Abstract
BACKGROUND
Hempseed is a novel functional food that contains several health-promoting polyunsaturated fatty acids (PUFAs). PUFAs, such as those found in flaxseed and fish, have been shown to protect the heart against arrhythmias following ischemia/reperfusion.
OBJECTIVE
To investigate the potential of dietary hempseed as a cardioprotective agent against global ischemia and subsequent reperfusion by assessing several measurements of cardiac performance: QT interval duration, left ventricular pressure, arrhythmia incidence and arrhythmia duration.
METHODS
Male New Zealand white rabbits were fed one of six diets: a control diet; or one supplemented with 10% hempseed, 10% delipidated hempseed, 0.5% cholesterol, 0.5% cholesterol plus 10% hempseed or 5% coconut oil. After eight weeks on their respective diets, the hearts were excised and subjected to 30 min of global ischemia and 45 min of reperfusion. Electrocardiogram traces were recorded throughout the experiment and were subsequently analyzed for QT interval duration, left ventricular pressure, arrhythmia incidence and arrhythmia duration. Plasma and cardiac tissue were analyzed for fatty acid content and composition.
RESULTS
Cholesterol-fed animals exhibited significantly higher PUFA levels in their plasma, but this did not directly translate into higher PUFA levels in their cardiac fractions. There were no significant differences among the groups in the incidence or duration of ischemia-derived arrhythmias. During reperfusion, there was a significant decrease in the incidence of fibrillation in the hearts obtained from cholesterol-fed and hempseed- plus cholesterol-fed rabbits compared with the hearts from delipidated hempseed-fed rabbits.
CONCLUSIONS
Dietary hempseed induced limited beneficial effects on cardiac function during ischemia/reperfusion challenge. The present study does not support the use of dietary hempseed to protect the heart during ischemic insult in this experimental model.
Keywords: Functional food, Ischemia, Polyunsaturated fatty acids, Reperfusion
Cardiac ischemia results from inadequate coronary artery flow and is commonly caused by coronary artery disease (CAD), a leading cause of death in the United States (1), Canada (2) and the United Kingdom (3). Because the myocardium is dependent on the blood supply delivered by the coronary arteries, a reduction or stop in blood flow as a result of CAD can trigger angina, arrhythmias and myocardial infarction. Restoration of blood flow through the coronary arteries is imperative but this can increase the generation of free radicals (4,5) and cause further ion disequilibrium (6,7), which may result in injury and increase the generation of arrhythmias.
Nutrition has been recognized as an important factor in CAD (8). Therefore, it is not surprising that the inclusion of several foods, such as fish or flaxseed, in the diet has been shown to be cardioprotective (9–13). The polyunsaturated fatty acid (PUFA) content in these foods has been suggested to be the basis for their cardioprotective effect (14–20). It is now thought to be important to identify foods that may possess similar cardio-protective effects due to their enriched PUFA composition.
Dietary hempseed contains omega-6 and omega-3 PUFAs in a 4:1 ratio, a proportion that has been recommended as having health benefits (21). This balance of PUFAs is typically lacking in the human diet due to the prevalence of omega-6 fatty acid consumption. While adequate sources of omega-3 PUFAs are available, these foods are not consumed regularly in many populations. There is limited scientific knowledge on the health-related benefits of hempseed. Its use has been documented in Arabic (22) and folk (23) medicine; however, this information must be viewed cautiously. In the past, hempseed most likely contained higher levels of tetrahydrocannabinol, a psychoactive substance, which may have given the impression of a beneficial effect. The hempseed that is available today has been selectively bred to contain very low levels of tetrahydrocannabinol (24). Thus, the existence of health benefits, specifically cardiovascular, remains unproven. The goal of the present preliminary study was to investigate the potential for dietary hempseed to provide cardioprotective effects during ischemia/reperfusion challenge.
METHODS
Animals and diets
All experiments conformed to the Canadian Council on Animal Care Guidelines. Individually housed male New Zealand white rabbits were randomly assigned to six experimental groups and maintained at a constant temperature in a 12 h light/dark cycle. The animals were fed a control diet (RG) or one of five supplemented diets for eight weeks. The supplemented diets were produced by adding either of the following to the RG diet before repelleting: 10% ground hempseed (HP); 10% partially delipidated hempseed (DHP); 0.5% cholesterol (OL); 0.5% cholesterol plus HP (OLHP); or 5% coconut oil (CO). The concentration of hempseed used in the present study (10%) is similar to that used in other animal studies to show health-related benefits of flaxseed (9). The food was air-dried and stored at 4°C until used. Each animal received 250 g/day of their respective diets and were continuously monitored for weight and health.
Ischemia/reperfusion protocol for isolated hearts
Hearts were mounted on the cannula of a custom perfusion apparatus where they were perfused at 20 mL/min with Tyrode’s solution, as previously described (25). Tyrode’s solution was continuously bubbled with 95% O2 and 5% CO2, and contained: 115 mM NaCl; 28 mM NaHCO3; 0.5 mM NaH2PO4; 20 mM dextrose; 4 mM KCl; 2 mM CaCl2; and 0.7 mM MgCl2. The right atrium was removed and the atrioventricular node crushed to allow external pacing. A hook electrode placed high in the right ventricle provided external pacing at 2 Hz. Left ventricular pressure was measured by placing a deflated latex balloon into the left ventricle, where it was inflated to a baseline of 5 mmHg. The balloon was connected to a pressure transducer that monitored left ventricular end-diastolic pressure (LVEDP) and left ventricular developed pressure (LVDP). LVEDP values were taken as the baseline pressure between stimulated left ventricular contractions. LVDP values were taken as the peak pressure of a stimulated contraction minus the LVEDP for that same contraction. Right ventricular and bath temperatures were monitored and maintained at 37±0.5°C. An equilibration period of 50 min ensured stable electrocardiogram (ECG) and pressure tracings. After equilibration, global ischemia was initiated by eliminating buffer flow to the heart and bubbling the bath continuously with 95% N2 and 5% CO2. Global ischemia lasted 30 min and was followed by a 45 min reperfusion period. The heart was snap frozen in liquid nitrogen and stored at −80°C after ischemia/reperfusion for further analysis.
ECG analysis
An ECG was recorded by immersing the heart into a circulating bath of Tyrode’s solution bubbled continuously with 95% O2 and 5% CO2. Three electrodes present in the walls of the bath formed Einthoven’s configuration and provided ECG leads I, II and III. For an ECG trace to be included for analysis, LVDP had to achieve 30 mmHg during the equilibration period. QT intervals were measured during the final 10 s of the equilibration period. Three separate measurements of this interval, from the beginning of the Q wave to the end of the T wave (26), were obtained for each heart. Each QRS-T complex measured was checked to ensure it was a stimulated beat (ie, fell at a 0.5 s interval), because the duration of the QT interval is dependent on heart rate (27). Animals used for measurement of the QT interval had to satisfy the same criteria as ECG analysis. In addition, the Q and T deflections had to have been readily identifiable to ensure correct quantification.
ECGs were analyzed for arrhythmias based on the parameters set at the Lambeth Conventions (28). Ventricular tachycardia was defined as a “run of 4 or more consecutive ventricular premature beats” (28). Ventricular fibrillation was defined as when “individual QRS deflections can no longer be distinguished from one another” and “a rate can no longer be measured” (28). The incidence of tachycardia and fibrillation was classified as continuous tachycardia or fibrillation 30 s or longer, respectively. The duration of tachycardia or fibrillation was the total length of tachycardia or fibrillation, respectively.
