1. INTRODUCTION
At a time when metabolic syndrome and the fat content of internal organs are increasingly linked to cardiovascular disease and mortality, the understanding of lipid content and dynamics in the myocardium remains vague, at best. Even more poorly understood are alterations in the intramyocardial triacylglycerol (TAG) dynamics of the failing heart. This study explores the storage, oxidation, and turnover of TAG in a rat model of pressure overload induced failure.
Exogenous fatty acids support 60−70% of the energy requirements of healthy myocardium [1]. Exogenous fats include those found in the blood, such as the long chain fatty acids bound to albumin, the various lipoproteins, and chylomicrons. Once these fats are transported into the cytoplasm they are either oxidized within the mitochondria or stored endogenously as acylglycerols. These internal lipid stores contribute an additional 10% to mitochondrial ATP production [2,3], increasing to 50% as exogenous fatty acids become limited [3]. Thus, the balance between exogenous fatty acid metabolism and fat storage in the myocardium is itself a dynamic process that may bear a reciprocal relationship to the development of maladaptive changes in cardiac metabolism in the hypertrophied and failing heart [4].
Importantly, recent studies suggest that changes in lipid homeostasis contribute to the development of various cardiomyopathies [5]. In the case of diabetes, cardiac triacylglycerol (TAG) pools increase dramatically in parallel to an increase in unbound free fatty acids (FFA) [6]. It is suggested that the accumulation of TAG contribute to reduced contractility [7], while the unesterfied FFA's have lipotoxic effects that, in part, trigger apoptotic pathways [8,9]. Conversely, patients and animal models with hypertensive cardiac hypertrophy have reduced myocardial fatty acid uptake and TAG depletion [10,11]. In humans with severe end-stage heart failure, myocardial TAG content is near normal levels, but the lipids increase significantly if accompanied by diabetes or obesity [12]. Beyond reporting tissue content levels in these earlier studies, little information exists about the oxidation or turnover rate of the endogenous TAG pool in heart failure.
As cardiac hypertrophy produced by pressure overload progresses to acute failure, metabolic processes revert to a fetal pattern [13,14,15,16]. There is less reliance on exogenous fats, and a greater dependence on carbohydrates. However, the balance between exogenous and endogenous lipid utilization in the failing heart remains relatively unexplored. We hypothesized that endogenous TAG oxidation and availability may be limited in failing heart. We used established 13C and 1H NMR methods to measure TAG oxidation in intact beating heart parallel to measures of glucose, glycogen, and exogenous palmitate oxidation. Indeed, the outcome reveals dramatic differences in TAG metabolism in the failing heart at basal workloads and during a β-adrenergic challenge. In addition, our measure of TAG turnover rate provides important insight into the mechanism for changes in TAG metabolism of failing heart.
2. MATERIAL AND METHODS
2.1 Aortic Banding Animal Model
Pressure-overload cardiac hypertrophy was induced by constricting the transverse aorta (hemoclip) of three week old male Sprague Dawley rats, as previously described [4,22]. This banding procedure relies on the natural growth of the animal to produce a gradually increasing degree of aortic constriction. At 10−12 weeks post-banding, the hearts were excised for metabolic studies. As previously described [17,22,28,32], this early-to-moderate stage of heart failure is characterized by a concentric cardiac hypertrophy, increased heart to body weight ratio, depressed LVDP and dP/dt, early signs of pulmonary edema, and atrial thrombi. The rats enter an acute end-stage heart failure at 4−6 months post banding. The protocol was approved by the Animal Care Policies and Procedures Committee at the University of Illinois at Chicago (Institutional Animal Care and Use Committee accredited), and animals were maintained in accordance with the Guide for the Care and Use of laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85−23, revised 1996).
2.2 Protocols
Three protocols were executed. The first protocol assessed the oxidative rate of exogenous palmitate in parallel to measures of the percent contribution of each substrate (glucose, glycogen, palmitate, endogenous TAG) to mitochondrial ATP production. At 10−12 weeks post banding, Langendorff perfused sham and hypertrophic rat hearts were positioned in a 9.4 T NMR spectrometer and perfused for 20 minutes at basal workloads with modified Krebs-Henseleit buffer (116 mM NaCl, 4 mM KCl, 1.5 mM CaCl2, 1.2 mM MgSO4 and 1.2 mM NaH2PO4, equilibrated with 95% O2/5% CO2) containing unlabeled 0.4 mM palmitate / albumin complex (3:1 molar ratio) and unlabeled 5 mM glucose as previously described [2,4,22]. During this equilibration period a 31P NMR signal was acquired to assess energy potential (PCr/βATP) followed by a 13C NMR spectrum of the natural abundance carbon signal. The perfusate was then switched to recirculated medium containing 0.4 mM [2,4,6,8,10,12,14,16-13C8] palmitate plus 5 mM unlabeled glucose. Sequential 13C NMR spectra were collected every 2 seconds and averaged every 2 minutes to follow the incorporation of labeled substrate into the TAG pool and glutamate pool for 25 minutes. Finally, a second 31P NMR signal was collected before freeze-clamping the hearts for additional extract analysis. This protocol was repeated to assess the oxidation of glucose and glycogen from isolated hearts perfused with buffer containing [1,6-13C] labeled glucose and unlabeled palmitate.
