Abstract
Sensorimotor adaptation, the ability to adjust motor output in response to persistent changes in sensory input, is a key function of the central nervous system. Although a great deal is known about vestibulo-ocular reflex and saccadic adaptation, relatively little is known about the behavior and neural mechanisms underlying gaze adaptation when the head is free to move. In an attempt to understand the mechanisms of gaze adaptation, and constrain hypotheses concerning the locus at which changes in gaze control may be implemented, we altered the size of large, head-unrestrained gaze shifts made to visual targets by surreptitiously moving the visual target forward (30→60°) or backwards (60→30°) during gaze shifts. In our 10 human subjects, after a few hundred back-step trials, gaze amplitudes were reduced by between 6° and 27°. Similarly, after a few hundred forward adaptation trials, our subjects increased gaze amplitude by between 0° and 26°. Changes in the amplitude of primary gaze shifts occurred regardless of the particular combinations of eye and head movements that made up the amplitude-altered gaze shifts. When gaze shifts were initiated with the eyes in systematically different positions relative to the head, the resulting changes in gaze, eye and head movement amplitudes were consistent with the hypothesis that gaze adaptation occurs at the level of a gaze shift command and not by altering separately the signals that produce eye and head movements.
Keywords: human, adaptation, gaze, head-unrestrained
Introduction
Saccades (high velocity, conjugate movements of the eyes) can redirect the line of sight such that the images of objects of interest fall near the fovea. These visual orienting movements, defined by a set of amplitude-velocity-duration relationships (Bahill, Clark, & Stark, 1975; Baloh, Sills, Kumley & Honrubia, 1975; VanGisbergen, Van Opstal, & Ottes, 1984), are accurate to within 5–10% of target displacement (Hyde, 1959; Becker, 1972; Becker & Fuchs, 1969; Henson, 1978, 1979; Prablanc, Masse, and Echallier, 1978; Kowler & Blaser, 1995) and precise: the standard deviation of saccade endpoints is ~3–6% of target eccentricity (Kowler & Blaser, 1995). Maintenance of this degree of accuracy and precision must be accomplished in the face of growth and development, as well as possible damage and senescence of the sensorimotor control apparatus. Investigation of the mechanisms of sensorimotor adaptation responsible for adjustment of motor output given persistent changes in sensory inflow (Oestreich, Dembrow, George, & Zakon, 2006) within the context of saccadic eye movements has centered on the experimental introduction of visual errors at the end of saccades. For example, McLaughlin (1967) demonstrated that the amplitude of a saccade to a visual target in a particular location can be gradually altered by repeatedly and surreptitiously shifting the location of the target. The resultant visual error (between the retinal image of the displaced target and the fovea) drives adaptation so that the amplitude of saccades become either smaller (“backward”) or larger (“forward”) than saccades made before adaptation (Wallman & Fuchs, 1998). The magnitude and rate of horizontal saccadic adaptation using the McLaughlin task has been characterized over a small range of visual errors (Hopp & Fuchs, 2004). The majority of experiments using this task attempt to elicit changes in saccade amplitude on the order of ~20–30% of the pre-adaptation movement amplitude (e.g. Straube, Robinson, & Fuchs, 1997; Deubel, Wolf, & Hauske, 1986). Since the preadaptation amplitude of saccades in these experiments is often between 8–15°, the 20–30% target shift amounts to approximately a 2–5° visual error at the end of the primary movement. In these experiments, adaptive mechanisms often fail to reduce this visual error to zero, and as a result saccade amplitudes are reduced or increased by ~2–4°. This type of saccadic adaptation has a roughly exponential time course with rate constants between 100–800 saccades in monkeys (Straube et al, 1997) and 30–60 saccades in humans (Albano, 1996; Deubel et al, 1986, Deubel, 1987; Frens & van Opstal, 1994). The rate and magnitude of saccadic adaptation is highly variable across subjects and across experiments in the same subject (Fuchs, Reiner, & Pong, 1996; Straube et al, 1997; Robinson, Noto, & Bevans, 2003).
The role of the imposed visual error at the end of the primary saccade in driving saccadic adaptation was tested by Robinson et al. (2003). In their experiment, the amplitude of the initial saccade and the visual error at the end of this saccade were systematically varied. The visual errors that resulted in the largest changes in saccade amplitude were between 15–45% of the initial target eccentricity. Visual errors larger than 45% were only slightly less effective in inducing saccadic adaptation. Despite these observations, the largest primary movement amplitude used in this experiment was 18°. Few saccadic adaptation experiments have attempted to increase or decrease the amplitude of larger initial movements (however, see Phillips, Fuchs, Ling, Iwamoto, & Votaw, 1997). To date, regardless of whether the head is restrained or allowed to move, it has been assumed that large primary gaze shifts cannot be systematically adjusted in response to large visual errors.
When the head is free to move, gaze shifts are often accomplished by combinations of saccades and simultaneous movements of the head. The relationships between gaze, eye, and head amplitude, peak velocity, and duration are predictable when gaze amplitude (or target displacement) and the initial positions of the eyes in the orbits are known (Volle & Guitton, 1993; Guitton & Volle, 1987; Delreux, Abeele, Lefevre, & Roucoux, 1991; Stahl, 1999; 2001; Freedman & Sparks, 1997; Freedman, 2005). Prior descriptions of head-unrestrained gaze adaptation have focused on the transfer of head-restrained saccadic adaptation to either head-only movements (Kroller, Pelisson, & Prablanc, 1996) or combined eye-head gaze shifts (Phillips et al., 1997). Kroller and colleagues (1996) did not observe transfer of saccadic adaptation to head only movements in humans. However, Phillips and colleagues observed the complete transfer of head-restrained, backward saccadic adaptation, induced using the McLaughlin task, to head-unrestrained gaze shifts in monkeys. Also, in one head-unrestrained subject, they were able to reduce gaze amplitude directly using this task. From these observations, Phillips and colleagues suggested that the gaze displacement command underlying both saccadic and combined eye-head gaze shifts had been adjusted by a visual error control mechanism. According to this hypothesis, a gaze displacement command, upstream of separate eye and head displacement commands, is altered during adaptation. This hypothesis predicts that the amplitude of a gaze shift will increase or decrease throughout the adaptation process independent of the relative amplitudes of the eyes and head used to shift the line of sight. In this case, the relative contributions of the eyes and head to a gaze shift are determined downstream from the locus of adaptation. An alternative hypothesis states that either the eyes and/or head are altered independently from each other during the adaptation process. For example, if adaptation resulted in a 20° reduction in gaze amplitude that was produced by reducing saccade amplitude by 15° and a concomitant 5° reduction in head contribution, these reductions in eye and head movements would persist in response to presentation of a target in the adapted location regardless of the initial conditions. These alternative hypotheses are dissociable when gaze shifts are initiated from a variety of initial eye positions.
The goals of the present study were to: 1) use the McLaughlin task to elicit large changes in gaze amplitude in head-unrestrained, human subjects; 2) describe the magnitude of forward and backward gaze adaptation; 3) quantify the relative contributions of the eyes and head to this adaptation, as well as 4) determine the amount of adaptation transfer between movements initiated with the eyes in different orbital positions. A preliminary account of this study has appeared elsewhere (Cecala & Freedman, 2005).
Material and Methods
Subjects
Ten neurologically normal human subjects (mean age 24.6± 2.4yr; 8 male and 2 female) served as subjects. With the exception of the first author (identified henceforth as subject A), subjects were naïve to the goals of the current investigation. Subjects participated in multiple data collection sessions that were separated by at least 2 days. Each data collection session is identified by a subject-specific letter and session number. For example, “H1” are data from the first adaptation experiment (regardless of adaptation direction) in which subject H participated, “H2” are data from the second adaptation experiment in which subject H participated, and so on.
All procedures were approved by the University of Rochester Institutional Review Board and were carried out in full accordance with the principles of the Declaration of Helsinki. The procedures were fully explained to the subjects, who gave written consent before the experiments and were compensated for their participation.
Visual Target Presentation
During all experiments subjects sat on a modified orthopedic chair in the center of 2.5m diameter, 0.5” plexiglas sphere (Capital Plastics, Beltsville, MD). The sphere was placed inside a 2m cube, housing the Helmholtz coils used for magnetic field generation (CNC Engineering, Seattle, WA). Care was taken to align the cube and sphere centers. The inside of the sphere was painted 18% gray, and served as the surface upon which visual targets were presented. Targets consisted of a fixed array of 43 lasers (650 nm, Calpac). Lasers were focused on the surface of the sphere and each spot subtended 0.1°. Laser spot locations were determined using a Fick gimbal, and referenced to a central (0,0) location. This reference location was defined as the intersection of the sphere and a horizontal line passing through the geometric center of the sphere and parallel to the sides of the field coil frame.
Eye and Head Measurements
The magnetic field used to measure horizontal eye and head positions was generated by two pairs of coils in spatial and phase quadrature (Collewijn, 1977). Signals from the eye coil (Skalar Delft, The Netherlands) and matching head-mounted coil were linear within ~2% over 360° in the horizontal plane. Vertical eye and head positions were proportional to the sine of the angle of the coils and the straight ahead position. Horizontal and vertical, eye and head position signals were filtered and sampled at 1kHz. Velocities were calculated from position signals using a parabolic differentiation (see Freedman, 2005 for description).
To insert the annular contact lens eye coils, a subject’s eye was anesthetized with proparacaine HCl (0.5%, Alcon Laboratories). To prevent irritation, eye coils were left in place for no longer than 40 minutes. The head coil was mounted on a lightweight head band securely fastened at the beginning of each experimental session. In addition to carrying the head coil, 3 diode lasers were secured to the top of the head band. The central laser of these three was aligned approximately in the midsagittal plane. The two other lasers pointed 30° to the left and right of this central laser. Head coil calibration was accomplished by asking subjects to align the central head-mounted laser spot with various static laser positions within the sphere; gains and offsets were adjusted appropriately. During experimental sessions the head-mounted lasers could be used to provide visual feedback of head position so that subjects could align their eyes and heads, or begin trials with the eyes deviated in the orbits either to the left or right of the head-centered position. This allowed the experimenter to control the initial position of the eyes at the onset of the gaze shift, which has been shown to systematically affect the relative eye and head amplitude associated with gaze shifts of particular amplitude and direction (Volle & Guitton, 1993; Guitton & Volle, 1987; Delreux et al, 1991; Stahl, 1999; 2001; Freedman & Sparks, 1997; Freedman, 2005).