Fatty acid methyl ester analysis
Fatty acids were extracted from the diet or cardiac tissue by an adaptation of the Folch et al (29) method. Fatty acids were esterified by the Lepage and Roy (30) method. Liberation of the organic solvent containing the fatty acid methyl esters resulted after centrifugation for 5 min at 4500 g at 22°C. An aliquot of the upper benzene layer was removed and analyzed by gas chromatography (GC) on a Varian GC-tandem mass spectrometry instrument (Varian Inc, USA) equipped with a CP-3800 GC, CP-8400 autosampler and a flame ionization detector.
Statistical analysis
Data were analyzed for statistical significance by ANOVA followed by a Student-Newman-Keuls post hoc test. Values are expressed as means ± SEM. Data on the incidence of arrhythmia were analyzed for significance by Fisher’s exact test. For graphs on the incidence of arrhythmia, no error bars can be presented because these graphs compare proportions, not means. Statistical significance was reached at P<0.05.
RESULTS
Fatty acid content of the diet
Each diet was sampled and extracted to analyze fatty acid composition (Table 1). The HP diet was significantly enriched in several fatty acids, including 16:0, 18:0, 20:0, 22:0, 18:1n-9, 20:1n-9, 18:2n-6 (linoleic acid [LA]), 18:3n-6 (gamma-linolenic acid [GLA]) and 18:3n-3 (alpha-linolenic acid [ALA]) fatty acids, compared with the RG diet. The DHP diet was significantly enriched in 20:0, 20:1n-9, LA, GLA and ALA fatty acids compared with the RG diet; however, the levels of these fatty acids in the DHP diet were approximately one-half those found in the HP diet, with the exception of 20:1n-9 fatty acids, which was approximately at the same level. The OL diet was significantly depleted in 16:0, 18:0, 16:1, 18:1n-9, 18:1n-7 and 20:1n-9 fatty acids compared with the RG diet. The OLHP diet was significantly enriched in 20:0, 22:0, 20:1n-9, LA, GLA and ALA fatty acids compared with the RG diet. In addition, the OLHP diet was significantly depleted in 16:1 fatty acids compared with the RG diet. The CO diet was significantly enriched in 14:0, 16:0, 18:0 and 18:1n-9 fatty acids compared with the RG diet.
TABLE 1.
Fatty acid content of the diet as a function of dietary intervention
| Control | Hempseed | Delipidated hempseed | Cholesterol | Cholesterol plus hempseed | Coconut oil | |
|---|---|---|---|---|---|---|
| Saturated fatty acid | ||||||
| 14:0 | 364.9±6.1* | 342.9±28.8* | 353.3±12.5* | 259.8±6.4* | 264.2±6.2* | 8259.8±295.9† |
| 16:0 | 9423.8±228.2* | 10938.7± 540.0† | 10127.0±280.2*† | 8095.2±154.2‡ | 9741.9±154.3*† | 13212.6±452.4§ |
| 18:0 | 3510.7±87.8* | 4290.5±219.1† | 3675.0±88.8* | 2817.7±76.4‡ | 3683.5±70.7* | 4657.1±159.2† |
| 20:0 | 119.9±4.9* | 440.0±29.3† | 240.6±19.0‡ | 102.3±2.2* | 382.5±9.3§ | 138.7±6.5* |
| 22:0 | 139.1±3.8* | 260.2±13.9† | 160.7±33.2* | 132.3±1.8* | 251.0±5.9† | 133.9±4.7* |
| Monounsaturated fatty acid | ||||||
| 14:1 | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* |
| 16:1 | 577.2±15.1* | 542.0±29.4* | 552.8±16.4* | 400.9±9.4† | 427.7±7.9† | 541.6±17.5* |
| 18:1n-9 | 13801.1±406.3* | 17361.1±828.8† | 14992.7±323.0*§ | 11586.7±116.6‡ | 15454.8±263.9*¶ | 16464.0±512.0†§¶ |
| 18:1n-7 | 1481.0±111.3* | 1511.2±144.8* | 1384.5±116.3* | 905.4±10.0† | 1230.0±29.6*† | 1159.1±39.4*† |
| 20:1n-9 | 249.8±8.9* | 348.5±21.2† | 292.6±7.2‡ | 170.3±2.7§ | 304.6±7.6‡ | 242.6±11.7* |
| 22:1 | 77.1±8.2*† | 79.5±18.1*† | 108.7±10.4† | 49.6±4.9* | 66.0±5.2*† | 48.8±9.6* |
| Polyunsaturated fatty acid | ||||||
| 18:2n-6 | 11687.8±304.7* | 31757.7±1738.8† | 18282.5±384.5‡ | 11453.9±274.3* | 30676.6±489.3† | 11381.3±341.8* |
| 18:3n-6 | 0.0±0.0* | 855.3±60.8† | 347.3±24.5‡ | 0.0±0.0* | 742.7±16.9§ | 0.0±0.0* |
| 18:3n-3 | 2631.0±75.7* | 8243.3±480.9† | 4334.5±71.7‡ | 2152.1±54.6* | 7860.3±137.7† | 2381.7±74.4* |
| 20:2n-6 | 45.6±4.6*† | 86.3±10.4* | 94.3±29.1* | 27.2±0.8† | 68.0±5.6*† | 56.4±2.6*† |
| 20:3n-6 | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* |
| 20:3n-3 | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* |
| 20:4n-6 | 10.0±2.7*‡ | 10.4±4.2*‡ | 12.0±3.1*‡ | 0.8±0.5*† | 5.6±1.5* | 19.6±1.8‡ |
| 20:5n-3 | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* |
| 22:6n-3 | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 9.6±9.6* | 10.4±10.4* | 0.0±0.0* |
Means ± SEM are shown for each group (n=3) and are represented as micrograms of fatty acid methyl ester per gram of diet. Significant differences among groups (P<0.05) are denoted by different superscripts. Conversely, if the superscript is the same between two or more groups then there was no statistically significant difference among those groups
Animal body weights
Animals were weighed before diet commencement and at termination. All animals included in the calculation of mean body weight completed the assigned diet for 56±3 days. One animal in the OLHP group ceased feeding and was removed from the data analysis. No statistically significant differences in animal weight were observed among the groups (data not shown).
Plasma cholesterol ester, triglyceride and fatty acid content
Plasma was isolated from blood collected at termination. The plasma was analyzed for cholesterol ester (CE), triglyceride (TG) and fatty acid content. Significant differences existed among groups for both plasma CE and TG levels (data not shown). The OL and OLHP groups had significantly elevated levels of plasma CE and TG compared with all other groups. In addition, the OLHP group had a significantly elevated plasma CE level compared with the OL group. Table 2 lists the plasma fatty acid composition as a function of the dietary interventions. Levels of 14:0 fatty acids were significantly elevated in the OL, OLHP and CO groups compared with the RG group. In addition, a significant elevation of 14:0 fatty acids was observed in the OL and OLHP groups compared with the CO group. Levels of 16:0, 18:0, 20:0 and 22:0 fatty acids were significantly elevated in the OL and OLHP groups compared with all other groups. In addition, the OLHP group had significant elevations in these fatty acids over the OL group. Levels of 14:1, 16:1, 18:1n-9, 18:1n-7, 20:1n-9 and 24:1n-9 fatty acids were significantly elevated in the OL and OLHP groups compared with all other groups. In addition, the level of 24:1n-9 fatty acids in the OLHP group was significantly elevated over the OL group. Levels of LA, ALA, 20:2n-6, 20:3n-6, 20:4n-6, 20:5n-3 and 22:6n-3 fatty acids were significantly elevated in the OL and OLHP groups compared with all other groups. In addition, the levels of these fatty acids in the OLHP group were significantly elevated over the levels in the OL group. Levels of 20:3n-6 fatty acids were also significantly elevated in the HP group compared with the RG, DHP and CO groups, but were significantly lower than the levels in the OL and OLHP groups. Levels of GLA were significantly elevated in the HP and OLHP groups compared with the RG, DHP, OL and CO groups, with the levels of this fatty acid in the OLHP group significantly elevated over the levels in the HP group.
