Abstract
OBJECTIVE
To compare adult rat cardiomyocytes in primary culture for up to 28 days with those in primary culture for 10 days plus up to 18 days in a coculture (CC) system. The phenomenon of ‘second-floor’ cells in primary cultures and the behaviour of CC myocytes were studied over varying times with regard to protein content and attachment to underlying cells.
METHODS
Qualitative confocal microscopy and quantitation using the Imaris program (Bitplane, Switzerland) for the measurement of the fluorescence intensity in the confocal microscope were used. The protein content of actin, myosin, desmin, tubulin, titin, myomesin, cadherin and connexin was determined. Cell death was evaluated using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling method for apoptosis and using propidium iodide applied to unfixed cultures to detect necrosis.
RESULTS
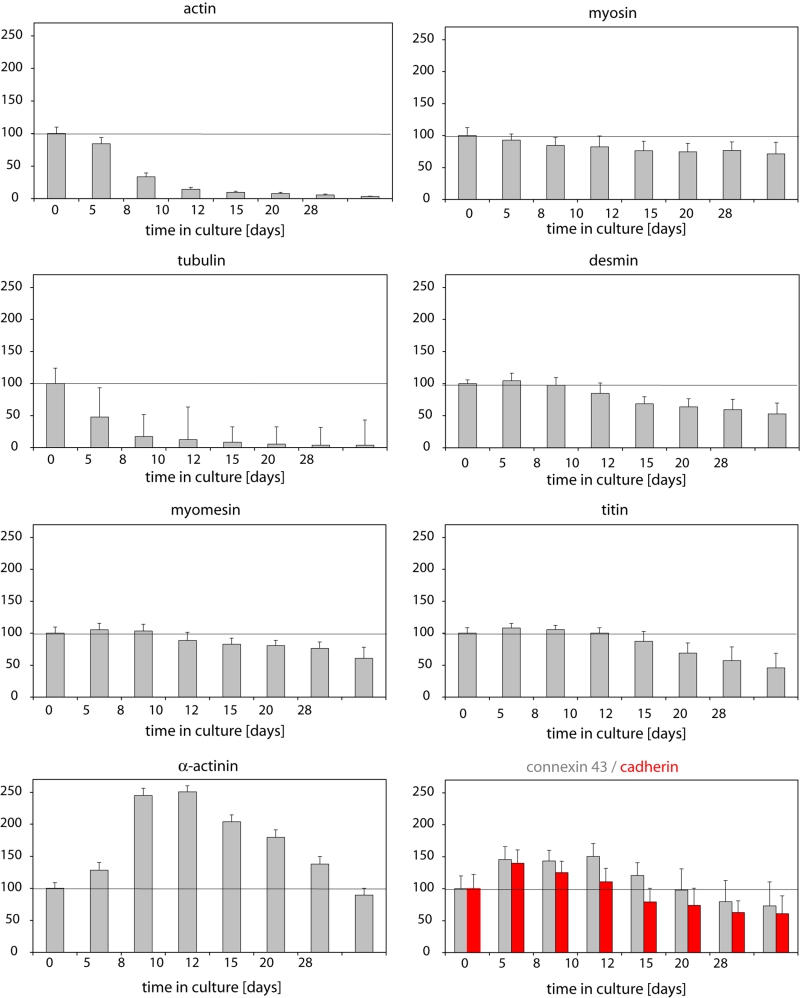
Compared with controls, second-floor cells contained only 14% of the actin and 4.9% of the tubulin at 10 days, whereas these proteins were well preserved in CC cells. All other proteins slowly declined in second-floor cells, whereas they were still present in normal amounts in CC cells. Cell death was evident in second-floor cells but absent in CC myocytes. Cellular attachment was still evident through original in vivo adherens junctions in second-floor cells but numerous newly developed cadherin- and connexin-containing junctions were visible in CC cells. It appears from the present study that second-floor cells are mummified dead cardiomyocytes, whereas CC myocytes survive and start to dedifferentiate.
CONCLUSION
The absence of actin and tubulin, together with nuclear changes, are indicators of loss of cell viability despite preservation of the cells’ rod shape and cross-striation, as observed in second-floor cells. In contrast, the establishment of a CC system of cardiomyocytes results in survival and organization of a three-dimensional cellular system, which may in the future be useful for tissue engineering attempts for replacement of lost tissue after myocardial infarction.
Keywords: Cardiomyocytes, Cell death, Coculture, Protein content, Second-floor cells
After pioneering work several decades ago (1–4), adult rat cardiomyocytes (ARC) in culture have become a widely used model to study myofibrillogenesis, cell substrate interactions, and cellular growth and adaptation under distinct experimental circumstances (this topic has been previously reviewed [5,6]).
The majority of ARC in long-term culture are characterized by the transition from an elongated rod-shape to polygonal spreading cells with numerous extensions, accompanied by dedifferentiation and redifferentiation processes (7–10). Another phenotype of myocytes is one in which cells remain small and rounded up, and where the cells will only spread at very late stages after initiation in culture (10). A third type of myocytes are those that do not attach to laminin-coated glass or plastic. These myocytes have been defined as ‘second-floor’ cells because of their topography (11). Although they are well known to other investigators (M Eppenberger, personal communication) (12), they have not yet been fully studied and their importance is unclear. However, the fact that these cells may show a behaviour different from that of spreading myocytes may be important under certain experimental conditions. Furthermore, because of the current interest in coculturing cardiomyocytes with mesenchymal stem cells (13), bone marrow stromal cells (14), skeletal myoblasts (15,16), fibroblasts (17) or endothelial progenitor cells (18), knowledge of the behaviour of cardiac myocytes in a three-dimensional culture is necessary.
Therefore, a morphological study that allowed for the examination of each individual cell in the culture dish was carried out to test the hypothesis that direct contact with the substratum is a prerequisite for adaptation, transformation and survival of adult cardiomyocytes in culture. Attachment of myocytes occurs by cell-matrix contact via the integrins (19–21), but other factors, such as seeding density, serum concentration, type of glass or plastic and its coating, influence survival, adhesion and the course of dedifferentiation and redifferentiation (4,10,22–24). From this, it is evident that these second-floor cells (ie, cells not adhering to the glass surface but to underlying myocytes) may show different biological properties and viability. To test this, quantitative immunocytochemistry using densitometry to determine myocyte protein content and viability was applied to ARC in culture.
In a second step, the possibility was tested that myocytes form direct cell-cell contacts when freshly isolated cells are seeded on a layer of cardiomyocytes already in the state of redifferentiation.
Therefore, the aim of the present study was to study the physiological properties of second-floor cells in a primary culture and in a coculture (CC) system of cardiomyocytes, to identify reliable indicators of myocyte viability, to determine and compare the protein content of second-floor cells in primary cultures with those in a myocyte CC system, and to evaluate the mechanism of cell-cell contact formation without interference from integrins.
