Abstract
Objective
The mouse serves as an important model for insulin-like (IGF)-related research. However, lack of homologous mouse assays has prevented studies of the normal ontology of the murine IGF-system. Therefore, we developed and validated immunoaassays for murine IGF-I, IGFBP-3 and ALS and studied levels of these analytes in mice.
Methods
Using commercially available reagents, we developed and validated specific enzyme-linked immunosorbent assays (ELISAs) for murine IGF-I, IGFBP-3, and ALS. Levels of these analytes were measured in sera from CD-1 mice, male and female, sampled at 1, 2, 4, 8, 20 and 32 wks of age. In addition, sera from pregnant and post-partum CD-1 mice were also studied.
Results
Validation of specific ELISAs for murine IGF-I, IGFBP-3 and ALS are described; all 3 assays were highly sensitive, precise and accurate. As measured by our homologous ELISA, IGF-I levels (ng/mL, mean±SD) in male mice were relatively low at 1 wk (53±8), rising sharply to peak at 8 wks of age (636±48), and gradually declining thereafter, reaching 395±64 at 32 wks. IGF-I levels in non-pregnant female mice peaked at 4 wks of age (548±77) declined at 8 wks (417±61), then recovered to plateau at 539±78 and 535±88 at 20 and 32 wks, respectively. In male mice, trends in IGFBP-3 were similar to the patterns of IGF-I. However, in non-pregnant female mice, the IGFBP-3 levels declined relatively slowly after peaking at 4-weeks of age, unlike IGF-I levels during this period. ALS levels followed the same pattern as IGF-I in both sexes. IGF-I to IGFBP-3 molar ratios (percent) were similar between sexes, rising continuously with age: ~30% at 1 wk, 80% at 4 wks, 135% at 32 wks. IGF-I was reduced in 8 wk old mice in mid-pregnancy (354±75 vs 417±61 in non-pregnant 8 wk females), reaching a nadir in late-term (146±40), and only partially recovering in the postpartum period (239±23). IGFBP-3 was also lower in late-pregnancy (1245±100 vs 1925±439) and remained depressed post-partum. In contrast to IGF-I and IGFBP-3, ALS increased more than 3-fold in mid-pregnancy (12180±1641 vs 3741±910), followed by a 4-fold decrease in late-pregnancy (2964±489), recovering postpartum (6104±1178).
Conclusions
We report the first ontological studies of IGF-I, IGFBP-3 and ALS in mice using newly-characterized sensitive, homologous immunoassays. Our results indicate that mice have a generally similar pattern in IGF-related axis components, with low levels early in life, increasing to peak during sexual maturation and declining thereafter. Significant gender differences in non-pregnant levels and dramatic changes during pregnancy were also found. Knowledge of the normal developmental changes in the murine IGF system and accurate tools for investigations of this system are a necessary foundation for research in this field.
Keywords: Acid-labile subunit, Insulin-like growth factor-I, Insulin-like growth factor binding-protein, Mouse insulin-like growth factor
INTRODUCTION
Insulin-like growth factor (IGF)-I and IGF-II are related peptides that mediate the mitogenic effects of growth hormone (GH) and, in addition, have important autocrine and paracrine actions [1]. In human serum, IGF-I and IGF-II are bound to soluble IGF-binding proteins, the most abundant of which is IGFBP-3 [2]. The major share of serum IGF is bound within a ternary complex which also consists of IGFBP-3 and the IGF-related acid-labile subunit (ALS). The IGFs have been characterized as important growth, differentiation and apoptotic factors for both normal and pathologic tissues; these activities are regulated to some extent by IGFBP-3 [3] and ALS [4]. In addition, IGF-independent actions of IGFBP-3 on apoptosis have been described [3].
Much of our understanding of IGF physiology is based on experimental manipulation of this system in mice [4–6]. Although mice appear to lack significant IGF-II production after the neonatal period, in most other respects, the IGF systems are largely concordant, with IGF-I serving as GH-regulated mitogen and most of the serum IGF-I present in a ternary complex with IGFBP-3 and ALS.
Although the mouse is an important experimental model for the IGF system, there are only limited quantitative data regarding normal serum levels of murine IGF-I and IGF-II, and virtually no data on IGFBP-3 and ALS. In addition, previous studies of serum IGF-related proteins have relied primarily on heterologous assays; e.g. assays or antibodies developed for use in another species. As shown previously for rat IGF-I [7], reliance on heterologous assays can lead to gross underestimation of actual levels.
To address these deficiencies, we studied developmental and pregnancy-related levels of murine serum IGF-I, IGFBP-3 and ALS using homologous, validated immunoassays. Technical descriptions of our IGF-I, IGFBP-3 and ALS assays have been recently reported [10, 11]; however, we provide additional detail in this report. Our results have important implications for understanding normal murine IGF physiology and for interpretation of experimental results.
METHODS
Materials
Mouse recombinant DNA-derived IGF-I [catalog #791], IGFBP-3 [catalog #775], ALS [catalog #1436], specific monoclonal antibodies to these proteins [MAB 791, MAB 775, MAB 1436, respectively], and biotinylated polyclonal detection antibodies [BAF 791, BAF 775, BAF 1436, respectively] were obtained from R&D Systems (Minneapolis, MN). Microtiter plates were obtained from Nalge Nunc (Rochester, NY). Streptavidin-horseradish peroxidase (HRP) conjugate, o-phenylenediamine hydrochloride (OPD) and hydrogen peroxide substrate were purchased from Pierce Chemicals (Rockford, IL). Other reagents were obtained from Sigma-Aldrich, St Louis, MO).
Animals
IGF-I and IGFBP-3 were assayed in the same samples. Blood was sampled cross-sectionally from healthy non-pregnant and 8 week old pregnant (10 and 18 days gestation) and ~4 day postpartum CD-1 mice (Charles River Laboratories, Wilmington, MA) by cardiac puncture under carbon dioxide anesthesia. Blood was collected into plastic tubes, allowed to clot, centrifuged, and the serum was then removed and stored at −70°C until assay. The use of CD-1 mice for this study was approved by the UCLA Animal Care and Use Committee.
ALS levels were run in separate samples provided by Dr. Shoshana Yakar, NIDDK, Bethesda, MD. For these samples, blood samples were obtained from CD-1 mice (Jackson Laboratory, Bar Harbor, ME) at the indicated ages and processed as described above. Frozen sera were shipped on dry ice to our laboratory at UCLA. The use of CD-1 mice for this portion of the study was approved by the Animal Care and Use Committee of the NIDDK, Bethesda, MD.