Behavioral Tasks
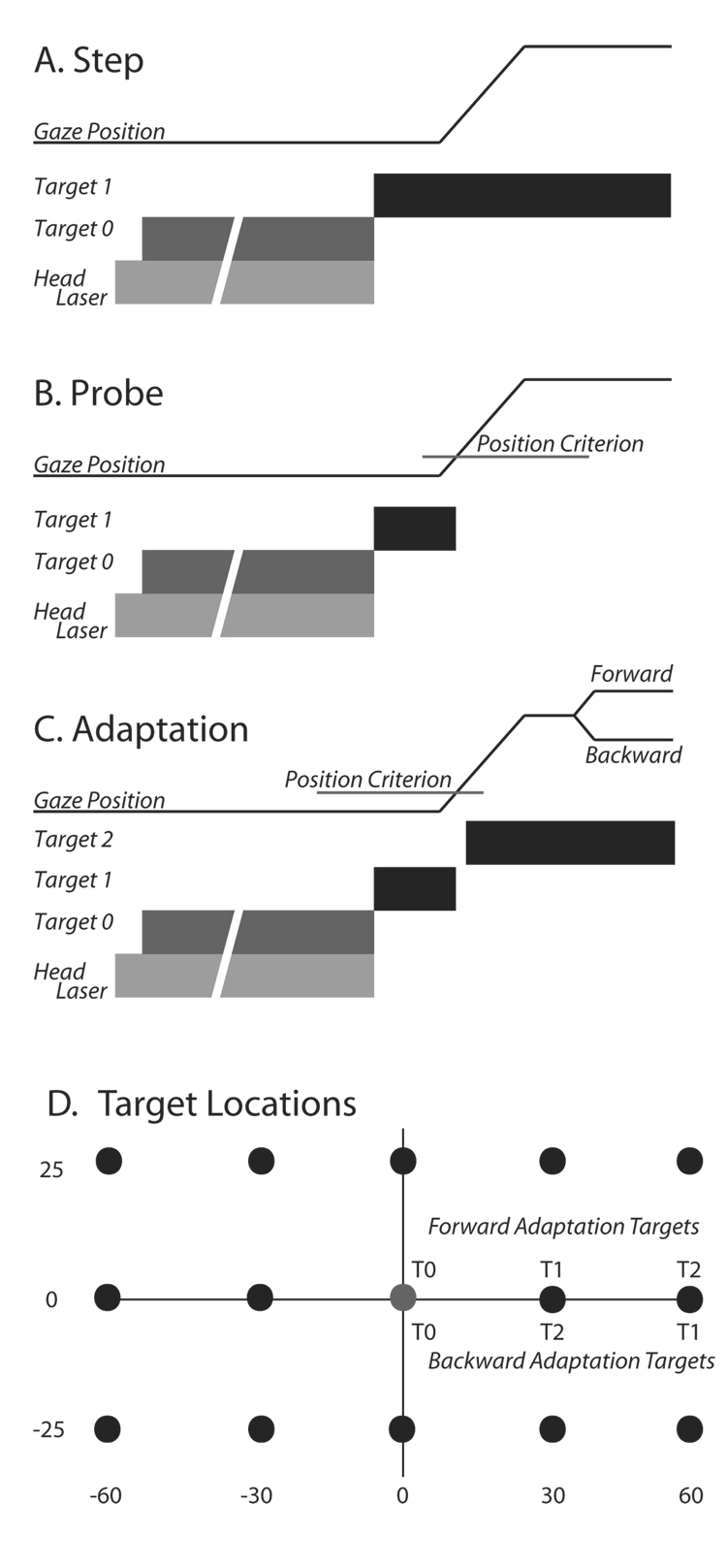
Subjects performed 3 tasks that each began with the illumination of one of the 3 head mounted lasers. This was followed by illumination of an initial fixation target (T0). Subjects were required to align the head-mounted laser spot with T0 and simultaneously fixate T0. T0 was typically located straight ahead of the subject (target location 0,0). If the central head-mounted laser was lit, the eyes and head began aligned in the straight-ahead position. If one of the other lasers was illuminated, in order to align the head-mounted spot with T0, subjects needed to rotate their heads 30° to the right or left of straight-ahead while keeping line of sight directed at T0. Thus, initial positions of the eyes in the orbits could be zero (aligned with the head) or 30° to the left or right while the initial direction of gaze was constant. Subjects were required to look at and align the head-mounted laser spot on T0 for between 600 and 1600 ms (200 ms increments). If either the line of sight or head position deviated beyond a computer defined window (6° radius) trials were aborted. Although the acceptance window was fairly large, alert subjects generally aligned the head-mounted laser and line of sight within ~2° of the illuminated target. If these conditions were met, the head-mounted laser and T0 were extinguished and a target in a new location (T1) was simultaneously illuminated. To this point all trial types were identical. During “Step” trials (Fig. 1A), T1 remained illuminated, and subjects were required to fixate this new target and maintain fixation within a computer defined window for a variable interval (1500–2000ms). During “Probe” trials (Fig. 1B), T1 remained illuminated until the position of the line of sight exited the computer window centered on the location of the no longer visible T0. T1 was never re-illuminated during probe trials; no visual feedback was available on these trials. Probe trials were used to reveal the current state of the adaptation process without potentially confounding sensory inputs that could arise from the presence of visual targets during movements, and/or the visual error at the end of primary movements.
Figure 1.
A–C: Schematic diagrams of trial types used. In each panel, gaze position is represented by a thin black line and plotted as a function of time. Below this trace, targets are represented by thick bars indicating when within the trial each target was illuminated and extinguished. In all case, trials began with the illumination of a head-mounted laser (light gray bar) followed by presentation of T0. If behavioral contingencies were satisfied, the head-mounted laser and T0 target were extinguished and a second target (T1) was simultaneously illuminated at one of the spatial locations shown in panel D (black circles). A: The T1 target remained illuminated for the duration of the “target step” trials. B: During the “probe” trials, the T1 target was extinguished when the line of sight moved beyond a computer defined window (“position criterion”) surrounding T0. C: “Adaptation” trials were similar to probe trials until 20ms after the position criterion was satisfied, at which time T1 was turned off and a target at location T2 was illuminated. D: target locations used during adaptation and probe trials.
“Adaptation” trials (Fig. 1C) were similar to probe trials except that 20 ms after T1 was extinguished, a target (T2) in a new location was illuminated (i.e. the target was relocated from the T1 to the T2 location with a 20ms blank period inserted before T2 illumination). The new location could be further away from (forward adaptation) or closer to (backward adaptation) T0. Targets at 14 locations (Fig. 1D) were used for probe and step trials. On any particular Probe trial, the position of T1 was randomly selected from these possible locations. On every step, adaptation, and probe trial T0 was located at (0,0). During forward adaptation T1 could be located at (30, 0) or (−30,0) and T2 could be located at (60,0) or (−60,0). During backward adaptation experiments, T1 could be located at (60,0) or (−60,0), and T2 at (30,0) or (−30,0). The location of “adaptation targets” systematically alternated (left or right) when successive recording sessions were separated by 5 days or less, except for one forward experiment (M2) and two backward experiments (I2 and H3). For illustrative purposes, all targets and data are discussed as if gaze shifts were directed to the right.
Subjects were instructed to “fixate the peripheral target as rapidly and accurately as possible”. No specific instructions were given regarding how or whether they should move their heads. However, subjects were instructed to minimize movements of their shoulders and to maintain an upright posture during the session. No feedback was provided concerning their performance at any point either during or after the experimental session. Before adaptation trials were presented subjects performed probe and step trials to all 14 possible target locations. This pre-adaptation portion of the experimental session was followed by the “adaptation” phase. During this portion of the session, probe trials to all 14 possible locations continued to be presented and these were randomly interspersed with adaptation trials in either the forward or backward configuration. Adaptation and probe trials were typically presented in a 3:1 ratio. Subjects performed between 77–187 adaptation trials per session (mean = 126.8±27).
Data Acquisition and Analysis
Horizontal and vertical gaze and head position signals were filtered to remove the magnetic field carrier frequencies and were digitized at 1kHz. All behavioral contingencies were accomplished in real-time with 1 ms resolution using custom software. Eye positions relative to the head were approximated by subtracting head from gaze position data. Data were analyzed off-line using Matlab (Natick, MA). Gaze beginning and end were defined using velocity criteria (60°/s onset; 35°/s offset); saccade amplitude was defined as the change in eye position that occurred between gaze onset and offset; head movement beginning and end were defined using 25°/s and 15°/s velocity criterion respectively. Movement amplitudes were defined as the change in position from the beginning to end of movements. Head contribution was defined as the change in head position that occurred during the gaze shift.
We compared the amplitudes of gaze, eye and head movements before and after adaptation. Means (±SD) were calculated using the last 5 probe trials to T1 prior to the introduction of adaptation trials (“pre-adaptation mean”) and these were quantitatively compared to the last 5 adaptation trials (“post-adaptation mean”). Probe trials during the adaptation phase are presented for qualitative comparison only (see Results section for details). When calculating mean differences between pre- and post-adaptation means, errors were propagated using the following formula: σdiff = srt(σpre2 + σpost2), where σpre represents the standard deviation of the preadaptation block and σpost the standard deviation of the post adaptation block.
Unless otherwise noted, comparisons of means were made using a two-tailed student’s t-test, significance was determined using the criterion p < 0.05.
Results
We measured 11,150 gaze shifts made during step, probe, and adaptation trials collected from 10 subjects in a total of 40 experimental sessions. Eighteen of the 40 sessions were “forward adaptation” sessions; the remaining 22 were “backward adaptation” sessions. It was immediately clear that using the McLaughlin task resulted in adaptation of gaze amplitude in human subjects free to move their heads. Each of our subjects showed clear adaptation of gaze amplitude, however, adaptation did not occur to the same extent during every experimental session. A statistically significant change in primary gaze amplitude occurred during 16 of 18 forward adaptation and 19 of 22 backward adaptation sessions. It is unclear why some subjects adapted during a particular session, but not another using the same stimuli. However, similar inter-and intra-subject variability have been reported during head-restrained, saccadic adaptation (e.g. monkey: Straube et al, 1997; human: Frens & van Opstal, 1994). Note also that during most experiments (exceptions are experiments A1, C1 and all “transfer” experiments) the onset of the each trial began with the random illumination of one of the three head lasers (see methods). Quantified data from a particular eye position (“Left”, “Centered”, or “Right”) presented below are from experiments in which there were a minimum of 5 pre-adaptation probe trials to the T1 used during adaptation trials and 5 post-adaptation trials from that eye position.
Eyes and Head Aligned – Forward Adaptation
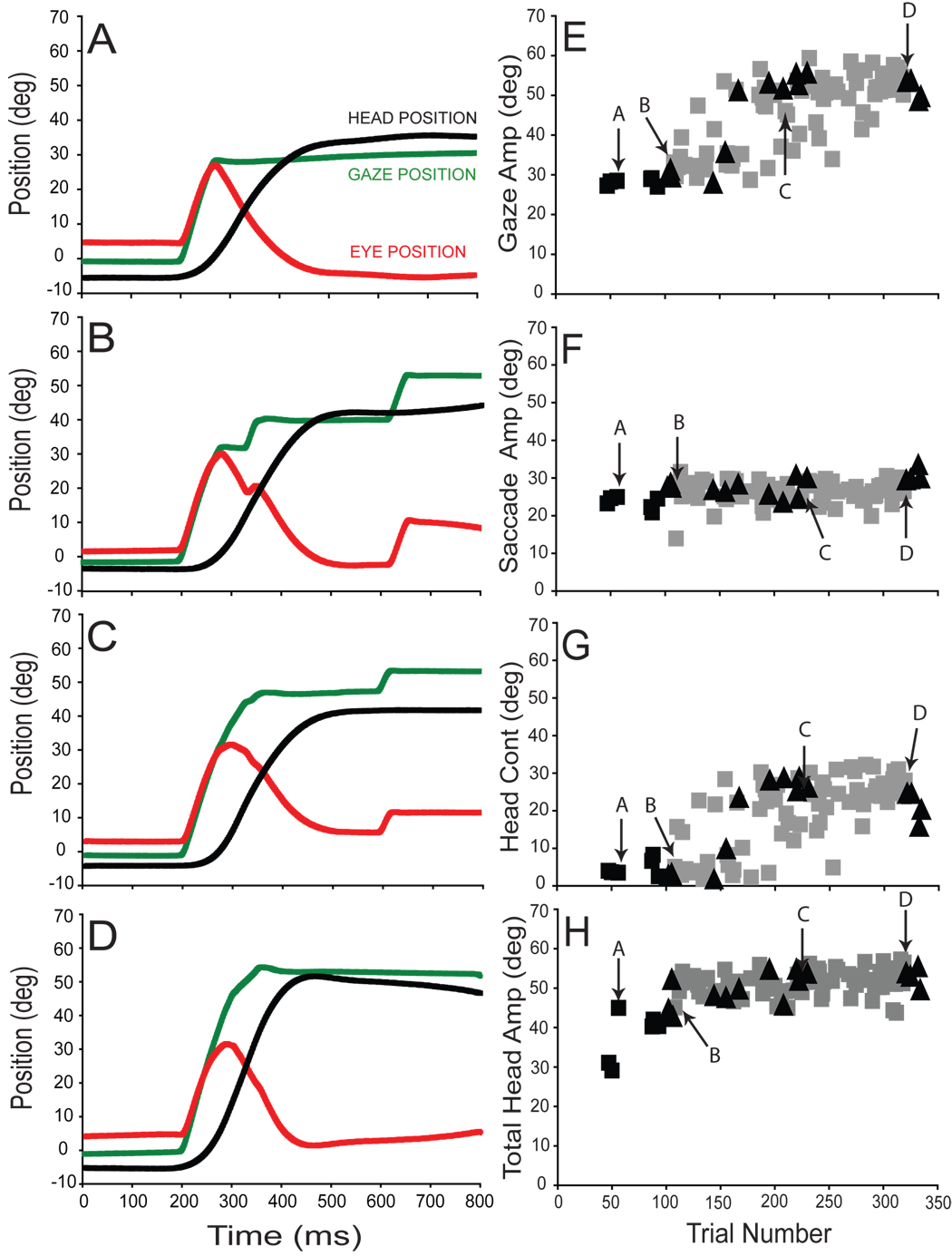
The amplitude of gaze shifts made in response to presentation of T1 increased for each subject during the adaptation phase of forward adaptation experiments. In panels 2A-D gaze (green), eye (red), and head (black) positions are plotted as functions of time for typical gaze shifts made by subject A during forward adaptation (session A7). The initial target displacement (T1−T0) was 30° in each case and movements began after subjects aligned the eyes and head (accomplished by aligning the central head-mounted laser with T0). Average eye position at the onset of movements was 1.5±2.1°.
