Abstract
Objective To assess the safety and effectiveness of eflornithine as first line treatment for human African trypanosomiasis.
Design Cohort study.
Setting Control programme in Ibba, southern Sudan.
Participants 1055 adults and children newly diagnosed with second stage disease in a 16 month period.
Main outcome measures Deaths, severe drug reactions, and cure at 24 months.
Results 1055 patients received eflornithine for 14 days (400 mg/kg/day in adults and 600 mg/kg/day in a subgroup of 96 children). Overall, 2824 drug reactions (2.7 per patient) occurred during hospital stay, 1219 (43.2%) after the first week. Severe reactions affected 138 (13.1%) patients (mainly seizures, fever, diarrhoea, and bacterial infections), leading to 15 deaths. Risk factors for severe reactions included cerebrospinal fluid leucocyte counts ≥100×109/l (adults: odds ratio 2.6, 95% confidence interval 1.5 to 4.6), seizures (adults: 5.9, 2.0 to 13.3), and stupor (children: 9.3, 2.5 to 34.2). Children receiving higher doses did not experience increased toxicity. Follow-up data were obtained for 924 (87.6%) patients at any follow-up but for only 533 (50.5%) at 24 months. Of 924 cases followed, 16 (1.7%) died during treatment, 70 (7.6%) relapsed, 15 (1.6%) died of disease, 403 (43.6%) were confirmed cured, and 420 (45.5%) were probably cured. The probability of event free survival at 24 months was 0.88 (0.86 to 0.91). Most (65.8%, 52/79) relapses and disease related deaths occurred after 12 months. Risk factors for relapse included being male (incidence rate ratio 2.42, 1.47 to 3.97) and cerebrospinal fluid leucocytosis: 20-99×109/l (2.35, 1.36 to 4.06); ≥100×109/l (1.87, 1.07 to 3.27). Higher doses did not yield better effectiveness among children (0.87 v 0.85, P=0.981).
Conclusions Eflornithine shows acceptable safety and effectiveness as first line treatment for human African trypanosomiasis. Relapses did occur more than 12 months after treatment. Higher doses in children were well tolerated but showed no advantage in effectiveness.
Introduction
Human African trypanosomiasis (sleeping sickness), caused by the protozoan Trypanosoma brucei gambiense and transmitted by the bite of the tsetse fly, represents an important threat in several sub-Saharan countries. In southern Sudan the disease is endemic in all of western Equatoria, the province bordering Uganda, Democratic Republic of Congo, and Central African Republic. In the past the disease has been controlled by active surveillance and treatment of cases and its prevalence was kept at low levels (0.3-0.5%) until 1989. Control activities disintegrated amid civil war, and massive population movements favoured the re-emergence of the disease in epidemic proportions.
Human African trypanosomiasis is invariably fatal unless treated. The disease progresses from haemolymphatic infection (first stage disease) to meningoencephalitic invasion (second stage). Most patients with second stage disease are treated with a series of intravenous injections of melarsoprol. This drug has been used since 1949 but is associated with severe toxic effects, in particular a reactive encephalopathy, which affects 5-10% of patients, with 10-70% of these cases dying.1 2 The encephalopathy cannot be predicted, prevented, or managed satisfactorily, despite half a century of investigations. Melarsoprol related death rates are about 6%.3 An additional concern is the increase in treatment failures with melarsoprol, as high as 30%, reported in several foci in Uganda, Sudan, Democratic Republic of Congo, and Angola.4 5 6 In southern Sudan, 18.5% of the first group of patients treated by Médecins Sans Frontières in 1999 with a 10 day course of melarsoprol (2.2 mg/kg/day) had relapsed after six months of follow-up.
Eflornithine (diethylfluoromethylornithine or DFMO), initially evaluated for the treatment of cancer, is the only alternative drug registered for the treatment of second stage human African trypanosomiasis. The drug exerts a trypanostatic effect by inhibiting the parasite’s ornithine decarboxylase, an enzyme needed for the synthesis of polyamines, themselves involved in cell multiplication and differentiation.7 8 9 After intravenous administration eflornithine has a short half-life (1.5-5 hours)10 11 12 13 and penetrates the blood-brain barrier well.
Adverse effects of eflornithine include seizures, gastrointestinal disorders, and myelosuppression. A major disadvantage of the drug is the labour intense mode of its administration, requiring one intravenous infusion every six hours for 14 days (56 infusions in total). Before 2001, when a five year donation from the manufacturer (Sanofi-Aventis) to the World Health Organization made eflornithine available to all African countries, its extensive use was unaffordable. For these reasons eflornithine has been used on a small scale, mostly reserved for second line treatment.
In view of the increasingly high relapse rates with melarsoprol and its known high toxicity, Médecins Sans Frontières adopted eflornithine as first line therapy for human African trypanosomiasis in its treatment centre based in Ibba, Maridi county, southern Sudan, in September 2001, as soon as the drug became financially accessible.
This centre was the first to use eflornithine on a large scale, offering a unique opportunity to document clinical results. We assessed the safety and effectiveness of first line treatment with eflornithine for second stage human African trypanosomiasis in a remote endemic focus.
The area was strongly affected by years of war and isolation. It lacked infrastructure, safe water, and an electricity and communications network; had poor roads, impractical to use for part of the year; insecurity (constant danger of air and land attacks by military forces or bandits); and low levels of education; and lacked trained human resources. The Médecins Sans Frontières team had gone through emergency evacuations repeatedly. The setting did not meet the minimum conditions for planning a rigorous clinical trial, ideally with a randomised controlled design and a good follow-up, which would have provided sounder data. In addition, a comparative study with the existing alternative treatment (melarsoprol) seemed unsustainable ethically because of the known higher death rate plus the low effectiveness of melarsoprol documented in this area.