TABLE 2.
Fatty acid content of plasma as a function of dietary intervention
| Control | Hempseed | Delipidated hempseed | Cholesterol | Cholesterol plus hempseed | Coconut oil | |
|---|---|---|---|---|---|---|
| Saturated fatty acid | ||||||
| 14:0 | 31.4±2.0* | 29.9±4.6* | 39.0±4.5* | 82.8±5.6† | 92.5±4.1† | 58.1±2.1‡ |
| 16:0 | 321.7±19.8* | 384.6±40.6* | 371.9±43.2* | 2633.9±287.0† | 3342.1±82.1‡ | 309.3±15.0* |
| 18:0 | 159.0±6.0* | 219.8±23.0* | 183.5±15.5* | 917.5±84.1† | 1167.5±52.8‡ | 184.7±18.7* |
| 20:0 | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 56.6±11.2† | 117.5±19.9‡ | 0.0±0.0* |
| 22:0 | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 60.7±4.7† | 81.1±0.9‡ | 0.0±0.0* |
| Monounsaturated fatty acid | ||||||
| 14:1 | 0.0±0.0* | 0.0±0.0* | 5.5±3.6* | 24.7±1.4† | 16.9±5.4† | 0.0±0.0* |
| 16:1 | 46.3±5.5* | 35.3±2.1* | 44.9±9.6* | 733.3±66.5† | 707.3±33.3† | 38.8±2.7* |
| 18:1n-9 | 445.5±22.0* | 458.4±47.6* | 456.0±58.9* | 5245.2±477.4† | 5856.9±78.8† | 327.5±16.9* |
| 18:1n-7 | 33.9±3.8* | 30.9±4.2* | 38.0±7.7* | 330.2±37.2† | 330.9±12.2† | 22.3±1.7* |
| 20:1n-9 | 5.5±3.5* | 7.9±3.0* | 6.7±4.4* | 33.3±3.5† | 37.1±1.1† | 0.0±0.0* |
| 24:1n-9 | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 108.0±9.9† | 123.1±1.5‡ | 0.0±0.0* |
| Polyunsaturated fatty acid | ||||||
| 18:2n-6 | 321.7±19.7* | 657.2±109.5* | 388.3±31.1* | 2620.7±283.0† | 6564.1±129.5‡ | 360.4±41.7* |
| 18:3n-6 | 0.0±0.0* | 11.6±3.1† | 0.0±0.0* | 4.5±1.7* | 52.3±1.5‡ | 0.0±0.0* |
| 18:3n-3 | 39.8±2.4* | 118.9±27.2* | 59.5±5.8* | 469.8±46.8† | 1656.3±27.5‡ | 36.0±1.4* |
| 20:2n-6 | 5.5±3.5* | 6.3±3.1* | 5.8±3.8* | 40.4±4.1† | 72.9±3.0‡ | 4.9±3.1* |
| 20:3n-6 | 0.0±0.0* | 19.9±2.9† | 6.4±4.2* | 45.5±5.1‡ | 104.6±4.3§ | 0.0±0.0* |
| 20:4n-6 | 41.9±2.2* | 42.1±5.3* | 41.0±5.3* | 223.8±22.8† | 313.3±13.0‡ | 50.0±6.8* |
| 20:5n-3 | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 13.7±3.0† | 19.4±0.3‡ | 0.0±0.0* |
| 22:6n-3 | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 26.4±5.8† | 36.0±0.9‡ | 0.0±0.0* |
Means ± SEM are shown for each group (n=3 for the control, cholesterol plus hempseed, and coconut oil diets, while n=4 for the hempseed, delipidated hempseed and cholesterol diets) and are represented as micrograms of fatty acid methyl ester per millilitre of plasma. Significant differences among groups (P<0.05) are denoted by different superscripts. Conversely, if the superscript is the same between two or more groups then there was no statistically significant difference among those groups
Cardiac fatty acid content
Table 3 lists cardiac fatty acid content as a function of the different dietary interventions. Levels of 14:0 fatty acids were significantly lower in the OL group compared with the RG and CO groups. In addition, the CO group had a significantly higher level of 14:0 fatty acids over all other groups. Levels of 16:0 fatty acids were significantly lower in the DHP and OL groups compared with the RG group. Levels of 20:0 fatty acids were significantly higher in the OL and OLHP groups compared with the HP, DHP and CO groups. Levels of 22:0 fatty acids were significantly higher in the OL and OLHP groups compared with all other groups. Levels of 14:1 fatty acids were significantly higher in the CO group compared with all other groups. Levels of 18:1n-9 fatty acids were significantly lower in the DHP and OL groups compared with the RG group. Levels of 18:1n-7 fatty acids were significantly lower in the OLHP group compared with the RG group. Levels of 20:1n-9 fatty acids were significantly higher in the OL group compared with the DHP group. Levels of LA were significantly higher in the HP and OLHP groups compared with the DHP and OL groups. Levels of GLA were significantly higher in the HP and OLHP groups compared with all other groups. In addition, levels of GLA in the OLHP group were significantly higher than in the HP group. Levels of ALA were significantly lower in the OL group compared with the HP and OLHP groups. Moreover, levels of ALA were significantly higher in the OLHP group compared with the DHP and CO groups. Levels of 20:2n-6 fatty acids were significantly higher in the OLHP group compared with the HP, DHP and CO groups. Levels of 20:3n-6 fatty acids were significantly higher in the HP, OL and OLHP groups compared with the RG, DHP and CO groups. The level of 20:3n-6 fatty acids was significantly elevated in the OL and OLHP groups compared with the HP group. The level of 20:3n-6 fatty acids was significantly elevated in the OLHP group compared with the OL group. Levels of 20:4n-6 fatty acids were significantly higher in the CO group compared with the DHP group. Levels of 22:6n-3 fatty acids were significantly higher in the OL group compared with the DHP and CO groups. Levels of 18:0, 16:1, 24:1n-9 and 20:5n-3 fatty acids were unchanged.
TABLE 3.