Figure 2A illustrates a gaze shift made during a pre-adaptation probe trial. The 28.5° gaze shift in this example was accomplished by combining a 25.0° saccadic eye movement with a head contribution of 3.5°. The head moved a total of 45.0° on this trial; most of this large head movement occurred after the line of sight was already directed toward T1. The vestibuloocular reflex allowed gaze to remain steady at this location during the ongoing head movement. In Fig. 2B, the first adaptation trial of the adaptation session is shown. The primary gaze shift in this example is similar to that shown in 2A. The 33.1° gaze shift was accomplished by a large saccade (28.0°) associated with a small head contribution (5.1°). However, the primary gaze shift was followed by two “corrective” gaze shifts which re-directed the line of sight so that the subject’s final gaze position was closer to T2 (T2−T0 = 60°).
Figure 2.
Forward adaptation session (A7) with the eyes and head aligned. Panels A–D plot gaze (green), eye (red) and head (black) positions observed at specific times during the adaptation. Primary gaze (E), saccade (F), head contribution (G) and total head (H) movement amplitudes are plotted as functions of trial number. Pre-adaptation probe trials (black squares), probe trials during the adaptation phase (black triangles), and adaptation trials (grey squares) are superimposed for direct comparison. Labeled arrows indicate trials presented in panels A–D.
In a later adaptation trial (Fig. 2C), the primary gaze shift (45.2°) was significantly larger than during pre-adaptation trials (2A). Note that the amplitude of the eye movement (28.4°) was similar to that seen during the movements in 2A and B. However, the head contribution increased substantially to 16.8°. This gaze shift was followed after a latency of more than 250ms, by a corrective movement. The primary gaze shift in the final adaptation trial of this session was 54.6° (2D). Saccade amplitude was 26.7° and the contribution of the head was 26.6°. Over the course of adaptation during this example session, eye movement amplitude increased only slightly from 25.0° to 28.4°. The observed 26° increase in gaze amplitude resulted from a dramatic increase in head contribution (from 3.5° to 26.6°).
For experimental session A7, changes in primary gaze, eye, head contribution, and total head movement amplitudes are summarized in panels 2E–H. Arrows in E–H indicate amplitude measurements from the individual trials illustrated in panels 2A–D. As shown, gaze amplitude and head contribution increased gradually as more adaptation trials were performed (gray squares in panels 2E & 2G). Total head movement also increase throughout the adaptation session (2H). In contrast, the amplitude of eye movements was relatively unchanged throughout the session (compare pre-adaptation (black) and peri-adaptation (gray) trials in Fig. 2F). During probe trials to T1, interleaved during adaptation session A7, the amplitudes of gaze, eye and head movements were indistinguishable from those seen during adaptation trials (compare gray squares and black triangles in panels 2E–H). Recall that probe trials are identical to adaptation trials except that the T2 target is never illuminated; no visual error is presented after the primary gaze shift. During probe trials a visual stimulus identical to that presented before adaptation evokes an altered motor output, revealing the current state of the adaptation process without potentially confounding sensory inputs that could arise from the presence of visual targets during movements, and/or the visual error at the end of primary movements.
Eyes and Head Aligned – Backward Adaptation
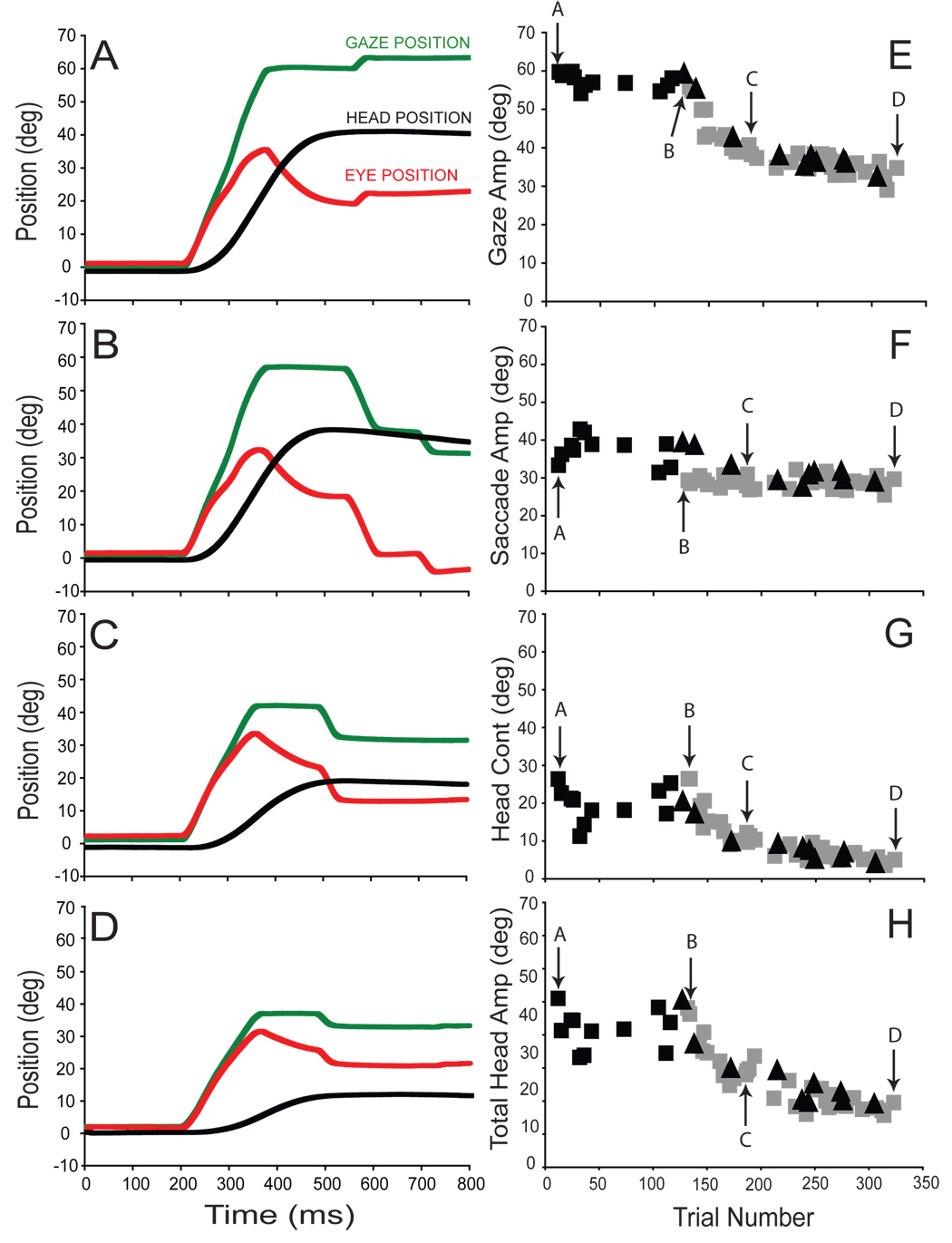
The amplitude of primary gaze shifts made towards T1 decreased for each subject during the adaptation phase of backward adaptation experiments. In panels 3A–D, gaze (green), eye (red), and head (black) positions are plotted as functions of time for typical gaze shifts made by subject H during backward adaptation (session H2). The initial target displacement (T1−T0) was 60° in each case and the average eye position at the onset of these movements was 1.5±0.6°.
Figure 3A illustrates a pre-adaptation trial in which primary gaze amplitude was 59.7°, saccade amplitude was 33.3°, and head contribution was 26.4°. The first adaptation trial (3B) was similar to pre-adaptation trials (gaze = 55.8°, eye = 29.3°, head contribution = 26.5°), the one notable difference being the two “corrective” saccades that followed the primary gaze shift by ~250ms. These secondary movements reduced the visual error introduced by shifting the target from the T1 to the T2 location (T2−T0 = 30°). Panels C and D illustrate trials part way through and at the end of the backward adaptation session. As shown, gaze amplitude significantly decreased over the course of this adaptation session. This gaze amplitude reduction was mediated primarily by a reduction in head contribution; whereas eye amplitude was reduced only slightly over the course of adaptation.
Figure 3.
Backward adaptation with the eyes and head aligned; experimental session (H2). Layout is identical to Fig. 2.
For this adaptation session, changes in gaze, eye, head contribution and total head movement amplitude are summarized in panels 3E–H. In this example, gaze amplitude clearly declined as the subject was increasingly exposed to adaptation trials (3E). The decrease in gaze amplitude was mediated largely by a decrease in the head contribution (3G). Total head amplitude declined concomitantly (3H). As previously noted for forward adaptation, the subject’s behavior during probe trials (to the T1 target location) strongly resembled behavior during adaptation trials throughout the session (compare gray squares with black triangles in panels 3E–H).
