Abstract
Myocardial depression in human sepsis was only unequivocally proven in the 1980s by the group of Parrillo, who used nuclear imaging techniques to measure heart volumes and function in intensive care patients. Heart failure in sepsis is frequently masked by a seemingly normal cardiac output. However, relative to the lowered systemic vascular resistance – resulting in a reduced afterload – cardiac outputs and ventricular ejection fractions are often not adequately enhanced. This septic cardiomyopathy (impairment of the heart within the scope of systemic sepsis) involves both the right and the left ventricles, and is potentially reversible. In response to volume substitution, the heart can be considerably enlarged. The cardiomyopathy is not primarily hypoxic in nature, but may be aggravated by ischemia. Autonomic dysfunction, documented by a reduced heart rate variability and impaired baroreflex and chemoreflex sensitivities, forms part of the disease entity. The severity of myocardial depression correlates with a poor prognosis. Noninfectious systemic inflammatory response syndrome can give rise to an analogous disease entity, namely, systemic inflammatory response syndrome cardiomyopathy.
The etiology of septic cardiomyopathy is multifactorial. Several candidates with a potential pathogenetic impact on the heart were identified: bacterial toxins; cytokines and mediators including tumour necrosis factor-alpha, interleukin-1 and nitric oxide; cardiodepressant factors; oxygen reactive species; and catecholamines. Symptomatic treatment consists of volume substitution and catecholamine support; causal therapeutic approaches aiming at an interruption of the proinflammatory mediator cascades are being tested.
Keywords: Autonomic dysfunction, Cardiomyopathy (septic), Heart failure (septic), Sepsis, Shock (septic)
Acute septic myocarditis in the preantibiotic era (1) was a purulent disease of the heart. Currently, the myocardium of patients whose heart failed in septic shock is merely characterized by nonspecific pathomorphological and pathohistological alterations (2). For decades, septic myocardial depression has been attributed to the release of cardiodepressant factors into the bloodstream, and the existence of human septic myocardial depression was only unequivocally proven in the early 1980s by Parrillo’s group (3), who used nuclear imaging techniques to examine patients in an intensive care unit. In recent years, the concept of septic cardiomyopathy was proposed (4,5), which emphasizes alterations of cardiac cellular phenotype as a basis of organopathy in response to a variety of agents acting on heart cells, such as bacterial toxins and endogenous cytokines, hormones, mediators and cardiodepressant factors.
The intention of the present review is to highlight newer aspects of cardiac involvement in systemic inflammation and especially in sepsis. Organ-related infectious heart diseases, such as viral myocarditis or bacterial endocarditis, are not the focus of the present paper, which instead focuses on the uniform reaction pattern of the heart (4–7) to generalized inflammatory processes such as sepsis and other systemic inflammatory response syndromes (SIRSs) (8,9).
Heart diseases of infectious systemic inflammatory origin are increasingly being paid attention to beyond the field of intensive care medicine; the pathogenetic mechanisms involved in this cardiomyopathy may also apply to common chronic heart disorders such as coronary artery disease and heart failure (4), which occur along with a moderate inflammatory response.
SEPTIC CARDIOMYOPATHY – A SECONDARY CARDIOMYOPATHY IN THE SCOPE OF A SYSTEMIC DISEASE
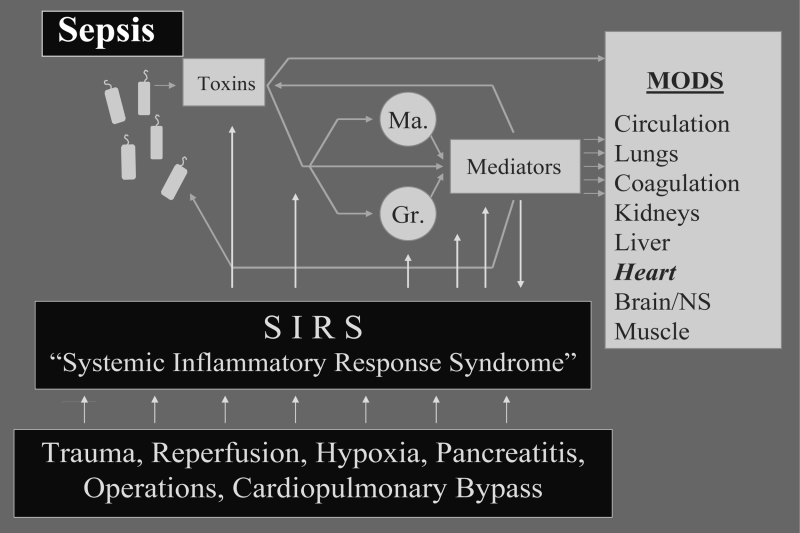
The present concept of the pathogenesis of bacterial sepsis is centred around the idea that numerous and very heterogeneous stimuli – including all classes of microorganisms or even isolated bacterial toxins such as endotoxin or superantigens – uniformly evoke the activation of mediator cells with a secondary release of cytokines and proinflammatory mediators (Figure 1). These primarily protective mechanisms are directed against invading microbes with the aim to inactivate toxins. However, the inflammatory response may take an exaggerated course and thereby be complicated by impairment of the patients’ organs. An overwhelming activation of mediator cascades has the clinical presentation of sepsis. In addition to the detrimental action of endogenous mediators, such as tumour necrosis factor-alpha (TNF-α) (10), a direct toxicity of the bacterial toxins –such as endotoxin (4,5,7,10), Pseudomonas exotoxin A (11) and lipoteichoic acid (12) – may potentially further aggravate malfunctioning of organs, including the heart.
Figure 1).
Pathophysiology of sepsis and escalating systemic inflammatory response syndrome (SIRS). For further explanation, see text. Gr Granulocytes; Ma Macrophages; MODS Multiple organ dysfunction syndrome; NS Nervous system
In recent years, it became evident that not only infectious but also noninfectious stimuli can trigger these defense mechanisms, and thus give rise to a clinical picture that may be indiscriminable from bacterial sepsis (Figure 1); examples of such noninfectious aggressions are major trauma, surgery with the assistance of cardiopulmonary bypass, pancreatitis, ischemia-reperfusion injuries or allograft rejection. Whatever the initial trigger may be – infectious or noninfectious – the sequence of events that follows adheres to a uniform pattern (13): there is a massive production and release of mediators interwoven in a complex network resulting in mediator-induced SIRS and multiple organ dysfunction syndrome (MODS), which includes the heart (4,5,7,14–21).
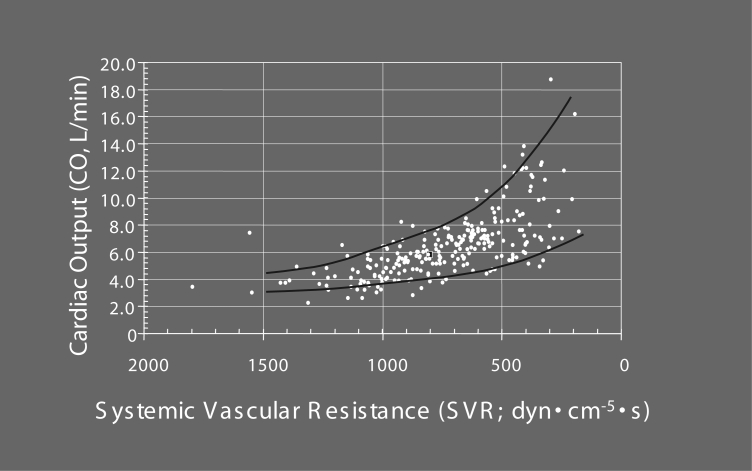
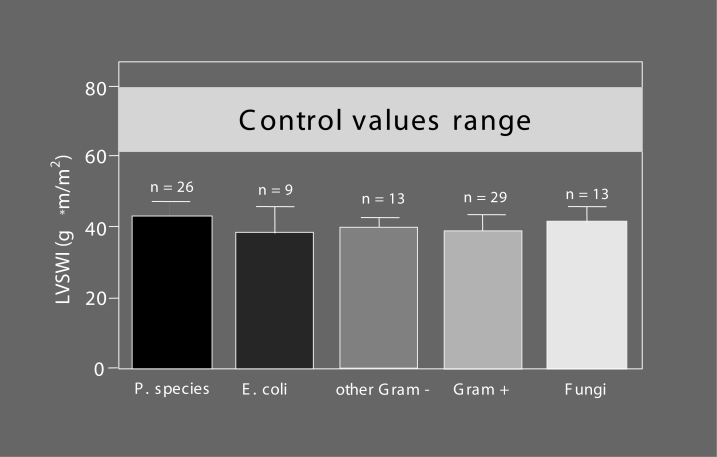
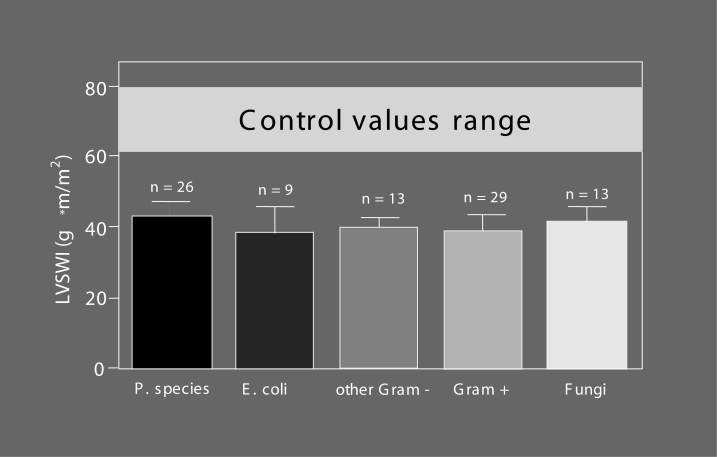
It has long been denied that cardiac involvement forms a part of septic MODS, because cardiac output values of septic patients usually are seemingly normal or may even be enhanced compared with the physiological range (Figure 2). However, heart failure becomes evident when cardiac output is considered in relation to systemic vascular resistance, which is severely lowered due to sepsis-induced vasodilation (Figure 3). A healthy heart may be able to compensate for the pathological fall in afterload (down to one-third or one-quarter of the normal value) by up to a three- or fourfold increase in cardiac output (Figure 3), while – very often – the observed values in septic patients are considerably lower (Figure 3); the compensatory increase in pump activity is not high enough to stabilize blood pressure. This is a consequence of septic cardiomyopathy (Table 1), a disease entity characterized by dominant left ventricular failure, which is not primarily hypoxic in nature because the coronary arteries are vasoplegic and dilated (22,23), and coronary blood flow is high (24,25). However, septic cardiomyopathy can be aggravated by myocardial ischemia (4,21,26), particularly in patients with pre-existing coronary artery disease, because the increased coronary blood flow in sepsis narrows the coronary reserve. Left ventricular stroke work indexes were found to be reduced to a similar degree (Figure 4) (7,27) in patients with various forms of Gram-negative, Gram-positive or fungal sepsis, indicating that it is not so much the bacterial virulence factors but rather the common mediator network that determines the occurrence and severity of the disease. Additional right ventricular dysfunction essentially belongs to septic myocardial depression and can be accentuated in the presence of pulmonary hypertension due to adult respiratory distress syndrome (ARDS) (28); right ventricular dilation and a reduced right ventricular ejection fraction can further impair left ventricular performance by a fall in left ventricular filling pressure and a mechanical compromise of the left ventricle by a septal shift. Although septic cardiomyopathy is potentially completely reversible –described as myocardial hibernation (29) – it still is a condition of high prognostic importance: it accounts for approximately 10% of the fatalities observed in sepsis and septic shock (3), with nonsurvivors having more depressed afterload-related myocardial depression than that of survivors (see next section “Septic cardiomyopathy – Not a rare organ dysfunction”).