MATERIALS AND METHODS
Isolation and cultivation of myocytes
Freshly isolated ARC were prepared from seven-week-old male Wistar rats, following standard methods. The animals were deeply anesthetized with ether. After an injection of heparin (300 U/kg) into the inferior vena cava, the heart was excised and the aorta was cannulated. The heart was perfused retrogradely with a Ca2+-free buffer followed by perfusion with 0.03% collagenase (CLS 2, Worthington Biochemical Corporation, USA) containing 0.004% pronase (Boehringer, Germany), 0.005% trypsin (1:250, Sigma, Germany) and 0.04 mM CaCl2 in perfusion buffer. After perfusion with collagenase solution for 20 min, the ventricles were minced in the above mentioned collagenase solution containing 1.2% bovine serum albumin. After mincing, the myocytes were filtered through a nylon mesh and centrifuged at 8 g for 3 min. The pellet was washed in perfusion buffer containing 0.1 mM CaCl2. After separation with 33% Percoll (Pharmacia, Germany) separation medium, the myocytes were washed with perfusion buffer. Calcium was then added stepwise up to a concentration of 1.0 mM. The cells were resuspended in medium 199 (Sigma) containing 5 mM creatine, 2 mM L-carnitine, 5 mM taurine, 0.1 mM insulin, 10 mM cytosine beta-D-arabinofuranoside, 100 U/mL penicillin-streptomycin and 10% fetal calf serum.
Cardiomyocytes were plated on culture chamber slides (Nunc, Germany) coated with 5 μg/mL laminin (Sigma) at 3×104 to 5×104 cells per well and incubated in a CO2 incubator at 37°C. After 3 h, the medium was replaced with the same fresh medium, and the cells were cultured up to 28 days.
Myocytes for the cocultures were isolated in the same way as described above. They were added to 10-day-old primary cultures and kept for a maximum of 18 days in coculture (ie, 28 days in total).
Fixation
At different time points, the cells were fixed in 4% freshly prepared paraformaldehyde (PFA) and prepared for immunomorphology as described below.
Propidium iodide for membrane integrity
Propidium iodide (PI) (Molecular Probes, Germany) was used as a fluorescence marker for membrane integrity. Unfixed ARC were incubated with PI (0.5 μg/mL in phosphate-buffered saline [PBS]) at room temperature for 30 min. After rinsing in PBS, cells were fixed in 4% paraformaldehyde and counterstained with fluorescein isothiocyanate-labelled phalloidin (Sigma) for 30 min to visualize the morphology of the contractile apparatus. Directly after mounting the culture plates with Mowiol (Hoechst, Germany), microscopic evaluation was performed to prevent fading of PI labelling.
Apoptosis
The ApoTag in situ apoptosis detection kit (Amersham, Germany) was used. Visualization of apoptotic cells was performed using an antidigoxigenin antibody conjugated with fluorescein isothiocyanate. One negative control for each culture was prepared and incubated in the absence of the enzyme terminal deoxynucleotidyl transferase. For a positive control, the myocytes were digested with 1 μg DNase-1 per millilitre of DNase buffer (Sigma) for 10 min.
Immunofluorescence
Cultured ARC were fixed in 4% PBS for 15 min, and permeabilized for 15 min in 0.05% Triton X (Sigma) in PBS. To ensure the total penetration of the fluorescent markers, the incubation of the primary and secondary antibody was carried out at 4°C in a moist chamber for 24 h using PBS with 0.005% Triton X. Incubation with Cy2-labelled streptavidin (Dianova, Germany) was performed at room temperature for 4 h. Between the incubations, unbound ligands were removed during three 3 min rinsing steps in PBS.
The different antibodies are shown in Table 1. For secondary and tertiary antibodies, antimouse biotin (Dianova) and Cy2-coupled streptavidin (Dianova) were used, respectively. Nuclei were stained red with 0.002% 7-aminoactinomycin D (Molecular Probes) for 30 min. After the last rinsing steps, the culture chamber slides were mounted with coverglasses using Mowiol. Cells were stored for another 12 h at 4°C in the dark to allow polymerization of the Mowiol. Omission of the primary antibody was performed as a negative control to observe unspecific labelling of the detection system.
TABLE 1.
Antibodies used in the present study
| Primary antibody | Clone | Source | Antigen |
|---|---|---|---|
| Beta-tubulin | Tub 2.1 | Sigma (Germany) | Rat brain tubulin |
| Titin | T 12 | Boehringer (Germany) | Titin from chicken skeletal muscle |
| Myosin | CCM52 | Kind gift from Dr Decker | MHC from chicken embryo heart |
| Myomesin | B4 | Kind gift from Drs Perriard and Eppenberger | Myomesin from cell culture |
| Desmin | DEU-10 | Sigma (Germany) | Desmin from pig stomach |
| Alpha-actinin | EA-53 | Sigma (Germany) | Alpha-actinin from rabbit skeletal muscle |
| N-Cadherin | GC-4 | Sigma (Germany) | N-Cadherin from chicken heart |
| Desmoplakin | DP 1&2–2.15 | Boehringer (Germany) | Desmoplakin from bovine epidermins |
| Connexin 43 | 1E9 | Biotrend (Germany) | Oligopeptide (amino acids 252 to 270 of rat connexin 43) |
MHC Myosin heavy chain
Electron microscopy
Myocytes were fixed overnight with 3% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) and embedded in Epon (Boehringer) following routine procedures. Semithin sections were stained with toluidine blue and viewed using a LEICA DM microscope (Leica Microsystems, Germany). Ultrathin sections were performed on a Reichert-Jung Ultracut E (Leica Microsystems) in different directions: in parallel or on a right angle to the substrate, or on the longitudinal axis of a particular myocyte. Sections were stained with uranyl acetate and lead citrate, and viewed and photographically recorded using a Philips CM 10 electron microscope (Philips Research Technologies, The Netherlands).
Confocal microscopy, image processing and intensity measurements
The imaging system consisted of a Leitz DM RBE fluorescence microscope, a Leica TCS 4D confocal scanner with an argon-krypton laser (Leica Microsystems) and a Motorola workstation (Motorola, Germany) for image recording. After data acquisition, the confocal series were transferred to a Silicon Graphics workstation (Silicon Graphics Inc, USA). Further restoration, three-dimensional visualization and archiving of the confocal images were performed using Imaris and Selima, respectively (Bitplane, Switzerland).
For quantification of the fluorescence, a volume of interest (VOI) was defined in a certain myocyte by using the section mode of Imaris to control the heterogenous distribution of the fluorescence labelling. VOI represents the number of voxels in the xy- and z-dimension, where size and resolution depends on the magnification, the numeral aperture of the objective lens and the number of layers defined at the time of confocal data acquisition. Data were expressed as arbitrary units obtained after calibration and the establishment of a brightness curve including 256 levels of intensity.