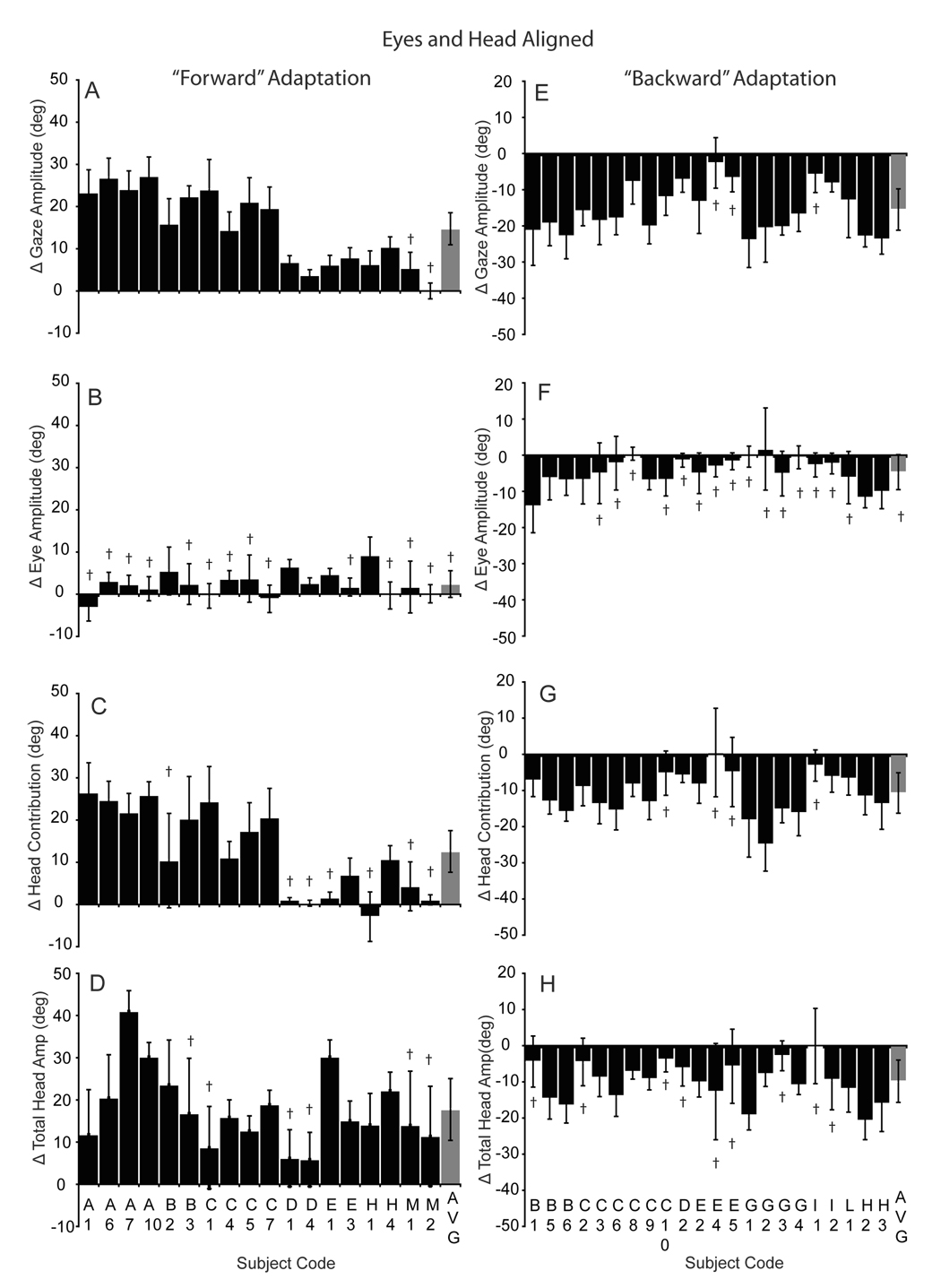
The illustrated forward (Fig 2) and backward (Fig 3) adaptation sessions demonstrate the general tendency of subjects to alter gaze amplitude during adaptation by changing the head contribution to the gaze shift when the eyes began centered in the orbits. The histograms in figure 4 plot the differences in gaze, eye, head contribution and total head amplitude before and after adaptation, during all forward (A–D) and backward (E–H) sessions initiated with the eyes and head aligned. Positive values indicate an increase in amplitude and negative values indicate a decrease. As shown, gaze amplitude increased significantly in 16 of 18 forward adaptation experiments (4A). However, there was variability within and across subjects; only one of our ten subjects failed to adapt significantly during these sessions (subject M). The average increase in gaze amplitude for all subjects (gray bar in 4A), including subject M, was 14.8±3.8° (range: 0 to 26.8°). Head contribution increased significantly in 11 sessions (range: −2.9 to 26.5°), while saccade amplitude increased significantly in only 5 sessions (range:−3.2 to 9.2°). Significant saccade amplitude increases associated with an increase in primary gaze amplitude tended to occur when gaze amplitude changed by less than 10° (D1, D4, E1, H1). On average, saccade amplitude did not change, whereas head contribution and total head movement increased significantly for the population (gray bars, 4B–D).
Figure 4.
Histograms summarizing adaptation-induced changes in amplitude during forward (A–D) and backward (E–H) adaptation with the eyes and head aligned. Each histogram bar indicates the mean (±SD) change in gaze (A, E), eye (B,F), head contribution (C,G) and total head movement amplitude (D, H) comparing the last 5 pre-adaptation trials with the last 5 adaptation trials in each session. Session/subject identifiers are listed on the abscissa. The group means (±SD) are indicated with the gray bar on the right of each panel. † indicates non-significant change in amplitude (p>0.05; two-tailed t-test); all other differences were statistically significant.
Gaze amplitude decreased significantly in 19 of 22 backward adaptation sessions (4E) and for the population (mean: −15.5±5.7; range: −2.6 to −23.9). A decline in gaze amplitude was associated with a significant decrease in head contribution in 15 sessions (4G). Total head movement also decreased significantly in 13 sessions and for the population (4H). There was a statistically significant decline in the saccadic component amplitude associated with the primary gaze shift in only 5 sessions (4F). Decreases in saccade amplitude occurred when gaze amplitude decreased by more than 15° (e.g. B1, B5, B6, H2, H3).
Eyes and Head Not Aligned
In the previous section, we described changes in gaze, eye, and head movements during forward and backward adaptation. At the beginning of these gaze shifts, the eyes and head were aligned. Data indicate that under these conditions, large changes in gaze amplitude induced during adaptation were not a result of dramatic changes in saccadic eye movement amplitude. Instead, changes in the degree to which the head contributed to gaze shifts accounted for most of the changes in gaze shift amplitude. One plausible hypothesis that can account for these data is that during head-unrestrained adaptation, the head command signal is altered. If this were the case, during forward adaptation, the visual error at the end of the primary gaze shift would lead to an increase in the amplitude of the head movement. As the amplitude of head movements and more importantly head contribution to the gaze shift increased over the course of many such trials, gaze amplitudes would also increase resulting in data similar to that described above. Alternatively, adaptive changes in gaze amplitude could result from visual-error-induced changes in the gaze shift command. If this were the case, gaze command changes would lead to increased (or decreased) gaze shift amplitude and the observed changes in head contribution would be a consequence of larger (or smaller) gaze shifts initiated with the eyes and head aligned. Put another way, the relative contributions of the eyes and head to gaze shifts altered by adaptation would be predictable based only on knowledge of the (new) amplitude of the gaze shift and the initial positions of the eyes.
For completeness, we mention a third alternative: that the adaptation process alters only a saccade amplitude command. This hypothesis, however, can be rejected based on the observations described above: gaze amplitude changes occur with little or no changes in saccade amplitude.
The alternative hypotheses outlined above make differential predictions about the amplitudes of gaze, eye and head movements during adaptation under conditions in which the eyes and head are initially not aligned. For example, consider a backward adaptation experiment in which T1 is 60° to the right of the initial fixation location and T2 is 30° also to the right of the initial fixation target. If the eyes began deviated 30° to the left in the orbits one would expect 60° gaze shifts to have a relatively small head contribution (in order to be explicit in the following argument assume the head contribution under these conditions would be 10°). On the other hand if the eyes began deviated in the orbits 30° to the right, the same 60° gaze shift would require a large head contribution (for example, 50°). During backward adaptation gaze amplitude would be expected to decline toward 30°. Because the head contribution during movements made when the eyes begin deviated to the left is small (only 10°), the hypothesis that adaptation alters only the head movement command predicts that gaze amplitude will be reduced only slightly; it could be reduced only by the amount that the head initially contributed – 10°. In contrast, given the same visual stimuli, if the eyes began deviated 30° to the right, changes in head contribution could result in gaze amplitude changes up to 50°. In this example T2 is only 30° from T1, and we would predict at most a 30° change in gaze amplitude mediated by a 30° change in head contribution. The alternative hypothesis that adaptive changes result from changes in gaze command signals predicts that gaze amplitude will be reduced by approximately 30° during backward adaptation regardless of the positions of the eyes in the orbits. As a result when the eyes begin 30° to the left, gaze amplitude changes will result from minor changes in head contribution and larger changes in saccade amplitude, whereas, when the eyes begin deviated to the right, the changes in gaze amplitude will result largely from changes in head contribution. After adaptation, the relative amplitudes of the eyes and head are predicted to be appropriate for the altered amplitude of the gaze shift given the initial conditions of each movement. Again, to be specific, eye and head contributions after adaptation should be similar to that observed during pre-adaptation control trials matched for gaze amplitude and initial eye position.
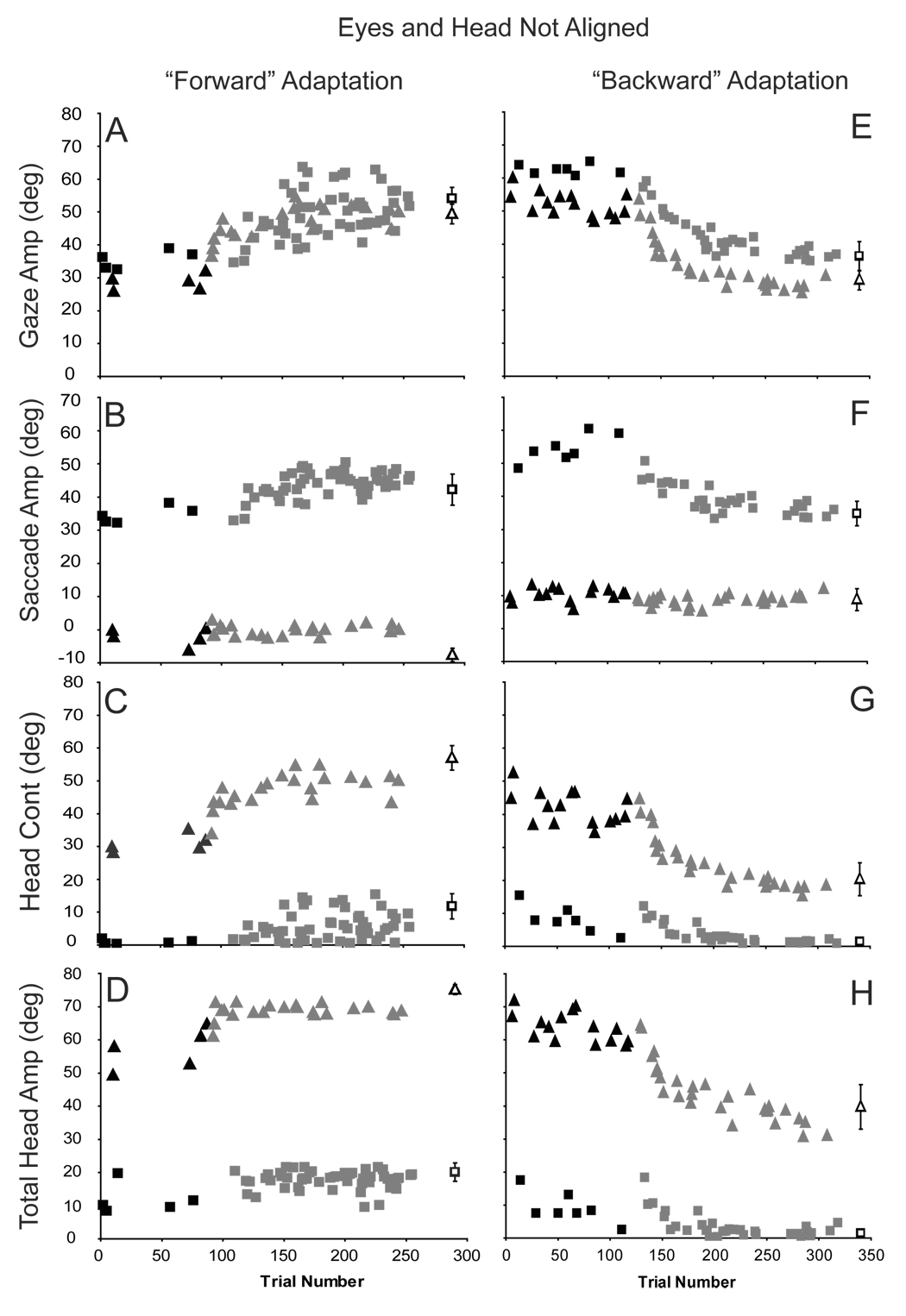
Using the 3 head-mounted lasers (see Methods) we were able to collect enough data to test the predictions of these alternatives in 6 of our 10 original subjects. The results of one forward adaptation session are illustrated in Fig. 5 (A–D). During this session, trials could be initiated with the eyes and head aligned or the eyes deviated in the orbits 30° to the left or to the right of center (on randomly interleaved trials). In panel 5A which plots the amplitude of gaze shifts as a function of trial number, squares represent movements initiated with the eyes deviated to the left (mean initial position = −27.9 ±0.7°), and triangles illustrate movements initiated with the eyes deviated to the right (mean initial position = 29.5 ±0.9°). Before adaptation trials were presented (black filled symbols) there were small differences in gaze shift amplitudes when comparing movements initiated from the drastically different initial eye positions; gaze shifts made when the eyes were nearly 30° to the right were systematically smaller (in the example ~7°) than those initiated with the eyes deviated to the left. Nonetheless, gaze amplitude increased consistently for both sets of movements during adaptation (gray symbols).
