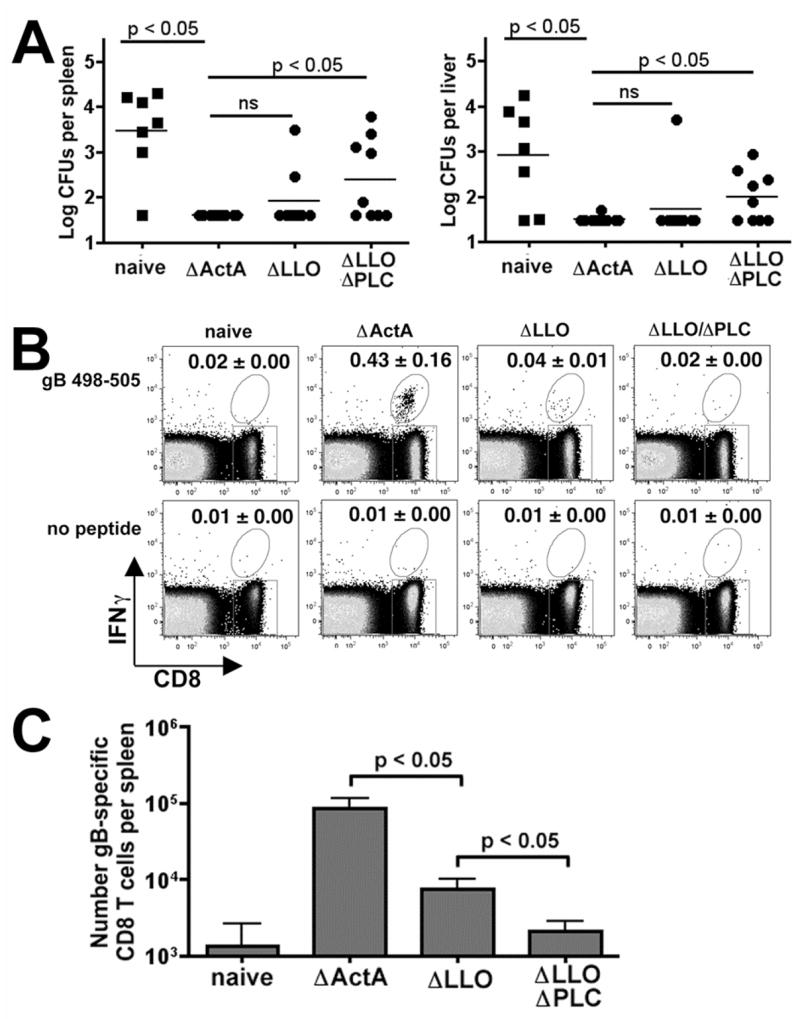
A. Recoverable Lm CFUs in the spleen and liver day 4 after infection with 2000 CFUs of wildtype Lm strain10403s containing the gB expression construct in either naïve mice or mice primed 28 days previously with the indicated Lm strains. These data are derived from seven to nine mice per group from two separate experiments. The difference between groups was analyzed using the Student’s T test (GraphPad software). FACS plot (B) demonstrating IFN-γ production by CD8+ splenocytes and total number (C) of IFN-γ producing CD8 T cells from these same mice after re-stimulation with gB498–505 peptide or no peptide. Numbers in the upper right hand quadrant indicate the mean percentage (± standard error) of IFN-γ+ cells of total CD8+ cells for 9–10 mice per group from two separate experiments.
In additional studies we compared the gB498–505 specific CD8 T cell response after wildtype Lm challenge in naïve mice or mice primed with Lm ΔactA, Lm ΔLLO, or Lm ΔLLO/ΔPLC. In both groups of protected mice (primed with Lm ΔactA or Lm ΔLLO), gB498–505 specific CD8 T cells that produce IFN-γ could be readily detected day 4 after challenge (Figure 2B,C), although the percentage and total number of these cells was significantly reduced in mice primed with Lm ΔLLO compared with Lm ΔactA. In contrast, the gB498–505 specific CD8 T cell response after challenge in mice primed with Lm ΔLLO/ΔPLC was comparable to that found in naïve mice and only at background levels (Figure 2B,C). These results indicate that while LLO-deficient Lm do not prime a detectable gB498–505 specific CD8 T cell response after primary inoculation, an antigen specific CD8 T cell response is primed that responds to secondary infection and confers protection from challenge with wildtype Lm. In contrast, Lm mutants defective in both LLO and PLC do not prime an antigen-specific CD8 T cell response and do not confer protection from wildtype Lm challenge.
DISCUSSION
Recombinant attenuated Lm strains are a promising class of vaccine vectors. However, the optimal means of attenuation that maximizes safety and immunogenicity has not been defined. An important virulence determinant in Lm is LLO; the LD50 for LLO deficient Lm mutant is increased > 4 log10 compared with wildtype Lm [17]. This extreme attenuation makes LLO-deficient Lm an attractive live attenuated vaccine vector, although the ability of LLO-deficient Lm strains to prime long-lasting antigen specific protection is unclear [12,13]. In this report, we demonstrate that priming with LLO-deficient Lm is able to confer protective immunity to subsequent Lm infection and that PLC expression by LLO-deficient Lm is required for these protective effects. Our findings that LLO-deficient Lm primes protective immunity to subsequent Lm infection are consistent with those of a recent study where the immune response and protective effects of LLO-deficient and wildtype recombinant Lm expressing an immune dominant H-2Kd antigen from LCMV were compared [13]. They report that prior infection with either LLO-deficient or WT Lm can trigger secondary expansion of antigen specific CD8 T cells and confer long-term protection to challenge with either virulent Lm or LCMV [13].