RESULTS
Second-floor cells
Morphological appearance
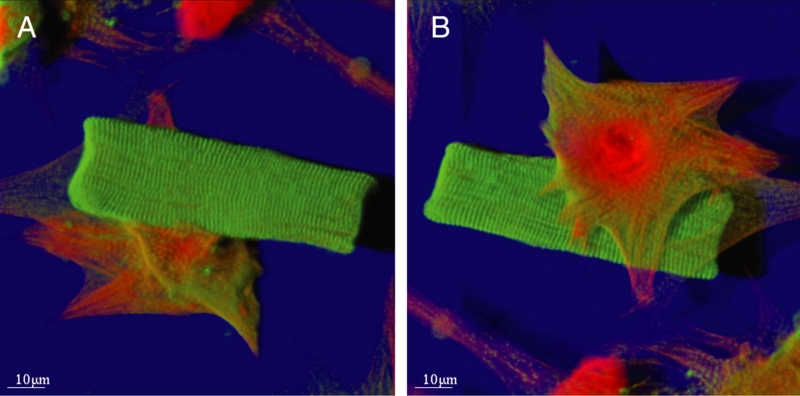
Figure 1 shows the typical appearance of a second-floor cell, which is rod-shaped and cross-striated. This cell is situated on top of a spreading cell that shows dedifferentiation. From the upside down projection shown in Figure 1B, it is evident that no contact with the glass exists for the cross-striated cell.
Figure 1).
Typical appearance of a ‘second-floor’ cell situated on a spreading myocyte. Ten days in culture. Shadow projection: Red represents actin and green represents alpha-actinin. Note the rod shape and distinct cross-striation of the second-floor cardiomyocyte, whereas the spreading cell is in the phase of dedifferentiation and contains a small amount of alpha-actinin and much more actin. A View from above. B View from the bottom
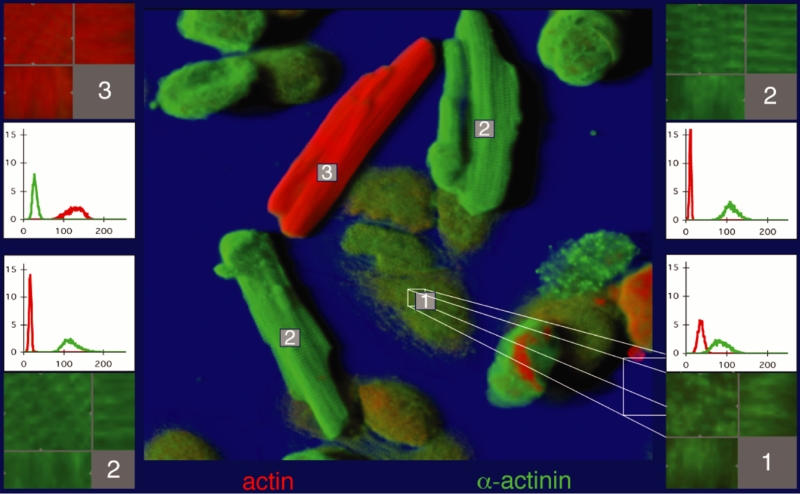
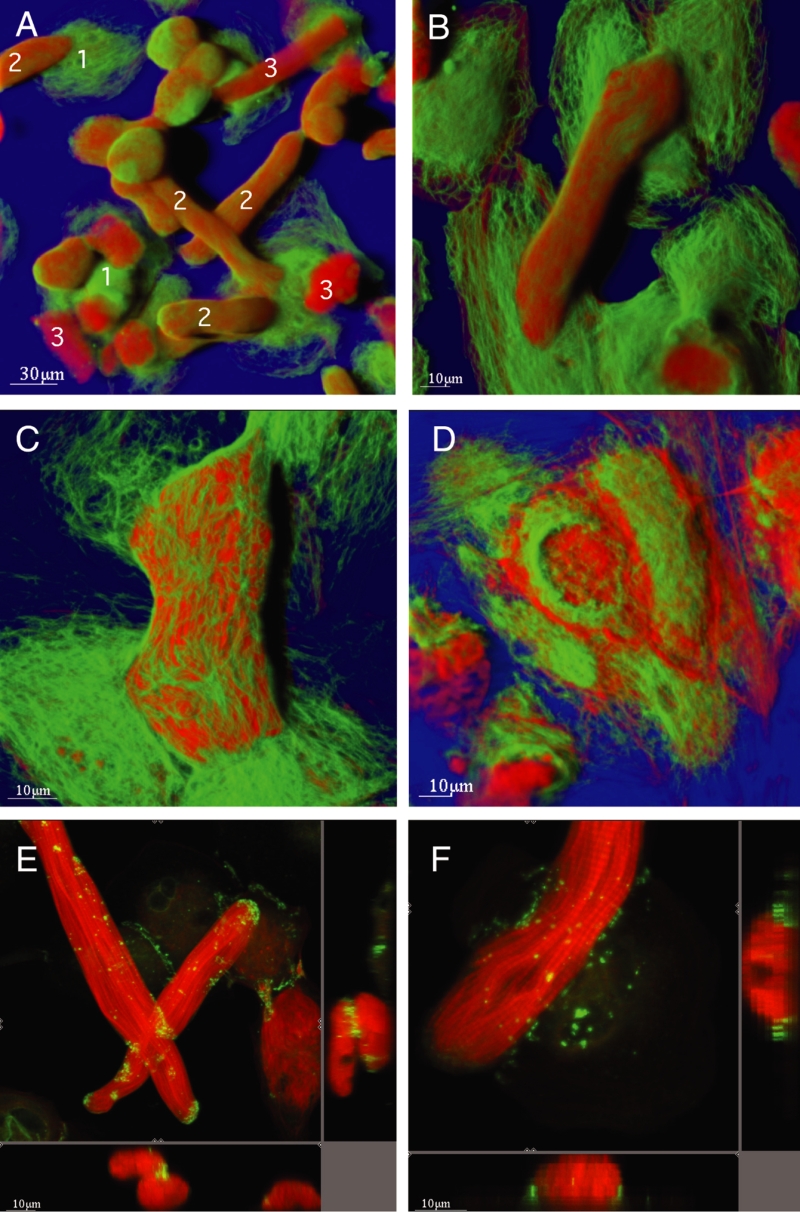
At early stages of cocultivation at 10 days plus 3 h, the appearance of the cardiomyocytes is heterogeneous (Figure 2). Cells attached to the glass show loss of cross-striation and the beginning of spreading, whereas cells located on attached myocytes show the typical second-floor appearance. The protein content of individual cells, depicted in the small graphs of Figure 2, was different from one cell to another; the spreading cells contained more alpha-actinin than actin, the second-floor cells contained much more alpha-actinin than actin, and the freshly added myocyte exhibited the highest actin content. In all measurements, the values obtained from this type of cell served as control or 100%. This is demonstrated in Figure 3. As an example, actin at 10 days was only 14% of control and tubulin was 4.9%, whereas alpha-actinin was 250%.
Figure 2).
Cardiomyocytes in culture at day 10 plus 3 h coculture. Shadow projection: Green represents alpha-actinin and red represents actin. (Cell 1) Spreading cells contain both actin and alpha-actinin, but more of the latter (histogram). (Cell 2) ‘Second-floor’ cells show only a small amount of actin but are strongly positive for alpha-actinin. (Cell 3) Cell was added at day 10 and shows plenty of actin; these cells served as a control for all measurements. The corresponding coloured boxes show the volume of interest (VOI) in section mode, with the corresponding xz-plane (below) and the yz-plane (right). The histograms (ordinate: brightness of fluorescence; abscissa: number of pixels) show the quantitative evaluation (red represents actin and green represents alpha-actinin)
Figure 3).