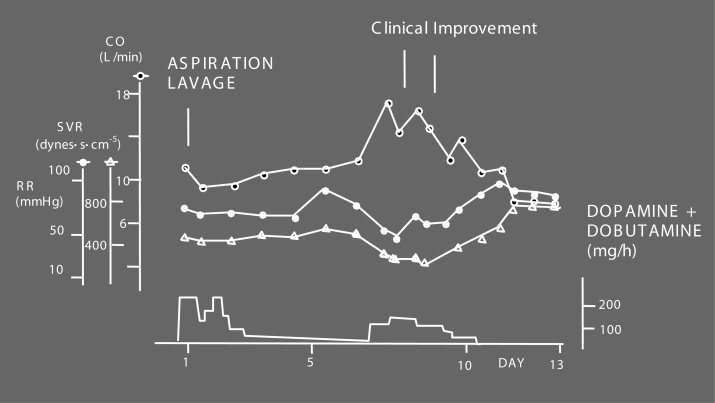
Figure 2).
Case report: cardiovascular changes in Pseudomonas sepsis. The patient suffered from an aspiration pneumonia on day 1. After initial stabilization, cardiovascular deterioration occurred, resulting in septic shock around day 7. Thereafter, the patient uneventfully recovered. This patient did not suffer from septic cardiomyopathy; notice the high cardiac output (CO) values of this patient even in the shock state. SVR Systemic vascular resistance. Reproduced from reference 4
Figure 3).
Correlation between cardiac output (CO) and systemic vascular resistance (SVR) in 31 patients with septic multiple organ dysfunction syndrome. In patients with septic multiple organ dysfunction syndrome, CO has been repeatedly measured during the course of the disease and plotted against the respective SVR. With decreasing after-load (fall in SVR), CO values increase, with considerable variation for each specific SVR value. The upper line of the graph represents the maximal achievable CO values for the respective SVR, while the values below indicate reduced CO values of different degrees
TABLE 1.
Features of acute septic cardiomyopathy
Pump failure
|
| Right ventricular compromise and dilation due to adult respiratory distress syndrome |
Superimposed hypoxic heart damage
|
The heart as a cytokine producer
|
Arrhythmias
|
Autonomic dysfunction
|
Figure 4).
Myocardial depression in patients with Gram-negative, Gram-positive and fungal septic shock. Left ventricular stroke work index (LVSWI) as a clinical marker of inotropy has been calculated in patients with septic shock of different etiologies. For further discussion, see text. E coli Escherichia coli; P species Pseudomonas species; Gram–Gram-negative pathogenic agents; Gram+ Gram-positive pathogenic agents; Fungi Fungal pathogenic agents. Modified from reference 4
In view of all these findings, cardiac impairment in sepsis can be classified as septic cardiomyopathy due to the involvement of the heart within the scope of the systemic disease sepsis. This classification is well accepted by intensivists, while cardiologists seemingly ignore this kind of cardiomyopathy; in a recent classification of cardiomyopathies (30), impairment of the heart in sepsis is not listed.
SEPTIC CARDIOMYOPATHY – NOT A RARE ORGAN DYSFUNCTION
Septic cardiomyopathy occurs more often than presently diagnosed. In a strict sense, 30% to 80% of patients with severe sepsis and septic shock suffer from a non-ST elevation myocardial infarction, with serum troponin T/troponin I values above the normal range. Septic patients with elevated troponin levels need high doses of noradrenaline and have an unfavourable prognosis (21,26). In addition, elevated serum levels of brain natriuretic peptides (BNPs) indicate cardiac impairment (15–17,20,31). Electrocardiographic changes in septic patients can document signs of ischemia, but in most cases they are nonspecific.
Echocardiography (32), on the other hand, very often underestimates the severity of septic cardiomyopathy, due to the dramatic afterload reduction in sepsis. In clinical practice, the diagnosis of septic cardiomyopathy is frequently hampered by the fact that all reference values for cardiac function parameters are normalized to an afterload of 1100 dynes·s·cm−5, but that reference values (eg, for echocardiography) for a systemic vascular resistance of 300 dynes·s·cm−5 have never been established. Despite this, in at least approximately 50% of all sepsis patients, a systolic pump failure can be documented by echocardiography (17).
An interesting new approach to characterize myocardial depression is calculating the cardiac power/cardiac power index (Cpi) as the product of mean arterial pressure (MAP) times cardiac flow as measured by the cardiac output/cardiac index (CI) (Cpi = MAP × CI × 0.0022 [W·m−2]) in relation to the systemic vascular resistance (33). Characteristic of septic shock are Cpi values of 0.5 W·m−2 to 1.0 W·m−2 and systemic vascular resistance index (SVRI) values of less than 1500 dynes·s·cm−3, while the normal range for Cpi is 0.5 W·m−2 to 0.7 W·m−2 and for SVRI it is 1000 dynes·s·cm−3 to 2500 dynes·s·cm−3 (33).
The method of choice for quantitating the severity of septic cardiomyopathy is the measurement of cardiac output/CI by a pulmonary artery catheter or a pulse contour cardiac output device and correlating these values to the systemic vascular resistance/SVRI data. According to Figure 3, an inverse correlation between afterload – represented best by the systemic vascular resistance (although it is a calculated and not measured value) – and cardiac performance can be demonstrated and quantitated (K Werdan, A Herklotz, U Muller-Werdan, unpublished results). If this inverse correlation is not taken into account, then data from echocardiographic or hemodynamic measurements may be misinterpreted.
Does quantification of septic cardiomyopathy really make sense? It does make sense if the severity of septic cardiomyopathy correlates with an unfavourable prognosis of these septic patients. And this is indeed the case: 70% of surviving patients with septic MODS have a normal or nearly normal pump function (cardiac output in relation to the respective SVR [COSVRrel] greater than 80% of normal range) and only 30% have a moderate cardiac impairment (COSVRrel 60% to 80% of expected value). On the other hand, only 25% of the nonsurviving patients had documented normal heart function (COSVRrel greater than 80%), while 50% had moderate (COSVRrel 60% to 80% of expected value) and 25% even severe cardiac impairment (COSVRrel 60% to less than 40% of expected value) (K Werdan, A Herklotz, U Muller-Werdan, unpublished results).
In addition, the elevated serum levels of natriuretic peptides document the cardiac damage in sepsis: BNP and N-terminal-pro-BNP (NT-proBNP) serum levels correlate inversely with left ventricular function (31) and discriminate very early between surviving and nonsurviving patients (15,17). Even in patients with normal echocardiographic findings, BNP levels can be astonishingly high (20).
MYOCARDIAL DEPRESSION IN SEPSIS –TRIGGERS AND MECHANISMS
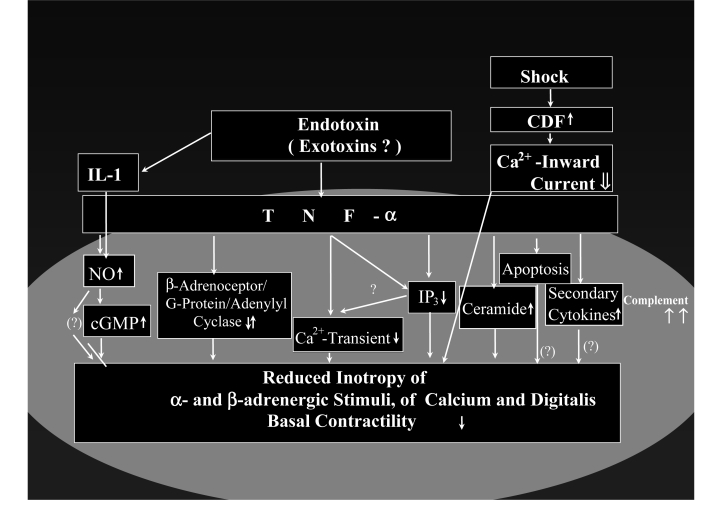
Many substances and mechanisms seem to be involved in myocardial depression in sepsis, including toxins, cytokines and further mediators, not yet identified cardiodepressant factors, complement activation and apoptosis (Figure 5).
Figure 5).