Figure 5.
Primary gaze (A,E), eye (B,F), head contribution (C,G) and total head amplitude (D,H) as a function of trial number for forward (C7) and backward (H2) adaptation sessions when the head and eyes were not aligned. Squares indicate data from gaze shifts made with the eyes initially deviated to the left. Triangles indicate movements made with the eyes initially deviated to the right. Black filled symbols are pre-adaptation trials; gray filled symbols are adaptation trials. Unfilled symbols show the mean (±SD) amplitudes during pre-adaptation trials made directly to the T2 target.
For comparison, this panel also presents mean (±SD) gaze shift amplitudes (black unfilled symbols) during pre-adaptation trials made directly to the T2 target location. Before adaptation with the eyes initially deviated in the orbits to the right (triangles) or to the left (squares), the amplitudes of gaze shifts made in response to presentation of the T2 target were indistinguishable from the amplitudes of post-adaptation gaze shifts made in response to presentation of the T1
When the eyes began deviated in the orbits to the left (Fig. 5B, black squares), saccade amplitude was approximately equal to gaze shift amplitude during pre-adaptation gaze shifts to T1. However, when the eyes were deviated to the right, saccade amplitudes during gaze shifts of similar amplitudes were quite small (5B, black triangles). In contrast, during pre-adaptation trials, when the eyes were deviated to the left, head contribution (5C) and total head movement amplitude (5D) were small whereas they were large when the eyes began deviated to the right (5C and D – black filled triangles). It is important to note that the relative contributions of the eyes and head after adaptation were quite similar to the relative contributions seen during amplitude-matched gaze shifts made before adaptation. In 5B–D, for example, only small differences were observed in saccade amplitude, head contribution, and total head movement amplitudes made with the eyes deviated either to the right (unfilled triangles) of left (unfilled squares) when gaze shift amplitudes were similar to post-adaptation movements.
An example of backward adaptation during gaze shifts initiated with the eyes in different orbital positions is illustrated in panels 5E–H. During backward adaptation, gaze shift amplitude declined systematically. This reduction in gaze amplitude was mediated primarily by a reduction in saccade amplitude when the eyes began deviated to the left, but was mediated primarily by a reduction in head contribution when the eyes began deviated to the right. Similar observations were made for all subjects who participated in data collection sessions in which the eyes and head were not initially aligned.
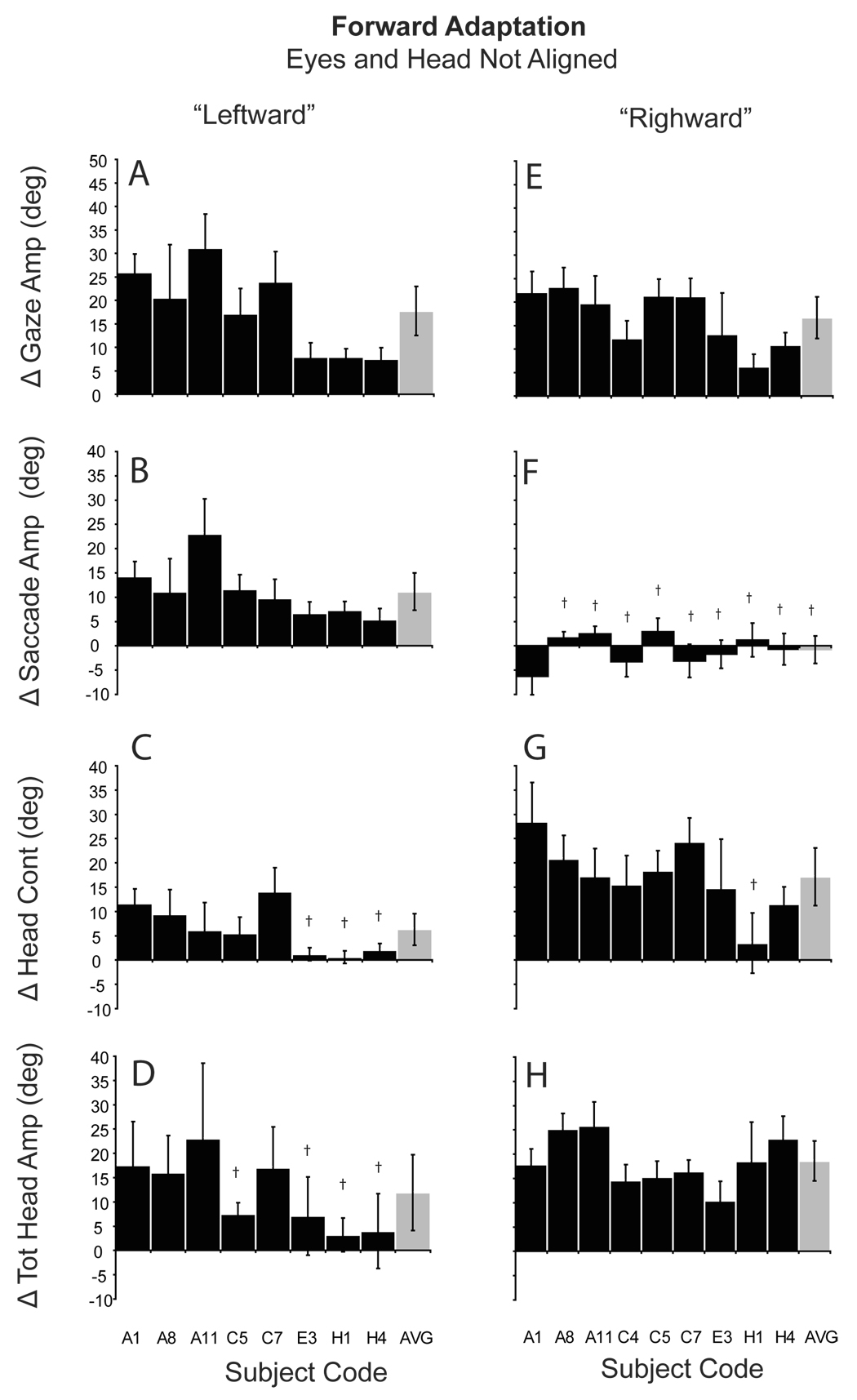
The hypothesis that adaptation occurs by alteration of a gaze command signal predicts that an increase in gaze amplitude during forward adaptation from the leftward eye position will result from primarily a change in saccade amplitude. Conversely, an increase in gaze amplitude when the gaze shift is initiated from the rightward eye position will result from an increase in head contribution. Figure 6 (A–D) illustrates the change in gaze, eye, head contribution, and total head movement amplitude during forward adaptation when gaze shifts were initiated with the eyes deviated to the left. Black bars in Fig. 6 represent means (±SD) from individual experiments and gray bars are population means. In each case the T1 target was 30° to the right of fixation and the T2 target was 60° to the right of initial fixation (30° further than the T1 location). As shown, with the eyes initially deviated to the left in the orbits, gaze amplitude increased during all sessions. During some sessions amplitude increased by more than 20° (A1, A8, A11, C5 and C7). However, there was inter- and intra-subject variability and during other similar sessions gaze amplitude increased by less than 10° (H4). Across sessions gaze amplitude increased by 17.7° (± 5.2). As shown in panel 7B, during gaze amplitude increases, when the eyes began deviated to the left, saccade amplitude increased by 11.2°. In contrast, the head contribution to gaze shifts (6C) increased only slightly (6.3°). For comparison, Fig. 6 (E−H) plots the results of forward adaptation when the eyes began deviated to the right. Under these conditions gaze shift amplitude increased by 16.6° (±4.4). This change in gaze amplitude was not different than the increase observed when the eyes began deviated to the left (t-test, p > 0.1). However, this change in gaze amplitude was not mediated by a large increase in saccade amplitude as seen with leftward deviation. As illustrated (6F), eye movement amplitude was essentially unchanged during these forward adaptation sessions. The large change in gaze amplitude was mediated by a large increase (17.2°) in head contribution (6G).
Figure 6.
Amplitude changes during forward adaptation when the eyes and head were not aligned. Histograms show the mean (±SD) difference between the last 5 adaptation trials and the last 5 pre-adaptation probe trials for gaze (A, E), saccade (B, F), head contribution (C, G), and total head amplitude (D, H). A–D: eyes initially deviated to the left; E–F: eyes initially deviated to the right. Gray bars indicate the group means (±SD). Subject/session codes indicated along the abscissa. † indicates non-significant change in amplitude (p>0.05; two-tailed t-test).
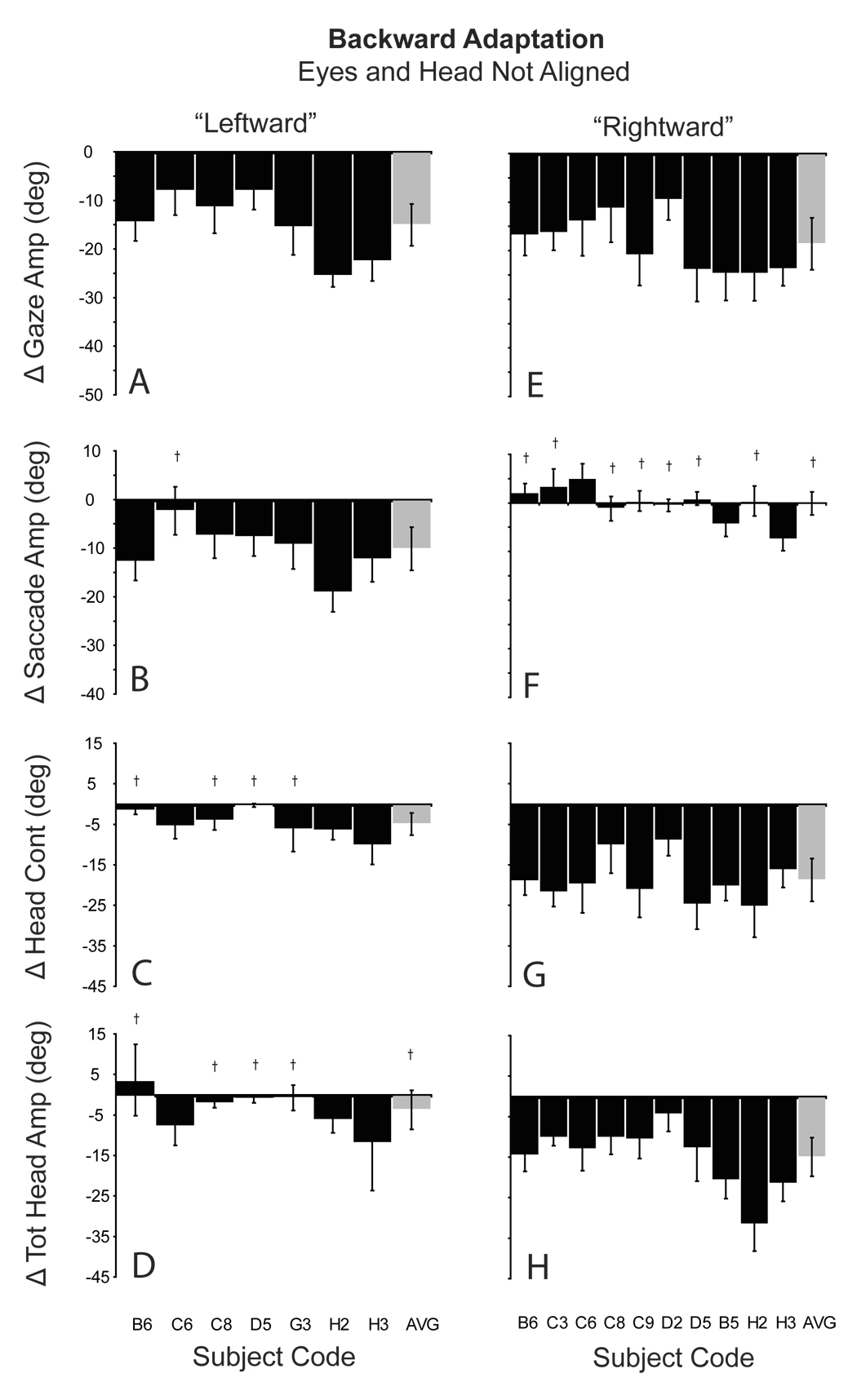
During backward adaptation with the eyes initially deviated to the left (Fig. 7A–D), gaze amplitude was reduced by 15.1° (±4.3). This reduction was caused by a significant reduction (10.1°) in the saccadic component of gaze shifts (7B), and a small decrease (4.9°) in head contribution (7C). When the eyes began deviated to the right, backward adaptation resulted in an 18.6° reduction in gaze amplitude. However, this reduction was not the result of a reduction in the amplitude of the saccadic portion of the movement. On average, saccade amplitude changed 0.1°. The large change in gaze amplitude occurred because of a large decrease (18.7°) in head contribution (7G).