Quantitative evaluation of the brightness of fluorescence at varying times (in per cent of control [control defined as 100%]). Measurements were carried out in ‘second-floor’ cells and cocultured cells served as controls (see Figure 2). Note that actin and tubulin show a steep decrease, while all other proteins with the exception of alpha-actinin show a late decline. The significant increase in alpha-actinin fluorescence intensity may not reflect the true amount of the protein but the fact that more alpha-actinin epitopes are accessible for the antibody, because the actin rapidly disappears (bare Z-line)
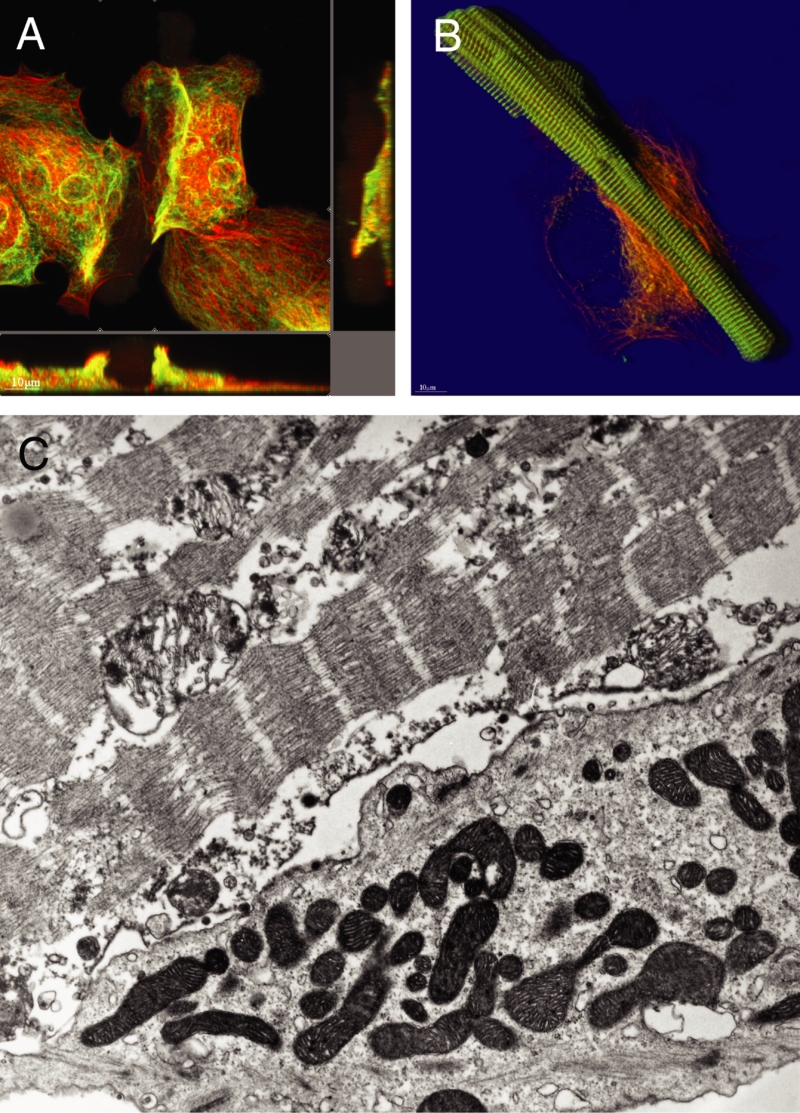
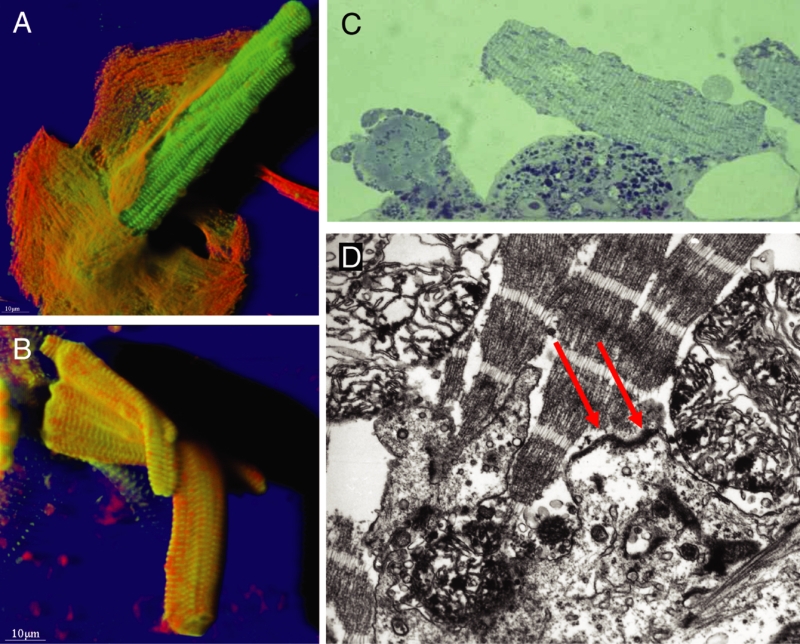
At late stages (ie, 28 days in culture), the difference in protein content was even more pronounced. The second-floor cells showed preserved rod shape and intact cross-striation (myomesin), whereas the underlying spreading cells contained a small amount of actin and almost no myomesin (Figure 4A). Another example is shown in Figure 4B, where the second-floor cells were almost devoid of tubulin and actin, but alpha-actinin was still preserved. Figure 4C shows that these differences in protein content correspond with the ultrastructural appearance of the cells; the spreading cell at the bottom is dedifferentiating with intact mitochondria, whereas the second-floor cell on top shows partially destroyed sarcomeres and mitochondria, which is typical of necrosis.
Figure 4).
Different protein composition of ‘second-floor’ cells compared with the underlying cardiomyocytes at 21 days of culture. Red represents actin and green represents tubulin in (A) and myomesin in (B). A Section mode. The second-floor cell is situated in the centre and barely visible because of the lack of actin and tubulin. The presence of a rod-shaped cell becomes more distinct in the xz-plane (below) in the centre of the micrograph. B Shadow projection. The rod-shaped second-floor cell shows intact cross-striation with intense alpha-actinin staining in green. C Electron microscopy. Different stages of preservation of myocytes. Bottom: Living cell in the state of dedifferentiation. Top: Necrotic second-floor cell (original magnification ×24,000)
From these observations, it becomes evident that second-floor cells are devoid of actin and tubulin very early, whereas all other proteins measured decreased very slowly over time, and alpha-actinin seemed to even increase and only fell at very late stages. The time courses of these observations were quantified as summarized in Figure 3.