Assay Methods
For the IGF-I enzyme-linked immunosorbent assay (ELISA), 96-well microtiter plates were coated with purified monoclonal hamster anti-mouse IGF-I antibody at 0.5 µg/well in 100 µl of phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.4), incubated overnight at room temperature on a shaker, then washed 3 times with 300 µL/well wash buffer (PBS, 0.05% Tween-20), followed by 1 hr incubation with 300 µL/well blocking buffer (PBS, 5% Tween-20, 5% sucrose, 0.05% sodium azide) and 3 final washes with wash buffer. Standards were prepared by diluting recombinant-DNA derived IGF-I in assay buffer (50 mM sodium phosphate, 150 mM NaCl, 0.1% Tween-20, 0.25% bovine serum albumin, pH 7.4) in concentrations ranging from 0 to 25 ng/mL.
Prior to IGF-I assay, 0.1 mL acid/ethanol reagent (12.5% 2N HCl, 87.5% ethanol, v/v) was added to 0.025 mL of each serum sample. The mixture was incubated at room temperature for 30 min. microcentrifuged at 10,000g for 10 min, and 0.05 mL supernatant was neutralized with 0.025 mL 1M Tris base and diluted with IGF-I assay buffer to a final dilution of 40–100 fold. For the acid-ethanol extraction procedure, recovery of added mouse IGF-I was >90%. Standards, controls or prepared samples (50 µL/well) and 50 ng/well (50 µL/well) biotinylated goat anti-mouse IGF-I antibody were added to the prepared assay plates and incubated at room temperature for 2 hr on a shaker.
Wells were then washed 3 times with wash buffer, followed by addition of streptavidin-HRP conjugate (100 µL/well) and further incubated for 20 min at room temperature. After 4 washes with wash buffer, 100 µL/well of OPD solution (1 mg/mL in hydrogen peroxide substrate) was added to each well and incubated for an additional 10–20 min. The reaction was stopped by the addition of 50 µL/well 2N H2SO4 and absorbance was determined on a plate spectrophotometer (Molecular Designs, Sunnyvale, CA) at 490 nm.
Assays for murine IGFBP-3 and ALS were constructed using the same method as for the IGF-I ELISA, utilizing purified monoclonal antibodies for microtiter plate coating and biotinylated goat-anti mouse antibody for detection. IGFBP-3 standards were prepared at 0–25 ng/mL and serum samples were diluted 100–400 fold with IGFBP3 assay buffer (PBS, 1% Tween-20, 10% normal goat serum) prior to assay.
The ALS standards were prepared at 0–250 ng/mL in ALS assay buffer (50 mM sodium phosphate, pH 7.6, 150 mM NaCl, 0.1% Tween-20, 0.2% BSA). Prior to the ALS assay, serum samples were acidified (1:4 v/v) with 0.2M glycine-HCl, pH 2.7, for 30 min at room temperature as previously described [8,9]. The acidified samples were then neutralized and diluted with ALS assay buffer to a final dilution of 20–400 fold. The acidification of the serum samples resulted in unfolding of ALS and dissociating from the ALS binding protein (e.g. IGFBP3) complex as previously reported [8,9], and thus eliminated the possible interference of binding proteins with the ALS assay.
Data Analysis
The ELISA standard curves were analyzed using 4-parameter logistic curvefit. Results are reported as the mean±SD. Median and range values are also presented. Differences between groups were analyzed by Student’s t-test, with significance defined as p<0.01.
RESULTS
Assay Performance
As previously reported [10], the sensitivity of the mouse IGF-I ELISA, as determined by 2SD from replicates of the 0 standard, was 0.1 ng/mL (10 ng/mL after correction for 100-fold sample dilution). There is no cross-reactivity with mouse IGF-II, human IGF-I or human IGF-II and no interference by mouse IGFBP-1, 2 or 3, human IGFBP-1, 2, 3, 4 (all substances tested at 200 ng/mL). Acid/ethanol extracted rat, human, rabbit, goat, horse and bovine sera are non-reactive in the assay. Intra-assay and inter-assay coefficients of variation (CVs) are <10% at IGF-I concentrations between 1 and 10 ng/mL. The linear range of sample dilution is from 40-fold to 400-fold and recovery of mouse IGF-I added to mouse serum prior to acid/ethanol extraction is 91–101% in the assay range from 1 to 10 ng/mL.
The mouse IGFBP-3 ELISA has a sensitivity of 0.2 ng/mL [11]. The assay has no cross-reactivity with human IGFBP-1, 2, 3, 4, 6 or human IGF-I or IGF-II. Rat, human, rabbit, horse and bovine sera are non-reactive in this assay. The inter and intra-assay CVs are <6% at 1 to 6 ng/mL and recoveries are 89 to 104% in the assay range from 2 to 10 ng/mL.
The mouse ALS assay [11] has a sensitivity of 1.0 ng/mL. There is no cross-reactivity with mouse IGFBP-1, 2 or 3, human IGFBP-1, 2, 3, or 4, mouse or human IGF-I and IGF-II, and no cross-reactivity with human ALS. The intra and inter-assay CVs are <10% at 10 to 60 ng/mL and recovery is 94–102% in the assay range from 15 to 85 ng/mL.