CD11c+ dendritic cells are essential for in vivo priming of antigen-specific CD8 T cells during Lm infection, although during infection the majority of dendritic cells are not directly infected with Lm [18,19]. These results suggest that, in addition to dendritic cells, cross presentation and indirect activation of other cell types have important roles in T cell priming during Lm infection. In vitro infection studies have demonstrated that while PLC can mediate cytoplasmic entry leading to the production of cytokines important in T cell priming such as type-I IFN in non-phagocytic cells, LLO is essential for cytoplasmic entry and activation of phagocytic cells such as dendritic cells or macrophages [7–9]. Accordingly, our findings that LLO-deficient Lm can confer protective immunity and that PLC is required for protective immunity primed by LLO-deficient Lm emphasize the overall importance of cytoplasmic entry into any cell type for T cell priming during Lm infection. Furthermore, these results imply that PLC-mediated cytoplasmic entry into non-phagocytic cells can bypass the requirement for LLO-mediated cytoplasmic entry into phagocytic cells allowing for priming of protective T cells during Lm infection. Additional studies are required to determine the specific cell type responsible for type-I IFN production during in vivo infection with WT and LLO-deficient Lm. Taken together, the results presented herein emphasize the potential of LLO-deficient Lm as vaccine vectors in targeting CD8 T cell immunity.
Acknowledgments
This work was supported in part by NIH-K08HD51584, a Wyeth Infectious Disease Society of America award, seed funding from Puget Sound Partners for Global Health, and a March of Dimes Basil O’Conner starter research award.
Footnotes
The authors have no commercial or other associations that might pose a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hamon M, Bierne H, Cossart P. Listeria monocytogenes: a multifaceted model. Nat Rev Microbiol. 2006;4:423–434. doi: 10.1038/nrmicro1413. [DOI] [PubMed] [Google Scholar]
- 2.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 3.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 6.Harty JT, Bevan MJ. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J Exp Med. 1992;175:1531–1538. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Riordan M, Yi CH, Gonzales R, Lee KD, Portnoy DA. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc Natl Acad Sci U S A. 2002;99:13861–13866. doi: 10.1073/pnas.202476699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brzoza KL, Rockel AB, Hiltbold EM. Cytoplasmic entry of Listeria monocytogenes enhances dendritic cell maturation and T cell differentiation and function. J Immunol. 2004;173:2641–2651. doi: 10.4049/jimmunol.173.4.2641. [DOI] [PubMed] [Google Scholar]
- 9.McCaffrey RL, Fawcett P, O’Riordan M, Lee KD, Havell EA, Brown PO, Portnoy DA. A specific gene expression program triggered by Gram-positive bacteria in the cytosol. Proc Natl Acad Sci U S A. 2004;101:11386–11391. doi: 10.1073/pnas.0403215101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berche P, Gaillard JL, Sansonetti PJ. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol. 1987;138:2266–2271. [PubMed] [Google Scholar]
- 11.Brunt LM, Portnoy DA, Unanue ER. Presentation of Listeria monocytogenes to CD8+ T cells requires secretion of hemolysin and intracellular bacterial growth. J Immunol. 1990;145:3540–3546. [PubMed] [Google Scholar]
- 12.Bahjat KS, Liu W, Lemmens EE, Schoenberger SP, Portnoy DA, Dubensky TW, Jr, Brockstedt DG. Cytosolic Entry Controls CD8+-T-Cell Potency during Bacterial Infection. Infect Immun. 2006;74:6387–6397. doi: 10.1128/IAI.01088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton SE, Badovinac VP, Khanolkar A, Harty JT. Listeriolysin O-deficient Listeria monocytogenes as a vaccine delivery vehicle: antigen-specific CD8 T cell priming and protective immunity. J Immunol. 2006;177:4012–4020. doi: 10.4049/jimmunol.177.6.4012. [DOI] [PubMed] [Google Scholar]
- 14.Brundage RA, Smith GA, Camilli A, Theriot JA, Portnoy DA. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc Natl Acad Sci U S A. 1993;90:11890–11894. doi: 10.1073/pnas.90.24.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orr MT, Orgun NN, Wilson CB, Way SS. Cutting Edge: Recombinant Listeria monocytogenes expressing a single immune-dominant peptide confers protective immunity to herpes simplex virus-1 infection. J Immunol. 2007;178:4731–4735. doi: 10.4049/jimmunol.178.8.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG. Early programming of T cell populations responding to bacterial infection. J Immunol. 2000;165:6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- 17.Barry RA, Bouwer HG, Portnoy DA, Hinrichs DJ. Pathogenicity and immunogenicity of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect Immun. 1992;60:1625–1632. doi: 10.1128/iai.60.4.1625-1632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muraille E, Giannino R, Guirnalda P, Leiner I, Jung S, Pamer EG, Lauvau G. Distinct in vivo dendritic cell activation by live versus killed Listeria monocytogenes. Eur J Immunol. 2005;35:1463–1471. doi: 10.1002/eji.200526024. [DOI] [PubMed] [Google Scholar]
- 19.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


