Abstract
Interleukin 17 (IL-17) family consists of six cytokines in mammals. Among them, IL-17 and IL-17F are expressed by a novel subset of CD4+ helper T (Th) cells and play critical function in inflammation and autoimmunity. On the other hand, IL-17E, also called IL-25, has been associated with allergic responses. Here we summarize recent work by us as well as other investigators in understanding the regulation and function of these three cytokines. From these studies, IL-17 family cytokines may serve as novel targets for pharmaceutical intervention of immune and inflammatory diseases.
Keywords: IL-17, IL-17F, IL-25, Inflammation, T-helper cells, autoimmune diseases
1. Introduction
Inflammation plays an important role in the resolution of pathogenic infections, tissue repair and modulation of innate and adaptive immune responses. However, chronic inflammation has been implicated in the pathogenesis of several autoimmune diseases and cancers. Cytokines such as IL-1β, TNFα and IL-6, refereed as proinflammatory cytokines, play critical roles in modulating inflammatory responses. Both non-immune and immune cells secrete these proinflammatory cytokines in response to infections or allergens. Upregulation of these cytokines have been associated with several autoimmune diseases and therapeutic strategies that block the action of these cytokines such as TNF blockade has been approved for treatment of Rheumatoid Arthritis (RA) and Crohn’s disease (CD) patients (Atzeni et al., 2005; Kyle et al., 2005; Lewis, 2007).
IL-17 is the founding member of a new cytokine family that has recently gained prominence due to its involvement in autoimmune and allergic diseases in both human and mouse. IL-17 family consists of six members including IL-17 (also called IL-17A), IL-17B, IL-17C, IL-17D, IL-17E (also called IL-25) and IL-17F (Aggarwal & Gurney, 2002; Gaffen, 2004; Huang et al., 2004; Kolls & Linden, 2004). Among these cytokines, IL-17, IL-25 and IL-17F are most investigated. IL-17 and IL-17F share the greatest similarity showing 50% identity at the amino acid level whereas IL-25 is most divergent in the family (Fossiez et al., 1998). In this review, we will discuss about the regulation and biological actions of these cytokines.
2. IL-17 and IL-17F: Expression, receptor signaling and biological functions
IL-17 was originally identified by Rouvier et al. from activated T cell clones and named CTLA-8 (Rouvier, Luciani, Mattei, Denizot, & Golstein, 1993; Yao et al., 1995). Bioinformatics approach combined with degenerative PCR strategies based on IL-17 sequence resulted in identification of five additional members of the IL-17 family (Kolls & Linden, 2004). Genes encoding IL-17 and IL-17F are located on chromosome 1-A4 region in mice and on chromosome 6p12 location in humans and are transcribed in opposite orientations. Considering their sequence similarity and proximity in the genome, IL-17 and IL-17F may have derived via a gene duplication event. Additionally, murine and human versions of IL-17 share 62% sequence similarity and IL-17F share 54% similarity. IL-17 and IL-17F are encoded in three exons and we have recently identified several conservative non-coding sequences in the locus (Akimzhanov, Yang, & Dong, 2007). Similar to chromatin changes identified in the locus containing IL-4, -5 and -13 genes in differentiating Th2 cells, IL-17 and IL-17F gene promoters and conserved non-coding sequence regions undergo coordinated chromatin modifications such as histone acetylation and methylation in Th cells (Akimzhanov et al., 2007). These data may provide mechanistic explanation for co-expression of IL-17 and IL-17F in T cells.
Except for IL-17B, all other IL-17 family members are N-glycosylated homodimers consisting of five highly conserved cystein residues forming characteristic cystein knot structure, similar to that found in TGF-β and nerve growth factor (Hymowitz et al., 2001). Additionally, it has been shown that IL-17 heterodimerizes with IL-17F, raising additional complexity in regulation of their biological functions (Chang & Dong, 2007; Wright et al., 2007).
2.1. Biological functions of IL-17 and IL-17F
IL-17 has long been implicated in several autoimmune diseases, primarily as a pro-inflammatory regulator (Langowski et al., 2006; Tartour et al., 1999; Witowski, Ksiazek, & Jorres, 2004). It was initially reported that CTLA-8 and Herpesvirus saimiri virus gene 13 product, named as IL-17 and vIL-17 respectively, induced NF-κB activity and IL-6 production in human fibroblasts (Yao et al., 1995; Yao et al., 1997). Later, it was shown that hematopoietic progenitor proliferation is sustained in the presence of fibroblasts treated with human IL-17, which stimulates the production of IL-6, IL-8, GM-CSF, and preferential maturation of neutrophils (Fossiez et al., 1996). These results suggest broader role for IL-17 as a potent inducer of other inflammatory cytokines. Moreover, IL-17 also synergizes with TNF-α in inflammatory regulation (Ruddy et al., 2004).
We further extended these observations and performed extensive analysis of IL-17-induced genes and found that several chemokines such as CXCL1 (Gro1), CXCL10, CCL2, CCL7, CCL20 (macrophage inflammatory protein-3α), and matrix metalloproteinases (MMP) 3 and 13 were upregulated upon IL-17 treatment (Park et al., 2005). We also showed that the expression of several chemokines that are required for mononuclear cell infiltration and pathogenesis of experimental autoimmune encephalomyelitis (EAE) was inhibited upon blocking IL-17 leading to reduced disease severity. Conversely, using transgenic mice overexpressing IL-17 in the lung, we demonstrated that IL-17 plays a direct role in induction of inflammatory reaction and IL-17 transgenic mice have increased expression of several chemokines and MMPs, which are implicated in patients with airway inflammation (Park et al., 2005).
Since IL-17 and IL-17F may bind to the same receptor(s), there is considerable overlap in the biological functions of these cytokines (Hizawa, Kawaguchi, Huang, & Nishimura, 2006; Numasaki, Tomioka, Takahashi, & Sasaki, 2004; Starnes et al., 2001). IL-17F also stimulates the production of IP-10 (interferon-gamma-inducible protein 10) in human bronchial epithelial cells which was enhanced by IFN-γ, IL-1β and TNF-α (Kawaguchi et al., 2007). We, as well as others, have shown that IL-17 and IL-17F form biologically active heterodimers with intermediate potency in inducing inflammatory genes (Chang & Dong, 2007; Wright et al., 2007). Taken together, IL-17 and related cytokines acts a potent proinflammatory cytokine responsible for the release of several chemokines and cytokines, synergizes with other proinflammatory cytokines in enhancing the inflammatory responses and contributing for pathogenesis of several autoimmune and inflammatory diseases.