Fatty acid content of cardiac tissue as a function of dietary intervention
| Control | Hempseed | Delipidated hempseed | Cholesterol | Cholesterol plus hempseed | Coconut oil | |
|---|---|---|---|---|---|---|
| Saturated fatty acid | ||||||
| 14:0 | 289.5±18.5* | 199.0±42.7*† | 154.3±13.6*† | 90.3±35.4† | 170.3±59.4*† | 1137.5±64.7‡ |
| 16:0 | 4769.9±287.7* | 3600.4±500.9*† | 2942.7±147.6† | 2837.5±383.5† | 3668.1±616.1*† | 3943.6±190.7*† |
| 18:0 | 2717.7±496.5* | 2321.4±111.4* | 2078.7±179.2* | 1849.6±136.8* | 2222.5±114.8* | 2610.6±287.5* |
| 20:0 | 14.8±9.1*† | 5.2±2.8* | 0.0±0.0* | 31.5±9.3† | 32.9±10.5† | 0.2±0.2* |
| 22:0 | 0.7±0.4* | 5.2±2.0* | 0.3±0.2* | 55.9±15.8† | 44.7±18.6† | 2.6±1.1* |
| Monounsaturated fatty acid | ||||||
| 14:1 | 4.8±2.3* | 3.4±2.3* | 0.0±0.0* | 0.0±0.0* | 3.3±2.1* | 18.7±3.8† |
| 16:1 | 506.1±31.6* | 312.6±69.8* | 261.4±19.1* | 273.7±71.0* | 295.5±85.2* | 364.5±74.8* |
| 18:1n-9 | 5539.8±365.5* | 3782.4±636.1*† | 3217.2±178.2† | 2876.7±443.8† | 3883.7±720.9*† | 4218.2±185.4*† |
| 18:1n-7 | 773.7±46.9* | 554.8±78.0*† | 553.9±30.3*† | 623.9±55.3*† | 507.3±67.0† | 620.8±31.5*† |
| 20:1n-9 | 49.0±13.0*† | 25.9±14.3*† | 14.4±4.3* | 60.3±3.5† | 47.6±5.5*† | 37.7±12.3*† |
| 24:1n-9 | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* | 0.0±0.0* |
| Polyunsaturated fatty acid | ||||||
| 18:2n-6 | 5908.6±371.5*† | 7084.6±551.0* | 5103.3±219.9† | 4259.0±371.8† | 7533.2±911.7* | 5676.1±116.6*† |
| 18:3n-6 | 0.0±0.0* | 34.0±7.2† | 0.4±0.4* | 0.0±0.0* | 62.2±9.4‡ | 0.0±0.0* |
| 18:3n-3 | 656.7±48.3*†‡ | 773.0±152.9*‡ | 386.3±39.0*† | 283.7±50.7† | 926.1±216.6‡ | 403.4±36.9*† |
| 20:2n-6 | 47.2±19.1*† | 29.5±7.9* | 19.8±3.5* | 59.5±6.2*† | 79.8±5.5† | 26.2±6.3* |
| 20:3n-6 | 45.0±10.2* | 80.8±6.5† | 54.0±4.8* | 111.1±8.4‡ | 156.4±14.8§ | 44.9±2.0* |
| 20:4n-6 | 2800.3±173.8*† | 2709.1±124.4*† | 2498.0±153.1* | 2721.6±137.8*† | 2688.1±54.7*† | 3186.7±84.5† |
| 20:5n-3 | 42.1±10.1* | 38.8±1.9* | 39.3±1.4* | 43.0±5.0* | 40.3±10.6* | 51.4±1.3* |
| 22:6n-3 | 81.9±12.0*† | 81.3±10.8*† | 48.9±7.5† | 105.0±10.8* | 70.8±3.0*† | 58.3±9.7† |
Means ± SEM are shown for each group (n=4) and are represented as micrograms of fatty acid methyl ester per gram of tissue. Significant differences among groups (P<0.05) are denoted by different superscripts. Conversely, if the superscript is the same between two or more groups then there was no statistically significant difference among those groups
Left ventricular pressure
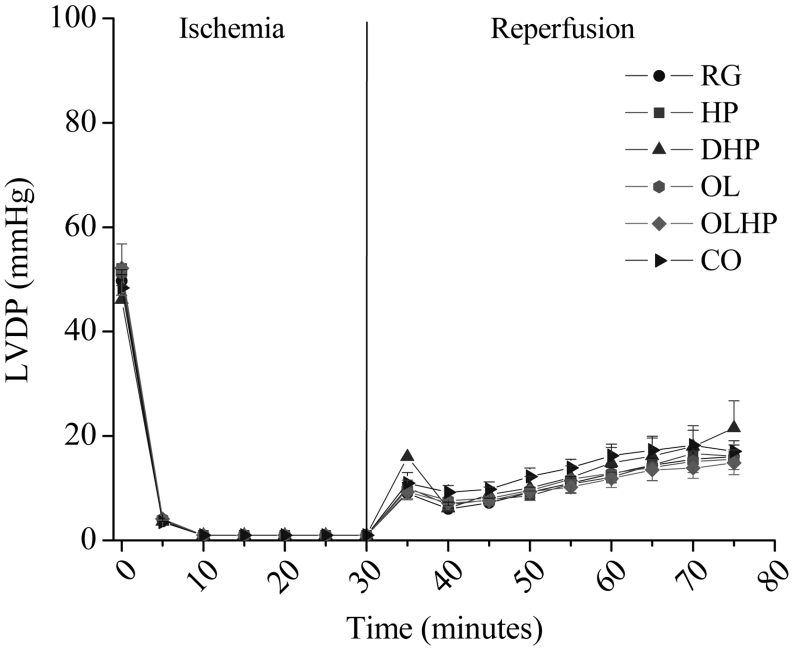
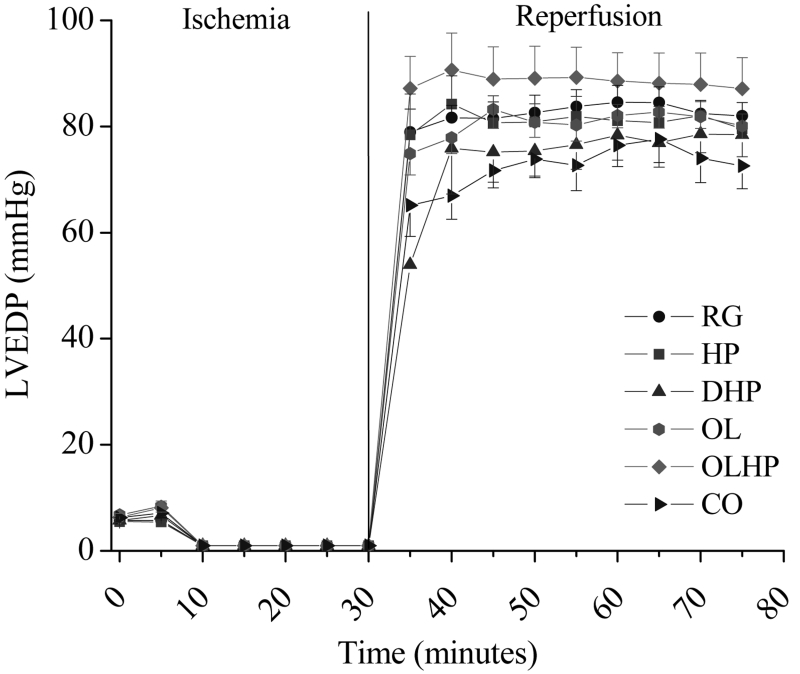
Left ventricular diastolic and systolic pressure readings were indistinguishable between 10 min and 30 min of ischemia. There were no significant differences detected among groups for either LVDP (Figure 1) or LVEDP (Figure 2) at any time point before ischemia, during ischemia or during reperfusion.
Figure 1).
Left ventricular developed pressure (LVD) in hearts exposed to ischemia and reperfusion as a function of dietary intervention. Mean ± SEM values are shown for each group at each time point (n= four to 10). There were no significant differences among the groups (P>0.05) at any time point. CO 5% coconut oil; DHP 10% partially delipidated hempseed; HP 10% ground hempseed; OL 0.5% cholesterol; OLHP OL plus HP; RG Control
Figure 2).
Left ventricular end-diastolic pressure (LVEDP) in hearts exposed to ischemia and reperfusion as a function of dietary intervention. Mean ± SEM values are shown for each group at each time point (n= four to 10). There were no significant differences among the groups (P>0.05) at any time point. CO 5% coconut oil; DHP 10% partially delipidated hempseed; HP 10% ground hempseed; OL 0.5% cholesterol; OLHP OL plus HP; RG Control
ECG analysis
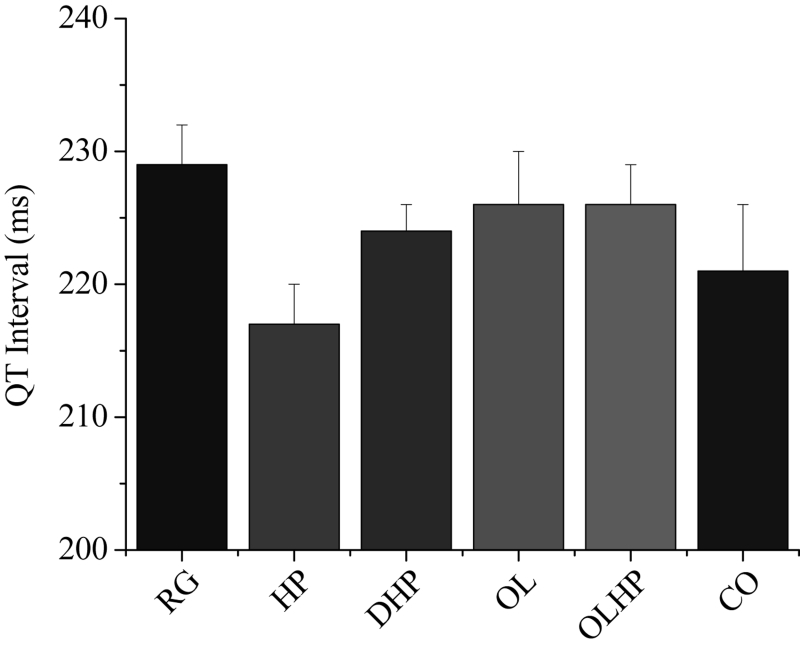
QT interval measurements were taken during the final 10 s of the equilibration period (before the onset of ischemia). Figure 3 shows the mean QT interval duration for each group. There were no significant differences among the groups.