Serum Levels
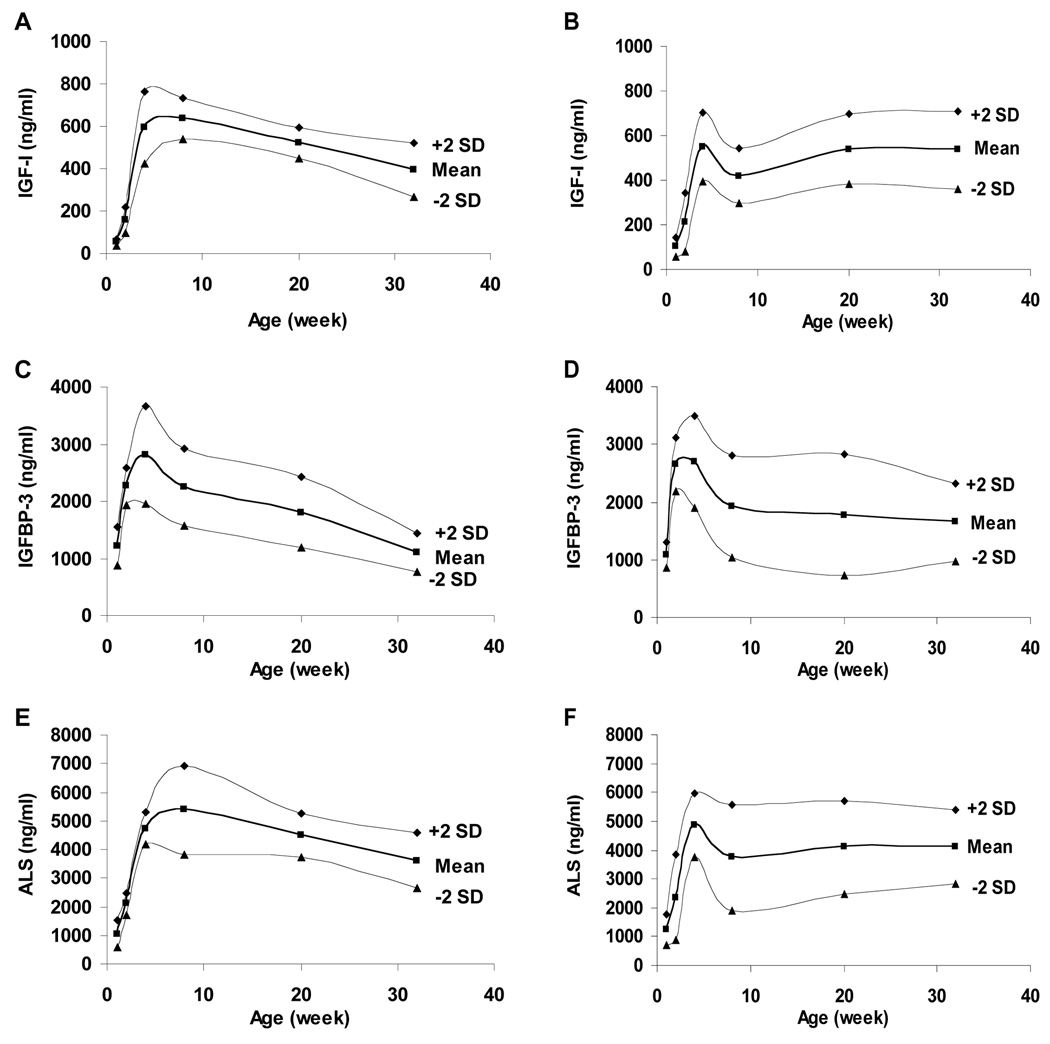
Serum levels of IGF-I, IGFBP-3 and ALS in healthy, non-pregnant CD-1 mice are detailed in Table 1 and depicted in Figure 2. In male CD-1 mice, serum levels of IGF-I were 53±8 ng/mL at 1 wk of age, peaking at 636±48 ng/mL at 8wks and declining thereafter, reaching 395±64 ng/mL at 32 weeks. Levels in 1 wk old female mice were 102±21 ng/mL, significantly higher than the male level, peaked at 548±77 ng/mL at 4 wks, declining to 417±61 ng/mL at 8 wks, then increasing to 539±78 and 535±88 ng/mL at 20 and 32 wks of age, respectively.
Table 1.
Levels and Molar Ratios for IGF-I, IGFBP-3 and ALS in Healthy, Non-Pregnant Mice
| Age (wks)1 |
|||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | 20 | 32 | ||
| IGF-I (ng/mL) | |||||||
| male | mean±SD | 53± 8 | 158±30 | 594±84 | 636±48 | 522±37 | 395±64 |
| median | 56 | 158 | 580 | 638 | 512 | 398 | |
| range | 42–63 | 123–192 | 475–694 | 550–692 | 483–589 | 315–517 | |
| female | mean±SD | 102±21 | 211±66 | 548±77 | 417±61 | 539±78 | 535±88 |
| median | 93 | 200 | 542 | 414 | 541 | 522 | |
| range | 87–126 | 156–288 | 418–666 | 348–537 | 389–655 | 396–664 | |
| IGFBP-3 (ng/mL) | |||||||
| male | mean±SD | 1213±173 | 2267±164 | 2800±428 | 2248±338 | 1808±314 | 1102±168 |
| median | 1245 | 2231 | 2707 | 2208 | 1673 | 1070 | |
| range | 946–1508 | 2048–2614 | 2205–3519 | 1781–2925 | 1407–2239 | 871–1352 | |
| female | mean±SD | 1078±108 | 2643±232 | 2699±398 | 1925±439 | 1776±522 | 1653±337 |
| median | 1097 | 2531 | 2672 | 1827 | 1687 | 1638 | |
| range | 867–1217 | 2444–3166 | 1998–3441 | 1469–2965 | 1212–3055 | 1108–3296 | |
| ALS (ng/mL) | |||||||
| male | mean±SD | 1052±237 | 2090±201 | 4721±281 | 5371±785 | 4484±385 | 3599±483 |
| median | 1001 | 2026 | 4654 | 5328 | 4408 | 3490 | |
| range | 731–1534 | 1858–2433 | 4443–5263 | 4469–6626 | 4004–5075 | 2910–4521 | |
| female | mean±SD | 1238±268 | 2360±738 | 4860±547 | 3741±910 | 4107±808 | 4121±638 |
| median | 1167 | 2199 | 4673 | 3596 | 4140 | 4074 | |
| range | 905–1753 | 1481–3826 | 4520–5934 | 2632–4738 | 2813–5132 | 3328–5225 | |
| IGF-1 to IGFBP-3 molar ratio (%) | |||||||
| male | mean±SD | 23±8 | 44±12 | 80±7 | 105±16 | 112±18 | 141±28 |
| median | 22 | 42 | 80 | 107 | 115 | 143 | |
| range | 16–40 | 27–59 | 71–94 | 85–128 | 84–135 | 105–182 | |
| female | mean±SD | 36±3 | 52±10 | 79±8 | 91±10 | 122±22 | 131±44 |
| median | 35 | 49 | 80 | 90 | 124 | 122 | |
| range | 33–40 | 44–66 | 64–91 | 78–109 | 83–156 | 93–232 | |
| IGFBP-3 to ALS molar ratio (%)2 | |||||||
| male | 270 | 254 | 139 | 98 | 95 | 72 | |
| female | 204 | 262 | 130 | 120 | 101 | 94 | |
| IGF-I to ALS molar ratio (%)2 | |||||||
| male | 46 | 69 | 114 | 107 | 106 | 100 | |
| female | 75 | 81 | 102 | 101 | 119 | 118 | |
n=6–8 for each sex at each age
ALS levels were from a different population. Therefore, the IGFBP-3 and IGF-I to ALS molar ratios are estimated from the mean levels for each age and sex.