2.2. Signaling mechanisms
Signaling of IL-17 family cytokines is primarily mediated by the IL-17 receptor family currently consisting of five individual members (Moseley, Haudenschild, Rose, & Reddi, 2003). Receptor for IL-17, IL-17R (also called IL-17RA), is a type 1 transmembrane receptor protein with a 525 amino acid long intracellular domain (Yao et al., 1997). Expression of IL-17R is detected ubiquitously in tissues including lung, kidney, liver, spleen etc. In humans, IL-17R mRNA has been shown in lymphocytes, fibroblasts, monocytes and vascular endothelial cells (Moseley et al., 2003). It was shown that IL-17R forms multimeric complexes even in the absence of ligand binding and receptors may undergo considerable conformational changes upon ligation by IL-17 (Kramer et al., 2006). Furthermore, IL-17R has been shown to associate with IL-17RC (Interleukin-17 receptor C) and acts as primary signal transducer of IL-17 and IL-17F (Toy et al., 2006; Zhou, Toh, Zrioual, & Miossec, 2007). Even though IL-17F binds to IL-17R, its affinity is ten-fold lower than IL-17, that may result in modulation of IL-17 biological responses.
How IL-17R signals has not been very clear. It was first shown that IL-6 induction by IL-17 in mouse embryonic fibroblasts (MEF) is dependent on TRAF6, TNF-receptor associated factor (Schwandner, Yamaguchi, & Cao, 2000). It was reported that TRAF6 is recruited to IL-17R upon IL-17F binding and ubiquitinate the receptor whereas IL-17-mediated ubiquitination does not require TRAF6 (Rong et al., 2007). These results indicate that despite using same receptor, mechanisms of signal transduction by IL-17 and IL-17F may use different intracellular signaling pathways. Both IL-17R and IL-17RC possess cytoplasmic domains containing conserved SEFIR (similar expression to fibroblast growth factor, IL-17 receptor and Toll-IL1R family) motifs which mediate homophilic interactions (Novatchkova, Leibbrandt, Werzowa, Neubuser, & Eisenhaber, 2003). However, our recent analysis indicated that Myd88 or IRAK4, essential components of TLR and IL-1R signaling, are not required for IL-17-induced IL-6 expression (Chang, Park, & Dong, 2006). Furthermore, the intracellular domain of IL-17R contains a novel TIR-like loop which is required for the activation of NF-κB, MAP Kinases and upregulation of C/EBPβ and C/EBPδ (Maitra et al., 2007).
Studies in our laboratory demonstrated the function of the SEFIR domain in IL-17R, which directly interacts with an NF-κB activator protein, Act1 (Chang et al., 2006). Act1 physically associates with the intracellular domain of IL-17R through homotypic interactions and knocking down Act1 expression leads to abrogation of IL-17-induced inflammatory gene expression and NFκB activation (Chang et al., 2006). It was further confirmed, using Act1-deficient mice, that IL-17-mediated inflammatory responses are indeed severely impaired in these mice (Qian et al., 2007). Act1 but not IL-17R contains a TRAF6 binding motif, raising the possibility of Act1-dependent recruitment of TRAF6 into the IL-17R signalosome. These studies identify important signaling regulators of IL-17R, which may be helpful in designing therapeutic targets to resolve inflammatory conditions associated with increased IL-17 signaling.
3. Regulation of IL-17 and IL-17F expression
3.1. Th17 as a new effector Th subset
Expression of IL-17A was first detected in memory CD4+ T cells from peripheral blood in humans (Aarvak, Chabaud, Miossec, & Natvig, 1999; Yao et al., 1995). In addition to CD4+ T cells, IL-17 is expressed by CD8+ T cells, NK cells, γδ T cells and neutrophils under certain conditions (Ferretti, Bonneau, Dubois, Jones, & Trifilieff, 2003; Kryczek et al., 2007; Shibata, Yamada, Hara, Kishihara, & Yoshikai, 2007). Recent studies from various groups have focused on the murine Th cells that produce IL-17 and have indicated that IL-17 is predominantly expressed by a Th cell lineage that is distinct from Th1 and Th2 cells (Dong, 2006).
For many years, Th1 and Th2 helper T cells represent two mutually exclusive differentiation programs undertaken by naïve CD4+ T cells during adaptive immune responses (Cher & Mosmann, 1987; Cherwinski, Schumacher, Brown, & Mosmann, 1987; Mosmann, Cherwinski, Bond, Giedlin, & Coffman, 1986; K. M. Murphy & Reiner, 2002; Szabo, Sullivan, Peng, & Glimcher, 2003). IFN-γ is the signature cytokine produced by Th1 cells and responsible for immunity against intracellular pathogens (Dong & Flavell, 2000). IL-4, IL-5 and IL-13 are secreted by Th2 cells, which provide immunity against extracellular pathogens and play an important role in allergic responses (Reiner, 2001).
Th1 cells have been historically implicated in autoimmune diseases such as murine experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis (CIA) models which are used to study human diseases multiple sclerosis (MS) and rheumatoid arthritis (RA), respectively (Constantinescu et al., 1998; Kageyama et al., 1998; Leonard, Waldburger, & Goldman, 1995, 1996; Matthys et al., 1998). However, mice deficient in IFN-γ or STAT1 (an important intracellular signaling component of IFNγ signaling pathway) were still susceptible to EAE and CIA, suggesting that an additional Th cell type other than Th1 cells plays a more important pathogenic role in these autoimmune diseases (Bettelli et al., 2004; Ferber et al., 1996; Kageyama et al., 1998; Willenborg, Fordham, Bernard, Cowden, & Ramshaw, 1996). A clue on IL-17 came from a study, in which mice deficient in inducible costimulator (ICOS) were protected against collagen-induced arthritis, which was associated with normal Th1 response but greatly reduced production of IL-17 (Dong & Nurieva, 2003). More conclusively, it was demonstrated that IL-23 rather than IL-12 is critical for the development of CIA and EAE (Y. Chen et al., 2006; Cua et al., 2003; Langrish et al., 2005; C. A. Murphy et al., 2003). In a series of experiments, these groups showed that IL-23 is important for IL-17 production and could function to expand antigen-primed IL-17-expressing T cells. Thus, the IL-17-producing Th cells appear to have distinct regulation than Th1 cells.
Generation of IL-17-producing CD4+ T cells in vitro had not been possible until recently, primarily due to strong inhibitory actions by Th1 and Th2 cytokines present during naïve CD4+ T cell differentiation. In studying the differentiation of IL-17-producing cells in vitro and in vivo, it was concluded that generation of these cells was independent of cytokines and transcription factors involved in Th1 or Th2 differentiation and thus provided first evidence of a novel subset of T-helper cells which produce IL-17 and later referred as Th17 cells (Harrington et al., 2005; Park et al., 2005).