Methods
Our cohort comprised patients with newly diagnosed second stage human African trypanosomiasis who were treated with eflornithine in the Ibba Sleeping Sickness Centre, Maridi county, southern Sudan between September 2001 and December 2002.
We defined patients as having second stage disease if trypanosomes were found in cerebrospinal fluid, if the number of leucocytes in cerebrospinal fluid was greater than 5×109/l with trypanosomes in blood or lymph, or if the number of leucocytes in cerebrospinal fluid was greater than 20×109/l with a card agglutination test for trypanosomiasis14 dilution titer of 1:4 or higher.
Patients were Sudanese from western Equatoria. They were diagnosed as having human African trypanosomiasis by active screening or passively at the Médecins Sans Frontières laboratories in Ibba, Maridi, and Kotobi (Maridi and Mundri counties). Diagnostic examinations included microscopy of lymph node fluid and blood (with capillary tube centrifugation15 and quantitative buffy coat16 techniques), cerebrospinal fluid for parasite detection and leucocyte counts, as well as serology with the card agglutination test for trypanosomiasis.
Patients testing positive for malaria (rapid test or microscopy) were treated with a combination of artesunate and sulfadoxine-pyrimethamine before starting the eflornithine regimen.
Eflornithine diluted in normal saline was administered in slow intravenous infusions over two hours. Adults received 100 mg/kg every six hours (400 mg/kg/day) for 14 days. Children received a higher dose—150 mg/kg every six hours (600 mg/kg/day) for 14 days—on the basis of previous reports17 18 that showed lower effectiveness among under 12s associated with lower concentrations of eflornithine in cerebrospinal fluid. This higher dose for children was adopted in January 2002 with an age cut-off point initially set at 15 years, modified later in 2002 to 12 years. Therefore among under 15s in the cohort only a subgroup received the higher dose.
We did not give eflornithine to pregnant women, and breastfed children of mothers receiving treatment were given a breast milk replacement. All patients were admitted to hospital for round the clock medical surveillance for the whole period of treatment.
The clinician in charge interrupted or stopped treatment according to the severity of adverse reactions. Treatments were resumed from the point of interruption when the patient’s condition improved.
We characterised all adverse events occurring in temporal association with treatment using the international common toxicity criteria,19 which grades adverse events by intensity from 1 to 4 (mild, moderate, severe, very severe), the relation between the drug and event (not related, unlikely, possible, probable, definite, unknown), and outcome (complete recovery, still present at discharge, sequelae, death). Drug reactions did not include adverse events classed in the “not related” category.
We graded adverse events retrospectively on the basis of medical records and discussion with patients’ clinicians.
After treatment we followed-up patients at 6, 12, and 24 months with routine clinical and laboratory parasitological assessments including blood concentration techniques and lymph node fluid and cerebrospinal fluid examinations. Patients who missed appointments were interviewed at home about their health status. We regarded those who were in good general health at 24 months or later but refused to travel for the final laboratory assessment as “probably cured.”
We considered a relapse to have occurred when trypanosomes were seen in any body fluid or if the cerebrospinal fluid leucocyte count had significantly increased. Patients who relapsed were re-treated for 10 days with a combination of melarsoprol 1.2 mg/kg and nifurtimox (15 mg/kg/day for adults and 20 mg/kg/day for under 15s).
To improve the follow-up rate, Médecins Sans Frontières strengthened its resources for patient tracing and community health education from July 2002 onwards.
Data sources
We obtained baseline and treatment data from medical records and entered these in an EpiData 3.0 (EpiData Association, Denmark) database. Data were cross verified against the Epitryps (Epicentre, France) database, admission to hospital registers, laboratory cards, and field programme lists and reports. The Médecins Sans Frontières team regularly entered follow-up laboratory data in the Epitryps database. Analysis was done with Stata 9.2.
Safety analysis
We focused the analysis of risk factors on major drug reactions (grade 3 or 4), given the broad spectrum of symptomatology with sleeping sickness and its invariable lethality without treatment. We also explored risk factors for reactions at injection sites.
Logistic regression was used for univariable and multivariable analysis of risk factors. For the multivariable analysis we used a backward elimination process and compared models with the likelihood ratio test. Further validation was done with automatic stepwise forward and backward selection methods, using maximum likelihood estimation tests (Wald test). We tested one degree interaction effects between variables with plausible biological links.
Effectiveness analysis
We carried out survival analysis with the Kaplan-Meier method. The follow-up time was calculated from the date of admission until the date of relapse, death, or last visit. We used Poisson regression analysis to test risk factors for relapse.
To permit comparisons with previous publications and with monitoring data from other treatment centres we also calculated proportions (a more customary approach). The follow-up time was calculated from the date of hospital discharge. Follow-up was considered complete for patients assessed at 22 months or later and they were not invited for a further visit.
Results
From September 2001 to December 2002, 1771 patients were treated for human African trypanosomiasis, of whom 1055 had newly diagnosed second stage disease and received eflornithine. This group comprised the study cohort.
Most patients (952, 90.2%) came from Mundri and Maridi counties and the rest from Yei and Yambio counties. Adults (median age 22) were the predominant group (829, 78.6%). Coinfection with malaria was detected in 445 (42.2%) patients. Trypanosomes were seen in lymph or blood in 1005 (95.3%) patients but in cerebrospinal fluid in only one third. Cerebrospinal fluid leucocyte counts were greater than 20×109/l in 605 (57.3%) patients (table 1). Nineteen patients (1.8%) were diagnosed without direct parasite evidence, having positive serology at dilutions of 1:4 or higher combined with more than 20 cells in cerebrospinal fluid.
Table 1.