Cardiodepressive factors (CDFs) in sepsis – experimentally proven concepts. Besides unspecific damages to cardiomyocytes (eg, by staphylococcal alpha-toxin [4], Pseudomonas exotoxin A [4,11] and lipoteichoic acid [12]), specific alterations to inotropic signal transduction pathways are induced by toxins and mediators; alterations occur in the positive inotropic pathways, namely, the beta-adrenoceptor-G-protein-adenylyl cyclase cascade, the alpha-adrenoceptor-phosphoinositol cascade and the Ca2+ transient, as well as in the negative inotropic pathways, namely, the nitric oxide (NO)-guanylyl cyclase cascade – a counterpart to the beta-adrenoceptor-G-protein-adenylyl cyclase cascade – and the sphingomyelin-ceramide cascade. As a consequence, there is a complex alteration of basal contractility as well as of stimulated contractility (by beta- and alpha-adrenoceptors, by digitalis and by Ca2+) in cardiomyocytes. Further deterioration of contractile function of the cardiomyocyte comes from the impairment of energy metabolism with disturbed oxygen utilization at the cellular level (cytopathic hypoxia [67]), the induction of inflammatory signal transduction pathways (nuclear factor kappa B and cytokines) in cardiomyocytes, the damage triggered by reactive oxygen species and peroxynitrite, as well as by the induction of apoptosis. For further information, see references 4 and 6, and for information on specific components, see the following references: CDFs (34,35,37); endotoxin (10); energy metabolism (44,56,65,66,108,109); complement (110); tumour necrosis factor-alpha (TNF-α) and interleukin (IL)-1-beta (10,38,40,41,48–53); NO (39,54,59,63,68–70,74,103,104); ceramide (42); phosphoinositol metabolism (43); apoptosis (45–47); reactive oxygen species and peroxynitrite (55–57); nuclear factor kappa B (58); NO-guanylyl cyclase pathway (64); and beta-adrenoceptor-G-protein-adenylyl cyclase pathway (70,72,74,111). cGMP Cyclic GMP; Complement Representative term referring to the activation of the complement pathway in cardiomyocytes; IP3 Inositol triphosphate
Pleiotropy and redundancy: Endotoxin, TNF-α and interleukin-1 as triggers
In the majority of cases, numerous bacterial toxins and primary, secondary and final mediators are involved in the pathogenesis of systemic inflammation. However, the sole administration of endotoxin or TNF-α is sufficient to induce the hemodynamic alterations of sepsis, including myocardial depression. Several cardiodepressant factors – some whose chemical composition is not fully unravelled (4,5,34–37) – have been described, with the combination of TNF-α and interleukin-1-beta (IL-1β) being extremely cardiodepressive (38).
TNF-α is regarded as the pertinent cardiodepressant cytokine in sepsis, with its negative inotropic impact frequently being ascribed to the induction of inducible nitric oxide (NO) synthase (iNOS) and an enhanced production of NO in the heart (4). Although iNOS induction was found in some myocardial specimens from patients with heart failure or sepsis, this was not a uniform finding (39). Results from in vitro studies suggest a complex interaction of TNF-α with the heart, with pleiotropic effects on cardiomyocyte performance (Figures 6 and 7) due to an interference with several inotropic pathways, as well as TNF-α-like cardiodepressive effects of other cytokines such as IL-1. The experimentally supported concepts of TNF-α cardiodepression include the induction of iNOS and the inhibition of constitutive NOS (cNOS) or endothelial NOS (eNOS) at high TNF-α concentrations (Figure 6), but also NO-independent cardiodepression at low, pathophysiologically more relevant concentrations (Figures 6 and 7) (40). The effects of TNF-α on the heart also vary with regard to the kinetics of the process (41): rapidly occuring cardiodepressant effects include the release of sphingosine (42) and the suppression of the calcium transient, while chronic administration of TNF-α was shown to depress the synthesis of precursors for the phosphoinositide pathway (43) and inhibit pyruvate dehydrogenase activity and mitochondrial function (Figure 8) (44). Cardiomyocytes are not only effector cells for cytokines, but on stimulation, they produce cytokines, which may be secreted (eg, IL-6) (10) or retained intracellularly (eg, IL-1) (10); it seems likely that secondary cytokines induced in cardiomyocytes by TNF-α or endotoxin (10,45) – via a CD14-dependent pathway (45) – contribute to cardiodepression. Whether apoptotic signals activated by TNF-α (46,47) are involved in the negative inotropic pathways is presently unknown.
Figure 6).
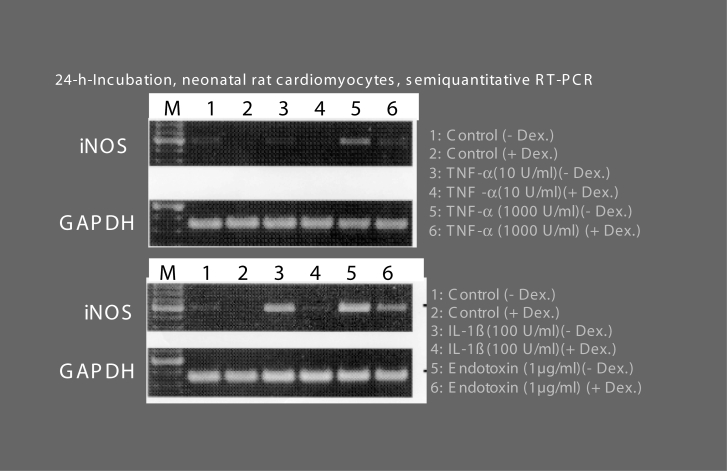
Induction of inducible nitric oxide synthase (iNOS) in beating neonatal rat cardiomyocytes in culture by endotoxin, tumour necrosis factor-alpha (TNF-α) and interleukin-1-beta (IL-1β) in cardiodepressive concentrations. Spontaneously beating neonatal rat cardiomyocytes in culture were incubated for 24 h with either TNF-α, IL-1β or endotoxin in cardiodepressive (see Figure 7) concentrations as indicated, in the absence or presence of dexamethasone (Dex) to suppress iNOS induction. Thereafter, semiquantitative reverse transcription polymerase chain reaction (RT-PCR) for iNOS was carried out to document the induction of messenger RNA, with the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) used for reference. For further information, see text and references 10 and 40. M Marker
Figure 7).
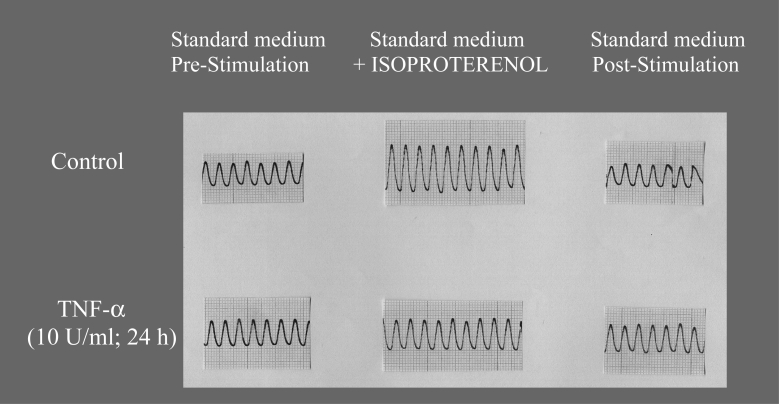
Preincubation of beating neonatal rat cardiomyocytes in culture with tumour necrosis factor-alpha (TNF-α) blocks beta-adrenoceptor-mediated increases in pulsation amplitude. Spontaneously beating neonatal rat cardiomyocytes were incubated for 24 h with 10 U/mL TNF-α (see Figure 6). Thereafter, cells were electrically driven and superfused with the beta-adrenergic agonist isoproterenol. In control cells, isoproterenol induced a reversible increase in pulsation amplitude, indicative of a positive inotropic effect of this catecholamine. In TNF-α-pretreated cells, however, no increase in pulsation amplitude could be seen, showing a block of the beta-adrenoceptor-mediated increase in pulsation amplitude by TNF-α. For further information, see text and references 10 and 40
Figure 8).
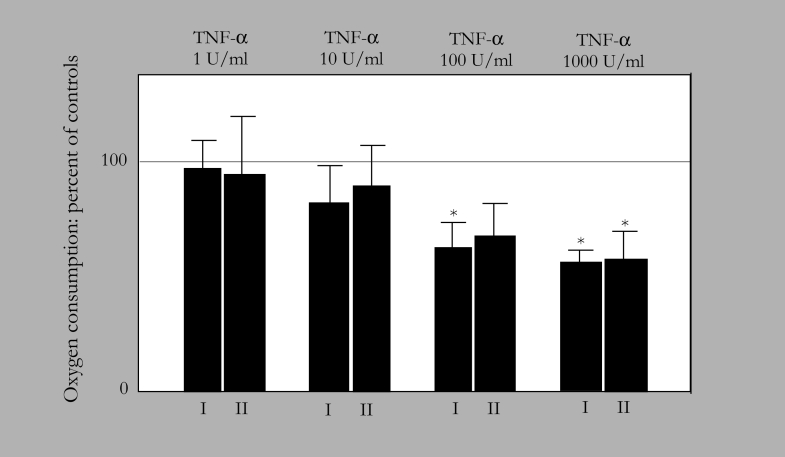
Inhibition of mitochondrial oxygen consumption in beating neonatal rat cardiomyocytes by tumour necrosis factor-alpha (TNF-α). Spontaneously beating neonatal rat cardiomyocytes in culture were incubated for 24 h with the indicated concentrations of TNF-α. Thereafter, mitochondria were isolated from the cells and oxygen consumption of complexes I and II were measured. A concentration-dependent inhibition of complexes I and II activity by TNF-α can be seen. For a further explanation, see text and reference 44. Modified from reference 44
Conclusive evidence for a pathogenetic impact of chronic TNF-α elevation on the heart comes from in vivo studies (48) and transgenic mice (49–51). However, TNF-α and IL-1 may not only have detrimental effects on the heart, but in myocytes they may also confer resistance to hypoxic injury (52) or alpha-adrenoceptor-induced arrhythmias (4,43).
NO and reactive oxygen species cause not only impairment of inotropic cascades, but also of mitochondrial function and cellular oxygen utilization
In the hierarchy of the proinflammatory mediator cascades, TNF-α and IL-1 (53) belong to the primary players, while NO (54) and oxygen free radicals (55) are well-known final effectors in the setting of SIRS cardiodepression. Endogenous peroxynitrite formation from NO and superoxide may be the most toxic compound – both with respect to contractility and respiration (see below) – in the concert of free radicals in systemic inflammation (56,57). The detection of an abundant expression of cyclooxygenase-2 in the myocardium in sepsis (58) insinuates a possible importance of prostanoids as final mediators of septic heart failure.