3.2. Cytokine regulation of Th17 cell differentiation
Although IL-23 is an important factor in regulation of Th17 cells in vivo, it does not appear to have a direct effect on naïve T cells to induce Th17 differentiation. It has now been shown by several groups that TGFβ and IL-6 together potently instruct T cells to differentiate into Th17 cells. TGFβ was identified as a cytokine responsible for the development of Th17 cells in vitro and in vivo and showed that TGFβ along with IL-6 are sufficient for the induction of Th17 differentiation (Bettelli et al., 2006; Mangan et al., 2006). Betelli et al. also demonstrated that IL-6 signaling is critical for the inhibition of FoxP3 expression induced by TGFβ and provided the crucial link for the divergence of T-regulatory cells and Th17 cell lineages. These results were consistent with an earlier report that showed that Th17 cells can be induced by coculturing with Treg cells, which serve as a source of TGF-β, and inflammatory cytokine milieu (Veldhoen, Hocking, Atkins, Locksley, & Stockinger, 2006). As demonstrated earlier, TLR signaling can relieve the suppressive effect of Treg cells by an IL-6-dependent mechanism (Pasare & Medzhitov, 2003), aforementioned studies indicate that infections and local inflammatory conditions may alleviate the suppressive effect of Treg cells which act as reservoirs of TGFβ and promote Th17 cell differentiation.
Th1 and Th2 differentiation is mediated not only by cytokines produced by the innate immune system, but also IFNγ and IL-4, respectively, as autocrine factors. Studies have shown that IL-21 is an essential cytokine produced by Th17 cells and functions in promoting Th17 differentiation. IL-6 induce the production of IL-21 which is further amplified by autocrine signaling and promotes the development of Th17 cells and inhibits generation of Foxp3+ regulatory T cells (Nurieva et al., 2007). IL-21-deficient mice exhibit deficiency of Th17 cells in vivo and are resistant to EAE pathogenesis mediated by Th17 cells. Independently, it was demonstrated that clearance of Treg cells in IL-6-deficient mice resulted in the reappearance of Th17 cells which was in part dependent on IL-21 (Korn et al., 2007); and that IL-6 is required for IL-21 induction which amplifies its own production and leads to IL-23R expression (L. Zhou et al., 2007). Furthermore, IL-21 and IL-23 signaling results in the upregulation of a critical transcriptional regulator of Th17 cells, RORγt (Retinoid-related Orphan Receptor gamma).
In addition to initial priming by TGFβ and IL-6 and further amplification by IL-21, effector Th17 cells are also regulated by other cytokines. Even though dispensable for initial commitment of Th17 cells, IL-23 has been shown to synergize with IL-6 in promoting Th17 differentiation (Yang et al., 2007). In addition, it acts on memory CD4 cells and enhance IL-17 secretion and may be involved in survival and expansion in vivo (Aggarwal, Ghilardi, Xie, de Sauvage, & Gurney, 2003). Moreover, IL-23 cooperates with IL-1α and IL-1β and enhances IL-17 secretion, independent of TCR stimulation (Sutton, Brereton, Keogh, Mills, & Lavelle, 2006). It has been shown that IL-18 also synergizes with IL-23 in inducing IL-17 production in CD4+ T cells (Mathur et al., 2007). These cytokine pathways may play a critical role in promoting inflammatory conditions by inducing IL-17 secretion from Th17 cells in the antigen-independent manner and contribute to disease pathogenesis.
Generation and function of Th17 cells are also negatively regulated by cytokines of which the role of IL-2 and IL-27 has been well described. IL-2 has been shown to negatively regulate the differentiation of naïve T cells into Th17 cells which is dependent on STAT5 (Laurence et al., 2007). STAT3 signaling enhances RORγt expression and Th17 differentiation whereas activation of the STAT5 pathway has an opposing effect on Th17 cells. Since IL-2 is critical for maintenance of Treg cells, IL-2 signaling may provide another checkpoint for the reciprocal differentiation of Treg and Th17 cells. Additionally, IL-27 has been shown to suppress both the differentiation of Th17 cells, cytokine secretion by Th17 cells and protect mice from Th17-mediated EAE disease (Batten et al., 2006; Y. Chen et al., 2006; Z. Chen et al., 2006; Fitzgerald et al., 2007). It has also been suggested that that IL-27 may offer protection against uveoretinitis in humans by suppressing Th17 cell expansion and function (Amadi-Obi et al., 2007). Hence, therapeutic intervention of these inhibitory cytokine pathways may be exploited in treating inflammatory diseases mediated by Th17 cells.
Even though TGFβ has been shown to be a cytokine potent for inducing Th17 differentiation in mice, the scenario is completely different in humans. In two independent recent studies, it has been shown that in the absence of TGF-β, IL-1β induces Th17 differentiation which was further enhanced by either IL-6 or IL-23 (Acosta-Rodriguez, Napolitani, Lanzavecchia, & Sallusto, 2007; Wilson et al., 2007). In fact, TGFβ showed a suppressive effect on Th17 differentiation induced by IL-1β and IL-6. IL-1β and IL-6 are produced by antigen-presenting cells stimulated by microbial products. Moreover, psoriatic lesions contain IL-23-producing DCs that may be involved in the expansion of Th17 cells in human skin. Even though the cytokine profiles secreted by human and murine Th17 cells overlap and may function similarly, their differentiation pathways and cytokine requirements may be somewhat different and require further characterization.
3.2 Transcriptional regulation of Th17 cells
During Th1 and Th2 differentiation, innate cytokines signal through particular STAT family members to establish lineage-specific transcriptional programs. Initial characterization of Th17 cells have shown that their differentiation is not dependent on STAT1, 4 or 6 and they do not express any conventional transcriptional factors such as T-bet, HLX and GATA3 which were involved in Th1 and Th2 differentiation (Harrington et al., 2005; Park et al., 2005). T cells deficient in Socs3 (suppressor of cytokine signaling 3), a negative regulator STAT3 phosphorylation, deficiency has resulted in great enhancement in IL-17 expression and Socs3-conditional knockout mice develop systemic autoimmune disease, similar to the phenotype observed in IL-17 and IL-23p19 transgenic mice (Z. Chen et al., 2006). Subsequently, we provided direct evidence that STAT3-deficiency resulted in impaired differentiation of Th17 cells (Yang et al., 2007). Furthermore, a hyper-active form of STAT3 can induce differentiation of Th17 cells and enhance the expression of Th17-associated genes. More importantly, STAT3 is involved in the expression of RORγt, a critical transcription factor required for Th17 differentiation. STAT3 mediated signals also repress Th1 associated transcription factor T-bet and FoxP3 that are required for Treg cell differentiation and these results are further confirmed by another independent study (Laurence et al., 2007).