The problem of cell attachment
Second-floor cells may be regarded as primarily intact cells that are situated by chance on the myocytes attached to the laminin-coated glass and that are not washed away because they stick to the underlying cell. Stickiness may be caused by still existing intercalated disc connections to the underlying cell (Figures 5A and 5D) or by mechanical engulfment of the second-floor cells by underlying spreading myocytes (Figure 5B). Figure 5C shows the appearance of cell arrangement in the light microscope. Second-floor cells still contained their original cadherin and connexin content and adhesion plaques (Figure 5D), which were being lost slowly over time – comparable with the loss of desmin or titin (Figure 3).
Figure 5).
Different modes of attachment of ‘second-floor’ cells to underlying spreading myocytes. Red represents actin and green represents alpha-actinin. A Shadow projection. The second-floor cell is attached with only a small tip of its cytoplasm to the underlying cell (top left corner). B Shadow projection. Attachment by engulfment of one second-floor cell by spreading myocytes. C Light microscopy of semithin sections shows the typical localization of a second-floor cell on a healthy, dedifferentiating cardiomyocyte (original magnification ×400). D Electron micrograph showing still existing attachment points between two myocytes (arrows), one living (bottom) and one dead (top) (original magnification ×39,000)
Viability of second-floor cells
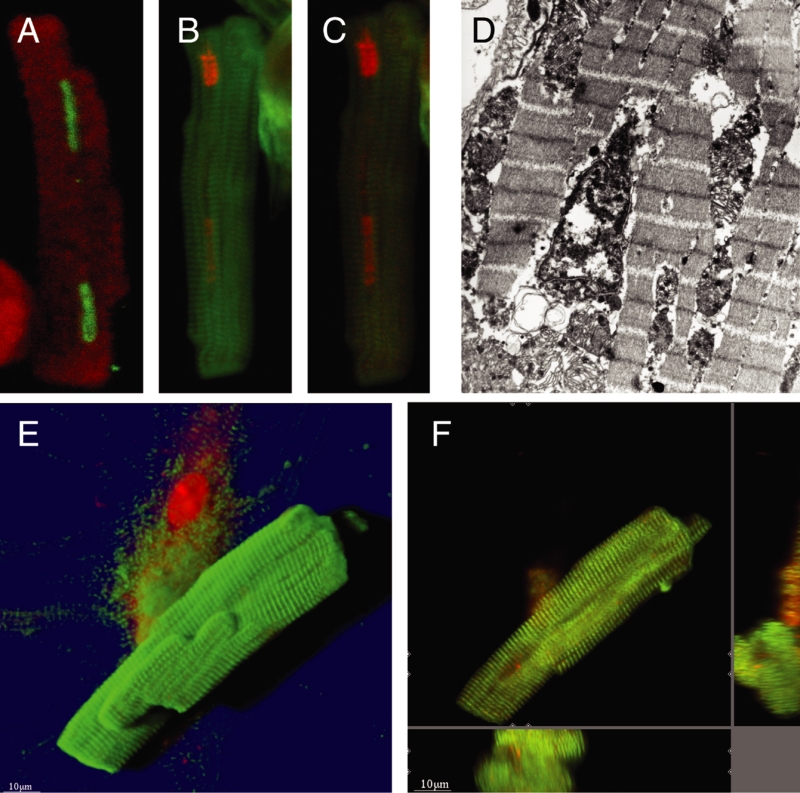
After isolation, the second-floor cells appeared to be normal for the first 48 h. However, between 72 h and 96 h, many cells were either positive for the terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling reaction, indicating apoptosis (Figure 6A), or myocytes showed uptake of PI, indicating necrosis accompanied by leakage of the sarcolemma (Figures 6B and 6C), which corresponds to the appearance of the nucleus on electron microscopy. Finally, numerous cells showed the absence of nuclei (Figures 6F and 6G). From these findings, it was concluded that second-floor cells, despite their apparently normal cross-striation for alpha-actinin, and their still preserved rod shape, are dead cells. However, markers for the loss of viability are not only nuclear changes, but also the significant and early loss of actin and tubulin. The term ‘mummified’ cells seems to be justified to describe these cells.
Figure 6).
Cell death in ‘second-floor’ cells. A A positive terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling reaction indicating apoptosis in the two nuclei of a second-floor cell at an early stage (72 h) in culture. Red represents actin. B Uptake of propidium iodide indicating necrosis of a second-floor cell. Red represents propidium iodide and green represents actin. C By reduction of the laser intensity in the confocal microscope, it became evident that the second nucleus was already fading. D Electron microscopy confirms the necrotic appearance of the nucleus (original magnification ×24,000). E Shadow projection. Absence of nuclei in a rod-shaped, cross-striated second-floor cell. The underlying spreading cell contains two intact nuclei. F This is more distinct in a section-mode presentation, where the xz-plane (below) and the yz-plane (right) clearly show the unstained nuclear region in the centre of the cell
CC experiments
To test the hypothesis that second-floor cells are already damaged cells, and therefore are doomed to die, freshly isolated cells (CC cells) were added to already spreading cells, that is, a CC system was used to study the behaviour of these cells by comparing them with second-floor cells.
Morphological appearance, attachment and viability
The CC cells were of normal appearance at early stages of coculture and they showed a rounded-up shape typical of spreading cells after three days (Figures 1, and 7A and 7B). As time progressed, it was evident that actin and tubulin remained present in CC cells, indicating viability (Figures 7B to 7D). Spreading in CC cells continued, although to a lesser extent than that observed in the underlying cell layer. Attachment to underlying cells occurred via newly formed connections containing cadherin and connexin (Figures 7D and 7F). These involved both cell layers, that is, the cells formed junctions with each other and with underlying cells wherever they made contact.
Figure 7).
Coculture (CC) of cardiomyocytes. Green represents tubulin and red represents actin. Shadow projection. A At 10 plus three days of CC, the underlying spreading cells show an intense tubulin network and smaller amounts of actin (cells marked ‘1’), whereas the myocytes on top are rounded-up and contain large amounts of actin (cells marked ‘2’). Note the necrotic cells from the first culture (cells marked ‘3’). B The higher magnification of cells at 10 plus three days of CC shows that actin and tubulin are present and the CC cell shows size and shape transformation. C At 10 plus five days of CC, these transformations are more evident than at 13 days. D At 10 plus 18 days of CC, the CC cell has spread and contains actin and tubulin (ie, it is viable). E Section mode. Red represents actin and green represents connexin 43. At 10 plus one day of CC, CC cells show slight rounding up at the ends, a strong actin labelling and a specific expression of connexin 43 at the crossing point, which is more obvious in the xz-plane (below) and the yz-plane (right). F Section mode. Red represents actin and green represents cadherin. At 10 plus three days of CC, the CC cell shows rounding up and multiple cadherin-containing connection points with the underlying cell
Markers for viability (ie, the presence of actin and tubulin) were positive, and apoptosis and necrosis were absent.
It was concluded that CC cells are living cells with the potential to survive in a three-dimensional, artificial in vitro system for at least 18 days.