Baseline characteristics of 1055 patients with newly diagnosed second stage human African trypanosomiasis treated with eflornithine in Ibba, southern Sudan. Values are numbers (percentages) of patients unless stated otherwise
| Characteristics | Patients with sleeping sickness (n=1055) |
|---|---|
| Women | 468 (44) |
| Median (interquartile range) age (years) | 22 (15-32) |
| Age <15 years | 226 (21) |
| Median (interquartile range) weight (kg) | 46 (38-54) |
| County of residence: | |
| Mundri | 676 (64) |
| Maridi | 276 (26) |
| Other | 103 (10) |
| Detected by active screening | 121 (12) |
| Malaria coinfection | 445 (42) |
| Axillary temperature ≥37.5°C | 357 (34) |
| Glasgow coma score* (n=1012): | |
| 10 | 7 (1) |
| 11 | 1 (0.1) |
| 12 | 2 (0.2) |
| 13 | 6 (1) |
| 14 | 22 (2) |
| 15 | 974 (96) |
| Localisation of trypanosomes: | |
| Lymph nodes | 683 (65) |
| Blood | 330 (31) |
| Lymph or blood | 1005 (95) |
| Cerebrospinal fluid | 351 (33) |
| Leucocyte count in cerebrospinal fluid: | |
| Geometric mean×109/l | 43.9 |
| ≤20×109/l | 450 (43) |
| 21-99×109/l | 256 (24) |
| ≥100×109/l | 349 (33) |
*Score is sum of points obtained in each of three criteria (best motor response, best verbal response, eye opening): 3-8=severe impairment; 9-12=moderate impairment; 13-14=mild impairment; and 15=normal.
Safety
Drug reactions
Overall, 2990 adverse events were recorded during hospital stay. In most cases the distinction between events provoked by the treatment, by the disease itself, or by concomitant conditions could not be clearly established. Thus the association with eflornithine treatment was classified as unrelated in 5.5% cases, unlikely in 9.0%, possible in 36.8%, probable in 35.5%, definite in 9.2%, and unknown in 4.0%. Excluding the 166 unrelated events, the remaining 2824 were regarded as drug reactions (table 2).
Table 2.
Description of drug reactions during eflornithine treatment in 1055 patients with newly diagnosed second stage human African trypanosomiasis. Values are numbers (percentages) of patients unless stated otherwise
| Drug reactions | No of reactions* | Median (range) onset (days) | Onset within 7 days† |
|---|---|---|---|
| Death | 15 (1.4) | 12 (1-23) | 2 (13) |
| Gastrointestinal: | |||
| Diarrhoea (major)‡ | 376 (17) (35.6) | 7 (0-23) | 169 (45) |
| Nausea, vomiting | 162 (15.4) | 5 (0-17) | 93 (57) |
| Anorexia | 22 (2.1) | 8 (0-14) | 9 (41) |
| Neurological: | |||
| Tremor | 140 (13.3) | 5 (1-17) | 87 (62) |
| Dizziness | 93 (8.8) | 6 (0-14) | 54 (58) |
| Seizures (major) | 51 (41) (4.8) | 3 (0-15) | 36 (71) |
| Confusion (major) | 38 (4) (3.6) | 5 (0-14) | 22 (58) |
| Coma (major) | 4 (4) (0.4) | 8 (2-13) | 2 (50) |
| Ataxia | 1 (0.1) | 11 | 0 (0) |
| Cardiovascular: | |||
| Arrhythmia | 12 (1.1) | 9.5 (3-13) | 4 (33) |
| Oedema (major) | 15 (10) (1.4) | 6 (2-14) | 8 (53) |
| Shock (major) | 1 (1) (0.1) | 14 | 0 (0) |
| Dermatological: | |||
| Reaction at injection site (major) | 261 (3) (24.7) | 6 (0-19) | 138 (53) |
| Pruritus | 67 (6.4) | 4 (0-17) | 48 (72) |
| Rash | 6 (0.6) | 12 (5-19) | 1 (17) |
| Infection: | |||
| Tissue infection (major) | 37 (6) (3.5) | 9 (0-19) | 11 (30) |
| Pneumonia (major) | 23 (3) (2.2) | 8 (0-29) | 10 (44) |
| Pain: | |||
| Abdominal pain | 398 (37.7) | 5 (0-22) | 241 (61) |
| Headache (major) | 329 (1) (31.2) | 4 (0-21) | 233 (71) |
| Muscle or joint pain | 91 (8.6) | 6 (0-17) | 52 (57) |
| Chest pain | 48 (4.5) | 5 (0-23) | 32 (67) |
| Other: | |||
| Fever (major) | 325 (67) (30.8) | 5 (0-19) | 200 (62) |
| Cough | 172 (16.3) | 6 (0-16) | 99 (58) |
| Other (major) | 137 (10) (13.0) | 8 (0-22) | 54 (40) |
| Total No of drug reactions | 2824 (2.7)§ | 6 (0-29) | 1605 (57) |
| No of patients with major reactions: | 138 (13.1) | ||
| 1 reaction | 116 (11) | — | — |
| ≥2 reactions | 22 (2) | — | — |
| Total No of major reactions | 167 (0.2)§ | 6 (0-23) | |
| Interruptions to treatment: | 109 (10.3) | ||
| Suspended for <24 hours | 57 (5.4) | — | — |
| Suspended for >24 hours | 42 (4.0) | — | — |
| Treatment stopped | 10 (0.9) | — | — |
*Percentage of entire cohort.
†Percentage of patients with reaction.
‡Grade 3 and 4 of National Cancer Institute common toxicity criteria.
§Incidence of reactions per patient in whole cohort during hospital stay.