Additional clues regarding the identification of a master transcription factor that directs Th17 differentiation came from Affymetrix gene chip analysis of Th1 and Th17 cells: it was observed that RORγt levels are elevated specifically in Th17 cells (Ivanov et al., 2006). Earlier, Littman and colleagues found that RORγt, a member of orphan nuclear hormone receptor superfamily, is expressed in lymphoid-tissue inducer cells (LTi) in the fetus and involved in the development of lymph nodes and payers patches. Curiously, it is also expressed in periphery by some of the TCRαβ+ and TCRγδ+ cells found in lamina propria (Eberl & Littman, 2003; Eberl et al., 2004). An important observation is that T cells from lamina propria that expresses RORγt are IL-17+ and these IL-17-expressing cells are greatly reduced in RORγt-deficient mice (Ivanov et al., 2006). These results suggest that RORγt may be required for the generation of Th17 in lamina propria. Consistent with this notion, retrovial-mediated forced expression of RORγt in the activated CD4+ T cells is sufficient to drive the differentiation of naïve CD4+ T cells into Th17 lineage of cells and induce the expression of IL-17A and IL-17F (Ivanov et al., 2006). Furthermore, RORγt-deficiency greatly reduces the differentiation of Th17 cells even in the presence of TGFβ and IL-6, establishing RORγt as a master transcriptional regulator of Th17 differentiation. Furthermore, RORγt-deficient mice are resistant to EAE pathogenesis mediated by Th17 cells (Ivanov et al., 2006).
Recent studies further elucidated the interplay between the cytokine pathways and the transcription factors involved in Th17 differentiation. RORγt synergizes with STAT3C, a critical signal transducer of IL-6 and IL-21, in enhancing IL-17 expression and increased the percentage of IL-17 producing cells obtained upon activated CD4+ T cells (L. Zhou et al., 2007). STAT3 is also required for upregulation of IL-21, another critical cytokine involved in Th17 differentiation (Nurieva et al., 2007; L. Zhou et al., 2007).
Additionally, the interferon regulatory factor-4 (IRF4) was identified as an important transcription factor necessary for Th17 lineage differentiation; IRF4 deficiency resulted in decreased RORγt expression and increased FoxP3 expression that may negatively impact Th17 differentiation (Brustle et al., 2007). Since IRF4 was previously identified as a critical factor in Th2 differentiation, it remains to be seen whether differentiation of Th2 and Th17 cells undergo some common changes before differentiating into respective lineages (Hu, Jang, Fanzo, & Pernis, 2002; Lohoff et al., 2002; Rengarajan et al., 2002).
How mechanistically the above transcription factors function to establish Th17 gene expression programs remain unknown. Detailed analysis of epigenetic changes in the IL-17 locus and the analysis of transcription factor binding will be helpful in understanding the mechanism of transcriptional regulation of Th17 cell differentiation. IL-17 and IL-17F gene promoters undergo lineage-specific chromatin remodeling, providing insights into the regulation of Th17 differentiation at epigenetic level (Akimzhanov et al., 2007). Moreover, several non-coding conserved sites have been identified in the IL-17-IL-17F locus and have been shown to undergo coordinated chromatin modifications such as histone acetylation and methylation in differentiating Th17 cells. Further analyses is required to establish the precise role of these transcription factors and to understand whether they act cooperatively or sequentially in imprinting and maintaining Th17 differentiation program in CD4+ T cells.
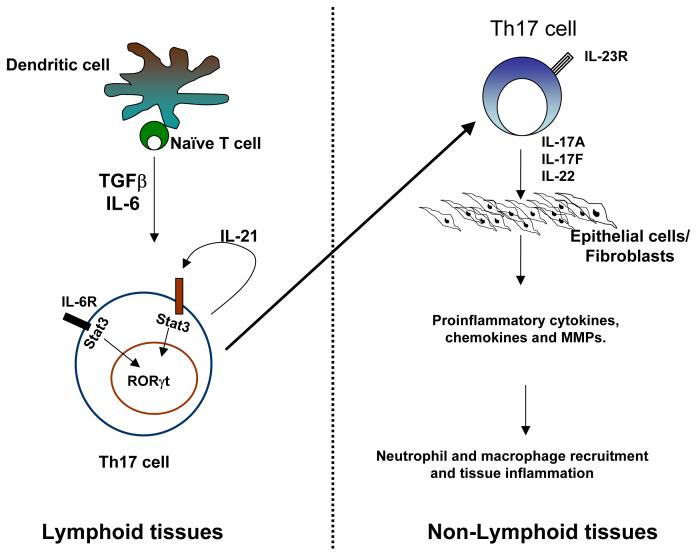
In summary, IL-17 and IL-17F are expressed by a novel subset of Th cells which are induced in secondary lymphoid organs and regulate inflammatory responses in a variety of nonlymphoid tissues (Figure 1). Further understanding of the regulation as well as function mechanisms may benefit treatment of inflammatory diseases.
Figure 1. Overview of Th17 lineage differentiation and function.
Naïve T cells activated in the presence of TGFβ and IL-6 differentiate into Th17 cells. IL-6 induces IL-21 production by Th17 cells and acts in an autocrine manner to amplify their differentiation. IL-23 is required for the maintenance, expansion and in vivo function of Th17 cells. IL-17 acts of epithelial cells, fibroblasts, microglial and other cell types and trigger the production of proinflammatory cytokines, several chemokines and MMPs which promote neutrophil and macrophage recruitment and result in tissue inflammation.
4. IL-25 (IL-17E)
IL-25/IL-17E was originally identified on the basis of sequence homology search of other IL-17 family members (Fort et al., 2001; Lee et al., 2001). However, unlike other IL-17 family member, IL-25 had a unique function in promoting type 2 immune response. The biological activity of this cytokine was initially described in vivo by exogenous administration of IL-25 protein (Fort et al., 2001; Hurst et al., 2002) or IL-25 transgenic mice (Kim et al., 2002; Pan et al., 2001). These mice showed upregulation of Th2 cytokine transcripts, including IL-4, IL-5 and IL-13 in several tissues, eosinophilia, mucus hyperplasia and epithelial cells hyperplasia, implicating that this particular IL-17 family cytokine is a new player in regulating type 2 immunity, which appears to be distinct from that by IL-17 or IL-17F.
4.1 The source of IL-25 and its cellular targets
Initially, IL-25 was described as a Th2 derived cytokine since its transcript expression was found in highly polarized Th2 cells in vitro (Fort et al., 2001). However, broad expression in several other tissues has implicated that there might be multiple cellular sources of IL-25 other than Th2 cells. Later on, several other cell types were reported to be potential IL-25 producers, including mast cells activated by IgE cross-linking (Ikeda et al., 2003), alveolar macrophage after particle inhalation (Kang et al., 2005) and activated eosinophils and basophils (Wang et al., 2007). IL-25 can also be produced by lung epithelial cells and alveolar macrophages upon allergen stimulation (Angkasekwinai et al., 2007). In addition to those studies, identification of the cellular source of IL-25 in vivo was examined; it was found that some gut-associated T cell subpopulation (Owyang et al., 2006) and brain microglia (Kleinschek et al., 2007) constitutively expressed IL-25 and their expression was associated with its protective role in limiting local inflammation.