Figure 3).
Duration of cardiac QT interval as a function of dietary intervention. Mean ± SEM values are shown for each group (n= five to eight). There were no significant differences among the groups (P>0.05). CO 5% coconut oil; DHP 10% partially delipidated hempseed; HP 10% ground hempseed; OL 0.5% cholesterol; OLHP OL plus HP; RG Control
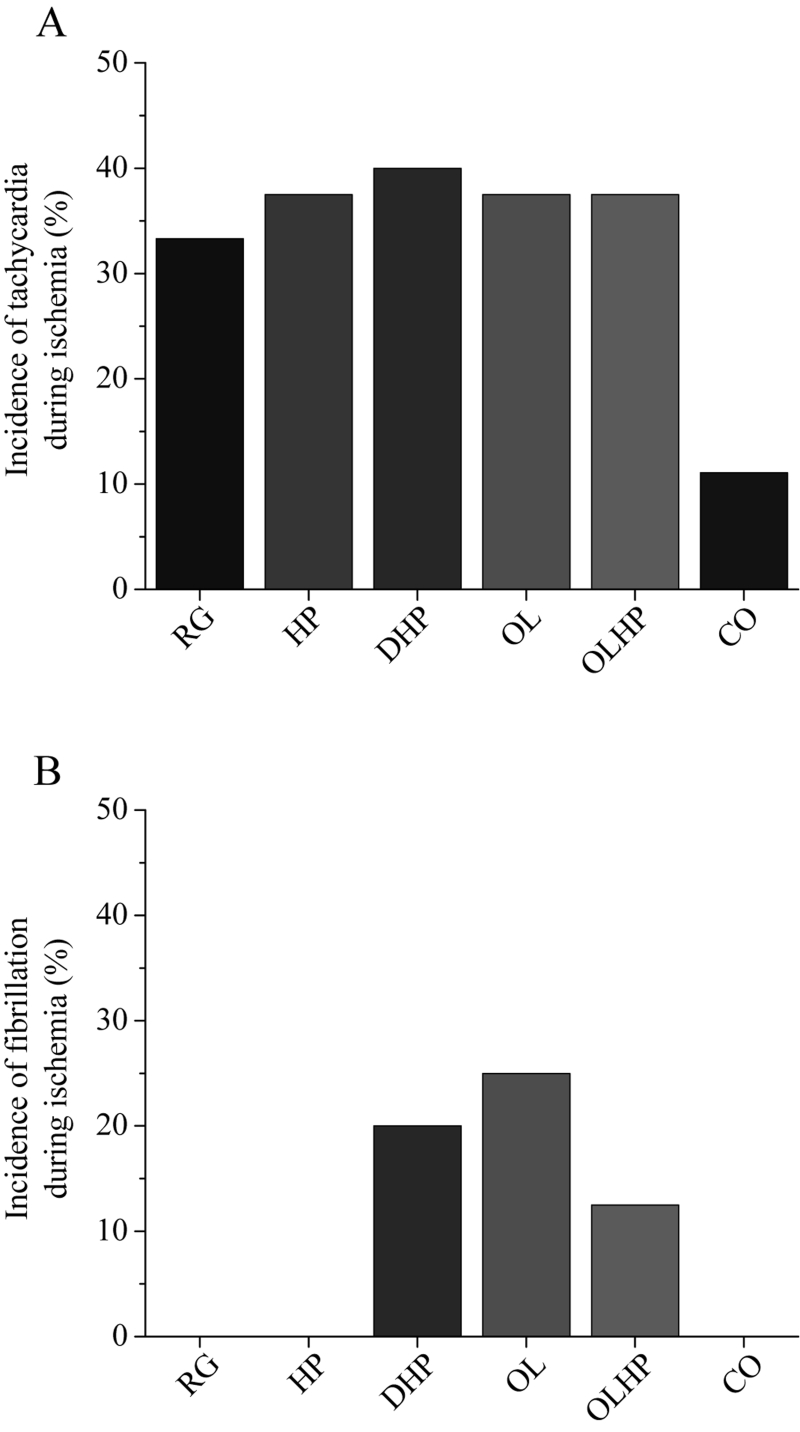
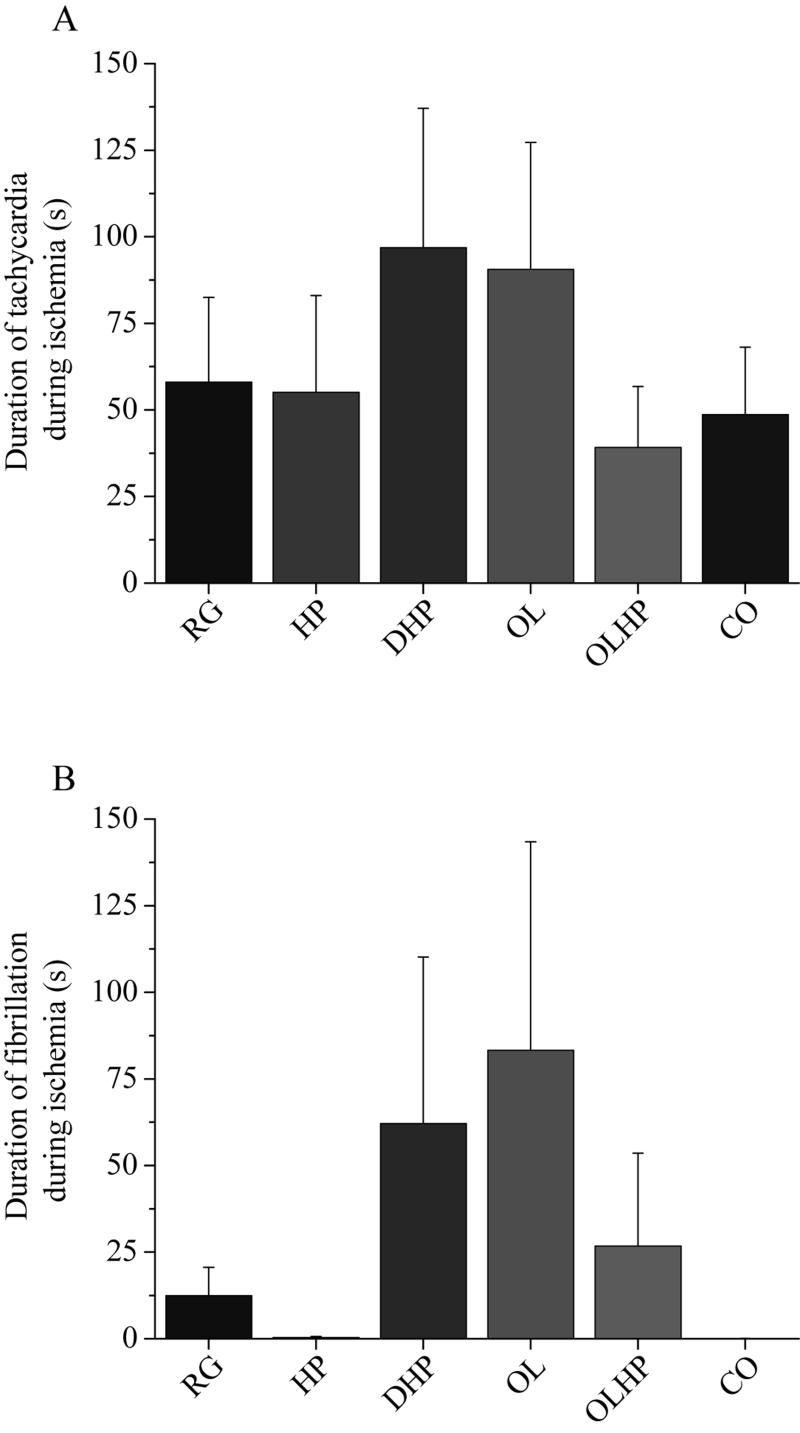
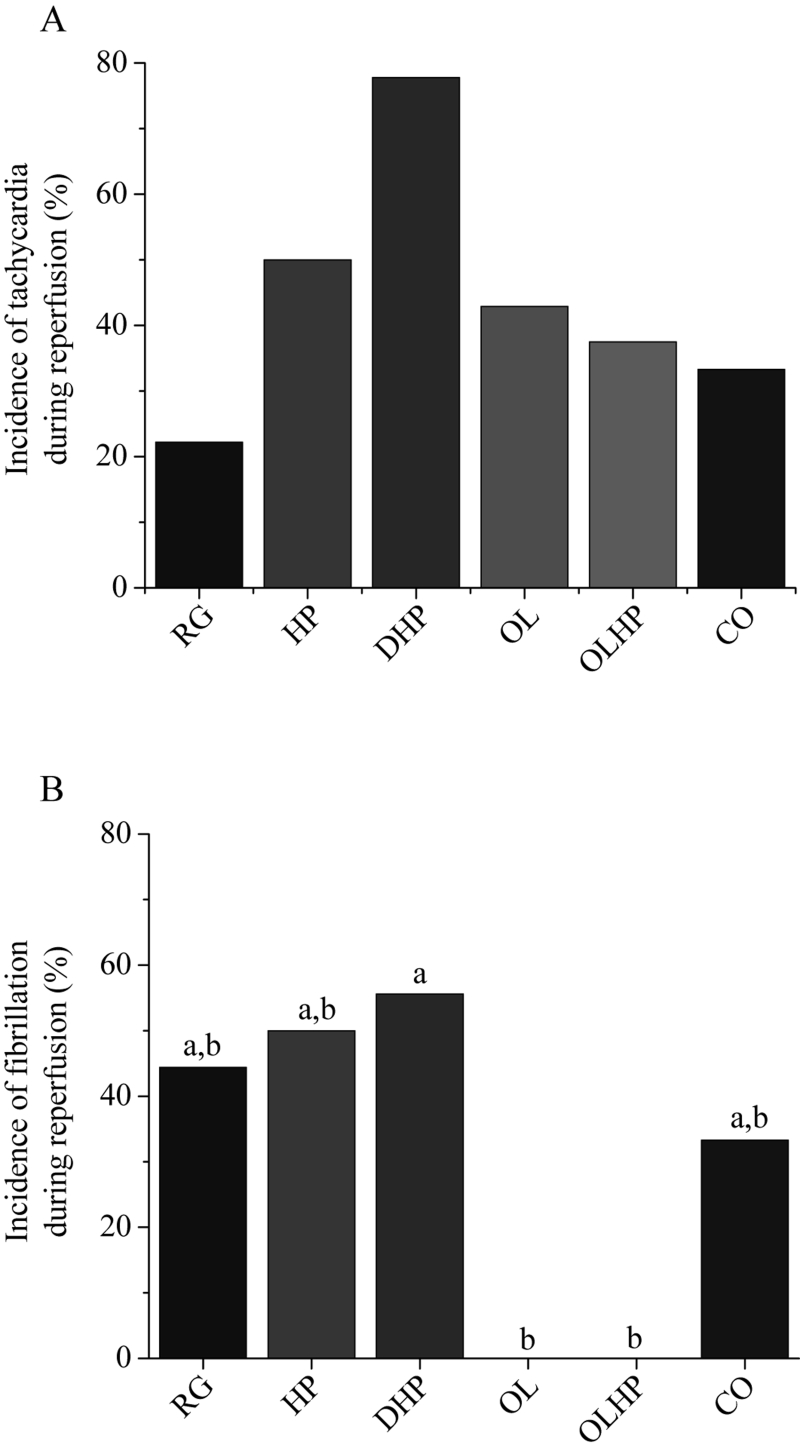
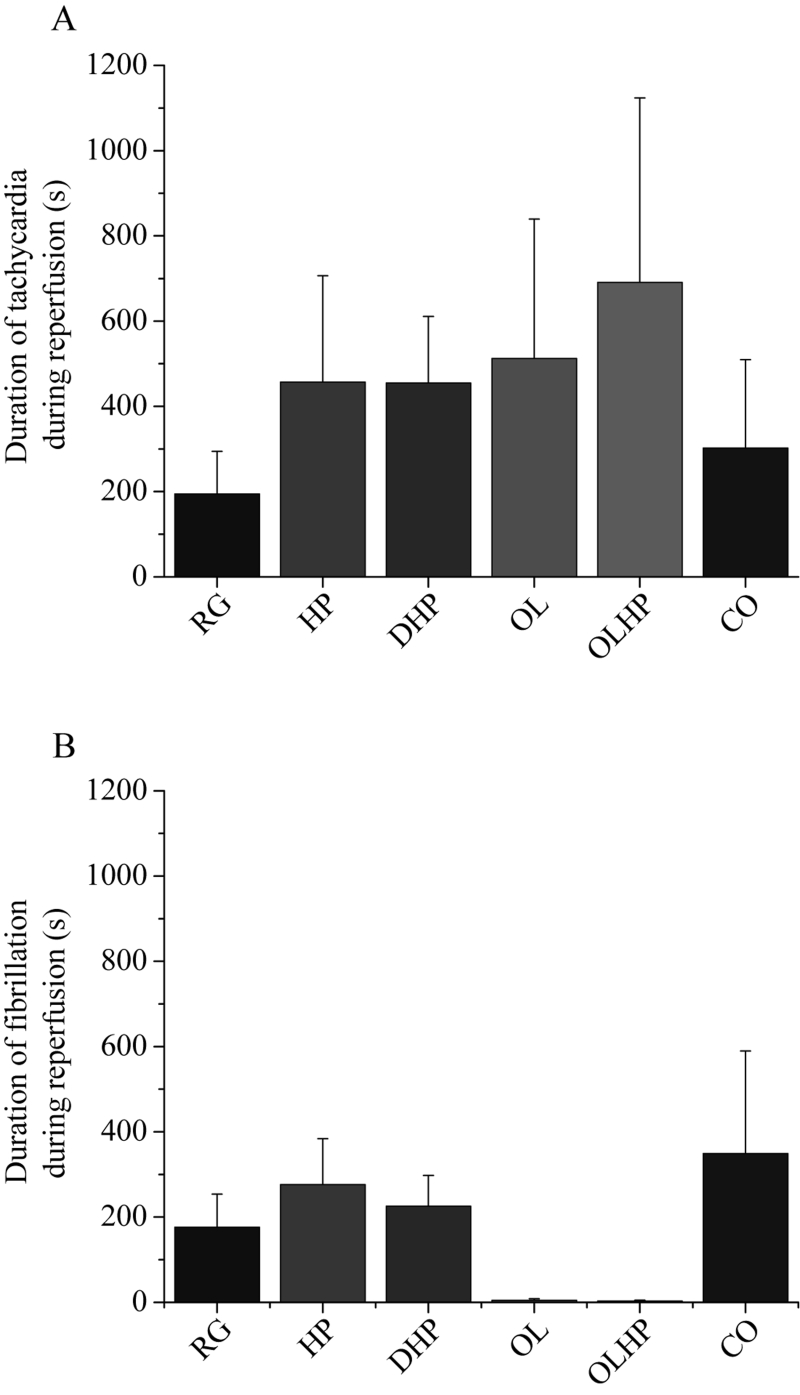
There were no significant differences in the incidence or duration of tachycardia or fibrillation during ischemia (Figures 4 and 5). There was no significant difference in the incidence of tachycardia for reperfusion-derived arrhythmias (Figure 6A). The incidence of reperfusion-derived fibrillation was significantly lower in the OL and OLHP groups than in the DHP group (Figure 6B), but there were no significant differences in any group compared with the animals fed the RG diet. There were no significant differences among the groups in the duration of tachycardia or fibrillation during reperfusion (Figure 7).