Protocol 2 repeated the first protocol at high workloads (inotropic challenge) to determine whether changes in endogenous lipid oxidation were workload dependent in the failing heart. As described above, isolated hearts were first perfused with unlabeled substrates. Ten minutes before switching to the 13C-enriched substrates, β-adrenergic challenge was induced with isoproterenol (0.01 μM).
Protocol 3 assessed endogenous triacylglycerol turnover rate in sham and failing heart at baseline workloads. As we previously reported [2], the rate is quantified by fitting a kinetic model of turnover to the dynamic 13C NMR enrichment data of TAG acquired from hearts oxidizing 13C labeled fatty acids. Protocol 3 was similar to Protocol 1, with the 13C labeling period extended to 90 minutes.
2.3 13C NMR labeling scheme
Hearts were isolated and retrograde perfused with buffer containing [2,4,6,8,10,12,14,16-13C8] palmitate + unlabeled glucose. Figure 1 illustrates the subsequent 13C labeling of several key metabolites. Palmitate is transported across the cell membrane and is either stored within the endogenous TAG pool or oxidized in the mitochondria. The oxidative rate of palmitate was determined using established 13C NMR kinetic models fit to the dynamic 13C NMR data for the 4- and 2- carbon positions of glutamate [2,4,25]. Experiments were repeated with buffer containing unlabeled palmitate + [1,6-13C2] labeled glucose. High resolution 13C and 1H NMR analysis of the heart extracts from both labeling schemes provided the fractional contribution of each substrate (palmitate, glucose, glycogen, and triacylglyerol) to mitochondrial ATP production as described below. NMR parameters required for acquisition of 13C, 31P, and 1H NMR spectra from isolated hearts and extracts have been extensively reported [2,4,22,25].
Figure 1.
Schematic representation of [2,4,6,8,10,12,14,16-13C8] palmitate and unlabeled glucose uptake and incorporation into the triacylglycerol (TAG) and glutamate pool.
2.4 Exogenous and Endogenous Substrate Oxidation
The high resolution NMR analysis of tissue extracts provided the relative contribution of glucose, palmitate, glycogen, and endogenous fats to acetyl-CoA formation and mitochondrial oxidative metabolism as described previously [2,4]. With both glucose and palmitate contributing to the formation of mitochondrial acetyl-CoA (see Figure 1), the oxidation of either substrate was assessed directly by following the incorporation of 13C label from either glucose or palmitate into the acetyl-CoA pool. The fractional enrichment of acetyl-CoA (Fc) was determined by standard isotopomer analysis from glutamate resonances [18].
The contribution of endogenous glycogen to mitochondrial metabolism was assessed based on 1H NMR analysis of alanine enrichment in hearts oxidizing [1,6-13C2] glucose and unlabeled palmitate as previously reported [2]. As shown in Figure 1, labeled glucose and endogenous glycogen contribute to the formation of both pyruvate and alanine via glycolysis. While intracellular pyruvate content is too low for NMR detection, isotopic equilibrium with the readily NMR detected alanine pool indicates enrichment of glycolytic end products (19,20). Thus, the labeled fraction of alanine (FA) corresponds to exogenous 13C-glucose utilization and the unlabeled fraction of alanine (1-FA) corresponds to the endogenous glycogen utilization, which was further corrected for incorporation of 13C glucose into glycogen (3.5−3.8% enrichment) using 13C NMR of glycogen extracts against a glucose concentration and enrichment standard [21]. Having measured the contribution of [1,6-13C2] glucose to mitochondrial metabolism based on acetyl-CoA enrichment (Fc, described above), the subsequent contribution of endogenous glycogen to mitochondrial acetyl-CoA and metabolism can be calculated as Fc(1-FA)/FA.
Having accessed the fractional contribution of exogenous palmitate, glucose, and endogenous glycogen stores to mitochondrial ATP production, the fractional balance was credited to the oxidation of endogenous TAG. Contributions from endogenous lactate and amino acids pools are negligible in isolated heart perfused under these conditions.
2.5 Lipid Extract data
Total myocardial acylglycerols (mono, di, tri) were extracted with chloroform and methanol as described previously [2,25], and quantified colorimetrically by enzymatic assay for glycerol (Wako Pure Chemical Industries). The fraction of fatty acid chains labeled with 13C was determined by mass-spec (Waters X-terra C18MS column; MS:scan m/z 100−600 Fragmentor 75V Negative ESI) [2,25]. Linear analysis of TAG turnover was calculated as TAG content multiplied by the 13C fractional enrichment of TAG / enrichment duration. Under steady state conditions the rate of TAG synthesis equals degradation.
2.6 Statistical Analysis
Data is presented as mean ± standard error unless otherwise stated. Data set comparisons were performed with Student's unpaired, two-tailed t-test. Differences in mean values were considered statistically significant at a probability level of less then 5% (P<0.05).