Figure 7.
Amplitude changes during backward adaptation when the eyes and head were not aligned. Histograms show the mean (±SD) difference between the last 5 adaptation trials and the last 5 pre-adaptation probe trials for gaze (A, E), saccade (B, F), head contribution (C, G), and total head amplitude (D, H). A–D: eyes initially deviated to the left; E–F: eyes initially deviated to the right. Gray bars indicate the group means (±SD). Subject/session codes indicated along the abscissa. † indicates non-significant change in amplitude (p>0.05; two-tailed t-test).
To summarize, during adaptation, changes in gaze shift amplitude were similar regardless of the initial positions of the eyes. However, these large changes in gaze amplitude were accomplished via large changes in head contribution when the eyes were deviated to the right (head deviated away from T1), and via changes in saccade amplitude when the eyes began deviated to the left (head deviated towards T1). Changes in head contribution and saccade amplitude depended not on the adaptation process directly, but instead on the position of the eyes in the orbits at the onset of gaze shifts.
Gaze Adaptation Transfer Experiments
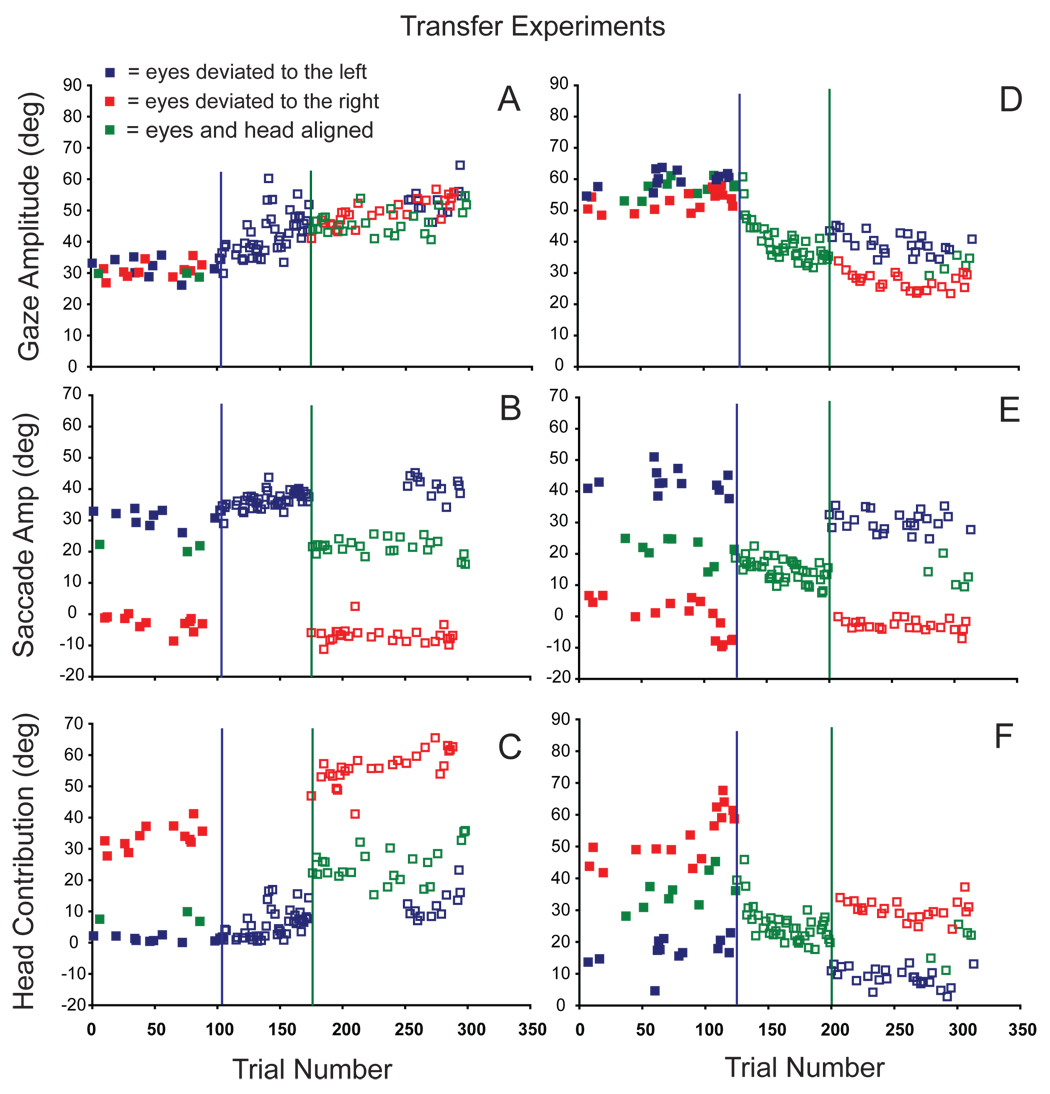
Alahyane and Pellisson (2004) demonstrated that forward and backward adaptation can occur simultaneously by driving forward adaptation when the eyes are initially deviated upwards and backward adaptation from downward eye positions. It is possible that data described above, illustrating that the amplitudes of eye and head movements were altered during adaptation in an initial-eye-position-dependent manner, could be due to a similar “context cue” effect. We tested this possibility by having a subset of our subjects (N=5) participate in gaze transfer experiments. During the pre-adaptation phase, three head-mounted lasers were used to elicit control gaze shifts initiated from three different initial eye positions. Then adaptation was induced with the eyes in one particular position, for example, with the eyes deviated 30° to the left. After the subject performed ~75–100 trials under these conditions, gaze shifts were made with the eyes in different (non-adapted) initial positions. Note that the subject may have still been adapting to the intra-gaze target displacement at the time that the new initial eye positions were introduced. Therefore, after ~75–100 gaze shifts initiated from the novel eye positions, the original eye position was often reintroduced. Two examples of transfer experiments are shown in Figure 8 which plots gaze (A), eye (B), and head contribution (C) amplitudes as functions of trial number during pre-adaptation probe (closed squares) and adaptation (open squares) trials. During this forward adaptation transfer experiment (C7) the eyes could be deviated to the left (blue), to the right (red), or aligned with the head (green). Regardless of initial eye position, the average pre-adaptation gaze amplitude was ~31° (filled symbols 8A). Different combinations of eye and head movements were used to accomplish these ~31° gaze shifts. As shown in 8B and C, when the eyes and head were aligned (green) head contributions were small (~10°), whereas when the eyes began deviated in the orbits to the right (in the direction of the ensuing gaze shifts), head contributions were large and accounted for nearly the entire gaze shift amplitude (saccade amplitudes were near 0° during these movements).
Figure 8.
During forward (A–C; experiment C7) and backward (D–F; experiment H3) adaptation, changes in gaze (A, D), eye (B, E) and head contribution (C, F) amplitudes are plotted as functions of trial number. Filled symbols illustrated movement amplitudes directed to T1 target locations during pre-adaptation trials when the eyes began either aligned with the head (green), or deviated in the orbits to the right (red), or left (blue). After ~100 trials adaptation began (blue vertical lines); adaptation trials were initiated with the eyes in one particular position (deviated to the left: A–C; aligned with the head: D–F). After ~75 adaptation trials (unfilled symbols) under these conditions, adaptation continued with the eyes in new (non-adapted) initial positions (green vertical lines). After an additional 100 adaptation trials initiated from these new eye positions, the original initial eye position was re-introduced.
After ~100 pre-adaptation control trials, adaptation trials were introduced (vertical blue line in 8A–C), and during the first 75 adaptation trials the eyes were always deviated to the left at the beginning of gaze shifts (blue unfilled symbols). As shown, gaze amplitude gradually increased from 30.9° ±3.6 to 46.2° ±3.5 during adaptation. The gaze amplitude increase was mediated by a significant increase in saccade amplitude (30.0° ±2.8 to 37.5° ±1.5) and head contribution (0.8° ±1.0 to 8.8° ±3.2).
After these initial adaptation trials, adaptation trials continued with the eyes either deviated to the right (red unfilled squares) or centered in the orbits (green unfilled squares). As clearly illustrated in 8A, the amplitude of gaze shifts made from initial eye positions not used during adaptation, were similar in amplitude to those made near the end of the primary adaptation. Comparing the amplitude of the last 5 adaptation trials made from the initial eye position (in this case with the eyes deviated to the left) with the first 5 trials after introducing the two new initial eye positions revealed no significant change in gaze amplitude (p > 0.05 t-test). The degree of adaptation that developed with the eyes in one initial position transferred immediately and completely to gaze shifts initiated with the eyes in different orbital positions.
However, eye amplitude and head contribution to these gaze shifts were markedly different than those observed during similar amplitude gaze shifts at the end of the initial adaptation. At the end of the first 75 adaptation trials, saccade amplitudes were ~40° (unfilled blue squares: 8B). However, immediately after introduction of the novel initial eye positions, saccade amplitudes were markedly different. As shown, saccade amplitudes were near 0° when the eyes were deviated to the right (8B red unfilled squares) and ~20° when the eyes and head were aligned (8B: green unfilled squares). During the initial period of adaptation, saccade amplitude increased ~8° (from ~32° to ~40°). However, even on the first adaptation trials initiated with the eyes in different positions, eye movement amplitudes were very different. In addition, eye movements were not ~8° larger than control saccades made before adaptation from the same starting positions. Changes in the amplitude of eye movements observed during adaptation did not transfer to movements made with the eyes in new initial positions. Similarly, the effects of adaptation on the contribution of the head did not transfer to movements initiated under different conditions.