High levels of nitrite and nitrate, the stable end products of NO metabolism, are found in patients with severe sepsis, and relate inversely to SVRIs and global oxygen extraction rates, but directly to blood lactate levels (59). It is well established that the reduced afterload in sepsis is determined by NO; inhibitors of NOS unmask a tonic pressor response of the counter-regulatory endothelin-1 (60), the blood levels of which are elevated in human sepsis (59,60). However, the role of NO in septic myocardial depression is less lucid. Striking similarities between the cardiac modifications in liver cirrhosis, which are related to NO in an animal model (61), and sepsis have been emphasized.
A pertinent intracellular target for NO with respect to contractile properties is guanylyl cyclase. While a low increase in cyclic GMP by low doses of NO was reported to improve the contractile response of myocytes (62), its activation by excessive amounts of NO results in negative inotropy, mainly due to an interference with compounds of the beta-adrenoceptor pathway (63). Additional cyclic GMP-related cardiac NO effects include the regulation of coronary flow (23), which is, however, maintained in eNOS-deficient mice (22), suggesting important compensatory mechanisms and enhanced cardiac adenosine production (64). However, cellular targets of NO other than guanylyl cyclase may be pathogenetically relevant; particularly, an interaction of NO with energy metabolism was found to be related to contractile dysfunction (65). Consistently, in the hearts of baboons with experimentally induced Escherichia coli sepsis, diminished activities of complexes I and II of the mitochondrial respiratory chain were found (Table 2) (66), which may be due to detrimental effects of sepsis mediators such as NO (65), TNF-α (Figure 8), IL-1 (44) and others. Interestingly, in patients with sepsis, skeletal partial oxygen pressure is not lowered but elevated in the very severe state of sepsis (67), being well in agreement with a toxin-and mediator-induced impairment of mitochondrial function leading to a disturbance of cellular oxygen utilization.
TABLE 2.
Septic cardiomyopathy in Escherichia coli sepsis in baboons – impairment of myocardial respiratory chain and glycolytic enzyme activity
|
E coli sepsis
|
|||
|---|---|---|---|
| Respiratory chain/enzymes | Controls (n=3) | Survivors (n=26) | Nonsurvivors (n=20) |
| Complex I/III | 5.1±4.1 | 3.4±2.9 | 0.6±0.6* |
| Complex II/III | 9.7±2.9 | 4.4±4.6 | 1.3±2.4* |
| Complex III | 153±57 | 140±114 | 90±56 |
| Cytochrome c oxidase | 77±11 | 61±42 | 39±29* |
| Citrate synthase | 424±81 | 584±451 | 507±307 |
| Succinate dehydrogenase | 25±6.4 | 22±16 | 15±13 |
| Phosphofructokinase | 288±168 | 312±175 | 47±32* |
| Glucose phosphate isomerase | 1768±214 | 1628±494 | 1082±480 |
| Lactate dehydrogenase | 1165±501 | 960±312 | 709±28 |
| Noncollagen protein | 164±26 | 159±60 | 138±43 |
Data are expressed as mean units per gram of noncollagen protein.
P<0.05. Data from reference 66
Several studies have questioned a possible role of myocyte NOS in nonseptic human heart failure (68,69), while another investigation confirmed the expression of cNOS and iNOS in failing human cardiac myocytes, but instead attributed inflammatory myocyte injury to abundant iNOS expression within infiltrating macrophages (70). However, another study questions an essential role for NO in contractile depression or beta-adrenoceptor desensitization in myocytes from failing human ventricle, because pharmacological inhibition of NOS did not increase contraction or beta-adrenoceptor sensitivity (71). During severe sepsis in humans, both beta-adrenergic receptor-dependent and -independent stimulation of adenylyl cyclase were shown to be impaired, suggesting heterologous desensitization (72). Evidence is available for an interplay of NO with sympathetic and parasympathetic signalling in the heart (73), but data from eNOS-deficient mice refute the idea that eNOS is obligatory for the normal autonomic control of cardiac muscle function (74). In summary, a very complex picture of the contribution of NO and NOS to septic and non-septic heart failure is evolving, and some more pieces of the puzzle are expected to emerge in the future.
THE COMPLEX CLINICAL SPECTRUM OF SEPTIC CARDIOMYOPATHY: MYOCARDIAL DEPRESSION IS NOT ALL
Pump failure
Myocardial depression is the most prominent feature of septic cardiomyopathy, resulting in right and left ventricular pump failure (Table 1). In relation to the dramatic afterload reduction (as indicated by the lowered systemic vascular resistance), cardiac output/CI, right and left ventricular ejection fractions and stroke volumes are not adequately raised or even depressed; both global and regional contractile disturbances can be found in septic cardiomyopathy, and not only systolic but also diastolic pump failure occurs. Due to an increase in left ventricular compliance with a shift of the pressure-volume curve to the left, considerable dilation of the left and right heart can be seen – a prognostically positive sign (3) (RR Flieger, medical thesis, Martin-Luther-University Halle-Wittenberg, Germany, 2005, unpublished). This pump failure is not primarily of hypoxic nature because coronary arteries are vasoplegic (22,23) and coronary blood flow, in relation to MAP, is higher than in nonseptic patients (24,25). However, one must assume that at the microcirculatory coronary level, similar disturbances may occur, as generally seen in patients with sepsis (75). Fortunately, myocardial depression is potentially reversible, in agreement with a described myocardial hibernation in sepsis (29).
The acute pump failure can be aggravated by additional right ventricular dysfunction due to ARDS-induced pulmonary hypertension (28). Especially in patients with pre-existing coronary artery disease, septic cardiomyopathy can be superimposed by a further ischemic pump failure due to severe coronary artery stenoses, with the ischemic region getting even less blood flow due to the steal phenomena, because of the dilated nonstenosed coronary arteries.
The heart as a cytokine producer
Proinflammatory cytokines not only depress contractility of the heart, they also get synthesized in the heart during the proinflammatory state, and thereby intensify myocardial depression and cardiac impairment (41,76). In addition, stimulation of myocardial beta-adrenoceptors and probably also of alpha-adrenoceptors by (excessive) catecholamines induces IL-6 production in the heart (76,77). Treatment with catecholamines can also add to cytokine production in the heart, which can be blocked by the beta-/alpha-blocker carvedilol (76,77). Consequently, all attempts to reduce catecholamine doses in sepsis treatment – for example, by the application of hydrocortisone (78) – are welcomed in their ability to reduce the proinflammatory burden of the heart.
Arrhythmias
In contrast to the well-documented myocardial depression in sepsis, only a few experimental and clinical studies on the occurrence of arrhythmias in sepsis have been described. A case of the coincidence of long QT syndrome and torsade de pointes with septic cardiomyopathy has been reported (79). In a retrospective analysis of score-evaluated patients, no increased incidence of ventricular and supraventricular arrhythmias was documented in 25 septic MODS patients compared with 15 nonseptic MODS patients (80). No higher rate of arrhythmias has been found in patients with septic MODS compared with those with nonseptic MODS, and MODS by itself increases the risk for atrial fibrillation (S Schaefer, medical thesis, Martin-Luther-University Halle-Wittenberg, 2006, unpublished). At present, no specific arrhythmogenic sepsis profile has been identified.
On the other hand, in postoperative intensive care unit patients, sepsis and SIRS have been identified as main triggers of tachyarrhythmias, with an adjusted odds ratio of 36.45 (81).
Autonomic dysfunction
A narrowed heart rate variability is an important feature of critical illness (Figure 9) (Table 3) and was found to be a marker of poor prognosis – associated with a severalfold increased mortality (Figure 10) (82,83). A reduction in heart rate variability is a measure of autonomic dysfunction, reflecting a loss of the balance between sympathetic and vagal tone (84,85). Sepsis and MODS in particular are coupled with an impairment of autonomic dysfunction, a finding that culminated in the hypothesis that a loss of the neurohumoral organ interaction (‘uncoupling of biological oscillators’) is a major pathogenetic factor in MODS (86–88). Interestingly, the administration of endotoxin to healthy humans reversibly induces narrowed heart rate variability (89).
Figure 9).
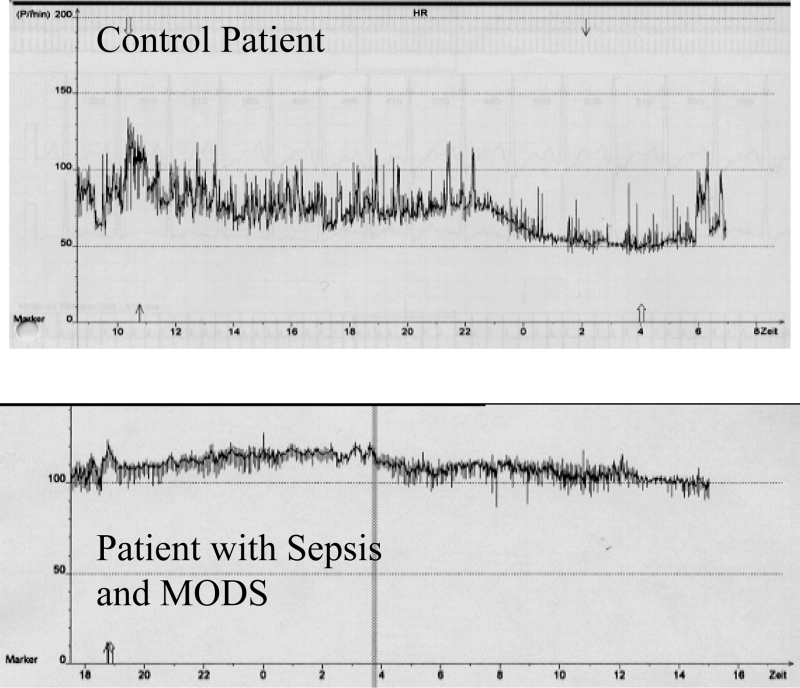
Twenty-four hour heart rate monitoring in a patient with, as well as in a patient without, sepsis and multiple organ dysfunction syndrome (MODS). A higher heart rate (ordinate) is clearly evident, as well as heart rate rigidity in the registration of the patient with sepsis and MODS. From these data, heart rate variability (HRV) parameters can be calculated (see Table 3), demonstrating a strong reduction of HRV (HRV rigidity) in a patient with sepsis and MODS. For a further explanation, see text and reference 85. Modified from reference 85
TABLE 3.