3. RESULTS
3.1 Animal Model
As expected for this well established model of hypertrophy [4,22], body mass of aortic–banded rats was lower than sham-operated control rats (HF = 372 ± 26 g, n=18; SHAM = 409 ± 26, n=16, p<0.0005), and the heart mass of the banded group was 37% greater (HF = 3.06 ± 0.07g, SHAM = 2.24 ± 0.06, p<0.0001). Heart-to-body ratio (mg/g) was 62% greater in HF rats (HF = 8.2 ± 0.2; SHAM = 5.1 ± 0.4, p<0.0001). At excision, TAG content in the hearts was 39% lower in the HF group compared to shams (HF 4.25 ± 0.45 μmol/gdw, n=5; Sham 6.99 ± 1.00, n=6, p<0.05).
3.2 Cardiac Function
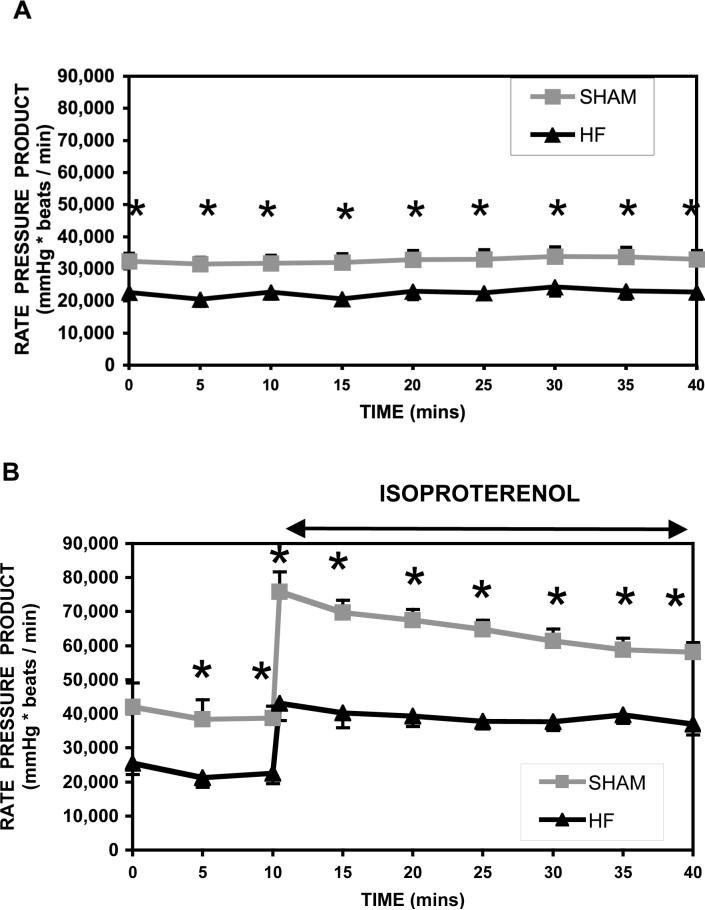
Heart rate, LVDP, -dP/dt, and +dP/dt are listed in Table 1, and rate-pressure-product is shown in Figure 2a and b, for Protocol 1 and 2 respectively. As expected for this stage of heart failure, LVDP and rate-pressure-product were significantly depressed (36%) at baseline workloads compared to shams. With β-adrenergic challenge, RPP doubled for both shams and HF groups.
TABLE I.
Isolated retrograde perfused heart function (midpoint) and energy potential at baseline workloads (Protocol 1) and during an adrenergic challenge (Isoproterenol; Protocol 2).
| SHAM | HEART FAILURE | |||
|---|---|---|---|---|
| Baseline | Isoproterenol | Baseline | Isoproternol | |
| Heart Rate, bpm | 282 ± 14 | 336 ± 9 † | 259 ± 26 | 326 ± 13 ¶ |
| LVDP, mm Hg | 116 ± 13 | 186 ± 18 † | 86 ± 14 * | 131 ± 8 §¶ |
| RPP, mmHg*beats/min | 33840 ± 4859 | 63801 ± 3834 † | 21665 ± 3530 * | 43010 ± 3051 §¶ |
| (+)dp/dt, mmHg/sec | 3151 ± 498 | 5306 ± 215 † | 2301 ± 390 * | 3122 ± 219 §¶ |
| (−)dp/dt, mmHg/sec | 2971 ± 433 | 5452 ± 344 † | 2284 ± 298 * | 3777 ± 233 §¶ |
| PCr/ATP (endpoint) | 2.11 ± 0.05 | 1.94 ± 0.09 | 1.43 ± 0.10 * | 1.34 ± 0.08 § |
Mean ± S.E.; Significant difference at p<0.05 for
Isoproterenol
Isoproterenol
Sham baseline vs HF baseline
Sham baseline vs Sham
Sham Isoproterenol vs HF
HF baseline vs HF Isoproterenol
Figure 2.
Rate-pressure-product (mmHg*beats/min ± S.E.) from isolated retrograde perfused rat hearts following 10 weeks aortic banded pressure-overload hypertrophy (σ HF, n=12) and sham operated healthy rats (ν sham, n=10). Data is presented for RPP at basal workloads (top) and during an adrenergic challenge (bottom). (*significant difference, p<0.05)
Protocol 3 duplicates Protocol 1 with an extended NMR acquisition period (90 min). At 30 minutes, cardiac function of shams and HF in Protocol 3 were similar to function for Protocol 1 at 30 min. By 90 minutes, RPP was 19,300 ± 1,800 (n=9) in the HF group and 26,900 ± 2,800 (n=6, p<0.05) in shams.