Most patients (91.2%, 962/1055) experienced drug reactions, with a mean of 2.7 reactions per patient (range 0-9; interquartile range 2-4). The overall death rate was 1.4% (n=15), and 0.4% (1/226) occurred among the under 15s.
Because the characterisation was done retrospectively, 242 drug reactions could not be graded owing to insufficient documentation in the medical records. Of the graded drug reactions, 6.5% (167/2582) were classified as major (grade 3 or 4), consisting mainly of 67 fever peaks with an axillary temperature of more than 39.5°C (6% of cohort), 41 seizures (4%), 17 episodes of diarrhoea (2%), and nine bacterial infections (1%).
Minor (grade 1 or 2) reactions were common (2.5 reactions per patient). Mild and moderate abdominal pain and headache were predominant, followed by fever and reactions at injection sites (including phlebitis). Soft tissue infections and pneumonia were notably common.
The median time to onset of drug reactions was at day 6 of treatment, and 43.2% (1219/2824) of reactions occurred after day 7. Some of the most predominant and clinically significant drug reactions were among those of late onset, such as diarrhoea and bacterial infections (55% and 65% started after day 7). Most neurological and dermatological reactions, however, occurred during the first week. Seizures appeared consistently at the beginning of treatment (median day 3). Patients recovered well from seizures and only two intractable cases necessitated stopping treatment.
Drug reactions led to treatment interruptions in 109 (10.3%) patients, but most were brief suspensions of less than 24 hours. Treatment was stopped for 10 patients (<1%).
At the end of hospital stay 88.0% of the drug reactions had fully subsided. Among all discharged patients, 19.3% (201/1039) had at least one disorder not yet fully resolved, the commonest being diarrhoea, abdominal pain, headache, and cough. No patients had long term sequelae.
Deaths
Overall, there were 15 deaths for which an association with eflornithine was possible to varying degrees. In 10 cases the cause of death was unclear; either the doctors could not distinguish between drug reactions, sleeping sickness related complications, and coexisting disease, or the information recorded in the files was insufficient. Eight deaths were attributed to complications of bacterial infections leading to sepsis, three of them characterised as a deterioration of pre-existing infections. Two deaths were associated with severe diarrhoea and dehydration, two were sudden deaths of suspected cardiogenic cause, and three were linked to complications of unexplained oedema, acute renal failure, and tuberculosis, respectively. One child (age 2 years) who received eflornithine 600 mg/kg/day died as a result of severe diarrhoea that started on day 11 of treatment.
Risk factors for drug reactions
In the initial multivariable analysis the risk factors on admission for major drug reactions, affecting 13.1% (n=138) of the cohort, included age less than 15 years, cerebrospinal fluid leucocyte counts of 100×109/l or higher, confusion, fever, a history of seizures, and absence of muscle or joint pain. An interaction effect was, however, observed between age and cerebrospinal fluid leucocyte counts, showing a differing association of cerebrospinal fluid leucocytes with drug reactions according to age group. Children and adults were therefore analysed separately and different risk factors were identified for the groups (table 3).
Table 3.
Risk factors for developing one or more major drug reactions among 1055 patients with newly diagnosed second stage human African trypanosomiasis included in cohort, by age group
| Risk factors | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| Children <15 years (n=226) | |||||
| Trypanosomes in cerebrospinal fluid | 2.2 (1.1 to 4.6) | 0.028 | — | NS | |
| Cerebrospinal fluid leucocytes ×109/l: | |||||
| 21-99 v ≤20 | 0.5 (0.2 to 1.6) | 0.269 | 0.4 (0.1 to 1.4) | 0.167 | |
| ≥100 v ≤20 | 2.2 (1.0 to 4.7) | 0.048 | 1.6 (0.7 to 3.8) | 0.249 | |
| Confusion or stupor | 8.3 (2.5 to 27.7) | 0.001 | 9.3 (2.5 to 34.2) | 0.001 | |
| Seizures | 5.6 (1.5 to 20.3) | 0.009 | 3.6 (0.9 to 14.6) | 0.078 | |
| Eflornithine 600 v 400 mg/kg/day | 1.1 (0.6 to 2.3) | 0.757 | — | NS | |
| Adults (n=829) | |||||
| Trypanosomes in lymph or blood | 0.3 (0.2 to 0.7) | 0.005 | 0.51 (0.2 to 1.2) | 0.107 | |
| Trypanosomes in cerebrospinal fluid | 2.3 (1.5 to 3.5) | <0.001 | — | NS | |
| Cerebrospinal fluid leucocytes ×109/l: | |||||
| 21-99 v ≤20 | 2.1 (1.2 to 3.9) | 0.013 | 2.1 (1.2 to 3.9) | 0.017 | |
| 100 v ≤20 | 3.4 (2.0 to 5.8) | <0.001 | 2.6 (1.5 to 4.6) | 0.001 | |
| Confusion or stupor | 2.9 (1.5 to 5.4) | 0.001 | 1.7 (0.8 to 3.4) | 0.151 | |
| Seizures | 7.3 (3.0 to 17.6) | <0.001 | 5.9 (2.0 to 13.3) | 0.001 | |
| Musculoskeletal pain | 0.5 (0.2 to 0.9) | 0.024 | 0.3 (0.2 to 0.7) | 0.003 | |
| Dehydration | 3.3 (1.0 to 11.0) | 0.049 | 2.6 (0.7 to 9.5) | 0.143 | |
NS=Not significant.
Among the 226 under 15s, 38 (16.8%) experienced major drug reactions. Multivariable analysis showed an increased risk for those presenting with confusion or stupor before treatment. All other factors were not significantly associated, including a dose of 600 mg/kg/day (96 children) compared with 400 mg/kg/day (130 children).