The receptor for IL-25, IL-17BR also called EVI27 (or IL17 receptor homolog 1, IL-17Rh1), was identified sharing the same receptor as IL-17B but with a higher affinity of binding (Lee et al., 2001; Shi et al., 2000; Tian et al., 2000). Unlike the broader expression of IL-25, its receptor has been shown to be more restricted in expression, highest being in kidney, liver and small intestine. The first IL-25 responder cell identified that exhibit Th2 cytokine production was a novel accessory population of non-T/non-B cells (NBNT), characterized as Lin-, MHC classIIhigh and CD11cdull (Fort et al., 2001). This population was further described to be important for the initiation of N. brasiliensis worm clearance in vivo (Fallon et al., 2006).
4.2 The role of IL-25 in immune mediated diseases
4.2.1 Allergic asthma disease
Allergic asthma disease is characterized by mucus hyperplasia, airway hyperresponsiveness (AHR), airway infiltration of Th2 cells, and eosinophils (Renauld, 2001). Systemic overexpression or adminitration of IL-25 or instillation of IL-25 into the lung consistently resulted in eosinophilia, an increase in serum IgE and IgG1, upregulation of tissue expression of IL-4, IL-5, and IL-13, and lung pathological changes, including epithelial cell hypertrophy and mucus hypersecretion (Fort et al., 2001; Hurst et al., 2002; Kim et al., 2002; Pan et al., 2001), implicating that IL-25 might play a role in regulating allergic asthma disease.
Several recent studies attempted to investigate the function of IL-25 in allergic asthma disease. By using a novel anti-IL-25 antibody, we found that blockade of IL-25 in an allergen-induced allergic inflammation resulted in decrease in antigen-specific Th2 cells and eosinophil infiltration in lung (Angkasekwinai et al., 2007). Conversely, transgenic overexpression of IL-25 by lung epithelial cells showed a pro-allergic type 2 phenotype, including hyper mucus secretion, increased infiltration of eosinophils and macrophages, and upregulation of several type 2 related chemokines, such as eotaxin, MDC (macrophage derived cytokine), and RANTES (regulated upon activation, normal T-cell expressed and secreted).
Considering that lung epithelial cells, when exposed to allergen such as Aspergillus oryzae and Ragweed, upregulate IL-25 gene expression, IL-25 may participate in innate immune responses to allergens and mediate in the initiation of allergic inflammation by recruiting Th2 cells and eosinophils. . It has been shown that IL-25 expression is upregulated during an Ova-induced airway inflammation, similar to allergic asthma, and promotes recruitment of Th2 cells and eosinophils in the airways (Tamachi et al., 2006). Tamachi et. al., found that IL-25 gene expression could be upregulated in the lung upon allergen challenging. Neutralization of IL-25 with a soluble IL-25 receptor resulted in the inhibition of infiltration of eosinophils and Th2 cells into lung, whereas overexpression could enhance the inflammation. The IL-25-mediated airway inflammation has been show to require CD4+T cells and the STAT6 signaling pathway. In addition, it was demonstrated that IL-25 instillation into lung not only induced pulmonary eosinophil infiltration and Th2 cytokine expression but also airway hyperreactivity (Sharkhuu et al., 2006). The mechanism by which IL-25 induce those allergic responses was suggested through the direct function of IL-25 on peribronchial lymph node T cells.
A possible association between IL-25 expression and human chronic allergic diseases such as chronic asthma and atopic dermatitis, a Th2 cell-mediated skin disease, was described (Wang et al., 2007). It was shown that eosinophils and basophils from normal subjects and asthmatic patients were the major IL-25 producers, and IL-25-expressing eosinophils enhanced the functions of activated Th2 memory cells in the presence of TSLP (Thymic stromal derived lymphopoietin)-treated DC by augmenting the expression of IL-5 and IL-13. The function of IL-25 in human allergic disease was suggested to further amplify Th2 function during disease progression.
4.2.2 Parasitic infection
While an inappropriate type 2 immune response may lead to allergic disease, type 2 immunity plays a protective role in defense against parasitic infection. Studies using different helminth infection models provided the evidence that IL-25 plays a critical role during parasitic infection (Fallon et al., 2006; Owyang et al., 2006). Owyang et al. found that exogenous IL-25 treatment induced protective type 2 immunity to Trichuris infection, while IL-25-deficient mice failed to protect against the infection which is associated with decreased antigen-specific Th2 cytokine responses (Owyang et al., 2006). Furthermore, IL-25 has been demonstrated to play an additional role during chronic infection by limiting the intestinal inflammation and expression of proinflammatory cytokines IFN-γ and IL-17. Using a helminth Nippostrongylus brasiliensis infection model, it was shown that IL-25 administration could induce rapid worm clearance (Fallon et al., 2006). Moreover, IL-25-deficient mice had delayed type 2 cytokine production, leading to inefficient worm clearance. Although IL-25 promotes type 2 cytokine response and immunity to Trichuris in a lymphocyte-dependent manner, IL-25-mediated Nippostronglus worm expulsion was shown to be T and B cell independent. Despite some differences in regulation of IL-25 during different parasitic infection models, the overall data so far implicate the important roles of IL-25 in parasitic immunity.
4.2.3 Experimental Autoimmune Encephalomyelitis disease
IL-17 has been shown to be an important cytokine initiating the development of EAE (Langrish et al., 2005; Park et al., 2005). The role of IL-25 in inhibiting IL-17-mediated EAE disease was reported: it was found that IL-25 is expressed by microglia in the brain, which may control the development of EAE (Kleinschek et al., 2007). Further more, IL-25-deficient mice had greater susceptibility to the disease which was associated with more infiltrated IL-17-producing CD4+T cells and less peripheral Th2 cells. Exogenous IL-25 treatment in EAE resulted in increase in IL-4, IL-5, and IL-13 levels, leading to the disease suppression in initiation, effector phase or relapsing period. The mechanism underlying disease protection was shown to be mediated by IL-13 since IL-25 administration in IL-13 deficient mice failed to protect from EAE. The inhibitory role of IL-13 was further delineated to be associated to the inhibition of IL-23, IL-1, and IL-6-expression by activated dendritic cells, suggesting that the function of IL-25 in suppressing the development of EAE may be due to the regulation of Th17 development indirectly through IL-13. Whether or not IL-25 plays a unique role in opposing other IL-17-mediated diseases remained to be clarified.
Since IL-25 plays crucial functions not only in initiating the allergic disease and regulating parasitic infection but also suppressing IL-17 mediated experimental autoimmune encephalitis disease, by neutralization or administration of this cytokine may provide the novel therapeutic approach in treatment of allergic asthma disease, multiple sclerosis and parasitic infections.
4.3 The regulation of IL-25 on Th2 differentiation and expansion
Since IL-25 is important for regulating type 2 immune response, several studies have attempted to investigate whether or not IL-25 may function in directing Th2 differentiation. Two studies clearly indicated that IL-25 works directly on T cells and regulate Th2 differentiation and Th2 effector/memory cells expansion in vitro (Angkasekwinai et al., 2007; Wang et al., 2007).