Figure 4).
Incidence of ischemia-derived arrhythmias as a function of dietary intervention. Values are shown as a percentage of positive incidence for either tachycardia (A) or fibrillation (B) (n= eight to 10). There were no significant differences among the groups (P>0.05). CO 5% coconut oil; DHP 10% partially delipidated hempseed; HP 10% ground hempseed; OL 0.5% cholesterol; OLHP OL plus HP; RG Control
Figure 5).
Duration of ischemia-derived arrhythmias as a function of dietary intervention. Mean ± SEM values are shown for each group for tachycardia (A) and fibrillation (B) (n= eight to 10). There were no significant differences among the groups (P>0.05). CO 5% coconut oil; DHP 10% partially delipidated hempseed; HP 10% ground hempseed; OL 0.5% cholesterol; OLHP OL plus HP; RG Control
Figure 6).
Incidence of reperfusion-derived arrhythmias as a function of dietary intervention. Values shown are the percentage of positive incidence for tachycardia (A) or fibrillation (B) (n= seven to nine). No significant differences exist in (A) (P>0.05); statistically significant differences among groups in (B) (P<0.05) are denoted by a unique letter. CO 5% coconut oil; DHP 10% partially delipidated hempseed; HP 10% ground hempseed; OL 0.5% cholesterol; OLHP OL plus HP; RG Control
Figure 7).
Duration of reperfusion-derived arrhythmias as a function of dietary intervention. Mean ± SEM values are shown for tachycardia (A) and fibrillation (B) (n= seven to nine). There were no significant differences among the groups (P>0.05). CO 5% coconut oil; DHP 10% partially delipidated hempseed; HP 10% ground hempseed; OL 0.5% cholesterol; OLHP OL plus HP; RG Control
DISCUSSION
The present study is the first to evaluate the impact of dietary hempseed on cardiac function. Three basic hypotheses were evaluated in the present study. Our first hypothesis was that dietary hempseed improves cardiac function and increases the arrhythmia threshold during ischemia/reperfusion challenge. Our second hypothesis was that dietary cholesterol decreases cardiac function and lowers the arrhythmia threshold during ischemia/reperfusion challenge. Our final hypothesis was that dietary hempseed reduces the detrimental effects of cholesterol on cardiac function and arrhythmia threshold during ischemia/reperfusion challenge. Specifically, we hypothesized that the unique composition of fatty acids present in hempseed would provide the mechanism responsible for any cardioprotective effect.
Our hypothesis that dietary hempseed would be cardio-protective was based on previous observations of a protective effect of PUFAs during ischemia/reperfusion challenge (14–16,18–20,31,32). However, we found no significant cardio-protective effect of dietary hempseed on five measurements of cardiac performance: QT interval duration before ischemia as well as LVEDP, LVDP, arrhythmia incidence and arrhythmia duration during ischemia and reperfusion. This is conclusive evidence that dietary hempseed did not offer significant beneficial effects under these experimental conditions.
The results and the conclusions obtained from the present study are limited to the duration of the dietary intervention used here. This was an eight-week dietary intervention and it is possible that a longer dietary treatment with hempseed may have induced protective effects. However, it is reasonable to expect eight weeks to induce significant cardioprotective effects based on a previous study that showed improved cardiovascular parameters at eight weeks using different dietary regimens (9). In addition, the sample size may have been too small to detect significant differences. We did observe a trend for the hearts of the HP group to have the shortest QT interval duration, which is similar to the study by Ander et al (9) who examined dietary flaxseed supplementation. Therefore, dietary hempseed may provide protection from arrhythmias by shortening the duration of the QT interval; however, a larger sample size may be needed to establish statistical significance. The present study is also limited to our experimental animal model – the rabbit. It is possible that the rabbit did not respond to hempseed as well as other animal species may have. One important limitation of the present study is that we examined cardiac performance in isolated hearts removed from the plasma fatty acid concentrations that would perfuse them in vivo. The PUFA-enriched plasma was replaced with a standard perfusate that did not contain PUFAs. This leads to an underestimate of the potential effects of PUFAs on cardiac performance during ischemia/reperfusion challenge. Finally, it is important to recognize that hempseed contains an enrichment of several different PUFAs, including LA, GLA and ALA. With such a complex mixture, it is difficult to dissect out the potential beneficial components. It is possible that although one or even two of the PUFAs found in hempseed may confer cardioprotection, one may inhibit these actions.
In regards to our second and third hypotheses (stated above), the OL group showed no significant increase in either incidence or duration of arrhythmias during ischemia or reperfusion compared with control levels. This is surprising because hypercholesterolemia, which our cholesterol-fed animals clearly demonstrated, increases susceptibility to ventricular arrhythmia (9,33). We found no significant differences in QT interval duration in the cholesterol-supplemented groups. This is in contrast to Ander et al (9), who found a significantly longer QT interval due to dietary cholesterol supplementation and a significantly shorter QT interval due to dietary flaxseed supplementation as well as dietary flaxseed plus cholesterol supplementation, compared with control values. The reason for this discrepancy between our findings with cholesterol-fed animals and those previously reported is not clear, but it may be due to the increased duration of the dietary intervention in the study by Ander et al (9). In contrast, we did observe a significant decrease in the incidence of fibrillation during reperfusion in the OL and OLHP groups compared with the DHP group. This finding demonstrates the possibility that hempseed may be proarrhythmic when it contains a reduced lipid level. The observation that hempseed and cholesterol cosupplementation significantly decreases the incidence of fibrillation compared with delipidated hempseed supplementation demonstrates the beneficial properties of hempseed-derived fatty acids. The OL and OLHP groups exhibited significant elevations in plasma levels of almost all fatty acids compared with the RG group, whereas the DHP group demonstrated no significant elevations in any plasma fatty acid levels compared with the RG group. It is unclear which specific PUFA may be responsible for the protective effects in the OL and OLHP groups. However, the hearts from the OLHP group had a significantly elevated level of GLA compared with the hearts from the DHP group. This elevated cardiac GLA level may have conferred the protection against reperfusion-derived arrhythmias observed in the OLHP hearts. Dietary evening primrose oil or borage oil supplementation (sources of GLA) has been shown to induce a significant reduction in ischemia/reperfusion-derived arrhythmias compared with a saturated fat diet (34).
CONCLUSIONS
Dietary hempseed induced limited beneficial effects on cardiac function during ischemia/reperfusion challenge. Our preliminary study does not support the use of dietary hempseed to protect the heart during ischemia/reperfusion challenge in this experimental model.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Canadian Institutes of Health Research.