Of the 829 adults, 100 (12.1%) had major drug reactions. Risk factors among this group after multivariable analysis included cerebrospinal fluid leucocyte counts greater than 20×109/l and seizures. Musculoskeletal pain was a protective factor.
The multivariable exploration of pretreatment risk factors for reactions at the injection site (261/1055 patients) identified as predictors: age less than 15 years (odds ratio 1.62, 95% confidence interval 1.16 to 2.26; P=0.005) and pruritus (odds ratio 1.60, 1.05 to 2.43; P=0.029).
Effectiveness
Follow-up results
Overall, 533 (50.5% of cohort) patients had a known outcome or at least 24 months of follow-up, 672 (63.7%) had at least 12 months of follow-up, and 924 (87.6%) had any follow-up. The cohort was followed-up for a median 16.5 months (interquartile range 7-26).
Relapses were established in 27 patients without detection of parasites in body fluids. Among them the median increase in leucocyte counts since the previous assessment was 65 cells (range 27-523).
Survival analysis
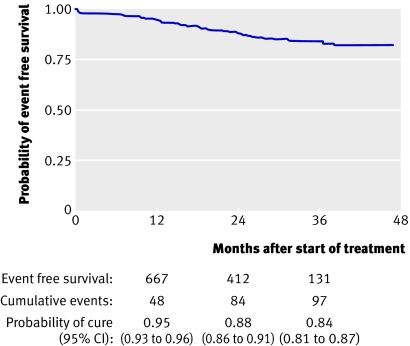
By survival analysis, the probability of event free survival for the whole cohort at 24 months was 0.88 (95% confidence interval 0.86 to 0.91; fig 1). At 12 months it was 0.95 (0.93 to 0.96) and at 36 months it was 0.84 (0.81 to 0.87).
Fig 1 Probability of event free survival among 1055 patients treated with eflornithine for newly diagnosed second stage human African trypanosomiasis in Ibba, southern Sudan
In an alternative survival analysis with no assumptions, 29 patients seen in apparent good health were not assumed to be “probably cured” but were instead censored at their last laboratory assessment. Fifteen patients who died of unverified causes during follow-up were not assumed to be “treatment failures” but were censored at their date of death. Under these conditions the probability of event free survival at 24 months was 0.90 (0.87 to 0.92).
Among the 226 under 15s the probability of event free survival at 24 months per dose group (600 v 400 mg/kg/day) was similar (0.87 v 0.85, log rank P=0.981). This finding did not change by multivariable analysis nor by selecting the 131 children aged less than 12 years (data not shown).
Customary analysis of proportions
To permit comparisons with other reports and with programme monitoring data, the effectiveness data are also presented in a more customary analysis (table 4). Of the 924 patients with a known final outcome or who were seen at least once during follow-up, 16 (1.7%) died during treatment, 70 (7.6%) relapsed, 15 (1.6%) died of disease during follow-up, 403 (43.6%) were confirmed cured, and 420 (45.5%) were probably cured (favourable progression until the last assessment). The cure rate among these patients was 89.1% (n=823). A more optimistic approach, taking into consideration those lost to follow-up as cured (12.4% of cohort), gave a cure rate of 90.4% (n=954).
Table 4.
Outcome after follow-up of 1055 patients with newly diagnosed second stage human African trypanosomiasis treated with eflornithine. Values are percentages unless stated otherwise
| Outcome | No of patients | Evaluable group (n=924) | Whole cohort (n=1055) |
|---|---|---|---|
| Cured at 24 months | 403 | 43.6 | 38.2 |
| Probably cured at 24 months* | 29 | 3.1 | 2.7 |
| Probably cured (partial follow-up)† | 391 | 42.3 | 37.1 |
| No follow-up | 131 | — | 12.4 |
| Died during treatment | 16‡ | 1.7 | 1.5 |
| Relapsed (all in second stage) | 70 | 7.6 | 6.6 |
| Probably relapsed (died of disease) | 15 | 1.6 | 1.4 |
*In good general health but refusing to travel for final laboratory assessment.
†11 patients died of non-disease causes (for example, war) during follow-up.
‡One death was unrelated to treatment.
Time to detection of relapse
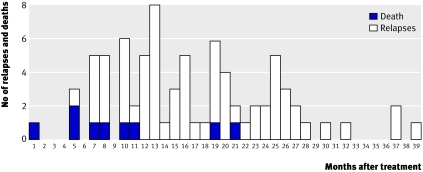
Figure 2 shows the time distribution of the detection of relapses and of deaths due to disease regarded as compatible with sleeping sickness. The date of death was not recorded for six patients. Of all 70 relapses plus the nine deaths with a known date regarded as relapses, 27 (34%) were detected within 12 months, 46 (58%) within 18 months, 63 (80%) within 24 months, and the remaining 16 (20%) afterwards.
Fig 2 Time to detection of relapses and deaths of disease with known date (n=79). Six patients with no date of death are excluded
To have a more realistic look at the dynamics of follow-up, taking into account that numerous patients attend their visits late, one month was added to each follow-up time point. As a result 35 (44%) patients relapsed at 13 months, 52 (66%) at 19 months, and 68 (86%) at 25 months.
Risk factors for relapse
A multivariable Poisson regression analysis (1039 patients discharged) revealed a higher risk of relapse for men than for women and for patients with high cerebrospinal fluid leucocytosis (table 5).
Table 5.