Figure 8 (D–F) illustrates a similar example during backward adaptation (H3). Like the forward adaptation described above, gaze shifts made from eye positions not used during adaptation were approximately the same amplitudes as gaze shifts made at the end of adaptation. However, the eye and head components of these gaze shifts were not the same as those observed at the end of adaptation. Nor did the changes in eye or head contribution that occurred during adaptation, transfer to movements made under different initial conditions.
The following ratios were used to quantify the degree to which changes in gaze(G), eye (E), and head contribution (HC) observed during the initial adaptation transferred to movement made from novel eye positions:
Gaze ratio = GIEP2 / GIEP1
Eye ratio = EIEP2 / EIEP1
Head contribution ratio = HCIEP2 / HCIEP1
Where IEP1 is the eye position used during initial adaptation and IEP2 indicates the novel eye positions introduced after initial adaptation. Average pre- transfer values were calculated from the last 5 adaptation trials before introducing new eye positions (data just prior to the green vertical line in figure 8). Post-transfer values were calculated from the first 5 adaptation trials after introduction of new positions (data just after the green vertical line in figure 8). Note that there could be more than one post transfer eye position. Table 1 contains the gaze, eye and head ratios for each of our 9 transfer experiments. These ratios were used to calculate the average values detailed below.
Table 1.
Transfer of Gaze, Saccade, and Head Contribution between Pre- and Post Transfer Phases
| Experiment | Condition | Eye Position | Gaze Ratio | Eye Ratio | Head Cont Ratio |
|---|---|---|---|---|---|
| B6 (Backward) | PreTrans | Left | |||
| PostTrans | Center | 0.82 | 0.68 | 9.49 | |
| PostTrans | Right | 0.87 | 0.22 | 41.69 | |
| C6 (Backward) | |||||
| PreTrans | Left | ||||
| PostTrans | Center | 0.89 | 0.48 | 2.32 | |
| PostTrans | Right | 0.84 | −0.15 | 4.31 | |
| C8 (Backward) | |||||
| PreTrans | Left | ||||
| PostTrans | Center | 0.79 | 0.45 | 2.47 | |
| PostTrans | Right | 0.77 | −0.13 | 5.27 | |
| G3 (Backward) | |||||
| PreTrans | Left | ||||
| PostTrans | Center | 1.26 | 0.52 | 5.05 | |
| PostTrans | Right | 1.20 | 0.09 | 6.84 | |
| C7 (Forward) | |||||
| PreTrans | Left | ||||
| PostTrans | Center | 1.02 | 0.57 | 2.80 | |
| PostTrans | Right | 0.97 | −0.21 | 6.02 | |
| E3 (Forward) | |||||
| PreTrans | Left | ||||
| PostTrans | Center | 1.06 | 0.87 | 3.45 | |
| PostTrans | Right | 1.03 | 0.31 | 10.28 | |
| C9 (Backward) | |||||
| PreTrans | Left | ||||
| PostTrans | Center | N/A | N/A | N/A | |
| PostTrans | Right | 0.83 | −0.10 | 1.78 | |
| H3 (Backward) | |||||
| PreTrans | Left | ||||
| PostTrans | Center | 1.44 | 2.72 | 0.50 | |
| PostTrans | Right | 0.85 | −0.19 | 1.37 | |
| H4 (Forward) | |||||
| PreTrans | Left | ||||
| PostTrans | Center | 1.12 | 3.53 | 0.28 | |
| PostTrans | Right | 1.06 | 0.41 | 2.80 | |
The hypothesis that adaptation occurs by alteration of a gaze command signal predicts that gaze amplitude before and after transfer should be the same regardless of initial eye position; in other words, the gaze ratio should be close to 1. The mean gaze ratio was 0.99 ±0.19 when combining gaze ratios from all conditions (n=17). No statistically significant difference between forward and backward gaze ratios was observed (p>0.05). This suggests that the degree of adaptation that developed with the eyes in one initial position transferred nearly completely to gaze shifts initiated with the eyes in different orbital positions.
A second prediction of the gaze hypothesis is that the changes in eye and head movement amplitude observed during the initial adaptation trials will not transfer to movements made from novel eye positions; rather the eye and head movement amplitudes will be appropriate for the gaze shifts of the adaptation-modified amplitude initiated from different eye positions. In 6 of 9 transfer experiments (the first 6 experiments listed in Table 1) initial adaptation trials were made when the eyes began deviated to the left (head deviated towards the target; e.g. Figure 8A–C). The mean eye ratios calculated from these experiments were 0.60±0.15 (when IEP2 was with eyes and head aligned) and 0.02±0.21 (when IEP2 was with the eyes deviated to the right). As expected, the saccadic eye movements associated with gaze shifts in the post-transfer phase from the centered and rightward eye positions were considerably smaller than those initiated from the leftward eye position in the post transfer phase. Conversely, the mean head contribution ratios were 4.3±2.7 (IEP2: centered) and 12.4±14.5 (IEP: rightward) indicating that the head contribution was much larger when movements were made under these conditions.
In the remaining 3 experiments, initial adaptation trials were produced with the eyes and head aligned (e.g. Figure 8D–F). In these experiments, the eye and head contribution ratios calculated using the leftward eye position (n=3) were 0.04±0.32 and 1.98±0.7 respectively. In contrast, the eye and head contribution ratios calculated using the rightward eye position (n=2) were 3.13±0.6 and 0.40 ±0.15. When adaptation was initially induced from the centered eye position, the saccade and head contribution in the post-transfer phase could be larger or smaller depending on the initial positions of the eyes in the orbits. In summary, although the transfer of gaze amplitude between pre- and post-transfer phases was almost 100%, the saccade and head contributions to gaze shifts were markedly different after gaze transfer and varied in an initial eye position dependent fashion.
Discussion
In animals that have retinal regions with high photoreceptor density (e.g. fovea), maintenance of gaze shift accuracy is critical for extracting high quality visual information from the environment. When the head is allowed to move, gaze shifts are often accomplished by coordinated movements of the eyes and head. Consequently, the adaptive mechanism that maintains gaze accuracy could affect the gaze command signal, or it could alter separately the independent signals driving the eyes and head. The goal of the present study was to describe changes in gaze, eye, and head movement amplitudes during a short-term adaptation task (McLaughlin, 1967) under conditions in which the alternative hypotheses make differential predictions.
Our data indicate that in human subjects with unrestrained heads: 1) large forward or backward target displacements during an ongoing gaze shift can gradually produce large changes in gaze amplitude, and that these changes persist during probe trials when targets are extinguished after gaze shift onset and not re-illuminated. The motor responses to visual stimuli presented in the adapted spatial location are altered during adaptation using the McLaughlin task when the head is free to move. 2) Data were inconsistent with changes to separate eye and/or head movement signals, but consistent with alterations of a gaze shift command. These observations are discussed in detail below.
The magnitude and time course of human saccade amplitude adaptation has been described extensively (for review see: Hopp & Fuchs, 2004). Typically initial target displacements ~10° have been used, and the target back-step (or forward-step) have been between 3–5° (30–50% of the primary movement; e.g. humans: Semmlow, Gauthier, & Vercher, 1987; monkeys: Straube et al, 1997). To our knowledge, the only example of saccade adaptation in which the primary gaze shift was 30° or more is a study in which Phillips and colleagues (1997), using rhesus monkeys as subjects, examined the transfer of head-restrained saccade adaptation to head-unrestrained gaze shifts. In their study, the initial target displacement during saccade adaptation was 50° and the target back-step was 20° (40%). On average, primary saccade amplitude was reduced by 8.4° (range: 5.6–12.4°) or 18.3% (range: 12.8–27.0%). Interestingly, the range of amplitude changes in the Phillips et al. study was similar to values reported by Erkelens and Hullerman (1993), as well as in other human (e.g. Miller, Anstis, & Templeton, 1981) and monkey adaptation studies (e.g. Straube et al., 1997) in which the initial target displacements were much smaller.
In the data presented here, the initial target displacement was either 60° during backward adaptation or 30° during forward adaptation. In both cases the target was moved 30° after the gaze shift was initiated. As a result, during backward adaptation, in order to eliminate the visual error at the end of the movement, gaze shift amplitudes would need to be reduced by half. To eliminate visual error during forward adaptation, movement amplitudes would need to be doubled. On average, our subjects decreased gaze amplitude by ~12° (range: 6–27°) or 20% (range: 10–45%) of the primary movement in response to the 30° back-step during backward adaptation experiments. During forward adaptation, gaze amplitudes increased by ~15° (range: 0–26°) or ~50% (range: 0–87%). Visual error was reduced by ~50% during backward and by ~40% during forward adaptation. These changes in gaze amplitude are of approximately the same magnitude as changes observed when the head is prevented from moving and when target displacements are much smaller. The ability to induce changes in movement amplitude using the McLaughlin task appears to be similar regardless the displacement of the targets, and independent of whether the head is free to move or not.
Prior studies of saccadic adaptation have also shown that primates can exhibit large variation in adaptation magnitude and rate for nearly identical conditions (humans: Erkelens and Hullerman, 1993; Albano and King, 1989; Frens and van Opstal, 1994; Fujita, Amagai, Minakawa, & Aoki, 2002; monkey: Fuchs et al, 1996; Straube et al, 1997). Similar to head restrained saccade adaptation, the magnitude of gaze adaptation in our subjects was highly variable both between (as noted above) and within subjects. For example, in response to a 30° target back-step, subject C decreased primary gaze amplitude between 13° and 24° (see figure 5, figure 7, figure 8). Short-term adaptation under the conditions used in these experiments produced consistent albeit variable changes in gaze shift amplitude. On a day-to-day basis, the test conditions were nearly identical. Despite efforts in this regard, given the same visual stimuli, on some occasions gaze amplitudes changed dramatically whereas during other sessions even with the same subject, adaptation-induced amplitude changes were much smaller. We have no explanation for this variability, although it is similar to the inter- and intra-subject variability observed when the head is restrained, and may represent the variability of the adaptation process using the McLaughlin task.
The data in this report are consistent with the hypothesis that gaze adaptation induced using the McLaughlin task when the head is allowed to move alters the command to change the direction of the line of sight (i.e. a gaze shift command). This conclusion is based on the systematic changes in gaze amplitude observed during both forward and backward adaptation sessions, coupled with the observation that particular changes in gaze amplitude during individual sessions could be mediated solely by changes in head contribution, solely by changes in eye movement amplitude or changes in both eye and head components. The relative contributions of the eyes and head were appropriate in all cases for the amplitude of the executed gaze shift both before and after adaptation, and depended on the starting positions of the eyes in the orbits. Thus, after adaptation had reduced (or increased) gaze amplitude, eye and head components were qualitatively similar to matched amplitude gaze shifts initiated under the same conditions but produced before adaptation. Specific amplitude changes to the eye or head components of gaze shifts were not observed. In addition during the adaptation transfer experiments, after the initial adaptation trials performed from one particular initial eye position, changes in gaze shift amplitude transferred immediately to gaze shifts made from novel (non-adapted) positions. In contrast neither the changes in eye or head movement amplitudes induced during the initial adaptation transferred to movements made when novel eye positions were introduced. These data are inconsistent with changes to eye- or head-specific commands, and indicate changes to a gaze command signal.
In the only previous investigation of head-unrestrained gaze adaptation, Phillips and colleagues (1997), using non-human primate subjects, describe the transfer of gaze amplitude changes induced when the head was prevented from moving to gaze shifts made after the head was released. In their report, reductions in saccade amplitude when the head was restrained transferred to head-unrestrained gaze shifts. Furthermore, the reduction in gaze amplitude (compared to pre-adaptation, head-unrestrained control movements to T1) resulted from changes in both eye and head movement amplitudes. As a result these authors concluded that the adaptation process did not alter saccade-specific signals, rather a gaze signal that drove both eye and head movements.