3.3 Energy Potential
As expected from previous reports, energy potential (PCr/β-ATP) (Table 1) was significantly lower in the HF group at baseline compared to shams (p<0.001) [23,24]. With β-adrenergic challenge, the ratio of PCr to β-ATP was maintained in both groups despite the two fold increase in workload for 25 min. Importantly, this finding indicates that the low energy reserve (PCr/ATP) in the HF group did not limit the ability of the heart to respond to the inotropic stimuli as previously hypothesized [24].
3.4 Dynamic 13C NMR Data
Sequential 13C NMR spectra were collected from shams and HF hearts to follow the incorporation of 13C label from [2,4,6,8,10,12,14,16-13C8] palmitate into the endogenous glutamate and triacylglycerol pools. The 13C NMR spectra acquired during the β-adrenergic challenge are shown in Figure 3. Consistent with the baseline spectra, are the observed resonances from glutamate 2-carbon (56 ppm), 4-carbon (34 ppm), and 3-carbon (27ppm), as well as the 13C signal from triacylglycerol at 15, 30, and 32 ppm.
Figure 3.
Dynamic mode 13C-NMR spectra obtained from an isolated perfused sham (left) and failing heart (right) oxidizing [2,4,6,8,10,12,14,16-13C8] palmitate and unlabeled glucose during an adrenergic challenge (0.01 μM isoproterenol).
Interestingly, with β-adrenergic stimulation, the HF group also revealed significant 13C enrichment of the aspartate 2- and 3-carbons, as seen in the 13C spectra at 53.4 ppm and 37.8 ppm, respectively. The 13C signals from aspartate resulted purely from increased enrichment and not from an increase in aspartate content. No aspartate labeling was observed in sham hearts at either workload, nor in the HF group at baseline. However, aspartate content was similar between all four groups (data not shown).
3.5 Palmitate Oxidation Rate and TCA Cycle Flux
Palmitate oxidation rate and the TCA (tricarboxylic acid) cycle flux were determined from kinetic analysis of the 13C isotopic enrichment data for glutamate [4,25]. At baseline workloads (Protocol 1), palmitate oxidation rate (SHAM = 1.7±0.2 μmol/min/g dw, HF = 1.0±0.1; p<0.05) and TCA cycle flux (SHAM = 17.9±1.9 μmol/min/g dw; HF = 11.0±1.7; p<0.05) were greater in the sham group compared to failing hearts. This increase is consistent with the higher workloads and energy demands of the shams. When metabolic rates were normalized to the workloads, both palmitate oxidation (Figure 4) and TCA cycle flux remained well coupled to workload in the HF group relative to Shams at baseline.
Figure 4.
Palmitate oxidative rate normalized to RPP for shams (white bar) and heart failure (HF, black bar) at basal and high workloads (isoproterenol). Palmitate oxidation rate was well coupled to workload in the failing group relative to the shams at baseline workloads but not with the adrenergic challenge.
With β-adrenergic challenge (Protocol 2), increases occurred in both palmitate oxidation rate (SHAMS = 3.0 ± 0.2 μmol/min/g dw, n=9; HF = 1.5 ± 0.3, n=9, p<0.05) and TCA cycle flux (SHAMS = 33.6 ± 1.6, HF=20.8±1.9, p<0.05). When normalized to rate pressure product TCA flux remained tightly coupled to work output in the HF group relative to shams. However, the rate of palmitate oxidation in the HF group did not keep pace with demands of high workload relative to shams (Figure 4).
3.6 13C NMR - Substrate Selection
In vitro 13C NMR analysis of tissue extracts provided the fractional contribution of each substrate to mitochondrial ATP production at both baseline and β-adrenergic challenge (Figure 5). In the sham group at baseline workload, the contribution of individual substrates (glucose 10%, glycogen 8%, palmitate 74%, TAG 8%) was comparable to previously reported values [3]. Surprisingly, there was no evidence of endogenous fat oxidation in the HF group at baseline workloads (glucose 14%, glycogen 13%, palmitate 72%, TAG 0%). This apparent loss in endogenous fatty acid oxidation was balanced by the increased contribution from carbohydrates.
Figure 5.

Percent contributions from each substrate to mitochondrial ATP production at basal and high workloads (isoproterenol).
It was unclear whether the loss in endogenous fatty acid oxidation could be attributed to the lower workload and energy demand of the HF group. For this reason, endogenous fatty acid oxidation was assessed during a β-adrenergic challenge in both HF and shams (Figure 5). In the sham group, the contribution of individual substrates (glucose 11%, glycogen 11%, palmitate 70%, TAG 8%) matched the contributions observed at baseline. This is not to be confused with oxidative rates. In fact, since the contribution of each substrate remained constant, oxidative rates would have doubled in parallel to the measured two-fold increase in palmitate oxidation.