Risk factors for relapse during follow-up among 1039 patients with newly diagnosed second stage human African trypanosomiasis discharged alive after treatment with eflornithine
| Risk factors | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| Incidence rate ratio (95% CI) | P value | Incidence rate ratio (95% CI) | P value | ||
| Male | 2.58 (1.58 to 4.23) | <0.0001 | 2.42 (1.47 to 3.97) | <0.0001 | |
| Age <15 years | 1.67 (1.06 to 2.62) | 0.027 | 1.52 (0.96 to 2.40) | 0.074 | |
| Trypanosomes in cerebrospinal fluid | 1.57 (1.02 to 2.42) | 0.040 | — | NS | |
| Cerebrospinal fluid leucocytes ×109/l: | |||||
| 21-99 v ≤20 | 2.37 (1.37 to 4.09) | 0.002 | 2.35 (1.36 to 4.06) | 0.002 | |
| ≥100 v ≤20 | 2.04 (1.19 to 3.51) | 0.010 | 1.87 (1.07 to 3.27) | 0.027 | |
| Confusion or stupor | 2.18 (1.09 to 4.35) | 0.027 | 1.74 (0.86 to 3.56) | 0.126 | |
| Oedema | 2.41 (0.59 to 9.79) | 0.219 | 3.74 (0.82 to 17.09) | 0.088 | |
| Skin diseases | 0.48 (0.15 to 1.51) | 0.209 | 0.36 (0.10 to 1.26) | 0.110 | |
NS=not significant.
Discussion
The use of eflornithine 400 mg/kg/day for 14 days as routine first line treatment for second stage human African trypanosomiasis in isolated foci was feasible and showed satisfactory safety and effectiveness. Relapses occurred more than 12 months after treatment and therefore long term follow-up of patients is important. A higher dose in children (600 mg/kg/day for 14 days) was well tolerated, but it was no more effective than the standard dose.
Strengths and weaknesses
Previously published clinical data on eflornithine concerned smaller cohorts with fewer than 300 participants, composed of a mix of naive and re-treated patients and different regimens. This cohort’s strengths include both its size and homogeneity: all patients were naive cases and received the same treatment regimen. With only one exception,20 the follow-up of our cohort was better than in previous studies. The sample size and acceptable follow-up provide a more robust measure of safety and effectiveness.
The safety data were collected retrospectively from routine medical records. We thought that severe clinical events were consistently reported but that inconsequential events tended to be under-reported. The high turnover of the team (doctors were asked to commit to six month terms in Ibba) leads to variability in the quality of reporting over time.
The limited medical equipment and laboratory capacity did not allow for more conclusive diagnosis of adverse events and causes of death.
Some caution is needed when interpreting the findings on effectiveness because of the incomplete follow-up after treatment, always a challenge in settings affected by war.
A few patients in the cohort (1.8%, 19/1055) were diagnosed without directly seeing trypanosomes, on the basis of serology combined with more than 20 cells in the cerebrospinal fluid, which are not universally accepted criteria. These criteria were used in this site (as is the case in other sites) because of the low sensitivity of current laboratory examinations to directly detect the parasites, coupled with the high level of disease transmission in the area at the time, plus the high probability of losing contact with the patients afterwards.
Safety
Our overall findings on safety are consistent with those reported in similar settings21 and reaffirm the advantage of eflornithine over melarsoprol. The case fatality rate of 1.4% is much lower than the fatality rate with melarsoprol.
Typically, hospital inpatients with second stage sleeping sickness have numerous adverse events, regardless of the regimen used. In most cases it cannot be conclusively established if the adverse events are provoked by the treatment, by the disease itself, or by concomitant conditions, and the same dilemma affects the data from this cohort.
The extraction of data from medical records showed that drug reactions with eflornithine were common. Major drug reactions were, however, relatively uncommon (13%). The commonest major drug reactions were fever spikes, generalised seizures, and diarrhoea. In most patients these conditions resolved favourably with routine management and/or temporary treatment suspensions.
The frequency of soft tissue infections and pneumonia was higher than anticipated. In the literature we found only one mention of three cases of pyoderma in a series of 207 patients.22 The late emergence of this phenomenon reaffirms its probable relation with the treatment. The fact that this was a first experience of a large number of patients treated over a sustained period, in challenging conditions where asepsis is difficult to ensure, could be a first general explanation. Infusions were prepared in large numbers, twice a day, every day. This repetitive routine may affect the rigour of the nursing technique. We hypothesised that these infections might be catheter related and that a contribution of eflornithine itself is possible, as a cytostatic drug interfering with the host’s immunity. After the first cases of serious infection in Ibba, infusion safety training was reinforced and a positive impact was observed thereafter. We stress that sustained vigilance needs to be maintained and the best possible safety materials and techniques must be introduced. Most of these soft tissue infections were successfully managed with antibiotics (cloxacillin or cephalosporins).
The observation that nearly half of the drug reactions emerged after day 7 of treatment is consistent with results of a previously reported trial,20 which compared eflornithine treatment for 14 days with seven days. It suggests that considerable drug related toxicity might be avoided with shorter eflornithine regimens when suitable. According to our data the main problems that could hypothetically be averted to some extent are gastrointestinal and cardiovascular disorders, injection site reactions, and bacterial infections. Only comparative studies could properly assess this hypothesis.
Risk factors for major drug reactions were estimated with odds ratios, which are considered good risk estimators if the phenomenon studied is infrequent, this being the case for our data. We found that high leucocyte counts in cerebrospinal fluid increased the risk of severe drug reactions in adults but not in under 15s. Children but not adults presenting with confusion or stupor developed more severe reactions. A history of seizures only in adults was an important risk factor for severe reactions.
Adults presenting with arthralgia or myalgia had a lower risk of major toxicity. An explanation might be that these symptoms are indicative of an earlier stage of the disease progression.