We found that the receptor for IL-25, IL-17BR transcript, is highly expressed by mouse Th2 cells but not Th1 or Th17 cell lineages (Angkasekwinai et al., 2007). Moreover, IL-25 treatment during CD4+ T cell differentiation enhanced Th2 cytokine production, including IL-4, IL-5 and IL-13 and inhibited IFN-γ production. This effect was greatly potentiated in the presence of anti-IFNγ antibody. These data indicate a novel role of IL-25 in regulating Th2 differentiation. IL-25 thus serves as an innate signal induced in response to allergen and parasitic infections to promote Th2 differentiation.
IL-25 not only plays a role during Th2 differentiation, but is also involved in functional regulation of effector Th2 cells and memory Th2 cells. IL-25 acts on in vitro differentiated effector Th2 cells by further enhancing their Th2 cytokine production (Wang et al., 2007). After finding high expression of IL-17BR transcript in Th2 memory cells, the role of IL-25 in direct regulation of Th2 memory cells was demonstrated (Wang et al., 2007). IL-25 acts on CRTH2+ (Chemoattractant receptor-homologous molecule expressed on T cells) human Th2 central memory cells that were previously activated with TSLP treated DCs, leading to their expansion and production of Th2 cytokines IL-4, IL-5, and IL-13 (Wang et al., 2007). These data clearly suggested that not only IL-25 could directly influence naïve T cell differentiation, but also enhance effector Th2 cell expansion, and memory Th2 cell polarization.
4.4 The molecular mechanism by which IL-25 regulates Th2 cells
IL-25 is a new player modulating CD4+ helper T cells differentiation. In attempting to identify the molecular mechanism by which IL-25 regulate Th2 differentiation, we found that IL-25 upregulated the expression of IL-4 gene transcript at day 2 after activation of naïve T cells and further enhanced its production by day 3 after activation (Angkasekwinai et al., 2007). These data indicated that IL-25 may induce Th2 differentiation by regulating early IL-4 gene expression. By using antagonistic anti-IL-4 antibody and IL-4 deficient mice, it was shown that Th2 cytokine production enhancement by IL-25 treatment is completely dependent on IL-4, indicating that endogenous IL-4 is required for IL-25-mediated Th2 differentiation enhancement. Investigating whether IL-25 may regulate the expression of transcription factors involved in Th2 lineage decision, we discovered that IL-25 treatment increased early IL-4 expression by upregulating the expression of transcription factor NFATc1 and JunB, which then possibly activate GATA-3 and STAT6 through the IL-4 signaling pathway. Unlike the early Th2 differentiation mediated by IL-25, it was found that IL-25-mediated regulation of CRTH2+CD4+Th2 memory cells was independent of IL-4 (Wang et al., 2007). In characterizing the expression of several transcription factors, it was found that IL-25 prevented the down-regulation of GATA-3, c-maf and JunB in Th2 memory cells during TCR triggering (Wang et al., 2007). Adding an antagonistic anti-IL-4 antibody could not inhibit the effects of IL-25 treatment. Collectively, these results suggested that IL-25 might utilize different molecular mechanism during different stages of Th2 differentiation. During naïve CD4+ helper T cell differentiation, IL-25 may regulate Th2 lineage commitment by enhancing the early IL-4 expression through the direct activation of NFATc1 and JUNB. IL-4 may further activate STAT-6 and upregulate GATA-3, leading to the Th2 differentiation. Unlike naïve CD4+ T cells, Th2 memory cells express high levels of IL-4 and transcription factors GATA-3, c-maf, and JUNB, and IL-25 may be involved in the maintenance of their expression that is independent of IL-4 signaling.
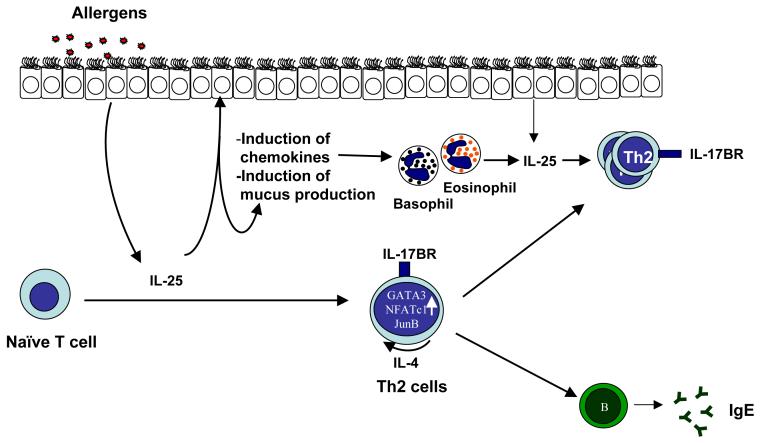
Therefore, compared to IL-17 and IL-17F, IL-25 has a unique function in promoting pro-allergic inflammatory responses (Fig. 2). Further understanding on the source as well as function of IL-25 will be important before designing therapeutic approaches to target IL-25 in allergic diseases.
Figure 2. The function of IL-25 in allergic inflammation.
IL-25 produced by lung epithelial cells, eosinophils and basophils upon allergen sensitization initiates allergic cascade by regulating both innate and adaptive immune response in responsible to the mucus production, effector cell recruitment, and Th2 cell differentiation and expansion.
5. Conclusions
Studies on IL-17 cytokines, especially on IL-17, IL-17F and IL-25 have greatly advanced our knowledge of immune responses, especially revealing novel pathways of T cell differentiation and regulation. IL-17 family cytokines have gradually come to the center stages of immune system performance, and play essential roles in type 1 and type 2 inflammation. Future work on these cytokines, especially on their regulation and signal transduction, will further benefit our understanding of the immune responses and may help to develop new treatments of immune diseases. In addition, other IL-17 cytokines- IL-17B, IL-17C and IL-17D await exploration. So far, these cytokines have not been shown to be expressed in T cells and perhaps are involved in innate regulation of inflammation. The proteins in IL-17R family are also poorly studied, and understanding of their expression, regulation and cell type-specific signaling may reveal complexity in orchestration of the inflammation symphony.