In the HF group, contributions from endogenous fatty acids remained negligible during the inotropic challenge, (glucose 21%, glycogen, 24%, palmitate 59%, TAG 0%) and contributions from palmitate dropped 18%. The lower contribution from palmitate was balanced by an increase in glucose and glycogen oxidation. To the best of our knowledge, this is the first report to assess endogenous fatty acid oxidation in pressure overload induced heart failure, and show that endogenous fat oxidation does not compensate for the known drop in exogenous fat oxidation of failing heart.
3.7 Triacylglycerol enrichment, content, and turnover
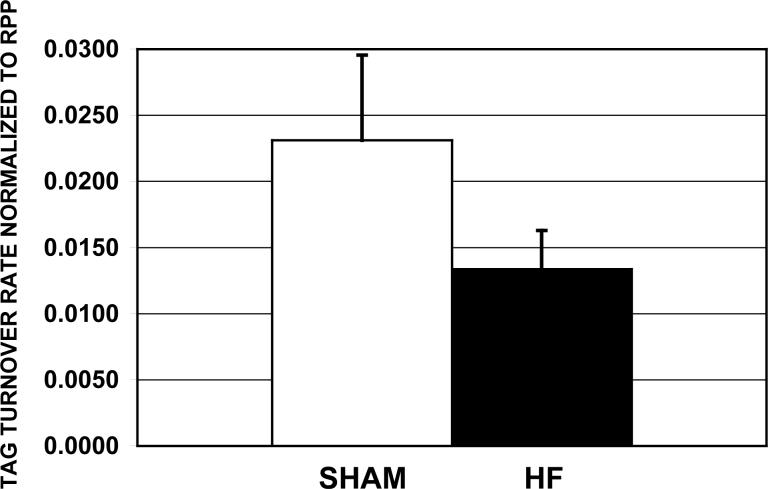
The final 13C fractional enrichment of the TAG pool was 2.15 ± 0.29 % for the HF group (n=10) and 4.13 ± 1.17 % for the Shams (n=7, p=0.074). TAG content was measured at the end of the 2 hr perfusion period, and the content was 29% lower in the HF group compared to shams (HF 9.76 ± 0.75 μmol/g dw; Shams 13.54 ± 0.99, p=0.01), even though both groups were perfused with buffer containing similar concentrations of palmitate and glucose. The turnover rate for the HF group was also significantly lower than the Shams (HF 3.1 ± 0.8 nmol/min/g dw, n=10; Shams 7.6 ± 2.2, n=7, p<0.05). With these rates normalized to the workloads (Figure 6), TAG turnover was uncoupled from workload in the HF group relative to Shams. Turnover was assessed only at basal workloads, because the HF group could not sustain high workloads for the 90 minutes necessary to acquire the TAG enrichment profile (preliminary data not shown).
Figure 6.
The turnover rate of triacylglycerol pool was measured in isolated perfused shams (white bar, n=7) and failing heart (black bar, n=7), and is shown here normalized to the rate-pressure-production (turnover rate/RPP; (μmol/min/gdw)/(mmHg*beats/min) ±S.E.).
4. DISCUSSION
The goal of the study was to determine the extent of endogenous fatty acid oxidation in heart failure, while examining potential mechanisms underlying any changes. The results show that, contrary to shams, endogenous fats (TAG) were not oxidized in failing hearts. This loss was not compensated by an increase in exogenous palmitate oxidation. Rather, palmitate contributions remained constant in this early stage of cardiac failure, while glucose and glycogen contributions increased to balance the loss of TAG oxidation. Notably, the loss of TAG oxidation was not linked to the lower workloads associated with the HF group. Increasing workloads through β-adrenergic challenge did not restore TAG oxidation despite a 100% increase in rate-pressure-product and energy demand. The mechanism for the loss is more closely linked to TAG storage and mobilization. This is supported by data showing both a lower concentration and turnover rate of the TAG pool in failing rat hearts.
4.1 Exogenous Palmitate Oxidation
It is well established that the hypertrophied heart shifts toward a fetal gene expression pattern that includes metabolic enzymes [14,26,27]. As we, and others have previously reported, there is a dramatic downregulation of exogenous fatty acid oxidation rate as the disease progresses towards end-stage failure [4,14,28,29]. Conversely, fatty acid oxidation rates are normal in a mild-to-moderate stage of heart failure [30,31,32]. In support of these reports, we also found palmitate oxidation rate to be similar between healthy shams and the HF group under baseline workloads (normalized to RPP). However, this was not true under conditions of stress. Palmitate oxidative rates did not increase proportionately with workload in our HF group during the β-adrenergic challenge, despite a two-fold increase in palmitate oxidation in shams. Failure to increase exogenous fatty acid (palmitate) oxidation with the β-adrenergic challenge indicates some underlying change has occurred at this stage and the change does limit FAO under conditions of stress. This finding opposes the hypothesis that abnormalities in the metabolic activity of the mitochondria represent a late, rather than early, phenomenon in the development of heart failure [27,31,32].