Impairment of heart rate variability (HRV) in patients with multiple organ dysfunction syndrome (MODS)
| Parameter | Normal | MODS patients | P |
|---|---|---|---|
| SDNN (ms) | 141±39 | 57.7±30.7 | <0.0001 |
| pNN50 (%) | 9±7 | 4.8±8.4 | <0.0001 |
| LF (ms2) | 791±563 | 129.3±405.1 | <0.0001 |
| HF (ms2) | 229±282 | 112.3±267.3 | <0.0001 |
| VLF (ms2) | 1782±965 | 191.3±661.1 | <0.0001 |
| LF/HF | 4.61±2.33 | 1.1±0.9 | <0.0001 |
MODS patients (n=85) show a strongly reduced HRV (HRV rigidity), as shown by the lower values of all HRV parameters. Normal parameter values were obtained from reference 83. The standard deviation of all normal-to-normal RR intervals (SDNN) represents global HRV activity. The percentage of differences of successive RR intervals differing by more than 50 ms (pNN50), the high frequency range (HF) and the very low frequency range (VLF) represent vagal activity. The low frequency range (LF) represents sympathetic as well as vagal activty. LF/HF represents the ratio of sympathetic to vagal activity. For a further explanation, see text and reference 83
Figure 10).
Predictive value of heart rate variability (HRV) in patients with multiple organ dysfunction syndrome. The Kaplan-Meier curve for 28-day survival uses the optimal cutoff point of the HRV parameter lnVLF (defined as the natural logarithm of HRV in the very low frequency range) (see Table 3) (3.9 lnms2) for the entire cohort of patients with multiple organ dysfunction syndrome (n=85). The dashed line indicates values above, and the solid line indicates values below, the cutoff point. The hazard ratio for 28-day mortality was 2.9 (95% CI 1.3 to 6.6). For a further explanation, see text and reference 83. Modified from reference 83
Not only is the reduction in heart rate variability long-lasting (90) in patients with MODS, but baroreflex sensitivity and chemoreflex sensitivity are impaired, all dysfunctions correlating with an unfavourable prognosis (83). However, the correlation between the severity of autonomic dysfunction and the severity of septic cardiomyopathy remains to be determined.
An interesting qualitative difference of autonomic dysfunction in MODS patients compared with cardiac patients is evident. In cardiac patients, the sympathetic activity dominates over the vagal tone, whereas in MODS patients, both the sympathetic and the vagal tone are attenuated (Table 3) (83). This led us to the hypothesis that in MODS patients, there is not only an impairment of the autonomic nervous system but also an attenuation of the sympathetic and vagal signal in the target cell, and in case of heart rate variability, there is narrowing at the level of the pacemaker cells in the sinus node of the heart (85). This attenuation of neuronal signals may be due either to a toxin- and/or mediator-induced alteration of the intracellular signal transduction pathways or to the pacemaker ion channel activity itself. In agreement with this hypothesis, preincubation of spontaneously beating neonatal rat cardiomyocytes in culture with endotoxin results in a narrowing of the beating rate variability of these pacemaker cells (85,91).
In patients with coronary artery disease, the reduced heart rate variability indicates an unfavourable prognosis due to the occurrence of lethal ventricular tachycardias. With an even more depressed heart rate variability, patients with septic MODS seldomly die from ventricular tachycardias. Therefore, this prognostic marker must be combined with – at present –not well understood additional risk factors for these patients. Because autonomic function and inflammation are tightly coupled (73) by the cholinergic anti-inflammatory reflex (92), the reduced vagal activity may lead to a prognostically relevant, overshooting proinflammatory response and thereby trigger a fatal course.
SIRS CARDIOMYOPATHY
While compromise of heart function due to bacterial sepsis has been thoroughly studied, few groups have clearly documented the presence of SIRS cardiomyopathy from noninfectious disease. Severe trauma occurs along with an abnormal left ventricular function (93). Prolonged hemorrhagic shock resulted in contractile impairment in an animal model (94).
Extracorporeal circulation unequivocally induces an inflammatory response. It has now been well documented by several groups that the impact of a cardiopulmonary bypass on cytokine release is very drastic (95,96) and relates to myocardial dysfunction (95,97). An escalating SIRS, as opposed to a nonescalating SIRS, in patients after cardiopulmonary bypass-assisted cardiac surgery is associated with an increased mortality, with exaggerated TNF-α and TNF receptor blood levels, and lowered left ventricular stroke work indexes (98), the latter not being due to different afterloads, but instead due an intrinsic myocardial depression, namely, SIRS cardiomyopathy (4).
CAUSAL THERAPEUTIC APPROACHES TOWARD THE TREATMENT OF ACUTE SEPTIC CARDIOMYOPATHY
Current consensus guidelines for the therapy of sepsis give detailed recommendations for fluid resuscitation and vasopressor and inotropic therapy (78,99). If cardiovascular stabilization can be achieved within the first hours, then the survival rate is relatively high (100,101). Dobutamine (an inotrope) and noradrenaline (a vasopressor) should be used if necessary, whereas dopamine use in patients with shock may be associated with increased mortality (102). Phosphodiesterase inhibitors, vasopressin and the calcium sensitizer levosimendan are not (yet) part of the standard treatment of septic shock. NO inhalation therapy – sometimes used for ARDS treatment – does not seem to have depressant effects on heart function (103,104).
Causal therapeutic strategies – aiming at an interruption of mediator cascades – to alleviate septic cardiomyopathy and vasculopathy are being tested, but do not form part of the standard regimens. Table 4 summarizes data that convey the notion that by antagonizing and eliminating pertinent proinflammatory mediators, septic vasculopathy is more treatable than septic cardiomyopathy.
TABLE 4.
Causal treatment approaches in septic circulatory shock and septic cardiomyopathy
| Treatment | CI | SVR | LVSWI |
|---|---|---|---|
| Endotoxin antibody (HA-1A) | Ø | Ø | Ø/↓ |
| TNF-α antibody/soluble receptors | Ø | Ø/↑ | Ø/↑ |
| Hemofiltration | Ø | ↑ | Ø |
| Plasma separation | Ø | Ø | Ø |
| Hydrocortisone | Ø | ↑ | Ø |
| NO synthase inhibitors | Ø | ↑ | Ø/↓/↑ |
| Methylene blue | Ø/↓ | ↑ | ↑ |
| Pentoxifylline | Ø | Ø | Ø |
| Hemoperfusion/endotoxin absorption | ↓ | ↑ | Ø |
| Activated protein C | Troponin ⇓ | NT-proBNP ⇓ | |
For a further explanation, see text and references 4 to 6. For information on specific treatments, see the following references: hemofiltration (112,113); hemoperfusion/endotoxin absorption (114,115); hydrocortisone (116); nitric oxide (NO) synthase inhibitors (60,71); and activated protein C (see text) (15).
⇓ No information is available on the effect of activated protein C on cardiac index (CI), systemic vascular resistance (SVR) and left ventricular stroke work index (LVSWI), but only on troponin and N-terminal-pro-brain natriuretic peptide (NT-proBNP), biomarkers representing ischemic damage and myocardial depression, respectively. Both biomarkers are lower in patients treated with activated protein C than in those patients not treated with activated protein C. Therefore, these findings indicate a beneficial effect of activated protein C on septic cardiomyopathy; ↓ Decrease; ↑ Increase; Ø No influence of the procedure on the measure indicated; TNF-α Tumour necrosis factor-alpha
The modulation of cardiac inflammatory reactions by phosphodiesterase inhibitors (105) or adenosine (106) has been reported. The options are limited to improve the reduced cardiac contractility, as well as the strongly impaired regulation of heart function. However, it is interesting to note that the beta-1-selective blocker esmolol improves septic myocardial dysfunction in rats, with an increase in cardiac output and cardiac efficiency as well as better myocardial oxygen utilization, in parallel with a reduction in the systemic proinflammatory state, as can be seen by the lower serum TNF-α levels (107).
Findings are encouraging in patients with severe sepsis as well as with septic shock when treated with activated protein C (drotrecogin alpha [activated], Xigris [Eli Lilly, USA]), a substance that has been shown to reduce mortality: patients treated with activated protein C have lower troponin and NT-proBNP serum levels than those not treated (15). This finding underscores a possible role for microcirculatory disturbances in septic cardiomyopathy.
SUMMARY
The original concept that septic myocardial depression involves a negative inotropic blood factor has undergone an evolution in recent years. Heart failure in severe sepsis and SIRS is currently regarded – although not yet classified (30) – as a symptom of a secondary cardiomyopathy, which is characterized by an altered cellular phenotype due to the impact of multiple mediators and toxins. Considerable progress has been made in characterizing the clinical spectrum, including autonomic dysfunction, and elucidating the pathogenetic mechanisms underlying septic and SIRS cardiomyopathy; however, trials to establish causal therapies for the disease have so far not yielded convincing results and necessitate continuing efforts in both basic and clinical research. A better understanding and treatment of septic cardiomyopathy will be of benefit beyond the scope of sepsis, because pathogenetic mechanisms underlying septic heart failure may also be operative in nonseptic heart failure, which occurs along with a measurable systemic inflammatory response.
An organ dysfunction not diagnosed will not be effectively treated. It is worthwhile to ‘discover’ septic cardiomyopathy.