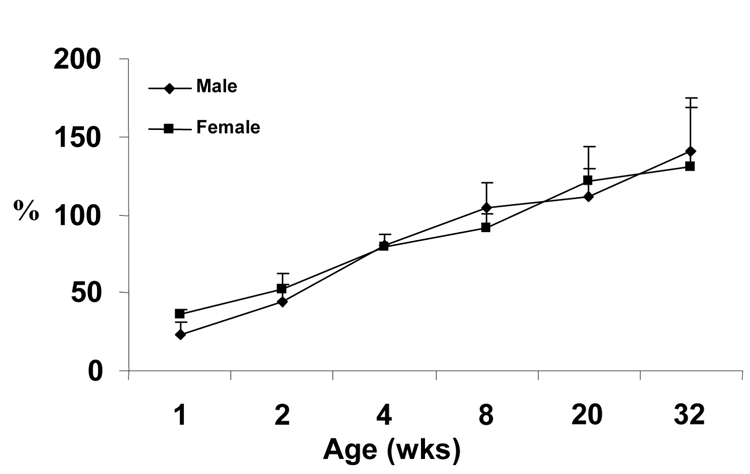
Figure 2. IGF-I to IGFBP-3 Molar Ratio (%).
Molar ratio of IGF-I to IGFBP-3 in serum of male and female mice by age.
In male mice, IGFBP-3 levels paralleled those of IGF-I: 1213±173 ng/mL at 1 wk of age, increasing to 2800±428 ng/mL at 8 wks and declining thereafter to 1102±168 ng/mL at 32 wks. IGFBP-3 levels in female mice showed the same pattern as in males, but was discordant with the increased IGF-I levels after 8 wks: 1078±108 ng/mL at 1 wk, peaking at 2699±398 at 4 wks, then declining to 1653±337 ng/mL by 32 wks of age.
ALS levels in both male and female mice generally paralleled changes in IGF-I. In male mice, ALS increased from 1052±237 at 1 wk of age, peaking at 5371±785 ng/mL at 8 wks and declining to 3599±483 ng/mL at 32 wks. In female mice, levels increased from 1238±268 ng/mL at 1 wk of age to 4860±547 ng/mL at 4 wks, then decreased to 3741±910 ng/mL, with a subsequent increase to 4140±808 and 4121±638 at 20 and 32 wks of age, respectively.
Molar Ratios
Concentrations of the analytes for each sample were converted to molar concentrations based on the predicted molecular weights of the unmodified proteins; i.e., 7.6 kDa for IGF-I, 29.4 kDa for IGFBP-3, and 68.9 kDa for ALS. Individual ratios were then calculated and averaged by age and sex, with results shown in Table 2. Despite the differences in mean IGF-I trends, the mean molar ratios of IGF-I to IGFBP-3 were similar between male and female mice at each age, except for a slightly lower ratio for males at 1 and 2 wks of age. By 4 wks, the molar ratio of IGF-I to IGFBP-3 for the population had increased to 80%, further increasing to ~135% at 32 wks.
Table 2.
Levels and Molar Ratios for IGF-I, IGFBP-3 and ALS in Pregnancy and Postpartum
| Pregnancy (8 wks old at impregnation) |
Non-pregnant | |||
|---|---|---|---|---|
| 10 d gestation n=5–8 | 18 d gestation n=5–8 | 4 d postpartum n=8 | 8 wk old female n=8 | |
| IGF-I (ng/mL) | ||||
| mean±SD | 354±75 | 146±40 | 239±23 | 417±61 |
| median | 363 | 129 | 243 | 414 |
| range | 240–459 | 129–192 | 201–262 | 348–537 |
| IGFBP-3 (ng/mL) | ||||
| mean±SD | 1803±294 | 1245±100 | 1481±197 | 1925±439 |
| median | 1824 | 1287 | 1537 | 1827 |
| range | 1307–2085 | 1131–1316 | 1145–1668 | 1469–2965 |
| ALS (ng/mL) | ||||
| mean±SD | 12180±1641 | 2964±489 | 6104±1178 | 3741±910 |
| median | 12579 | 3087 | 5891 | 3596 |
| range | 9656–14288 | 1832–3312 | 4510–7646 | 2632–4738 |
| IGF-I to IGFBP-3 molar ratio (%) | ||||
| mean±SD | 78±12 | 45±8 | 63±8 | 91±10 |
| median | 71 | 44 | 61 | 90 |
| range | 68–107 | 34–58 | 58–82 | 78–109 |
| IGFBP-3 to ALS molar ratio (%)1 | ||||
| 35 | 98 | 57 | 101 | |
| IGF-I to ALS molar ratio (%)1 | ||||
| 26 | 45 | 36 | 120 | |
ALS levels were from a different population. Therefore, the IGFBP-3 and IGF-I to ALS molar ratios are estimated from the mean levels for each age and sex.
Molar ratios of IGFBP-3 and IGF-I to ALS could not be directly calculated since the individual samples were obtained from different mice. Therefore, ratios were estimated from the means of the IGFBP-3, IGF-I and ALS levels. The estimated IGFBP-3 to ALS molar ratio indicate a 2 to 3-fold molar excess of IGFBP-3 to ALS at 1 and 2 weeks of age, declining sharply to ~1.5-fold at 4 weeks, and declining further to approximate parity thereafter. In contrast, the estimated molar ratio of IGF-I to ALS shows an initial relative deficit of IGF-I, reaching parity by 4 wks of age.
Pregnancy
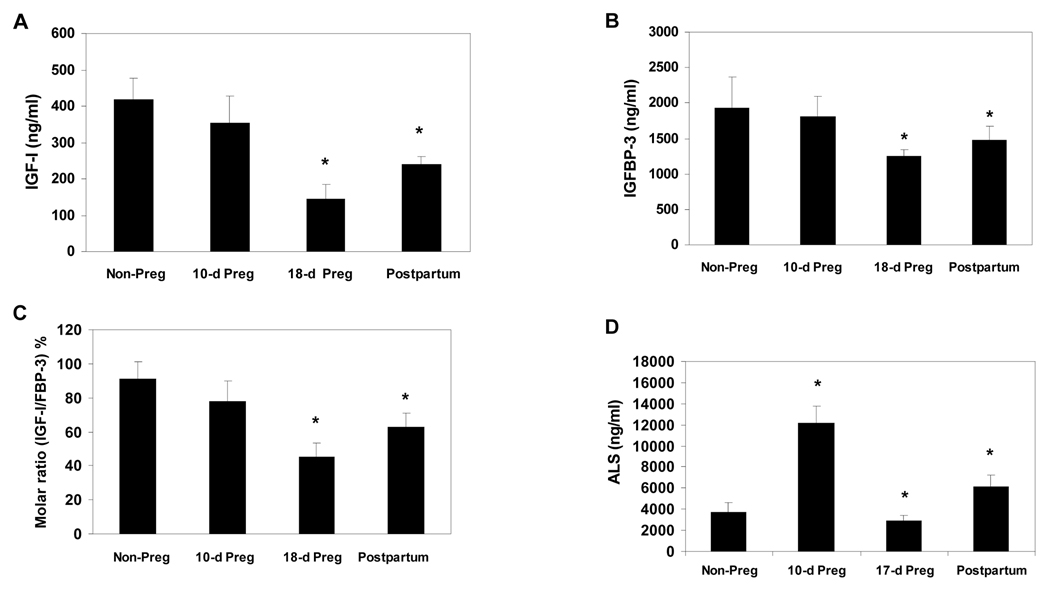
IGF-I, IGFBP-3 and ALS levels were studied in mice impregnated at about 8 wks of age and sampled at mid-term (10 day gestation), late-term (18 day gestation) and ~4 days postpartum. Results, including molar ratios, are shown in Table 3 and Figure 3, with values for non-pregnant 8 wk old female mice for comparison.