In parallel to the measured decrease in palmitate oxidation in the HF group during β-adrenergic stimulation, we also observed 13C-labeling of the aspartate pool. This unexpected finding supports our earlier report that anaplerosis of the mitochondria increases significantly in failing heart [4]. We showed in cardiac hypertrophy, when palmitate oxidation rates fall and are uncoupled from TCA cycle flux, there is a recruitment of compensatory pathways (ie., anaplerosis) to maintain TCA flux. An increase in exchange between labeled substrates of the mitochondria and cytosol, via the aspartate-oxaloacetate transporter, would result in an increase in total tissue enrichment of the aspartate pool. Indeed, at basal workloads palmitate oxidation was not depressed relative to both workload and TCA cycle flux in the HF group, and aspartate labeling was not detected. With the β-adrenergic challenge, palmitate oxidation was uncoupled from TCA cycle flux in the HF group, and aspartate labeling was observed. Thus, the increase in aspartate labeling is consistent with an increase in anaplerosis via the unidirectional aspartate-oxaloacetate transporter of the mitochondria.
4.2 Endogenous TAG oxidation
There have been few studies to address the oxidation of endogenous fats in normal heart [3,33], and no study addressing that oxidation in hypertrophy or failing heart. In the normal hearts, Saddick reports an 11% contribution from endogenous fats to mitochondrial energy production in rat hearts perfused with buffer containing glucose and palmitate [3]. We find a similar level of endogenous fat contributions in normal hearts perfused with similar substrates. However, no TAG was oxidized in the heart failure group at baseline.
The baseline rate-pressure-products of the HF group were lower than the healthy shams. For this reason, we hypothesized that TAG oxidation would recover in the HF group if workload and energy demands were increased with a β-adrenergic challenge (Protocol 2). An adrenergic challenge has already been shown by several groups to increase TAG lipolysis [34,35,36]. With an increase in lipolysis, or turnover, TAG availability for oxidation would be enhanced. Indeed, the oxidative rate for TAG doubled in the shams as the percent contribution of TAG to mitochondrial oxidation remained constant. However, the inotropic challenge did not increase TAG oxidation in failing heart. No oxidation of TAG was detected in the HF group. Thus, the observed loss in TAG oxidation was not workload dependent in HF.
4.3 Carbohydrate Oxidation
It is unclear whether the loss in palmitate and TAG oxidation in heart failure is the cause or effect for the observed increase in carbohydrate oxidation. It is well established that carbohydrate utilization increases as hypertrophy progresses to acute heart failure [13,14,15,16]. These changes occur to compensate for a maladaptive and damaged mitochondrion. However, the loss in FAO we observed in the HF group with β-adrenergic challenge was compensated by the increased oxidation of carbohydrates by the mitochondria. Therefore, the oxidative processes of the mitochondria are still functional and responsive to demands at this stage of failure (at least downstream from β-oxidation).
4.4 TAG content and turnover
Triacylglycerol content and turnover were measured to assess whether the mechanism for reduced TAG oxidation in HF could be linked to endogenous lipid availability. Myocardial TAG content was 30−40 % lower in the HF group relative to shams at both the time of excision and at the end of the 2 hour perfusion period (Protocol 3).
The concentration of TAG reported for other models of hypertrophy have been varied. In the spontaneously hypertensive rat (SHR), TAG was reduced significantly [11,37] in keeping with reduced fatty acid transport via FATP and CD36. In the hypertensive Dahl salt-sensitive rat, TAG was reportedly unchanged [38] or elevated [39]. In humans of normal body weight, TAG content was normal in failing heart, but increased significantly if the disease was accompanied by obesity or diabetes [12].
The exact mechanism for the reduced [TAG] is unclear. In two recent studies utilizing transgenic mice, the knock-out of lipoprotein lipase (hLpL0) in heart resulted in a drop in TAG content and a compensatory increase in glucose uptake, glycolysis, and glucose oxidation [40], whereas the accumulation of TAG in heart was linked to the knock-out of adipose TAG lipase (ATGL) [41]. Whether changes in either of these regulatory enzymes occurred in our heart failure model, and account for the loss of TAG content and oxidation, remains to be determined.
Kinetic analysis of the 13C NMR enrichment data indicated TAG turnover rates were significantly lower in the HF group at baseline workloads compared to shams. However, because workloads were also lower for the HF group, we normalized turnover to RPP. This is illustrated in Figure 6, and the results indicate that turnover is, in fact, uncoupled from workload in the HF group relative to shams. With TAG turnover, content, and the 13C fractional enrichments lower in the HF group compared to shams, we conclude that TAG storage and mobilization are reduced which leads to limited availability of endogenous fats.
4.5 Conclusion
In this study we report a decrease in endogenous TAG (a) content, (b) turnover, and (c) oxidation in pressure-overloaded early cardiac failure. The loss was not associated with a change in exogenous palmitate oxidation at basal workloads, but rather an increase in carbohydrate oxidation. Increasing energy demands by a β-adrenergic challenge did not recruit or restore TAG oxidation in the failing heart, despite a two-fold increase in TAG oxidation in healthy hearts. Indeed, this is surprising given the mitochondria were still functional as demonstrated by the increase in carbohydrate oxidation. Furthermore, the mechanism for the change in TAG metabolism was linked to substrate availability, as storage and mobilization of the TAG pool were reduced. These factors of reduced contributions from endogenous lipid to oxidative ATP synthesis during an increased work demand of the failing heart appear to present yet one more indication of impaired energy conversion pathways in the onset of hypertrophic cardiomyopathy.