REFERENCES
- 1.Thom T, Haase N, Rosamond W, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics – 2006 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. (Erratum in 2006;113:e696) [DOI] [PubMed] [Google Scholar]
- 2.Heart and Stroke Foundation of Canada. The Growing Burden of Heart Disease and Stroke in Canada. Ottawa: Heart and Stroke Foundation of Canada; 2003. [Google Scholar]
- 3.British Heart Foundation Health Promotion Research Group. Coronary Heart Disease Statistics 2006. < http://www.bhf.org.uk/professionals/index.asp?SecID=15&secondlevel=519> (Version current at August 16, 2006)
- 4.Lefer DJ, Granger DN. Oxidative stress and cardiac disease. Am J Med. 2000;109:315–23. doi: 10.1016/s0002-9343(00)00467-8. [DOI] [PubMed] [Google Scholar]
- 5.Levine RL. Ischemia: From acidosis to oxidation. FASEB J. 1993;7:1242–6. doi: 10.1096/fasebj.7.13.8405809. [DOI] [PubMed] [Google Scholar]
- 6.Leifert WR, Jahangiri A, McMurchie EJ. Antiarrhythmic fatty acids and antioxidants in animal and cell studies. J Nutr Biochem. 1999;10:252–67. doi: 10.1016/s0955-2863(99)00011-x. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari R. Metabolic disturbances during myocardial ischemia and reperfusion. Am J Cardiol. 1995;76:17B–24B. [PubMed] [Google Scholar]
- 8.Yusuf S, Hawken S, Ounpuu S, et al. INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 9.Ander BP, Weber AR, Rampersad PP, Gilchrist JS, Pierce GN, Lukas A. Dietary flaxseed protects against ventricular fibrillation induced by ischemia-reperfusion in normal and hypercholesterolemic rabbits. J Nutr. 2004;134:3250–6. doi: 10.1093/jn/134.12.3250. [DOI] [PubMed] [Google Scholar]
- 10.Albert CM, Hennekens CH, O’Donnell CJ, et al. Fish consumption and risk of sudden cardiac death. JAMA. 1998;279:23–8. doi: 10.1001/jama.279.1.23. [DOI] [PubMed] [Google Scholar]
- 11.Hu FB, Bronner L, Willett WC, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–21. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 12.Oomen CM, Feskens EJ, Rasanen L, et al. Fish consumption and coronary heart disease mortality in Finland, Italy, and The Netherlands. Am J Epidemiol. 2000;151:999–1006. doi: 10.1093/oxfordjournals.aje.a010144. [DOI] [PubMed] [Google Scholar]
- 13.Yuan JM, Ross RK, Gao YT, Yu MC. Fish and shellfish consumption in relation to death from myocardial infarction among men in Shanghai, China. Am J Epidemiol. 2001;154:809–16. doi: 10.1093/aje/154.9.809. [DOI] [PubMed] [Google Scholar]
- 14.Billman GE, Kang JX, Leaf A. Prevention of sudden cardiac death by dietary pure omega-3 polyunsaturated fatty acids in dogs. Circulation. 1999;99:2452–7. doi: 10.1161/01.cir.99.18.2452. [DOI] [PubMed] [Google Scholar]
- 15.McLennan PL, Abeywardena MY, Charnock JS. Dietary fish oil prevents ventricular fibrillation following coronary artery occlusion and reperfusion. Am Heart J. 1988;116:709–17. doi: 10.1016/0002-8703(88)90328-6. [DOI] [PubMed] [Google Scholar]
- 16.McLennan PL, Abeywardena MY, Charnock JS. The influence of age and dietary fat in an animal model of sudden cardiac death. Aust N Z J Med. 1989;19:1–5. doi: 10.1111/j.1445-5994.1989.tb01662.x. [DOI] [PubMed] [Google Scholar]
- 17.McLennan PL, Barnden LR, Bridle TM, Abeywardena MY, Charnock JS. Dietary fat modulation of left ventricular ejection fraction in the marmoset due to enhanced filling. Cardiovasc Res. 1992;26:871–7. doi: 10.1093/cvr/26.9.871. [DOI] [PubMed] [Google Scholar]
- 18.McLennan PL, Bridle TM, Abeywardena MY, Charnock JS. Dietary lipid modulation of ventricular fibrillation threshold in the marmoset monkey. Am Heart J. 1992;123:1555–61. doi: 10.1016/0002-8703(92)90809-a. [DOI] [PubMed] [Google Scholar]
- 19.McLennan PL, Dallimore JA. Dietary canola oil modifies myocardial fatty acids and inhibits cardiac arrhythmias in rats. J Nutr. 1995;125:1003–9. doi: 10.1093/jn/125.4.1003. [DOI] [PubMed] [Google Scholar]
- 20.Pepe S, McLennan PL. Dietary fish oil confers direct antiarrhythmic properties on the myocardium of rats. J Nutr. 1996;126:34–42. doi: 10.1093/jn/126.1.34. [DOI] [PubMed] [Google Scholar]
- 21.Holub BJ. Clinical nutrition: 4. Omega-3 fatty acids in cardiovascular care. CMAJ. 2002;166:608–15. [PMC free article] [PubMed] [Google Scholar]
- 22.Lozano I. The therapeutic use of Cannabis sativa (L) in Arabic medicine. J Cannabis Ther. 2001;1:63–70. [Google Scholar]
- 23.Grigoriev OV. Application of hempseed (Cannabis sativa L) oil in the treatment of ear, nose and throat (ENT) disorders. J Industrial Hemp. 2002;7:5–15. [Google Scholar]
- 24.Leson G, Pless P, Grotenhermen F, Kalant H, ElSohly MA. Evaluating the impact of hemp food consumption on workplace drug tests. J Anal Toxicol. 2001;25:691–8. doi: 10.1093/jat/25.8.691. [DOI] [PubMed] [Google Scholar]
- 25.Botsford MW, Lukas A. Ischemic preconditioning and arrhythmogenesis in the rabbit heart: Effects on epicardium versus endocardium. J Mol Cell Cardiol. 1998;30:1723–33. doi: 10.1006/jmcc.1998.0735. [DOI] [PubMed] [Google Scholar]
- 26.Berne RM. Physiology. St Louis: Mosby; 1998. [Google Scholar]
- 27.Katz AM. Physiology of the Heart. New York: Raven Press; 2001. [Google Scholar]
- 28.Walker MJ, Curtis MJ, Hearse DJ, et al. The Lambeth Conventions: Guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovasc Res. 1988;22:447–55. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- 29.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 30.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986;27:114–20. [PubMed] [Google Scholar]
- 31.Lepran I, Szekeres L. Effect of dietary sunflower seed oil on the severity of reperfusion-induced arrhythmias in anesthetized rats. J Cardiovasc Pharm. 1992;19:40–4. doi: 10.1097/00005344-199201000-00006. [DOI] [PubMed] [Google Scholar]
- 32.McLennan PL, Abeywardena MY, Charnock JS. Influence of dietary lipids on arrhythmias and infarction after coronary artery ligation in rats. Can J Physiol Pharmacol. 1985;63:1411–7. doi: 10.1139/y85-232. [DOI] [PubMed] [Google Scholar]
- 33.Liu YB, Wu CC, Lu LS, et al. Sympathetic nerve sprouting, electrical remodeling, and increased vulnerability to ventricular fibrillation in hypercholesterolemic rabbits. Circ Res. 2003;92:1145–52. doi: 10.1161/01.RES.0000072999.51484.92. [DOI] [PubMed] [Google Scholar]
- 34.Charnock JS. Gamma-linolenic acid provides additional protection against ventricular fibrillation in aged rats fed linoleic acid rich diets. Prostaglandins Leukot Essent Fatty Acids. 2000;62:129–34. doi: 10.1054/plef.1999.0132. [DOI] [PubMed] [Google Scholar]