Figure 3. IGF-I, IGFBP-3 and ALS Levels in Pregnant and Postpartum Mice.
Serum levels of IGF-I (A), IGFBP-3 (B), Molar ratio of IGF-I to IGFBP-3 (C) and ALS (D) in non-pregnant 8-wk old female (n=8), midterm-pregnant (n=5–8), late-term pregnant (n=5–8) and 4-day postpartum (n=8) mice.
IGF-I levels were decreased at mid-term as compared to the non-pregnant levels (354±75 vs 417±61 ng/mL, respectively, p=0.06) and further decreased to 146±40 ng/mL by late-term (p<0.001 vs 8 wk old non-pregnant females). Levels only partially recovered in the immediate postpartum period.
IGFBP-3 levels showed a similar pattern. Mid-term levels were not significantly lower in pregnant mice as compared to 8 wk old non-pregnant mice (p=0.29), but decreased significantly by late-term (1245±100 vs 1925±439 ng/mL in non-pregnant, p<0.01), increasing in the postpartum period to 1481±197 ng/mL (p<0.02 vs non-pregnant).
In contrast, ALS levels at mid-term were >3-fold higher than in non-pregnant mice (12180±1641 vs 3741±910 ng/mL, p<0.001), dramatically deceasing to 2964±489 (p=0.03 vs non-pregnant, p<0.001 vs mid-term) by late gestation and rebounding to high levels postpartum 6104±1189 (p<0.001 vs non-pregnant, mid-term and late-term).
Molar ratios of IGF-I to IGFBP-3 were decreased in mid-term (78±12%, p=0.05 vs non-pregnant) and decreased further in late-term (45±8%, p<0.001 vs non-pregnant and p<0.01 vs mid-term). On the other hand, estimated IGFBP-3 to ALS ratios were substantially lower during pregnancy and in the immediate postpartum period as compared to similarly aged non-pregnant females. The estimated molar ratio of IGFBP-3 to ALS showed a relative deficit of IGFBP-3 in early pregnancy at a level 4-fold lower than for non-pregnant 8 wk old females. Levels increase to approximate parity, and approximately equal to non-pregnancy, in late gestation, then decrease to approximately 2-fold lower than non-pregnancy in the immediate postpartum period.
DISCUSSION
Our report provides the first quantitative assessment of developmental changes in murine serum IGF-I, IGFBP-3 and ALS using homologous, validated immunoassays. In addition, we provide the first report of these analytes in murine pregnancy. For these studies, we selected the CD-1 strain, which is commonly used in experimental protocols involving the IGF system.
The importance of using a homologous immunoassay for rodent IGF studies was detailed in a previous publication in which rat serum IGF-I levels were found to be 3-fold higher in a homologous RIA as compared to levels measured using a standard heterologous RIA for human IGF-I (896±319 vs 308±154 ng/mL, respectively) [7]. The correlation between the 2 assays had a wide scatter, indicating that the heterologous assay inaccurately quantified experimental changes in endogenous IGF-I levels. The poor performance of the heterologous RIA was somewhat surprising since rat and human IGF-I different at only 3 non-contiguous amino acid residues, positions 20, 35 and 56 of the 70 amino acid sequence.
There are no previous reports of normal murine IGF-I levels using a homologous assay. Measurements using assays for human IGF-I vary considerably between methods and across strains, ranging from ~140 ng/mL in 6–8 week old male mice with mixed background [12] to ~900 ng/mL in 5 and 10 week old mice selected for increased body fat [13]. IGF-I levels have been reported at ~300 ng/mL in 6-day old C57BL/6J male mice [14], ~400 ng/mL in 1 to 23 month old male and female 120/Ola/BalbC mice [15], ~600–700 ng/mL in 1 to 10 month old C3HHeJ female mice [16] and ~450 ng/mL in C57BL/6J females [16].
Results using rat IGF-I assays also give variable results. In 3 mixed murine strains used for transgenic studies, controls had IGF-I levels of 500–900 ng/mL at 6 wks of age [17]. Significantly lower levels of ~250 ng/mL have been reported [18], but ages were not specified in these studies. IGF-I levels of ~900 ng/mL have been reported in male and female CD-1 mice at 5 months [19]; nearly 2-fold higher than those observed in our studies. Finally, levels of ~400 ng/mL were found in 8 wk old CD-1 mice [20], which is similar to our findings.
Relative to our results, it is evident that some heterologous assays probably underestimate murine IGF-I levels, while others give approximately equivalent or higher levels. These discrepancies could be related to the assay methodology, with competitive displacement assays (e.g. RIA, competitive EIA) generally underestimating levels. Direct detection assays, such as the 2-site ELISAs used in this study, may be theoretically less likely to be affected by interspecies sequence differences, unless the epitope(s) for one or both antibodies include the inter-species amino acid difference(s).
Although IGFBP-3 is the major serum IGF-binding protein in rodents, as it is in humans, and ALS is a crucial component of the ternary complex, there are limited data regarding quantitative estimation of these proteins in mouse serum. Immuno and ligand blots used in previous studies are only semi-quantitative [14,15]. A previous study using a heterologous (human) IGFBP-3 assay found levels of ~300 ng/mL in 8 wk old CD-1 mice [20], approximately 7-fold lower than in our investigations. Prior to the first report of our ALS assay [11], there were no published quantitative data regarding murine ALS levels.