FUNDING SOURCES
This work was supported in part by the National Institutes of Health Grant (Lewandowski) RO1HL62702, R37HL049244, and (O'Donnell) RO1HL79415.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol. 1974;36:413–459. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- 2.O'Donnell JM, Zampino M, Alpert NM, Fasano MJ, Geenen DL, Lewandowski ED. Accelerated triacylglycerol turnover kinetics in hearts of diabetic rats include evidence for compartmented lipid storage. Amer J Physiol Endocrinol Metab. 2006;290:E448–455. doi: 10.1152/ajpendo.00139.2005. [DOI] [PubMed] [Google Scholar]
- 3.Saddik M, Lopaschuk GD. Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J Biol Chem. 1991;266(13):8161–8170. [PubMed] [Google Scholar]
- 4.Sorokina N, O'Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, Lanoue KF, et al. Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase I activity characterized inefficiency in energy metabolism in hypertrophied hearts. Circulation. 2007;115(15):2033–2041. doi: 10.1161/CIRCULATIONAHA.106.668665. [DOI] [PubMed] [Google Scholar]
- 5.Schaffer JE. Lipotoxicity: when tissues overeat. Curr. Opin. Lipidol. 2003;14(3):281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res. 1997;34:25–33. doi: 10.1016/s0008-6363(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 7.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, et al. The cardiac phenotype induced by PPAR[alpha] overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2001;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabkin SW, Huber M, Krystal G. Modulation of palmitate-induced cardiomyocyte cell death by interventions that alter intracellular calcium. Prostaglandins Leukot Essent Fatty Acids. 1999;61:195. doi: 10.1054/plef.1999.0090. [DOI] [PubMed] [Google Scholar]
- 9.Sparagna GC, Hickson-Bick DL, Buja LM, McMillin JB. A metabolic role for mitochondria in palmitate-induced cardiac myocyte apoptosis. Am J Physiol. Heart Circ Physiol. 2000;279:H2124–H2132. doi: 10.1152/ajpheart.2000.279.5.H2124. [DOI] [PubMed] [Google Scholar]
- 10.De las Fuentes L, Herrero P, Peterson LR, Kelly DP, Gropler RJ, Davila-Roman VG. Myocardial fatty acid metabolism: independent predictor of left ventricular mass in hypertensive heart disease. Hypertension. 2003;41(1):83–87. doi: 10.1161/01.hyp.0000047668.48494.39. [DOI] [PubMed] [Google Scholar]
- 11.Perona JS, Ruiz-Gutierrez V. Triacylglycerol molecular species are depleted to different extents in the myocardium of SHR fed two oleic acid–rich oils. Am J Hypertens. 2005;18:72–80. doi: 10.1016/j.amjhyper.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18:1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 13.Wittels B, Spann JF. Defective lipid metabolism in the failing heart. J Clin Invest. 1968;47:1787–1794. doi: 10.1172/JCI105868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razeghi P, Young ME, Alcorn JL, Moravec CS, Frazier OH, Taegtmeyer H. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104:2923–2931. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- 15.Bishop SP, Altschuld RA. Increased glycolytic metabolism in cardiac hypertrophy and congestive failure. Am J Physiol. 1970;218:153–159. doi: 10.1152/ajplegacy.1970.218.1.153. [DOI] [PubMed] [Google Scholar]
- 16.Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol. 1994;267:H742–H750. doi: 10.1152/ajpheart.1994.267.2.H742. [DOI] [PubMed] [Google Scholar]
- 17.Vatner DE, Ingwall JS. Effects of moderate pressure-overload cardiac hypertrophy on the distribution of creatine kinase isozymes. Proc Soc Exp Biol Med. 1984;175(1):5–9. doi: 10.3181/00379727-175-1-rc2. [DOI] [PubMed] [Google Scholar]
- 18.Malloy CR, Sherry AD, Jeffrey FM. Evaluation of carbon flux and substrate selection through alternate pathways involving the citric acid cycle of the heart by 13C NMR spectroscopy. J Biol Chem. 1988;263:6964–71. [PubMed] [Google Scholar]
- 19.Damico LA, White LT, Yu X, Lewandowski ED. Chemical versus isotopic equilibrium and the metabolic fate of glycolytic end products in the heart. J Mol Cell Cardiol. 1996;28:989–999. doi: 10.1006/jmcc.1996.0092. [DOI] [PubMed] [Google Scholar]
- 20.Peuhkurinen KJ, Nuutinen EM, Pietilainen EP, Hiltunen JK, Hassinen IE. Role of pyruvate carboxylation in the energy-linked regulation of pool sizeds of tricarboxylic acid-cycle intermediates in the myocardium. Biochem J. 1982;208:577–81. doi: 10.1042/bj2080577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalrymple RH, Hamm R. A method for the extraction of glycogen and metabolites from a single muscle sample. Int J Food Sci and Technol. 1973;8:439–444. [Google Scholar]