DISCUSSION
Quantification of fluorescence
The present study reports the use of the Imaris program for the measurement of fluorescence intensity (FI) in cardiomyocytes being examined with confocal microscopy (8). This method allows for multiple determination of FI in small well-defined volumes (VOI) in single cells, whereby a comparison of different cellular regions is possible. In addition, this method permits the comparison of FI from different cells for the same proteins, and allows conclusions to be made about the functional state and the viability of cells, as discussed below. Furthermore, one of the major advantages of this quantitative method is the possibility of detecting low FI values, and examining these values in correlation with the predominant fluorescence.
The prerequisite for using this method is the acquisition and transmission of confocal data sets under strictly defined, standardized conditions. In the confocal microscope, the laser power, the size of the pinhole, the voltage and the deviations in the Z-level must be fixed in their dimensions and have to remain unchanged during the confocal data set acquisition. Another essential step is a standardized staining procedure, which requires identical antibody concentrations, permeation procedures and the use of the same secondary detection system. When used properly with all precautions taken, this method is accurate and precise in providing data of individual cells or a group of cells.
Second-floor cells in primary cultures
The occurrence of so-called second-floor cells in long-term cultures of ARC is described in the present report. Approximately up to 2% of all cells maintained their original cylindrical shape, did not participate in the processes of dedifferentiation and redifferentiation (1,25,26) and adhered strongly to underlying healthy myocytes. These myocytes preserved their cross-striation for proteins of the sarcomeric skeleton, including myomesin, alpha-actinin and titin, desmin and the membrane-associated proteins vinculin, dystrophin and talin, but they lacked tubulin. The contractile protein actin was significantly reduced, whereas myosin labelling was present.
FI measurements revealed that the actin content was reduced compared with normal cells, and that tubulin was virtually absent. Unfixed second-floor cells took up PI, indicating leakage of the sarcolemma and necrosis, or they were positive for DNA fragmentation, indicating apoptosis. Electron microscopy confirmed these findings.
There was no evidence of new formation of intercalated disc components connecting the second-floor cells with underlying cardiomyocytes, but the original cadherin or connexin molecules were still visible. It appears that the second-floor cells, despite their preserved cross-striation for alpha-actinin, myomesin or other proteins, are mummified dead cells. They are kept on top of the cultured cells either by still remaining cell-cell contacts to underlying spreading myocytes or by engulfment by spreading myocytes through a mechanism still unknown. It was concluded from this part of the study that myocytes need contact with the substratum (eg, laminin-coated culture dishes) to survive. Consecutively, this hypothesis was tested in the CC system.
Myocyte viability
Several methods have been recommended for the determination of myocyte viability (for an overview, see previous work by our group [27,28]). Even now, with the availability of immunological or molecular markers for the identification of cell death, electron microscopy is still the absolute gold standard because typical ultrastructural alterations of nuclei, mitochondria and the cell membrane can be easily and doubtlessly identified. In our experience, exclusion of trypan blue seems to be unreliable and does not provide a clear-cut, yes-or-no answer (26,29). The uptake of PI, on the other hand, indicates leaky membranes and cell death (30). Changes of several other different proteins have been implicated in the diagnosis of cell damage. Jennings and coworkers (31) reported that vinculin disappeared at an early time point of ischemic tissue injury and thereby offered an explanation for the appearance of blebs in injured myocytes. However, in our experience, vinculin seems to be a protein that is somewhat resistant to ischemic injury (32). As described below, strong labelling of alpha-actinin in a cross-striation pattern is not necessarily a reliable parameter of cell viability, as was reported earlier (33). Our group has observed that the sensitivity to ischemia is most pronounced in myosin and tubulin (32). However, most of the studies cited here were carried out in myocardial tissue but not in isolated or cultured cells, and often by the use of polyclonal antibodies with a high and unspecific background staining pattern. Although these methods probably allow conclusions to be made about the ischemic tolerance of cardiac proteins, they do not permit a clear-cut diagnosis of cell death.
Using quantitative confocal microscopy and the comparison of the structural appearance of the cells by electron microscopy, it was possible to define changes in two proteins that indicated a loss of cellular viability; specifically, the disappearance of tubulin and the significant reduction of the actin content were reliable signs that the myocytes suffered severe injury and were no longer viable. This was evident in the second-floor cells in primary cultures, as confirmed by electron microscopy and PI uptake. The alpha and beta heterodimers of tubulin, with a molecular weight of 55 kDa each, polymerize and form microtubuli that show an extremely fast turnover of approximately 10 min by polymerization at the plus-end and degradation at the minus-end (34). However, the molecule itself shows a half-life of approximately 24 h. The disappearance of tubulin is caused by either a higher rate of degradation or a slower rate of polymerization, or both (ie, an imbalance between synthesis and breakdown). The antibody used in the present study detects the presence of beta-tubulin. Because the staining pattern for alpha-tubulin is similar and its disappearance due to injury and cell death is identical, it is sufficient to stain for only one of the two heterodimers.
Tubulin and desmin are the major components of the cytoskeleton in myocytes (35). The fact that tubulin disappears in ischemic canine myocardium has been described by Iwai et al (36), and Ganote and Vander Heide (37) reported that vinculin and alpha-actinin showed diminished staining after prolonged ischemia, whereas the cytoskeletal component desmin remained relatively unchanged. The role of the cytoskeleton in myocardial injury is still not entirely clear, as pointed out by Ganote and Armstrong in a comprehensive review (38). Our group reported that tubulin in myocytes is sensitive to ischemic injury but that it persisted in vascular structures (32). Another study showed that adult cardiomyocytes were unable to spread in the absence of microtubules or microfilaments (39), which confirmed our own findings (40).
Compared with control, the actin content was reduced in cells lacking tubulin, which is in accordance with Rothen-Rutishauser et al (39), who showed the lack of sarcomerogenesis in cells lacking microfilaments (39). The degradation of the thin filament complex has been reported in globally ischemic rat hearts using Western blot (41), but quantitative confocal data are lacking. The present work is the first to report such changes in myocytes on both qualitative and quantitative levels.
Myocytes in a CC system
When freshly isolated cardiomyocytes were added to cells already 10 days in culture, they adhered strongly to the already spreading cells. In contrast to the mummified second-floor cells, which were also evident in this culture, the newly added cells formed cell-cell contacts by expressing cadherin, desmoplakin and, in a later stage, connexins that appeared on electron microscopy as intercalated disc-like structures. Connections could be formed between newly added myocytes or with the spreading underlying cells. These junctional proteins were apparent in any part of the sarcolemma wherever contact was established, indicating that a site of predetermination for the formation of the intercalated disc is nonexistent in these three-dimensional cultures. The cause for this phenomenon may be the lack of a directed tension on these quiescent non-contracting myocytes (42).
These newly added cells started to spread after a few days in culture but spreading was somewhat limited and did not reach the dimensions of the underlying cardiomyocytes. Actin and tubulin were preserved in these cells, even at later stages, and these cardiomyocytes were fully viable cells. In general, these findings indicate that myocytes can directly attach to other myocytes by segmental specialization of the sarcolemma, which is facilitated because isolated cardiomyocytes are free of any glycocalix material.