Abbreviations
- CRTH
Chemoattractant receptor-homologous molecule expressed on T cells
- CTLA-8
Cytotoxic T lymphocyte-associated Antigen 8
- EAE
Experimental autoimmune encephalomyelitis
- GATA3
GATA binding protein 3
- ICOS
Inducible costimulator
- NF-κB
Nuclear factor of kappa-B
- NFAT
Nuclear factor of activated T cells
- RA
Rheumatoid arthritis
- ROR
Retinoid-related Orphan Receptor
- STAT
Signal transducer and activator of transcription
- T-bet
T-box expressed in T cells
- TGFβ
Transforming growth factor beta
- TNFα
Tumor necrosis factor alpha
- TRAF
TNF-receptor associated factor
- TSLP
Thymic stromal derived lymphopoietin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarvak T, Chabaud M, Miossec P, Natvig JB. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J Immunol. 1999;162(3):1246–1251. [PubMed] [Google Scholar]
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007 doi: 10.1038/ni1496. doi:10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278(3):1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71(1):1–8. [PubMed] [Google Scholar]
- Akimzhanov AM, Yang XO, Dong C. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J Biol Chem. 2007;282(9):5969–5972. doi: 10.1074/jbc.C600322200. [DOI] [PubMed] [Google Scholar]
- Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13(6):711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- Angkasekwinai P, Park H, Wang YH, Chang SH, Corry DB, Liu YJ, et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204(7):1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzeni F, Turiel M, Capsoni F, Doria A, Meroni P, Sarzi-Puttini P. Autoimmunity and anti-TNF-alpha agents. Ann N Y Acad Sci. 2005;1051:559–569. doi: 10.1196/annals.1361.100. [DOI] [PubMed] [Google Scholar]
- Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7(9):929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200(1):79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, et al. The development of inflammatory TH-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007 doi: 10.1038/ni1500. doi:10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17(5):435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem. 2006;281(47):35603–35607. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116(5):1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, et al. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103(21):8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cher DJ, Mosmann TR. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987;138(11):3688–3694. [PubMed] [Google Scholar]
- Cherwinski HM, Schumacher JH, Brown KD, Mosmann TR. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu CS, Wysocka M, Hilliard B, Ventura ES, Lavi E, Trinchieri G, et al. Antibodies against IL-12 prevent superantigen-induced and spontaneous relapses of experimental autoimmune encephalomyelitis. J Immunol. 1998;161(9):5097–5104. [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6(4):329–334. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- Dong C, Flavell RA. Cell fate decision: T-helper 1 and 2 subsets in immune responses. Arthritis Res. 2000;2(3):179–188. doi: 10.1186/ar85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Nurieva RI. Regulation of immune and autoimmune responses by ICOS. J Autoimmun. 2003;21(3):255–260. doi: 10.1016/s0896-8411(03)00119-7. [DOI] [PubMed] [Google Scholar]
- Eberl G, Littman DR. The role of the nuclear hormone receptor RORgammat in the development of lymph nodes and Peyer’s patches. Immunol Rev. 2003;195:81–90. doi: 10.1034/j.1600-065x.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5(1):64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203(4):1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156(1):5–7. [PubMed] [Google Scholar]
- Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170(4):2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Sarma JD, et al. Suppressive Effect of IL-27 on Encephalitogenic Th17 Cells and the Effector Phase of Experimental Autoimmune Encephalomyelitis. J Immunol. 2007;179(5):3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15(6):985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- Fossiez F, Banchereau J, Murray R, Van Kooten C, Garrone P, Lebecque S. Interleukin-17. Int Rev Immunol. 1998;16(56):541–551. doi: 10.3109/08830189809043008. [DOI] [PubMed] [Google Scholar]
- Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183(6):2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL. Biology of recently discovered cytokines: interleukin-17--a unique inflammatory cytokine with roles in bone biology and arthritis. Arthritis Res Ther. 2004;6(6):240–247. doi: 10.1186/ar1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hizawa N, Kawaguchi M, Huang SK, Nishimura M. Role of interleukin-17F in chronic inflammatory and allergic lung disease. Clin Exp Allergy. 2006;36(9):1109–1114. doi: 10.1111/j.1365-2222.2006.02550.x. [DOI] [PubMed] [Google Scholar]
- Hu CM, Jang SY, Fanzo JC, Pernis AB. Modulation of T cell cytokine production by interferon regulatory factor-4. J Biol Chem. 2002;277(51):49238–49246. doi: 10.1074/jbc.M205895200. [DOI] [PubMed] [Google Scholar]
- Huang SH, Frydas S, Kempuraj D, Barbacane RC, Grilli A, Boucher W, et al. Interleukin-17 and the interleukin-17 family member network. Allergy Asthma Proc. 2004;25(1):17–21. [PubMed] [Google Scholar]
- Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169(1):443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- Hymowitz SG, Filvaroff EH, Yin JP, Lee J, Cai L, Risser P, et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. Embo J. 2001;20(19):5332–5341. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Nakajima H, Suzuki K, Kagami S, Hirose K, Suto A, et al. Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood. 2003;101(9):3594–3596. doi: 10.1182/blood-2002-09-2817. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Kageyama Y, Koide Y, Yoshida A, Uchijima M, Arai T, Miyamoto S, et al. Reduced susceptibility to collagen-induced arthritis in mice deficient in IFN-gamma receptor. J Immunol. 1998;161(3):1542–1548. [PubMed] [Google Scholar]
- Kang CM, Jang AS, Ahn MH, Shin JA, Kim JH, Choi YS, et al. Interleukin-25 and interleukin-13 production by alveolar macrophages in response to particles. Am J Respir Cell Mol Biol. 2005;33(3):290–296. doi: 10.1165/rcmb.2005-0003OC. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Kokubu F, Huang SK, Homma T, Odaka M, Watanabe S, et al. The IL-17F signaling pathway is involved in the induction of IFN-gamma-inducible protein 10 in bronchial epithelial cells. J Allergy Clin Immunol. 2007;119(6):1408–1414. doi: 10.1016/j.jaci.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Kim MR, Manoukian R, Yeh R, Silbiger SM, Danilenko DM, Scully S, et al. Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood. 2002;100(7):2330–2340. doi: 10.1182/blood-2002-01-0012. [DOI] [PubMed] [Google Scholar]
- Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, et al. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204(1):161–170. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448(7152):484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JM, Yi L, Shen F, Maitra A, Jiao X, Jin T, et al. Evidence for ligand-independent multimerization of the IL-17 receptor. J Immunol. 2006;176(2):711–715. doi: 10.4049/jimmunol.176.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Wei S, Vatan L, Escara-Wilke J, Szeliga W, Keller ET, et al. Cutting Edge: Opposite Effects of IL-1 and IL-2 on the Regulation of IL-17+ T Cell Pool IL-1 Subverts IL-2-Mediated Suppression. J Immunol. 2007;179(3):1423–1426. doi: 10.4049/jimmunol.179.3.1423. [DOI] [PubMed] [Google Scholar]