- 22.Lewandowski ED, O'Donnell JM, Scholz TD, Sorokina N, Buttrick PM. Recruitment of NADH shuttling in pressure-overloaded and hypertrophic rat hearts. Am J Physiol Cell Physiol. 2007;292(5):C1880–1886. doi: 10.1152/ajpcell.00576.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Donnell JM, Narayan P, Bailey MQ, Abduljalil AM, Altschuld RA, McCune SA, Robitaille PML. 31P-NMR analysis of congestive heart failure in the SHHF/Mcc-FA rat heart. J Mol Cell Cardiol. 1998;30:235–241. doi: 10.1006/jmcc.1997.0587. [DOI] [PubMed] [Google Scholar]
- 24.Tian R, Ingwall JS. The molecular energetics of the failing heart from animal models - small animal models. Heart Fail Rev. 1999;4:245–253. [Google Scholar]
- 25.O'Donnell JM, Alpert NM, White LT, Lewandowski ED. Coupling of mitochondrial fatty acid uptake to oxidative flux in the intact heart. Biophysical J. 2002;82:11–18. doi: 10.1016/S0006-3495(02)75369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 27.van Bilsen M, Smeets PJH, Gilde AJ, van der Vusse GJ. Metabolic remodeling of the failing heart: the cardiac burn-out syndrome? Cardiovasc Res. 2004;61:218–226. doi: 10.1016/j.cardiores.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 28.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation. 1996;94(11):2837–42. doi: 10.1161/01.cir.94.11.2837. [DOI] [PubMed] [Google Scholar]
- 29.Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, et al. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation. 2002;106:606–612. doi: 10.1161/01.cir.0000023531.22727.c1. [DOI] [PubMed] [Google Scholar]
- 30.el Alaoui-Talibi Z, Landormv S, Loireau A, Maravec J. Fatty acid oxidation and mechanical performance of volume-overloaded rat hearts. Am J Physiol. 1992;262(4):H1068–H1074. doi: 10.1152/ajpheart.1992.262.4.H1068. [DOI] [PubMed] [Google Scholar]
- 31.Sambandam N, Lopaschuk GD, Brownsey RW, Allard MF. Energy metabolism in the hypertrophied heart. Heart Fail Rev. 2002;7:161–173. doi: 10.1023/a:1015380609464. [DOI] [PubMed] [Google Scholar]
- 32.Chandler MP, Kerner J, Huang H, Vazquez E, Reszko A, Martini WZ, et al. Moderate severity heart failure does not involve a downregulation of myocardial fatty acid oxidation. Am J Physiol Heart Circ Physiol. 2004;287:H1538–H1543. doi: 10.1152/ajpheart.00281.2004. [DOI] [PubMed] [Google Scholar]
- 33.Goodwin GW, Taylor CS, Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem. 1998;273(45):29530–29539. doi: 10.1074/jbc.273.45.29530. [DOI] [PubMed] [Google Scholar]
- 34.Crass MF. Heart triglyceride and glycogen metabolism: effects of catecholamines, dibutyryl cyclic AMP, theophylline, and fatty acids. Recent Adv Stud Cardiac Struct Metab. 1973;3:275–290. [PubMed] [Google Scholar]
- 35.Goodwin GW, Taegtmeyer H. Improved energy homeostasis of the heart in the metabolic state of exercise. Am J Physiol. Heart Circ Physiol. 2000;279(4):H1490–1501. doi: 10.1152/ajpheart.2000.279.4.H1490. [DOI] [PubMed] [Google Scholar]
- 36.Swanton EM, Saggerson ED. Effects of adrenaline on triacylglycerol synthesis and turnover in ventricular myocytes from adult rats. Biochem J. 1997;328(3):913–922. doi: 10.1042/bj3280913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajri T, Ibrahimi A, Coburn CT, Knapp FF, Kurtz T, Pravenec M, Abumrad NA. Defective fatty acid uptake in the spontaneously hypertensive rat is a primary determinant of altered glucose metabolism, hyperinsulinemia, and myocardial hypertrophy. J Biol Chem. 2001;276(26):23661–23666. doi: 10.1074/jbc.M100942200. [DOI] [PubMed] [Google Scholar]
- 38.Okere IC, Chess DJ, McElfresh TA, Johnson J, Rennison J, Ernsberger P, Hoit BD, et al. High-fat diet prevents cardiac hypertrophy and improves contractile function in the hypertensive dahl salt-sensitive rat. Clin Exper Pharmacol Physiol. 2005;32:825–831. doi: 10.1111/j.1440-1681.2005.04272.x. [DOI] [PubMed] [Google Scholar]
- 39.Yonekura Y, Brill AB, Som P, Yamamoto K, Srivastava SC, Iwai J, Elmaleh DR, Livni E, Strauss HW, Goodman MM, et al. Regional myocardial substrate uptake in hypertensive rats: a quantitative autoradiographic measurement. Science. 1985;227(4693):1494–1496. doi: 10.1126/science.3975623. [DOI] [PubMed] [Google Scholar]
- 40.Augustus AS, Buchanan J, Park TS, Hirata K, Noh HL, Sun J, et al. Loss of lipoprotein lipase-derived fatty acids leads to increased cardiac glucose metabolism and heart dysfunction. J Biol Chem. 2006;281(13):87168723. doi: 10.1074/jbc.M509890200. [DOI] [PubMed] [Google Scholar]
- 41.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]