In contrast to these cocultured ARC, second-floor cells are mummified cells. Second-floor cells die very quickly after only a few hours in culture, retaining their original shape and cross-striation, whereas myocytes added to an already existing culture of cardiomyocytes survive and start to dedifferentiate. There are different possible explanations for this varying behaviour. First, cardiomyocytes in culture for 10 days are adapted to this situation, they spread and resynthesize contractile and cytoskeletal as well as junctional proteins, and therefore, they may provide a better environment for newly added cells attempting to attach. Second, these adapted cardiomyocytes may secrete survival-promoting growth factors and cytokines as a conditioned medium, similar to other cell cultures, especially endothelial cells.
The fact that myocytes survive and undergo remodelling resulting in a three-dimensional structure may be useful for establishing cell grafts for the treatment of myocyte loss due to myocardial infarction. It may be possible that by using tissue engineering techniques, groups of cells may be cultivated in large numbers and transplanted into endangered regions such as the border zone of infarcts (33,43).
CONCLUSION
A small number of mummified myocytes can be observed in the cardiomyocyte cell culture system. The absence of actin and tubulin, together with nuclear changes, are indicators of the loss of cell viability, despite preservation of their rod-shape and cross-striation. On the other hand, the addition of isolated myocytes to cardiac cells already in culture results in survival and the organization of a three-dimensional cellular system, which may in the future be useful for replacement of dead tissue in the myocardium.
REFERENCES
- 1.Claycomb WC, Palazzo MC. Culture of the terminally differentiated adult cardiac muscle cell: A light and scanning electron microscope study. Dev Biol. 1980;80:466–82. doi: 10.1016/0012-1606(80)90419-4. [DOI] [PubMed] [Google Scholar]
- 2.Piper HM, Probst I, Schwartz P, Hutter FJ, Spieckermann PG. Culturing of calcium stable adult cardiac myocytes. J Mol Cell Cardiol. 1982;14:397–412. doi: 10.1016/0022-2828(82)90171-7. [DOI] [PubMed] [Google Scholar]
- 3.Decker ML, Simpson DG, Behnke M, Cook MG, Decker RS. Morphological analysis of contracting and quiescent adult rabbit cardiac myocytes in long-term culture. Anat Rec. 1990;227:285–99. doi: 10.1002/ar.1092270303. [DOI] [PubMed] [Google Scholar]
- 4.Eppenberger HM, Hertig C, Eppenberger-Eberhardt M. Adult rat cardiomyocytes in culture: A model system to study the plasticity of the differentiated cardiac phenotype at the molecular and cellular levels. Trends Cardiovasc Med. 1994;4:187–93. doi: 10.1016/1050-1738(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 5.Piper HM, Volz A, Schwartz P. Adult ventricular rat heart cells. In: Piper HM, editor. Cell Culture Techniques in Heart and Vessel Research. Berlin: Springer-Verlag; 1990. pp. 36–60. [Google Scholar]
- 6.Mitcheson JS, Hancox JC, Levi AJ. Cultured adult cardiac myocytes: Future applications, culture methods, morphological and electrophysiological properties. Cardiovasc Res. 1998;39:280–300. doi: 10.1016/s0008-6363(98)00128-x. [DOI] [PubMed] [Google Scholar]
- 7.Nag AC, Cheng M. Biochemical evidence for cellular dedifferentiation in adult rat cardiac muscle cells in culture: Expression of myosin isozymes. Biochem Biophys Res Commun. 1986;137:855–62. doi: 10.1016/0006-291x(86)91158-7. [DOI] [PubMed] [Google Scholar]
- 8.Messerli JM, Eppenberger-Eberhardt ME, Rutishauser BM, et al. Remodelling of cardiomyocyte cytoarchitecture visualized by three-dimensional (3D) confocal microscopy. Histochemistry. 1993;100:193–202. doi: 10.1007/BF00269092. [DOI] [PubMed] [Google Scholar]
- 9.Messerli JM, Perriard JC. Three-dimensional analysis and visualization of myofibrillogenesis in adult cardiomyocytes by confocal microscopy. Microsc Res Tech. 1995;30:521–30. doi: 10.1002/jemt.1070300609. [DOI] [PubMed] [Google Scholar]
- 10.Person V, Kostin S, Suzuki K, Labeit S, Schaper J. Antisense oligonucleotide experiments elucidate the essential role of titin in sarcomerogenesis in adult rat cardiomyocytes in long-term culture. J Cell Sci. 2000;113:3851–9. doi: 10.1242/jcs.113.21.3851. [DOI] [PubMed] [Google Scholar]
- 11.Hein S, Bauer E, Klövekorn WP, Schaper J. Cocultures of isolated rat ventricular adult cardiomyocytes rapidly rebuild specific cell-cell contracts and form a 3-dimensional network. Circulation. 1998;98(Suppl):4276. (Abst) [Google Scholar]
- 12.Nag AC, Schultz DE, Lee ML. Expression of atrial natriuretic peptide in cultured adult cardiac ventricular muscle cells as studied by immunofluorescence microscopy. Cell Mol Biol (Noisy-le-grand) 1995;41:813–25. [PubMed] [Google Scholar]
- 13.Yoon J, Shim WJ, Ro YM, Lim DS. Transdifferentiation of mesenchymal stem cells into cardiomyocytes by direct cell-to-cell contact with neonatal cardiomyocyte but not adult cardiomyocytes. Ann Hematol. 2005;84:715–21. doi: 10.1007/s00277-005-1068-7. [DOI] [PubMed] [Google Scholar]
- 14.Xu M, Wani M, Dai YS, et al. Differentiation of bone marrow stromal cells into the cardiac phenotype requires intercellular communication with myocytes. Circulation. 2004;110:2658–65. doi: 10.1161/01.CIR.0000145609.20435.36. [DOI] [PubMed] [Google Scholar]
- 15.Reinecke H, Minami E, Poppa V, Murry CE. Evidence for fusion between cardiac and skeletal muscle cells. Circ Res. 2004;94:e56–60. doi: 10.1161/01.RES.0000125294.04612.81. [DOI] [PubMed] [Google Scholar]
- 16.Formigli L, Francini F, Tani A, et al. Morphofunctional integration between skeletal myoblasts and adult cardiomyocytes in coculture is favored by direct cell-cell contacts and relaxin treatment. Am J Physiol Cell Physiol. 2005;288:C795–804. doi: 10.1152/ajpcell.00345.2004. [DOI] [PubMed] [Google Scholar]