Our results indicate that mice have developmental changes in the IGF system similar to humans. A dramatic increase in IGF-I levels between 2 and 4 wks of age, coincident with sexual maturation, are generally consistent with limited data using heterologous assays. In Jcl-ICR male mice, IGF-I levels based on a human heterologous assay with an arbitrary mouse serum standard were ~7.5 U/mL at 1 wk of age, doubling at 2 wks, then declining to a stable level of ~12 U/mL at 4 to 20 wks [21]. Using a human IGF-I assay, wild-type MR-1X129/Sv male mice had plasma IGF-I levels of ~50 ng/mL at 1 wk of age, peaking at ~350 ng/mL at 24 days of age, then declining to ~200 ng/mL on day 30 [22]. In this latter study, testosterone levels rose only after 35 days of age. Conversely, no significant developmental changes were observed between 1 and 10 wks of age in C4H or C57BL6 mice, with both having levels of ~500 ng/mL [16] throughout this period. Finally, using a rat IGF-I RIA, IGF-I levels increased from ~100 ng/mL at 1 wk of age, increasing to ~500 ng/mL at 4 wks, with no significant change thereafter to 52 wks in lit/+ (heterozygous GH deficient) mice [23]. There are no previous quantitative data regarding developmental changes in murine IGF-I using a homologous assays except for a limited previous report using our IGF-I assay in studies of cross-bred wild-type and AFP-BP1 transgenic mice [10]. At 0, 4 and 8 wks of age, IGF-I levels were 23± 9 and 21± 6, 375±32 and 325±48, and 393±15 and 336±38, for the wild-type and transgenic strains, respectively. These results are lower than we observed for CD-1 mice at the same ages, indicating possible inter-strain differences. However, published normal-range data for IGF-system components in non-CD-1 strains are inadequate for valid inter-strain comparisons
Quantitative levels of murine IGFBP-3 and ALS levels, measured in our laboratory, were previously reported for 12 wk old mixed strain male mice and liver IGF-I (LID), ALS (ALSKO) and combined (LA) knockout models treated with parathyroid hormone (PTH) [11]. In the control mice, IGFBP-3 and ALS levels were 294±66 and 824±68 ng/mL, substantially lower than the levels for CD-1 male mice at 8 and 20 weeks, possibly due to interspecies differences.
Our data regarding developmental changes in IGFBP-3 are consistent with previous limited semi-quantitative data in rodents. A densitometric analysis showed an approximate doubling of IGFBP-3 concentrations between 4 to 8 and 12 to 16 wks of age, followed by an ~25% decline at 20–52 wks [23]. Using a specific rat IGFBP-3 assay, Frystyk et al [24] showed that rat IGFBP-3 levels increase from 273 to 3091 ng/mL, and from 236 to 2154 ng/mL in male and female Wistar rats, respectively, with most of the increase occurring between 20 and 30 days of age. In this latter study, male IGF-I levels are clearly higher than female levels at 80 and 130 days, in contrast to our findings in mice.
Factors controlling the developmental changes in murine IGF-I, IGFBP-3 and ALS levels are not yet defined. In humans, pubertal increases in these GH-dependent serum proteins have been related to increased GH and sex steroid production [25, 26]. In mice, IGF-I levels appear to be regulated by caloric intake and weight gain; therefore, the increased levels at 4 wks may be correlated with the major increase in body weight that occurs at this age. Both male and female fertility also typically occur at or shortly after 4 wks of age, and may be related to body fat and leptin production. Further studies will be needed to define the normal relationships between the IGF system, nutritional status and gonadal maturation in mice, and to determine whether these are mechanistically similar to human physiology.
We also characterized levels of IGF-I, IGFBP-3 and ALS during pregnancy and in the immediate postpartum period. We found that both periods are associated with declines in IGF-I and IGFBP-3, and with significant increases in ALS in mid-gestation and postpartum. Our findings in relation to IGF-I confirm previous work by Travers et al [27], who reported a 70% decline in maternal IGF-I levels during mid-pregnancy using a heterologous IGF-I RIA. Levels remained below non-pregnant female levels during lactation and normalized thereafter. Quantitative values for IGF-I were not reported, but the changes were correlated with maternal hepatic IGF-I mRNA levels. A similar decrease in serum IGF-I was subsequently reported in pregnant and lactating rats, although total hepatic IGF-I mRNA increased during this period [28]. In humans, maternal IGF-I levels decrease in early gestation, followed by a gradual increase to term [29]. This appears to be different from our findings in mice; however, mice are born at a relatively earlier stage of gestation. Therefore, murine mid-gestation may be comparable to human early gestation. Our findings of low IGF-I levels in the immediate postpartum period consistent with studies in other species, particularly ruminants, in which maternal IGF-I levels are low during lactation [30].
Pregnant maternal IGFBP-3 and ALS levels have not been completely characterized in rodents, although semi-quantitative analyses of IGFBPs have been reported [31, 32]. In humans, total immunoreactive IGFBP-3 levels increase during pregnancy, although intact levels fall or show no significant change [33]. This discrepancy is due to increased IGFBP-3 protease activity, leading to accumulation of fragments that may be immunoreactive in a competitive RIA but undetectable in a 2-site assay for intact protein. Our results using a 2-site mouse IGFBP-3 ELISA suggest that a similar phenomenon occurs in mice, with immunoreactive IGFBP-3 levels falling during pregnancy. Serum IGFBP-3 protease activity has been characterized in pregnant rats [32] and non-pregnant mice [33].
In a woman with GH deficiency, maternal IGF-I and ALS were reported to be low before pregnancy, with both increasing during the second half of pregnancy and decreasing post-partum [34]. These results are similar to mice, in which hypophysectomy led to decreased maternal IGF-I levels in the non-pregnant state, but IGF-I levels increased during pregnancy [32]. Therefore, in both humans and rodents, it appears probable that the increased IGF-I and ALS increases during pregnancy are mediated by placental somatotropin.
In our investigations, the molar ratio of IGF-I to IGFBP-3 is low at 1 and 2 wks of age, then increases progressively through the 32 wk period, exceeding parity by 8 wks of age. The relative deficit in IGF-I prior to 4 wks of age could be due to residual IGF-II production in the neonatal period. A similar progressive increase in the molar ratio of IGF-I to IGFBP-3 has been observed in humans, with a relative molar excess of IGF-I during human puberty [25, 35]. A study in rats appears to show that the IGF-I to IGFBP-3 molar ratio decreases with age in rats [24]; contrasting with our findings in mice. However, this previous study in rats used a heterologous IGF-I RIA, which may have underestimated rat IGF-I levels. Interestingly, our estimates indicate a significant molar excess of IGFBP-3 to ALS before 4 to 8 weeks of age, which may be discordant with human data [26, 36].
The increased molar ratio of IGF-I to IGFBP-3 during pregnancy and postpartum (lactation) has been reported in other species [37], but not previously in mice. It has been postulated that this phenomenon may increase the bioavailability of IGF-I despite the lower total levels. In contrast to the non-pregnant state, we found a molar excess of ALS to IGFBP-3 in early pregnancy and in the postpartum period, and of ALS to IGF-I throughout pregnancy and postpartum.