The Ibba programme was the first to administer a considerably (50%) higher dose to children, which had been empirically suggested to improve eflornithine effectiveness in children but that had not yet been implemented or described. This cohort included 96 under 15s who received this higher dose. Because we also had a group of 130 children who for independent reasons received the standard dose, we examined the association of the higher dose with major drug reactions but found no significant increase in toxicity in children receiving eflornithine 600 mg/kg/day. Our data did not show a difference in effectiveness between the two doses, which is in contradiction with the conclusions of previous reports.17 18 20
Effectiveness
Considering the difficult conditions in southern Sudan and the typical low compliance with follow-up schedules of patients with human African trypanosomiasis, the level of follow-up for this cohort was good.
The issue of losses to follow-up in longitudinal studies of human African trypanosomiasis remains a dilemma. Patients have a noticeable tendency to miss follow-up assessments (which always include a lumbar puncture), especially those in remote, impoverished, and war stricken regions. Our field observations indicate that patients who are relapsing tend to come for assessment because of symptoms; asymptomatic patients tend to miss assessments owing to lack of motivation to travel, lack of transportation, subsistence priorities, displacement (sometimes war related), and fear of the lumbar puncture; and a small proportion may have died (resulting from relapses of human African trypanosomiasis or other causes) without this information reaching the programme.
Our experience during the effort of active tracing in this and other cohorts has shown that most patients not attending spontaneously for assessment are in fact cured or in good health. We believe therefore that the lost to follow-up group is different to the followed-up group. Assuming that relapses are less common among the lost to follow-up group than among the followed-up group, excluding them from the analysis leads to overestimation of failure rates.
Survival analysis is affected by the same dilemma because patients who are partially or totally lost to follow-up contribute less to the analysis than those who complete the schedule, among whom are all the treatment failures.
Most clinicians and researchers in the field consider two years to be an adequate time to assess “cure” and hence it is possible to refer to the 0.88 probability of event free survival at 24 months as the cure rate using eflornithine in this study. This value is in line with the 89.1% (823/924) cure rate by customary analysis (see table 4) in which patients who were lost to follow-up were excluded.
The time to relapse in our cohort underlines the importance of following patients for more than one year, since only a third of all relapses and deaths with a known date were detected within 12 months.
Before its application in Ibba, eflornithine had not been used on a large scale as first line treatment for second stage human African trypanosomiasis. Although the drug has been donated by the manufacturer since mid-2001, the application in the field remains costly because of the need for injection fluids, catheterisation materials, the logistics of transport and storage, long hospital stays, and round the clock nursing care. Since 2007 WHO with funding from Sanofi-Aventis have provided national programmes with a kit containing the drug and all materials needed for treating patients. This initiative alleviates some aspects of the problem, but large scale access to patients in safe conditions remains unmet.
Conclusions
Adverse events were common during treatment with eflornithine although most were not serious. Major events were infrequent. Those of clinical concern included seizures, soft tissue infections, and diarrhoea. Safety can be improved by the prevention and early detection of soft tissue infections.
Adults with a history of seizures or high cerebrospinal fluid leucocytosis before treatment, as well as children admitted with confusion or stupor, should be monitored closely for adverse events during eflornithine treatment.
We believe that treatment programmes for human African trypanosomiasis should use eflornithine as first line treatment because the safety and efficacy of this drug compares favourably to results reported with melarsoprol under similar conditions. This is possible if the capacity of treatment centres is strengthened in terms of nursing staff and materials needed for safe intravenous infusions, as shown in this isolated site.
The occurrence of late relapses emphasises the need for long term follow-up. The administration of a higher dose in children was well tolerated but did not show an advantage in effectiveness.
The complex administration mode of eflornithine remains a barrier to its implementation, and its extensive use in monotherapy could bring about the emergence of parasite resistance. For these reasons, mid-term and long term alternatives are urgently needed, such as the development of drug combinations and new molecules.
What is already known on this topic
The relatively good safety and effectiveness profiles of eflornithine have been poorly documented in the field
The difficult mode of its administration is a barrier for extensive access to patients
What this study adds
Eflornithine shows satisfactory safety and effectiveness as routine first line treatment for second stage human African trypanosomiasis
Children tolerated considerably higher doses but no advantage on effectiveness was seen
We thank the Sudanese health authorities; the international and local clinical and laboratory field team members of Médecins Sans Frontières, based in Ibba and Kotobi (Sudan), Lokichokio and Nairobi (Kenya), and Paris (France) whose hard work permitted this research to happen; Xavier Guinotte, Christophe Fournier, Pauline Horrill, Suna Balkan, Laurence Bonte, Greg Elder, Damien McCarthy, and Darin Portnoy for their significant support; Florence Thomas, Dominique Legros, Xavier de Radiguès, and Sofia Stegmann for their pioneer data analysis in the field and headquarters; and Philippe Guerin and Rebecca Grais for advice on the manuscript.
Contributors: GP designed and coordinated the study, managed and analysed the data, and wrote the manuscript. He is a guarantor. LP managed and led the main data analysis. She is a guarantor. IBF and BB managed patients, collected clinical data, and characterised medical retrospective data. NN extracted, managed, and analysed retrospective data. GG managed patients, collected clinical data, and extracted and characterised medical retrospective data. CH supervised field medical activities, and extracted and characterised medical retrospective data. MB managed patients, collected clinical data, and participated in data interpretation and preparation of the manuscript. All authors declare that they accept full responsibility for the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: French section of Médecins Sans Frontières.
Competing interests: None declared.