- 17.Driesen RB, Dispersyn GD, Verheyen FK, et al. Partial cell fusion: A newly recognized type of communication between dedifferentiating cardiomyocytes and fibroblasts. Cardiovasc Res. 2005;68:37–46. doi: 10.1016/j.cardiores.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 18.Gruh I, Beilner J, Blomer U, et al. No evidence of transdifferentiation of human endothelial progenitor cells into cardiomyocytes after coculture with neonatal rat cardiomyocytes. Circulation. 2006;113:1326–34. doi: 10.1161/CIRCULATIONAHA.105.559005. [DOI] [PubMed] [Google Scholar]
- 19.Ross RS, Borg TK. Integrins and the myocardium. Circ Res. 2001;88:1112–9. doi: 10.1161/hh1101.091862. [DOI] [PubMed] [Google Scholar]
- 20.Springer TA, Wang JH. The three-dimensional structure of integrins and their ligands, and conformational regulation of cell adhesion. Adv Protein Chem. 2004;68:29–63. doi: 10.1016/S0065-3233(04)68002-8. [DOI] [PubMed] [Google Scholar]
- 21.Brancaccio M, Hirsch E, Notte A, Selvetella G, Lembo G, Tarone G. Integrin signalling: The tug-of-war in heart hypertrophy. Cardiovasc Res. 2006;70:422–33. doi: 10.1016/j.cardiores.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Eppenberger M, Hauser I, Baechi T, et al. Immunocytochemical analysis of the regeneration of myofibrils in long-term cultures of adult cardiomyocytes of the rat. Dev Biol. 1988;130:1–15. doi: 10.1016/0012-1606(88)90408-3. [DOI] [PubMed] [Google Scholar]
- 23.Bugaisky LB, Zak R. Differentiation of adult rat cardiac myocytes in cell culture. Circ Res. 1989;64:493–500. doi: 10.1161/01.res.64.3.493. [DOI] [PubMed] [Google Scholar]
- 24.Bird SD, Doevendans PA, van Rooijen MA, et al. The human adult cardiomyocyte phenotype. Cardiovasc Res. 2003;58:423–34. doi: 10.1016/s0008-6363(03)00253-0. [DOI] [PubMed] [Google Scholar]
- 25.Moses RL, Claycomb WC. Ultrastructure of terminally differentiated adult rat cardiac muscle cells in culture. Am J Anat. 1982;164:113–31. doi: 10.1002/aja.1001640203. [DOI] [PubMed] [Google Scholar]
- 26.Nag AC, Cheng M, Fischman DA, Zak R. Long-term cell culture of adult mammalian cardiac myocytes: Electron microscopic and immunofluorescent analyses of myofibrillar structure. J Mol Cell Cardiol. 1983;15:301–17. doi: 10.1016/0022-2828(83)91342-1. [DOI] [PubMed] [Google Scholar]
- 27.Kostin S, Pool L, Elsasser A, et al. Myocytes die by multiple mechanisms in failing human hearts. Circ Res. 2003;92:715–24. doi: 10.1161/01.RES.0000067471.95890.5C. [DOI] [PubMed] [Google Scholar]
- 28.Kostin S. Pathways of myocyte death: Implications for development of clinical laboratory biomarkers. Adv Clin Chem. 2005;40:37–98. doi: 10.1016/s0065-2423(05)40002-5. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki K, Kostin S, Person V, Elsässer A, Schaper J. Time course of the apoptotic cascade and effects of caspase inhibitors in adult rat ventricular cardiomyocytes. J Mol Cell Cardiol. 2001;33:983–94. doi: 10.1006/jmcc.2001.1364. [DOI] [PubMed] [Google Scholar]
- 30.Taimor G, Hofstaetter B, Piper HM. Apoptosis induction by nitric oxide in adult cardiomyocytes via cGMP-signaling and its impairment after simulated ischemia. Cardiovasc Res. 2000;45:588–94. doi: 10.1016/s0008-6363(99)00272-2. [DOI] [PubMed] [Google Scholar]
- 31.Steenbergen C, Hill ML, Jennings RB. Cytoskeletal damage during myocardial ischemia: Changes in vinculin immunofluorescence staining during total in vitro ischemia in canine heart. Circ Res. 1987;60:478–86. doi: 10.1161/01.res.60.4.478. [DOI] [PubMed] [Google Scholar]
- 32.Hein S, Scheffold T, Schaper J. Ischemia induces early changes to cytoskeletal and contractile proteins in diseased human myocardium. J Thorac Cardiovasc Surg. 1995;110:89–98. doi: 10.1016/S0022-5223(05)80013-3. [DOI] [PubMed] [Google Scholar]
- 33.Scorsin M, Marotte F, Sabri A, et al. Can grafted cardiomyocytes colonize peri-infarct myocardial areas? Circulation. 1996;94(Suppl 9):II337–40. [PubMed] [Google Scholar]
- 34.Sammak PJ, Borisy GG. Direct observation of microtubule dynamics in living cells. Nature. 1988;332:724–6. doi: 10.1038/332724a0. [DOI] [PubMed] [Google Scholar]
- 35.Kostin S, Heling A, Hein S, Scholz D, Klövekorn WP, Schaper J. The protein composition of the normal and diseased cardiac myocyte. Heart Fail Rev. 1998;2:245–60. [Google Scholar]
- 36.Iwai K, Hori M, Kitabatake A, et al. Disruption of microtubules as an early sign of irreversible ischemic injury. Immunohistochemical study of in situ canine hearts. Circ Res. 1990;67:694–706. doi: 10.1161/01.res.67.3.694. [DOI] [PubMed] [Google Scholar]
- 37.Ganote CE, Vander Heide RS. Cytoskeletal lesions in anoxic myocardial injury. A conventional and high-voltage electron-microscopic and immunofluorescence study. Am J Pathol. 1987;129:327–44. [PMC free article] [PubMed] [Google Scholar]
- 38.Ganote C, Armstrong S. Ischaemia and the myocyte cytoskeleton: Review and speculation. Cardiovasc Res. 1993;27:1387–403. doi: 10.1093/cvr/27.8.1387. [DOI] [PubMed] [Google Scholar]
- 39.Rothen-Rutishauser BM, Ehler E, Perriard E, Messerli JM, Perriard JC. Different behaviour of the non-sarcomeric cytoskeleton in neonatal and adult rat cardiomyocytes. J Mol Cell Cardiol. 1998;30:19–31. doi: 10.1006/jmcc.1997.0596. [DOI] [PubMed] [Google Scholar]
- 40.Maeno Y, Kostin S, Hein S. Multifactorial origin of the reduction of myofilaments causing contractile dysfunction in failing hearts. Circulation. 1997;96(Suppl I):3865. (Abst) [Google Scholar]
- 41.Westfall MV, Solaro RJ. Alterations in myofibrillar function and protein profiles after complete global ischemia in rat hearts. Circ Res. 1992;70:302–13. doi: 10.1161/01.res.70.2.302. [DOI] [PubMed] [Google Scholar]
- 42.Zeevi-Levin N, Barac YD, Reisner Y, et al. Gap junctional remodeling by hypoxia in cultured neonatal rat ventricular myocytes. Cardiovasc Res. 2005;66:64–73. doi: 10.1016/j.cardiores.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Naito H, Melnychenko I, Didie M, et al. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation. 2006;114(Suppl 1):I72–8. doi: 10.1161/CIRCULATIONAHA.105.001560. [DOI] [PubMed] [Google Scholar]