In summary, utilizing newly-characterized homologous ELISAs, we provide the first comprehensive study of ontological changes in IGF-I, IGFBP-3 and ALS in healthy mice, as well as levels of these IGF system components in pregnancy. Developmental and pregnancy-related trends in these analytes generally mirror those observed in humans and other species. Experimental protocols that include murine serum IGF-I, IGFBP-3 or ALS levels as endpoint measures should take into account the dramatic variability in normal levels that can occur during the relatively short murine lifespan.
Figure 1. IGF-I, IGFBP-3 and ALS Levels.
Serum levels of IGF-I in male mice (A), female mice (B); IGFBP-3 levels in male mice (C), female mice (D); ALS levels in male mice (E), female mice (F) by age. The smoothed lines are the mean ± 2SD.
ACKNOWLEDGEMENTS
Supported in part by NIH grants P30DK063491, 1R01CA100938, P50CA92131, 1R01HD047013, and 2R01AG018381 (PC). We thank Dr. Orlando Gutierrez, and Dr. Shoshana Yakar for their contributions to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Denley A, Cosgrove LJ, Booker GW, Wallace JC, Forbes BE. Molecular interactions of the IGF system. Cytokine Growth Factor Rev. 2005;16:421–439. doi: 10.1016/j.cytogfr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Firth S, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocrin. Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 3.Cohen P. Insulin-like growth factor binding protein-3: insulin-like growth factor independence comes of age. Endocrinology. 2006;147:2109–2111. doi: 10.1210/en.2006-0195. [DOI] [PubMed] [Google Scholar]
- 4.Domene HN, Bengolea SV, Jasper HG, Boisclair YR. Acid-labile subunit deficiency: phenotypic differences and similarities between human and mouse. J. Endocrinol. Invest. 2005;28 suppl 5:43–46. [PubMed] [Google Scholar]
- 5.Silha JV, Murphy LJ. Insights from insulin-like growth factor binding protein transgenic mice. Endocrinology. 2002;143:35–47. doi: 10.1210/en.2002-220116. [DOI] [PubMed] [Google Scholar]
- 6.Yakar S, Sun H, Zhao H, Pennisi P, Toyoshima Y, Setser J, Stannard B, Scavo L, LeRoith D. Metabolic effects of IGF-I deficiency: lessons from mouse models. Pediatr. Endocrinol. Rev. 2005;3:11–19. [PubMed] [Google Scholar]
- 7.Lee PD, Baker BK, Liu F, Kwan EY, Hintz RL. A homologous radioimmunoassay for rat insulin-like growth factor-I (IGF-I): implications for studies of human IGF-I physiology. J. Clin. Endocrinol. Metab. 1996;81:2002–2005. doi: 10.1210/jcem.81.5.8626873. [DOI] [PubMed] [Google Scholar]
- 8.Liu F, Hintz RL, Khare A, Diaugustine RP, Powell DR, Lee PDK. Immunoblot studies of the IGF-related acid-labile subunit. J. Clin. Endocrinol. Metab. 1994;79:1883–1886. doi: 10.1210/jcem.79.6.7527417. [DOI] [PubMed] [Google Scholar]
- 9.Stadler S, Wu Z, Dressendorfer RA, Morrison KM, Khare A, Lee PDK, Strasburger CJ. Monoclonal anti-acid-labile subunit oligopeptide antibodies and their use in a two-site immunoassay for ALS measurement in humans. J. Immuno. Methods. 2001;252:73–82. doi: 10.1016/s0022-1759(01)00335-0. [DOI] [PubMed] [Google Scholar]
- 10.Watson CS, Bialek P, Anzo M, Khosravi J, Han VK. Elevated circulating insulin-like growth factor binding protein-1 is sufficient to cause fetal growth restriction. Endocrinology. 2006;147:1175–1186. doi: 10.1210/en.2005-0606. [DOI] [PubMed] [Google Scholar]
- 11.Yakar S, Bouxsein ML, Canalis E, et al. The ternary IGF complex influences postnatal bone acquisition and the skeletal response to intermittent parathyroid hormone. J. Endocrinology. 2006;189:289–299. doi: 10.1677/joe.1.06657. [DOI] [PubMed] [Google Scholar]
- 12.Yakar S, Rosen CJ, Beamer WG, et al. Circulating levels of IGF-I directly regulate bone growth and density. J. Clin. Invest. 2002;110:771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKnight BJ, Goddard C. The effect of food restriction on circulating insulin-like growth factor-I in mice divergently selected for high or low protein or fat to body mass ratios. Comp.Biochem. Physiol. A. 1989;92:565–569. doi: 10.1016/0300-9629(89)90366-6. [DOI] [PubMed] [Google Scholar]
- 14.Moriwake T, Abribat T, Brazeau P, Ecarot B. Serum insulin-like growth factor finding protein-3 in the hypophosphatemic mouse: decreased activituy and abnormal modulation by dietary phosphate. J. Bone Mineral Res. 1995;10:1698–1704. doi: 10.1002/jbmr.5650101112. [DOI] [PubMed] [Google Scholar]
- 15.Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- 16.Rosen CJ, Dimai HP, Vereault D, et al. Circulating and skeletal insulin-like growth factor-I (IGF-I) concentrations in two inbred strains of mice with different bone mineral densities. Bone. 1997;21:217–223. doi: 10.1016/s8756-3282(97)00143-9. [DOI] [PubMed] [Google Scholar]
- 17.Yakar S, Liu JL, Stannard B, A, et al. Normal growth and development in the absence of hepatic insulin-like growth factor-I. Proc. Natl. Acad. Sci. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sotelo AI, Bartke A, Kopchick JJ, Knapp JR, Turyn D. Growth hormone (GH) receptors, binding protein and IGF-I concentrations in the serum of transgenic mice expressing bovine GH agonist or antagonist. J. Endocrinol. 1998;158:53–59. doi: 10.1677/joe.0.1580053. [DOI] [PubMed] [Google Scholar]
- 19.Cisneros FJ, Wilson R, Travlos G, Anderson LM, Branch S. Susceptibility to postnatal growth retardiaton induced by 5-AZA-2’-deoxycytidine in utero: gender specificity and correlation with reduced insulin-like growth factor I. Life Sci. 2003;72:2887–2894. doi: 10.1016/s0024-3205(03)00229-7. [DOI] [PubMed] [Google Scholar]