REFERENCES
- 1.Romberg E. Die septische akute Myokarditis. In: Romberg E, editor. Lehrbuch der Krankheiten des Herzens und der Blutgefässe. Stuttgart: Enke-Verlag; 1921. p. 494. [Google Scholar]
- 2.Müller-Hoecker J, Haerty W. Pathomorphological aspects of the heart in septic patients. In: Schlag G, Redl H, editors. Pathophysiology of Shock, Sepsis, and Organ Failure. Berlin: Springer-Verlag; 1993. pp. 853–8. [Google Scholar]
- 3.Parrillo JE. The cardiovascular pathophysiology of sepsis. Annu Rev Med. 1989;40:469–85. doi: 10.1146/annurev.me.40.020189.002345. [DOI] [PubMed] [Google Scholar]
- 4.Mueller-Werdan U, Reithmann C, Werdan K. Cytokines and the Heart: Molecular Mechanisms of Septic Cardiomyopathy. Berlin: Springer-Verlag; 1996. [Google Scholar]
- 5.Müller-Werdan U, Werdan K. Septic cardiomyopathy. Curr Opin Crit Care. 1999;5:415–21. [Google Scholar]
- 6.Taiberg L, Wong J, Kumar A. Myocardial depression in sepsis and septic shock. Advances in Sepsis. 2005;4:82–94. [Google Scholar]
- 7.Briassoulis G, Narlioglou M, Zavras N, Hatzis T. Myocardial injury in meningococcus-induced purpura fulminans in children. Intensive Care Med. 2001;27:1073–82. doi: 10.1007/s001340100957. [DOI] [PubMed] [Google Scholar]
- 8.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–8. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]; Crit Care Med. 2003;31:1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 9.Alberti C, Brun-Buisson C, Chevret S, et al. European Sepsis Study Group. Systemic inflammatory response and progression to severe sepsis in critically ill infected patients. Am J Respir Crit Care Med. 2005;171:461–8. doi: 10.1164/rccm.200403-324OC. [DOI] [PubMed] [Google Scholar]
- 10.Müller-Werdan U, Schumann H, Loppnow H, et al. Endotoxin and tumor necrosis factor alpha exert a similar proinflammatory effect in neonatal rat cardiomyocytes, but have different cardiodepressant profiles. J Mol Cell Cardiol. 1998;30:1027–36. doi: 10.1006/jmcc.1998.0667. [DOI] [PubMed] [Google Scholar]
- 11.Müller-Werdan U, Pfeifer A, Hübner G, et al. Partial inhibition of protein synthesis by Pseudomonas exotoxin A deranges catecholamine sensitivity of cultured rat heart myocytes. J Mol Cell Cardiol. 1997;29:799–811. doi: 10.1006/jmcc.1996.0324. [DOI] [PubMed] [Google Scholar]
- 12.Grandel U, Hopf M, Buerke M, et al. Mechanisms of cardiac depression caused by lipoteichoic acids from Staphylococcus aureus in isolated rat hearts. Circulation. 2005;112:691–8. doi: 10.1161/CIRCULATIONAHA.104.503938. [DOI] [PubMed] [Google Scholar]
- 13.Singer M, De Santis V, Vitale D, Jeffcoate W. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet. 2004;364:545–8. doi: 10.1016/S0140-6736(04)16815-3. [DOI] [PubMed] [Google Scholar]
- 14.Brander L, Weinberger D, Henzen C. Heart and brain: A case of focal myocytolysis in severe pneumococcal meningoencephalitis with review of the contemporary literature. Anaesth Intensive Care. 2003;31:202–7. doi: 10.1177/0310057X0303100212. [DOI] [PubMed] [Google Scholar]
- 15.Brueckmann M, Huhle G, Lang S, et al. Prognostic value of plasma N-terminal pro-brain natriuretic peptide in patients with severe sepsis. Circulation. 2005;112:527–34. doi: 10.1161/CIRCULATIONAHA.104.472050. [DOI] [PubMed] [Google Scholar]
- 16.Castillo JR, Zagler A, Carrillo-Jimenez R, Hennekens CH. Brain natriuretic peptide: A potential marker for mortality in septic shock. Int J Infect Dis. 2004;8:271–4. doi: 10.1016/j.ijid.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Charpentier J, Luyt CE, Fulla Y, et al. Brain natriuretic peptide: A marker of myocardial dysfunction and prognosis during severe sepsis. Crit Care Med. 2004;32:660–5. doi: 10.1097/01.ccm.0000114827.93410.d8. [DOI] [PubMed] [Google Scholar]
- 18.Guest TM, Ramanathan AV, Tuteur PG, Schechtman KB, Ladenson JH, Jaffe AS. Myocardial injury in critically ill patients. A frequently unrecognized complication. JAMA. 1995;273:1945–9. [PubMed] [Google Scholar]
- 19.Kuhn C, Muller-Werdan U, Schmitt DV, et al. Improved outcome of APACHE II score-defined escalating systemic inflammatory response syndrome in patients post cardiac surgery in 1996 compared to 1988–1990: The ESSICS-study pilot project. Eur J Cardiothorac Surg. 2000;17:30–7. doi: 10.1016/s1010-7940(99)00345-0. [DOI] [PubMed] [Google Scholar]
- 20.Maeder M, Ammann P, Kiowski W, Rickli H. B-type natriuretic peptide in patients with sepsis and preserved left ventricular ejection fraction. Eur J Heart Fail. 2005;7:1164–7. doi: 10.1016/j.ejheart.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Spies C, Haude V, Fitzner R, et al. Serum cardiac troponin T as a prognostic marker in early sepsis. Chest. 1998;113:1055–63. doi: 10.1378/chest.113.4.1055. [DOI] [PubMed] [Google Scholar]
- 22.Gödecke A, Decking UK, Ding Z, et al. Coronary hemodynamics in endothelial NO synthase knockout mice. Circ Res. 1998;82:186–94. doi: 10.1161/01.res.82.2.186. [DOI] [PubMed] [Google Scholar]
- 23.Kostic MM, Petronijevic MR, Jakovljevic VL. Role of nitric oxide (NO) in the regulation of coronary circulation. Physiol Res. 1996;45:273–8. [PubMed] [Google Scholar]
- 24.Cunnion RE, Schaer GL, Parker MM, Natanson C, Parrillo JE. The coronary circulation in human septic shock. Circulation. 1986;73:637–44. doi: 10.1161/01.cir.73.4.637. [DOI] [PubMed] [Google Scholar]
- 25.Dhainaut JF, Huyghebaert MF, Monsallier JF, et al. Coronary hemodynamics and myocardial metabolism of lactate, free fatty acids, glucose, and ketones in patients with septic shock. Circulation. 1987;75:533–41. doi: 10.1161/01.cir.75.3.533. [DOI] [PubMed] [Google Scholar]
- 26.Wu AH. Increased troponin in patients with sepsis and septic shock: Myocardial necrosis or reversible myocardial depression? Intensive Care Med. 2001;27:959–61. doi: 10.1007/s001340100970. [DOI] [PubMed] [Google Scholar]
- 27.Pilz G, McGinn P, Boekstegers P, Kääb S, Weidenhöfer S, Werdan K. Pseudomonas sepsis does not cause more severe cardiovascular dysfunction in patients than non-Pseudomonas sepsis. Circ Shock. 1994;42:174–82. [PubMed] [Google Scholar]
- 28.Dhainaut JF, Pinsky MR, Nouria S, Slomka F, Brunet F. Right ventricular function in human sepsis: A thermodilution study. Chest. 1997;112:1043–9. doi: 10.1378/chest.112.4.1043. [DOI] [PubMed] [Google Scholar]
- 29.Levy RJ, Piel DA, Acton PD, et al. Evidence of myocardial hibernation in the septic heart. Crit Care Med. 2005;33:2752–6. doi: 10.1097/01.ccm.0000189943.60945.77. [DOI] [PubMed] [Google Scholar]
- 30.Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 31.Witthaut R, Busch C, Fraunberger P, et al. Plasma atrial natriuretic peptide and brain natriuretic peptide are increased in septic shock: Impact of interleukin-6 and sepsis-associated left ventricular dysfunction. Intensive Care Med. 2003;29:1696–702. doi: 10.1007/s00134-003-1910-0. [DOI] [PubMed] [Google Scholar]
- 32.McLean AS, Huang SJ. Intensive care echocardiography. In: Vincent JL, editor. Yearbook of Intensive Care and Emergency Medicine 2006. Heidelberg, New York, Berlin: Springer-Verlag; 2006. pp. 131–41. [Google Scholar]
- 33.Cotter G, Moshkovitz Y, Kaluski E, et al. The role of cardiac power and systemic vascular resistance in the pathophysiology and diagnosis of patients with acute congestive heart failure. Eur J Heart Fail. 2003;5:443–51. doi: 10.1016/s1388-9842(03)00100-4. [DOI] [PubMed] [Google Scholar]
- 34.Hallström S, Koidl B, Müller U, Werdan K, Schlag G. A cardiodepressant factor isolated from blood blocks Ca2+ current in cardiomyocytes. Am J Physiol. 1991;260:H869–H76. doi: 10.1152/ajpheart.1991.260.3.H869. [DOI] [PubMed] [Google Scholar]
- 35.Hallström S, Bernhart E, Müller U, et al. A cardiodepressant factor (CDF) isolated from hemofiltrates of patients in septic and/or cardiogenic shock blocks calcium inward current in cardiomyocytes. Shock. 1994;2(Suppl):15. (Abst) [Google Scholar]
- 36.Hoffmann JN, Werdan K, Hartl WH, Jochum M, Faist E, Inthorn D. Hemofiltrate from patients with severe sepsis and depressed left ventricular contractility contains cardiotoxic compounds. Shock. 1999;12:174–80. doi: 10.1097/00024382-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Pathan N, Sandiford C, Harding SE, Levin M. Characterization of a myocardial depressant factor in meningococcal septicemia. Crit Care Med. 2002;30:2191–8. doi: 10.1097/00003246-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med. 1996;183:949–58. doi: 10.1084/jem.183.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thoenes M, Förstermann U, Tracey WR, et al. Expression of inducible nitric oxide synthase in failing and non-failing human heart. J Mol Cell Cardiol. 1996;28:165–9. doi: 10.1006/jmcc.1996.0016. [DOI] [PubMed] [Google Scholar]
- 40.Müller-Werdan U, Schumann H, Fuchs R, et al. Tumor necrosis factor alpha (TNF alpha) is cardiodepressant in pathophysiologically relevant concentrations without inducing inducible nitric oxide-(NO)-synthase (iNOS) or triggering serious cytotoxicity. J Mol Cell Cardiol. 1997;29:2915–23. doi: 10.1006/jmcc.1997.0526. [DOI] [PubMed] [Google Scholar]