Ethical approval: Ethical approval was not required because this study did not involve any experimental intervention. The treatment administered, the laboratory examinations, and patient management and follow-up were all part of the routine of the human African trypanosomiasis control programme, as agreed between Médecins Sans Frontières and the Sudanese authorities. The study is limited to analysing data that were collected within the standard medical data recording and programme monitoring system.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Pepin J, Milord F, Khonde AN, Niyonsenga T, Loko L, Mpia B, et al. Risk factors for encephalopathy and mortality during melarsoprol treatment of Trypanosoma brucei gambiense sleeping sickness. Trans R Soc Trop Med Hyg 1995;89:92-7. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Control and surveillance of African trypanosomiasis: report of a WHO expert committee WHO Technical Report Series 881. Geneva: WHO, 1998 [PubMed]
- 3.Schmid C, Richer M, Bilenge CM, Josenando T, Chappuis F, Manthelot CR, et al. Effectiveness of a 10-day melarsoprol schedule for the treatment of late-stage human African trypanosomiasis: confirmation from a multinational study (IMPAMEL II). J Infect Dis 2005;191:1922-31. [DOI] [PubMed] [Google Scholar]
- 4.Legros D, Evans S, Maiso F, Enyaru JC, Mbulamberi D. Risk factors for treatment failure after melarsoprol for Trypanosoma brucei gambiense trypanosomiasis in Uganda. Trans R Soc Trop Med Hyg 1999;93:439-42. [DOI] [PubMed] [Google Scholar]
- 5.Burri C, Keiser J. Pharmacokinetic investigations in patients from northern Angola refractory to melarsoprol treatment. Trop Med Int Health 2001;6:412-20. [DOI] [PubMed] [Google Scholar]
- 6.Stanghellini A, Josenando T. The situation of sleeping sickness in Angola: a calamity. Trop Med Int Health 2001;6:330-4. [DOI] [PubMed] [Google Scholar]
- 7.Metcalf BW, Bey P, Danzin C, Jung MJ, Casara P, Vevert JP. Catalytic irreversible inhibition of mammalian ornithine decarboxylase (E.C.4.1.1.17) by substrate and product analogues. J Am Chem Soc 1978;100:2551-3. [Google Scholar]
- 8.Bacchi CJ, Nathan HC, Hutner SH, McCann PP, Sjoerdsma A. Polyamine metabolism: a potential therapeutic target in trypanosomes. Science 1980;210:332-4. [DOI] [PubMed] [Google Scholar]
- 9.Bacchi CJ, Garofalo J, Mockenhaupt D, McCann PP, Diekema KA, Pegg AE, et al. In vivo effects of alpha-DL-difluoromethylornithine on the metabolism and morphology of Trypanosoma brucei brucei. Mol Biochem Parasitol 1983;7:209-25. [DOI] [PubMed] [Google Scholar]
- 10.Haegele KD, Alken RG, Grove J, Schechter PJ, Koch-Weser J. Kinetics of alpha-difluoromethylornithine: an irreversible inhibitor of ornithine decarboxylase. Clin Pharmacol Ther 1981;30:210-7. [DOI] [PubMed] [Google Scholar]
- 11.Abeloff MD, Slavik M, Luk GD, Griffin CA, Hermann J, Blanc O, et al. Phase I trial and pharmacokinetic studies of alpha-difluoromethylornithine—an inhibitor of polyamine biosynthesis. J Clin Oncol 1984;2:124-30. [DOI] [PubMed] [Google Scholar]
- 12.Griffin CA, Slavik M, Chien SC, Hermann J, Thompson G, Blanc O, et al. Phase I trial and pharmacokinetic study of intravenous and oral alpha-difluoromethylornithine. Invest New Drugs 1987;5:177-86. [DOI] [PubMed] [Google Scholar]
- 13.Pepin J, Milord F. The treatment of human African trypanosomiasis. Adv Parasitol 1994;33:1-47. [DOI] [PubMed] [Google Scholar]
- 14.Magnus E, Vervoort T, Van Meirvenne N. A card-agglutination test with stained trypanosomes (CATT) for the serological diagnosis of T B gambiense trypanosomiasis. Ann Soc Belg Med Trop 1978;58:169-76. [PubMed] [Google Scholar]
- 15.Woo PT. The haematocrit centrifuge technique for the diagnosis of African trypanosomiasis. Acta Trop 1970;27:384-6. [PubMed] [Google Scholar]
- 16.Bailey JW, Smith DH. The use of the acridine orange QBC technique in the diagnosis of African trypanosomiasis. Trans R Soc Trop Med Hyg 1992;86:630. [DOI] [PubMed] [Google Scholar]
- 17.Van Nieuwenhove S. Advances in sleeping sickness therapy. Ann Soc Belg Med Trop 1992;72:39-51. [PubMed] [Google Scholar]
- 18.Milord F, Loko L, Ethier L, Mpia B, Pepin J. Eflornithine concentrations in serum and cerebrospinal fluid of 63 patients treated for Trypanosoma brucei gambiense sleeping sickness. Trans R Soc Trop Med Hyg 1993;87:473-7. [DOI] [PubMed] [Google Scholar]
- 19.NCI-CTEP-NIH. Common toxicity criteria, version 2.0. National Cancer Institute. Cancer therapy evaluation program 1999. http://ctep.info.nih.gov
- 20.Pepin J, Khonde N, Maiso F, Doua F, Jaffar S, Ngampo S, et al. Short-course eflornithine in Gambian trypanosomiasis: a multicentre randomized controlled trial. Bull World Health Organ 2000;78:1284-95. [PMC free article] [PubMed] [Google Scholar]
- 21.Chappuis F, Udayraj N, Stietenroth K, Meussen A, Bovier PA. Eflornithine is safer than melarsoprol for the treatment of second-stage Trypanosoma brucei gambiense human African trypanosomiasis. Clin Infect Dis 2005;41:748-51. [DOI] [PubMed] [Google Scholar]
- 22.Milord F, Pepin J, Loko L, Ethier L, Mpia B. Efficacy and toxicity of eflornithine for treatment of Trypanosoma brucei gambiense sleeping sickness. Lancet 1992;340:652-5. [DOI] [PubMed] [Google Scholar]