- 20.Modric T, Silha JV, Shi Z, et al. Phenotypic manifestations of insulin-like growth factor-binding protein-3 overexpression in transgenic mice. Endocrinology. 2001;142:1958–1967. doi: 10.1210/endo.142.5.8165. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto T, Matsuo S, Ueshima Y, Inoue F, Kinugasa A, Sawada T. Plasma levels of insulin-like growth factor-I are reduced at one week of age in monosodium L-glutamate-treated mice. Endocrine J. 1993;40:461–465. doi: 10.1507/endocrj.40.461. [DOI] [PubMed] [Google Scholar]
- 22.Wang G-M, O’Shaughnessy PJ, Chubb C, Bobaire B, Hardy MP. Effects of insulin-like growth factor-I on steroidogenic enzyme expression levels in mouse Leydig cells. Endocrinology. 2003;144:5058–5064. doi: 10.1210/en.2003-0563. [DOI] [PubMed] [Google Scholar]
- 23.Donahue LR, Beamer WG. Growth hormone deficiency in “little” mice results in aberrant body composition, reduced insulin-like growth factor-I and insulin-like growth factor-binding protein-3 (IGFBP-3), but does not affect IGFBP-2, -1, or -4. J. Endocrinol. 1993;136:91–104. doi: 10.1677/joe.0.1360091. [DOI] [PubMed] [Google Scholar]
- 24.Frystyk J, Grønbæk H, Skjærbæk C, Flyvbjerg A, Ørskov H, Baxter RC. Developmental changes in serum levels of free and total insulin-like growth factor-I (IGF-I), IGF-binding protein-1 and -3, and the acid-labile subunit in rats. Endocrinology. 1998;139:4286–4292. doi: 10.1210/endo.139.10.6273. [DOI] [PubMed] [Google Scholar]
- 25.Juul A, Dalgaard P, Blum WF, et al. Serum levels of insulin-like growth factor (IGF)-binding protein-3 (IGFBP-3) in healthy infants, children, and adolescents: the relation to IGF-I, IGF-II, IGFBP-1, IGFBP-2, age, sex, body mass index, and pubertal maturation. J. Clin. Endocrinol. Metab. 1995;80:2534–2542. doi: 10.1210/jcem.80.8.7543116. [DOI] [PubMed] [Google Scholar]
- 26.Juul A, Møller S, Mosfeldt-Laursen E, et al. The acid-labile subunit of human ternary insulin-like growth factor binding protein complex in serum: hepatosplanchnic release, diurnal variation, circulating concentrations in healthy subjects, and diagnostic use in patients with growth hormone deficiency. J. Clin. Endocrinol. Metab. 1998;83:4408–4415. doi: 10.1210/jcem.83.12.5311. [DOI] [PubMed] [Google Scholar]
- 27.Travers MT, Madon RJ, Flint DJ. Regulation of serum insulin-like growth factor-I (IGF-I), hepatic growth hormone binding and IGF-I gene expression in the rat during pregnancy and lactation. J. Endocrinol. 1993;139:89–95. doi: 10.1677/joe.0.1390089. [DOI] [PubMed] [Google Scholar]
- 28.Travers MT, Madon RJ, Vallance AJ, Barber MC. Circulating concentrations and hepatic expression of IGF-I during pregnancy and lactation in the mouse. Biochem. Soc. Trans. 1990;18:1268. doi: 10.1042/bst0181268. [DOI] [PubMed] [Google Scholar]
- 29.Monaghan JM, Godber IM, Lawson N, et al. Longitudinal changes of insulin-like growth factors and their binding proteins throughout normal pregnancy. Ann. Clin. Biochem. 2004;41:220–226. doi: 10.1258/000456304323019596. [DOI] [PubMed] [Google Scholar]
- 30.Heidler B, Parvizi N, Sauerwein H, et al. Effects of lactation on metabolic and reproductive hormones in Lipizzaner mares. Domestic Anim. Endocrinol. 2003;25:47–59. doi: 10.1016/s0739-7240(03)00044-4. [DOI] [PubMed] [Google Scholar]
- 31.Gargosky SE, Nanto-Salonen K, Tapanainen P, Rosenfeld RG. Pregnancy in growth hormone-deficient rats: assessment of insulin-like growth factors (IGFs), IGF-binding proteins (IGFBPs) and IGFBP protease activity. J. Endocrinology. 1993;136:479–486. doi: 10.1677/joe.0.1360479. [DOI] [PubMed] [Google Scholar]
- 32.Fielder PJ, Thordarson G, Talamantes F, Rosenfeld RG. Characterization of insulin-like growth factor binding proteins (IGFBPs) during gestation in mice: effect of hypophysectomy and an IGFBP-specific serum protease activity. Endocrinology. 1990;127:2270–2280. doi: 10.1210/endo-127-5-2270. [DOI] [PubMed] [Google Scholar]
- 33.Giudice LC, Farrell EM, Pham H, Lamson G, Rosenfeld RG. Insulin-like growth factor binding proteins in maternal serum throughout gestation and in the puerperium: effects of a pregnancy-associated serum protease activity. J. Clin. Endocrinol. Metab. 1990;71:806–816. doi: 10.1210/jcem-71-4-806. [DOI] [PubMed] [Google Scholar]
- 34.Wiesli P, Zwimpfer C, Zapf J, Schmid C. Pregnancy-induced changes in insulin-like growth factor I (IGF-I), insulin-like growth factor binding protein 3 (IGFBP-3) and acid-labile subunit (ALS) in patients with growth hormone (GH) deficiency and excess. Acta Obstet. Gynecol. Scand. 2006;85:900–905. doi: 10.1080/00016340600676532. [DOI] [PubMed] [Google Scholar]
- 35.Juul, Main K, Blum WF, Lindholm J, Ranke MB, Skakkebaek NE. The ratio between serum levels of insulin-like growth factor (IGF)-I and the IGF binding proteins (IGFBP-1, 2 and 3) decreases with age in healthy adults and is increased in acromegalic patients. Clin. Endocrinology (Oxf) 1994;41:85–93. doi: 10.1111/j.1365-2265.1994.tb03788.x. [DOI] [PubMed] [Google Scholar]
- 36.Baxter RC, Svejkar M, Khosravi MJ, et al. Measurement of the acid-labile subunit of the insulin-like growth factor binding protein complex in human serum: a comparison of four immunoassays. J. Endocrinology. 2000;165:271–279. doi: 10.1677/joe.0.1650271. [DOI] [PubMed] [Google Scholar]
- 37.Kim JW, Rhoads RP, Segoale N, Kristensen NB, Bauman DE, Boisclair YR. Isolation of the cDNA encoding the acid labile subunit (ALS) of the 150 kDa IGF-binding protein comples in cattle and ALS regulation during the transition from pregnancy to lactation. J. Endocrinology. 2006;189:583–593. doi: 10.1677/joe.1.06824. [DOI] [PubMed] [Google Scholar]