- 41.Müller-Werdan U, Engelmann H, Werdan K. Cardiodepression by tumor necrosis factor-alpha. Eur Cytokine Netw. 1998;9:689–91. [PubMed] [Google Scholar]
- 42.Oral H, Dorn GW, II, Mann DL. Sphingosine mediates the immediate negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian cardiac myocyte. J Biol Chem. 1997;272:4836–42. doi: 10.1074/jbc.272.8.4836. [DOI] [PubMed] [Google Scholar]
- 43.Reithmann C, Werdan K. Tumor necrosis factor alpha decreases inositol phosphate formation and phosphatidylinositol-bisphosphate (PIP2) synthesis in rat cardiomyocytes. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:175–82. doi: 10.1007/BF00169834. [DOI] [PubMed] [Google Scholar]
- 44.Zell R, Geck P, Werdan K, Boekstegers P. TNF-alpha and IL-1 alpha inhibit both pyruvate dehydrogenase activity and mitochondrial function in cardiomyocytes: Evidence for primary impairment of mitochondrial function. Mol Cell Biochem. 1997;177:61–7. doi: 10.1023/a:1006896832582. [DOI] [PubMed] [Google Scholar]
- 45.Comstock KL, Krown KA, Page MT, et al. LPS-induced TNF-alpha release from and apoptosis in rat cardiomyocytes: Obligatory role for CD14 in mediating the LPS response. J Mol Cell Cardiol. 1998;30:2761–75. doi: 10.1006/jmcc.1998.0851. [DOI] [PubMed] [Google Scholar]
- 46.Krown KA, Page MT, Nguyen C, et al. Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest. 1996;98:2854–65. doi: 10.1172/JCI119114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Natoli G, Costanzo A, Guido F, Moretti F, Levrero M. Apoptotic, non-apoptotic, and anti-apoptotic pathways of tumor necrosis factor signalling. Biochem Pharmacol. 1998;56:915–20. doi: 10.1016/s0006-2952(98)00154-3. [DOI] [PubMed] [Google Scholar]
- 48.Bozkurt B, Kribbs SB, Clubb FJ, Jr, et al. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 1998;97:1382–91. doi: 10.1161/01.cir.97.14.1382. [DOI] [PubMed] [Google Scholar]
- 49.Bryant D, Becker L, Richardson J, et al. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1998;97:1375–81. doi: 10.1161/01.cir.97.14.1375. [DOI] [PubMed] [Google Scholar]
- 50.Kubota T, McTiernan CF, Frye CS, Demetris AJ, Feldman AM. Cardiac-specific overexpression of tumor necrosis factor-alpha causes lethal myocarditis in transgenic mice. J Card Fail. 1997;3:117–24. doi: 10.1016/s1071-9164(97)90045-2. [DOI] [PubMed] [Google Scholar]
- 51.Kubota T, McTiernan CF, Frye CS, et al. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res. 1997;81:627–35. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- 52.Nakano M, Knowlton AA, Dibbs Z, Mann DL. Tumor necrosis factor-alpha confers resistance to hypoxic injury in the adult mammalian cardiac myocyte. Circulation. 1998;97:1392–400. doi: 10.1161/01.cir.97.14.1392. [DOI] [PubMed] [Google Scholar]
- 53.Loppnow H, Werdan K, Reuter G, Flad HD. The interleukin-1 and interleukin-1 converting enzyme families in the cardiovascular system. Eur Cytokine Netw. 1998;9:675–80. [PubMed] [Google Scholar]
- 54.Kelly RA, Balligand JL, Smith TW. Nitric oxide and cardiac function. Circ Res. 1996;79:363–80. doi: 10.1161/01.res.79.3.363. [DOI] [PubMed] [Google Scholar]
- 55.Singal PK, Khaper N, Palace V, Kumar D. The role of oxidative stress in the genesis of heart disease. Cardiovasc Res. 1998;40:426–32. doi: 10.1016/s0008-6363(98)00244-2. [DOI] [PubMed] [Google Scholar]
- 56.Xie YW, Kaminski PM, Wolin MS. Inhibition of rat cardiac muscle contraction and mitochondrial respiration by endogenous peroxynitrite formation during posthypoxic reoxygenation. Circ Res. 1998;82:891–7. doi: 10.1161/01.res.82.8.891. [DOI] [PubMed] [Google Scholar]
- 57.Szabo C. The pathophysiological role of peroxynitrite in shock, inflammation, and ischemia-reperfusion injury. Shock. 1996;6:79–88. doi: 10.1097/00024382-199608000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Wong SC, Fukuchi M, Melnyk P, Rodger I, Giaid A. Induction of cyclooxygenase-2 and activation of nuclear factor-kappaB in myocardium of patients with congestive heart failure. Circulation. 1998;98:100–3. doi: 10.1161/01.cir.98.2.100. [DOI] [PubMed] [Google Scholar]
- 59.Groeneveld AB, Hartemink KJ, de Groot MC, Visser J, Thijs LG. Circulating endothelin and nitrate-nitrite relate to hemodynamic and metabolic variables in human septic shock. Shock. 1999;11:160–6. doi: 10.1097/00024382-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 60.Avontuur JA, Boomsma F, van den Meiracker AH, de Jong FH, Bruining HA. Endothelin-1 and blood pressure after inhibition of nitric oxide synthesis in human septic shock. Circulation. 1999;99:271–5. doi: 10.1161/01.cir.99.2.271. [DOI] [PubMed] [Google Scholar]
- 61.van Obbergh L, Vallieres Y, Blaise G. Cardiac modifications occurring in the ascitic rat with biliary cirrhosis are nitric oxide related. J Hepatol. 1996;24:747–52. doi: 10.1016/s0168-8278(96)80272-8. [DOI] [PubMed] [Google Scholar]
- 62.Kojda G, Kottenberg K, Nix P, Schluter KD, Piper HM, Noack E. Low increase in cGMP induced by organic nitrates and nitrovasodilators improves contractile response of rat ventricular myocytes. Circ Res. 1996;78:91–101. doi: 10.1161/01.res.78.1.91. [DOI] [PubMed] [Google Scholar]
- 63.Balligand JL. Molecular mechanisms of action of nitric oxide. In: Vincent JL, editor. Yearbook of Intensive Care and Emergency Medicine 1998. Heidelberg, New York, Berlin: Springer-Verlag; 1998. pp. 107–24. [Google Scholar]
- 64.Obata T, Sato T, Yamanaka Y, Arita M. NO and cGMP facilitate adenosine production in rat hearts via activation of ecto-5′-nucleotidase. Pflugers Arch. 1998;436:984–90. doi: 10.1007/s004240050733. [DOI] [PubMed] [Google Scholar]
- 65.Kelm M, Schäfer S, Dahmann R, et al. Nitric oxide induced contractile dysfunction is related to a reduction in myocardial energy generation. Cardiovasc Res. 1997;36:185–94. doi: 10.1016/s0008-6363(97)00149-1. [DOI] [PubMed] [Google Scholar]
- 66.Gellerich FN, Trumbeckaite S, Hertel K, et al. Impaired energy metabolism in hearts of septic baboons: Diminished activities of Complex I and Complex II of the mitochondrial respiratory chain. Shock. 1999;11:336–41. [PubMed] [Google Scholar]
- 67.Boekstegers P, Weidenhöfer S, Kapsner T, Werdan K. Skeletal muscle partial pressure of oxygen in patients with sepsis. Crit Care Med. 1994;22:640–50. doi: 10.1097/00003246-199404000-00021. [DOI] [PubMed] [Google Scholar]
- 68.Drexler H, Kastner S, Strobel A, Studer R, Brodde OE, Hasenfuss G. Expression, activity and functional significance of inducible nitric oxide synthase in the failing human heart. J Am Coll Cardiol. 1998;32:955–63. doi: 10.1016/s0735-1097(98)00336-2. [DOI] [PubMed] [Google Scholar]
- 69.Paulus WJ. Myocardial inducible nitric oxide synthase and left ventricular performance in the human heart. In: Vincent JL, editor. Yearbook of Intensive Care and Emergency Medicine 1999. Heidelberg, New York, Berlin: Springer-Verlag; 1999. pp. 497–503. [Google Scholar]
- 70.Fukuchi M, Hussain SN, Giaid A. Heterogeneous expression and activity of endothelial and inducible nitric oxide synthases in end-stage human heart failure: Their relation to lesion site and beta-adrenergic receptor therapy. Circulation. 1998;98:132–9. doi: 10.1161/01.cir.98.2.132. [DOI] [PubMed] [Google Scholar]
- 71.Harding SE, Davies CH, Money-Kyrle AM, Poole-Wilson PA. An inhibitor of nitric oxide synthase does not increase contraction or beta-adrenoceptor sensitivity of ventricular myocytes from failing human heart. Cardiovasc Res. 1998;40:523–9. doi: 10.1016/s0008-6363(98)00188-6. [DOI] [PubMed] [Google Scholar]
- 72.Bernardin G, Strosberg AD, Bernard A, Mattei M, Marullo S. Beta-adrenergic receptor-dependent and -independent stimulation of adenylate cyclase is impaired during severe sepsis in humans. Intensive Care Med. 1998;24:1315–22. doi: 10.1007/s001340050768. [DOI] [PubMed] [Google Scholar]
- 73.Scrogin KE, Hatton DC, Chi Y, Luft FC. Chronic nitric oxide inhibition with L-NAME: Effects on autonomic control of the cardiovascular system. Am J Physiol. 1998;274:R367–R74. doi: 10.1152/ajpregu.1998.274.2.R367. [DOI] [PubMed] [Google Scholar]
- 74.Vandecastelle G, Eschenhagen T, Scholz H, Stein B, Verde I, Fischmeister R. Muscarinic and beta-adrenergic regulation of heart rate, force of contraction and calcium current is preserved in mice lacking endothelial nitric oxide synthase. Nat Med. 1999;5:331–4. doi: 10.1038/6553. [DOI] [PubMed] [Google Scholar]
- 75.De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166:98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- 76.Müller-Werdan U, Werdan K. Immune modulation by catecholamines – A potential mechanism of cytokine release in heart failure? Herz. 2000;25:271–3. doi: 10.1007/s000590050019. [DOI] [PubMed] [Google Scholar]
- 77.Müller-Werdan U, Jacoby J, Loppnow H, et al. Noradrenaline stimulates cardiomyocytes to produce interleukin-6, indicative of a proinflammatory action, which is suppressed by carvedilol. Eur Heart J. 1999;20(Suppl):P1721. (Abst) [Google Scholar]