- Kyle S, Chandler D, Griffiths CE, Helliwell P, Lewis J, McInnes I, et al. Guideline for anti-TNF-alpha therapy in psoriatic arthritis. Rheumatology (Oxford) 2005;44(3):390–397. doi: 10.1093/rheumatology/keh514. [DOI] [PubMed] [Google Scholar]
- Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442(7101):461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Lee J, Ho WH, Maruoka M, Corpuz RT, Baldwin DT, Foster JS, et al. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J Biol Chem. 2001;276(2):1660–1664. doi: 10.1074/jbc.M008289200. [DOI] [PubMed] [Google Scholar]
- Leonard JP, Waldburger KE, Goldman SJ. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J Exp Med. 1995;181(1):381–386. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard JP, Waldburger KE, Goldman SJ. Regulation of experimental autoimmune encephalomyelitis by interleukin-12. Ann N Y Acad Sci. 1996;795:216–226. doi: 10.1111/j.1749-6632.1996.tb52671.x. [DOI] [PubMed] [Google Scholar]
- Lewis JD. Anti-TNF antibodies for Crohn’s disease--in pursuit of the perfect clinical trial. N Engl J Med. 2007;357(3):296–298. doi: 10.1056/NEJMe078111. [DOI] [PubMed] [Google Scholar]
- Lohoff M, Mittrucker HW, Prechtl S, Bischof S, Sommer F, Kock S, et al. Dysregulated T helper cell differentiation in the absence of interferon regulatory factor 4. Proc Natl Acad Sci U S A. 2002;99(18):11808–11812. doi: 10.1073/pnas.182425099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra A, Shen F, Hanel W, Mossman K, Tocker J, Swart D, et al. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc Natl Acad Sci U S A. 2007;104(18):7506–7511. doi: 10.1073/pnas.0611589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O’Malley JT, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178(8):4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- Matthys P, Vermeire K, Mitera T, Heremans H, Huang S, Billiau A. Anti-IL-12 antibody prevents the development and progression of collagen-induced arthritis in IFN-gamma receptor-deficient mice. Eur J Immunol. 1998;28(7):2143–2151. doi: 10.1002/(SICI)1521-4141(199807)28:07<2143::AID-IMMU2143>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14(2):155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198(12):1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2(12):933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- Novatchkova M, Leibbrandt A, Werzowa J, Neubuser A, Eisenhaber F. The STIR-domain superfamily in signal transduction, development and immunity. Trends Biochem Sci. 2003;28(5):226–229. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- Numasaki M, Tomioka Y, Takahashi H, Sasaki H. IL-17 and IL-17F modulate GM-CSF production by lung microvascular endothelial cells stimulated with IL-1beta and/or TNF-alpha. Immunol Lett. 2004;95(2):175–184. doi: 10.1016/j.imlet.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203(4):843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G, French D, Mao W, Maruoka M, Risser P, Lee J, et al. Forced expression of murine IL-17E induces growth retardation, jaundice, a Th2-biased response, and multiorgan inflammation in mice. J Immunol. 2001;167(11):6559–6567. doi: 10.4049/jimmunol.167.11.6559. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8(3):247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- Reiner SL. Helper T cell differentiation, inside and out. Curr Opin Immunol. 2001;13(3):351–355. doi: 10.1016/s0952-7915(00)00226-0. [DOI] [PubMed] [Google Scholar]
- Renauld JC. New insights into the role of cytokines in asthma. J Clin Pathol. 2001;54(8):577–589. doi: 10.1136/jcp.54.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengarajan J, Mowen KA, McBride KD, Smith ED, Singh H, Glimcher LH. Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J Exp Med. 2002;195(8):1003–1012. doi: 10.1084/jem.20011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Z, Cheng L, Ren Y, Li Z, Li Y, Li X, et al. Interleukin-17F signaling requires ubiquitination of interleukin-17 receptor via TRAF6. Cell Signal. 2007;19(7):1514–1520. doi: 10.1016/j.cellsig.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150(12):5445–5456. [PubMed] [Google Scholar]
- Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, et al. Functional Cooperation between Interleukin-17 and Tumor Necrosis Factor-{alpha} Is Mediated by CCAAT/Enhancer-binding Protein Family Members. J. Biol. Chem. 2004;279(4):2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- Schwandner R, Yamaguchi K, Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J Exp Med. 2000;191(7):1233–1240. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkhuu T, Matthaei KI, Forbes E, Mahalingam S, Hogan SP, Hansbro PM, et al. Mechanism of interleukin-25 (IL-17E)-induced pulmonary inflammation and airways hyper-reactivity. Clin Exp Allergy. 2006;36(12):1575–1583. doi: 10.1111/j.1365-2222.2006.02595.x. [DOI] [PubMed] [Google Scholar]
- Shi Y, Ullrich SJ, Zhang J, Connolly K, Grzegorzewski KJ, Barber MC, et al. A novel cytokine receptor-ligand pair. Identification, molecular characterization, and in vivo immunomodulatory activity. J Biol Chem. 2000;275(25):19167–19176. doi: 10.1074/jbc.M910228199. [DOI] [PubMed] [Google Scholar]
- Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178(7):4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- Starnes T, Robertson MJ, Sledge G, Kelich S, Nakshatri H, Broxmeyer HE, et al. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J Immunol. 2001;167(8):4137–4140. doi: 10.4049/jimmunol.167.8.4137. [DOI] [PubMed] [Google Scholar]
- Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203(7):1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, et al. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J Allergy Clin Immunol. 2006;118(3):606–614. doi: 10.1016/j.jaci.2006.04.051. [DOI] [PubMed] [Google Scholar]
- Tartour E, Fossiez F, Joyeux I, Galinha A, Gey A, Claret E, et al. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 1999;59(15):3698–3704. [PubMed] [Google Scholar]
- Tian E, Sawyer JR, Largaespada DA, Jenkins NA, Copeland NG, Shaughnessy JD., Jr. Evi27 encodes a novel membrane protein with homology to the IL17 receptor. Oncogene. 2000;19(17):2098–2109. doi: 10.1038/sj.onc.1203577. [DOI] [PubMed] [Google Scholar]
- Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, et al. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177(1):36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC activated Th2 memory cells. J Exp Med. 2007;204(8):1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157(8):3223–3227. [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin-17 producing helper T cells. Nat Immunol. 2007 doi: 10.1038/ni1497. doi:10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- Witowski J, Ksiazek K, Jorres A. Interleukin-17: a mediator of inflammatory responses. Cell Mol Life Sci. 2004;61(5):567–579. doi: 10.1007/s00018-003-3228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JF, Guo Y, Quazi A, Luxenberg DP, Bennett F, Ross JF, et al. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J Biol Chem. 2007;282(18):13447–13455. doi: 10.1074/jbc.M700499200. [DOI] [PubMed] [Google Scholar]
- Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282(13):9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155(12):5483–5486. [PubMed] [Google Scholar]
- Yao Z, Spriggs MK, Derry JM, Strockbine L, Park LS, VandenBos T, et al. Molecular characterization of the human interleukin (IL)-17 receptor. Cytokine. 1997;9(11):794–800. doi: 10.1006/cyto.1997.0240. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8(9):967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Toh ML, Zrioual S, Miossec P. IL-17A versus IL-17F induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in AGS gastric adenocarcinoma cells. Cytokine. 2007;38(3):157–164. doi: 10.1016/j.cyto.2007.06.002. [DOI] [PubMed] [Google Scholar]