In a small number of cases (n = 2), these authors also report changes in gaze, eye and head movements produced during head-unrestrained gaze adaptation. The authors concluded that the most straight-forward explanation of these results was that adaptation induced changes to motor commands before gaze signals were separated into eye and head specific commands. However, the alternative, that changes to eye and head specific signals could not be ruled out based on their results.
Due to the very large target displacements used in this study, it is tempting to assume that subjects were consciously aware of the target jump, whereas this explanation has largely been rejected (perhaps prematurely) when small target displacements and head restrained subjects are used. This presumed “awareness” of the target displacement could potentially influence the eye and head movements observed during these trials. We view this account with skepticism, particularly in its attempt to characterize head restrained saccadic adaptation using the McLaughlin task as a “true” adaptation, whereas in this view, head unrestrained adaptation using the same task is tainted by subjects’ conscious choices regarding the amplitude of their eye and head movements. There are several points to be raised in discussion of this issue. First, our conclusion that a gaze command signal is being modified by the altered visual stimuli presented during this task arises from considering the changes in gaze amplitude when the eyes begin in different orbital positions. Our data show that although the gaze shift amplitude has been altered regardless of initial eye position, the relative amplitudes of eye and head movements depend only on the amplitude of the gaze shift that is made, and the starting positions of the eyes in the orbits. When gaze shifts of matched amplitude are made with the eyes in similar starting positions, but not in the context of adaptation, the relative amplitudes of the eyes and head are the same as observed during the adaptation process (Fig. 5). Second, randomly interleaved with adaptation trials are trials in which the T1 target is turned off when the gaze shift begins but the T2 target is not illuminated (Fig. 2 and 3). During these trials there is no displacement of the target during the ongoing gaze shift and therefore any potential “conscious awareness” of the target jump is eliminated. The relative amplitudes of eye and head movements on these trials are nearly identical to that seen during adaptation trials. If the subjects were aware of the target step it did not seem to have an impact on the relative amplitudes of the eye and head movements during adaptation. While it is not possible to rule out explanations for these results based on covert processes that the experiment was not designed to address, such hypotheses have little explanatory value, and apply equally to head unrestrained and head restrained adaptation data using the McLaughlin task. Importantly, the observation that changes in gaze shift amplitude are independent of the relative eye and head movement amplitudes support our conclusions that under the conditions of our experiment a gaze signal is being altered, and this conclusion does not depend upon assumptions regarding the conscious awareness (or lack thereof) of the displacement of the target.
In summary, a large intra-gaze target displacement during human, head-unrestrained gaze shifts towards visual targets can produce large increases or decreases in primary gaze amplitude. Under these conditions, changes in gaze amplitude induced using the McLaughlin task were independent of the amplitudes of eye and/or head movements. This leads to the suggestion that the neural structures responsible for altering the motor output in response to a persistent visual error, are likely structures that encode the redirection of the line of sight (gaze); the superior colliculus seems like a good candidate for having some role in this process (Takeichi et al. 2007). The alternative, that adaptation under these conditions alters eye and/or head specific commands can be rejected based on these data.
Acknowledgements
This work is supported in part by the following grants P30-EY01319, T32-EY07125 (ALC), EY-13239 (EGF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alahyne N, Pellison D. Eye position specificity of saccadic adaptation. Invest Ophthalmol Vis Sci. 2004;45:123–130. doi: 10.1167/iovs.03-0570. [DOI] [PubMed] [Google Scholar]
- Albano J. Adaptive changes in saccade amplitude: oculocentric or orbtiocentric. Vision Res. 1996;36:2087–2098. doi: 10.1016/0042-6989(96)89627-1. [DOI] [PubMed] [Google Scholar]
- Albano J, King W. Rapid adaptation of saccade amplitude in humans and monkeys. Invest. Opthal. Vis. Sci. 1989;30:1883–1893. [PubMed] [Google Scholar]
- Bahill AT, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Math. Biosci. 1975;24:191–204. [Google Scholar]
- Baloh RW, Sills AW, Kumley WE, Honrubia V. Quantiative measurement of saccade amplitude, duration, and velocity. Neurology. 1975;25:1065–1070. doi: 10.1212/wnl.25.11.1065. [DOI] [PubMed] [Google Scholar]
- Becker W. The control of eye movements in the saccadic system. Biblioteca Ophthalmologica. 1972;82:233–243. [PubMed] [Google Scholar]
- Becker W, Fuchs AF. Further properties of the human saccadic system: Eye movements and correction saccadic with and without visual fixation points. Vision Research. 1969;9:1247–1258. doi: 10.1016/0042-6989(69)90112-6. [DOI] [PubMed] [Google Scholar]
- Cecala AL, Freedman EG. Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2005. Amplitude Changes In Response To Target Displacements During Human Eye--Head Movements Program No. 858.13. Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H. Eye- and head movements in freely moving rabbits. J. Physiol. 1977;266:471–498. doi: 10.1113/jphysiol.1977.sp011778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delreux V, Abeele SV, Lefevre P, Roucoux A. Eye-head coordination: influence of eye position on the control of head movement amplitude. In: Paillard J, editor. Brain and Space. London: Oxford Univ. Press; 1991. pp. 38–48. [Google Scholar]
- Deubel H, Wolf W, Hauske G. Adaptive gain control of saccadic eye movements. Human Neurobiology. 1986;5:245–253. [PubMed] [Google Scholar]
- Deubel H. Adaptivity of gain and direction in oblique saccades. In: O’Regan J, Levy-Schoen J, Amsterdam A, editors. Eye Movements: from Physiology to Cognition. Amsterdam: Elsevier; 1987. pp. 181–191. [Google Scholar]
- Erkelens C, Hulleman J. Selective adaptation of internally triggered saccades made to visual targets. Exp. Brain Res. 1993;93:157–164. doi: 10.1007/BF00227790. [DOI] [PubMed] [Google Scholar]
- Freedman EG. Head-eye interactions during vertical gaze shifts made by rhesus monkeys. Exp. Brain Res. 2005;167:557–570. doi: 10.1007/s00221-005-0051-9. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Eye-head coordination during head-unrestrained gaze shifts in rhesus monkey. J. Neurophysiol. 1997;77:2328–2348. doi: 10.1152/jn.1997.77.5.2328. [DOI] [PubMed] [Google Scholar]
- Frens M, van Opstal A. Transfer of short-term adaptation in human saccadic eye movements. Exp. Brain Res. 1994;100:293–306. doi: 10.1007/BF00227199. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Reiner D, Pong M. Transfer of gain changes from targeting to other types of saccade in the monkey: constraints on possible sites of saccadic gain adaptation. J. Neurophysiol. 1996;74:2522–2535. doi: 10.1152/jn.1996.76.4.2522. [DOI] [PubMed] [Google Scholar]
- Fujita M, Amagai A, Minakawa F, Aoki M. Selective and delay adaptation of human saccades. Cogn. Brain Res. 2002;13:41–52. doi: 10.1016/s0926-6410(01)00088-x. [DOI] [PubMed] [Google Scholar]
- Guitton D, Volle M. Gaze control in humans: eye-head coordination during orienting movements to targets within and beyond the oculomotor range. J. Neurophysiol. 1987;58:427–459. doi: 10.1152/jn.1987.58.3.427. [DOI] [PubMed] [Google Scholar]
- Henson D. Corrective saccades: effects of altering visual feedback. Vision Res. 1978;18:63–67. doi: 10.1016/0042-6989(78)90078-0. [DOI] [PubMed] [Google Scholar]
- Henson D. Investigation into corrective saccadic eye movements for refixation amplitudes of 10 degrees and below. Vision Research. 1979;19:57–61. doi: 10.1016/0042-6989(79)90121-4. [DOI] [PubMed] [Google Scholar]
- Hopp JJ, Fuchs AF. The characteristics and neuronal substrate of saccadic eye movement plasticity. Prog. Neurobiol. 2004;72:27–53. doi: 10.1016/j.pneurobio.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Hyde JE. Some characteristics of voluntary human ocular movements in the horizontal plane. Am. J. Opthalmol. 1959;48:85–94. doi: 10.1016/0002-9394(59)90290-9. [DOI] [PubMed] [Google Scholar]
- Kowler E, Blaser E. The accuracy and precision of saccades to small and large targets. Vision Res. 1995;35:1741–1754. doi: 10.1016/0042-6989(94)00255-k. [DOI] [PubMed] [Google Scholar]
- Kröller J, Pélisson D, Prablanc C. On the short-term adaptation of eye saccades and its transfer to head movements. Exp Brain Res. 1996 Oct;111(3):477–482. doi: 10.1007/BF00228738. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. Parametric adjustment in saccadic eye movements. Percept Psychophys. 1967;2:359–362. [Google Scholar]
- Miller J, Anstis T, Templeton W. Saccadic plasticity: parametric adaptive control by retinal feedback. J. Exp. Psychol. 1981;7:356–366. doi: 10.1037//0096-1523.7.2.356. [DOI] [PubMed] [Google Scholar]
- Oestreich J, Dembrow NC, George AA, Zakon HH. A “sample-and-hold” pulse-counting integrator as a mechanism for graded memory underlying sensorimotor adaptation. Neuron. 2006;49:577–588. doi: 10.1016/j.neuron.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Phillips JO, Fuchs AF, Ling L, Iwamoto Y, Votaw S. Gain adaptation of eye and head movement components of simian gaze shifts. J. Neurophysiol. 1997;78:2817–2821. doi: 10.1152/jn.1997.78.5.2817. [DOI] [PubMed] [Google Scholar]
- Prablanc C, Masse D, Echallier JF. Error-correcting mechanisms in large saccades. Vision Res. 1978;18:557–560. doi: 10.1016/0042-6989(78)90202-x. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Noto CT, Bevans SE. Effect of visual error size on saccade adaptation in monkey. J. Neurophysiol. 2003;90:1235–1244. doi: 10.1152/jn.00656.2002. [DOI] [PubMed] [Google Scholar]
- Semmlow J, Gauthier G, Vercher JL. Mechanisms of short-term saccadic adaptation. J. Exp. Psychol. 1989;15:249–258. doi: 10.1037//0096-1523.15.2.249. [DOI] [PubMed] [Google Scholar]
- Stahl J. Amplitude of human head movements associated with horizontal saccades. Exp. Brain Res. 1999;126:41–54. doi: 10.1007/s002210050715. [DOI] [PubMed] [Google Scholar]
- Stahl J. Eye-head coordination and the variation of eye-movement accuracy with orbital eccentricity. Exp. Brain Res. 2001;136:200–210. doi: 10.1007/s002210000593. [DOI] [PubMed] [Google Scholar]
- Straube A, Robinson F, Fuchs A. Characteristics of saccadic gain adaptation in Rhesus macaques. J. Neurophysiol. 1997;77:874–895. doi: 10.1152/jn.1997.77.2.874. [DOI] [PubMed] [Google Scholar]
- Takeichi N, Kaneko CR, Fuchs AF. Activity changes in monkey superior colliculus during saccade adaptation. J. Neurophysiol. 2007;97:4096–4107. doi: 10.1152/jn.01278.2006. [DOI] [PubMed] [Google Scholar]
- Van Ginsbergen JAM, Van Opstal AJ, Ottes FP. Parameterization of saccadic velocity profiles in man. In: Gale AG, Johnson F, editors. Theoretical and Applied Aspects of Eye Movement Research. Amsterdam: Elsevier; 1984. pp. 87–94. [Google Scholar]
- Volle M, Guitton D. Human gaze shifts in which the head and eyes are not initially aligned. Exp. Brain Res. 1993;94:463–470. doi: 10.1007/BF00230204. [DOI] [PubMed] [Google Scholar]
- Wallman J, Fuchs A. Saccadic gain modification: visual error drives motor adaptation. J. Neurophysiol. 1998;80:2405–2416. doi: 10.1152/jn.1998.80.5.2405. [DOI] [PubMed] [Google Scholar]