- 78.Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign Management Guidelines Committee. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–73. doi: 10.1097/01.ccm.0000117317.18092.e4. (Errata in 2004;32:1448 and 2004;32:2169–70) [DOI] [PubMed] [Google Scholar]
- 79.Varriale P, Ramaprasad S. Septic cardiomyopathy as a cause of long QT syndrome. J Electrocardiol. 1995;28:327–9. doi: 10.1016/s0022-0736(05)80051-2. [DOI] [PubMed] [Google Scholar]
- 80.Prondzinsky R, Stache N, Witthaut R, et al. Multiorgan-failure (MOF) with and without sepsis: Differences in incidence and pattern of detected arrhythmias. Crit Care. 1997;1(Suppl 1):P30. (Abst) [Google Scholar]
- 81.Knotzer H, Mayr A, Ulmer H, et al. Tachyarrhythmias in a surgical intensive care unit: A case-controlled epidemiologic study. Intensive Care Med. 2000;26:908–14. doi: 10.1007/s001340051280. [DOI] [PubMed] [Google Scholar]
- 82.Winchell RJ, Hoyt DB. Spectral analysis of heart rate variability in the ICU: A measure of autonomic function. J Surg Res. 1996;63:11–6. doi: 10.1006/jsre.1996.0214. [DOI] [PubMed] [Google Scholar]
- 83.Schmidt H, Müller-Werdan U, Hoffmann T, et al. Autonomic dysfunction predicts mortality in patients with multiple organ dysfunction syndrome of different age groups. Crit Care Med. 2005;33:1994–2002. doi: 10.1097/01.ccm.0000178181.91250.99. [DOI] [PubMed] [Google Scholar]
- 84.Stein PK, Kleiger RE. Insights from the study of heart rate variability. Annu Rev Med. 1999;50:249–61. doi: 10.1146/annurev.med.50.1.249. [DOI] [PubMed] [Google Scholar]
- 85.Schmidt H, Werdan K, Mueller-Werdan U. Autonomic dysfunction in the ICU patient. Curr Opin Crit Care. 2001;7:314–22. doi: 10.1097/00075198-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 86.Godin PJ, Buchman TG. Uncoupling of biological oscillators: A complementary hypothesis concerning the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med. 1996;24:1107–16. doi: 10.1097/00003246-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 87.Seely AJ, Christou NV. Multiple organ dysfunction syndrome: Exploring the paradigm of complex nonlinear systems. Crit Care Med. 2000;28:2193–200. doi: 10.1097/00003246-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 88.Marshall JC. Complexity, chaos, and incomprehensibility: Parsing the biology of critical illness. Crit Care Med. 2000;28:2646–8. doi: 10.1097/00003246-200007000-00080. [DOI] [PubMed] [Google Scholar]
- 89.Godin PJ, Fleisher LA, Eidsath A, et al. Experimental human endotoxemia increases cardiac regularity: Results from a prospective, randomized, crossover trial. Crit Care Med. 1996;24:1117–24. doi: 10.1097/00003246-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 90.Schmidt H, Flieger RR, Hennen R, et al. Reversible autonomic dysfunction in a young woman with septic multiple organ dysfunction syndrome. Dtsch Med Wochenschr. 2005;130:648–51. doi: 10.1055/s-2005-865075. [DOI] [PubMed] [Google Scholar]
- 91.Schmidt HB, Mueller-Werdan U, Saworski J, Werdan K. Can LPS of TNF-alpha narrow the beating rate variability of contracting cardiomyocytes? Shock. 2000;13:79. doi: 10.1177/0968051907086233. (Abst) [DOI] [PubMed] [Google Scholar]
- 92.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 93.Smail N, Descorps Declere A, Duranteau J, Vigue B, Samii K. Left ventricular function after severe trauma. Intensive Care Med. 1996;22:439–42. doi: 10.1007/BF01712161. [DOI] [PubMed] [Google Scholar]
- 94.McDonough KH, Giaimo M, Quinn M, Miller H. Intrinsic myocardial function in hemorrhagic shock. Shock. 1999;11:205–10. doi: 10.1097/00024382-199903000-00009. [DOI] [PubMed] [Google Scholar]
- 95.Menasché P. The inflammatory response to cardiopulmonary bypass and its impact on postoperative myocardial function. Curr Opin Cardiol. 1995;10:597–604. [PubMed] [Google Scholar]
- 96.Prondzinsky R, Knüpfer A, Loppnow H, et al. Surgical trauma affects the proinflammatory status after cardiac surgery to a higher degree than cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2005;129:760–6. doi: 10.1016/j.jtcvs.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 97.Hennein HA, Ebba H, Rodriguez JL, et al. Relationship of the proinflammatory cytokines to myocardial ischemia and dysfunction after uncomplicated coronary revascularization. J Thorac Cardiovasc Surg. 1994;108:626–35. [PubMed] [Google Scholar]
- 98.Pilz G, Kääb S, Kreuzer E, Werdan K. Evaluation of definitions and parameters for sepsis assessment in patients after cardiac surgery. Infection. 1994;22:8–17. doi: 10.1007/BF01780757. [DOI] [PubMed] [Google Scholar]
- 99.Dellinger RP. Cardiovascular management of septic shock. Crit Care Med. 2003;31:946–55. doi: 10.1097/01.CCM.0000057403.73299.A6. [DOI] [PubMed] [Google Scholar]
- 100.Rivers E, Nguyen B, Havstad S, et al. Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. (Comment in 2001;345:1417–8 and 2002;346:1025–6,1377) [DOI] [PubMed] [Google Scholar]
- 101.Levy MM, Macias WL, Vincent JL, et al. Early changes in organ function predict eventual survival in severe sepsis. Crit Care Med. 2005;33:2194–201. doi: 10.1097/01.ccm.0000182798.39709.84. [DOI] [PubMed] [Google Scholar]
- 102.Sakr Y, Reinhart K, Vincent JL, et al. Does dopamine administration in shock influence outcome? Results of the Sepsis Occurrence in Acutely Ill Patients (SOAP) Study. Crit Care Med. 2006;34:589–97. doi: 10.1097/01.CCM.0000201896.45809.E3. [DOI] [PubMed] [Google Scholar]
- 103.Hayward CS, Kalnins WV, Rogers P, Feneley MP, MacDonald PS, Kelly RP. Effect of inhaled nitric oxide on normal human left ventricular function. J Am Coll Cardiol. 1997;30:49–56. doi: 10.1016/s0735-1097(97)00143-5. [DOI] [PubMed] [Google Scholar]
- 104.Argenziano M, Dean DA, Moazami N, et al. Inhaled nitric oxide is not a myocardial depressant in a porcine model of heart failure. J Thorac Cardiovasc Surg. 1998;115:700–8. doi: 10.1016/S0022-5223(98)70336-8. [DOI] [PubMed] [Google Scholar]
- 105.Takeuchi K, del Nido PJ, Ibrahim AE, et al. Vesnarinone and amrinone reduce the systemic inflammatory response syndrome. J Thorac Cardiovasc Surg. 1999;117:375–82. doi: 10.1016/S0022-5223(99)70436-8. [DOI] [PubMed] [Google Scholar]
- 106.Wagner DR, McTiernan C, Sanders VJ, Feldman AM. Adenosine inhibits lipopolysaccharide-induced secretion of tumor necrosis factor-alpha in the failing human heart. Circulation. 1998;97:521–4. doi: 10.1161/01.cir.97.6.521. [DOI] [PubMed] [Google Scholar]
- 107.Suzuki T, Morisaki H, Serita R, et al. Infusion of the beta-adrenergic blocker esmolol attenuates myocardial dysfunction in septic rats. Crit Care Med. 2005;33:2294–301. doi: 10.1097/01.ccm.0000182796.11329.3b. [DOI] [PubMed] [Google Scholar]
- 108.Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–23. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 109.Brealey D, Karyampudi S, Jacques TS, et al. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol. 2004;286:R491–R7. doi: 10.1152/ajpregu.00432.2003. [DOI] [PubMed] [Google Scholar]
- 110.Niederbichler AD, Hoesel LM, Westfall MV, et al. An essential role for complement C5a in the pathogenesis of septic cardiac dysfunction. J Exp Med. 2006;203:53–61. doi: 10.1084/jem.20051207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boillot A, Massol J, Maupoil V, et al. Alterations of myocardial and vascular adrenergic receptor-mediated responses in Escherichia coli-induced septic shock in the rat. Crit Care Med. 1996;24:1373–80. doi: 10.1097/00003246-199608000-00017. [DOI] [PubMed] [Google Scholar]
- 112.Dhainaut JF, Vinsonneau C, Journois D. Hemofiltration and left ventricular function in sepsis: Mechanisms and clinical implications. Crit Care Med. 1999;27:473–4. doi: 10.1097/00003246-199903000-00017. [DOI] [PubMed] [Google Scholar]
- 113.Hoffmann JN, Hartl WH, Deppisch R, Faist E, Jochum M, Inthorn D. Effect of hemofiltration on hemodynamics and systemic concentrations of anaphylatoxins and cytokines in human sepsis. Intensive Care Med. 1996;22:1360–7. doi: 10.1007/BF01709552. [DOI] [PubMed] [Google Scholar]
- 114.Iwama H, Komatsu T. Effect of an endotoxin-removing column containing immobilized polymyxin B fiber in a patient with septic shock from gram-positive infection. Acta Anaesthesiol Scand. 1998;42:590–3. doi: 10.1111/j.1399-6576.1998.tb05172.x. [DOI] [PubMed] [Google Scholar]
- 115.Reinhart K, Meier-Hellmann A, Beale R, et al. EASy-Study Group. Open randomized phase II trial of an extracorporeal endotoxin adsorber in suspected Gram-negative sepsis. Crit Care Med. 2004;32:1662–8. doi: 10.1097/01.ccm.0000132902.54925.b5. [DOI] [PubMed] [Google Scholar]
- 116.Briegel J, Forst H, Haller M, et al. Stress doses of hydrocortisone reverse hyperdynamic septic shock: A prospective, randomized, double-blind, single-center study. Crit Care Med. 1999;27:723–32. doi: 10.1097/00003246-199904000-00025. [DOI] [PubMed] [Google Scholar]